Submitted:
11 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1. Material & Methods
1.1. Participants, Study Design, and Variables
1.1. Daily Intake of Food and Polyamines
1.1. Determination of Polyamines in Feces via High Performance Liquid Chromatography (HPLC)
1.1. Statistical Analyses
1. Results
1.1. Study Participants and Types of Colorectal Lesion
1.1. Association of Nutritional Survey Data and the Presence of Colorectal Lesions
1.1. Association of Dietary Intake of Polyamines with Colorectal Lesions in Patients
1.1. Association of Biogenic Amines in Feces with Colorectal Lesions in Patients
1.1. Correlation between Dietary Intake of Polyamines and Their Fecal Content in Relation to Colorectal Lesion Types
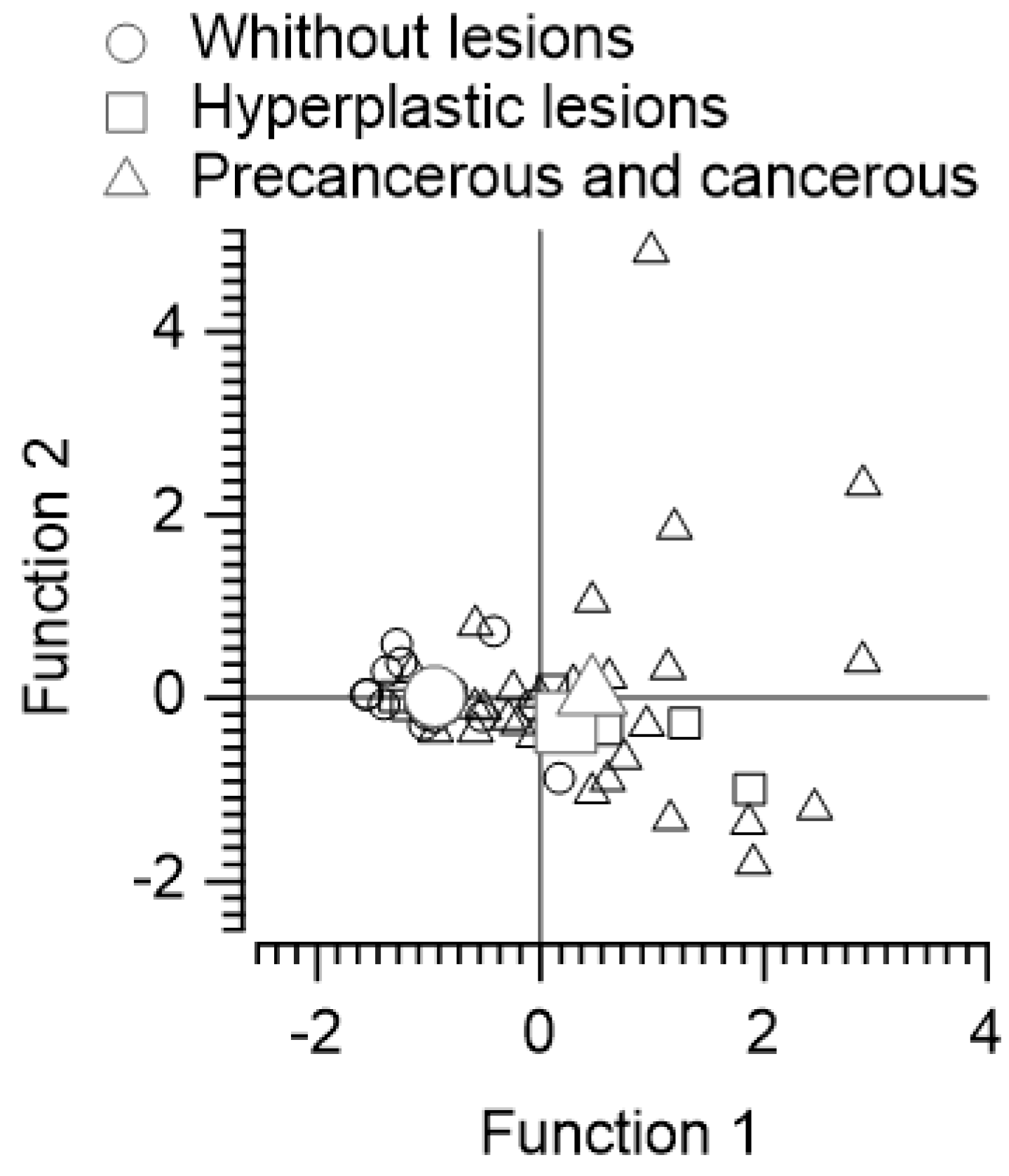
1.1. Discriminant Function Analysis of Subjects without Lesions, with Benign Lesions, and with Precancerous or Malignant Lesions Based on Polyamine Intake and Fecal Content of N-acetyl Putrescine and Cadaverine
1.1. Lineal Regression Analysis on Fecal Polyamines as Dependent Variables
1.1. Logistic Regression Analysis to Predict the Presence of Colorectal Lesions
1. Discussion
Acknowledgments and Fundings
Author Contributions
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Beaumont, M.; Andriamihaja, M.; Davila, A.M.; Lan, A.; Grauso, M.; Armand, L.; Benamouzig, R.; Tomé, D. Changes in the Luminal Environment of the Colonic Epithelial Cells and Physiopathological Consequences. The American journal of pathology 2017, 187, 476–486. [Google Scholar] [CrossRef]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Advances in nutrition (Bethesda, Md.) 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Cantabrana, B.; Peña-Iglesias, P.; Castro-Estrada, P.; Suarez, L.; Bordallo, J.; Barreiro-Alonso, E.; Sánchez, M. Dietary intake of polyamines in the Spanish adult population showed a direct correlation with the healthy dietary index score and inverse with the dietary inflammatory index score. unpublished.
- Liu, B.; Jiang, X.; Cai, L.; Zhao, X.; Dai, Z.; Wu, G.; Li, X. Putrescine mitigates intestinal atrophy through suppressing inflammatory response in weanling piglets. Journal of animal science and biotechnology 2019, 10, 69. [Google Scholar] [CrossRef]
