1. Introduction
The combined impact of obstetric syndromes related to placental dysfunction make a significant contribution to global maternal and perinatal morbidity and mortality [
1,
2,
3]. As a result, considerable international effort has been directed at understanding the pathogenesis and pathophysiology of these related conditions to inform prevention and management strategies. Given the heterogeneity of causes of placental dysfunction, it is recognized that a clinically effective approach to prevention and treatment will involve a multimodal intervention strategy [
4,
5]. The introduction of a combined approach using lifestyle, screening, and medical management, as outlined in this review, has the potential to significantly reduce morbidity and mortality, decrease transgenerational transmission of chronic disease, and improve long-term maternal and neonatal health following pregnancy.
The causes of placental dysfunction can be genetic, epigenetic, or environmental. In recent times, there has been increasing interest in the contribution of pre-existing maternal pathophysiological processes, such as insulin resistance, low-grade chronic inflammation, and hyperandrogenism, to placental disorders [
4,
6]. This has resulted in renewed interest in the role of lifestyle interventions for the prevention and treatment of pregnancy complications. Lifestyle-based interventions encompass a variety of domains including nutrition, exercise, smoking cessation, weight management, sleep, stress, and community support. Most of the research has been directed to interventions related to diet and exercise, both of which have been recommended by international obstetric societies to promote healthy pregnancy and reduce adverse outcomes [
7,
8].
First and second trimester screening strategies have been developed following rigorous pre-clinical, case control, observational, intervention, and real-world evaluation studies [
9,
10]. These screening models have been found to have good predictive value for early diagnosis of some obstetric syndromes, such as pre-eclampsia (PE) and fetal growth restriction (FGR), and result in increased surveillance of all women identified as high-risk [
10,
11]. Multivariate screening can be performed at 11-14 weeks gestation using a combination of maternal, biophysical, and serum biochemical markers [
9]. Women identified as high-risk for PE on first trimester multivariate screening can be offered prophylactic treatment with low-dose aspirin [
12]. High-risk women can be monitored by measurement of the serum angiogenic ratio, which can be used in conjunction with ultrasound scans, blood tests, fetal monitoring, and clinical assessment, commencing at 22 weeks gestation [
10]. Following delivery, women identified as high-risk can be assessed for ongoing cardiometabolic risk factors and offered lifestyle support [
13,
14].
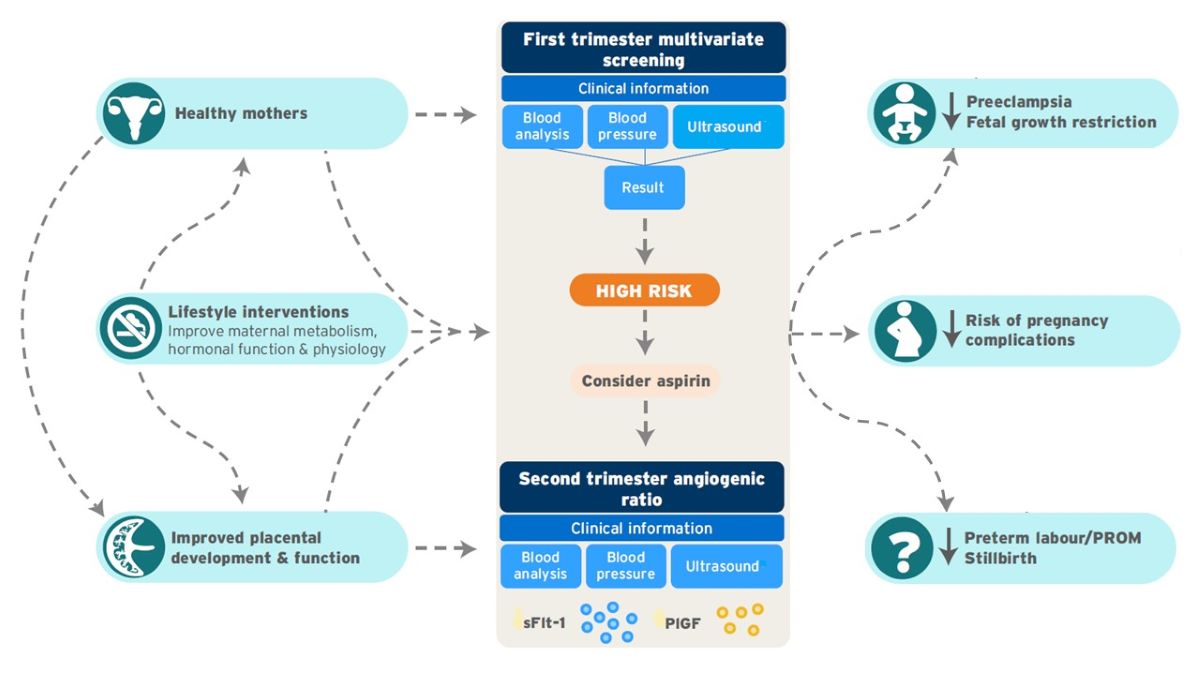
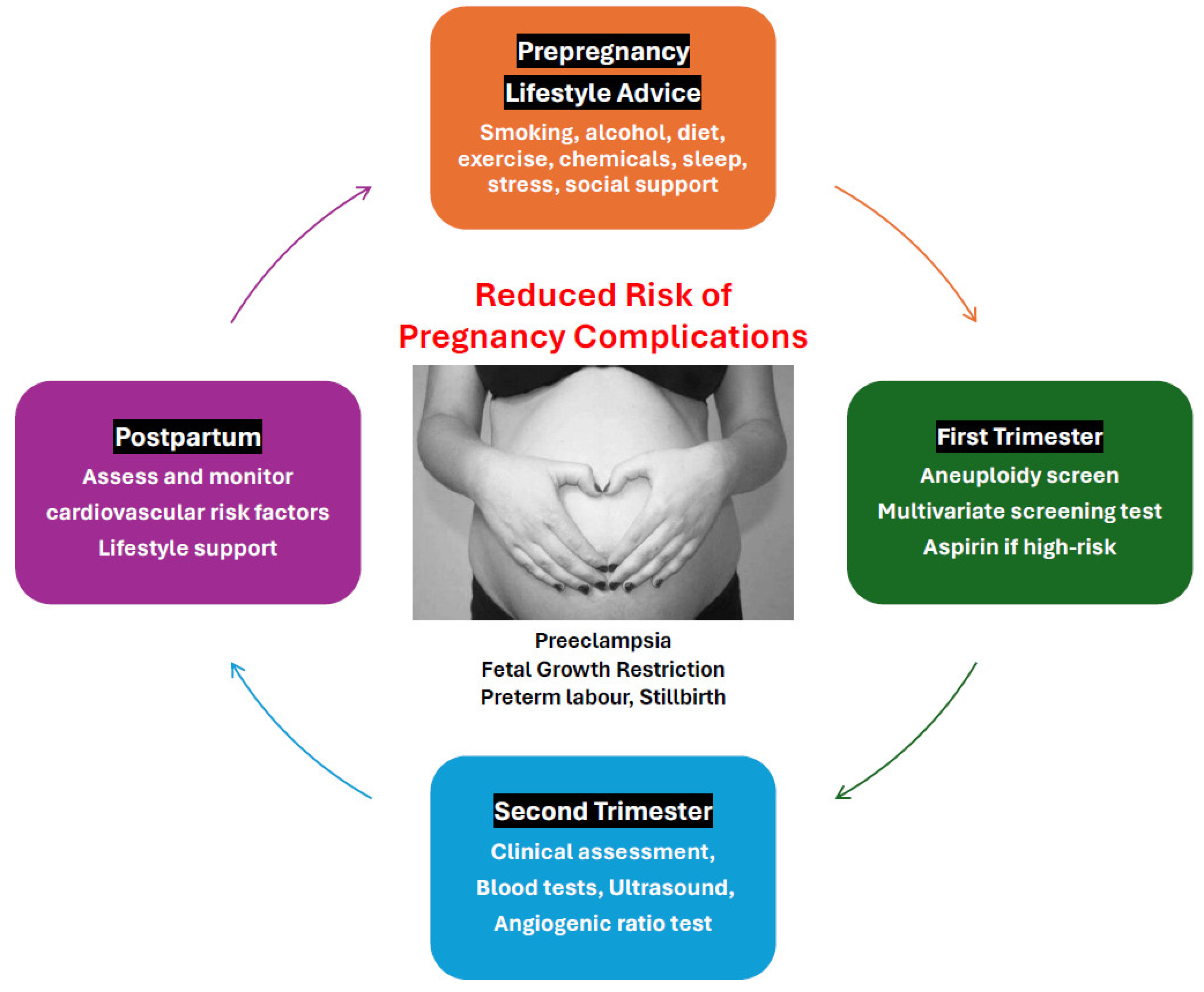
This review outlines a multimodal approach that aims to reduce the risk of pregnancy complications, decrease social disruption, hospitalization, and time away from work and family, and reduce health-care costs. The recommended strategy involves coordinated preconception, antenatal, and postpartum interventions (
Figure 1).
