Submitted:
14 June 2024
Posted:
17 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Defining Radioactive Iodine Refractory Differentiated Thyroid Cancer
3. Identifying RAI-R
4. Current Molecular Imaging and Care Options
5. Future Diagnostic and Therapeutic Perspectives
6. A Case of Re-Differentiation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Klain, M.; Nappi, C.; Zampella, E.; Cantoni, V.; Green, R.; Piscopo, L.; Volpe, F.; Manganelli, M.; Caiazzo, E.; Petretta, M.; et al. Ablation Rate after Radioactive Iodine Therapy in Patients with Differentiated Thyroid Cancer at Intermediate or High Risk of Recurrence: A Systematic Review and a Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4437–4444. [Google Scholar] [CrossRef]
- Worden, F.; Rajkovic-Hooley, O.; Reynolds, N.; Milligan, G.; Zhang, J. Real-World Treatment Patterns and Clinical Outcomes in Patients with Radioiodine-Refractory Differentiated Thyroid Cancer (RAI-R DTC) Treated with First Line Lenvatinib Monotherapy in the United States. Endocrine 2023. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Dana, T.; Brent, G.A.; Goldner, W.; Haymart, M.; Leung, A.M.; Ringel, M.D.; Sosa, J.A. Serum Thyroglobulin Measurement Following Surgery Without Radioactive Iodine for Differentiated Thyroid Cancer: A Systematic Review. Thyroid Off. J. Am. Thyroid Assoc. 2022, 32, 613–639. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Leboulleux, S. Current Practice in Patients with Differentiated Thyroid Cancer. Nat. Rev. Endocrinol. 2021, 17, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, R.; Shen, X.; Zhu, G.; Li, B.; Xing, M. The Genetic Duet of BRAF V600E and TERT Promoter Mutations Robustly Predicts Loss of Radioiodine Avidity in Recurrent Papillary Thyroid Cancer. J. Nucl. Med. 2020, 61, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kikumori, T.; Miyajima, N.; Takano, Y.; Noda, S.; Takeuchi, D.; Iwano, S.; Kodera, Y. Impact of Patient Age and Histological Type on Radioactive Iodine Avidity of Recurrent Lesions of Differentiated Thyroid Carcinoma. Clin. Nucl. Med. 2018, 43, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Simões-Pereira, J.; Mourinho, N.; Ferreira, T.C.; Limbert, E.; Cavaco, B.M.; Leite, V. Avidity and Outcomes of Radioiodine Therapy for Distant Metastasis of Distinct Types of Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2021, 106, e3911–e3922. [Google Scholar] [CrossRef] [PubMed]
- Pace, L.; Klain, M.; Salvatore, B.; Nicolai, E.; Zampella, E.; Assante, R.; Pellegrino, T.; Storto, G.; Fonti, R.; Salvatore, M. Prognostic Role of 18F-FDG PET/CT in the Postoperative Evaluation of Differentiated Thyroid Cancer Patients. Clin. Nucl. Med. 2015, 40, 111. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, B.; Klain, M.; Nicolai, E.; D’Amico, D.; De Matteis, G.; Raddi, M.; Fonti, R.; Pellegrino, T.; Storto, G.; Cuocolo, A.; et al. Prognostic Role of FDG PET/CT in Patients with Differentiated Thyroid Cancer Treated with 131-Iodine Empiric Therapy. Medicine (Baltimore) 2017, 96, e8344. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Bulbul, B.Y.; Ayturk, S.; Durmus, Y.; Gurkan, H.; Can, N.; Tastekin, E.; Ustun, F.; Sezer, A.; Guldiken, S. The Relation between BRAFV600E Mutation and Clinicopathological Characteristics of Papillary Thyroid Cancer. Med. Glas. Ljek. Komore Zenicko-Doboj. Kantona 2020, doi:10.17392/1086-20. [CrossRef]