- Sánchez, M.; Suárez, L.; Andrés, M.T.; Flórez, B.H.; Bordallo, J.; Riestra, S.; Cantabrana, B. Modulatory effect of intestinal polyamines and trace amines on the spontaneous phasic contractions of the isolated ileum and colon rings of mice. Food & nutrition research 2017, 61, 1321948. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Hu, S.; Wang, X. Eflornithine for chemoprevention in the high-risk population of colorectal cancer: a systematic review and meta-analysis with trial sequential analysis. Frontiers in oncology 2023, 13, 1281844. [Google Scholar] [CrossRef]
- Kaur, H.; Das, C.; Mande, S.S. In Silico Analysis of Putrefaction Pathways in Bacteria and Its Implication in Colorectal Cancer. Frontiers in microbiology 2017, 8, 2166. [Google Scholar] [CrossRef]
- Sittipo, P.; Shim, J.W.; Lee, Y.K. Microbial Metabolites Determine Host Health and the Status of Some Diseases. International journal of molecular sciences 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Adomako-Bonsu, A.G.; Wang, M.; Li, J. Three specific gut bacteria in the occurrence and development of colorectal cancer: a concerted effort. PeerJ 2023, 11, e15777. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.H.; Agarwal, D.; Singh, J.K.; Traiki, T.B.; Pandey, M.K.; Ahmad, R.; Srivastava, S.K. Association of the microbiome with colorectal cancer development (Review). International journal of oncology 2021, 58. [Google Scholar] [CrossRef]
- Lichtenstern, C.R.; Lamichhane-Khadka, R. A tale of two bacteria – Bacteroides fragilis, Escherichia coli, and colorectal cancer. Frontiers in Bacteriology 2023, 2. [Google Scholar] [CrossRef]
- Hanus, M.; Parada-Venegas, D.; Landskron, G.; Wielandt, A.M.; Hurtado, C.; Alvarez, K.; Hermoso, M.A.; López-Köstner, F.; De la Fuente, M. Immune System, Microbiota, and Microbial Metabolites: The Unresolved Triad in Colorectal Cancer Microenvironment. Frontiers in immunology 2021, 12, 612826. [Google Scholar] [CrossRef]
- Ye, C.; Liu, X.; Liu, Z.; Pan, C.; Zhang, X.; Zhao, Z.; Sun, H. Fusobacterium nucleatum in tumors: from tumorigenesis to tumor metastasis and tumor resistance. Cancer biology & therapy 2024, 25, 2306676. [Google Scholar] [CrossRef]
- Doyle, D.J.; Hendrix, J.M.; Garmon, E.H. American Society of Anesthesiologists Classification [Updated 2023 Aug 17]. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan: 2024.
