Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Phenotypic Data Acquisition
2.3. Genotypic Data Acquisition
2.4. Phenotypic Data Processing and Analysis
2.5. Functional Mapping
2.6. Construction of Genetic Effect Networks
2.7. Functional Annotation
2.8. Genetic Effects Analysis of Significant Genes
3. Results
3.1. Analysis of Phenotypic Variation under Different Light Conditions
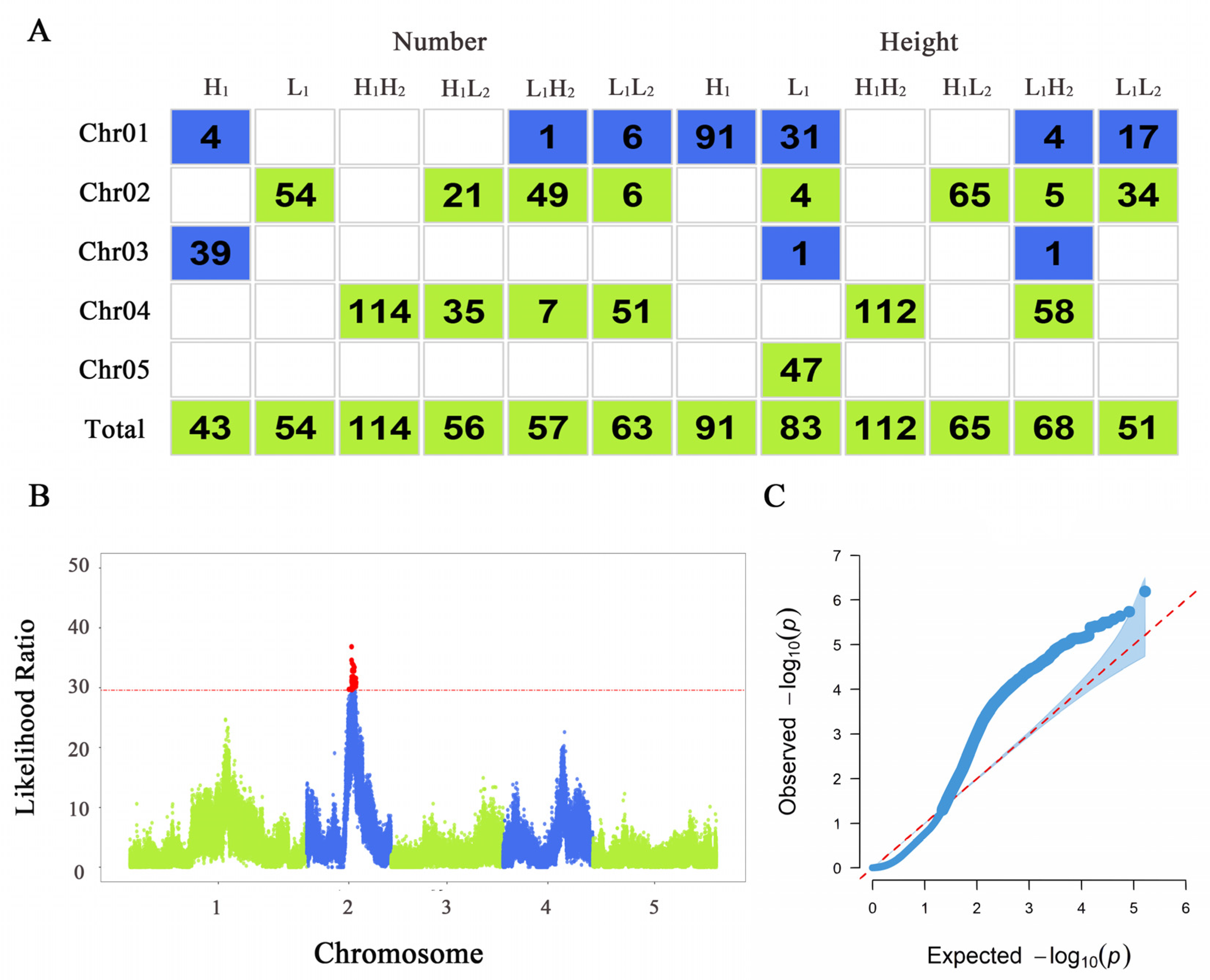
3.2. Significant SNPs Located via Functional Mapping
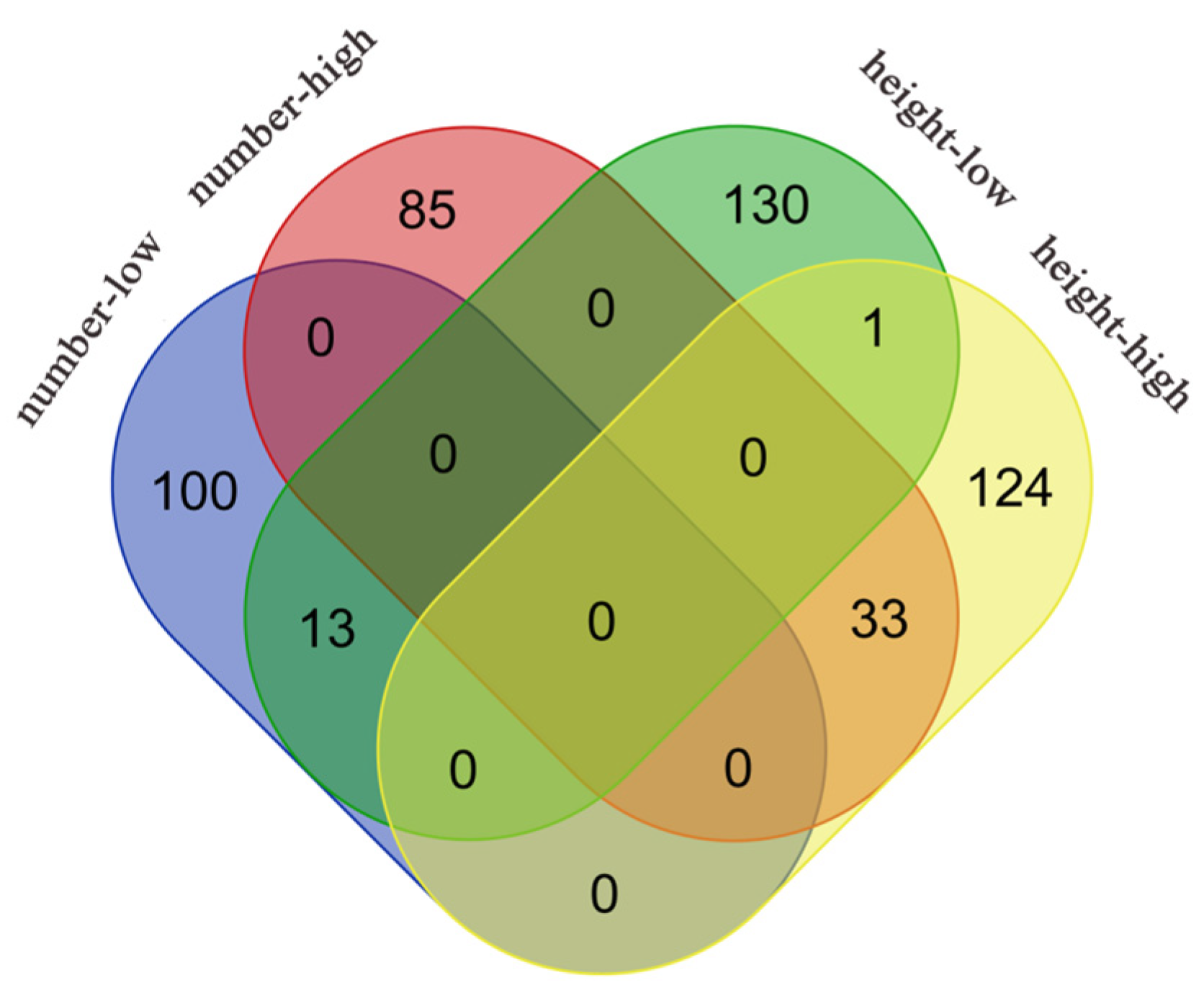
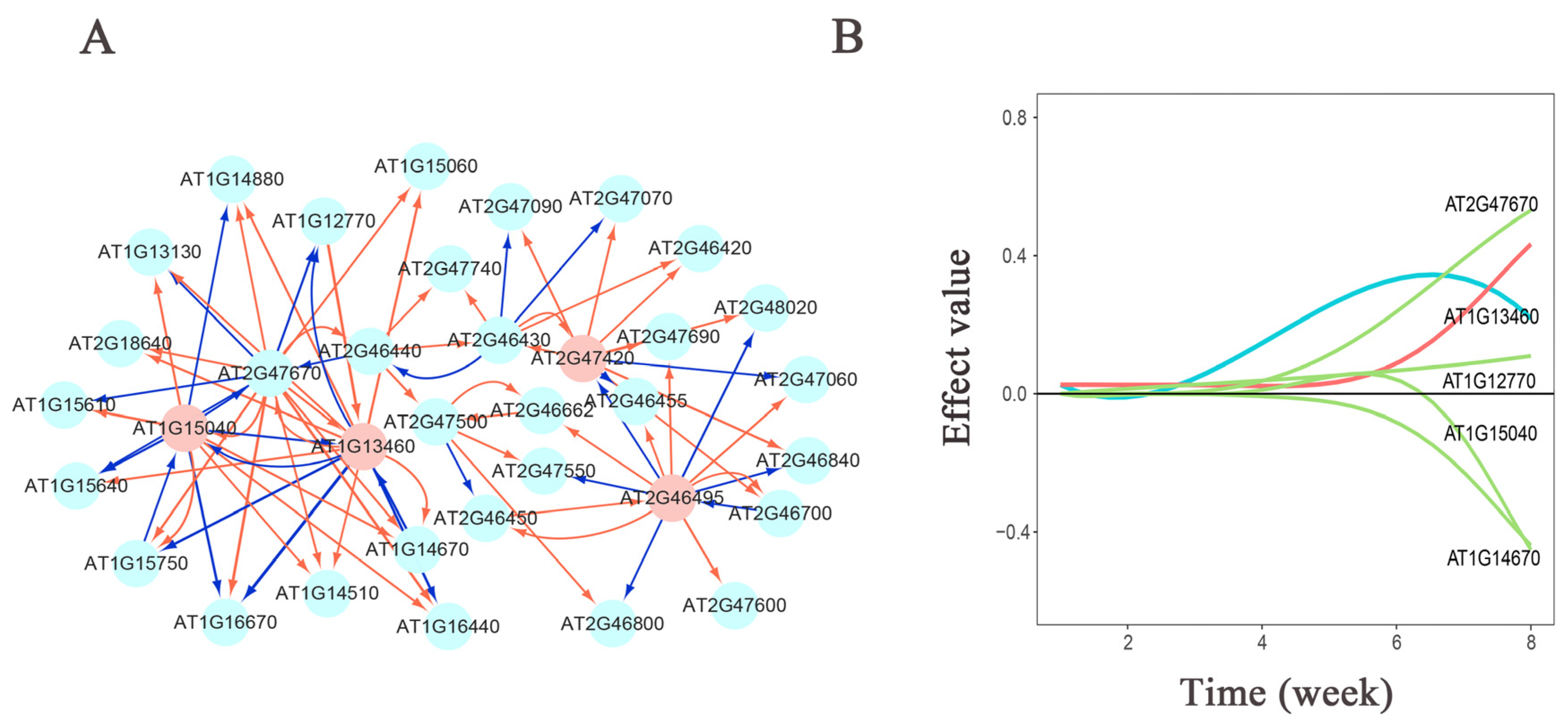
3.3. Genetic Effect Network Analysis
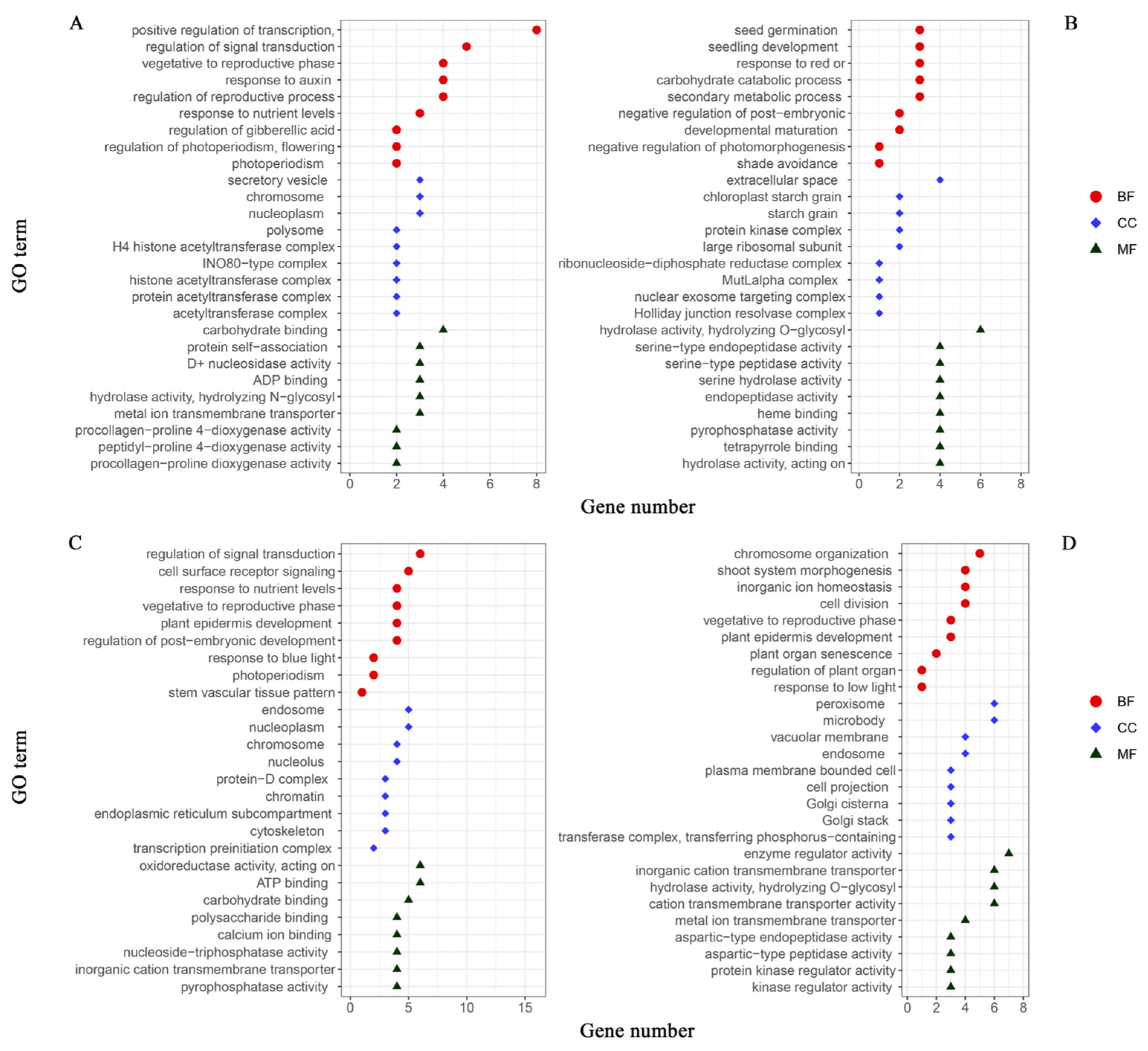
3.4. Gene Ontology (GO) Analysis
3.5. Genetic Effects Analysis of Significant Genes Across Different Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibanez, C.P.Y.P. Ambient temperature and genotype differentially affect developmental and phenotypic plasticity in Arabidopsis thaliana. BMC Plant Biol. 2017, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Galloway, L.F.; Etterson, J.R. Transgenerational plasticity is adaptive in the wild. Science 2007, 318, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Miska, E.A.; Ferguson-Smith, A.C. Transgenerational inheritance: models and mechanisms of non-DNA sequence-based inheritance. Science 2016, 354, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.H. Transgenerational epigenetics: the role of maternal effects in cardiovascular development. Integr. Comp. Biol. 2014, 54, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Whittle, C.A.; Otto, S.P.; Johnston, M.O.; Krochko, J.E. Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany 2009, 87, 650–657. [Google Scholar] [CrossRef]
- Deng, Y.; Bossdorf, O.; Scheepens, J.F. Transgenerational effects of temperature fluctuations in Arabidopsis thaliana. Aob Plants 2021, 13, plab064. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Tian, D.; Han, W.; Ji, C.; Hou, X.; Guo, Y.; Fang, J. Weak transgenerational effects of ancestral nitrogen and phosphorus availabilities on offspring phenotypes in Arabidopsis thaliana. J. Plant Res. 2023, 136, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.I.; Robinson, J.W. Digest: Transgenerational stress memory mechanisms in Arabidopsis thaliana. Evolution 2020, 74, 2423–2424. [Google Scholar] [CrossRef] [PubMed]
- Latzel, V.; Klimešová, J. Transgenerational plasticity in clonal plants. Evol. Ecol. 2010, 24, 1537–1543. [Google Scholar] [CrossRef]
- Weinhold, A. Transgenerational stress-adaption: an opportunity for ecological epigenetics. Plant Cell Reports 2018, 37, 3–9. [Google Scholar] [CrossRef]
