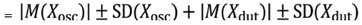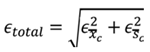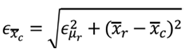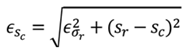1. Introduction
Cardiovascular disease is the number one cause of death worldwide, and hypertension is a major risk factor. An estimated 1.28 billion adults aged 30–79 have hypertension, with two-thirds living in low- and middle-income countries. An estimated 46% of adults with hypertension are unaware that they have the disease, while only 21% have hypertension under control. One of the global targets for non-communicable diseases is to reduce hypertension prevalence by 33% between 2010 and 2030. In recognizing hypertension as a public health problem, the World Health Organization has provided evidence-based recommendations for the initiation of treatment, including blood pressure (BP) monitoring [
1,
2].
Hypertension monitoring requires measurement accuracy and usability. Simple, inexpensive, easy-to-use devices have been adopted throughout the years. The most reliable device is the cuff-based sphygmomanometer, but its use may be difficult due to problems in finding the handling for correct measurement and attachment of the cuff. A cuff-based sphygmomanometer is also used in secondary healthcare settings, such as home monitoring of BP after a physical examination or following the suggestion of the patient’s physician. However, many healthy people are also interested in daily health monitoring, including daily BP measurements. The control of BP through exercise, food intake, and sleep can prevent hypertension. As high BP is not a subjective symptom, it can be diagnosed during a health checkup, after which daily BP monitoring is recommended. Thus, a simple, easy-to-use device for monitoring BP that is also affordable for people in low- and middle-income counties would be of value. Cuffless sphygmomanometers are useful devices for this purpose. With the increasing popularity of cuffless blood sphygmomanometers, studies to determine their safety and reliability are needed with methods for the post-marketing surveillance of these devices.
Several studies have identified the major issues to be considered in validating cuffless sphygmomanometers [
3,
4,
5,
6]. For example, their accuracy at rest should be similar to that of cuff-based sphygmomanometers. Currently, there are no regulations regarding cuffless sphygmomanometers' dynamic response and long-term stability. Thus, while the clinical application of these devices has been discussed, a number of issues regarding their home use remain. For instance, whereas in clinical practice, cuffless sphygmomanometers must demonstrate accuracy and validation, ease of handling is also essential for home healthcare use.
The European Society of Hypertension (ESH) recently suggested several regulatory categories for cuffless sphygmomanometers (see
Section 3) depending on the intended use. A cuffless sphygmomanometer intended for clinical practice will require stricter regulations than one intended for use in home healthcare or during a physical checkup screening. This study focused on the intermittent use of cuffless sphygmomanometers as an alternative to cuff-based sphygmomanometers.
2. Currently Approved Cuffless Sphygmomanometers
Cuffless sphygmomanometers are popular devices and are readily available. The principles underlying their operation have been described in several review articles that examined their tonometry, pulse transit time, and pulse contour methods [
7,
8,
9,
10,
11], while aspects such as ultrasound, seismocardiography, microwave, and impedance are of research interest.
The principles of the currently approved device are briefly explained. An alternative approach to continuous and non-invasive indirect BP measurement is based on changes in pulse wave velocity (PWV), which is the velocity of a pressure pulse propagating along the arterial wall; this can be calculated from the pulse transit time (PTT), which refers to the interval required for the blood pressure wave to transit from the proximal to distal arterial sites during the same cardiac cycle shown in
Figure 1(a).
The pulse arrival time (PAT) obtained from the interval between the electrocardiography (ECG) R wave and the starting point or peak of the photoplethysmographic (PPG) wave, which is the sum of the PTT and the pre-ejection period (PEP), is gaining popularity for tracking BP because it is easy to estimate. Faithfully, PAT and PTT are different; PAT includes PEP, which is the time elapsed between the electrical depolarization of the left ventricle (Q-, R-, and S-wave complex (QRS) on the ECG) and the beginning of ventricular ejection and represents the period of left ventricular contraction with the cardiac valves closed. When blood pressure rises, the pre-ejection period (PEP) occasionally exhibits a prolongation, contrary to the expected reduction. Therefore, the pulse arrival time (PAT), which is equated with the pulse transit time (PTT) in some studies, does not consistently mirror changes in blood pressure [
12]. Nevertheless, if both methods accept the accuracy of blood pressure change, the device can be used as a blood pressure monitor.
The most popular direction is now based on the pulse contour and the second derivative of the PPG signal (SDPPG) methods shown in
Figure 1(b). The measured PPG signal (solid line) and its second derivative (grey line) indicate systolic and diastolic peaks. They represent alternatives for estimating the BP. Pulse demodulation analysis (PDA) was developed to evaluate the arterial pressure pulse, and estimation has confirmed the existence of two major reflection sites in the central arteries.
The downward traveling primary pressure pulse (P1) gives rise to upward traveling pulses (P2 and P3), originating from the renal and iliac reflection sites, respectively, where pulse P1 impinges. The time difference between the arrival of P1 and the second reflection pulse (P3), referred to as T1-3, corresponds to the changes in arterial pulse pressure.
In PDA models, lumped parameter models of the cardiovascular system are commonly used to simulate arterial blood pressure waveform and wave propagation, which consist of resistor impedance and capacitors to fit systolic and diastolic pressures. Then, the second derivative of the PPG signal (SDPPG) is analyzed based on the amplitudes of waves “a”, “b”, “c”, “d”, and “e”, which arise in the systolic phase of the heart cycle. The amplitudes of the waves are normalized as b/a, c/a, d/a, and e/a. The SDPPG contains information on aortic compliance and stiffness, which are highly correlated with BP. To make use of the PPG real waveform and SDPPG, the BP must be analyzed numerically using a neural network and/or support vector machine.
