Level of Evidence IV.
1. Introduction
Skin defects in the head, face and neck resulting from chronic wounds, traumatic injuries, or oncological conditions require effective closure. A tailored approach must be developed for each defect based on the patient’s requirements and the extent of the affected tissues. [
1] The reconstructive ladder is a tool used in Plastic surgery to evaluate the extent of a defect and select the most appropriate method for treatment. This ladder begins with primary closure, followed by free skin grafts, local flaps, and pedicled flaps, and ends with free flaps, depending on the location and severity of the defect. [
2,
3] When reconstructing defects in the face, head, and neck, surgeons often use regional or distant pedicled flaps. Regional flaps are a suitable option for treating areas where the damage is not too severe and where it is crucial to maintain the natural look of the face. An understanding of the biomechanics of soft tissue and the vascular supply to skin flaps is elementary for assessing skin-/ and flap perfusion.[
4]
Despite significant advancements in medical, surgical and technical fields, no technique has yet been found that allows for a consistent, simple, reliable and cost-effective assessment of perfusion in cutaneous flaps. Therefore, direct clinical evaluation remains the gold standard in the monitoring of skin flaps. [
5,
6] Most head and neck surgeons evaluate flap perfusion based on parameters such as colour, reperfusion, scratch testing (quality of the blood), and flap surface temperature. [
6,
7]
Thermal Imaging (TI), smartphone-based TI (SBTI) and dynamic infrared thermography (DIRT) have now shown promise as an innovative tool in several medical specialties. Using the detection of infrared radiation emitted by the body, thermography produces accurate visual representations of surface temperatures, allowing areas of increased or decreased blood flow to be highlighted. [
8]
As an advanced, non-invasive and low-cost, mostly smartphone-based (Teledyne FLIR LLC, Wilsonville, Oregon, United States) [
9,
10,
11,
12,
13] imaging technique, SBTI offers valuable insights into graduation of burn depths [
9,
10], as well as thermal physiology[
14].
This paper aims to deliver an implementable algorithm for the use of SBTI in the perioperative setting and a prior insight into the accuracy of this TI technique as a reliable assessment for cutaneous perfusion in pedicled flaps in the face, head and neck.
2. Materials and Methods
2.1. Ethics Statement
This study involving human participants adheres to ethical standards. Approval was obtained from the Ethics Committee of the University of Heidelberg (Votum: S-630/2023) on 06.11.2023. The research conforms to the Declaration of Helsinki.
Publication of images and individual participant information is contingent on the authors securing free prior informed consent. Authors confirm consent during the author’s license to publish without submitting the actual form. A standard patient consent form from Thieme© was obtained by all patients.
2.2. Thermal Imaging Technique
TI and dynamic infrared thermography (DIRT), have now shown promise as an innovative tool in several medical specialties. [
14] Thermal imaging technology utilises electromagnetic radiation in the near-infrared (NIR) range, specifically wavelengths ranging from 780 to 1400 nm. Unlike visible light, which is perceived by the human eye, NIR radiation is invisible and requires technology-based recording, analysis, and interpretation. By employing specialized cameras sensitive to NIR wavelengths, such as infrared cameras (e.g. the FLIR One System), it becomes possible to capture and visualize NIR radiation. [
15] Using the detection of infrared radiation emitted by the body, thermography produces accurate visual representations of surface temperatures, allowing areas of increased or decreased blood flow to be highlighted. [
8] (see
Error! Reference source not found. and
Error! Reference source not found., blue=cold, red=warm) Over these colour-coded heat-emission differences indirect perfusion patterns can be drawn.