- Kristal, A.R.; Kolar, A.S.; Fisher, J.L.; Plascak, J.J.; Stumbo, P.J.; Weiss, R.; Paskett, E.D. Evaluation of web-based, self-administered, graphical food frequency questionnaire. Journal of the Academy of Nutrition and Dietetics 2014, 114, 613–621. [Google Scholar] [CrossRef]
- Basiotis, P.; Carlson, A.; Gerrior, S.; Juan, W.; Lino, M. The Healthy Eating Index: 1999–2000. Washington. DC: USDA, Center for Nutrition Policy and Promotion, CNPP-12: 2002.
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E. , et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PloS one 2012, 7, e43134. [Google Scholar] [CrossRef]
- Suárez, L.; Moreno-Luque, M.; Martínez-Ardines, I.; González, N.; Campo, P.; Huerta-Cima, P.; Sánchez, M. Amine variations in faecal content in the first weeks of life of newborns in relation to breast-feeding or infant formulas. The British journal of nutrition 2019, 122, 1130–1141. [Google Scholar] [CrossRef]
- Castelló, A.; Amiano, P.; Fernández de Larrea, N.; Martín, V.; Alonso, M.H.; Castaño-Vinyals, G.; Pérez-Gómez, B.; Olmedo-Requena, R.; Guevara, M.; Fernandez-Tardon, G. , et al. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. European journal of nutrition 2019, 58, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.S.; Kirsh, V.A.; Rohan, T.E. The association of the healthy eating index with risk of colorectal cancers (overall and by subsite) among Canadians. Cancer epidemiology 2023, 87, 102454. [Google Scholar] [CrossRef]
- Nardone, O.M.; Zammarchi, I.; Santacroce, G.; Ghosh, S.; Iacucci, M. Inflammation-Driven Colorectal Cancer Associated with Colitis: From Pathogenesis to Changing Therapy. Cancers 2023, 15. [Google Scholar] [CrossRef]
- Castelló, A.; Rodríguez-Barranco, M.; Fernández de Larrea, N.; Jakszyn, P.; Dorronsoro, A.; Amiano, P.; Chirlaque, M.D.; Colorado-Yohar, S.; Guevara, M.; Moreno-Iribas, C. , et al. Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Nutrients 2022, 14. [Google Scholar] [CrossRef]
- ter Steege, J.C.; Buurman, W.A.; Forget, P.P. Spermine induces maturation of the immature intestinal immune system in neonatal mice. Journal of pediatric gastroenterology and nutrition 1997, 25, 332–340. [Google Scholar] [CrossRef]