The principle of applanation tonometry of the radial artery is that when a radial artery is partially compressed or splinted against a bone, the pulsations are proportional to the intra-arterial pressure shown in Figure 1(c).
However, the transducer needs to be situated directly over the center of the artery; hence, the signal is very position-sensitive; this has been dealt with by using an array of transducers placed across the artery. Although the technique has been developed for beat-to-beat wrist blood pressure monitoring, it requires calibration in each patient and is unsuitable for routine clinical settings.
Like any newly developed device, regulatory authorities must approve cuffless sphygmomanometers. However, the standards established for cuff-based sphygmomanometers are currently applied, as thus far, the International Organization for Standardization (ISO) has not developed specific standards
for cuffless sphygmomanometers. The Institute of Electrical and Electronics Engineers (IEEE) has published standards for intermittently used cuffless sphygmomanometers. Consequently, while several wearable cuffless sphygmomanometers are available on the market, only a few devices have been approved for medical use. These are briefly described in
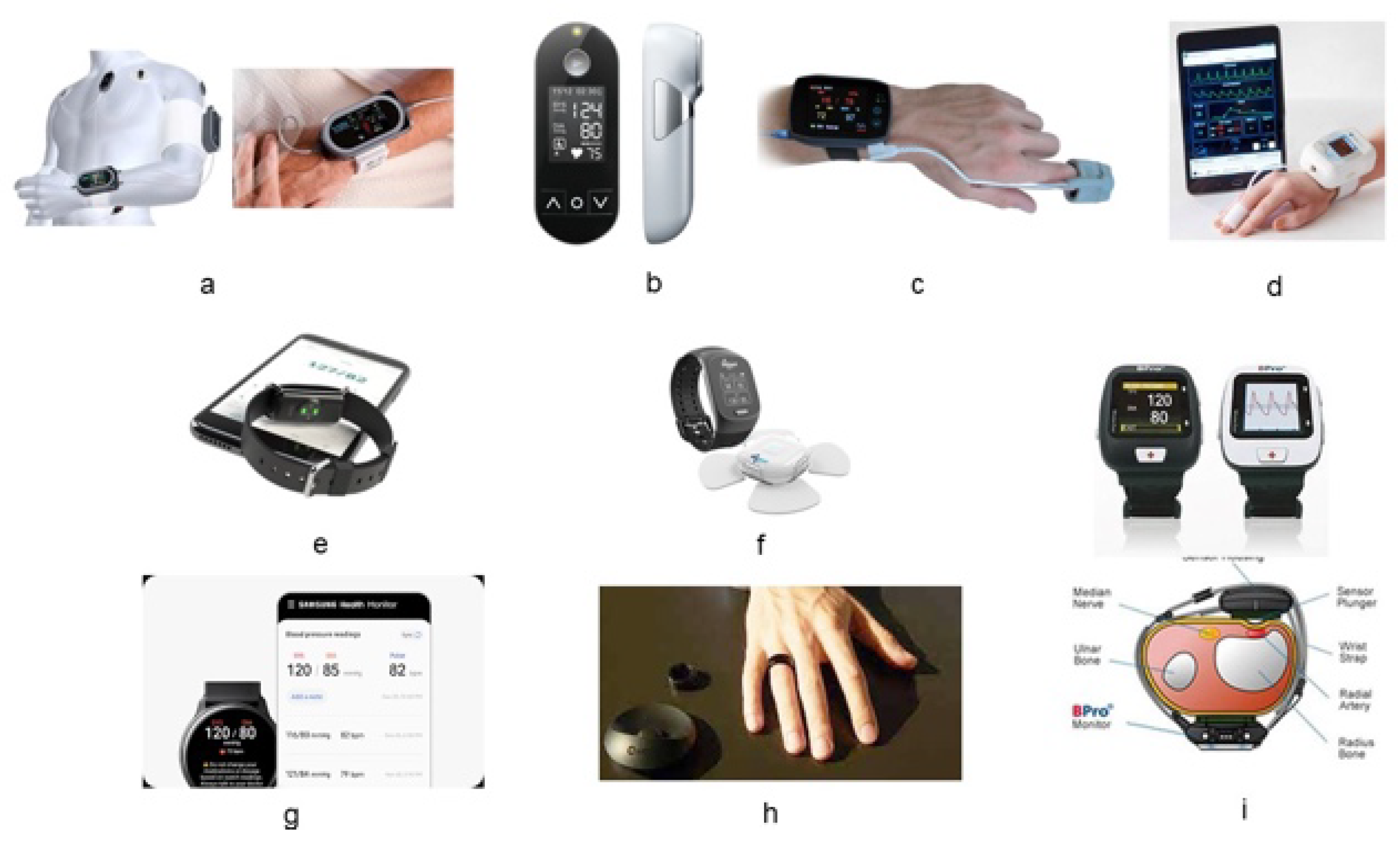
Table 1, with a few examples shown in
Figure 2.
The first cuffless sphygmomanometer to be approved by the U.S. Food and Drug Administration (FDA) for medical use was the BPro in 2006 (FDA 510(K), number K060315), followed by the ViSi Mobile System, Caretaker, and Biobeat devices in 2012 (K112478), 2016 (K151499), and 2019 (K190792), respectively. These devices require a prescription for clinical practice in the USA. In addition, several devices have received CE approval. In Korea, the Ministry of Food and Drug Safety has approved two Korean products: the Galaxy A watch and the CART-1 Plus ring sensor. Despite the absence of standards for cuffless sphygmomanometers, the devices have been approved for static measurements with a required accuracy of 5 ± 8 mmHg. The intended use of these cuffless sphygmomanometers is within a hospital setting and for continuous monitoring during surgery or in an intensive care unit (ICU).
