Submitted:
25 June 2024
Posted:
26 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Computational Method and Details
3. Results
3.1. Micromagnetic Simulation of Coercivity in (Fe0.7Co0.3)2B Nanowires
3.1.1. Coercivity of Defect-Free (Fe0.7Co0.3)2B Nanowires
3.1.2. Coercivity of (Fe0.7Co0.3)2B Nanowires with Surface Defect
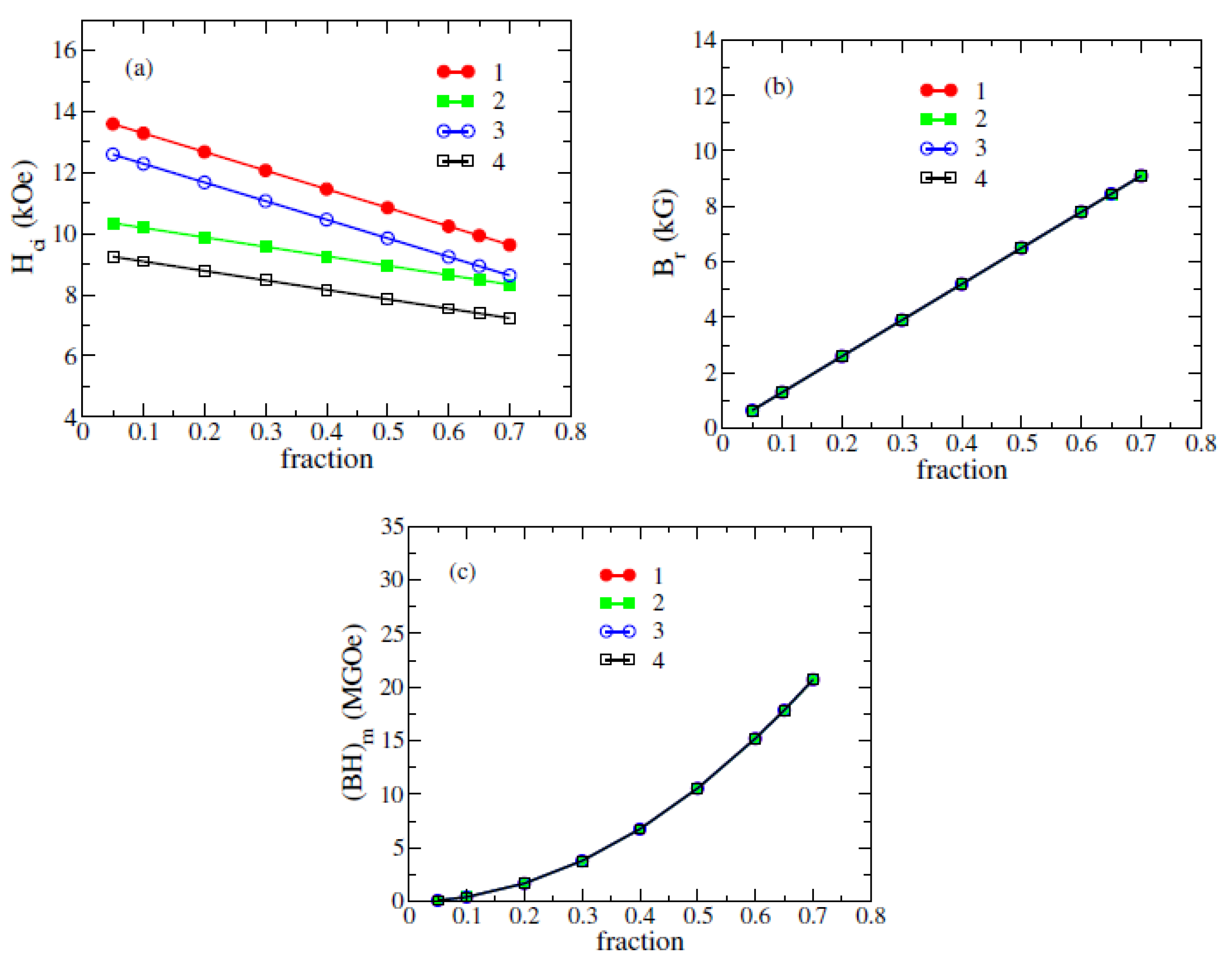
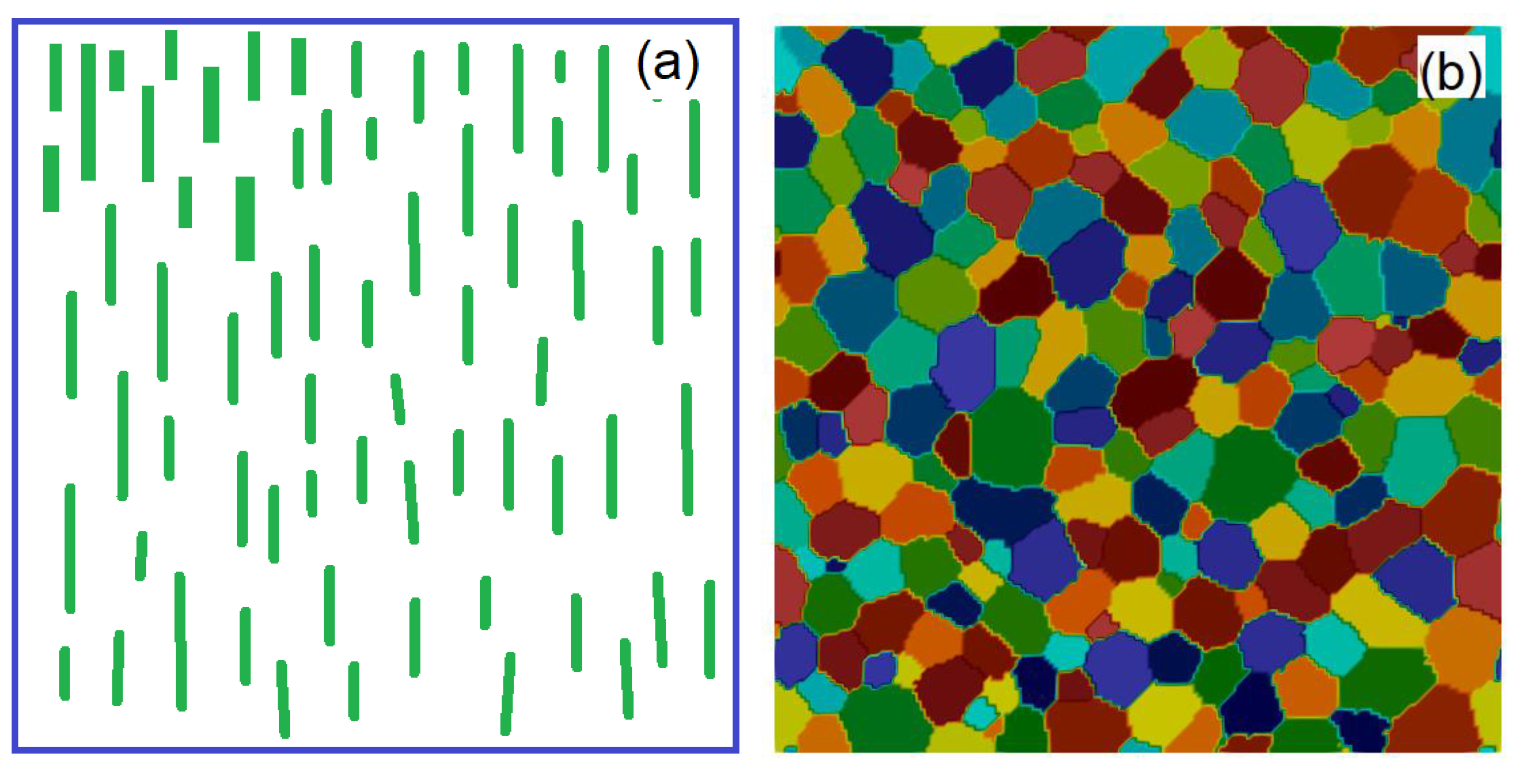
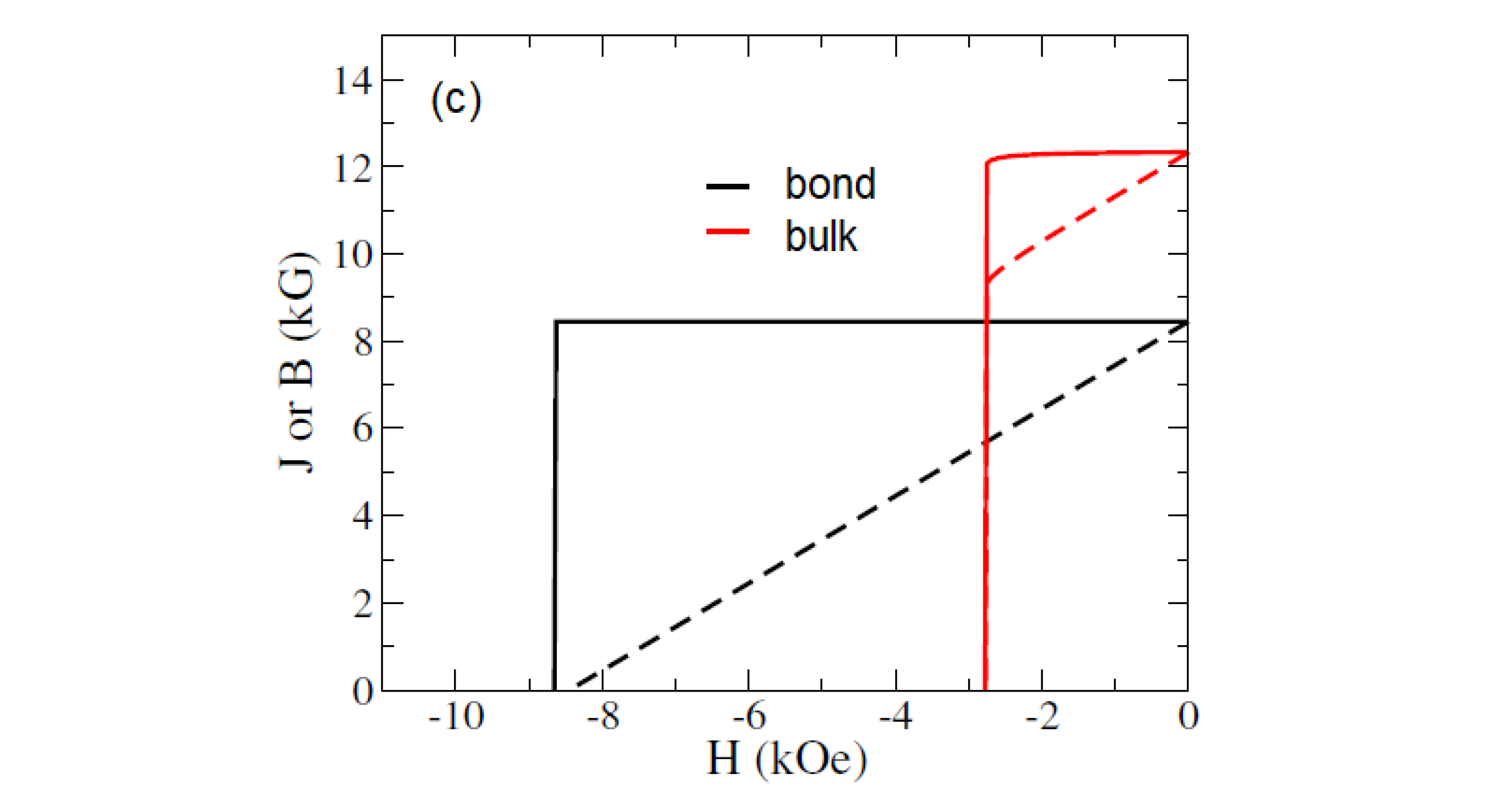
3.2. Coercivity of (Fe0.7Co0.3)2B Bonded Magnet from Nanowires
3.3. Challenge and Roadmap for Developing (Fe0.7Co0.3)2B Based Permanent Magnets
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coey, J.M.D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic Materials and Devices for the 21st Century: Stronger, Lighter, and More Energy Efficient. Advanced Materials 2011, 23, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Coey, J.M.D. Permanent Magnets: Plugging the Gap. Scr Mater 2012, 67, 524–529. [Google Scholar] [CrossRef]
- Kramer, M.J.; McCallum, R.W.; Anderson, I.A.; Constantinides, S. Prospects for Non-Rare Earth Permanent Magnets for Traction Motors and Generators. JOM 2012, 64, 752–763. [Google Scholar] [CrossRef]
- Cui, J.; Kramer, M.; Zhou, L.; Liu, F.; Gabay, A.; Hadjipanayis, G.; Balasubramanian, B.; Sellmyer, D. Current Progress and Future Challenges in Rare-Earth-Free Permanent Magnets. Acta Mater 2018, 158, 118–137. [Google Scholar] [CrossRef]
- Mohapatra, J.; Liu, X.; Joshi, P.; Liu, J.P. Hard and Semi-Hard Fe-Based Magnetic Materials. J Alloys Compd 2023, 955, 170258. [Google Scholar] [CrossRef]
- Yibole, H.; Lingling-Bao, B.; Xu, J.Y.; Alata, H.; Tegus, O.; Hanggai, W.; van Dijk, N.H.; Brück, E.; Guillou, F. (Fe,Co)2(P,Si) Rare-Earth Free Permanent Magnets: From Macroscopic Single Crystals to Submicron-Sized Particles. Acta Mater 2021, 221, 117388. [Google Scholar] [CrossRef]