- Holdsworth, M.J.; Bentsink, L.; Soppe, W. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008, 179, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Robertson, F.C.; Skeffington, A.W.; Gardner, M.J.; Webb, A.A. Interactions between circadian and hormonal signalling in plants. Plant Mol.Biol. 2009, 69, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; He, L.; Zhang, X.; Jin, Y.; Chen, J. Differential effects of transgenerational plasticity on morphological and photosynthetic properties between an invasive plant and its congeneric native one. Biol. Invasions 2023, 25, 115–123. [Google Scholar] [CrossRef]
- Yan, Y.; Stoddard, F.L.; Neugart, S.; Oravec, M.; Urban, O.; Sadras, V.O.; Aphalo, P.J. The transgenerational effects of solar short-UV radiation differed in two accessions of Vicia faba L. from contrasting UV environments. J. Plant Physiol. 2020, 248, 153145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, R.; Zeng, X.Y.; Lee, S.; Ye, L.H.; Tian, S.L.; Zhang, Y.J.; Busch, W.; Zhou, W.B.; Zhu, X.G.; et al. GLK transcription factors accompany ELONGATED HYPOCOTYL5 to orchestrate light-induced seedling development in Arabidopsis. Plant Physiol. 2024. [Google Scholar] [CrossRef]
- Hou, L.Y.; Sommer, F.; Poeker, L.; Dziubek, D.; Schroda, M.; Geigenberger, P. The impact of light and thioredoxins on the plant thiol-disulfide proteome. Plant Physiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Fang, K.; Qiao, K.; Xiong, J.; Lan, J.; Chen, J.; Tian, Y.; Kang, X.; Lei, W.; Zhang, D.; et al. Cooperative transcriptional regulation by ATAF1 and HY5 promotes light-induced cotyledon opening in Arabidopsis thaliana. Sci. Signal. 2024, 17, eadf7318. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.L.; Ma, C.X.; Hou, W.; Corva, P.; Medrano, J.F. Functional mapping of quantitative trait loci that interact with the hg mutation to regulate growth trajectories in mice. Genetics 2005, 171, 239–249. [Google Scholar] [CrossRef] [PubMed]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for ontogenetic growth. Nature 2001, 413, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hou, W.; Littell, R.C.; Wu, R. Structured antedependence models for functional mapping of multiple longitudinal traits. Stat. Appl. Genet. Mol. Biol. 2005, 4. [Google Scholar] [CrossRef]
- Gao, F.; Han, L. Implementing the Nelder-Mead simplex algorithm with adaptive parameters. Comput. Optim. Appl. 2012, 51, 259–277. [Google Scholar] [CrossRef]
- Yuan, G.; Sheng, Z.; Wang, B.; Hu, W.; Li, C. The global convergence of a modified BFGS method for nonconvex functions. J. Comput. Appl. Math. 2018, 327, 274–294. [Google Scholar] [CrossRef]