Regulatory issues have impeded physicians' interest in using cuffless sphygmomanometers in clinical practice. In response, the EHS working group developed and published a set of recommendations for cuffless sphygmomanometers [
26].
3. Standards for Cuffless Sphygmomanometers
According to the ISO and regulatory authorities, standards for BP monitors address quantitative measurements, weight, range, and clinical utility. However, no ISO standards or other regulations are yet available
for cuffless sphygmomanometers. Using cuffless sphygmomanometers in clinical practice has elicited positive [
27,
28,
29,
30,
31,
32] and negative [
33,
34,
35,
36,
37,
38] reactions. In response, the ESH has recommended six validation tests to evaluate the safety and reliability of cuffless sphygmomanometers for clinical use
: static testing, testing of accuracy at different measurement sites, testing in patients using antihypertensive drugs, testing during different sleep stages and during exercise, and long-term variability. In clinical practice, sphygmomanometers are used mainly for short-term monitoring. While cuff-based sphygmomanometers provide intermittent (snapshot) measurements, cuffless sphygmomanometers have the additional advantages of beat-to-beat and continuous monitoring, which can be used in the ICU and operation room and for nocturnal monitoring and the detection of sudden changes in BP. However, in hypertension management for home healthcare, BP is typically only measured early in the morning and before bedtime. For this intermittent use, the six validation tests may not be necessary; rather, fundamental tests of static accuracy, dynamic response, and long-term stability may suffice.
Many over-the-counter (OTC) medical devices, defined as those not requiring a prescription and sold directly to the consumer in physical or online stores, are available for use at home and in nursing homes or other long-term care facilities. They allow patients to self-monitor and, thus, potentially self-treat their condition. The device's instructions must be written to allow patients to use it without any help from a healthcare provider. OTC cuffless sphygmomanometers are safe and effective for standardized BP measurements in the home healthcare setting. Doubts regarding their accuracy can be resolved by clinical confirmation. Therefore, the following section proposes validation tests for OTC cuffless sphygmomanometers.
4. Proposed Regulation for OTC Cuffless Sphygmomanometer
A simple validation protocol is required for OTC devices used for home screening and simple physical checkups. The proposed tests were developed with reference to the ISO standards for cuff-based sphygmomanometers (ISO 81060-2:2018; Non-Invasive Sphygmomanometers Part 2: Clinical Investigation of Intermittent Automated Measurement Type) and continuous BP monitors (ISO81060-3:2022; Non-Invasive Sphygmomanometers Part 3: Clinical Investigation of Continuous Automated Measurement Type), as well as the IEEE standards for wearable cuffless blood pressure monitoring systems (IEEE 1708:2014 and 1798a:2019). The three validation tests described herein refer to standards for static accuracy, dynamic accuracy, and long-term stability,
