Submitted:
08 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
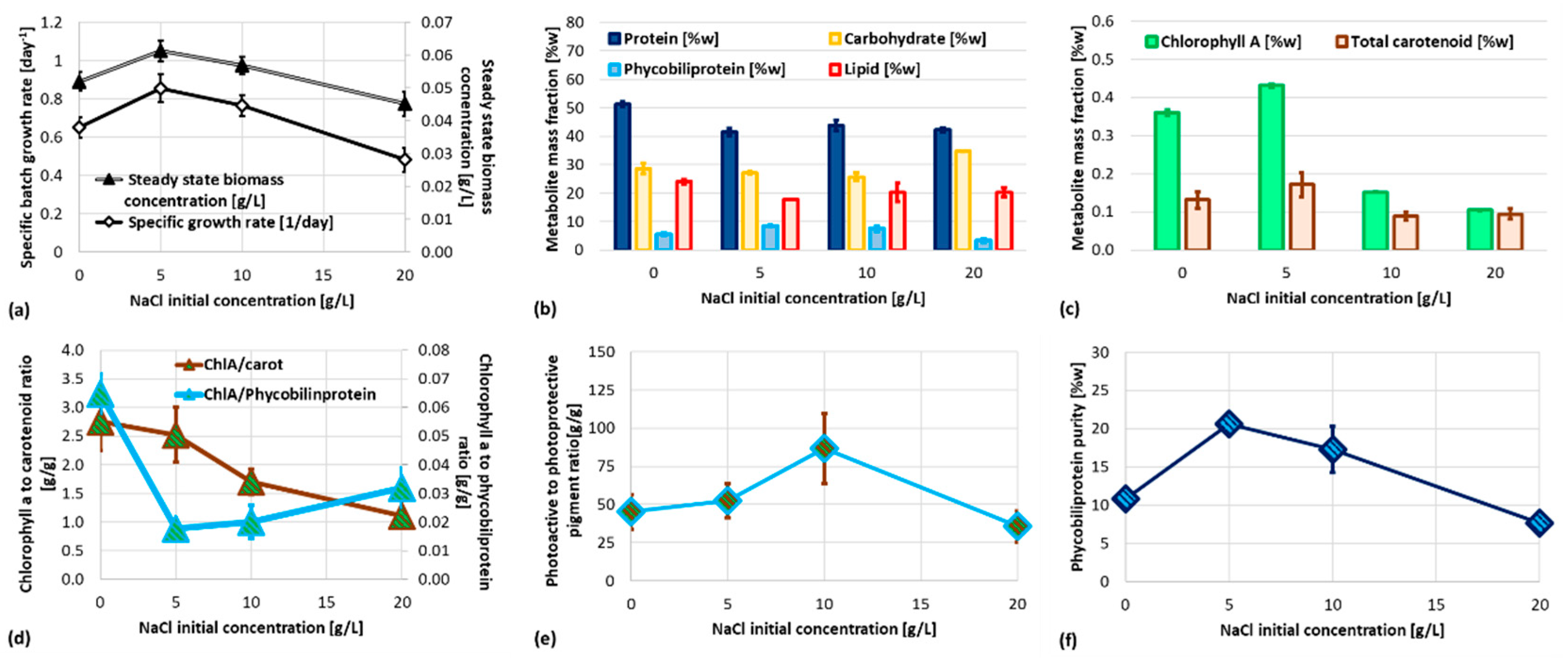
2.1. Assessment of NaCl Effect on Galdieria sulphuraria
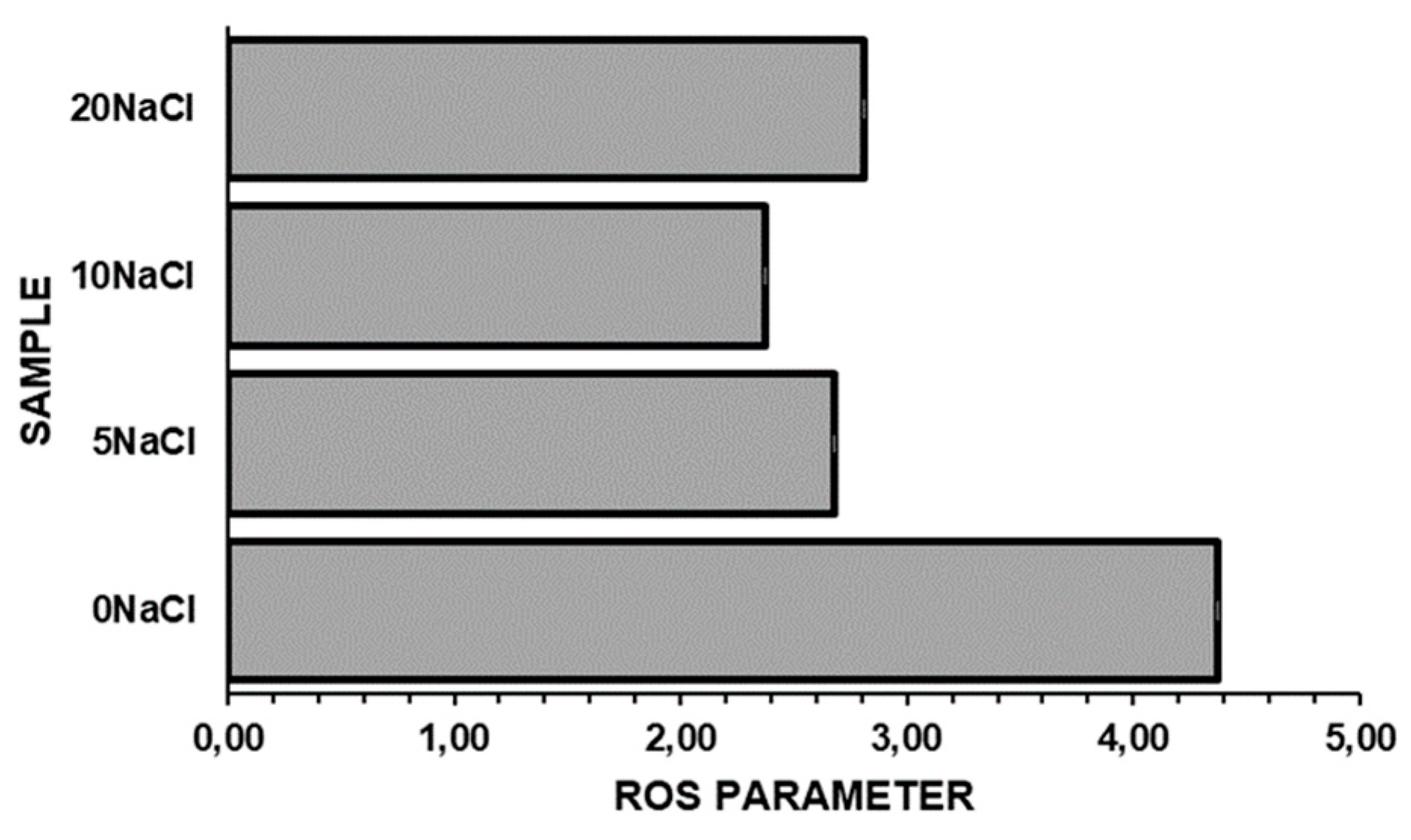
2.1.1. Growth, Metabolites Accumulation, and Intracellular Redox State
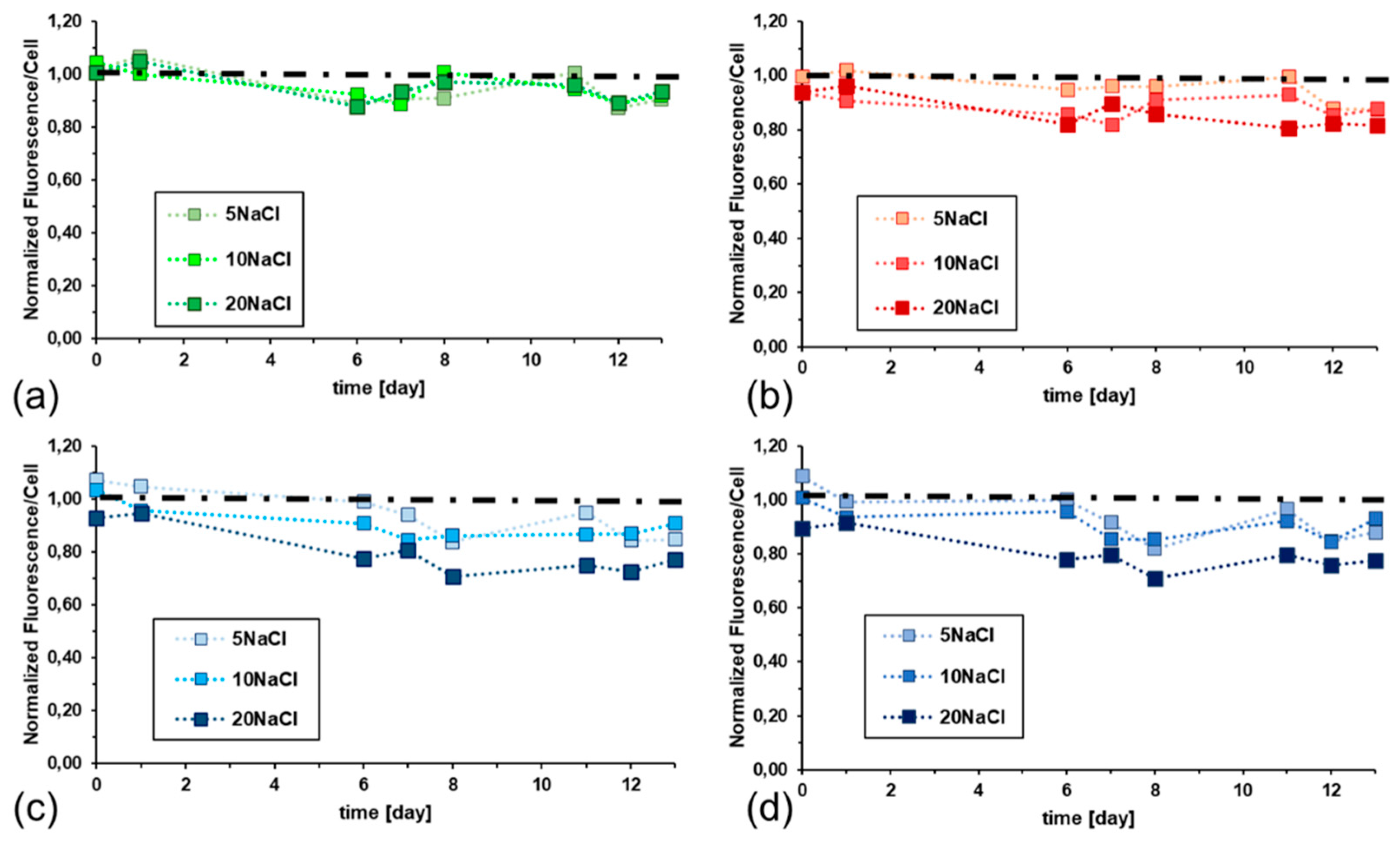
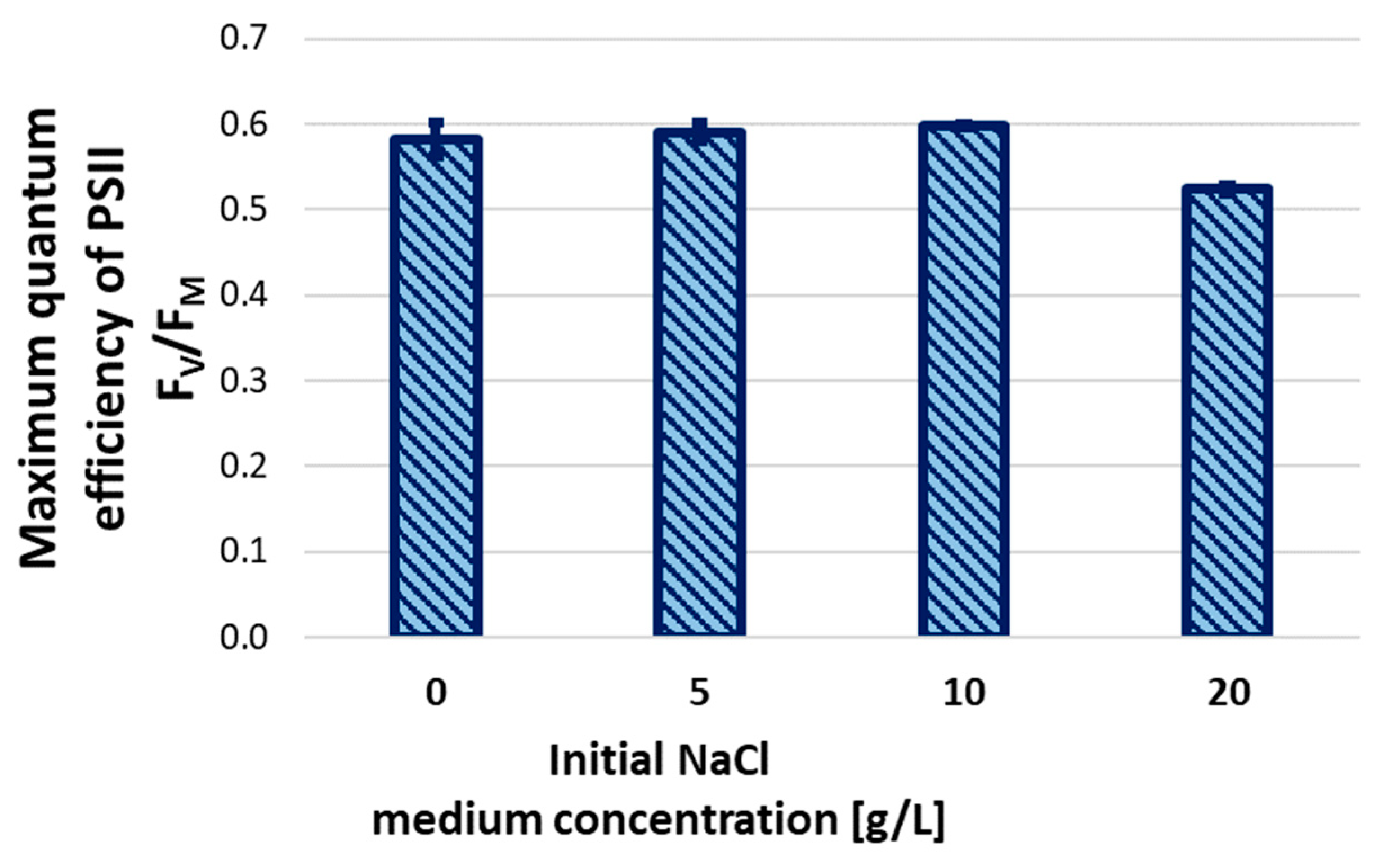
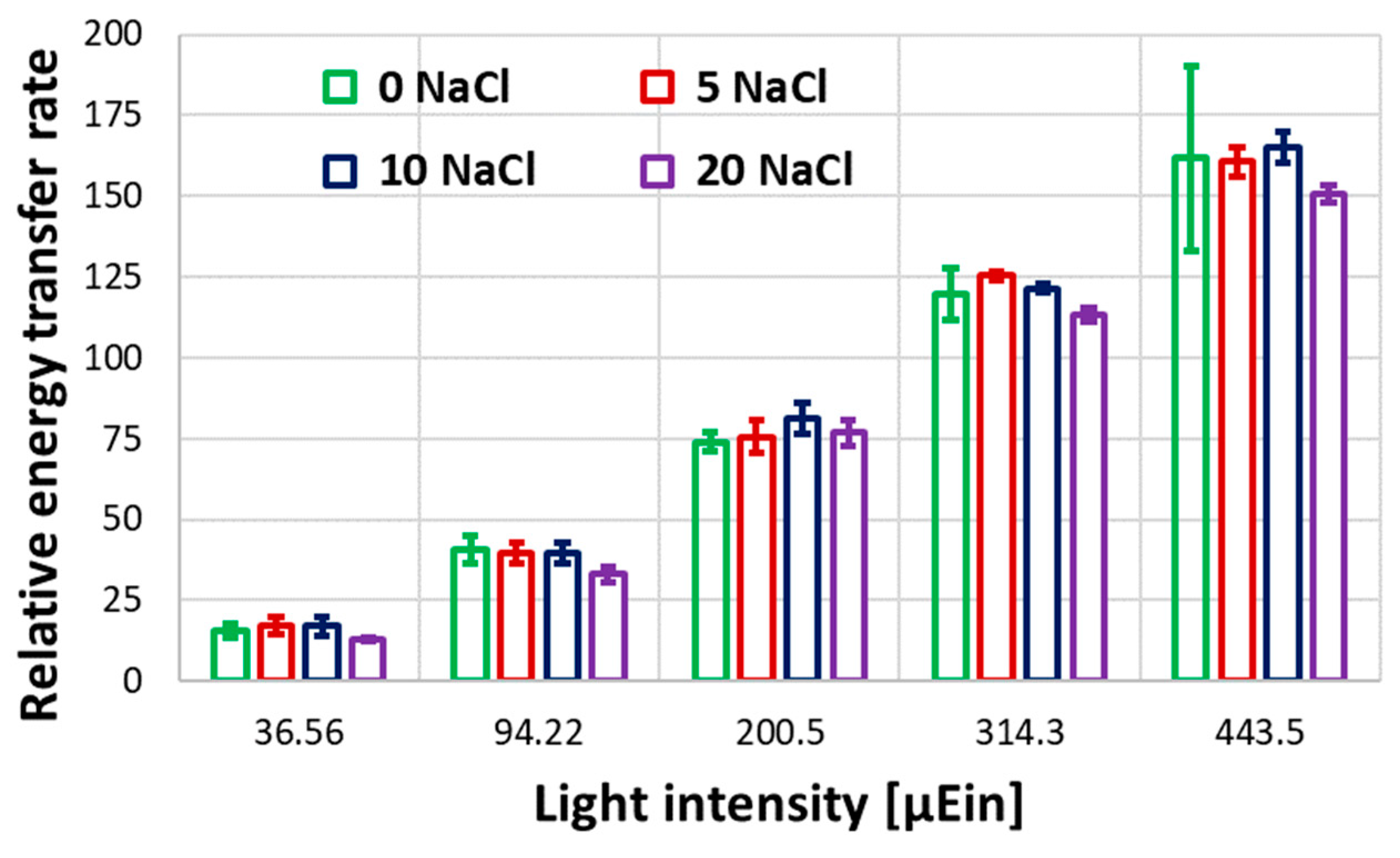
2.1.2. Dynamic of Light Harvesting System and Photosystem Efficiency
2.2. Artificial Formation Water (AFW) Cultivation Test
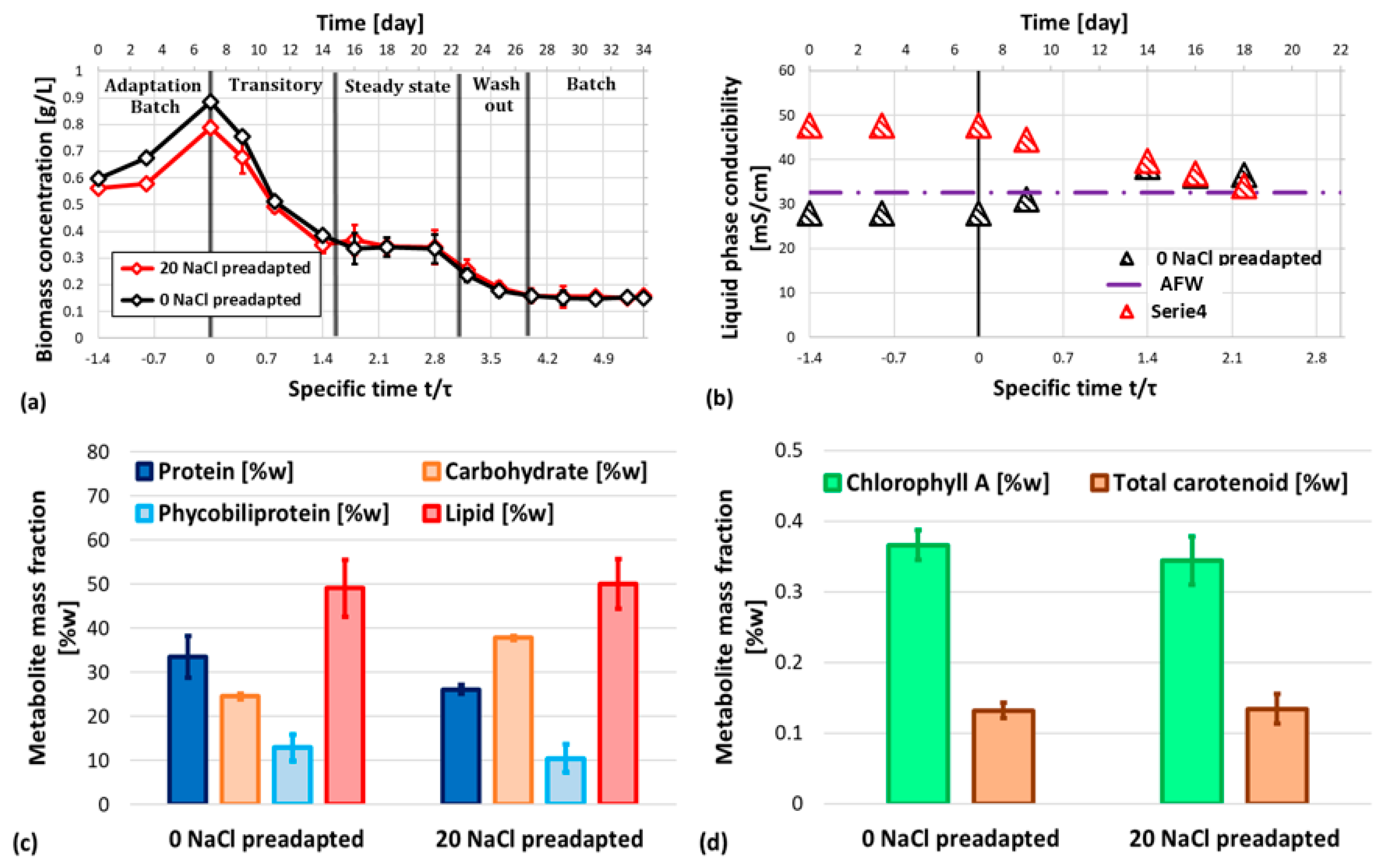
2.2.1. Dynamics of Cell Growth and Accumulation of Primary Metabolites and Pigments
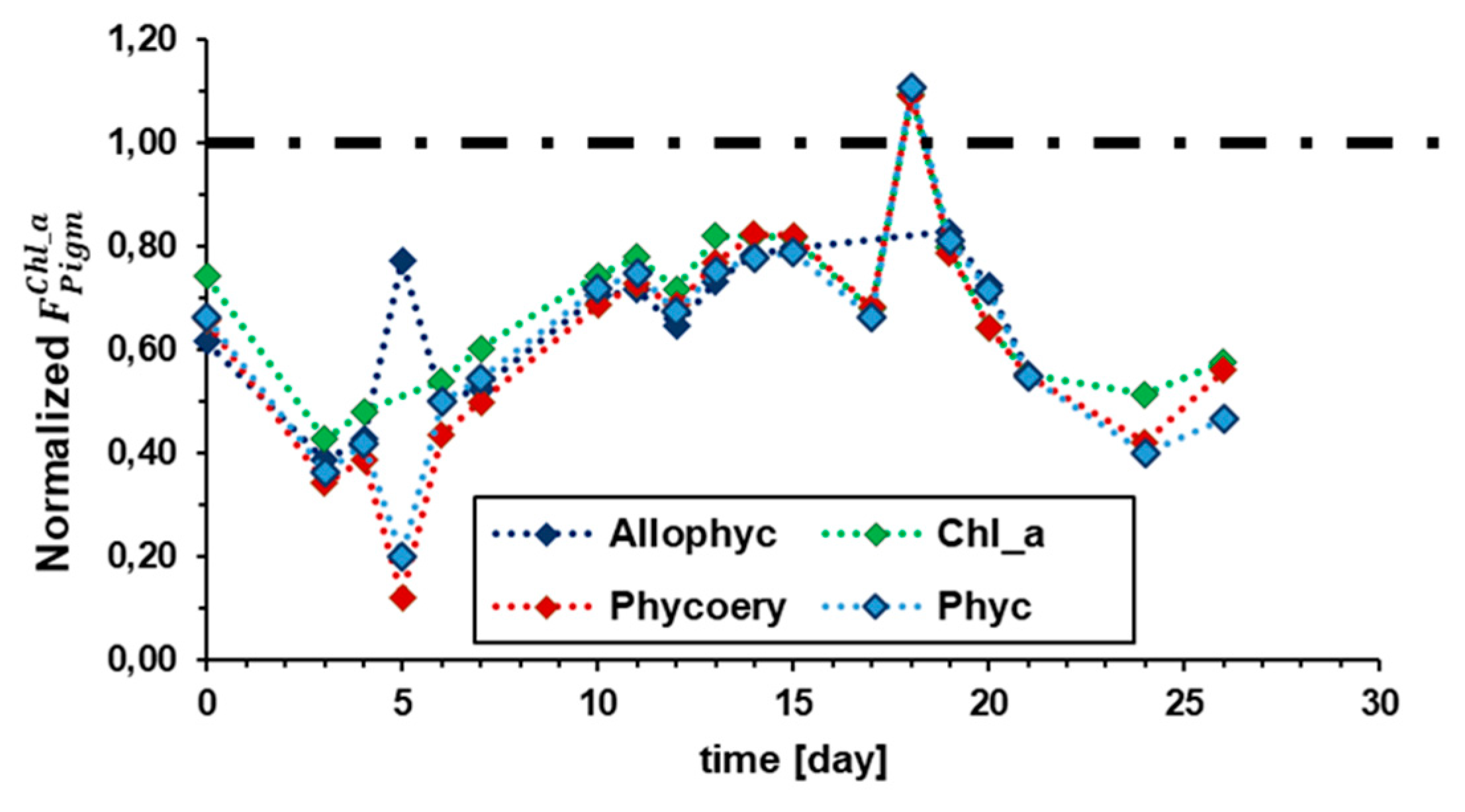
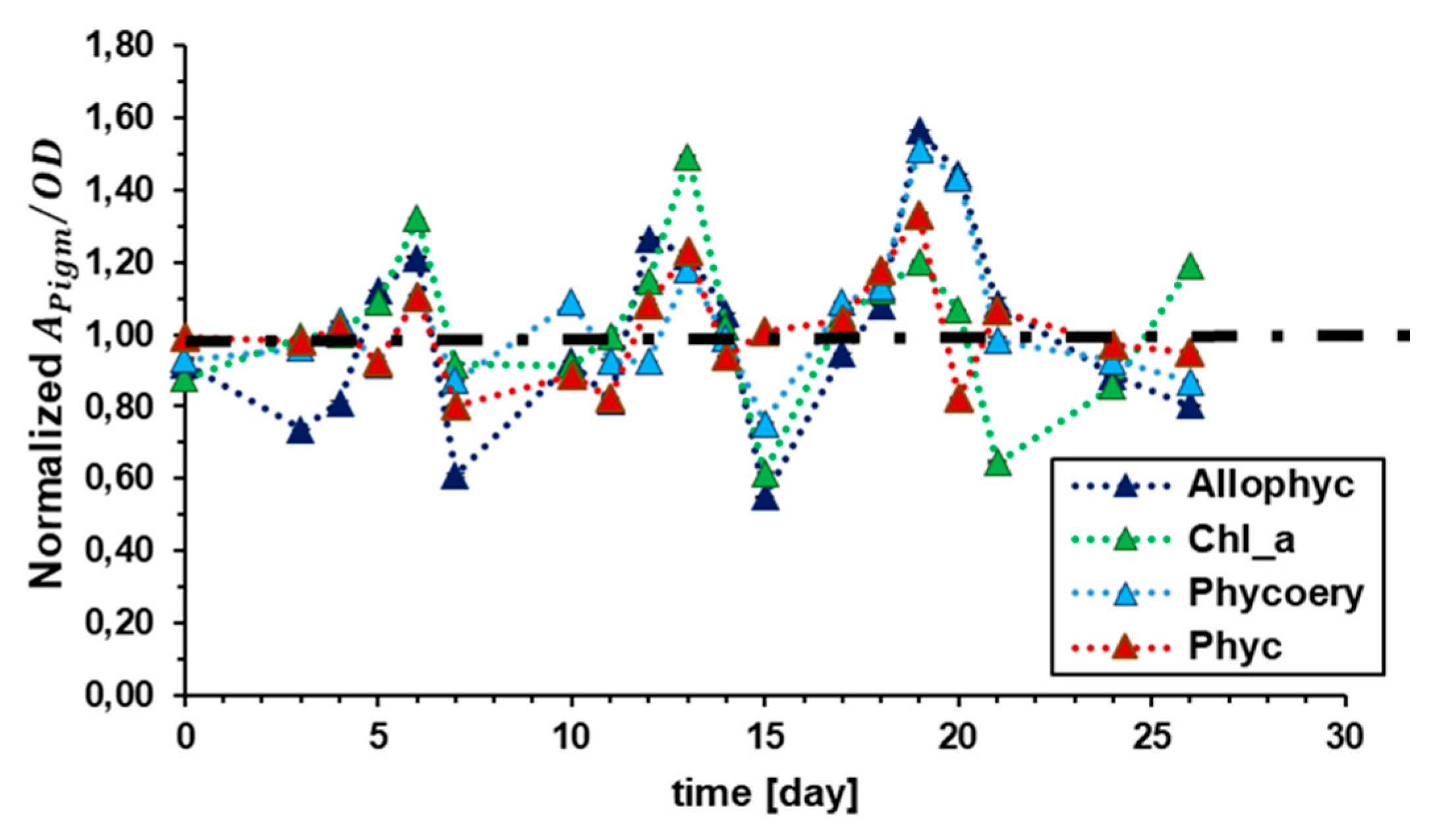
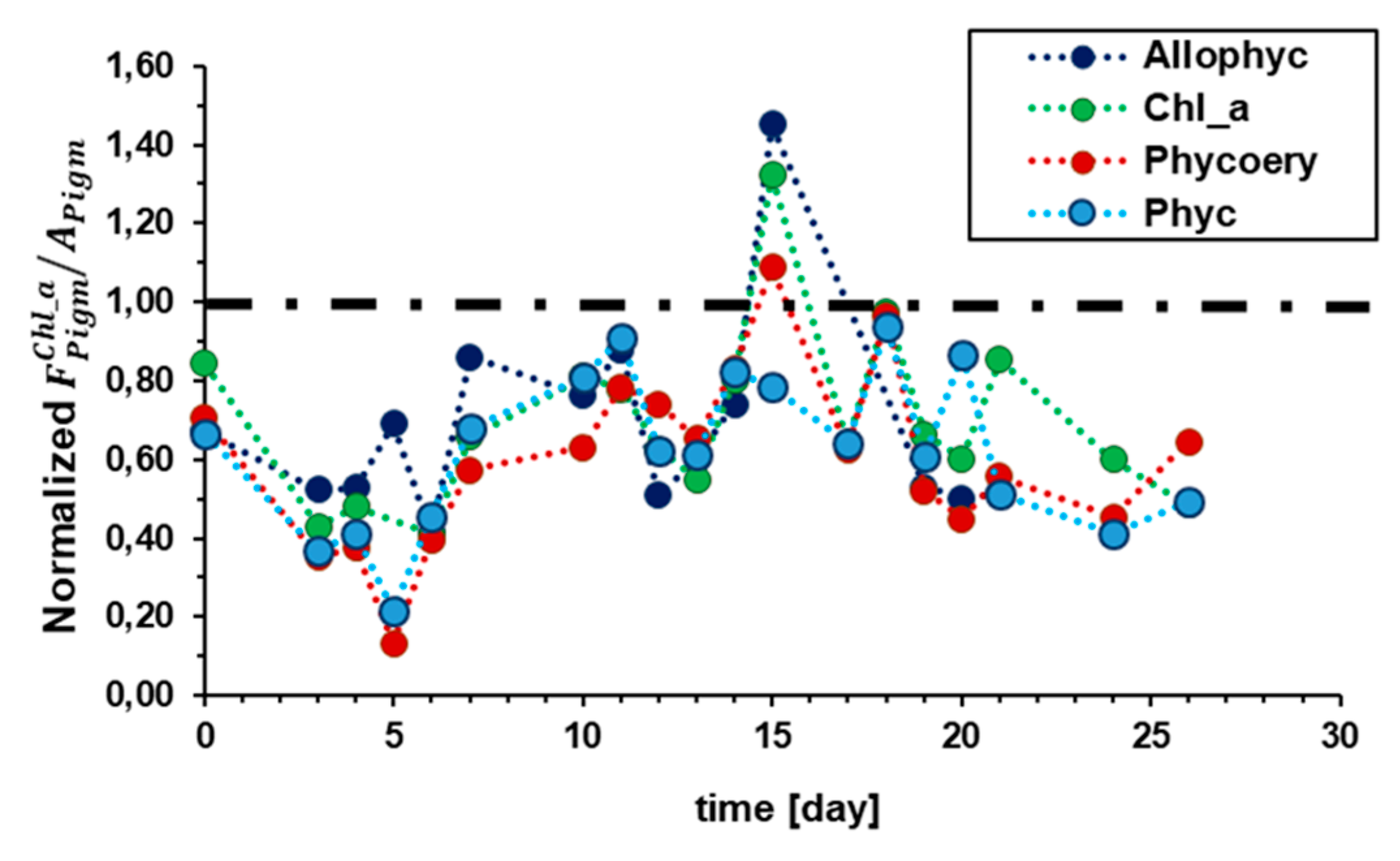
2.2.2. Dynamic of Light Harvesting System: Artificial Formation Water Test
3. Discussion
4. Materials and Methods