- Dam, P.; Rodriguez-R, L.M.; Luo, C.; Hatt, J.; Tsementzi, D.; Konstantinidis, K.T.; Voit, E.O. Model-based comparisons of the abundance dynamics of bacterial communities in two lakes. Sci. Rep. 2020, 10, 2423. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jin, Y.; He, X.; Dong, A.; Wang, J.; Wu, R. Inferring multilayer interactome networks shaping phenotypic plasticity and evolution. Nat. Commun. 2021, 12, 5304–5317. [Google Scholar] [CrossRef] [PubMed]
- van Nocker, S.; Ludwig, P. The WD-repeat protein superfamily in Arabidopsis: conservation and divergence in structure and function. BMC Genomics 2003, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Mindrebo, J.T.; Nartey, C.M.; Seto, Y.; Burkart, M.D.; Noel, J.P. Unveiling the functional diversity of the alpha/beta hydrolase superfamily in the plant kingdom. Curr. Opin. Struct. Biol. 2016, 41, 233–246. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, M.; Snoek, L.B.; van Eck-Stouten, E.; Proveniers, M.C.G.; Torii, K.U.; Voesenek, L.A.C.J.; Millenaar, F.F.; Peeters, A.J.M. ERECTA controls low light intensity-induced differential petiole growth independent of phytochrome B and cryptochrome 2 action in Arabidopsis thaliana. Plant Signal. Behav. 2010, 5, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Hershko, A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005, 12, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.L.; Callis, J. Ubiquitin ligases mediate growth and development by promoting protein death. Curr. Opin. Plant Biol. 2007, 10, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Vierstra, R.D. The ubiquitin - 26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009, 10, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Taganna, J. Genome-wide analysis of the U-box E3 ubiquitin ligase enzyme gene family in tomato. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Mazzucotelli, E.; Belloni, S.; Marone, D.; De Leonardis, A.M.; Guerra, D.; Di Fonzo, N.; Cattivelli, L.; Mastrangelo, A.M. The E3 ubiquitin ligase gene family in plants: regulation by degradation. Curr. Genomics 2006, 7, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Boycheva, I.; Vassileva, V.; Revalska, M.; Zehirov, G.; Iantcheva, A. Cyclin-like F-box protein plays a role in growth and development of the three model species Medicago truncatula, Lotus japonicus, and Arabidopsis thaliana. Research and reports in biology 2015, 6, 117–130. [Google Scholar] [CrossRef]