4.1. Static Accuracy
The validation protocol developed to measure resting-state BP using an OTC cuffless sphygmomanometer followed that of a cuff-based sphygmomanometer, i.e., the auscultation method. The protocol was tested by measuring the resting-state BP of 35 volunteers with a wide distribution of BP values, as recommended by the ESH.
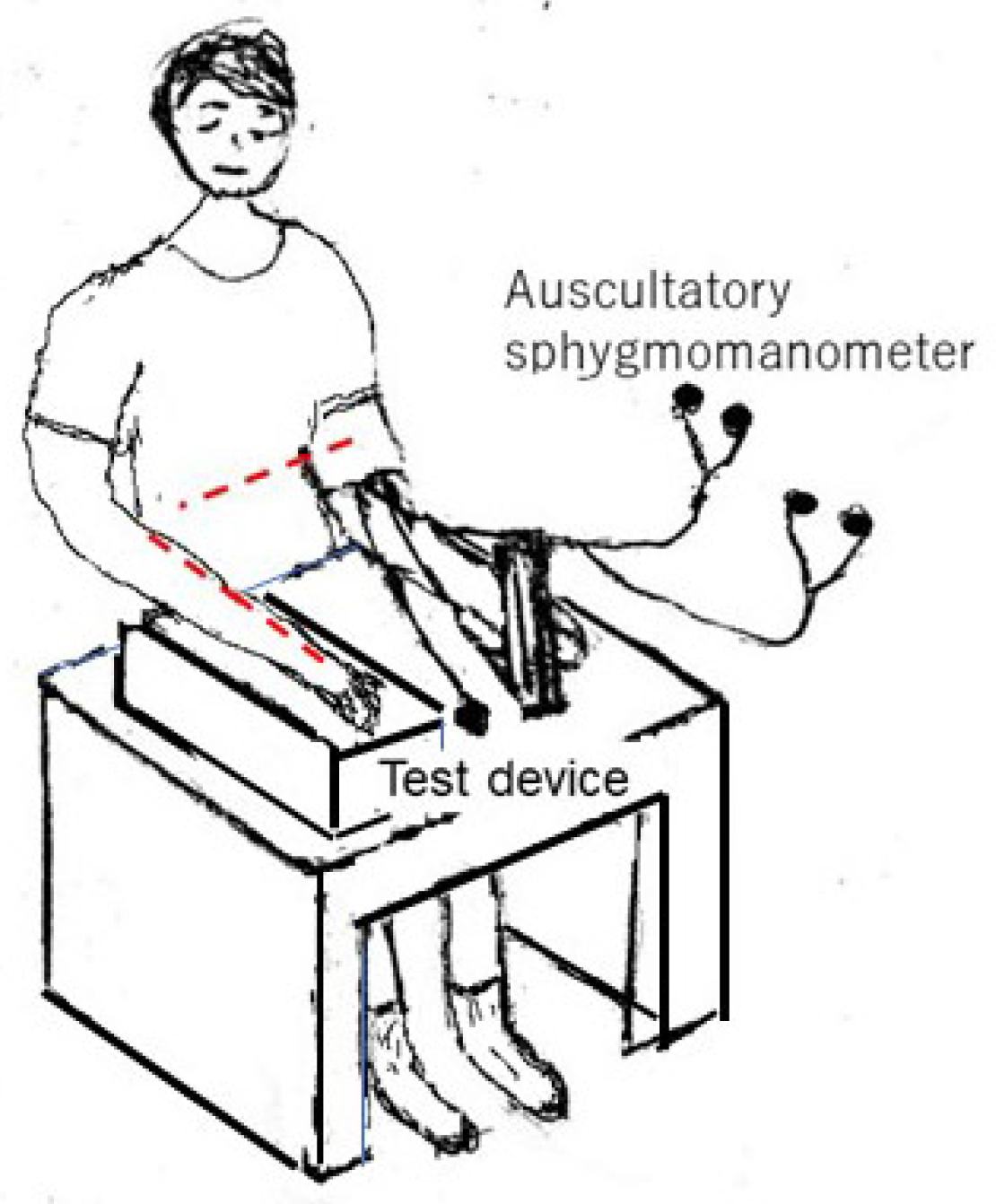
The static test is conducted as illustrated in Figure 3, with the individual sitting comfortably in a chair with his or her back straight. A cuff-based reference BP monitor—in this study, an auscultation sphygmomanometer—is attached to either the left or right arm, and the test device is attached to the same or the opposite arm. The sensor of the test device should be placed at the same height as the cuff on the upper arm, i.e., at the heart level (red dotted line in Figure 3). The height differences are critical [
40]. The height of the smartwatch and wristbands must be the same as the heart level. Manufacturers must emphasize the importance of maintaining this height equivalence in their instructions, particularly for static measurements, and provide clear guidelines on measuring and adjusting for any height discrepancies. Two observers measure the resting-state BP by following the instructions of the reference and test devices. The reference and test measurements can be performed simultaneously or consecutively. If the measurements are simultaneous, the absence of interference between the two devices must be ensured. If the measurements are consecutive, there should be an interval of at least 60 s between measurements.
The data are excluded if the reference systolic BP (SBP) range is > 20 mmHg or if the reference diastolic BP (DBP) range is > 12 mmHg during or before determination by the test device. For the test device, three pairs of measurements are obtained for snapshot BP measurement devices, and three recordings of 60 s each are obtained for beat-to-beat measurement devices.
4.2. Dynamic BP Changes
While the measurement of dynamic BP changes is not required for snapshot monitoring of BP during home healthcare, it may be useful in other settings. The current standard for cuff-based sphygmomanometers also includes a protocol for determining dynamic BP changes. A cuffless sphygmomanometer can be used in the continuous beat-to-beat measurement of BP changes, as is often desirable during a clinical setting. Simultaneous recordings with a cuffless sphygmomanometer and a direct BP sensor can provide accurate and valuable BP data. For OTC devices, simultaneous recordings with a continuous volume-clamp device are recommended. The ISO81060-3 standard also recommends direct BP monitoring, but this method is problematic in terms of both ethics and safety. For ethical reasons, the specialized techniques required for direct BP measurement are not approved for use in clinically healthy individuals in some countries. Moreover, the FDA has not approved the use of volume-clamp devices for standard continuous BP monitoring. However, the accuracy of OTC devices is sufficient for healthcare purposes.