- Wu, Y.; Shi, L.; Zhao, Y.; Chen, P.; Cui, R.; Ji, M.; He, N.; Wang, M.; Li, G.; Hou, P. Synergistic Activation of Mutant TERT Promoter by Sp1 and GABPA in BRAFV600E-Driven Human Cancers. Npj Precis. Oncol. 2021, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Lacroix, L.; Russo, D.; Filetti, S.; Bidart, J.-M. Defects in Iodide Metabolism in Thyroid Cancer and Implications for the Follow-up and Treatment of Patients. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Namwongprom, S.; Dejkhamron, P.; Unachak, K. Success Rate of Radioactive Iodine Treatment for Children and Adolescent with Hyperthyroidism. J. Endocrinol. Invest. 2021, 44, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Shogbesan, G.; Muzahir, S.; Bridges, A. Radioiodine Refractory Differentiated Thyroid Cancer: Albatross of Patients and Physicians. J. Nucl. Med. 2022, 63, 2693–2693. [Google Scholar]
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and Management of Radioactive Iodine-Refractory Differentiated Thyroid Cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Finessi, M.; Liberini, V.; Deandreis, D. Definition of Radioactive Iodine Refractory Thyroid Cancer and Redifferentiation Strategies. In Integrated Diagnostics and Theranostics of Thyroid Diseases; Giovanella, L., Ed.; Springer International Publishing: Cham, 2023; pp. 143–156. ISBN 978-3-031-35213-3. [Google Scholar]
- Kiyota, N.; Robinson, B.; Shah, M.; Hoff, A.O.; Taylor, M.H.; Li, D.; Dutcus, C.E.; Lee, E.K.; Kim, S.-B.; Tahara, M. Defining Radioiodine-Refractory Differentiated Thyroid Cancer: Efficacy and Safety of Lenvatinib by Radioiodine-Refractory Criteria in the SELECT Trial. Thyroid 2017, 27, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrand, D. Selected Controversies of Radioiodine Imaging and Therapy in Differentiated Thyroid Cancer. Endocrinol. Metab. Clin. North Am. 2017, 46, 783–793. [Google Scholar] [CrossRef]
- Deandreis, D.; Rubino, C.; Tala, H.; Leboulleux, S.; Terroir, M.; Baudin, E.; Larson, S.; Fagin, J.A.; Schlumberger, M.; Tuttle, R.M. Comparison of Empiric Versus Whole-Body/-Blood Clearance Dosimetry–Based Approach to Radioactive Iodine Treatment in Patients with Metastases from Differentiated Thyroid Cancer. J. Nucl. Med. 2017, 58, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.-Z.; Zhang, Y.-Q.; Sun, D.; Lu, T.; Lin, Y.-S. Effect of BRAFV600E and TERT Promoter Mutations on Thyroglobulin Response in Patients With Distant-Metastatic Differentiated Thyroid Cancer. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2022, 28, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical Therapy in Cancer: Clinical Advances and Challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Dotinga, M.; Vriens, D.; van Velden, F.H.P.; Stam, M.K.; Heemskerk, J.W.T.; Dibbets-Schneider, P.; Pool, M.; Rietbergen, D.D.D.; de Geus-Oei, L.-F.; Kapiteijn, E. Reinducing Radioiodine-Sensitivity in Radioiodine-Refractory Thyroid Cancer Using Lenvatinib (RESET): Study Protocol for a Single-Center, Open Label Phase II Trial. Diagnostics 2022, 12, 3154. [Google Scholar] [CrossRef] [PubMed]