- Yin, L.; Juneja, R.; Lindsay, L.; Pandey, T.; Parker, D.S. Semihard Iron-Based Permanent-Magnet Materials. Phys Rev Appl 2021, 15, 024012. [Google Scholar] [CrossRef]
- Wang, J.-P. Environment-Friendly Bulk Fe16N2 Permanent Magnet: Review and Prospective. J Magn Magn Mater 2019. [Google Scholar] [CrossRef]
- Coene, W.; Hakkens, F.; Coehoorn, R.; de Mooij, D.B.; de Waard, C.; Fidler, J.; Grössinger, R. Magnetocrystalline Anisotropy of Fe3B, Fe2B and Fe1.4Co0.6B as Studied by Lorentz Electron Microscopy, Singular Point Detection and Magnetization Measurements. J Magn Magn Mater 1991, 96, 189–196. [Google Scholar] [CrossRef]
- Iga, A. Magnetocrystalline Anisotropy in (Fe 1- x Co x ) 2 B System. Jpn J Appl Phys 1970, 9, 415–416. [Google Scholar] [CrossRef]
- Kuz’min, M.D.; Skokov, K.P.; Jian, H.; Radulov, I.; Gutfleisch, O. Towards High-Performance Permanent Magnets without Rare Earths. Journal of Physics: Condensed Matter 2014, 26, 064205. [Google Scholar] [CrossRef]
- Edström, A.; Werwiński, M.; Iuşan, D.; Rusz, J.; Eriksson, O.; Skokov, K.P.; Radulov, I.A.; Ener, S.; Kuz’Min, M.D.; Hong, J.; et al. Magnetic Properties of (Fe1-XCox)2 B Alloys and the Effect of Doping by 5d Elements. Phys Rev B Condens Matter Mater Phys 2015, 92. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Palasyuk, O.; Antropov, V.P.; Zhuravlev, I.A.; Belashchenko, K.D.; Nlebedim, I.C.; Dennis, K.W.; Jesche, A.; Kramer, M.J.; Bud’ko, S.L.; et al. Reinvestigation of the Intrinsic Magnetic Properties of (Fe1-XCox)2B Alloys and Crystallization Behavior of Ribbons. J Magn Magn Mater 2020, 513, 167214. [Google Scholar] [CrossRef]