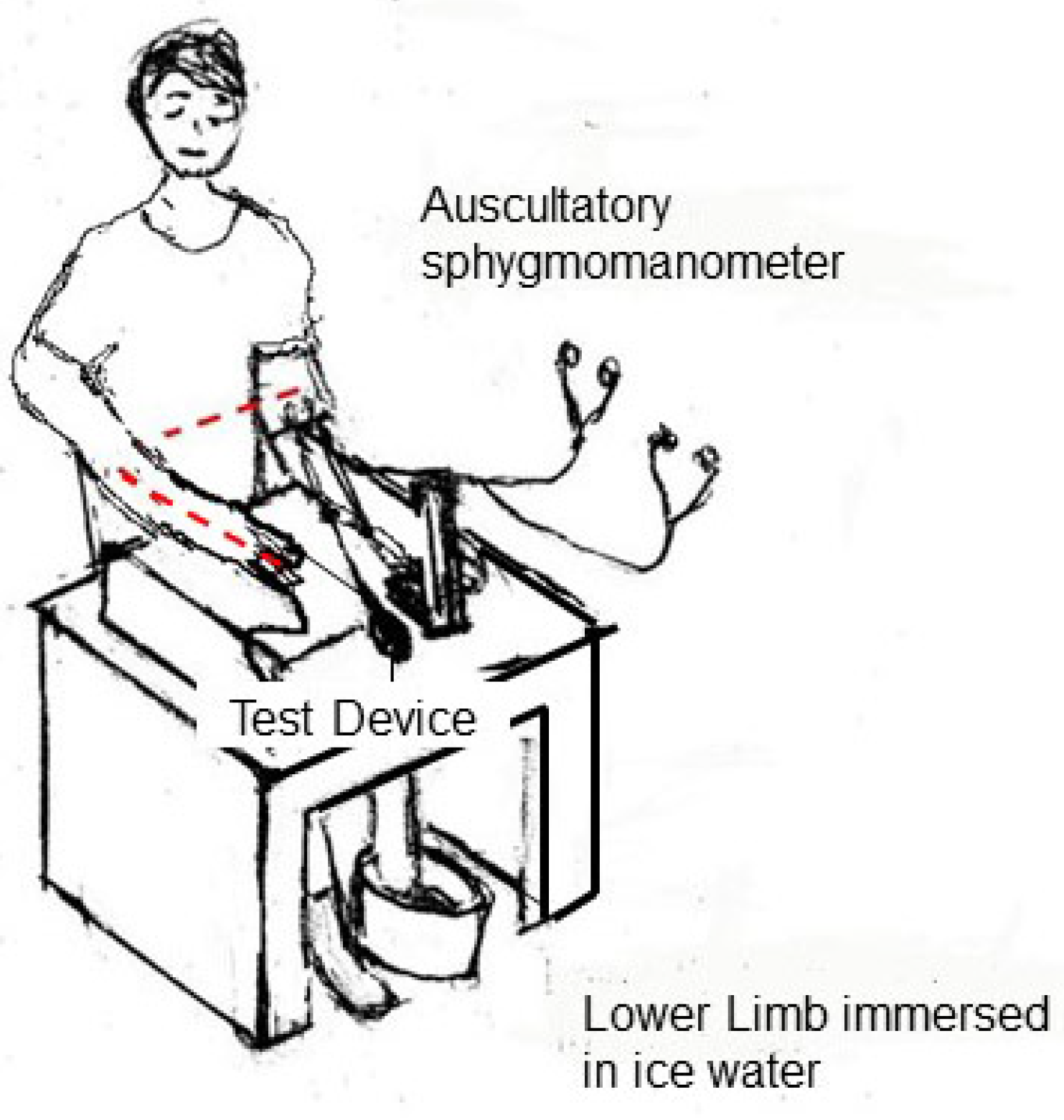
Methods for measuring BP changes are not included in current standards, but testing during exercise [
41,
42] or cold stress is recommended. The cold stress test demonstrates increased blood pressure during a one- to two-minute immersion of one foot in ice water at 4–10 °C shown in
Figure 4. Three pairs of measurements for snapshot BP measurement devices and three recordings of 60 s each for beat-to-beat measurement devices are made using observers 2 min after the induction of a BP change. The highest BP reading obtained while the foot is in the ice water is recorded as the response index.
4.3. Long-Term Stability
Long-term stability is the most important issue in measuring BP at home, as hypertension management requires the long-term tracking of BP values. For hypertension patients prescribed an antihypertensive drug, testing is usually conducted after 1 month. Thus, accuracy validation for OTC devices at 1 month is sufficient.
This study's time interval between measurements used to evaluate long-term stability followed the current standard. We recommend a medically approved device, such as an oscillometric automated sphygmomanometer. The long-term accuracy of the test device can then be evaluated based on the propagation error, defined as the sum of the error of the standard device, in this case, the oscillometric automated sphygmomanometer (Xosc), and that of the test device (Xdut), with each error containing the mean ± standard deviation (SD). Thus:
Total error
Let us assume that the specifications of reference device
Xosc are errors in mean measurement
nd errors in standard deviation measurement
, measured mean
, and measured SD
. To derive the total error
f the cuffless device, as shown in (1), the deterministic error in mean
and SD
are calculated by (2) and (3), respectively, where
nd
are the measured mean and measured SD of the cuffless device.
The error of the reference device
ould be within 5 ± 8 mmHg, as reported by the manufacturer or recommended sphygmomanometers from two organizations: dabl Educational Trust and STRIDE BP [
45]. For the test device, the error is based on its static error. If the static error of the test device is 5 ± 8 mmHg, then the total device error is 10 ± 11.3 mmHg. However, most cuffless sphygmomanometers are more accurate when used under resting conditions. The error criteria are smaller than the above error. Regardless, if the test device's long-term stability error is satisfied within the above errors, the device must be accepted as an OTC device.
The differences between the reference BP monitor and the test device during the test, specifically whether the differences are within 5, 10, 15, or > 15 mmHg, are determined. This flexibility aids in the evaluation of accuracy. For high-grade sphygmomanometers, a difference ≤ 10 mmHg is defined as a slight but acceptable inaccuracy. The number of absolute BP differences (i.e., test BP minus mean versus reference BP readings) within 5, 10, and 15 mmHg for SBP and DBP is also determined. A device is considered acceptable if its estimated probability of a tolerable error (≤ 10 mmHg) is at least 85% [
46].
For a recalibration period between 1 and 10 days, readings should be taken twice daily for the first 3 days (9 ± 3 h apart) and then once daily thereafter, including on day 10, or as specified by the manufacturer. Thereafter, recalibration should be performed once every 10 days until day 30 or as specified by the manufacturer (up to 30 days).