2. Obstetric Syndromes
2.1. Pre-Eclampsia
Hypertensive disorders of pregnancy are a leading cause of maternal-fetal morbidity and mortality worldwide. Pre-eclampsia affects 2-7% of pregnancies globally and is responsible for 70,000 maternal deaths and 500,000 fetal/neonatal deaths every year [
9]. Severe PE occurs in 1-2% of pregnancies and complications associated with this condition account for 15% of direct maternal deaths and 10% of perinatal deaths. Pre-eclampsia is the indication for 20% of labour inductions and 15% of Caesarean sections and accounts for 5-10% of preterm deliveries. Early-onset PE (< 34 weeks) is less common than late-onset but accounts for significantly greater PE-related morbidity, mortality, and health-care costs [
15]. Women with a history of PE have a significantly increased risk of cardiovascular disease, metabolic disorders, chronic hypertension, renal disease, and dementia, later in life [
13,
16,
17,
18,
19]. In addition, the experience of PE can be traumatic to women, their partners, and their support networks [
20].
Many of the features of PE are non-specific (headache, visual disturbance, abdominal pain) and diagnosis based on clinical signs (hypertension, proteinuria) and symptoms is subjective and a poor predictor of adverse outcomes [
21]. Approximately 30% of all pregnancies will be evaluated for PE and require repeat hospital admissions and increased antenatal surveillance, that add significantly to health-care cost [
10]. Early identification of PE using predictive screening models and prophylactic treatment with lifestyle interventions and aspirin, represent a significant advance in perinatal care [
10,
22].
2.1.1. Screening for Pre-eclampsia
Screening models for the detection of obstetric syndromes have primarily focused on identifying women at high-risk of developing PE [
23] and FGR [
24]. First trimester multivariate screening (maternal factors, mean arterial pressure [MAP], uterine artery pulsatility index [UtAPI], and pregnancy-associated plasma protein A or placental growth factor [PlGF]) are effective predictors of PE, having detections rates of 90% for early-onset PE (<34 weeks gestation), 75% for preterm PE (<37 weeks), and 42% for term PE, with a false-positive rate of 10% [
9,
24,
25,
26]. The effectiveness of the multivariate algorithm for detecting FGR is 50% [
24,
27].
Circulating angiogenic proteins, soluble fms-like tyrosine kinase-1 (sFlt-1) and PlGF, have an important biological role in the pathophysiology of PE, are expressed prior to the onset of clinical signs and symptoms, and can be used in the prediction of early-onset PE [
10,
28]. The serum angiogenic factors can be assessed from 22 weeks gestation and have a high negative predictive value to rule out PE in asymptomatic high-risk women and in women suspected of having PE [
10]. The positive predictive value to predict PE and/or adverse outcomes within 4 weeks is 65% when the cut-off of 38 is exceeded [
10,
28]. The angiogenic ratio test (sFlt-1/PlGF) can be used to exclude other conditions that mimic PE (eg non-HELLP thrombocytopenia, chronic hypertension, chronic kidney disease) [
10,
29,
30], and can help discriminate between constitutional small-for-gestational-age and growth-restricted fetuses [
31]. Prediction of adverse outcomes is improved when angiogenic markers are combined with clinical, laboratory, and ultrasonographic data, to guide management [
32].
2.1.2. Placental Pathology in Pre-Eclampsia
The characteristic features of defective placentation in PE, such as incomplete remodeling of the junctional zone segment, atherosis of the decidual basal arterioles, and spiral artery thrombosis, are also found in other obstetric syndromes such as FGR, preterm labour with intact membranes, preterm prelabour rupture of the membranes, placental abruption, preterm labour, and stillbirth [
2,
3,
33,
34,
35,
36,
37]. These changes were initially identified in morphological studies, then more recently by functional investigations (doppler flow, uteroplacental perfusion, and biochemical and immunological studies), and electron microscopy [
2]. Spiral artery remodeling commences in the first trimester of pregnancy when decidual natural killer cells and macrophages initiate disorganization and fragmentation of the vascular smooth muscle resulting in vessel dilatation [
38,
39]. This is followed by endovascular trophoblast invasion and more proximal vessel dilatation, in the second trimester, that extends into the myometrial segment of the spiral arteries and terminal radial arteries [
39]. Failure of deep placentation during the second trimester is a common pathological feature in obstetric syndromes [
2,
3,
40].
2.1.3. Impact of Maternal Pathophysiology on Placentation in Pre-eclampsia
Underlying maternal pathophysiological states before and during pregnancy, such as chronic inflammation [
41,
42,
43,
44], insulin resistance [
45,
46,
47], and hyperandrogenemia [
48,
49,
50,
51,
52], alter placental metabolism and physiology and have a significant impact on placental development and function [
4,
6,
53]. Laboratory cell culture, animal, molecular, human epidemiological, and interventional studies demonstrate clear associations and mechanistic links between maternal pathophysiology and placental dysfunction in PE and other obstetric syndromes [
4,
6,
53].
Normal placental development is dependent on bidirectional feto-maternal communication signals such as cytokines, exosomes, extracellular vesicles, transcription factors, and hormones [
6,
54]. These signals are influenced by sperm and oocyte genetics, epigenetics, and metabolic factors [
55,
56,
57], the physiological state of the maternal decidua, and the underlying maternal systemic metabolic and hormonal environment [
6,
58]. Disturbance of any of these components of normal physiology can lead to abnormal feto-maternal dialogue, deficient trophoblast invasion, altered spiral artery remodeling, and metabolic dysregulation that may all contribute to the common pathophysiological changes seen in obstetric syndromes [
59,
60,
61,
62].
2.1.4. Lifestyle Factors in Pre-Eclampsia
Lifestyle factors have been suspected to play a role in increasing the risk of pregnancy complications for decades [
4,
63]. Over 70 maternal risk factors have been associated with the development of PE [
64]. There is significant overlap in the risk factors for PE and other obstetric syndromes [
65]. Obesity is recognized as the most significant risk for PE and is associated with chronic inflammation, insulin resistance, and hyperandrogenemia, all of which can contribute to placental dysfunction [
4,
7]. International and National Guidelines recommend lifestyle interventions, such as diet [
7] and exercise [
8] for the management of women with PE. The previous emphasis on smoking, alcohol, diet, and exercise has expanded to include stress, sleep, community engagement, social supports, environmental chemicals, and the effects of climate [
66,
67,
68,
69,
70,
71].
2.1.5. Population Attributable Risk of Pre-Eclampsia from Modifiable Risk Factors
Although some of the risk factors associated with PE are clinically important and identify women at significantly increased individual risk, they may only make a small contribution to the total burden of PE in the population [
72]. One way to assess the broader impact of risk factors is to assess the population attributable risk or proportional contribution of a risk factor to the entire population [
73]. For example, it is important to identify women with a history of antiphospholipid syndrome for individual assessment and surveillance, but antiphospholipid syndrome was found to have one of the lowest population attributable risk fractions of 0.18% with a 95% confidence interval (CI) of 0.08 to 0.33%, for PE. The determination of the population attributable risk can also provide important information that informs public health policy and prevention programs [
64,
73].
It is therefore important to identify women at increased individual risk, determine the population attributable risk, and assess whether the relationship between the risk factor and PE is modifiable [
64]. A large systematic review of cohort studies with greater than 1000 participants evaluated the risk of PE in relation to common clinical risk factors in pooled data from 25 million women [
72]. The investigators emphasized the importance of population attributable risk and found that many common risk factors have a modifiable component. The pooled relative risk was used to calculate the population attributable fraction for PE in relation to 16 common clinical risk factors. Nulliparity had the greatest population attributable fraction (32.3%, 95% CI: 27.4-37%). When considered as a group, modifiable risk factors including prepregnancy body mass index (BMI) >25 (23.8%, CI: 22 to 25.6%) and pregestational diabetes (3.7%, 95% CI: 3.1 to 4.3%) made up 27.5% of population attributable risk. It was noted that other risk factors that are linked to obesity, such as chronic hypertension, could also be reduced by a reduction in prepregnancy BMI, which would increase the proportion of modifiable risk for the development of PE [
72]. In addition, other common lifestyle and metabolic-associated risk factors not assessed in this review, such as insulin resistance, polycystic ovary syndrome, and metabolic-dysfunction associated fatty liver disease, suggest that the modifiable population attributable risk for PE would be significant.
A subsequent hierarchical review of the relationship between 78 risk factors and PE by an expert advisory group also emphasized the importance of population attributable fraction related to modifiable risk factors [
64]. It is recognized that modifiable risk factors also contribute to fetal growth restriction [
74,
75], preterm labour [
76,
77], premature rupture of the membranes [
78,
79], and stillbirth [
80,
81]. The implementation of a multimodal intervention model that includes lifestyle advice, multivariate screening, aspirin prophylaxis, and assessment of the serum angiogenic ratio, therefore has the potential to detect and reduce morbidity and mortality related to many obstetric syndromes, as has been demonstrated with PE and FGR (
Figure 2).