- van Wettere, W.H.; Willson, N.L.; Pain, S.J.; Forder, R.E. Effect of oral polyamine supplementation pre-weaning on piglet growth and intestinal characteristics. Animal: An international journal of animal bioscience 2016, 10, 1655–1659. [Google Scholar] [CrossRef]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-Promoting Effects of Dietary Polyamines. Medical sciences (Basel, Switzerland) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Hofer, S.J.; Pendl, T.; Bauer, M.A.; Eisenberg, T.; Carmona-Gutierrez, D.; Kroemer, G. Nutritional Aspects of Spermidine. Annual review of nutrition 2020, 40, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.J.; Ashbeck, E.L.; Wertheim, B.C.; Wallace, R.B.; Neuhouser, M.L.; Thomson, C.A.; Thompson, P.A. Dietary polyamine intake and colorectal cancer risk in postmenopausal women. The American journal of clinical nutrition 2015, 102, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.J.; Wertheim, B.C.; Gerner, E.W.; Thomson, C.A.; Rock, C.L.; Thompson, P.A. Dietary polyamine intake and risk of colorectal adenomatous polyps. The American journal of clinical nutrition 2012, 96, 133–141. [Google Scholar] [CrossRef]
- Huang, C.Y.; Fang, Y.J.; Abulimiti, A.; Yang, X.; Li, L.; Liu, K.Y.; Zhang, X.; Feng, X.L.; Chen, Y.M.; Zhang, C.X. Dietary Polyamines Intake and Risk of Colorectal Cancer: A Case-Control Study. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Gobert, A.P.; Latour, Y.L.; Asim, M.; Barry, D.P.; Allaman, M.M.; Finley, J.L.; Smith, T.M.; McNamara, K.M.; Singh, K.; Sierra, J.C. , et al. Protective Role of Spermidine in Colitis and Colon Carcinogenesis. Gastroenterology 2022, 162, 813–827. [Google Scholar] [CrossRef]
- Bekebrede, A.F.; Keijer, J.; Gerrits, W.J.J.; Boer, V.C.J. The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Milovic, V. Polyamines in the gut lumen: bioavailability and biodistribution. European journal of gastroenterology & hepatology 2001, 13, 1021–1025. [Google Scholar] [CrossRef]
- Pugin, B.; Barcik, W.; Westermann, P.; Heider, A.; Wawrzyniak, M.; Hellings, P.; Akdis, C.A.; O'Mahony, L. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microbial Ecology in Health and Disease 2017, 28, 1353881. [Google Scholar] [CrossRef]
- Pugin, B.; Barcik, W.; Westermann, P.; Heider, A.; Wawrzyniak, M.; Hellings, P.; Akdis, C.A.; O’Mahony, L. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microbial Ecology in Health and Disease 2017, 28, 1353881. [Google Scholar] [CrossRef]