- Kuroda, H.; Takahashi, N.; Shimada, H.; Seki, M.; Shinozaki, K.; Matsui, M. Classification and expression analysis of Arabidopsis F-box-containing protein genes. Plant Cell Physiol. 2002, 43, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cardozo, T.; Lovering, R.C.; Elledge, S.J.; Pagano, M.; Harper, J.W. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004, 18, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Gagne, J.M.; Downes, B.P.; Shiu, S.H.; Durski, A.M.; Vierstra, R.D. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 11519–11524. [Google Scholar] [CrossRef] [PubMed]
- Baute, J.; Polyn, S.; De Block, J.; Blomme, J.; Van Lijsebettens, M.; Inze, D. F-Box protein FBX92 affects leaf size in Arabidopsis thaliana. Plant Cell Physiol. 2017, 58, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Gusti, A.; Baumberger, N.; Nowack, M.; Pusch, S.; Eisler, H.; Potuschak, T.; De Veylder, L.; Schnittger, A.; Genschik, P. Schnittger, A.; Genschik, P. The Arabidopsis thaliana F-Box protein FBL17 is essential for progression through the second mitosis during pollen development. PLoS One 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Majee, M.; Kumar, S.; Kathare, P.K.; Wu, S.; Gingerich, D.; Nayak, N.R.; Salaita, L.; Dinkins, R.; Martin, K.; Goodin, M.; et al. KELCH F-BOX protein positively influences Arabidopsis seed germination by targeting PHYTOCHROME-INTERACTING FACTOR1. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E4120–E4129. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.Y.; Lv, T.X.; Shen, H.; Luong, P.; Wang, J.; Wang, Z.Y.; Huang, Z.G.; Xiao, L.T.; Engineer, C.; Kim, T.H.; et al. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol. 2014, 164, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Koops, P.; Pelser, S.; Ignatz, M.; Klose, C.; Marrocco-Selden, K.; Kretsch, T. EDL3 is an F-box protein involved in the regulation of abscisic acid signalling in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 5547–5560. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Li, D.; Liu, Z.; Wang, J.; Li, X.; Yang, Y. The Arabidopsis F-box E3 ligase RIFP1 plays a negative role in abscisic acid signalling by facilitating ABA receptor RCAR3 degradation. Plant Cell Environ. 2016, 39, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Mo, J.; Chen, H.; Hu, Z.; Wang, B.; Wu, J.; Liang, Z.; Xie, W.; Du, K.; Peng, M.; et al. Structural insights into molecular mechanism for N 6-adenosine methylation by MT-A70 family methyltransferase METTL4. Nat. Commun. 2022, 13. [Google Scholar] [CrossRef]