2.2. Fetal Growth Restriction (FGR)
Fetal growth restriction is influenced by maternal, fetal, and placental factors, and is a significant cause of perinatal morbidity and mortality [
85,
86]. Between 5–10% of pregnancies are complicated by FGR making it the second leading cause of perinatal mortality in babies without congenital anomalies. In addition, FGR is responsible for 30% of stillbirths [
85]. Placental insufficiency is considered the main cause of FGR, and a variety of screening models related to placental function have been investigated [
87,
88,
89]. Both first trimester multivariate and second trimester angiogenic assessment are predictive of early-onset FGR (< 34 weeks), albeit at lower detection rates than those found in PE [
24]. Early-onset FGR is a significant cause of iatrogenic preterm delivery and early detection of FGR is also important for reducing the incidence of stillbirth.
Many lifestyle interventions have been investigated for their effect on fetal growth and well-being. A systematic review found that unhealthy dietary patterns (high intakes of refined grains, processed meat, high saturated fat or sugar) were associated with lower birth weight (mean difference: −40 g; 95% CI: −61 to −20 g) [
90]. Physical activity during pregnancy has been shown to reduce the risk of gestational diabetes (by reducing blood sugar levels), decrease the risk of PE, lower the risk of Caesarean section, and reduce the severity of prenatal depression [
91,
92,
93,
94]. However, it is recognized that some modification of exercise routines may be necessary to accommodate maternal anatomical and physiological changes in pregnancy [
95]. Low to moderate intensity endurance and resistance training are associated with beneficial maternal and fetal effects [
95]. The evidence suggests that high intensity and volume weight-bearing and aerobic activity should be avoided, particularly during the third trimester, as it may contribute to lower birth-weight [
95,
96,
97]. Many national guidelines now contain specific advice regarding the frequency, duration, and intensity of exercise that is recommended in pregnancy [
8,
98]. These recommendations form part of a comprehensive multimodal intervention model.
2.3. Preterm Labour and Premature Rupture of the Membranes
Preterm birth, defined as delivery before 37 weeks gestation, occurs in 10.6% of pregnancies globally and is the leading cause of perinatal morbidity and mortality in the absence of congenital anomalies [
99]. Preterm labour, with intact membranes or following premature rupture of the membranes, results in two-thirds of preterm births, and PE and FGR account for one-third [
100]. Acute chorioamnionitis, as a cause or consequence, is the most common placental lesion in women with spontaneous preterm labour and vascular lesions are the second most common [
3]. Preterm labour and premature rupture of the membranes are associated with defective placentation in common with other obstetric syndromes [
2,
3]. Since there is significant overlap in the incidence of these conditions with other obstetric syndromes, interventions that reduce the impact of PE and FGR may also lower the incidence of preterm birth.
Maternal nutrition is a major determinant of birth outcomes and offspring health later in life [
101]. A systematic review of observational studies that investigated the effect of dietary patterns in pregnancy found that healthy dietary patterns (high intakes of vegetables, fruits, wholegrains, low-fat dairy, and lean protein foods) were significantly associated with a lower risk of preterm birth with an odds ratio (OR) of 0.79 (95% CI: 0.68 to 0.91) [
90]. The investigators noted that the healthy diet patterns identified in the review were similar to current dietary recommendations in many countries (United Kingdom, United Sates, Canada, China). This data supports the recommendations of the current multimodal model that lifestyle and dietary advice should be aligned with national food and nutrition guidelines.
2.4. Stillbirth
It has been estimated that there are two million stillbirths in the world each year [
102]. The majority of stillbirths (84%) occur in low/middle-income countries, and the causes differ due to socioeconomic factors, both between and within countries [
80,
103,
104,
105]. These include genetic and environmental factors, maternal and fetal co-morbidities, and placental dysfunction. Of these, placental dysfunction, be it acute (abruptio placentae) or chronic (placental insufficiency), is the largest and most clearly defined risk factor. Forty percent of stillbirths occur intrapartum and are probably preventable [
80]. Nineteen percent of stillbirths are associated with maternal risk factors (nulliparity, pre-existing hypertension, increased BMI) that are known to be associated with maternal and placental vascular dysfunction [
81]. Over 50% of stillbirths are therefore preventable and multivariable prediction models and angiogenic factor assessments have the potential to improve early detection of fetal problems and facilitate preventative intervention in this group of women [
5].
An umbrella review of 69 systematic reviews examining factors associated with stillbirth found that maternal age, BMI, and prior adverse pregnancy outcomes (stillbirth, preterm birth, small-for-gestational-age) were better predictors than ultrasound or biochemical markers [
5]. Nevertheless, components of the multivariate model were found to be associated with an increased risk of stillbirth. Placental growth factor had a strong association with stillbirth with an OR of 49.2 (95% CI: 12.7 to 191) and second trimester UtAPI had an OR of 8.3 (95% CI: 3.0–22.4) [
5]. A prospective real-world study of 979 high-risk pregnant women found low PlGF levels (<100 pg/mL) were associated with an increased risk of preterm birth, early-onset PE, and stillbirth (OR 15.9, CI: 7.6-33.3). In addition, low PlGF levels were found to distinguish between placental and fetal causes of stillbirth [
106].
Angiogenic factors have also been found to be of value in risk assessment for predicting stillbirth [
106,
107]. Chaiworapongsa et al. performed a prospective cohort study of 12 pregnant women and found that a reduced PlGF to soluble vascular endothelial growth factor receptor-1 (sVEGFR-1: also known as soluble fms-like tyrosine kinase-1) ratio at 34 weeks had a likelihood ratio of 14 for the prediction of subsequent stillbirth [
107]. A cross-sectional study that included 44 women with unexplained fetal death, found a significantly higher concentration of plasma sVEGFR-1 (p=0.04) than in normal pregnant women [
108]. Future prospective studies will be required to investigate the predictive ability of combined multivariate first trimester screening with second trimester monitoring using angiogenic ratios to predict and reduce rates of both unexplained and syndrome-related stillbirth.
Population level interventions, such as control of malaria and syphilis and optimizing nutrition, may play a significant role in stillbirth prevention at a global level [
109]. A systematic review of behavioural and nutritional interventions before and during pregnancy, concluded that many antepartum stillbirths are preventable through dietary and environmental interventions, and improved antenatal management of high-risk women [
109]. A large cohort study from the United Kingdom found that potentially modifiable risk factors (maternal obesity, smoking in pregnancy, FGR) were associated with over half of all stillbirths [
110]. Therefore, the available evidence suggests that a combined multimodal approach that includes lifestyle and dietary advice also has the potential to reduce the incidence of stillbirth.
Additionally, there have been suggestions that aspirin may have a role in the prevention of stillbirth, however, reported clinical trials have so far been underpowered and have not detected a reduction in risk [
12,
80]. The identification of women at increased risk of pregnancy complications on first and second trimester screening, facilitates increased surveillance and would be expected to help identify fetal compromise prior to stillbirth in some women. Taken together, the available evidence suggests that implementation of the multimodal model may reduce the incidence of stillbirth. This important area of perinatal research should be a priority in future large prospective trials.
3. Medical Management with Acetylsalicylic Acid (Aspirin)
Prophylactic low-dose aspirin therapy has been shown to be both efficacious and cost-effective for preventing pregnancy complications [
15]. When aspirin is initiated in early pregnancy (<16 weeks gestation), it is associated with a significant reduction in early-onset PE [
12], early-onset FGR [
111], and preterm birth [
15]. Women identified as high-risk on first trimester multivariable screening can be offered prophylactic low-dose aspirin, which can be taken at night and continued until 37 weeks gestation. High-risk women can be followed with angiogenic factor assessment from 22 weeks gestation in conjunction with current standard of care. Studies have shown that the reduction in the risk of complications is dependent on high rates of compliance with aspirin treatment [
112]. Women with less than 90% adherence have a greater rate of PE (OR 2.3, 95% CI: 1.2-11.6, p=0.03), FGR (OR 5.8, 95% CI: 1.2-8.3, p=0.001), and preterm birth (OR 5.2, 95% CI: 1.5-8.7, p=0.008) [
113]. Effective education and compliance-aiding strategies are therefore of utmost importance in clinical practice.
Recent preliminary studies have investigated the possibility of ceasing aspirin therapy at 24 to 28 weeks’ gestation in high-risk women, if the angiogenic ratio and/or UtAPI are normal, in an effort to reduce side-effects, and increase compliance and convenience [
114,
115]. A multicentre randomized trial (StopPRE) investigated whether aspirin (150 mg) could be discontinued at 24-28 weeks’ gestation if the angiogenic ratio was normal (<38) [
114]. There was no significant difference in the incidence of pre-term PE in women who ceased aspirin at 24-28 weeks’ (1.48%, 7/473) compared with women who continued aspirin until 37 weeks’ gestation (1.73%, 8/463) (absolute difference -0.25%; 95% CI: -1.86% to 1.36%). The investigators found a higher incidence of minor antepartum hemorrhage in the group that continued aspirin until 37 weeks compared with those who discontinued treatment at 24-28 weeks’ gestation (12.31% vs 7.61%; absolute difference, -4.70; 95% CI: -8.53 to -0.87) [
114]. A secondary analysis of the StopPRE trial showed that discontinuation of aspirin at 24-28 weeks in women with a UtAPI less than the 90th percentile was not inferior to continuing aspirin until 37 weeks’ gestation [
115]. These data also suggests that there is a significant therapeutic effect of aspirin during the second trimester of pregnancy that corresponds to the period of deep placentation that is known to be a common pathological feature in obstetric syndromes [
2]. Further intervention studies are required to investigate the reproducibility and generalizability of these results in diverse population groups [
116].