2.3. Implementation of SBTI in the Operating Room
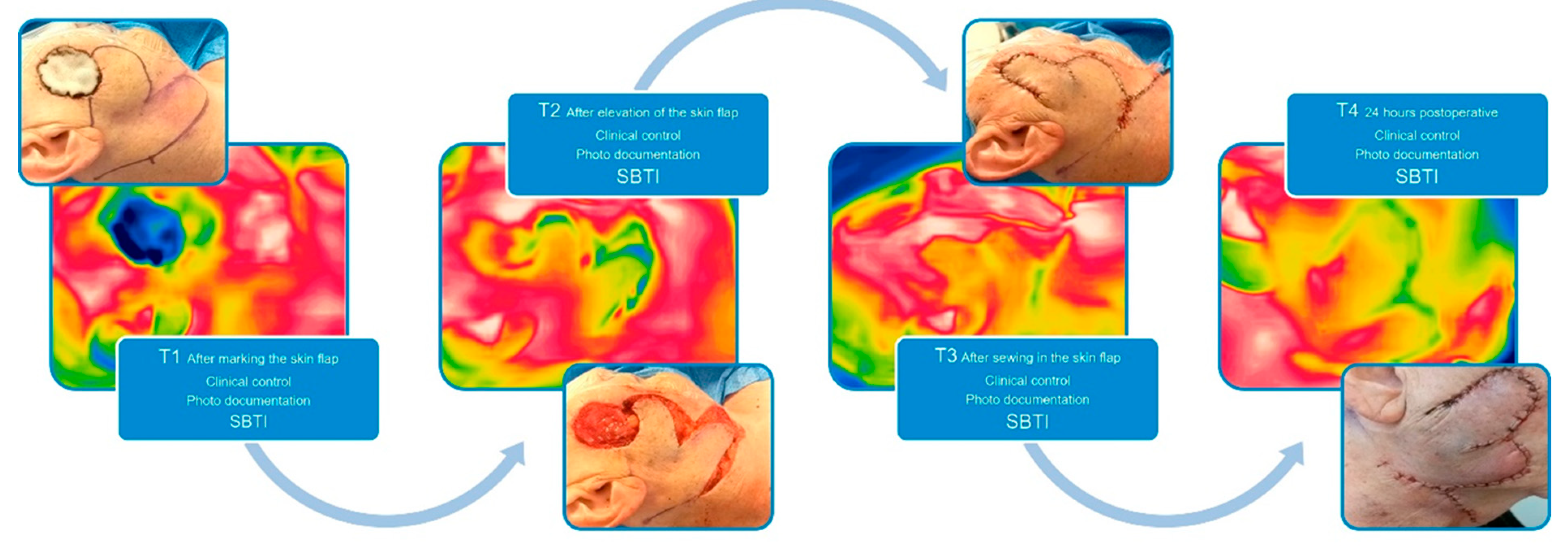
Before the evaluation of the SBTI technique in cutaneous flaps, we established an imaging protocol as given in Error! Reference source not found., where we defined definite points of interest (T1-T4). The ROI (region of interest) was defined by the centre of the flap with an imaging distance taken 30 cm away from the central point of the flap. The room temperature in our OR is controlled by a thermoregulation system which holds it constantly around 23°C. The humidity of the room is constantly around 30%.
Figure 1.
SBTI algorithm.
Figure 1.
SBTI algorithm.
2.4. Study Course
Our study aimed to integrate thermal imaging into the assessment of patients undergoing reconstructive surgery for defects in the face and neck following tumour resections of varying extents. This investigation was conducted at SLK Kliniken Heilbronn, Germany, within the Department for Otorhinolaryngology/Head and Neck, Plastic Surgery, over four months from November 2023 to March 2024. We integrated various data such as age, gender, diagnosis, disease stage, diabetes mellitus status, smoking history (pack years), hypertension, anticoagulants, history of previous surgeries in the operative area, details of the surgical procedure (including technique and date), location and size of the defect, intra-operative clinical assessment and Doppler signal, imaging details, postoperative complications, exact timing of post-operative assessments (T4) and the need for revision surgery followed up for two months.
Drawing from findings in prior research, we found that incorporating the FLIR One camera into our surgical protocol was straightforward, albeit requiring a learning curve for both its application and the interpretation of images about the indirect visualization of blood flow based on temperature differentials. Hence, we opted for the FLIR One System (Teledyne FLIR LLC, Wilsonville, Oregon, United States) for this pilot study. This multispectral camera features a near-infrared camera unit with a resolution of 160 x 120 pixels, covering a temperature range of 0°C to 400°C, with a thermal resolution of 70 millikelvin.
For our investigation, we took thermal images from a total of n=11 axial pattern and random pattern pedicled skin flaps of the face and neck (e.g. bilobed flaps, Rieger flaps, Hatchet flaps, island flaps, …) using the FLIR One camera, resulting in a total of n=44 images. These images were captured at various stages: after marking (T1), after flap elevation (T2), upon completion of surgery (T3), and 24 hours postoperatively (T4). Subsequently, the clinical outcome was evaluated by a single surgeon one week postoperatively, with assessments made in correlation with the captured thermal images. (see Error! Reference source not found.)
The thermal imaging data was then retrospectively graded by two surgeons with a grading system from 1-5 from “1” showing perfect perfusion of the flap and ”5” showing no perfusion. When grading a “0”, flap perfusion was not evaluable. The grading was then averaged with a mean of both surgeons.
Figure 2.
Implemented SBTI algorithm T1-4, showing the intraoperative picture in correlation to the SBTI picture.