- Kim, K.M.; Kwon, H.W.; Lee, J.G.; Yu, J.H. Coercivity and Phase Evolution in Mechanically Milled (FeCo) 2 B-Type Hard Magnetic Alloy. IEEE Trans Magn 2018, 54, 1–5. [Google Scholar] [CrossRef]
- Wallisch, W.; Fidler, J.; Toson, P.; Sassik, H.; Svagera, R.; Bernardi, J. Synthesis and Characterisation of (Fe,Co)2–3B Microcrystalline Alloys. J Alloys Compd 2015, 644, 199–204. [Google Scholar] [CrossRef]
- Campbell, R.B.; Julien, C.A. Structure of Alnico v. J Appl Phys 1961, 32, S192–S194. [Google Scholar] [CrossRef]
- Zhou, L.; Miller, M.K.; Lu, P.; Ke, L.; Skomski, R.; Dillon, H.; Xing, Q.; Palasyuk, A.; McCartney, M.R.; Smith, D.J.; et al. Architecture and Magnetism of Alnico. Acta Mater 2014, 74, 224–233. [Google Scholar] [CrossRef]
- Ortega, E.; Reddy, S.M.; Betancourt, I.; Roughani, S.; Stadler, B.J.H.; Ponce, A. Magnetic Ordering in 45 Nm-Diameter Multisegmented FeGa/Cu Nanowires: Single Nanowires and Arrays. J Mater Chem C Mater 2017, 5, 7546–7552. [Google Scholar] [CrossRef]
- Zighem, F.; Mercone, S. Magnetization Reversal Behavior in Complex Shaped Co Nanowires: A Nanomagnet Morphology Optimization. J Appl Phys 2014, 116. [Google Scholar] [CrossRef]
- Maurer, T.; Ott, F.; Chaboussant, G.; Soumare, Y.; Piquemal, J.-Y.; Viau, G. Magnetic Nanowires as Permanent Magnet Materials. Appl Phys Lett 2007, 91, 172501. [Google Scholar] [CrossRef]
- Gandha, K.; Elkins, K.; Poudyal, N.; Liu, X.; Liu, J.P. High Energy Product Developed from Cobalt Nanowires. Sci Rep 2014, 4. [Google Scholar] [CrossRef]
- Dumestre, F.; Chaudret, B.; Amiens, C.; Fromen, M.-C.; Casanove, M.-J.; Renaud, P.; Zurcher, P. Shape Control of Thermodynamically Stable Cobalt Nanorods through Organometallic Chemistry. Angewandte Chemie International Edition 2002, 41, 4286–4289. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, J.; Xing, M.; Elkins, J.; Beatty, J.; Liu, J.P. Extraordinary Magnetic Hardening in Nanowire Assemblies: The Geometry and Proximity Effects. Adv Funct Mater 2021, 31, 2010157. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, H.; Jiang, M.; Kang, Q.; Zhou, W.; Wang, P.; Zhou, F. Boron Enhances Oxygen Evolution Reaction Activity over Ni Foam-Supported Iron Boride Nanowires. J Mater Chem A Mater 2020, 8, 13638–13645. [Google Scholar] [CrossRef]
- Beron, F.; Clime, L.; Ciureanu, M.; Menard, D.; Cochrane, R.W.; Yelon, A. First-Order Reversal Curves Diagrams of Ferromagnetic Soft Nanowire Arrays. IEEE Trans Magn 2006, 42, 3060–3062. [Google Scholar] [CrossRef]
- Brown W.F., Jr. Micromagnetics; Wiley: New York, 1963. [Google Scholar]
- Fidler, J.; Schrefl, T. Micromagnetic Modelling - The Current State of the Art. J Phys D Appl Phys 2000, 33, R135–R156. [Google Scholar] [CrossRef]
- Durst, K.-D.; Kronmüller, H. The Coercive Field of Sintered and Melt-Spun NdFeB Magnets. J Magn Magn Mater 1987, 68, 63–75. [Google Scholar] [CrossRef]
- Vansteenkiste, A.; De Wiele, B. V; de Wiele B., V. Mumax: A New High-Performance Micromagnetic Simulation Tool. J Magn Magn Mater 2011, 323, 2585–2591. [Google Scholar] [CrossRef]
- Vansteenkiste, A.; Leliaert, J.; Dvornik, M.; Helsen, M.; Garcia-Sanchez, F.; van Waeyenberge, B. The Design and Verification of MuMax3. AIP Adv 2014, 4, 107133. [Google Scholar] [CrossRef]
- Brown, W.F. THE FUNDAMENTAL THEOREM OF THE THEORY OF FINE FERROMAGNETIC PARTICLES. Ann N Y Acad Sci 1969, 147, 463–488. [Google Scholar] [CrossRef]
- Liu, X.B.; Nlebedim, I.C. Robustness of Magnetocrystalline Anisotropy and Coercivity in Fe–Co–B. Physica B Condens Matter 2024, 683, 415914. [Google Scholar] [CrossRef]
- Coey, J. 2010.