5. Preliminary Results
In our preliminary study, a 1-month validation test of the modified Checkme device (Sanei Medisys Ltd., Japan) was performed according to the proposed protocol perspective. This case report was conducted with the approval of the Ethical Committee of Sanei Medisys Ltd. The static errors were −0.12 ± 5.49 mmHg for SBP and −1.17 ± 5.06 mmHg for DBP, respectively. Based on the propagation error, the accuracy criteria for the long-term stability of SBP and DBP measurements obtained using the oscillometric automated sphygmomanometer were −5.12 ± 9.70 mmHg and −6.17 ± 9.48 mmHg. In particular, the total error for SBP was |5|±8 mmHg for the assumed oscillometric automated sphygmomanometer + |−0.12| ± 5.49 mmHg for the static error of the test device, and then it was -5.12± 9.70 mmHg. The total error for DBP was |5|±8 mmHg for the assumed oscillometric automated sphygmomanometer + |−1.17| ± 5.06 mmHg and then it was −6.17 ± 9.48 mmHg.
For the test device in our long-term stability test, the errors were −3.38 ± 7.10 mmHg for SBP and −1.38 ± 5.40 mmHg for DBP, which met the accuracy criterion.
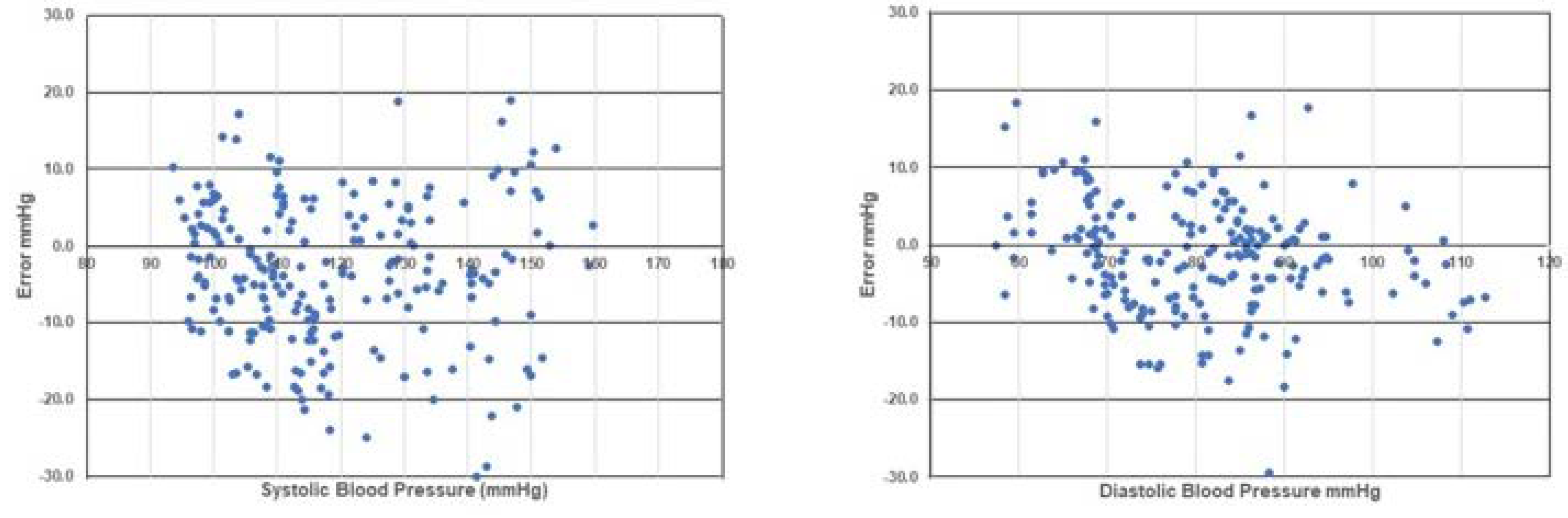
Figure 5 shows the number of absolute BP differences between 10 and 20 mmHg for SBP and DBP, depicted using standardized Bland–Altman scatterplots. According to the above criteria, the test device was considered acceptable.
6. Limitations
We proposed and evaluated new standards for using cuffless sphygmomanometers in home healthcare. Although the cuffless sphygmomanometer is a medically approved device in the hospital setting, its long-term stability is the most important operating factor for home healthcare purposes. In this study, a commercially available cuffless sphygmomanometer was tested by comparison with a standard device. Not all OTC devices for home use require approval by regulatory authorities, but OTC sphygmomanometers should be medically approved to allow BP monitoring at home or for screening and physical checkups. During COVID-19, OTC pulse oximeters without medical approval became popular and were sold directly as cutometers to estimate oxygen saturation. However, OTC devices could not be used for medical purposes. The OTC cuffless sphygmomanometer must be approved by regulatory agencies because of its safety and accurate long-term stability. Accurate trends are important for hypertension management. Additionally, medically approved OTC device reviews cover general healthcare and wellness purposes.
7. Future Prospective
Most current literatures on cuffless blood pressure monitoring focuses either on mathematical models based on cardiovascular parameters or on artificial intelligence (AI) and Neural Networks (NN) with non-calibrated devices. Since the publication of the ESH recommendation, only a few articles have addressed validation, regulation, and standards in this area. The challenge of long-term stability remains, with no specific solutions proposed yet. Evidence, regulation, and reliable validation tests still require time to develop to a point where they are market-ready [
47,
48].
Long-term validation in routine practice remains a topic of discussion, especially since the validation study presented in ISO81060-3 standard is complex and may not be suitable for home healthcare protocols. Our study proposes simpler and more easily acceptable standards. It's noteworthy that the standard for cuffless blood pressure is now available in IEEE1708, and new standards in both IEEE and ISO are expected in the near future. We anticipate the establishment of one unified standard for clinical practice through the collaboration of ISO and IEEE, which will further the discourse on home healthcare applications.
Furthermore, the role of AI and NN in cuffless blood pressure measurement needs to be discussed beyond existing protocols. We have added relevant references to support these points, including both positive and negative comments on current research in the field.