- Yang, Y.; Misra, B.B.; Liang, L.; Bi, D.; Weng, W.; Wu, W.; Cai, S.; Qin, H.; Goel, A.; Li, X. , et al. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics 2019, 9, 4101–4114. [Google Scholar] [CrossRef]
- Coradduzza, D.; Arru, C.; Culeddu, N.; Congiargiu, A.; Azara, E.G.; Scanu, A.M.; Zinellu, A.; Muroni, M.R.; Rallo, V.; Medici, S. , et al. Quantitative Metabolomics to Explore the Role of Plasma Polyamines in Colorectal Cancer. International journal of molecular sciences 2022, 24. [Google Scholar] [CrossRef]
- Venäläinen, M.K.; Roine, A.N.; Häkkinen, M.R.; Vepsäläinen, J.J.; Kumpulainen, P.S.; Kiviniemi, M.S.; Lehtimäki, T.; Oksala, N.K.; Rantanen, T.K. Altered Polyamine Profiles in Colorectal Cancer. Anticancer research 2018, 38, 3601–3607. [Google Scholar] [CrossRef]
- Kuwabara, H.; Katsumata, K.; Iwabuchi, A.; Udo, R.; Tago, T.; Kasahara, K.; Mazaki, J.; Enomoto, M.; Ishizaki, T.; Soya, R. , et al. Salivary metabolomics with machine learning for colorectal cancer detection. Cancer science 2022, 113, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacological reviews 2018, 70, 549–620. [Google Scholar] [CrossRef] [PubMed]
| Number of colorectal lesions | Number of patients | Percentage |
|---|---|---|
| 1 | 14 | 34.15 |
| 2 | 11 | 26.83 |
| 3-5 | 11 | 26.83 |
| 6-10 | 5 | 12.20 |
| Location of colorectal lesions | Number of lesions | Percentage |
| Ascending colon | 20 | 18.87 |
| Transverse colon | 11 | 10.38 |
| Descending colon | 9 | 8.49 |
| Sigmoid colon | 47 | 44.34 |
| Rectum | 19 | 17.92 |
| Anatomical pathology classification | Number of lesions | Percentage |
| Hyperplastic lesions | 23 | 21.70 |
| Tubular adenomas | 69 | 65.09 |
| Tubulovillous adenomas | 11 | 10.38 |
| Adenocarcinomas | 3 | 2.83 |
| Variable | Without colorectal lesions | With colorectal lesions | Hyperplastic lesions | Precancerous and adenocarcinomas |
|---|---|---|---|---|
| Characteristics of participants and of diets | ||||
| Age (years) | 58.42 (52.23 - 65.41) | 61.25 (56.24 - 64.93) | 58.73 (54.86 - 61.16) | 62.17 (56.44 - 65.22) |
| Calories (kcal/day) | 1973.83 (1642.46 - 2162.3) | 2120.13 (1909.81 - 2618.88) | 2918.25 (1923.8 - 3070.51) | 2108.4 (1907.34 - 2470.14) |