4.1. Assessment of NaCl Effect on Galdieria sulphuraria Growth
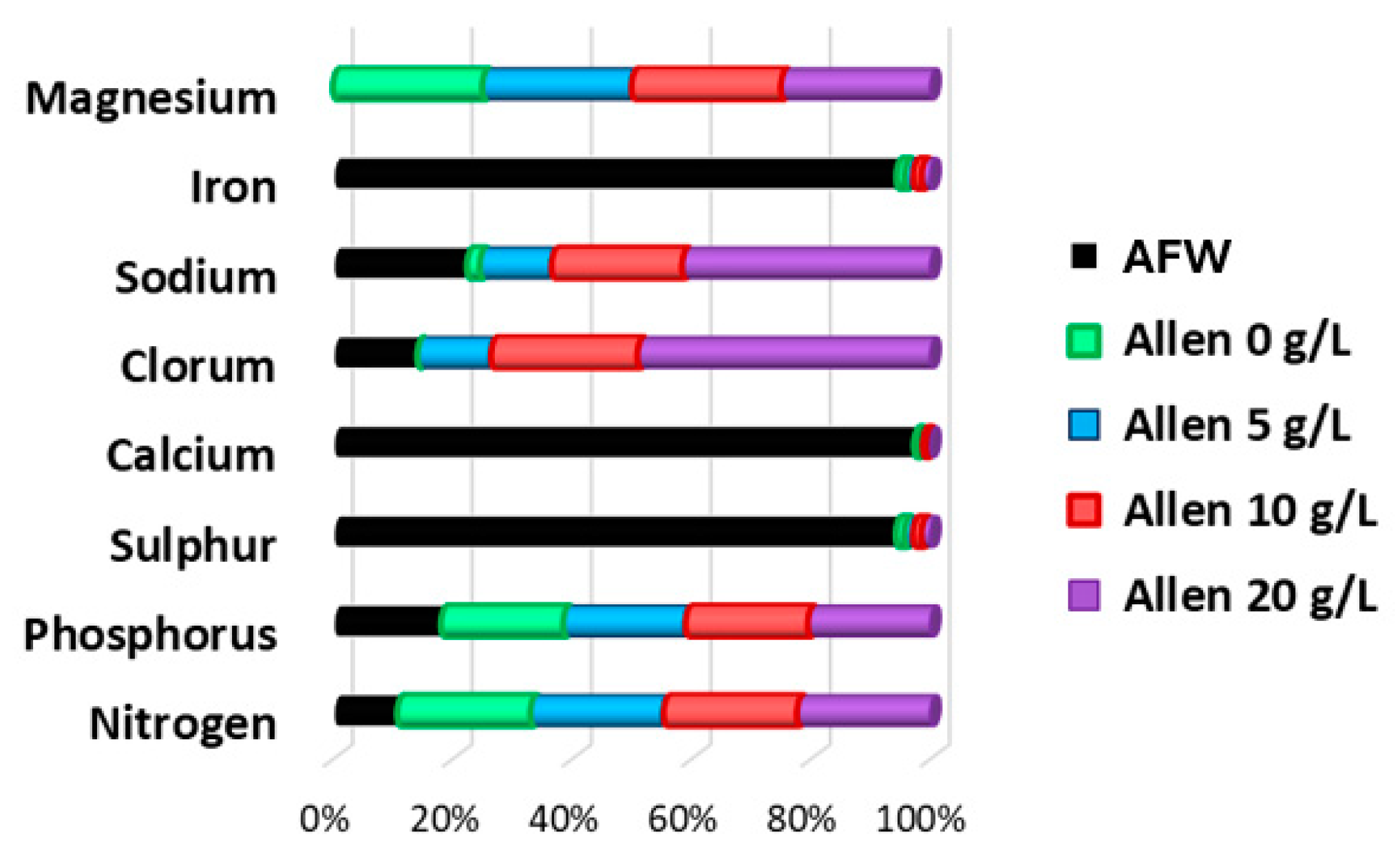
4.1.1. Culture Media
| Chemical species | Amount [mg/Kg] |
| NH4+ | 154 |
| NO3- | 7,48 |
| NO2- | <1,2 |
| SO42- | 652 |
| SO32- | 16 |
| S2- | 94 |
| PO42- | 18 |
| Ca2+ | 969 |
| Cl- | 6990 |
| Na+ | 4480 |
| Fe | 1,34 |
| F- | 6,44 |
4.1.2. Cultivation Conditions
4.1.3. Cultivation Monitoring
4.1.4. Intracellular Redox Equilibrium
4.1.5. Effect of Salinity on Dynamic of Light Harvesting System
4.1.6. Error Propagation and Representation
4.1.7. PAM Fluorometry Assay
4.1.8. Proximate Analysis of Biomass
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- M. Frenkel-Pinter, V. Rajaei, J. B. Glass, N. V. Hud, and L. D. Williams, “Water and Life: The Medium is the Message,” J. Mol. Evol., vol. 89, no. 1–2, pp. 2–11, 2021. [CrossRef]