- Frye, M.; Harada, B.T.; Behm, M.; He, C. RNA modifications modulate gene expression during development. Science 2018, 361, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, L.; Huang, Y.; Ma, J.; Min, J. Readers, writers and erasers of N(6)-methylated adenosine modification. Curr. Opin. Struct. Biol. 2017, 47, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kranz, R.G. A nitrogen-regulated glutamine amidotransferase (GAT1_2.1) represses shoot branching in Arabidopsis. Plant Physiol. 2012, 160, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, H.; Guo, F.; Yadav, V.K.; Mcilwain, S.J.; Rowse, M.; Choudhary, A.; Lin, Z.; Li, Y.; Gu, T.; et al. PP2A-B’ holoenzyme substrate recognition, regulation and role in cytokinesis. Cell Discov. 2017, 3. [Google Scholar] [CrossRef]
- Lechward, K.; Awotunde, O.S.; Swiatek, W.; Muszynska, G. Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochim. Pol. 2001, 48, 921–933. [Google Scholar] [PubMed]
- Noda, S.; Takahashi, Y.; Tsurumaki, Y.; Yamamura, M.; Nishikubo, N.; Yamaguchi, M.; Sakurai, N.; Hattori, T.; Suzuki, H.; Demura, T.; et al. ATL54, a RING-H2 domain protein selected by a gene co-expression network analysis, is associated with secondary cell wall formation in Arabidopsis. Plant Biotechnol. 2013, 30, 169–174. [Google Scholar] [CrossRef]
- Riaño-Pachón, D.M.; Kleessen, S.; Neigenfind, J.; Durek, P.; Weber, E.; Engelsberger, W.R.; Walther, D.; Selbig, J.; X, S.W.; Birgit, K. Proteome-wide survey of phosphorylation patterns affected by nuclear DNA polymorphisms in Arabidopsis thaliana. BMC Genomics 2010, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wieckowski, Y.; Schiefelbein, J. Nuclear ribosome biogenesis mediated by the DIM1A rRNA dimethylase is required for organized root growth and epidermal patterning in Arabidopsis. Plant Cell 2012, 24, 2839–2856. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kang, H. Emerging roles of RNA-binding proteins in plant growth, development, and stress responses. Mol. Cells 2016, 39, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Shuchao Dong, D.T.M.S. An HB40 - JUNGBRUNNEN1 - GA 2-OXIDASE regulatory module for gibberellin 2 homeostasis in Arabidopsis. BioRxiv 2021. [Google Scholar] [CrossRef]
- Shahnejat-Bushehri, S.; Tarkowska, D.; Sakuraba, Y.; Balazadeh, S. Arabidopsis NAC transcription factor JUB1 regulates GA / BR metabolism and signalling. Nat. Plants 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zawadzka, A.; Czarnocki, Z.; Reiter, R.J.; Back, K. Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J. Pineal Res. 2016, 61, 470–478. [Google Scholar] [CrossRef]