Figure 2.
Implemented SBTI algorithm T1-4, showing the intraoperative picture in correlation to the SBTI picture.
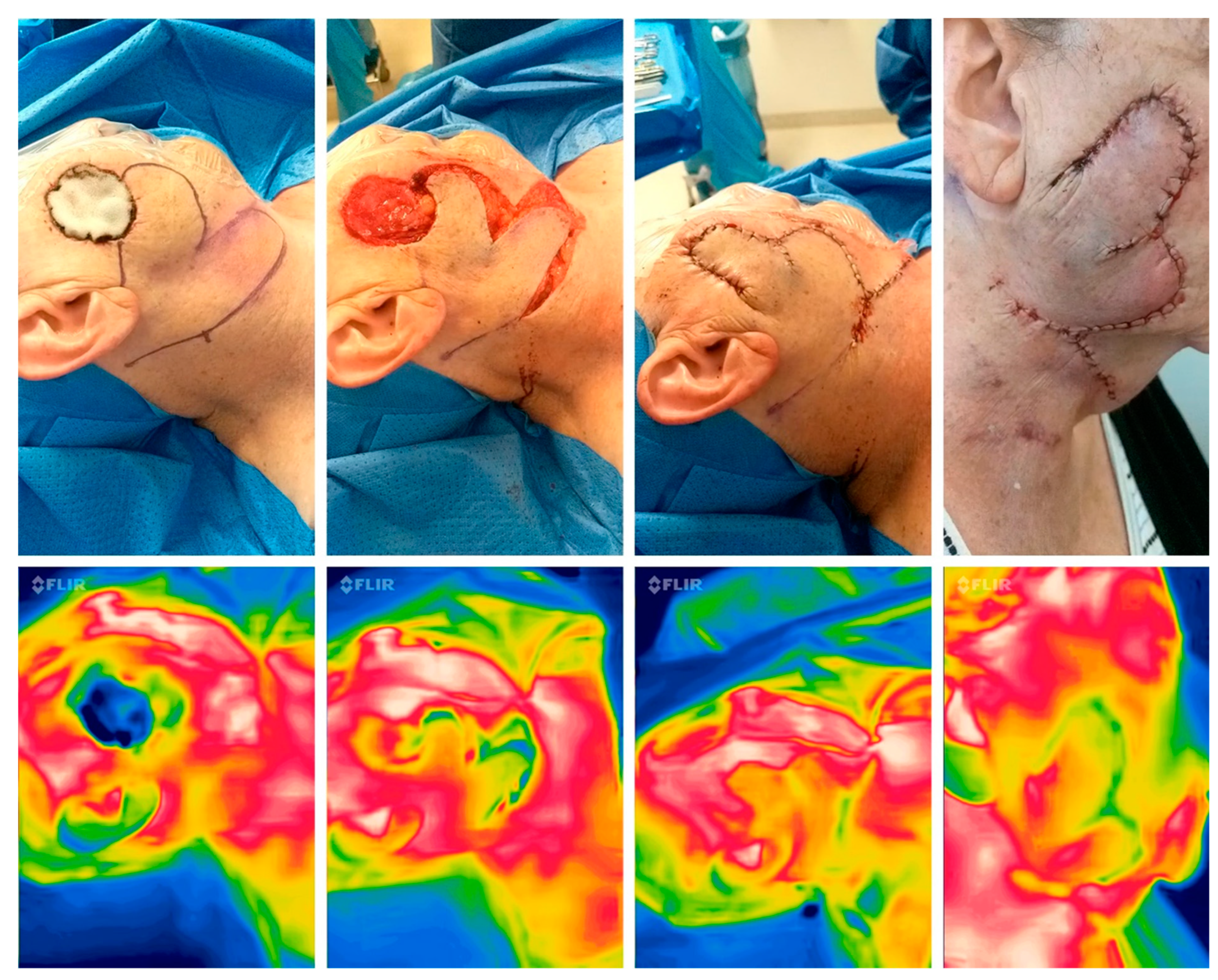
Figure 3.
Perioperative thermal imaging in a random pattern flap with the FLIR one Pro Camera preoperatively (left), after flap elevation, after reconstruction and 24 hours after surgery (right) showing good perfusion due to the red colour and minor yellow area in the periphery, which shows a low delta between normal and flap temperature.
Figure 3.
Perioperative thermal imaging in a random pattern flap with the FLIR one Pro Camera preoperatively (left), after flap elevation, after reconstruction and 24 hours after surgery (right) showing good perfusion due to the red colour and minor yellow area in the periphery, which shows a low delta between normal and flap temperature.