4. Integrated Clinical Management to Reduce Pregnancy Complications
The proposed model is only one component of comprehensive clinical management strategy to ensure high-quality pregnancy care and prevent complications. Lifestyle recommendations need to be easy to understand, succinct, and follow national and international guidelines [
7,
8]. Antenatal caregivers will need to be informed about the performance and interpretation of the new screening tests, protocols for the measurement of MAP need to be implemented [
117], ultrasonographers will be required to learn techniques for assessing UtAPI [
118], serum biochemical tests need standardization and monitoring for compliance [
119], and clinicians will be required to learn how to integrate components of the model into routine clinical practice. The angiogenic ratio test should be used in conjunction with ultrasound scans, usual blood tests, fetal monitoring, and clinical assessment (
Table 1) [
8].
4.1. Practical aspects of Implementing First Trimester Multivariate Screening
The Fetal Medicine Foundation (FMF) first trimester multivariate risk assessment model is generally used worldwide for the prediction of PE and FGR [
9,
24,
89,
126]. This model has been extensively studied, has undergone successful internal and external validation, and is continually re-evaluated using real-world data [
119,
127]. Multivariate testing is superior to risk factor-based models for identifying high-risk women and allows individualized antenatal care [
128,
129]. Maternal risk factors and biomarkers can be entered into an on-line risk calculator free of charge at
https://fetalmedicine.org/research/assess/preeclampsia.
The FMF screening test has been endorsed by the International Federation of Gynecology and Obstetrics [
26], the International Society for the Study of Hypertension in Pregnancy [
9] and many National Obstetric Societies [
8]. In order to maintain optimal screening performance, it is important to follow standardized methods for performing the required biophysical (MAP, UtAPI) and biochemical (PlGF) measurements.
4.1.1. Measurement of Mean Arterial Blood Pressure (MAP)
Determining MAP antenatally is inexpensive, non-invasive, quick and can be performed with minimal training. However, its effectiveness depends on various factors such as the population studied, user training, measurement accuracy, and the protocols for intervention based on the results [
119]. Inaccurate measurements of MAP affect the performance of the screening test and impact the risk estimate given to the patient [
117]. Standardized measurement protocols have therefore been developed to limit data errors entered into the FMF risk calculator.
Mean arterial blood pressure is measured with women sitting with their back against the seat, legs uncrossed, and arms supported at the level of the heart. The correct cuff size is selected, and blood pressure is measured in both arms simultaneously using a validated automated device. Two readings are taken from each arm, 1 minute apart, and MAP is calculated from the average of the 4 measurements [
117]. Automated blood pressure devices require calibration at regular intervals to ensure reliable measurements over time [
130].
4.1.2. Measurement of Uterine Artery Pulsatility Index (UtAPI)
The reproducibility and reliability of UtAPI assessment is dependent on the use of standardized protocols that take measurements at defined anatomical locations using specific ultrasound machine settings [
118]. A transabdominal ultrasound transducer is used to obtain a sagittal section of the uterus at the level of the internal cervical os. The uterine arteries are identified using Color Doppler flow mapping followed by pulsed wave Doppler measurement of UtAPI and peak systolic velocity, when 3 consecutive waveforms are obtained. Standardized transducer positions and Doppler settings are employed [
119,
131].
Ultrasonographers require specific training in UtAPI measurement technique followed by regular assessment of their results for continued accreditation [
118]. This procedure has been shown to reduce operator- and technique-dependent measurement variability, and improve detection rates for PE [
132,
133]. Measurement of UtAPI can be taken between 11- and 14-weeks’ gestation at the same time as the first trimester scan.
4.1.3. Measurement of Placental Growth Factor (PlGF) and Compliance Monitoring
Standardized protocols are required to minimize variations in the measurement of PlGF that can arise due to changes in reagent batches, fluctuations in temperature, deviations from manufacturer protocols, and the absence of a continuous quality control process [
119,
134,
135]. Automated assays allow standardized measurements with rapid availability of results [
134]. Measurement of PlGF can be conducted on the same blood sample as routine blood tests for first trimester aneuploidy screening.
A comparison of 3 commercially available automated immunoassays found that there was considerable difference between raw data values between different platforms, that was likely to be clinically significant [
134]. The authors recommended that reference ranges specific for each platform should be reported with raw data values, when PlGF measurement is used in clinical practice. Conversion of raw data to multiples of median values allows direct comparison of results between different platforms. Analyzers need frequent calibration and results are regularly monitored to ensure consistency.
4.1.4. Integrating Angiogenic Ratio Testing into Clinical Practice
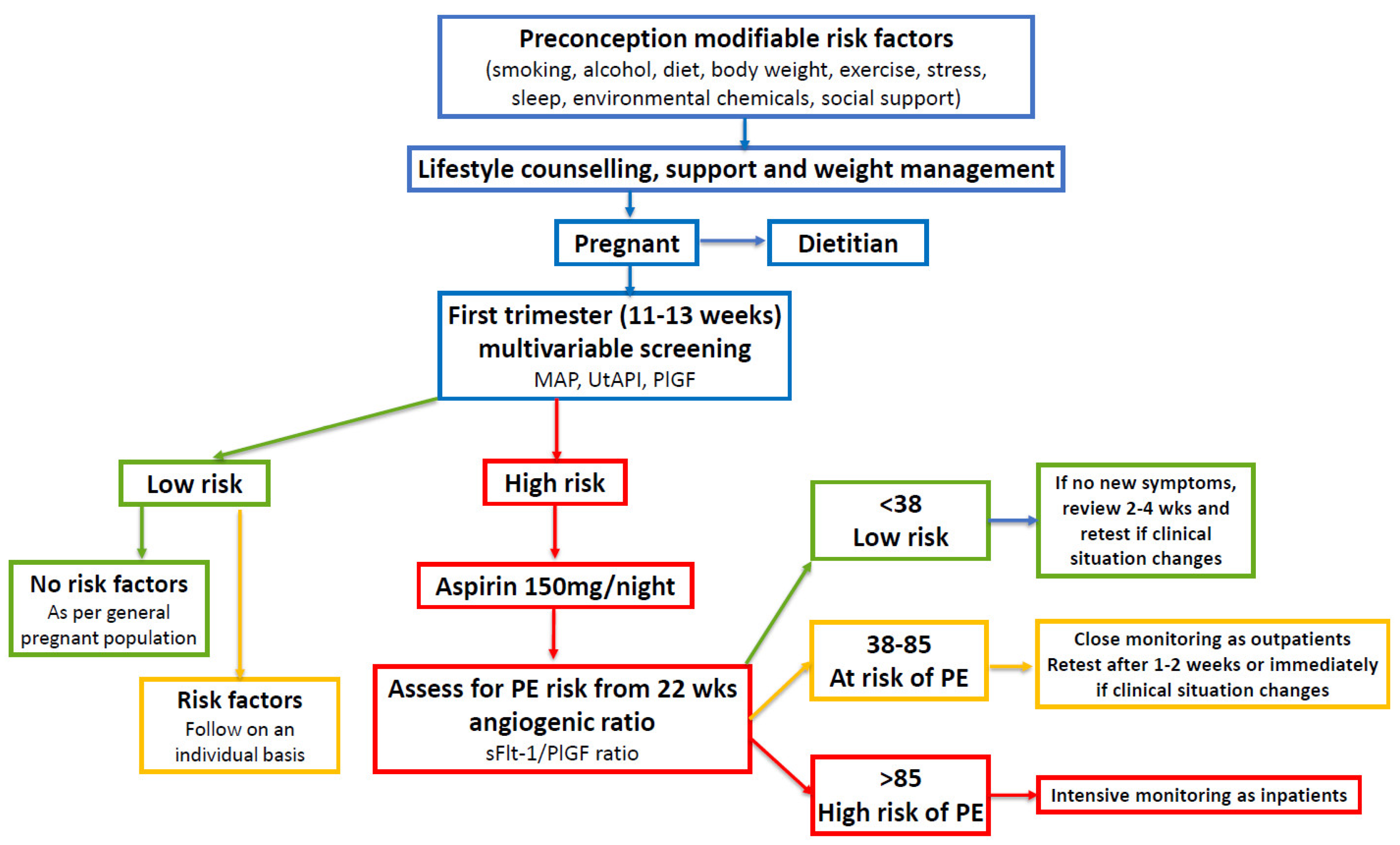
There is now international consensus that all pregnant women should be offered first trimester multivariate screening [
9,
26]. Approximately 10% of women will be classified as high-risk on the basis of maternal factors, placental biomarkers, MAP, and UtAPI. The data are entered into the FMF risk calculator and high-risk women can be offered prophylactic treatment with low-dose aspirin [
12]. Asymptomatic high-risk women may then be followed with monthly sFlt-1/PlGF ratio tests from 22 weeks (figure 2) [
10].