- Yu, Y.; Lv, Y.; Shi, Y.; Li, T.; Chen, Y.; Zhao, D.; Zhao, Z. The role of phyto-melatonin and related metabolites in response to stress. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Hagel, J.M.; Facchini, P.J. Expanding the roles for 2-oxoglutarate-dependent oxygenases in plant metabolism. Nat. Prod. Rep. 2018, 35, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, T.; Kitashiba, H.; Zou, Z.; Li, F.; Fukino, N.; Ohara, T.; Nishio, T.; Ishida, M. A 2-oxoglutarate-dependent dioxygenase mediates the biosynthesis of glucoraphasatin in Radish. Plant Physiol. 2017, 173, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, W.; Fu, R.; Zhang, Y. Genome-wide characterization of 2-oxoglutarate and Fe (II)- dependent dioxygenase family genes in tomato during growth cycle and their roles in metabolism. BMC Genomics 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wan, W.; Jiang, G.; Xi, Y.; Huang, H.; Cai, J.; Chang, Y.; Duan, C.; Mangrauthia, S.K.; Peng, X.; et al. Nucleocytoplasmic trafficking of the Arabidopsis WD40 repeat protein XIW1 regulates ABI5 stability and abscisic acid responses. Mol. Plant. 2019, 12, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Mitchum, M.G.; Yamaguchi, S.; Hanada, A.; Kuwahara, A.; Yoshioka, Y.; Kato, T.; Tabata, S.; Kamiya, Y.; Sun, T.P. Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J. 2006, 45, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, A.; Furumoto, T.; Ishida, S.; Takahashi, Y. AGF1, an AT-hook protein, is necessary for the negative feedback of AtGA3ox1 encoding GA 3-oxidase. Plant Physiol. 2007, 143, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Krupnova, T.; Sasabe, M.; Ghebreghiorghis, L.; Gruber, C.W.; Hamada, T.; Dehmel, V.; Strompen, G.; Stierhof, Y.; Lukowitz, W.; Kemmerling, B.; et al. Microtubule-associated kinase-like protein RUNKEL needed for cell plate expansion in Arabidopsis cytokinesis. Curr. Biol. 2009, 19, 536. [Google Scholar] [CrossRef]
- Nowack, M.K.; Harashima, H.; Dissmeyer, N.; Zhao, X.; Bouyer, D.; Weimer, A.K.; De Winter, F.; Yang, F.; Schnittger, A. Genetic framework of cyclin-dependent kinase function in Arabidopsis. Dev. Cell 2012, 22, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, L.; Ye, H.; Yu, X.; Algreen, A.; Yin, Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 7648–7653. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Chen, X.M. HUA ENHANCER3 reveals a role for a cyclin-dependent protein kinase in the specification of floral organ identity in Arabidopsis. Development 2004, 131, 3147–3156. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Suorsa, M.; Rantala, M.; Lundin, B.; Aro, E. Serine and threonine residues of plant STN7 kinase are differentially phosphorylated upon changing light conditions and specifically influence the activity and stability of the kinase. Plant J. 2016, 87, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