- Rosignolo, F.; Sponziello, M.; Giacomelli, L.; Russo, D.; Pecce, V.; Biffoni, M.; Bellantone, R.; Lombardi, C.P.; Lamartina, L.; Grani, G.; et al. Identification of Thyroid-Associated Serum microRNA Profiles and Their Potential Use in Thyroid Cancer Follow-Up. J. Endocr. Soc. 2017, 1, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Celano, M.; Rosignolo, F.; Maggisano, V.; Pecce, V.; Iannone, M.; Russo, D.; Bulotta, S. MicroRNAs as Biomarkers in Thyroid Carcinoma. Int. J. Genomics 2017, 2017, 6496570. [Google Scholar] [CrossRef] [PubMed]
- Pecce, V.; Sponziello, M.; Verrienti, A.; Grani, G.; Abballe, L.; Bini, S.; Annunziata, S.; Perotti, G.; Salvatori, M.; Zagaria, L.; et al. The Role of miR-139-5p in Radioiodine-Resistant Thyroid Cancer. J. Endocrinol. Invest. 2023, 46, 2079–2093. [Google Scholar] [CrossRef]
- Huang, I.-C.; Chou, F.-F.; Liu, R.-T.; Tung, S.-C.; Chen, J.-F.; Kuo, M.-C.; Hsieh, C.-J.; Wang, P.-W. Long-Term Outcomes of Distant Metastasis from Differentiated Thyroid Carcinoma. Clin. Endocrinol. (Oxf.) 2012, 76, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Klain, M.; Zampella, E.; Piscopo, L.; Volpe, F.; Manganelli, M.; Masone, S.; Pace, L.; Salvatore, D.; Schlumberger, M.; Cuocolo, A. Long-Term Prognostic Value of the Response to Therapy Assessed by Laboratory and Imaging Findings in Patients with Differentiated Thyroid Cancer. Cancers 2021, 13, 4338. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Yoon, J.-K.; Lee, S.J.; Soh, E.Y.; Lee, J.; An, Y.-S. Risk Factors for Indeterminate Response After Radioactive Iodine Therapy in Patients With Differentiated Thyroid Cancer. Clin. Nucl. Med. 2019, 44, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Tramontin, M.Y.; Nobre, G.M.; Lopes, M.; Carneiro, M.P.; Alves, P.A.G.; de Andrade, F.A.; Vaisman, F.; Corbo, R.; Bulzico, D. High Thyroglobulin and Negative Whole-Body Scan: No Long-Term Benefit of Empiric Radioiodine Therapy. Endocrine 2021, 73, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W.; Mineiro Filho, A.F.C.; Lacerda, R.X.; dos Santos, D.A.; Calsolari, M.R. The Value of Diagnostic Whole-Body Scanning and Serum Thyroglobulin in the Presence of Elevated Serum Thyrotropin during Follow-up of Anti-Thyroglobulin Antibody-Positive Patients with Differentiated Thyroid Carcinoma Who Appeared to Be Free of Disease after Total Thyroidectomy and Radioactive Iodine Ablation. Thyroid Off. J. Am. Thyroid Assoc. 2012, 22, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Al Hatmi, A.; Jain, A.; Mittal, A.K.; Hussain, S. Evaluation of Diagnostic Value of SPECT/CT Imaging in Post-Radioiodine Therapy in Thyroid Cancer. Sultan Qaboos Univ. Med. J. 2022, 22, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Spanu, A.; Nuvoli, S.; Marongiu, A.; Gelo, I.; Mele, L.; Piras, B.; Madeddu, G. Neck Lymph Node Metastasis Detection in Patients with Differentiated Thyroid Carcinoma (DTC) in Long-Term Follow-up: A 131I-SPECT/CT Study. BMC Cancer 2020, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Zilioli, V.; Peli, A.; Panarotto, M.B.; Magri, G.; Alkraisheh, A.; Wiefels, C.; Rodella, C.; Giubbini, R. Differentiated Thyroid Carcinoma: Incremental Diagnostic Value of 131I SPECT/CT over Planar Whole Body Scan after Radioiodine Therapy. Endocrine 2017, 56, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Tiu, S.; Chu, M.; Goel, S.; Friedman, K. I-131 SPECT/CT Elucidates Cryptic Findings on Planar Whole-Body Scans and Can Reduce Needless Therapy with I-131 in Post-Thyroidectomy Thyroid Cancer Patients. Thyroid Off. J. Am. Thyroid Assoc. 