- Gong, M.; Dai, Q.; Ren, S. Magnetic Dipolar Interaction Induced Cobalt Nanowires. Nanotechnology 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Nlebedim, I.C.; Ucar, H.; Hatter, C.B.; McCallum, R.W.; McCall, S.K.; Kramer, M.J.; Paranthaman, M.P. Studies on in Situ Magnetic Alignment of Bonded Anisotropic Nd-Fe-B Alloy Powders. J Magn Magn Mater 2017, 422, 168–173. [Google Scholar] [CrossRef]
- Knowles, J. Packing Factor and Coercivity in Tapes: A Monte Carlo Treatment. IEEE Trans Magn 1985, 21, 2576–2582. [Google Scholar] [CrossRef]
- Lyberatos, A.; Wohlfarth, E.P. A Monte Carlo Simulation of the Dependence of the Coercive Force of a Fine Particle Assembly on the Volume Packing Factor. J Magn Magn Mater 1986, 59, L1–L4. [Google Scholar] [CrossRef]
- Chang, C.-R.; Shyu, J.-P. Particle Interaction and Coercivity for Acicular Particles. J Magn Magn Mater 1993, 120, 197–199. [Google Scholar] [CrossRef]
- Skomski, R.; Liu, Y.; Shield, J.E.; Hadjipanayis, G.C.; Sellmyer, D.J. Permanent Magnetism of Dense-Packed Nanostructures. J Appl Phys 2010, 107, 09A739. [Google Scholar] [CrossRef]
- Knowles, J.E. Coercivity and Packing Density in Acicular Particles. J Magn Magn Mater 1981, 25, 105–112. [Google Scholar] [CrossRef]
- Panagiotopoulos, I.; Fang, W.; Ott, F.; Boué, F.; Aït-Atmane, K.; Piquemal, J.-Y.; Viau, G. Packing Fraction Dependence of the Coercivity and the Energy Product in Nanowire Based Permanent Magnets. J Appl Phys 2013, 114. [Google Scholar] [CrossRef]
- Donev, A.; Stillinger, F.H.; Chaikin, P.M.; Torquato, S. Unusually Dense Crystal Packings of Ellipsoids. Phys Rev Lett 2004, 92, 255506. [Google Scholar] [CrossRef] [PubMed]