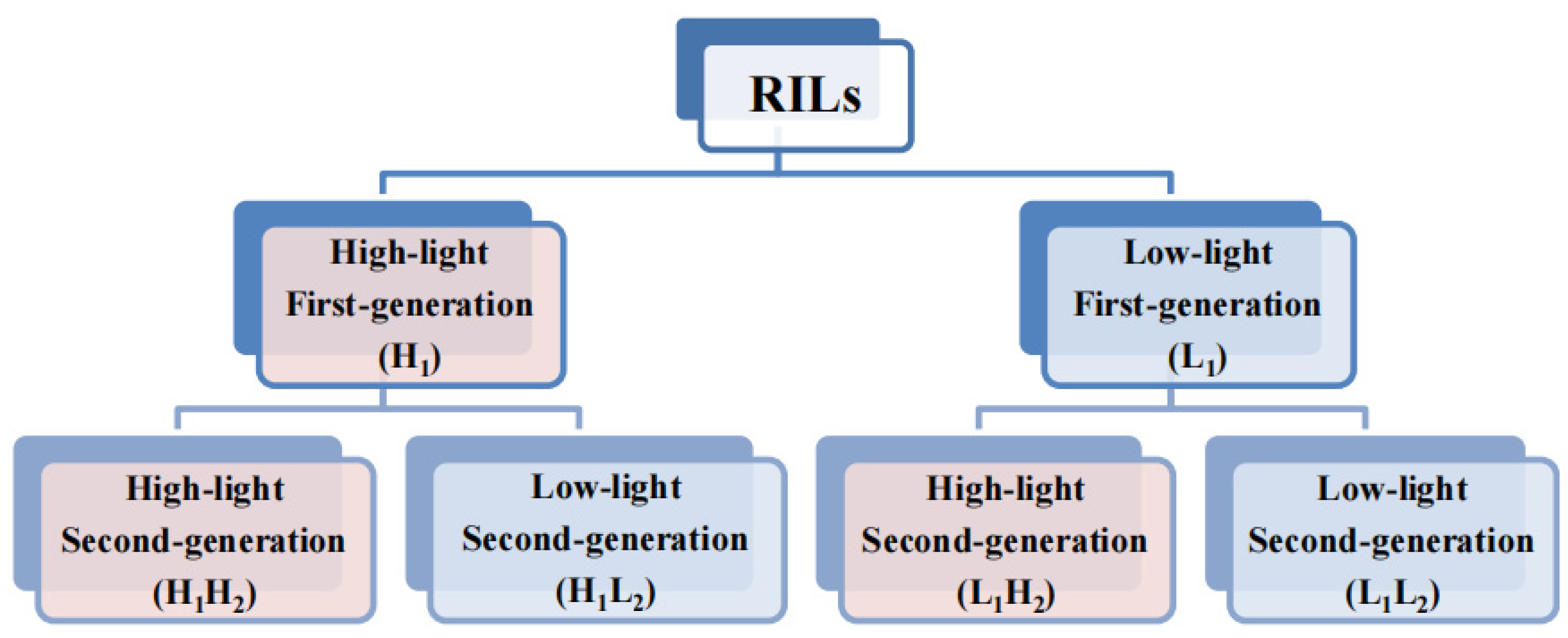
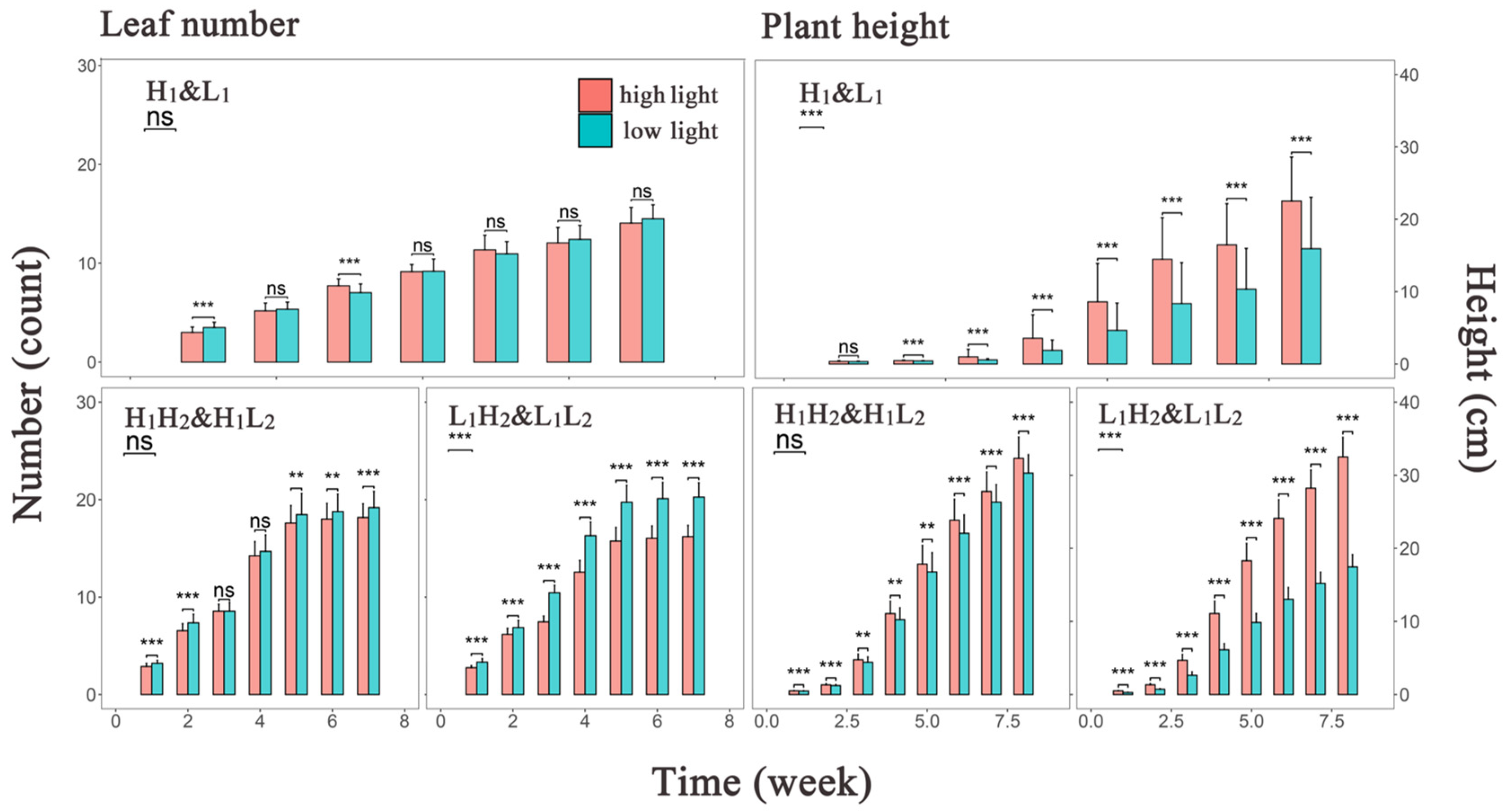
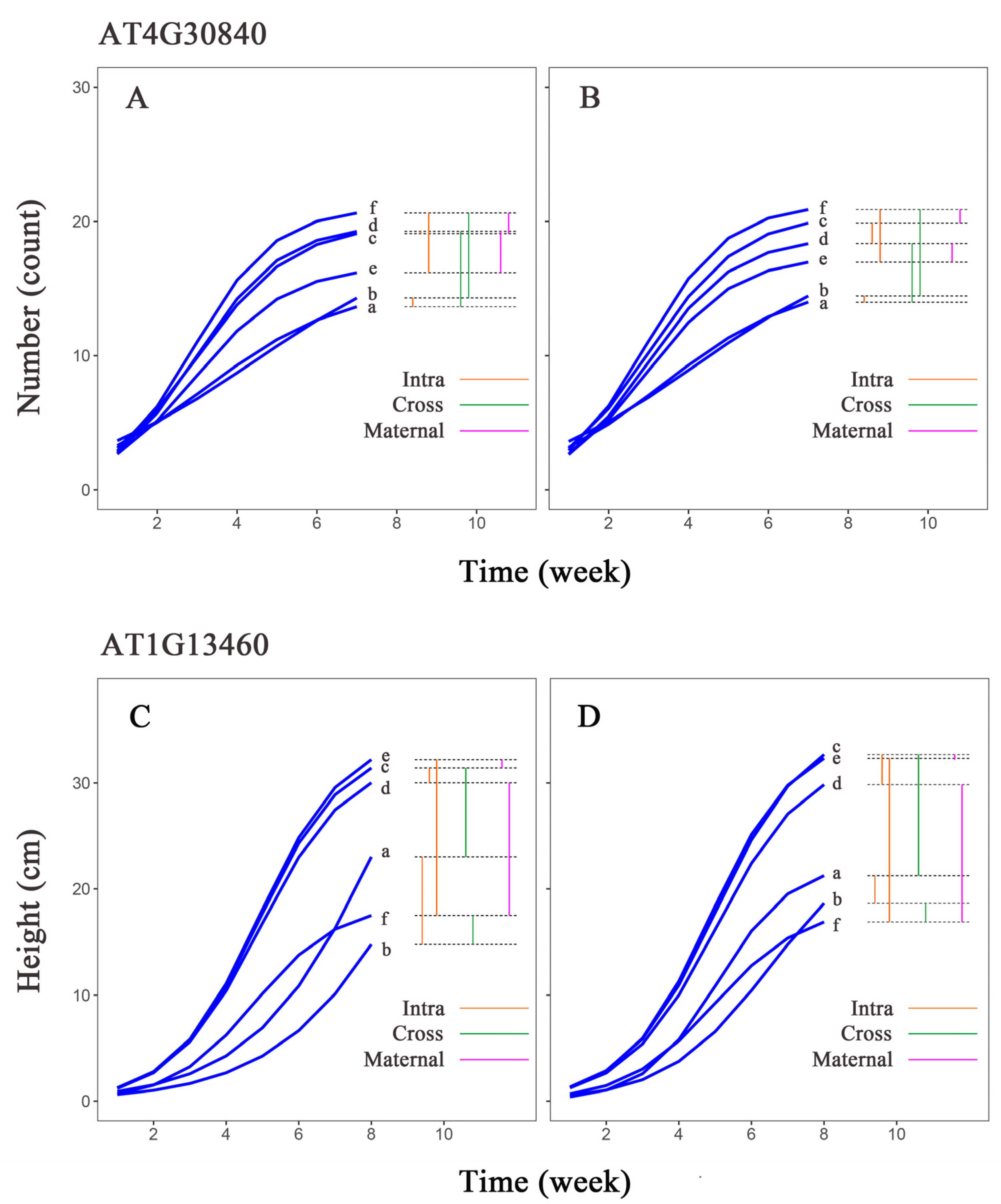
| Category | Phenotypic Analysis Group | Abbreviation |
| Intragenerational plasticity | High-light and low-light | H1&L1 |
| High-light–high-light and high-light–low-light | H1H2&H1L2 | |
| Low-light–high-light and low-light–low-light | L1H2&L1L2 | |
| Cross-generational plasticity | High-light and high-light–high-light | H1&H1H2 |
| Low-light and low-light–low-light | L1&L1L2 | |
| Maternal effect | High-light–high-light and low-light–high-light | H1H2&L1H2 |
| High-light–low-light and low-light–low-light | H1H2&L1H2 |
| Category | Gene ID | Position | Chr | Alle | Variation type | Gene Description |
|---|---|---|---|---|---|---|
| Developmental regulation |
AT4G30840 | 15023742 | 4 | T/T | upstream_gene | Transducin/WD40 repeat-like superfamily protein |
| AT4G34220 | 16388827 | 4 | A/A | upstream_gene | Leucine-rich repeat protein kinase family protein | |
| AT4G35165 | 16739054 | 4 | C/C | downstream_gene | Egg cell-secreted-like protein (DUF1278) | |
| AT4G35370 | 16812606 | 4 | A/A | upstream_gene | Transducin/WD40 repeat-like superfamily protein | |
| AT4G35390 | 16829046 | 4 | C/C | upstream_gene | AT-hook protein of GA feedback 1 | |
| AT4G35840 | 16982215 | 4 | C/C | synonymous | RING/U-box superfamily protein | |
| AT4G35860 | 16989499 | 4 | T/T | upstream_gene | GTP-binding 2 | |
| AT4G35900 | 17005228 | 4 | G/G | missense | Basic-leucine zipper (bZIP) transcription factor family protein | |
| AT4G35910 | 17010429 | 4 | G/G | synonymous | Adenine nucleotide alpha hydrolases-like superfamily protein | |
| AT4G35950 | 17026971 | 4 | A/A | upstream_gene | RAC-like 6 | |
| AT4G36080 | 17065428 | 4 | G/G | synonymous | phosphotransferases/inositol or phosphatidylinositol kinase | |
| AT4G36160 | 17118705 | 4 | T/T | upstream_gene | NAC domain containing protein 76 | |
| AT4G36630 | 17273803 | 4 | C/C | synonymous | Vacuolar sorting protein 39 | |
| Stress response |
AT2G14210 | 6018220 | 2 | T/T | upstream_gene | AGAMOUS-like 44 |
| AT4G35920 | 17013293 | 4 | C/C | splice_region&synonymous | PLAC8 family protein | |
| AT4G36150 | 17107236 | 4 | C/C | missense | disease resistance protein (TIR-NBS-LRR class) family | |
| AT4G36140 | 17109354 | 4 | T/T | upstream_gene | disease resistance protein (TIR-NBS-LRR class) | |
| AT4G37640 | 17688360 | 4 | G/G | upstream_gene | calcium ATPase 2 | |
| Transcriptional regulation | AT4G35890 | 16995463 | 4 | C/C | upstream_gene | winged-helix DNA-binding transcription factor family protein |
| AT4G36590 | 17265352 | 4 | C/C | upstream_gene | MADS-box transcription factor family protein | |