Institutional Review Board Statement
Not applicable to this study.
Informed Consent Statement
Not applicable to this study.
Acknowledgments
This research was funded by a Grant-in-Aid for Scientific Research (KAKENHI #17K01440, #21K12760), the Japan Agency for Medical Research and Development (Grant no. s20dk0310111), and a project research grant from the Institute for Healthcare Robotics, Future Robotic Organization, Waseda University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou B, Carrillo-Larso R.M., Danaei G.et al. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1,201 population representative studies with 104 million participants. The Lancet 2021, 398, 10304, 957-980. [CrossRef]
- Heidenreich PA., Bozkurt B., Aguilar D et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2022,145, e895–e1032. [CrossRef]
- Moon, J.H., Kang, MK., Choi, CE. et al. Validation of a wearable cuff-less wristwatch-type blood pressure monitoring device. Sci Rep 2020, 10, 19015. [CrossRef]
- Mukkamala R., Yavarimanesh M., Keerthana Natarajan K et al. Evaluation of the Accuracy of Cuffless Blood Pressure Measurement Devices: Challenges and Proposals Hypertension 2021 78 (5), 1161-1167. [CrossRef]
- Hu, J-R, Martin, G, Iyengar, S. et al. Validating cuffless continuous blood pressure monitoring deviceValidating cuffless continuous blood pressure monitoring devices Cardiovascular Digital Health J. 2023 4(1), 9-20. [CrossRef]
- Stergiou GS, Avolio AP, Palatini P. et al European Society of Hypertension recommendations for the validation of cuffless blood pressure measuring devices: European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. J Hypertens. 2023, 41(12) , 2074-2087. https://do.org/10.1097/HJH.0000000000003483 .
- Elgendi, M., Fletcher, R., Liang, Y. et al. The use of photoplethysmography for assessing hypertension. npj Digit. Med 2019, 2, 60 . [CrossRef]
- Pandit JA, Lores E, Batlle D. Cuffless Blood Pressure Monitoring: Promises and Challenges. Clin J Am Soc Nephrol. 2020, 15(10):1531-1538. [CrossRef]
- Tamura T, Cuffless blood pressure monitors: Principles, standards and approval for medical use, IEICE Transactions on Communications, 2021, E104B(6), pp.580-586. [CrossRef]
- Schutte AE, Kollias A, Stergiou GS. Blood pressure and its variability: classic and novel measurement techniques. Nat Rev Cardiol. 2022, 19(10), 643-654. [CrossRef]
- Mukkamala R, Stergiou G.S., Alberto P. Cuffless Blood Pressure Measurement Annual Review of Biomedical Engineering 2022. 24:1, 203-230. [CrossRef]
- Krohova J, Czippelova B, Turianikova Z, Lazarova Z, Tonhajzerova I, Javorka M. Preejection period as a sympathetic activity index: a role of confounding factors. Physiol Res. 2017 66(Suppl 2), S265-S275. [CrossRef]
- Welch J, Kanter B, Skora B, McCombie S, Henry I, McCombie D, Kennedy R, Soller B. Multi-parameter vital sign database to assist in alarm optimization for general care units. J Clin Monit Comput. 2016 , 30(6):895-900. [CrossRef]
- Boubouchairopoulou N, Kollias A, Chiu B, et al. A novel cuffless device for self-measurement of blood pressure: concept, performance and clinical validation. J Hum Hypertens. 2017, 31, 479–82. [CrossRef]
- Bilo G, Zorzi C, Ochoa Munera JE, et al. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015, 20(5), 291-4. [CrossRef]
- Baruch, M.C., Warburton, D.E., Bredin, S.S. et al. Pulse Decomposition Analysis of the digital arterial pulse during hemorrhage simulation. Nonlinear Biomed Phys 2011, 5, 1. [CrossRef]
- Elgendi, Mohamed. On the Analysis of Fingertip Photoplethysmogram Signals. Curr Cardiol Rev. 2012 ,8(1), 14-25. [CrossRef]
- Shin H, Min S.D. Feasibility study for the non-invasive blood pressure estimation based on ppg morphology: normotensive subject study. Biomed Eng Online. 2017, 16(1), 10. [CrossRef]
- Gratz, I., Deal, E., Spitz, F. et al. Continuous Non-invasive finger cuff CareTaker® comparable to invasive intra-arterial pressure in patients undergoing major intra-abdominal surgery. BMC Anesthesiol 2017, 17, 48 . [CrossRef]
- Pellaton, C, Vybornova, A; Fallet, S, et al ; Accuracy testing of a new optical device for noninvasive estimation of systolic and diastolic blood pressure compared to intra-arterial measurements. Blood Pressure Monit. 2020 25(2), p 105-109,. [CrossRef]
- Nachman D, Gepner Y, Goldstein N, et al. Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Sci Rep. 2020, 10(1),16116. [CrossRef]
- Han, M., Lee, YR., Park, T. et al. Feasibility and measurement stability of smartwatch-based cuffless blood pressure monitoring: A real-world prospective observational study. Hypertens Res 2023, 46, 922–931. [CrossRef]
- Joung, J., Jung, CW., Lee, HC. et al. Continuous cuffless blood pressure monitoring using photoplethysmography-based PPG2BP-net for high intrasubject blood pressure variations. Sci Rep 2023, 8605. [CrossRef]