- Kusner, W. Upper Bounds on Packing Density for Circular Cylinders with High Aspect Ratio. Discrete Comput Geom 2014, 51, 964–978. [Google Scholar] [CrossRef]
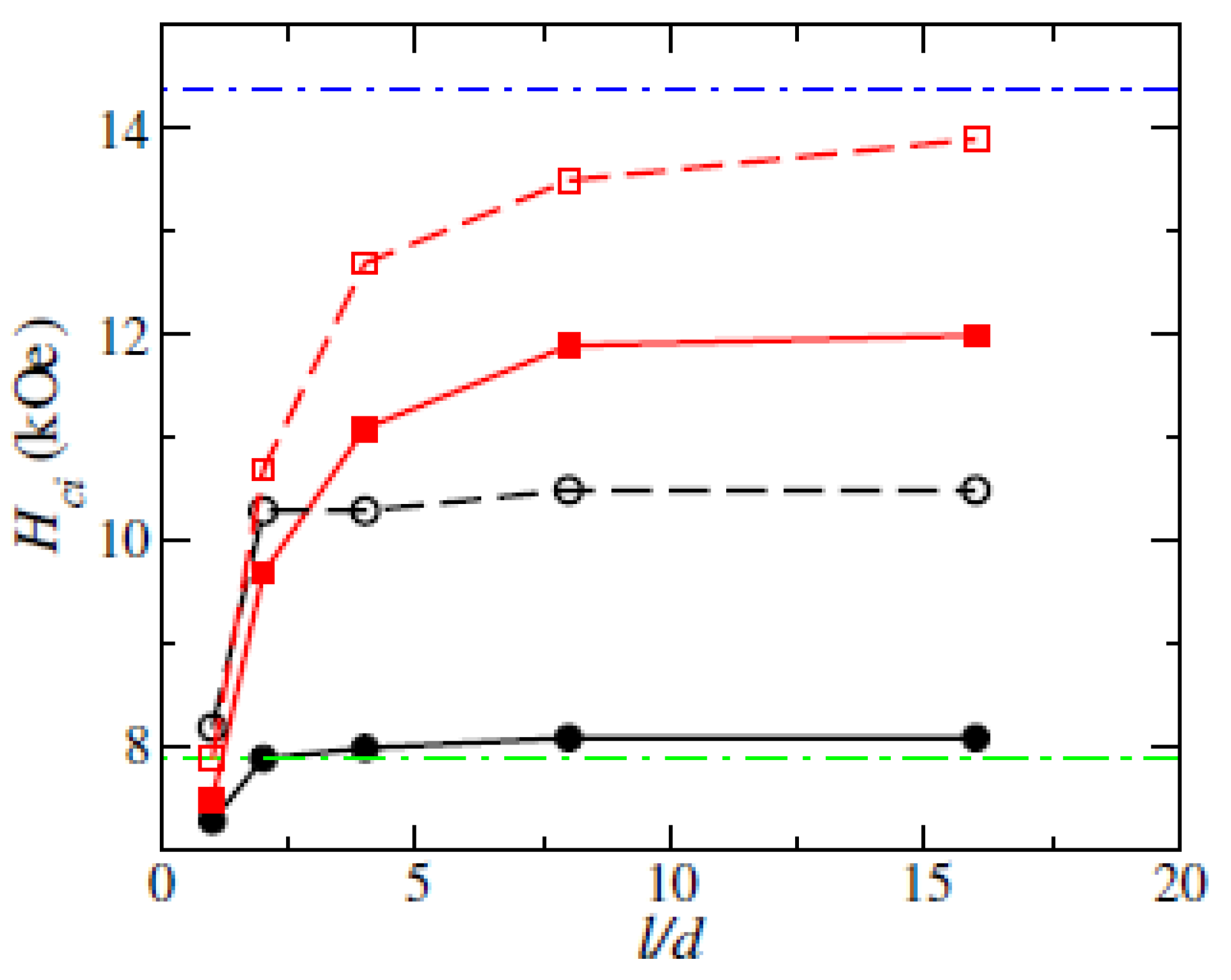
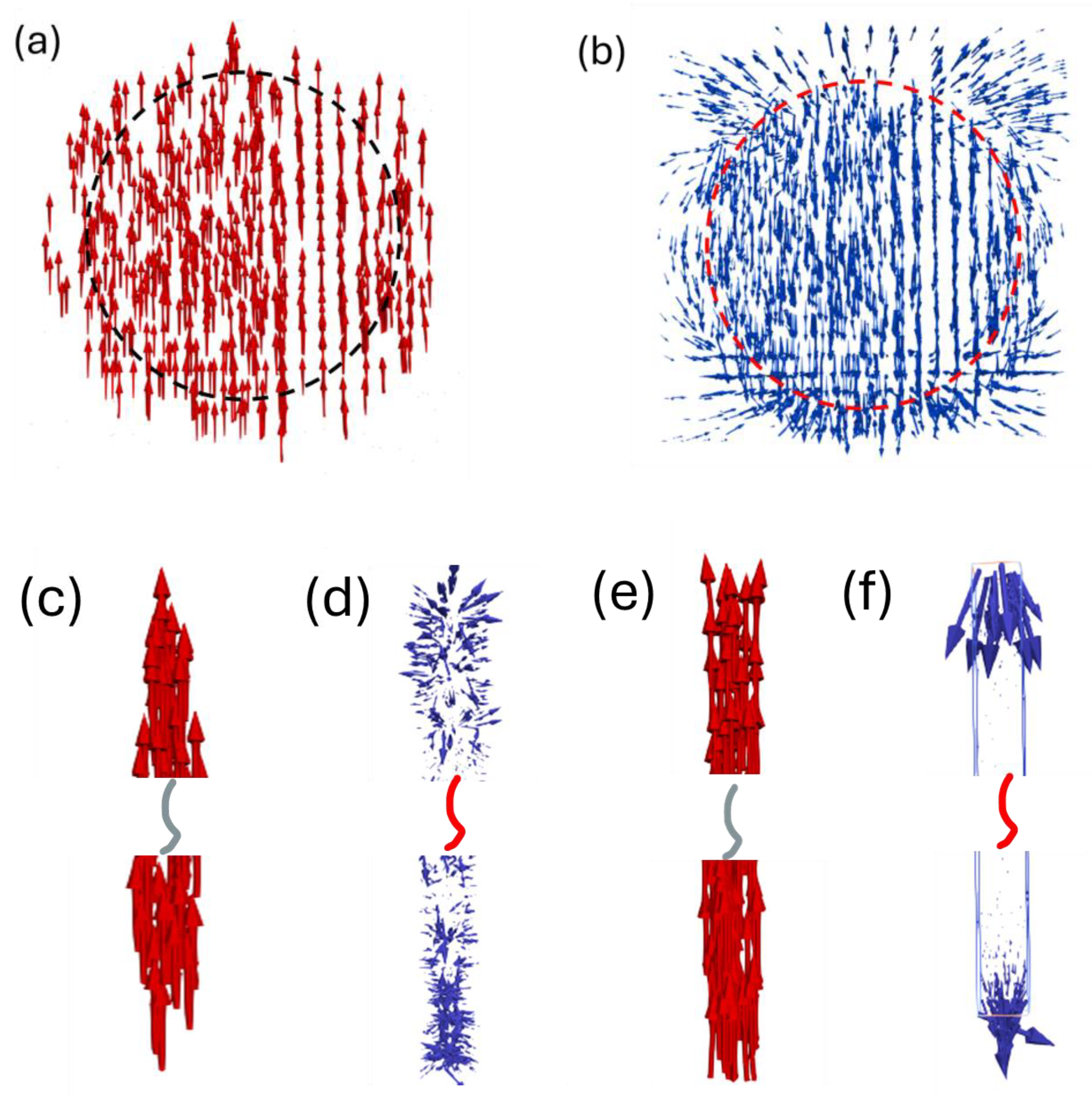
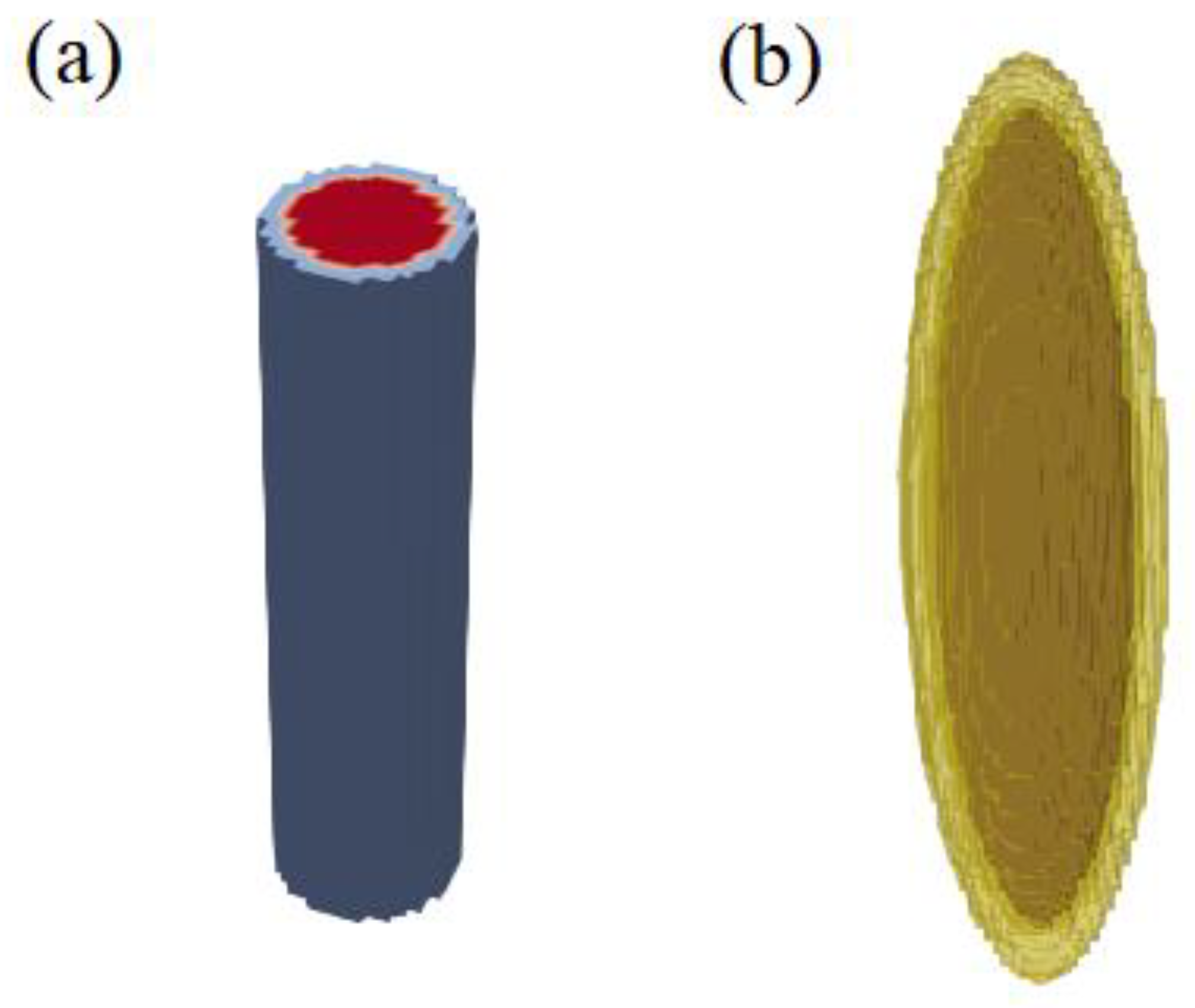
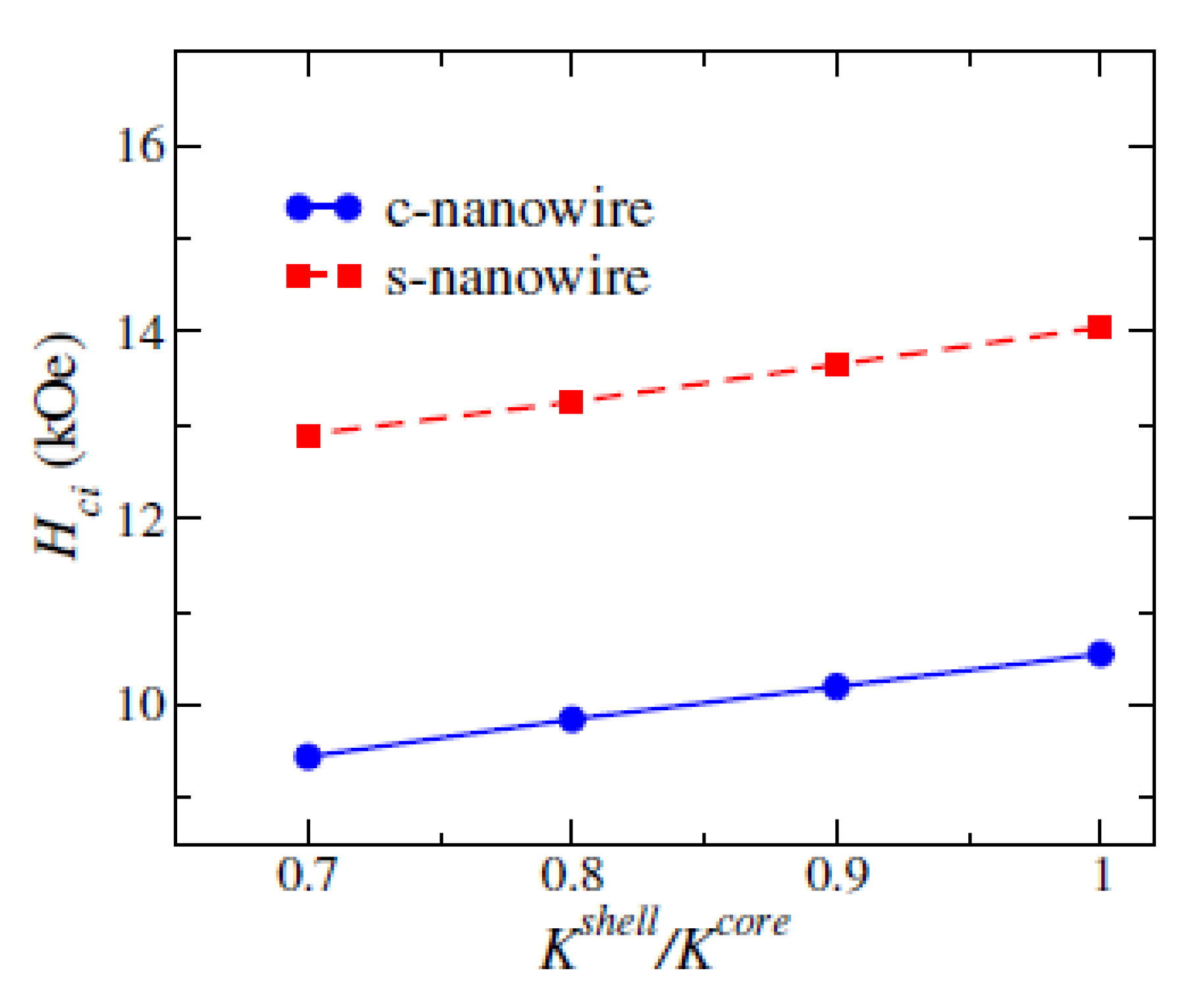
| Size (nm) | SFL Thickness (nm) | Br (kG) | Hci (kOe) | (BH)m (MGOe) | |
|---|---|---|---|---|---|
| c-nanowire | Φ16×256 | 0 | 13.0 | 10.5 | 42 |
| c-nanowire | Φ16×256 | 1 | 13.0 | 9.9 | 42 |
| c-nanowire | Φ16×256 | 2 | 13.0 | 9.4 | 42 |
| c-nanowire | Φ32×256 | 0 | 13.0 | 8.2 | 42 |
| s-nanowire | Φ16×256 | 0 | 13.0 | 14.1 | 42 |
| s-nanowire | Φ16×256 | 1 | 13.0 | 13.3 | 42 |
| s-nanowire | Φ16×256 | 2 | 13.0 | 12.9 | 42 |
| s-nanowire | Φ32×256 | 0 | 13.0 | 12.0 | 42 |
| nanowires | Shape | Size (nm) | A (kOe) | B (kOe) | |
| 1 | s-nanowire | Φ16×256 | 7.9 | 6.1 | |