An angiogenic ratio <38 can rule out the onset of PE for 1 week with a NPV of 99.3% and up to 4 weeks with a NPV of 94.3% [
10]. This can provide reassurance to clinicians and women for continued outpatient management [
136]. Women with an intermediate ratio result of 38-85 require increased outpatient monitoring. This may include clinical assessment, ultrasound, cardiotocography, blood tests, repeat blood pressure measurement, and a repeat angiogenic ratio test in 1-2 weeks, or sooner, if the clinical situation changes. Women with a sFlt-1/PlGF ratio >85 require intensive monitoring, usually as inpatients.
Most of the research on angiogenic factors has so far been related to predicting, diagnosing, and/or managing PE and its complications, as well as assessing the severity and the associated rate of clinical deterioration in patients with PE. However, emerging evidence suggests that altered levels of the sFlt-1/PlGF ratio or PlGF itself are also associated with FGR, preterm birth and stillbirth [
137,
138,
139]. This reflects the common pathogenesis of such outcomes as often being related to placental dysfunction, of which angiogenic biomarker imbalance is a feature.
Additionally, angiogenic factor measurement may be of clinical value in the differential diagnosis of PE-like conditions which may occur during pregnancy [
10,
29,
30,
82]. These include presentations involving exacerbations of chronic hypertension, systemic lupus erythematosus, diabetic nephropathy, renal transplant rejection and other chronic kidney diseases, as well as new presentations of conditions manifesting as hypertension (eg phaeochromocytoma), liver dysfunction (eg hepatitis), proteinuria (eg nephrotic syndrome) or thrombocytopaenia (eg idiopathic thrombocytopaenic purpura).
At a research level, these angiogenic biomarkers will also be valuable in helping the selection of suitable trial entrants for studies examining the treatment and/or management of PE and related disorders. For example, an sFlt-1/PlGF ratio above a certain cut-off level could be an inclusion criterion for recruitment to a study, thereby optimizing the number of suitable at-risk patients enrolled and facilitating sufficient sample size achievement in a more cost-effective manner than might otherwise be the case.
Also, it is worthy of note that support for the use of angiogenic biomarkers by national authorities has been gathering momentum [
140,
141] and health economic evaluations of their use in practice have been consistently positive [
142]. A review of 9 studies that investigated the cost-effectiveness of the use of diagnostic angiogenic biomarkers in women suspected of having PE found that all studies demonstrated cost savings [
142].
Overall, the expanded use of angiogenic factor measurement as a standard part of antenatal care has the potential to improve maternal and perinatal outcomes in pregnancies complicated by placental dysfunction. An elevated angiogenic ratio facilitates early diagnosis of placental dysfunction and effective intervention can mean a cost-beneficial use of limited health funds. Equally important, having a normal angiogenic ratio excludes a diagnosis of placental dysfunction and can mean avoiding ineffective overuse of scarce health resources.
6. Strengths and Limitations of the Current Review
6.1. Strengths
Individual components of the proposed model have been extensively evaluated in case control, prospective, randomized, and real-world implementation studies. There is a large body of research investigating the role of nutrition in promoting healthy pregnancy and preventing complications. In summary, systematic reviews of observational studies, expert reviews, and national and international guidelines, support dietary and exercise recommendations for lifestyle-based interventions before, during, and after pregnancy. Multivariate and angiogenic factor screening strategies have been extensively evaluted over two decades in a variety of ethnic populations and clinical environments. Over 75 randomized trials have consistently shown a significant reduction in pregnancy complications, such as PE and fetal growth restriction, using prophylactic low-dose aspirin. The proposed multimodal model is the first time all of the individual components have been integrated into a sequential algorithm that can be integrated into existing clinical practice structures.
6.2. Limitations
The implementation of this model has some limitations. Education and training of health-care practitioners in various aspects of the proposed model will be required. Protocols for the measurement of mean arterial blood pressure need to be implemented. Ultrasonographers will need to be upskilled and accredited in techniques for assessing uterine artery pulsatility index, which is a measurement taken at the time of the first trimester ultrasound. Protocols to ensure that laboratories comply with quality control of serum biomarker assays will also be needed. Doctors managing pregnant women will require information about how to integrate angiogenic ratio results into existing clinical management practices. The multivariable model has a 10% false positive rate, so some low-risk women will be assessed as high-risk, and adequate counselling will be required. Equity of access to qualified professionals will take time during the training and implementation phase and real world evaluation of the combined multimodal model will be essential.
7. Conclusions
Pregnancy conditions resulting from placental dysfunction may complicate up to 30 million pregnancies worldwide annually. Implementation of a multimodal integrated management strategy using lifestyle, screening, and medical treatment has the potential to significantly reduce pregnancy complications, decrease maternal and perinatal morbidity and mortality, limit transgenerational transmission of chronic disease, reduce future maternal cardiometabolic risk, decrease health-care related costs, and improve quality of life. Translation of validated components of this model into clinical practice should be a global health-care priority.
Author Contributions
Conceptualization, JP, PH, SB; writing - original draft preparation, JP, PH, SB; writing – review and editing, JP, PH, SB. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hoffman MK. The great obstetrical syndromes and the placenta. BJOG An Int J Obstet Gynaecol. 2023;130(S3):8–15.
- Brosens I, Puttemans P, Benagiano G. Placental bed research: I. The placental bed: From spiral arteries remodeling to the great obstetrical syndromes. Am J Obstet Gynecol. 2019;221(5):437–56.
- Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol [Internet]. 2011;25(3):313–27. Available from. [CrossRef]
- Parker J, O’Brien C, Yeoh C, Gersh FL, Brennecke S. Reducing the Risk of Pre-Eclampsia in Women with Polycystic Ovary Syndrome Using a Combination of Pregnancy Screening, Lifestyle, and Medical Management Strategies. J Clin Med. 2024;13(1774):1–33.
- Townsend R, Sileo FG, Allotey J, Dodds J, Heazell A, Jorgensen L; et al. Prediction of stillbirth: An umbrella review of evaluation of prognostic variables. BJOG An Int J Obstet Gynaecol. 2021;128(2):238–50.
- Burton GJ, Jauniaux E. The human placenta: New perspectives on its formation and function during early pregnancy. Proc R Soc B Biol Sci. 2023;290:20230191.
- Kinshella MLW, Pickerill K, Bone JN, Prasad S, Campbell O, Vidler M; et al. An evidence review and nutritional conceptual framework for pre-eclampsia prevention. Br J Nutr. 2023;130(6):1065–76.
- Society of Obstetric Medicine Australia and New Zealand, Hypertension in Pregnancy Guideline, Sydney; 2023. [Internet]. Available from: https://www.somanz.org/hypertension-in-pregnancy-guideline-2023/.
- Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens [Internet]. 2022;27(October 2021):148–69. Available from. [CrossRef]
- Verlohren S, Brennecke SP, Galindo A, Karumanchi SA, Mirkovic LB, Schlembach D; et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens [Internet]. 2022;27(August 2021):42–50. Available from. [CrossRef]
- Serrano B, Bonacina E, Rodo C, Garcia-Manau P, Sanchez-Duran MÁ, Pancorbo M; et al. First-trimester screening for pre-eclampsia and small for gestational age: A comparison of the gaussian and Fetal Medicine Foundation algorithms. Int J Gynecol Obstet. 2023;160(1):150–60.
- Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377(7):613–22.
- Lewey J, Beckie TM, Brown HL, Brown SD, Garovic VD, Khan SS; et al. Opportunities in the Postpartum Period to Reduce Cardiovascular Disease Risk after Adverse Pregnancy Outcomes: A Scientific Statement from the American Heart Association. Circulation. 2024;149(7):E330–46.
- Hinkle SN, Schisterman EF, Liu D, Pollack AZ, Yeung EH, Mumford SL; et al. Pregnancy Complications and Long-Term Mortality in a Diverse Cohort. Circulation. 2023;147(13):1014–25.
- Ortved D, Hawkins TLA, Johnson JA, Hyett J, Metcalfe A. Cost-effectiveness of first-trimester screening with early preventative use of aspirin in women at high risk of early-onset pre-eclampsia. Ultrasound Obstet Gynecol. 2019;53(2):239–44.
- Olié V, Lailler G, Torres MJ, Regnault N, Carcaillon-Bentata L, Blacher J. Young-Onset Dementia Among Individuals With History of Preeclampsia. JAMA Netw open. 2024;7(5):e2412870.
- Schliep KC, Shaaban CE, Meeks H, Fraser A, Smith KR, Majersik JJ; et al. Hypertensive disorders of pregnancy and subsequent risk of Alzheimer’s disease and other dementias. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2023;15(2):1–10.