| BMI (kg/m²) | 26.45 (22.78 - 34.23) | 27 (25 - 29.7) | 24.85 (24.05 - 34.05) | 27.1 (25.4 - 29.4) |
| HEI Score | 75.8 (67.05 - 80.23) | 68.4 (61.85 - 79.65) | 65.7 (56.25 - 79.4) | 72.1 (61.9 - 80) |
| DII Score | -2.09 (-3.4 - -0.44) | -1.32 (-3.01 - 0.59) | -1.1 (-3.48 - 1.15) | -1.32 (-2.9 - -0.19) |
| Components of the diet per day | ||||
| Alcohol Calories (kcal) | 26.82 (13.84 - 39.71) | 105.92 (20.13 - 260.43)* | 228.7 (9.62 - 362.92) | 105.02 (20.76 - 253.83) |
| Alcohol Servings | 0.2 (0.11 - 0.28) | 0.85 (0.17 - 2.19)** | 1.93 (0.1 - 2.84) | 0.73 (0.18 - 2.16)ɸ |
| Cholesterol (mg) | 211.22 (150.75 - 303.79) | 296.86 (183.75 - 341.12) | 365.47 (288.64 - 453.69)ɸ | 265.89 (167.32 - 337.76) |
| Number of citrus, melon, berry cup equivalents (cups) | 0.56 (0.18 - 0.64) | 0.77 (0.3 - 1.36)* | 0.93 (0.3 - 2.69) | 0.77 (0.27 - 1.3) |
| Fish Servings | 1.92 (0.87 - 2.94) | 2.21 (1.31 - 3.43) | 4.64 (2.19 - 7.79) | 1.7 (1.16 - 3.08) |
| Fructose (g) | 18.22 (14.97 - 22.41) | 22.36 (17.74 - 28.15) | 27.94 (23.39 - 34.54)ɸ | 20.68 (17.09 - 25.75) |
| Galactose (g) | 0.26 (0.13 - 0.47) | 0.48 (0.21 - 1.96)* | 1.22 (0.23 - 2.12) | 0.42 (0.21 - 1.95) |
| Glucose (g) | 16.71 (15.12 - 18.49) | 21.17 (16.09 - 27.94)* | 28.64 (23.74 - 32.92)ɸ ɸ | 20.44 (15.26 - 24.7) |
| Inositol (g) | 0.42 (0.33 - 0.52) | 0.56 (0.4 - 0.85)* | 0.65 (0.39 - 1.33) | 0.56 (0.4 - 0.83) |
| Lignan Secoisolariciresinol | 65.21 (54.38 - 102.47) | 103.97 (65.44 - 139.35)* | 132.23 (90.22 - 151.01) | 91.99 (59.79 - 139.95) |
| Low Fat Dairy Servings | 1.62 (0.49 - 2.41) | 0.53 (0.06 - 1.5)* | 0.64 (0.2 - 0.98) | 0.45 (0.05 - 1.5) |
| Eggs (equivalents to 28.3 g of lean meat) | 0.38 (0.29 - 0.63) | 0.63 (0.41 - 1.04)* | 1.05 (0.52 - 1.11) | 0.6 (0.41 - 0.86) |
| Cooked lean meat from fish, other seafood low in Omega-3 (28.3 g) | 0.78 (0.31 - 1.06) | 0.84 (0.42 - 1.21) | 1.6 (0.94 - 2.62)¥ | 0.63 (0.35 - 1.08) |
| Cooked lean meat from meat, poultry, fish (28.3 g) | 3.36 (2.15 - 5.47) | 3.38 (2.47 - 4.88) | 5.57 (4.27 - 6.98)¥ | 3 (2.38 - 4.3) |
| MUFA 16:1 (palmitoleic acid)(g) | 0.86 (0.54 - 1.07) | 0.98 (0.75 - 1.23) | 1.36 (0.97 - 1.57)ɸ | 0.94 (0.72 - 1.2) |
| MUFA 20:1 (gadoleic acid)(g) | 0.2 (0.16 - 0.28) | 0.22 (0.18 - 0.32) | 0.35 (0.25 - 0.49)ɸ | 0.21 (0.17 - 0.27) |