- C. J. Vorosmarty and D. Sahagian, “Anthropogenic disturbance of the terrestrial water cycle,” Bioscience, vol. 50, no. 9, pp. 753–765, 2000. [CrossRef]
- UNESCO, Water in a Changing World. 2009.
- World Health Organization (WHO), Guidelines for drinking-water quality, vol. 21, no. 6. 2004.
- American Public Health Association, American Water Works Association, and Water Environment Federation, “Standard Methods for the Examination of Water and Waste Water,” Stand. Methods Exam. Water Waste Water, pp. 3–104, 1992.
- A. ISRA-CNR, Analytical Methods for Water. Agenzia per la protezione dell’ambiente e per i servizi tecnici (APAT) Istituto di Ricerca sulle Acque - Consiglio Nazionale delle Ricerche (ISRA-CNR). 2003.
- UNESCO; WMO; IAEA;, “The State of the Resource,” in Water: a shared responsibility; the United Nations world water development report 2, UNESCO-WWAP, Ed. 2006, pp. 130–172.
- United Nations World Water Assessment, “Wastewater The Untapped Resource,” Paris, 2017.
- A. Panagopoulos, “Study and evaluation of the characteristics of saline wastewater (brine) produced by desalination and industrial plants,” Environ. Sci. Pollut. Res., vol. 29, no. 16, pp. 23736–23749, 2022. [CrossRef]
- A. G. Collins, Geochemistry of oilfield waters, vol. $40. 1975.
- J. Wang et al., “Geochemistry of Formation Water and Its Implications for Petroleum Source Rocks in the Fengcheng Formation, Mahu Depression, Xinjiang, China,” Front. Earth Sci., vol. 9, no. March, pp. 1–14, 2022. [CrossRef]
- M. S. Shipaeva et al., “Geochemical analysis of formation water as a tool for better understanding of water flooding,” IOP Conf. Ser. Earth Environ. Sci., vol. 1087, no. 1, 2022. [CrossRef]
- K. L. Benko and J. E. Drewes, “Produced water in the Western United States: Geographical distribution, occurrence, and composition,” Environ. Eng. Sci., vol. 25, no. 2, pp. 239–246, 2008. [CrossRef]