- Boucheron P, Lailler G, Moutengou E, Regnault N, Gabet A, Deneux-Tharaux C; et al. Hypertensive disorders of pregnancy and onset of chronic hypertension in France: The nationwide CONCEPTION study. Eur Heart J. 2022;43(35):3352–61.
- Wu R, Wang T, Gu R, Xing D, Ye C, Chen Y; et al. Hypertensive Disorders of Pregnancy and Risk of Cardiovascular Disease-Related Morbidity and Mortality: A Systematic Review and Meta-Analysis. Cardiol. 2020;145(10):633–47.
- East C, Conway K, Pollock W, Frawley N, Brennecke S. Women’s experiences of preeclampsia: Australian action on preeclampsia survey of women and their confidants. J Pregnancy. 2011;2011:375653.
- Creswell L, O’gorman N, Palmer KR, Costa F da S, Rolnik DL. Perspectives on the Use of Placental Growth Factor (PlGF) in the Prediction and Diagnosis of Pre-Eclampsia: Recent Insights and Future Steps. Int J Womens Health. 2023;15(February):255–71.
- Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am J Obstet Gynecol [Internet]. 2018;218(3):287-293.e1. Available from. [CrossRef]
- Wright D, Wright A, Nicolaides KH. The competing risk approach for prediction of preeclampsia. Am J Obstet Gynecol [Internet]. 2020;223(1):12-23.e7. Available from. [CrossRef]
- Tousty P, Fraszczyk-Tousty M, Golara A, Zahorowska A, Sławiński M, Dzidek S; et al. Screening for Preeclampsia and Fetal Growth Restriction in the First Trimester in Women without Chronic Hypertension. J Clin Med. 2023;12(17):5582.
- O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC; et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol. 2016;214(1):103.e1-103.e12.
- Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynecol Obstet. 2019;145(S1):1–33.
- Karagiannis G, Akolekar R, Sarquis R, Wright D, Nicolaides KH. Prediction of small-for-gestation neonates from biophysical and biochemical markers at 11-13 weeks. Fetal Diagn Ther. 2011;29(2):148–54.
- Dröge LA, Perschel FH, Stütz N, Gafron A, Frank L, Busjahn A; et al. Prediction of Preeclampsia-Related Adverse Outcomes With the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor)-Ratio in the Clinical Routine: A Real-World Study. Hypertension. 2021;77(2):461–71.
- Hernández-Pacheco JA, Rosales-Zamudio CI, Borboa-Olivares H, Espejel-Núñez A, Parra-Hernández S, Estrada-Gutiérrez G; et al. The sFlt-1/PlGF ratio as a triage tool to identify superimposed preeclampsia in women with chronic hypertension in emergency rooms. Pregnancy Hypertens [Internet]. 2020;21(April):38–42. Available from. [CrossRef]
- Perni U, Sison C, Sharma V, Helseth G, Hawfield A, Suthanthiran M; et al. Angiogenic factors in superimposed preeclampsia: A longitudinal study of women with chronic hypertension during pregnancy. Hypertension. 2012;59(3):740–6.
- Rajiv P, Cade T, Dean J, Jones GD, Brennecke SP. Maternal serum soluble fms-like tyrosine kinase-1–to–placental growth factor ratio distinguishes growth-restricted from non–growth-restricted small-for-gestational-age fetuses. AJOG Glob Reports. 2024;4(1):1–10.
- Dragan I, Georgiou T, Prodan N, Akolekar R, Nicolaides KH. Screening for pre-eclampsia using sFlt-1/PlGF ratio cut-off of 38 at 30–37 weeks’ gestation. Ultrasound Obstet Gynecol. 2017;49(1):73–7.
- McMaster-Fay RA. Failure of physiologic transformation of the spiral arteries of the uteroplacental circulation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2004;191(5):1837–8.
- Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S; et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187(5):1137–42.
- Kim JY, Kim YM. Acute atherosis of the uterine spiral arteries: Clinicopathologic implications. J Pathol Transl Med. 2015;49(6):462–71.
- Labarrere CA, DiCarlo HL, Bammerlin E, Hardin JW, Kim YM, Chaemsaithong P; et al. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am J Obstet Gynecol [Internet]. 2017;216(3):287.e1-287.e16. Available from:. [CrossRef]
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol [Internet]. 2011;204(3):193–201. Available from:. [CrossRef]
- Bacon SJ, Zhu Y, Ghosh P. Early spiral arteriole remodeling in the uterine–placental interface: A rat model. J Anat. 2024;(October 2023):1–13.
- Pijnenborg R, Vercruysse L, Hanssens M. The Uterine Spiral Arteries In Human Pregnancy: Facts and Controversies. Placenta. 2006;27(9–10):939–58.
- Pijnenborg, R. Brosens, I. Romero R. Placental bed disorders: Basic science and it’s translation to obstetrics [Internet]. Pijnenborg, R. Brosens, I. Romero R, editor. Cambridge Medicine; 2010. 1–309 p. Available from. [CrossRef]
- Murthi P, Pinar AA, Dimitriadis E, Samuel CS. Inflammasomes—A molecular link for altered immunoregulation and inflammation mediated vascular dysfunction in preeclampsia. Int J Mol Sci. 2020;21:1406.
- Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211(1):165–79.
- Matteo M, Serviddio G, Massenzio F, Scillitani G, Castellana L, Picca G; et al. Reduced percentage of natural killer cells associated with impaired cytokine network in the secretory endometrium of infertile women with polycystic ovary syndrome. Fertil Steril [Internet]. 2010;94(6):2222-2227.e3. Available from:. [CrossRef]
- Redman CWG, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 I):499–506.
- Vega M, Mauro M, Williams Z. Direct toxicity of insulin on the human placenta and protection by metformin. Fertil Steril [Internet]. 2019;111(3):489-496.e5. Available from. [CrossRef]
- Lassance L, Haghiac M, Leahy P, Basu S, Minium J, Zhou J; et al. Identification of early transcriptome signatures in placenta exposed to insulin and obesity. Am J Obstet Gynecol [Internet]. 2015;212(5):647.e1-647.e11. Available from:. [CrossRef]
- Tarkun I, Arslan BC, Cantürk Z, Türemen E, Şahin T, Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: Relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89(11):5592–6.
- Koster MPH, DeWilde MA, Veltman-Verhulst SM, Houben ML, Nikkels PGJ, Van Rijn BB; et al. Placental characteristics in women with polycystic ovary syndrome. Hum Reprod. 2015;30(12):2829–37.
- Naver K V., Grinsted J, Larsen SO, Hedley PL, Jørgensen FS, Christiansen M; et al. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG An Int J Obstet Gynaecol. 2014;121(5):575–81.
- Sathish Kumar, Geoffrey H Gordon, David H Abbott, Mishra J. Androgens in maternal vascular and placental function: Implications for Preeclampsia Pathogenesis. Reproduction. 2018;156:R155–67.
- Gopalakrishnan K, Mishra JS, Chinnathambi V, Vincent KL, Patrikeev I, Motamedi M; et al. Elevated Testosterone Reduces Uterine Blood Flow, Spiral Artery Elongation, and Placental Oxygenation in Pregnant Rats. Hypertension. 2016;67(3):630–9.
- Frolova AI, O’Neill K, Moley KH. Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol Endocrinol. 2011;25(8):1444–55.
- Aplin JD, Myers JE, Timms K. John D. Aplin , Jenny E. Myers , Kate Timms and Melissa Westwood. Nat Rev |Endocrinology [Internet]. 2020;16(9):479–94. Available from: https://www.nature.com/articles/s41574-020-0372-6.
- Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV; et al. Pre-eclampsia. Nat Rev Dis Prim. 2023;9(1):1–22.
- Trigg NA, Skerrett-Byrne DA, Xavier MJ, Zhou W, Anderson AL, Stanger SJ; et al. Acrylamide modulates the mouse epididymal proteome to drive alterations in the sperm small non-coding RNA profile and dysregulate embryo development. Cell Rep [Internet]. 2021;37(1):109787. Available from. [CrossRef]
- Schjenken JE, Sharkey DJ, Green ES, Chan HY, Matias RA, Moldenhauer LM; et al. Sperm modulate uterine immune parameters relevant to embryo implantation and reproductive success in mice. Commun Biol [Internet]. 2021;4(1):1–14. Available from:. [CrossRef]
- Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol [Internet]. 2011;31(3):363–73. Available from:. [CrossRef]
- Burton GJ, Cindrova-Davies T, Turco MY. Review: Histotrophic nutrition and the placental-endometrial dialogue during human early pregnancy. Placenta [Internet]. 2020;102(February):21–6. Available from. [CrossRef]
- Rabaglino MB, Conrad KP. Evidence for shared molecular pathways of dysregulated decidualization in preeclampsia and endometrial disorders revealed by microarray data integration. FASEB J. 2019;33(11):11682–95.
- Pollheimer J, Vondra S, Baltayeva J, Beristain AG, Knöfler M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol. 2018;9(NOV):1–18.
- Doshani A, Konje JC. Placental dysfunction in obese women and antenatal surveillance. Best Pract Res Clin Obstet Gynaecol [Internet]. 2023;91:102407. Available from. [CrossRef]
- Kim DW, Young SL, Grattan DR, Jasoni CL. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol Reprod. 2014;90(6):1–11.