- Nair D, Tan SY, Gan H.W. et al. The use of ambulatory tonometric radial arterial wave capture to measure ambulatory blood pressure: the validation of a novel wrist-bound device in adults. J Hum Hypertens. 2008 22, p220–2. . [CrossRef]
- Komori T, Eguchi K, Hoshide S, Williams B, Kario K. Comparison of wristtype and armtype 24-h blood pressure monitoring devices for ambulatory use. Blood Press Monit. 2013, 18, p57–62. [CrossRef]
- Stergiou GS, Mukkamala R, Avolio A et al. European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. Cuffless blood pressure measuring devices: review and statement by the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. J Hypertens. 2022 , 40(8), 1449-1460. [CrossRef]
- Almeida, T.P., Cortés, M., Perruchoud, D. et al. Aktiia cuffless blood pressure monitor yields equivalent daytime blood pressure measurements compared to a 24-h ambulatory blood pressure monitor: Preliminary results from a prospective single-center study. Hypertens Res 2023, 46, 1456–1461. [CrossRef]
- Yano, Y., Kario, K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res 2012 35, p695–701. [CrossRef]
- Vybornova, A, Polychronopoulou, E, Wurzner-Ghajarzadeh, A et al. Blood pressure from the optical Aktiia Bracelet: a 1-month validation study using an extended ISO81060-2 protocol adapted for a cuffless wrist device. Blood Pressure Monit. 2021, 26(4), p 305-311,. [CrossRef]
- Kachel E, Constantini K, Nachman D, Carasso S, Littman R, Eisenkraft A, Gepner Y. A Pilot Study of Blood Pressure Monitoring After Cardiac Surgery Using a Wearable, Non-invasive Sensor. Front Med (Lausanne). 2021 5, 8:693926. [CrossRef]
- Yoon Y, Kim J, Lee KJ, et al. Blood Pressure Measurement Based on the Camera and Inertial Measurement Unit of a Smartphone: Instrument Validation Study JMIR Mhealth Uhealth 2023;11:e44147. [CrossRef]
- 20 November; 44, 32. De Almeida TP, Sola J, Theiler K, Performance of a cuffless blood pressure monitor in the adult and elderly population - a comparative validation study, European Heart Journal, Volume 44, Issue Supplement_2, November 2023. [CrossRef]
- Falter M, Scherrenberg M, Driesen K, et al. Smartwatch-Based Blood Pressure Measurement Demonstrates Insufficient Accuracy. Frontiers in Cardiovascular Medicine 2022, 9 https://www.frontiersin.org/articles/10.3389/fcvm.2022.958212.
- Heimark S, Bøtker-Rasmussen KG, Stepanov A, et al. Accuracy of non-invasive cuffless blood pressure in the intensive care unit: Promises and challenges. Front Med (Lausanne). 2023, 7, 10:1154041. [CrossRef]
- Mukkamala R, Shroff S.G, Landry C, et. al. The Microsoft Research Aurora Project: Important Findings on Cuffless Blood Pressure Measurement Hypertension. 2023; 80: 534–540. [CrossRef]
- Tan I, Gnanenthiran SR, Chan J et al. Evaluation of the ability of a commercially available cuffless wearable device to track blood pressure changes. J Hypertens. 2023;41(6):1003-1010. [CrossRef]
- Heimark S., Hove C. , Stepanov A, et al. Accuracy and User Acceptability of 24-hour Ambulatory Blood Pressure Monitoring by a Prototype Cuffless Multi-Sensor Device Compared to a Conventional Oscillometric Device, Blood Pressure 2023, 32:1,. [CrossRef]
- Hu, JR., Park, D.Y., Agarwal, N. et al. The Promise and Illusion of Continuous, Cuffless Blood Pressure Monitoring. Curr Cardiol Rep 2023 25, 1139–1149. [CrossRef]
- BaHammam, A.S., Alshahrani, M., Aleissi, S.A. et al. Blood pressure dipping during REM and non-REM sleep in patients with moderate to severe obstructive sleep apnea. Sci Rep 2021 11, 7990. [CrossRef]
- Sola, J., Vybornova, A., Fallet, S. et al. Validation of the optical Aktiia bracelet in different body positions for the persistent monitoring of blood pressure. Sci Rep 2021 11, 20644. [CrossRef]
- Franz IW. Ergometry in the assessment of arterial hypertension. Cardiology. 1985, 72(3), 147-59. [CrossRef]
- Wielemborek-Musial K, Szmigielska K, Leszczynska J, Jegier A. Blood Pressure Response to Submaximal Exercise Test in Adults. Biomed Res Int. 2016, 5607507. [CrossRef]
- Silverthorn DU, Michael J. Cold stress and the cold pressor test. Adv Physiol Educ. 2013, 37(1), 93-6. [CrossRef]
- Dabl education trust http://www.dableducational.org (accessed on November 8, 2023).
- STRIDE BP https://www.stridebp.org/ (accessed on November 8, 2023).
- Stergiou GS, Alpert B, Mieke S, et al A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens. 2018 36(3), 472-478. [CrossRef]
- Tamura, T., Shimizu, S., Nishimura, N. et al. Long-term stability of over-the-counter cuffless blood pressure monitors: a proposal. Health Technol. 2023 13, 53–63 (2023). [CrossRef]
- Hu, JR, Martin G, Iyengar S. et al. Validating cuffless continuous blood pressure monitoring devices, Cardiovascular Digital Health Journal, 4(1), 2023, 9-20. [CrossRef]
- Lui ZD Li y., Zhang Y-T. et al., Cuffless Blood Pressure Measurement Using Smartwatches: A Large-Scale Validation Study, IEEE Journal of Biomedical and Health Informatics, 2023 vol. 27, no. 9, pp. 4216-4227,. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).