| 2 | Core-shell s-nanowire | Core Φ 12×256 Shell thickness: 2 |
6.9 | 6.1 | K1 shell =0.7*K1 core |
| 3 | c-nanowire | Φ16×256 | 7.45 | 3.1 | |
| 4 | Core-shell c-nanowire | Core Φ 12×256 Shell thickness: 2 |
6.85 | 3.1 | K1 shell =0.7*K1 core |
| Type | MCA Fluctuation |
Volume content | Br (kG) | Hci (kOe) | (BH)m (MGOe) |
| Bonded, c-nanowire Φ16×256 nm | 0 | 70% | 9.09 | 8.3 | 20.6 |
| Bonded, c-nanowire Φ16×256 nm | 0 | 50% | 6.5 | 8.9 | 10.5 |
| Bonded, c-nanowire Φ16×256 nm | 30% | 70% | 9.09 | 7.3 | 20.6 |
| Bonded, c-nanowire Φ16×256 nm | 30% | 50% | 6.5 | 7.9 | 10.5 |
| Bonded, s-nanowire Φ16×256 nm | 0 | 70% | 9.10 | 9.6 | 20.7 |
| Bonded, s-nanowire Φ16×256 nm | 0 | 50% | 6.5 | 10.8 | 10.5 |
| Bonded, s-nanowire Φ16×256 nm | 30% | 70% | 9.10 | 8.6 | 20.7 |
| Bonded, s-nanowire Φ16×256 nm | 30% | 50% | 6.5 | 9.8 | 10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