- Belkacemi L, Michael Nelson D, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biol Reprod. 2010;83(3):325–31.
- Elawad T, Scott G, Bone JN, Elwell H, Lopez CE, Filippi V; et al. Risk factors for pre-eclampsia in clinical practice guidelines: Comparison with the evidence. BJOG An Int J Obstet Gynaecol. 2022;(September):1–17.
- National Academies of Sciences Engineering and Medicine. Systemic Influences on Outcomes in Pregnancy and Childbirth [Internet]. Birth Settings in America: Outcomes, Quality, Access, and Choice. 2020. Available from. [CrossRef]
- Yüzen D, Graf I, Diemert A, Arck PC. Climate change and pregnancy complications: From hormones to the immune response. Front Endocrinol (Lausanne). 2023;14(April):1–11.
- Sampathkumar S, Parkhi D, Ghebremichael-Weldeselassie Y, Sukumar N, Saravanan P. Effectiveness of pre-pregnancy lifestyle in preventing gestational diabetes mellitus—A systematic review and meta-analysis of 257,876 pregnancies. Nutr Diabetes. 2023;13(1):1–12.
- Stephenson J, Heslehurst N, Hall J, Schoenaker DAJM, Hutchinson J, Cade JE; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet [Internet]. 2018;391(10132):1830–41. Available from:. [CrossRef]
- Romero R, Badr MS. A role for sleep disorders in pregnancy complications: Challenges and opportunities. Am J Obstet Gynecol [Internet]. 2014;210(1):3–11. Available from:. [CrossRef]
- Traylor CS, Johnson JD, Kimmel MC, Manuck TA. Effects of psychological stress on adverse pregnancy outcomes and nonpharmacologic approaches for reduction: An expert review. Am J Obstet Gynecol MFM. 2020;2(4):100229.
- Padula AM, Monk C, Brennan PA, Borders A, Barrett ES, McEvoy CT; et al. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors—Implications for research on perinatal outcomes in the ECHO program. J Perinatol. 2020;40(1):10–24.
- Bartsch E, Medcalf KE, Park AL, Ray JG, Al-Rubaie ZTA, Askie LM; et al. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:1753.
- Askari M, Namayandeh SM. The difference between the population attributable risk (Par) and the potentioal impact fraction (pif). Iran J Public Health. 2020;49(10):2018–9.
- Lewandowska M. Maternal obesity and risk of low birth weight, fetal growth restriction, and macrosomia: Multiple analyses. Nutrients. 2021;13(4):1213.
- Prabhu N, Smith N, Campbell D, Craig LC, Seaton A, Helms PJ; et al. First trimester maternal tobacco smoking habits and fetal growth. Thorax. 2010;65(3):235–40.
- Nisar MI, Yoshida S. Population-based rates, risk factors and consequences of preterm births in South-Asia and sub-Saharan Africa: A multi-country prospective cohort study. J Glob Health. 2022;12:04011.
- Mitrogiannis I, Evangelou E, Efthymiou A, Kanavos T, Birbas E, Makrydimas G; et al. Risk factors for preterm birth: An umbrella review of meta-analyses of observational studies. BMC Med [Internet]. 2023;21(1):1–17. Available from. [CrossRef]
- Bouvier D, Forest JC, Blanchon L, Bujold E, Pereira B, Bernard N; et al. Risk factors and outcomes of preterm premature rupture of membranes in a cohort of 6968 pregnant women prospectively recruited. J Clin Med. 2019;8(11):1987.
- Garg A, Jaiswal A. Evaluation and Management of Premature Rupture of Membranes: A Review Article. Cureus. 2023;15(3):e36615.
- Atkins B, Kindinger L, Mahindra MP, Moatti Z, Siassakos D. Stillbirth: Prevention and supportive bereavement care. BMJ Med. 2023;2(1):e000262.
- The Stillbirth Collaborative Research Network Writing Group. Association Between Stillbirth and Risk Factors. JAMA. 2011;306(22):2469–79.
- Verlohren S, Dröge LA. The diagnostic value of angiogenic and antiangiogenic factors in differential diagnosis of preeclampsia. Am J Obstet Gynecol [Internet]. 2022;226(2):S1048–58. Available from. [CrossRef]
- Rolnik DL, Nicolaides KH, Poon LC. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol [Internet]. 2022;226(2):S1108–19. Available from. [CrossRef]
- Chaemsaithong P, Gil MM, Chaiyasit N, Cuenca-Gomez D, Plasencia W, Rolle V; et al. Accuracy of placental growth factor alone or in combination with soluble fms-like tyrosine kinase-1 or maternal factors in detecting preeclampsia in asymptomatic women in the second and third trimesters: A systematic review and meta-analysis. Am J Obstet Gynecol. 2023;229(3):222–47.
- Nardozza LMM, Caetano ACR, Zamarian ACP, Mazzola JB, Silva CP, Marçal VMG; et al. Fetal growth restriction: Current knowledge. Arch Gynecol Obstet. 2017;295(5):1061–77.
- Pels A, Beune IM, van Wassenaer-Leemhuis AG, Limpens J, Ganzevoort W. Early-onset fetal growth restriction: A systematic review on mortality and morbidity. Acta Obstet Gynecol Scand. 2020;99(2):153–66.
- Bonacina E, Mendoza M, Farràs A, Garcia-Manau P, Serrano B, Hurtado I; et al. Angiogenic factors for planning fetal surveillance in fetal growth restriction and small-for-gestational-age fetuses: A prospective observational study. BJOG An Int J Obstet Gynaecol. 2022;129(11):1870–7.
- Zheng C, Ji C, Wang B, Zhang J, He Q, Ma J; et al. Construction of prediction model for fetal growth restriction during first trimester in an Asian population. Ultrasound Obstet Gynecol. 2024;63(3):321–30.
- Hromadnikova I, Kotlabova K, Krofta L. First-Trimester Screening for Fetal Growth Restriction and Small-for-Gestational-Age Pregnancies without Preeclampsia Using Cardiovascular Disease-Associated MicroRNA Biomarkers. Biomedicines. 2022;10(3):1–16.
- Chia AR, Chen LW, Lai JS, Wong CH, Neelakantan N, Van Dam RM; et al. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv Nutr. 2019;10(4):685–95.
- Laredo-Aguilera JA, Gallardo-Bravo M, Rabanales-Sotos JA, Cobo-Cuenca AI, Carmona-Torres JM. Physical activity programs during pregnancy are effective for the control of gestational diabetes mellitus. Int J Environ Res Public Health. 2020;17(17):1–14.
- Cilar Budler L, Budler M. Physical activity during pregnancy: A systematic review for the assessment of current evidence with future recommendations. BMC Sports Sci Med Rehabil [Internet]. 2022;14(1):1–14. Available from. [CrossRef]
- Sánchez-Polán M, Franco E, Silva-José C, Gil-Ares J, Pérez-Tejero J, Barakat R; et al. Exercise During Pregnancy and Prenatal Depression: A Systematic Review and Meta-Analysis. Front Physiol. 2021;12:640024.
- Spracklen CN, Ryckman KK, Triche EW, Saftlas AF. Physical Activity During Pregnancy and Subsequent Risk of Preeclampsia and Gestational Hypertension: A Case Control Study. Matern Child Health J. 2016;20(6):1193–202.
- Bauer I, Hartkopf J, Kullmann S, Schleger F, Hallschmid M, Pauluschke-Fröhlich J; et al. Spotlight on the fetus: How physical activity during pregnancy influences fetal health: A narrative review. BMJ Open Sport Exerc Med. 2020;6(1):1–12.
- Salvesen KA, Hem E, Sundgot-Borgen J. Fetal wellbeing may be compromised during strenuous exercise among pregnant elite athletes. Br J Sports Med. 2012;46(4):279–83.
- Szymanski LM, Satin AJ. Strenuous exercise during pregnancy: Is there a limit? Am J Obstet Gynecol [Internet]. 2012;207(3):179.e1-179.e6. Available from:. [CrossRef]
- Mottola MF, Davenport MH, Ruchat SM, Davies GA, Poitras V, Gray C; et al. No. 367-2019 Canadian Guideline for Physical Activity throughout Pregnancy. J Obstet Gynaecol Canada. 2018;40(11):1528–37.
- Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob Heal. 2019;7(1):e37–46.
- Goldenberg RL, Culhane JF, Iams JD RR. Preterm birth: Epidemiology and causes of preterm birth. 2020;Lancet 200(January):75–84.
- Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev. 2010;32(1):5–25.
- You D, Hug L, Mishra A et al. A neglected tragedy the global burden of stillbirths report of the UN inter-agency group for child mortality estimation. [Internet]. New York; 2020. Available from: https://www.unicef.org/%0Amedia/84851/file/UN-IGME-the-global-burden-of-stillbirths-2020.
- Conde-Agudelo A, Bird S, Kennedy SH, Villar J, Papageorghiou AT. First- and second-trimester tests to predict stillbirth in unselected pregnant women: A systematic review and meta-analysis. BJOG An Int J Obstet Gynaecol. 2015;122(1):41–55.