| Niacin equivalents (mg) | 39.02 (29.09 - 51.83) | 39.14 (33.86 - 47.22) | 51.12 (43.27 - 57.46)¥ | 38.08 (33.29 - 45.98) |
| Non-Fried Fish Servings | 1.31 (0.59 - 2.58) | 2.08 (0.99 - 3.32) | 4.35 (2 - 6.46)ɸ | 1.7 (0.87 - 2.81) |
| PUFA 18:3 n-6 (g) | 0.02 (0.01 - 0.02) | 0.02 (0.01 - 0.03) | 0.03 (0.02 - 0.04)¥ | 0.01 (0.01 - 0.02) |
| PUFA 20:4 (arachidonic acid)(g) | 0.11 (0.06 - 0.14) | 0.12 (0.09 - 0.16) | 0.17 (0.14 - 0.21)ɸ | 0.11 (0.08 - 0.16) |
| SFA 17:0 (margaric acid)(g) | 0.08 (0.06 - 0.12) | 0.1 (0.07 - 0.14) | 0.16 (0.1 - 0.21)ɸ | 0.1 (0.06 - 0.12) |
| SFA 4:0 (butyric acid)(g) | 0.28 (0.12 - 0.43) | 0.4 (0.23 - 0.74) | 0.66 (0.33 - 0.99)ɸ | 0.37 (0.19 - 0.69) |
| Variable | Without colorectal lesions | With colorectal lesions | Hyperplastic lesions | Precancerous and adenocarcinomas |
|---|---|---|---|---|
| Dietary intake of polyamines in mg per person and day | ||||
| Putrescine | 15.58 (11.11 - 20.66) | 23.81 (12.6 - 33.88)* | 27.51 (14.52 - 35.63) | 22.17 (11.88 - 34.09) |
| Spermidine | 10.93 (8.1 - 14.4) | 11.22 (8.47 - 12.5) | 11.24 (10.16 - 14.24) | 11.19 (8.05 - 12.5) |
| Spermine | 5.98 (4.2 - 11.45) | 7.35 (5.47 - 9.01) | 8.89 (7.96 - 12.91) | 7.09 (4.98 - 8.5) |
| Polyamines Total | 33.37 (24.71 - 46.66) | 42.91 (28.09 - 50.61) | 46.88 (35.56 - 58.25) | 42.19 (27.36 - 50.61) |
| Ratios of dietary intake of polyamines | ||||
| Putrescine/Spermidine Ratio | 1.46 (0.97 - 1.85) | 2.12 (1.46 - 3.06)** | 2.47 (1.18 - 3.04) | 2.01 (1.47 - 3.12)ɸ |
| Putrescine/Spermine Ratio | 1.91 (1.41 - 3.14) | 3.17 (2.13 - 4.28)* | 2.71 (1.66 - 3.47) | 3.4 (2.15 - 4.33) |
| Spermidine/Spermine Ratio | 1.56 (1.24 - 1.86) | 1.44 (1.31 - 1.81) | 1.3 (1.01 - 1.47) | 1.48 (1.35 - 1.86) |
| Dietary intake of polyamines in mg per kcal per person and day | ||||
| Putrescine | 8.25 (5.34 - 10.48) | 10.46 (5.96 - 14.95) | 9.43 (5.33 - 15.99) | 10.49 (6.13 - 14.12) |
| Spermidine | 5.76 (4.38 - 7.19) | 5.01 (3.87 - 6.01) | 4.96 (3.55 - 5.95) | 5.01 (3.9 - 6.06) |
| Spermine | 3.83 (2.22 - 5.25) | 3.29 (2.81 - 4.19) | 3.88 (2.86 - 4.84) | 3.21 (2.73 - 4.18) |
| Polyamines Total | 18.73 (12.18 - 22.65) | 18.27 (14.09 - 25.87) | 16.16 (14.77 - 26.6) | 19.06 (13.74 - 25.35) |
| Feces polyamines in nmol per mg of sample | ||||