- U.S. Energy Information Administration, “Crude oil including lease condensate production (Mb/d),” 2023.
- A. McGenity, T. J.; Oren, “Hypersaline environments,” in Life at Extremes: Environments, Organisms and Strategies for Survival, no. 9781402092114, ed. E.M. Bell, Ed. 2012, pp. 402–437.
- M. F. Edbeib, R. A. Wahab, and F. Huyop, “Halophiles: biology, adaptation, and their role in decontamination of hypersaline environments,” World J. Microbiol. Biotechnol., vol. 32, no. 8, pp. 1–23, 2016. [CrossRef]
- T. D. Sharkey, “Discovery of the canonical Calvin–Benson cycle,” Photosynth. Res., vol. 140, no. 2, pp. 235–252, 2019. [CrossRef]
- R. Kaňa and Govindjee, “Role of ions in the regulation of light-harvesting,” Front. Plant Sci., vol. 7, no. DECEMBER2016, pp. 1–17, 2016. [CrossRef]
- R. A. Andersen, Algal Culturing Techniques. Phycological Society of America, 2005.
- A. A, Martins et al., “Water footprint of microalgae cultivation in photobioreactor,” Energy Procedia, vol. 153, pp. 426–431, 2018. [CrossRef]
- E. Uggetti, J. García, J. A. Álvarez, and M. J. García-Galán, “Start-up of a microalgae-based treatment system within the biorefinery concept: From wastewater to bioproducts,” Water Sci. Technol., vol. 78, no. 1, pp. 114–124, 2018. [CrossRef]
- F. Wollmann et al., “Microalgae wastewater treatment: Biological and technological approaches,” Eng. Life Sci., vol. 19, no. 12, pp. 860–871, 2019. [CrossRef]
- Z. Y. Yang et al., “Improving sedimentation and lipid production of microalgae in the photobioreactor using saline wastewater,” Bioresour. Technol., vol. 347, no. July 2021, p. 126392, 2022. [CrossRef]
- G. Schönknecht et al., “Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote,” Science (80-. )., vol. 339, no. 6124, pp. 1207–1210, 2013. [CrossRef]
- S. I. Park et al., “Revised classification of the Cyanidiophyceae based on plastid genome data with descriptions of the Cavernulicolales ord. nov. and Galdieriales ord. nov. (Rhodophyta),” J. Phycol., vol. 59, no. 3, pp. 444–466, 2023. [CrossRef]
- Lang, S. Bashir, M. Lorenz, S. Rader, and G. Weber, “Exploiting the potential of Cyanidiales as a valuable resource for biotechnological applications,” Appl. Phycol., vol. 3, no. 1, pp. 199–210, 2022. [CrossRef]
- M. Sirakov et al., “Cyanidiophyceae (Rhodophyta) tolerance to precious metals: Metabolic response to palladium and gold,” Plants, vol. 10, no. 11, pp. 1–8, 2021. [CrossRef]
- A. Musacchio, G. Pinto, S. Sabato, and R. Taddei, “Aloresistenza in diversi ceppi di Cyanidium caldarium forma A 3 forma B,” Delpinoa. Nuova Ser., 1978.