- Flenady V, Middleton P, Smith GC, Duke W, Erwich JJ, Khong TY; et al. Stillbirths: The way forward in high-income countries. Lancet. 2011;377(9778):1703–17.
- McClure EM, Saleem S, Pasha O, Goldenberg RL. Stillbirth in developing countries: A review of causes, risk factors and prevention strategies. J Matern Neonatal Med. 2009;22(3):183–90.
- McLaughlin K, Snelgrove JW, Audette MC, Syed A, Hobson SR, Windrim RC; et al. PlGF (Placental Growth Factor) Testing in Clinical Practice: Evidence From a Canadian Tertiary Maternity Referral Center. Hypertension. 2021;77(6):2057–65.
- Chaiworapongsa T, Romero R, Korzeniewski SJ, Kusanovic JP, Soto E, Lam J; et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol [Internet]. 2013;208(4):287.e1-287.e15. Available from:. [CrossRef]
- Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK; et al. Unexplained fetal death: Another anti-angiogenic state. J Matern Neonatal Med. 2007;20(7):495–507.
- Bhutta ZA, Darmstadt GL, Haws RA, Yakoob MY, Lawn JE. Delivering interventions to reduce the global burden of stillbirths: Improving service supply and community demand. BMC Pregnancy Childbirth. 2009;9(SUPPL. 1):1–37.
- Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: Population based study. BMJ. 2013;346(7893):1–14.
- Loussert L, Vidal F, Parant O, Hamdi SM, Vayssiere C, Guerby P. Aspirin for prevention of preeclampsia and fetal growth restriction. Prenat Diagn. 2020;40(5):519–27.
- Wright D, Poon LC, Rolnik DL, Syngelaki A, Delgado JL, Vojtassakova D; et al. Aspirin for Evidence-Based Preeclampsia Prevention trial: Influence of compliance on beneficial effect of aspirin in prevention of preterm preeclampsia. Am J Obstet Gynecol [Internet]. 2017;217(6):685.e1-685.e5. Available from:. [CrossRef]
- Shanmugalingam R, Wang XS, Motum P, Fulcher I, Lee G, Kumar R; et al. Clinical Influence of Nonadherence With Prophylactic Aspirin in Preventing Preeclampsia in High-Risk Pregnancies: A Multicenter, Prospective, Observational Cohort Study. Hypertension. 2020;75(4):1125–32.
- Mendoza M, Bonacina E, Garcia-Manau P, López M, Caamiña S, Vives À; et al. Aspirin Discontinuation at 24 to 28 Weeks’ Gestation in Pregnancies at High Risk of Preterm Preeclampsia: A Randomized Clinical Trial. Jama. 2023;329(7):542–50.
- Bonacina E, Garcia-Manau P, López M, Caamiña S, Vives À, Lopez-Quesada E; et al. Mid-trimester uterine artery Doppler for aspirin discontinuation in pregnancies at high risk for preterm pre-eclampsia: Post-hoc analysis of StopPRE trial. BJOG An Int J Obstet Gynaecol. 2023;00:1–9.
- Emeruwa UN, Gyamfi-Bannerman C LL. Biomarkers and the Risk of Preeclampsia. JAMA - J Am Med Assoc. 2023;329(7):539–41.
- Roberts L, Chaemsaithong P, Sahota DS, Nicolaides KH, Poon LCY. Protocol for measurement of mean arterial pressure at 10–40 weeks’ gestation. Pregnancy Hypertens [Internet]. 2017;10(May):155–60. Available from:. [CrossRef]
- Chaemsaithong P, Ting YH, Cheng KYY, Poon CYL, Leung TY, Sahota DS. Uterine artery pulsatility index in the first trimester: Assessment of intersonographer and intersampling site measurement differences. J Matern Neonatal Med [Internet]. 2018;31(17):2276–83. Available from. [CrossRef]
- Chaemsaithong P, Sahota DS, Poon LC. First trimester preeclampsia screening and prediction. Am J Obstet Gynecol [Internet]. 2022;226(2):S1071-S1097.e2. Available from:. [CrossRef]
- American College of Obstetricians and Gynecologists. Practice advisory: Low-dose aspirin use for the prevention of preeclampsia and related morbidity and mortality. [Internet]. 2021. Available from: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/12/low-dose-aspirin-use-for-the-prevention-of-preeclampsia-and-related-morbidity-and-mortality.
- Public Health Agency of Canada. Your guide to a healthy pregnancy. [Internet]. 2021. 1–88 p. Available from: https://www.canada.ca/content/dam/phac-aspc/documents/services/health-promotion/healthy-pregnancy/healthy-pregnancy-guide.pdf.
- The Association of UK Dietitians. Food Fact Sheet: Pregnancy and diet. Br Diet Assoc [Internet]. 2021; Available from: https://www.bda.uk.com/resource/pregnancy-diet.html.
- Wang S shan, Lay S, Yu H ning, Shen S rong. Dietary Guidelines for Chinese Residents (2016): Comments and comparisons. J Zhejiang Univ Sci B. 2016;17(9):649–56.
- Buelt A, Richards A, Jones AL. Hypertension: New Guidelines from the International Society of Hypertension. Am Fam Physician [Internet]. 2021;103(12):763–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34128614.
- Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM; et al. Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: US Preventive Services Task Force Recommendation Statement. JAMA - J Am Med Assoc. 2021;326(12):1186–91.
- Tan MY, Syngelaki A, Poon LC, Rolnik DL, O’Gorman N, Delgado JL; et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2018;52(2):186–95.
- Park FJ, Leung CHY, Poon LCY, Williams PF, Rothwell SJ, Hyett JA. Clinical evaluation of a first trimester algorithm predicting the risk of hypertensive disease of pregnancy. Aust New Zeal J Obstet Gynaecol. 2013;53(6):532–9.
- O’Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, de Alvarado M; et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: Comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017;49(6):756–60.
- Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D; et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: Results of SPREE. Ultrasound Obstet Gynecol. 2018;51(6):743–50.
- Reinders A, Cuckson AC, Lee JTM, Shennan AH. An accurate automated blood pressure device for use in pregnancy and pre-eclampsia: The Microlife 3BTO-A. BJOG An Int J Obstet Gynaecol. 2005;112(7):915–20.
- Khalil A, Nicolaides KH. How to record uterine artery Doppler in the first trimester. Ultrasound Obstet Gynecol. 2013;42(4):478–9.
- Ridding G, Hyett JA, Sahota D, McLennan AC. Assessing quality standards in measurement of uterine artery pulsatility index at 11 to 13 + 6 weeks’ gestation. Ultrasound Obstet Gynecol. 2015;46(3):299–305.
- Rolnik DL, da Silva Costa F, Sahota D, Hyett J, McLennan A. Quality assessment of uterine artery Doppler measurement in first-trimester combined screening for pre-eclampsia. Ultrasound Obstet Gynecol. 2019;53(2):245–50.
- Black C, Al-Amin A, Rolnik DL, Kane SC, Stolarek C, White A; et al. Midpregnancy testing for soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF): An inter-assay comparison of three automated immunoassay platforms. Placenta [Internet]. 2019;86(February):11-14. Available from. [CrossRef]
- Agrawal S, Shinar S, Cerdeira AS, Redman C, Vatish M. Predictive performance of PlGF (Placental Growth Factor) for screening preeclampsia in asymptomatic women: A systematic review and meta-analysis. Hypertension. 2019;74(5):1124–35.
- Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med. 2016;374(1):13–22.
- Sherrell H, Dunn L, Clifton V, Kumar S. Systematic review of maternal Placental Growth Factor levels in late pregnancy as a predictor of adverse intrapartum and perinatal outcomes. Eur J Obstet Gynecol Reprod Biol [Internet]. 2018;225:26–34. Available from. [CrossRef]
- Benton SJ, McCowan LM, Heazell AEP, Grynspan D, Hutcheon JA, Senger C; et al. Placental growth factor as a marker of fetal growth restriction caused by placental dysfunction. Placenta. 2016;42:1–8.
- Hong J, Crawford K, Cavanagh E, Clifton V, Kumar S. Prediction of preterm birth in women with fetal growth restriction – Is the weekly change in sFlt-1/PlGF ratio or PlGF levels useful? Acta Obstet Gynecol Scand. 2024;(February):1112–9.
- NICE. PLGF-based testing to help diagnose suspected preterm pre-eclampsia. Diagnostics guidance [DG49] [Internet]. 2022. Available from: https://www.nice.org.uk/guidance/dg49.
- US Food and Drug Administration. FDA Roundup. FDA News Release [Internet]. :Accessed 18 June 2024. Available from: https://www.fda.gov/news-events/press-announcements/fda-roundup-may-19-2023.
- Schlembach D, Hund M, Wolf C, Vatish M. Diagnostic utility of angiogenic biomarkers in pregnant women with suspected preeclampsia: A health economics review. Pregnancy Hypertens [Internet]. 2019;17(December 2018):28–35. Available from. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).