2011, 21, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xiang, Y.; Huang, R.; Tian, R.; Liu, B. Clinical Applications of Single-Photon Emission Computed Tomography/Computed Tomography in Post-Ablation 131iodine Scintigraphy in Children and Young Adults with Differentiated Thyroid Carcinoma. Pediatr. Radiol. 2021, 51, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Jannin, A.; Lamartina, L.; Moutarde, C.; Djennaoui, M.; Lion, G.; Chevalier, B.; Vantyghem, M.C.; Deschamps, F.; Hadoux, J.; Baudin, E.; et al. Bone Metastases from Differentiated Thyroid Carcinoma: Heterogenous Tumor Response to Radioactive Iodine Therapy and Overall Survival. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2401–2413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, M.; Wang, Q.; Jen, J.; Liu, B.; Guo, M. Intratumor Epigenetic Heterogeneity-A Panel Gene Methylation Study in Thyroid Cancer. Front. Genet. 2021, 12, 714071. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, H.; Li, Q.; Shen, G.; Shi, L.; Tian, R. Investigating 18F-FDG PET/CT Parameters as Prognostic Markers for Differentiated Thyroid Cancer: A Systematic Review. Front. Oncol. 2021, 11, 648658. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Dondi, F.; Mazzoletti, A.; Bellini, P.; Rodella, C.; Bertagna, F. Prognostic Role of 2-[18F]FDG PET/CT Metabolic Volume Parameters in Patients Affected by Differentiated Thyroid Carcinoma with High Thyroglobulin Level, Negative 131I WBS and Positive 2-[18F]-FDG PET/CT. Diagn. Basel Switz. 2021, 11, 2189. [Google Scholar] [CrossRef]
- Terroir, M.; Borget, I.; Bidault, F.; Ricard, M.; Deschamps, F.; Hartl, D.; Tselikas, L.; Dercle, L.; Lumbroso, J.; Baudin, E.; et al. The Intensity of 18FDG Uptake Does Not Predict Tumor Growth in Patients with Metastatic Differentiated Thyroid Cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Bikas, A.; Garcia, C.A.; Desale, S.; Wartofsky, L.; Burman, K.D. 18F-FDG-PET SUV as a Prognostic Marker of Increasing Size in Thyroid Cancer Tumors. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2017, 23, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Klain, M.; Maurea, S.; Gaudieri, V.; Zampella, E.; Volpe, F.; Manganelli, M.; Piscopo, L.; De Risi, M.; Cuocolo, A. The Diagnostic Role of Total-Body 18F-FDG PET/CT in Patients with Multiple Tumors: A Report of the Association of Thyroid Cancer with Lung or Renal Tumors. Quant. Imaging Med. Surg. 2021, 11, 4211–4215. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, P.; Khthir, R.; Solnes, L.B.; Ladenson, P.W. The Relationship of Brafv600e Mutation Status to Fdg Pet/Ct Avidity in Thyroid Cancer: A Review and Meta-Analysis. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2018, 24, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Park, K.W.; Heo, J.H.; Jung, S.-N.; Liu, L.; Kim, S.M.; Kwon, I.S.; Koo, B.S. Relationship Between 18F-Fluorodeoxyglucose Accumulation and the BRAF V600E Mutation in Papillary Thyroid Cancer. World J. Surg. 2018, 42, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.-Y.; Ehya, H.; et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef] [PubMed]