| AT4G36650 | 17285093 | 4 | T/T | synonymous | plant-specific TFIIB-related protein | |
| Anabolism | AT4G35650 | 16910021 | 4 | T/T | 3_prime_UTR | Isocitrate dehydrogenase III |
| AT4G35640 | 16911160 | 4 | A/A | upstream_gene | Serine acetyltransferase 3;2 | |
| AT4G36090 | 17079871 | 4 | T/T | synonymous | oxidoreductase, 2OG-Fe(II) oxygenase family protein | |
| AT4G36250 | 17151190 | 4 | A/A | synonymous | aldehyde dehydrogenase 3F1 | |
| AT4G36360 | 17183722 | 4 | T/T | upstream_gene | beta-galactosidase 3 | |
| Other | AT2G14310 | 6070613 | 2 | A/A | upstream_gene | pseudo |
| AT4G35660 | 16912804 | 4 | T/T | missense | selection/upkeep of intraepithelial T-cell protein, putative (DUF241) | |
| AT4G35837 | 16984099 | 4 | C/C | upstream_gene | hypothetical protein | |
| AT4G36120 | 17098217 | 4 | C/C | upstream_gene | filament-like protein (DUF869) | |
| AT4G35980 | 17032032 | 4 | T/T | upstream_gene | uncharacterized protein | |
| AT4G36197 | 17129567 | 4 | T/T | upstream_gene | tRNA-Glu | |
| AT4G36648 | 17286112 | 4 | C/C | upstream_gene | ncRNA |
| Category | Gene ID | Position | Chr | Alle | Variation type | Gene Description |
|---|---|---|---|---|---|---|
| Developmental regulation |
AT1G16440 | 5616819 | 1 | G/G | missense | root hair specific 3 |
| AT2G27520 | 11762957 | 2 | T/T | synonymous | F-box and associated interaction domains-containing protein | |
| Stress response |
AT1G16670 | 5694727 | 1 | G/G | upstream_gene | Protein kinase superfamily protein |
| AT2G27580 | 11777295 | 2 | A/A | 5_prime_UTR | A20/AN1-like zinc finger family protein | |
| AT2G28060 | 11952520 | 2 | T/T | upstream_gene | 5’-AMP-activated protein kinase beta-2 subunit protein | |
| Transcriptional regulation | AT2G27700 | 11818626 | 2 | C/C | upstream_gene | eukaryotic translation initiation factor 2 family protein / eIF-2 family protein |
| AT2G27880 | 11878388 | 2 | A/A | downstream_gene | Argonaute family protein | |
| Anabolism | AT2G18640 | 8086595 | 2 | T/T | upstream_gene | geranylgeranyl pyrophosphate synthase 4 |
| AT2G27570 | 11775564 | 2 | G/G | stop_gained | P-loop containing nucleoside triphosphate hydrolases superfamily protein | |
| AT2G27650 | 11801094 | 2 | G/G | upstream_gene | Ubiquitin carboxyl-terminal hydrolase-related protein | |
| Other | AT1G15610 | 5371155 | 1 | G/G | missense | uncharacterized protein |
| AT2G27740 | 11821497 | 2 | G/G | upstream_gene | RAB6-interacting golgin (DUF662) | |
| AT2G28180 | 12017798 | 2 | C/C | upstream_gene | cation/hydrogen exchanger family protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