- L. D’Elia, A. Del Mondo, M. Santoro, A. De Natale, G. Pinto, and A. Pollio, “Microorganisms from harsh and extreme environments: A collection of living strains at ACUF (Naples, Italy),” Ecol. Quest., vol. 29, no. 3, pp. 63–74, 2018. [CrossRef]
- H. Foyer, “Reactive oxygen species, oxidative signaling and the regulation of photosynthesis,” Environ. Exp. Bot., vol. 154, no. May, pp. 134–142, 2018. [CrossRef]
- L. Wang, T. Yang, Y. Pan, L. Shi, Y. Jin, and X. Huang, “The Metabolism of Reactive Oxygen Species and Their Effects on Lipid Biosynthesis of Microalgae,” Int. J. Mol. Sci., vol. 24, no. 13, 2023. [CrossRef]
- H. L. Macintyre, E. Lawrenz, and T. L. Richardson, Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications, no. January 1970. 2010.
- Ministero Dello Sviluppo Economico, “Crude oil production in Italy in 2018, by region,” 2018.
- Sherwood Lollar, O. Warr, and P. M. Higgins, “The Hidden Hydrogeosphere: The Contribution of Deep Groundwater to the Planetary Water Cycle,” Annu. Rev. Earth Planet. Sci., vol. 52, no. 1, pp. 1–24, 2024. [CrossRef]
- F. van Weert, J. van der Gun, and J. Reckman, “Global Overview of Saline Groundwater Occurrence and Genesis (Report number: GP 2009-1),” Utr. IGRAC - U. N. Int. Groundw. Resour. Assess. Cent., no. July, pp. 1–32, 2009.
- V. Reeb and D. Bhattacharya, “The Thermo-Acidophilic Cyanidiophyceae (Cyanidiales),” in Red Algae in the Genomic Age, J. Seckbach and D. J. Chapman, Eds. Dordrecht: Springer Netherlands, 2010, pp. 409–426.
- J. Barber, “An explanation for the relationship between salt-induced thylakoid stacking and the chlorophyll fluorescence changes associated with changes in spillover of energy from photosystem II to photosystem I,” FEBS Lett., vol. 118, no. 1, pp. 1–10, 1980. [CrossRef]
- Peter Mitchell, “Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic type of Mechanism,” Nature, vol. 191, pp. 144–148, 1944.
- M. Martinez-Garcia and M. J. E. C. van der Maarel, “Floridoside production by the red microalga Galdieria sulphuraria under different conditions of growth and osmotic stress,” AMB Express, vol. 6, no. 1, pp. 0–7, 2016. [CrossRef]
- G. Q. Chen, Y. Jiang, and F. Chen, “Salt-induced alterations in lipid composition of diatom Nitzschia laevis (Bacillariophyceae) under heterotrophic culture condition,” J. Phycol., vol. 44, no. 5, pp. 1309–1314, 2008. [CrossRef]
- W. Elloumi, A. Jebali, A. Maalej, M. Chamkha, and S. Sayadi, “Effect of mild salinity stress on the growth, fatty acid and carotenoid compositions, and biological activities of the thermal freshwater microalgae Scenedesmus sp.,” Biomolecules, vol. 10, no. 11, pp. 1–17, 2020. [CrossRef]
- S. Fal, A. Aasfar, R. Rabie, A. Smouni, and H. EL Arroussi, “Salt induced oxidative stress alters physiological, biochemical and metabolomic responses of green microalga Chlamydomonas reinhardtii,” Heliyon, vol. 8, no. 1, 2022. [CrossRef]
- X. Ji et al., “The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002,” Sci. Total Environ., vol. 633, pp. 593–599, 2018. [CrossRef]
- A. Gomes, E. Fernandes, and J. L. F. C. Lima, “Fluorescence probes used for detection of reactive oxygen species,” J. Biochem. Biophys. Methods, vol. 65, no. 2–3, pp. 45–80, 2005. [CrossRef]
- G. G. Martinovich, I. V. Martinovich, S. N. Cherenkevich, and H. Sauer, “Redox Buffer Capacity of the Cell: Theoretical and Experimental Approach,” Cell Biochem. Biophys., vol. 58, no. 2, pp. 75–83, 2010. [CrossRef]
- G. Fantner, “A brief introduction to error analysis and propagation,” no. February, pp. 1–13, 2011.
- S. White, A. Anandraj, and F. Bux, “PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids,” Bioresour. Technol., vol. 102, no. 2, pp. 1675–1682, 2011. [CrossRef]
- N. R. Baker, “Chlorophyll fluorescence: A probe of photosynthesis in vivo,” Annu. Rev. Plant Biol., vol. 59, pp. 89–113, 2008. [CrossRef]
- Y. Chen and S. Vaidyanathan, “Simultaneous assay of pigments, carbohydrates, proteins and lipids in microalgae,” Anal. Chim. Acta, vol. 776, pp. 31–40, 2013. [CrossRef]
- M. M. Bradford, “A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.,” Anal. Biochem., 1976. [CrossRef]
- A. Bennett and L. Bogobad, “Complementary chromatic adaptation in a filamentous blue-green alga,” J. Cell Biol., vol. 58, no. 2, pp. 419–435, 1973. [CrossRef]
- W. J. Bligh, E.G. and Dyer, “Canadian Journal of Biochemistry and Physiology,” Can. J. Biochem. Physiol., vol. 37, no. 8, 1959.
- J. A. Knight, S. Anderson, and J. M. Rawle, “Chemical basis of the sulfo-phospho-vanillin reaction for estimating total serum lipids.,” Clin. Chem., vol. 18, no. 3, pp. 199–202, 1972. [CrossRef]
- A. R. Wellburn, “The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution,” J. Plant Physiol., vol. 144, no. 3, pp. 307–313, 1994. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