- Cortas, C.; Charalambous, H. Tyrosine Kinase Inhibitors for Radioactive Iodine Refractory Differentiated Thyroid Cancer. Life 2024, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, P.; Yu, Y.; Zhi, J.; Zheng, X.; Yu, J.; Gao, M. Comparison of Diagnostic Methods for the Detection of a BRAF Mutation in Papillary Thyroid Cancer. Oncol. Lett. 2019. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Hechtman, J.F. Detection of NTRK Fusions: Merits and Limitations of Current Diagnostic Platforms. Cancer Res. 2019, 79, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Lin, Z.; Chen, W. Can 99 Tc m -3PRGD 2 (α ν β 3 ) and 18 F-FDG Dual-Tracer Molecular Imaging Change the Therapeutic Strategy for Progressive Refractory Differentiated Thyroid Cancer: Case Report. Medicine (Baltimore) 2023, 102, e32751. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhang, G.-J.; Wang, Y.-B.; Liu, Y.; Wang, F.; Jia, X.; Liang, Y.-Q.; Yang, A.-M. Clinical Value of 99mTc-3PRGD2 SPECT/CT in Differentiated Thyroid Carcinoma with Negative 131I Whole-Body Scan and Elevated Thyroglobulin Level. Sci. Rep. 2018, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jia, X.; Wang, Y.; Liu, Y.; Yao, X.; Bai, Y.; Han, P.; Chen, S.; Yang, A.; Gao, R. Evaluation of Integrin Avβ3-Targeted Imaging for Predicting Disease Progression in Patients with High-Risk Differentiated Thyroid Cancer (Using 99mTc-3PRGD2). Cancer Imaging 2022, 22, 72. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.S.; Mittal, B.R.; Kumar, R.; Shukla, J.; Bhattacharya, A. 68 Ga-DOTA-RGD 2 Positron Emission Tomography/Computed Tomography in Radioiodine Refractory Thyroid Cancer: Prospective Comparison of Diagnostic Accuracy with 18 F-FDG Positron Emission Tomography/Computed Tomography and Evaluation Toward Potential Theranostics. Thyroid 2020, 30, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Chernaya, G.; Mikhno, N.; Khabalova, T.; Svyatchenko, S.; Mostovich, L.; Shevchenko, S.; Gulyaeva, L. The Expression Profile of Integrin Receptors and Osteopontin in Thyroid Malignancies Varies Depending on the Tumor Progression Rate and Presence of BRAF V600E Mutation. Surg. Oncol. 2018, 27, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Coerts, H.I.; De Keizer, B.; Verburg, F.A. Advances in the Development of Positron Emission Tomography Tracers for Improved Detection of Differentiated Thyroid Cancer. Cancers 2024, 16, 1401. [Google Scholar] [CrossRef] [PubMed]
- Volpe, F.; Nappi, C.; Piscopo, L.; Zampella, E.; Mainolfi, C.G.; Ponsiglione, A.; Imbriaco, M.; Cuocolo, A.; Klain, M. Emerging Role of Nuclear Medicine in Prostate Cancer: Current State and Future Perspectives. Cancers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer (TheraP): A Randomised, Open-Label, Phase 2 Trial. Lancet Lond. Engl. 2021, 397, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Alan-Selcuk, N.; Beydagi, G.; Demirci, E.; Ocak, M.; Celik, S.; Oven, B.B.; Toklu, T.; Karaaslan, I.; Akcay, K.; Sonmez, O.; et al. Clinical Experience with [225Ac]Ac-PSMA Treatment in Patients with [177Lu]Lu-PSMA–Refractory Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2023, 64, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Demirci, E.; Ocak, M.; Kabasakal, L.; Decristoforo, C.; Talat, Z.; Halaç, M.; Kanmaz, B. (68)Ga-PSMA PET/CT Imaging of Metastatic Clear Cell Renal Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1461–1462. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Reuter, V.E.; Heston, W.D.; Bander, N.H.; Grauer, L.S.; Gaudin, P.B. Five Different Anti-Prostate-Specific Membrane Antigen (PSMA) Antibodies Confirm PSMA Expression in Tumor-Associated Neovasculature. Cancer Res. 1999, 59, 3192–3198. [Google Scholar] [PubMed]