| Putrescine | 0.55 (0.21 - 1.02) | 0.64 (0.38 - 1.95) | 0.91 (0.33 - 2.62) | 0.58 (0.35 - 1.9) |
| Spermidine | 0.61 (0.43 - 0.94) | 0.99 (0.55 - 1.43) | 1.15 (0.56 - 1.51) | 0.87 (0.53 - 1.4) |
| Spermine | 0.03 (0.01 - 0.04) | 0.03 (0.02 - 0.05) | 0.04 (0.03 - 0.07) | 0.03 (0.02 - 0.05) |
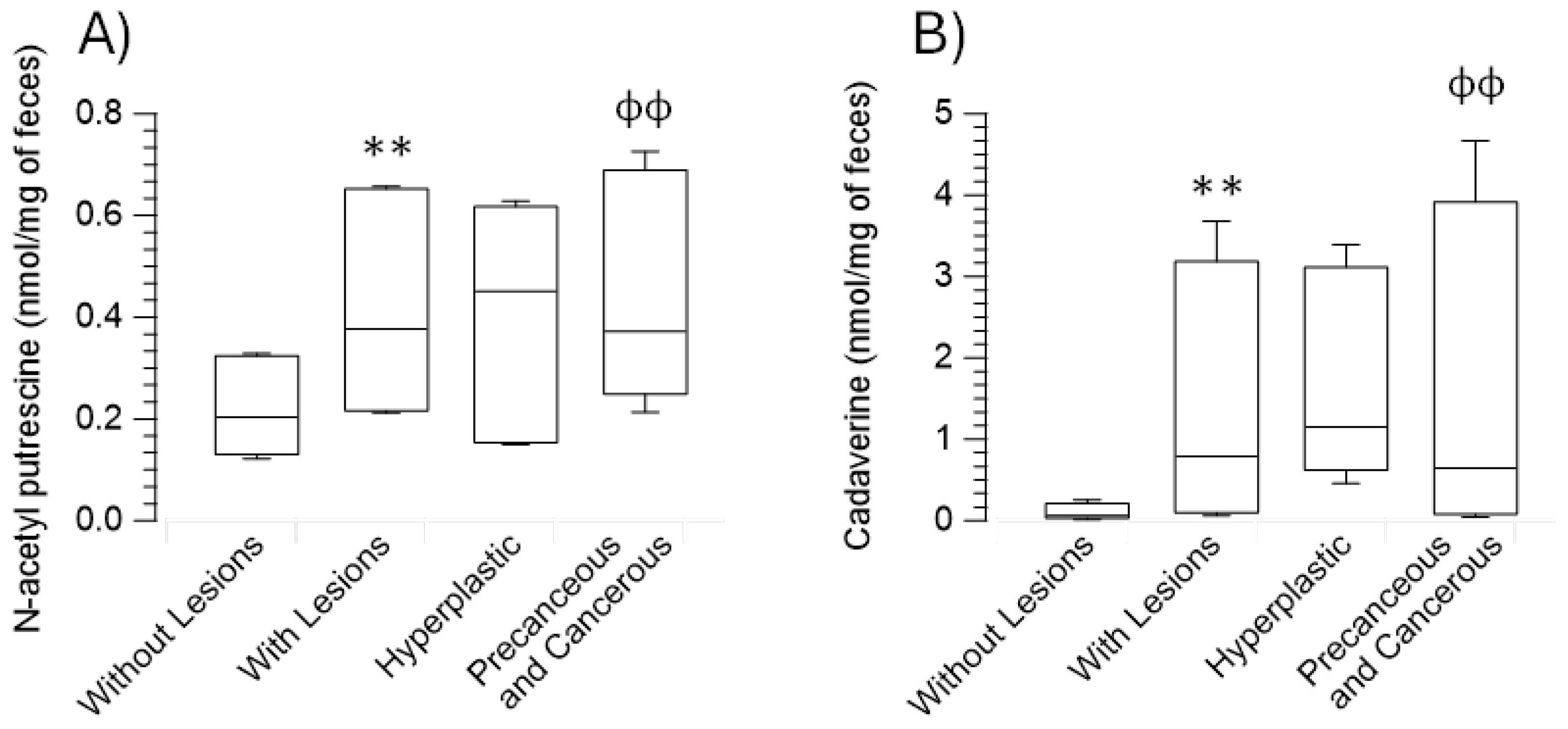
| N-Acetyl putrescine | 0.89 (0.56 - 1.39) | 1.58 (0.92 - 2.7)*** | 1.88 (0.67 - 2.59) | 1.56 (0.93 - 2.98)ɸ ɸ |
| N-Acetyl spermidine | 0.17 (0.11 - 0.22) | 0.29 (0.16 - 0.43) | 0.27 (0.14 - 0.54) | 0.29 (0.16 - 0.43) |
| Cadaverine | 0.24 (0.06 - 1.02) | 3.14 (0.25 - 14.71)*** | 4.6 (1.83 - 13.55) | 2.58 (0.18 - 18.66)ɸ ɸ |
| Tyramine | 0.08 (0.06 - 0.13) | 0.09 (0.06 - 0.16) | 0.1 (0.07 - 0.13) | 0.08 (0.06 - 0.21) |
| Isoamylamine | 2.04 (1.37 - 2.72) | 2.1 (0.38 - 2.74) | 1.51 (0.18 - 2.76) | 2.1 (0.77 - 2.81) |
| Ratios of feces content of polyamines | ||||
| Putrescine/Spermidine Ratio | 0.8 (0.36 - 1.41) | 0.93 (0.32 - 2.67) | 0.8 (0.27 - 2.65) | 1.08 (0.35 - 2.87) |
| Putrescine/Spermine Ratio | 19.56 (10.47 - 32.67) | 22.45 (7.89 - 50.18) | 23.35 (5.14 - 67.38) | 22.45 (7.92 - 55.05) |
| Spermidine/Spermine Ratio | 25.53 (17.99 - 35.76) | 22.85 (16.46 - 35.87) | 23.34 (18.64 - 31.82) | 22.85 (16.11 - 38.78) |
| Putrescine/Cadaverine Ratio | 1.77 (0.45 - 9.22) | 0.52 (0.1 - 1.47)* | 0.19 (0.07 - 2.76) | 0.67 (0.13 - 1.55) |
| Putrescine/N-Acetyl putrescine Ratio | 0.55 (0.28 - 0.85) | 0.6 (0.23 - 1.22) | 0.78 (0.14 - 1.34) | 0.53 (0.24 - 1.23) |
| N-Acetyl putrescine/Cadaverine Ratio | 2.88 (0.79 - 14.67) | 0.7 (0.21 - 5.19)** | 0.33 (0.15 - 2.59) | 0.79 (0.21 - 5.2)¥ |
| Cadaverine/Tyramine Ratio | 2.31 (0.92 - 11.23) | 20.04 (3.67 - 112.14)*** | 48.8 (27.41 - 153.41)ɸ | 14.26 (3.55 - 111.52)ɸ ɸ |
| Regression Model Variables for each Amine | B | SE B | β | p |
|---|---|---|---|---|
| N-acetyl putrescine in feces (R2 = 0.41, ANOVA p < 0.001) | ||||
| Constant | 0.579 | 0.101 | < 0.001 | |
| Whole grain servings | 0.177 | 0.030 | 0.709 | < 0.001 |
| Lignane secoisolariciresinol (mg) | -0.001 | 0.000 | -0.357 | 0.005 |
| Cadaverine in feces (R2 = 0.1, ANOVA p = 0.021) | ||||
| Constant | 10.148 | 0.403 | 0.06 | |
| Whole grain servings | 0.476 | 0.200 | 0.316 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