- Bychkov, A.; Vutrapongwatana, U.; Tepmongkol, S.; Keelawat, S. PSMA Expression by Microvasculature of Thyroid Tumors – Potential Implications for PSMA Theranostics. Sci. Rep. 2017, 7, 5202. [Google Scholar] [CrossRef] [PubMed]
- Heitkötter, B.; Steinestel, K.; Trautmann, M.; Grünewald, I.; Barth, P.; Gevensleben, H.; Bögemann, M.; Wardelmann, E.; Hartmann, W.; Rahbar, K.; et al. Neovascular PSMA Expression Is a Common Feature in Malignant Neoplasms of the Thyroid. Oncotarget 2018, 9, 9867–9874. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Malhotra, G.; Agrawal, R.; Sonavane, S.; Meshram, V.; Asopa, R.V. Evidence of Prostate-Specific Membrane Antigen Expression in Metastatic Differentiated Thyroid Cancer Using 68Ga-PSMA-HBED-CC PET/CT. Clin. Nucl. Med. 2018, 43, e265–e268. [Google Scholar] [CrossRef]
- Verburg, F.A.; Krohn, T.; Heinzel, A.; Mottaghy, F.M.; Behrendt, F.F. First Evidence of PSMA Expression in Differentiated Thyroid Cancer Using [68Ga]PSMA-HBED-CC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1622–1623. [Google Scholar] [CrossRef] [PubMed]
- Lütje, S.; Gomez, B.; Cohnen, J.; Umutlu, L.; Gotthardt, M.; Poeppel, T.D.; Bockisch, A.; Rosenbaum-Krumme, S. Imaging of Prostate-Specific Membrane Antigen Expression in Metastatic Differentiated Thyroid Cancer Using 68Ga-HBED-CC-PSMA PET/CT. Clin. Nucl. Med. 2017, 42, 20–25. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.H.; Lodewijk, L.; Braat, A.J.A.T.; Krijger, G.C.; Valk, G.D.; Lam, M.G.E.H.; Borel Rinkes, I.H.M.; Vriens, M.R.; de Keizer, B. 68Ga-PSMA PET/CT in Radioactive Iodine-Refractory Differentiated Thyroid Cancer and First Treatment Results with 177Lu-PSMA-617. EJNMMI Res. 2020, 10, 18. [Google Scholar] [CrossRef]
- Pishdad, R.; Treglia, G.; Mehta, A.; Santhanam, P. Somatostatin Receptor Imaging of Thyroid Tissue and Differentiated Thyroid Cancer Using Gallium-68-Labeled Radiotracers—a Review of Clinical Studies. Endocrine 2024. [Google Scholar] [CrossRef]
- Sancak, S.; Hardt, A.; Singer, J.; Klöppel, G.; Eren, F.T.; Güllüoglu, B.M.; Sen, L.S.; Sever, Z.; Akalin, N.S.; Eszlinger, M.; et al. Somatostatin Receptor 2 Expression Determined by Immunohistochemistry in Cold Thyroid Nodules Exceeds That of Hot Thyroid Nodules, Papillary Thyroid Carcinoma, and Graves’ Disease. Thyroid Off. J. Am. Thyroid Assoc. 2010, 20, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Pisarek, H.; Stepień, T.; Kubiak, R.; Borkowska, E.; Pawlikowski, M. Expression of Somatostatin Receptor Subtypes in Human Thyroid Tumors: The Immunohistochemical and Molecular Biology (RT-PCR) Investigation. Thyroid Res. 2009, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Klagge, A.; Krause, K.; Schierle, K.; Steinert, F.; Dralle, H.; Fuhrer, D. Somatostatin Receptor Subtype Expression in Human Thyroid Tumours. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 2010, 42, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, J.J.M.; Kwekkeboom, D.J.; Kooij, P.P.M.; Bakker, W.H.; Krenning, E.P. Peptide Receptor Radionuclide Therapy for Non-Radioiodine-Avid Differentiated Thyroid Carcinoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2005, 46 Suppl 1, 107S–14S. [Google Scholar]
- Piscopo, L.; Zampella, E.; Pellegrino, S.; Volpe, F.; Nappi, C.; Gaudieri, V.; Fonti, R.; Vecchio, S.D.; Cuocolo, A.; Klain, M. Diagnosis, Management and Theragnostic Approach of Gastro-Entero-Pancreatic Neuroendocrine Neoplasms. Cancers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Subramaniam, R.M. Diagnosis and Treatment of Lung Neuroendocrine Neoplasms: Somatostatin Receptor PET Imaging and Peptide Receptor Radionuclide Therapy. PET Clin 2023, 18, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, R.; Giordano, A.; Milella, M.; Storto, R.; Pellegrino, T.; Nardelli, A.; Nappi, A.; Tarricone, L.; Storto, G. Ga-68-Edotreotide Positron Emission Tomography/Computed Tomography Somatostatin Receptors Tumor Volume Predicts Outcome in Patients With Primary Gastroenteropancreatic Neuroendocrine Tumors. Cancer Control 2023, 30, 10732748231152328. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Bonazzi, N.; Zanoni, L.; Fanti, S.; Ambrosini, V. Molecular Imaging Theranostics of Neuroendocrine Tumors. Semin. Nucl. Med. 2023, 53, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Ferri, V.; Fisher, G.; Shaheen, S.; Davidzon, G.; Moradi, F.; Nguyen, J.; Franc, B.; Iagaru, A.; Aparici, C.M. Evaluation of Interim 68Ga-Dotatate PET after Two Cycles of Peptide Receptor Radionuclide Therapy (PRRT) in Neuroendocrine Tumors (NET). Clin. Nucl. Med. 2023, 48, e276. [Google Scholar]
- Donohoe, K.J.; Aloff, J.; Avram, A.M.; Bennet, K.G.; Giovanella, L.; Greenspan, B.; Gulec, S.; Hassan, A.; Kloos, R.T.; Solórzano, C.C.; et al. Appropriate Use Criteria for Nuclear Medicine in the Evaluation and Treatment of Differentiated Thyroid Cancer. J. Nucl. Med. 2020, 61, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Bertagna, F.; Albano, D.; Giovanella, L.; Giubbini, R.; Treglia, G. F18-Choline/C11-Choline PET/CT Thyroid Incidentalomas. Endocrine 2019, 64, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Durmo, R.; Bertagna, F.; Giubbini, R. 18F-Choline PET/CT Incidental Thyroid Uptake in Patients Studied for Prostate Cancer. Endocrine 2019, 63, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Trimboli, P.; Puntoni, M.; Foppiani, L.; Treglia, G.; Naseri, M.; Bottoni, G.L.; Massollo, M.; Sola, S.; Ferrarazzo, G.; et al. Role of 18F-Choline Positron Emission Tomography/Computed Tomography to Detect Structural Relapse in High-Risk Differentiated Thyroid Cancer Patients. Thyroid Off. J. Am. Thyroid Assoc. 2019, 29, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Fozzatti, L.; Cheng, S. Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer. Endocrinol. Metab. 2020, 35, 673–680. [Google Scholar] [CrossRef]
- Dvorak Harold, F. Tumors: Wounds That Do Not Heal. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, S.; Zhang, J.; Yao, S.; Miao, W. 68Ga-DOTA-FAPI-04 PET/CT Imaging in Radioiodine-Refractory Differentiated Thyroid Cancer (RR-DTC) Patients. Ann. Nucl. Med. 2022, 36, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Roesch, F.; Satapathy, S.; Moon, E.S.; Martin, M.; Wakade, N.; Sheokand, P.; Tripathi, M.; Chandekar, K.R.; et al. Head-to-Head Comparison of [68Ga]Ga-DOTA.SA.FAPi with [18F]F-FDG PET/CT in Radioiodine-Resistant Follicular-Cell Derived Thyroid Cancers. Eur. J. Nucl. Med. Mol. Imaging 2023. [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Rösch, F.; ArunRaj, S.T.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Bal, C. First-in-Human Experience With 177Lu-DOTAGA.(SA.FAPi)2 Therapy in an Uncommon Case of Aggressive Medullary Thyroid Carcinoma Clinically Mimicking as Anaplastic Thyroid Cancer. Clin. Nucl. Med. 2022, 47, e444–e445. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Huang, J.; Zhao, T.; Wang, H.; Chen, Y.; Xu, W.; Pang, Y.; Guo, W.; Sun, L.; Wu, H.; et al. Fibroblast Activation Protein-Targeted Radioligand Therapy with 177Lu-EB-FAPI for Metastatic Radioiodine-Refractory Thyroid Cancer: First-in-Human, Dose-Escalation Study. Clin. Cancer Res. 2023, 29, 4740–4750. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, T.; Luongo, C.; Sessa, F.; Klain, M.; Masone, S.; Troncone, G.; Bellevicine, C.; Schlumberger, M.; Salvatore, D. Long-Term Management of Lenvatinib-Treated Thyroid Cancer Patients: A Real-Life Experience at a Single Institution. Endocrine 2021, 73, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Durante, C.; Topliss, D.J.; Winquist, E.; Robenshtok, E.; Iwasaki, H.; Luster, M.; Elisei, R.; Leboulleux, S.; Tahara, M. Lenvatinib for the Treatment of Radioiodine-Refractory Differentiated Thyroid Cancer: Treatment Optimization for Maximum Clinical Benefit. The Oncologist 2022, 27, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, L.; Worden, F. Novel Therapeutics for Advanced Differentiated Thyroid Cancer. Endocrinol. Metab. Clin. North Am. 2022, 51, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Solomon, B.; Pattison, D.A.; Jackson, P.; Ravi Kumar, A.; Kong, G.; Hofman, M.S.; Akhurst, T.; Hicks, R.J. Mitogen-Activated Protein Kinase Pathway Inhibition for Redifferentiation of Radioiodine Refractory Differentiated Thyroid Cancer: An Evolving Protocol. Thyroid 2019, 29, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Leboulleux, S.; Benisvy, D.; Taieb, D.; Attard, M.; Bournaud, C.; Terroir, M.; Ghuzlan, A.A.; Lamartina, L.; Schlumberger, M.J.; Godbert, Y.; et al. MERAIODE: A Redifferentiation Phase II Trial with Trametinib Followed by Radioactive Iodine for Metastatic Radioactive Iodine Refractory Differentiated Thyroid Cancer Patients with a RAS Mutation. Ann. Oncol. 2021, 32, S1204. [Google Scholar] [CrossRef]
- Balakirouchenane, D.; Seban, R.; Groussin, L.; Puszkiel, A.; Cottereau, A.S.; Clerc, J.; Vidal, M.; Goldwasser, F.; Arrondeau, J.; Blanchet, B.; et al. Pharmacokinetics/Pharmacodynamics of Dabrafenib and Trametinib for Redifferentiation and Treatment of Radioactive Iodine-Resistant Mutated Advanced Differentiated Thyroid Cancer. Thyroid® 2023, 33, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
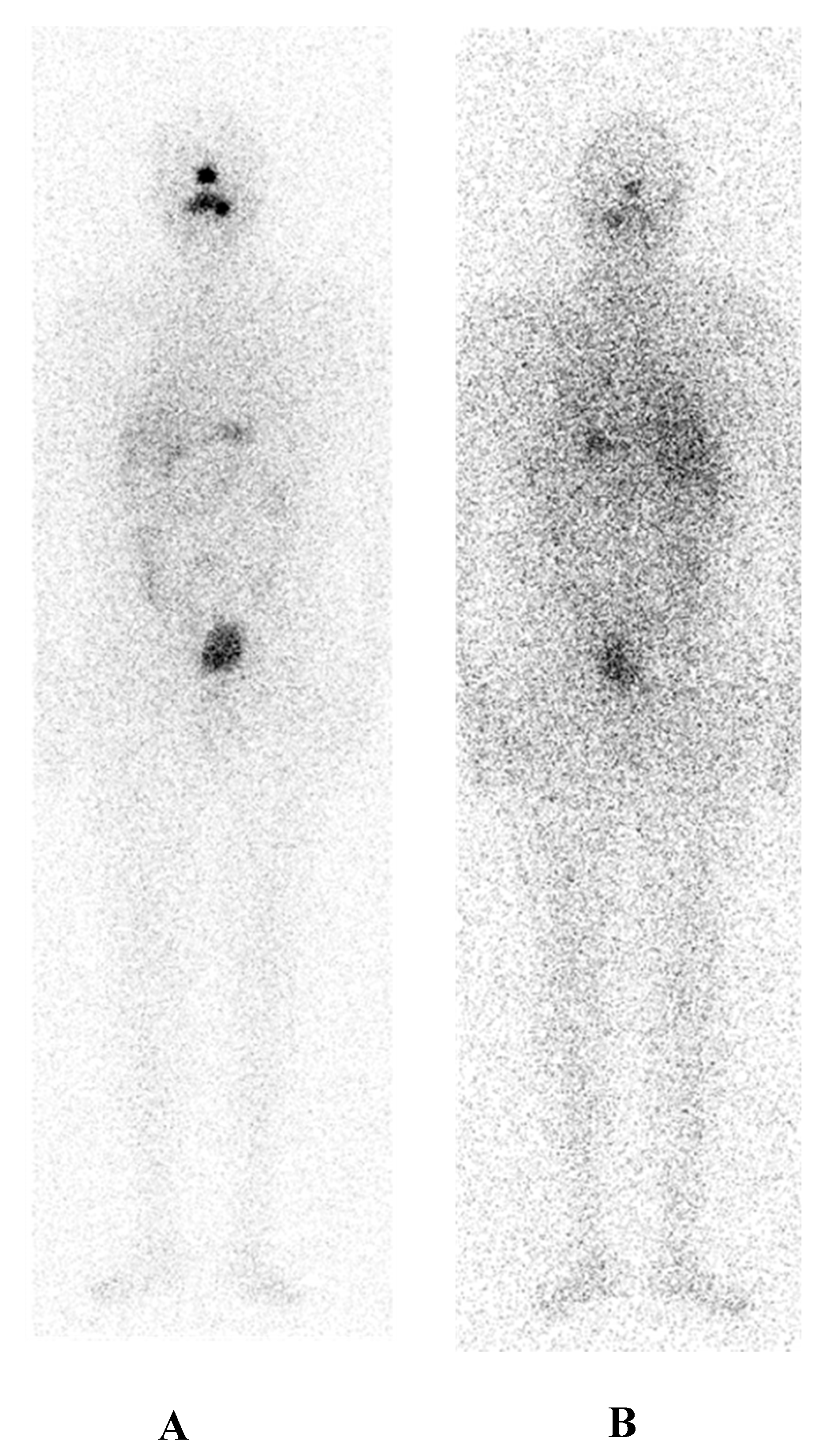
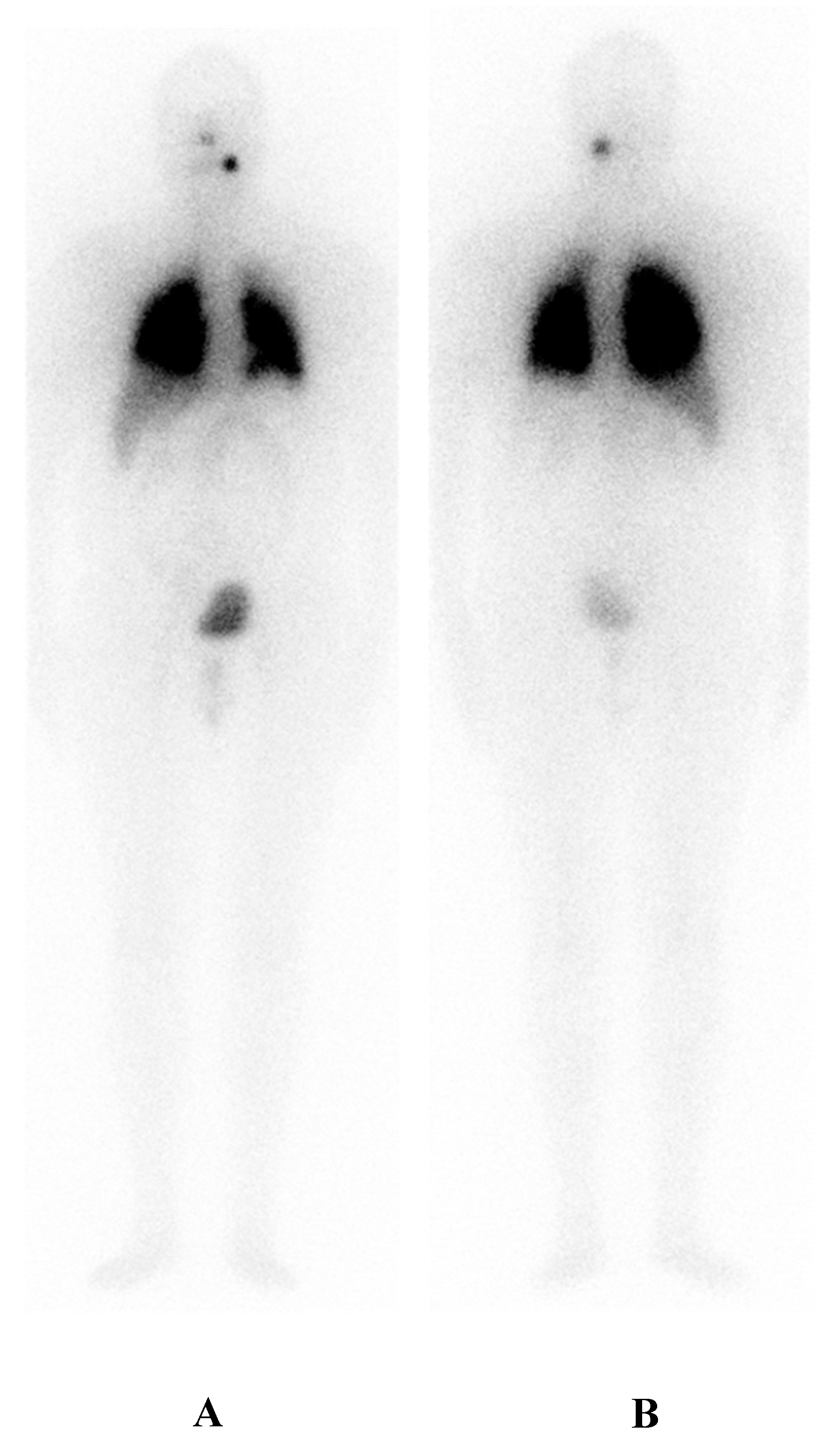
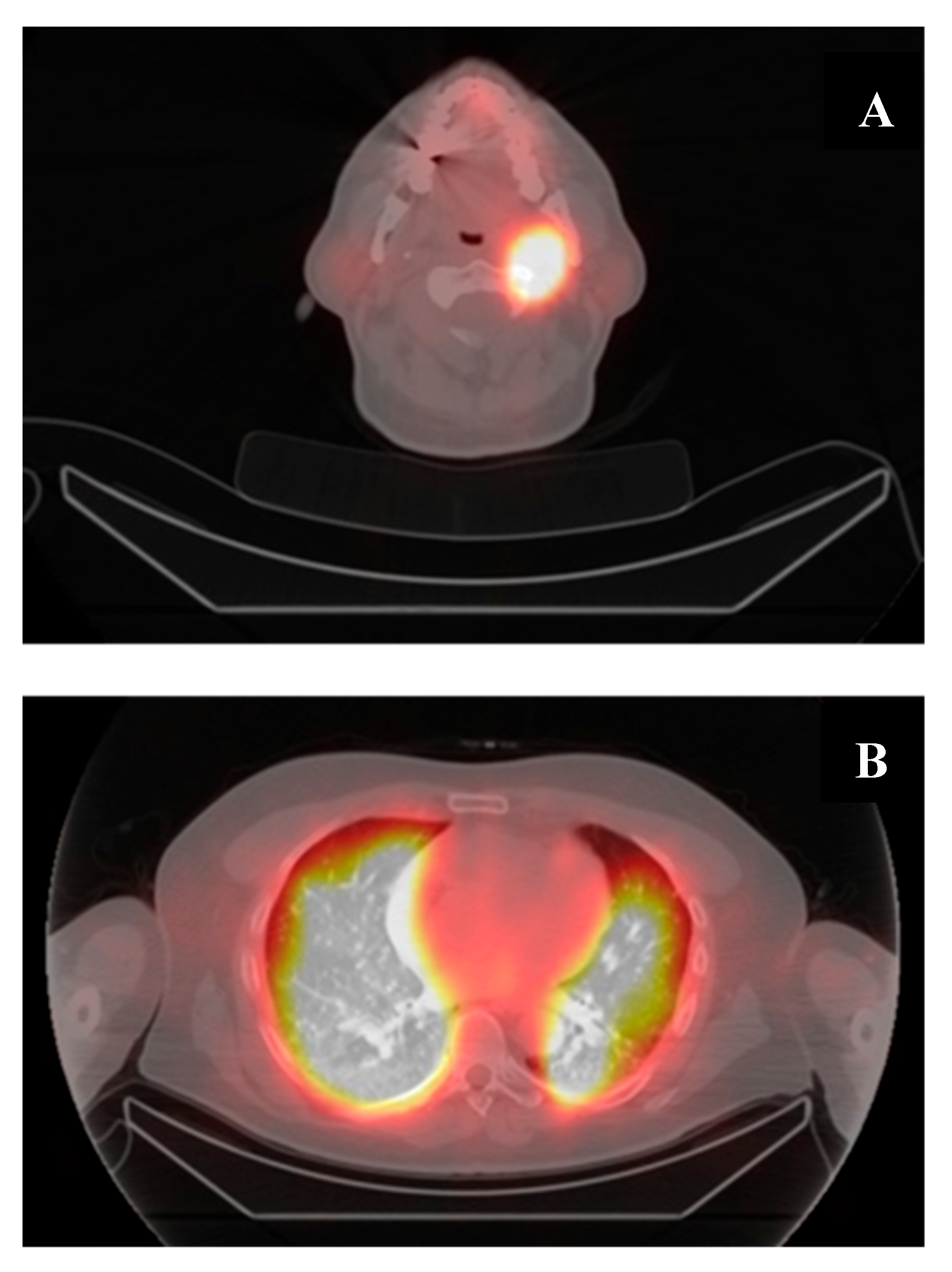
| I. | Malignant/metastatic tissue cannot concentrate RAI on a diagnostic radioiodine scan. |
| II. | Malignant tissue cannot concentrate RAI on a post-131I therapy scan. |
| III. | The tumor loses the ability to concentrate RAI after previous evidence of RAI-avid disease. |
| IV. | RAI is concentrated in some lesions only. |
| V. | Metastasis progression even with significant RAI uptake. |
| VI. | > 600 mCi of cumulated 131I therapy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