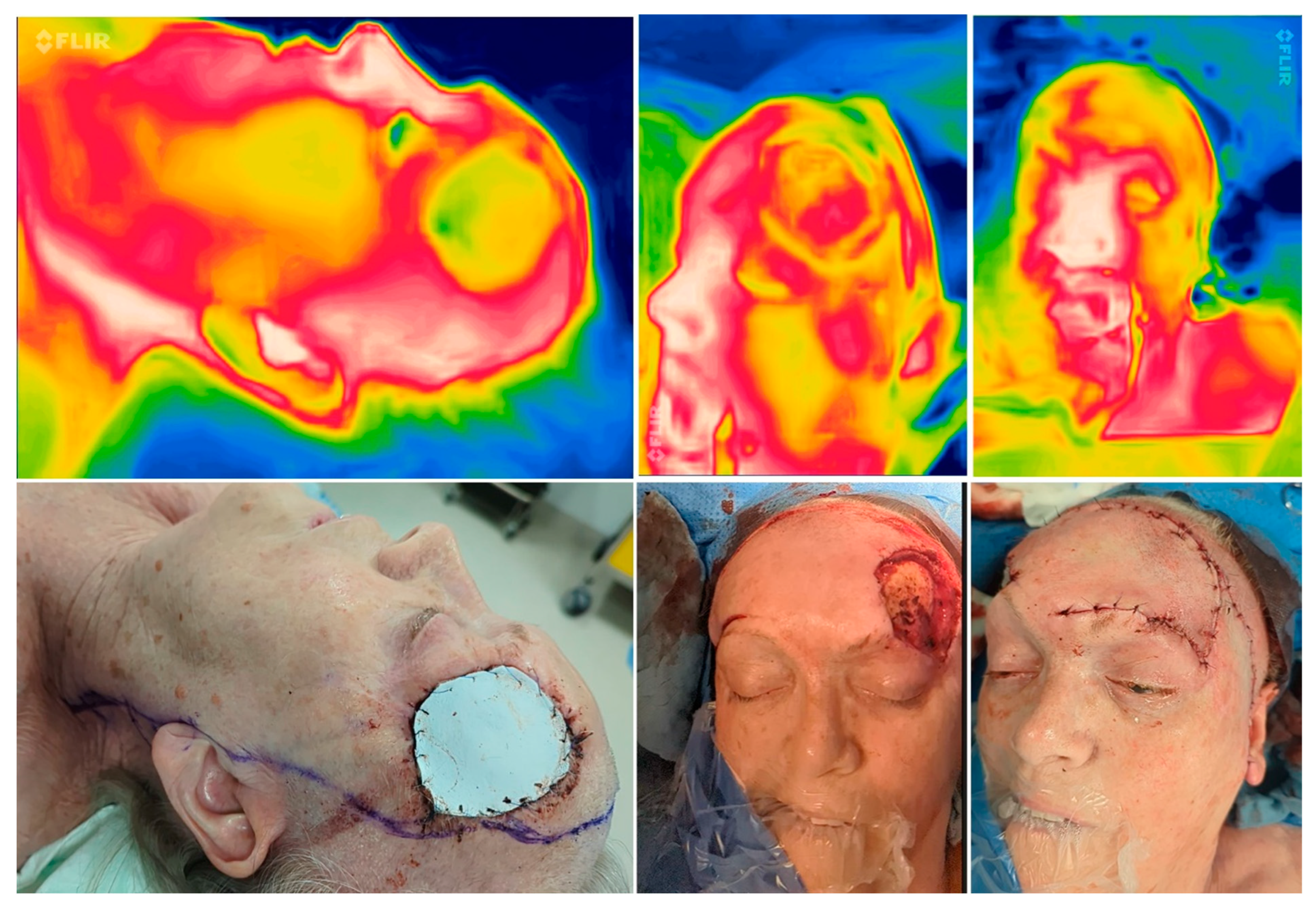
Figure 4.
Perioperative thermal imaging in a combined reconstruction preoperatively (left), after flap elevation (middle), and after reconstruction (right) showing good perfusion due to a homogenous yellowish colour emitted by the flaps (modified Hatchet and Esser-flap).
Figure 4.
Perioperative thermal imaging in a combined reconstruction preoperatively (left), after flap elevation (middle), and after reconstruction (right) showing good perfusion due to a homogenous yellowish colour emitted by the flaps (modified Hatchet and Esser-flap).
3. Results
3.1. Implementation
The implementation process for the study began with planning and drafting the study protocol inspired by Nischwitz et al. [
1], without a cooling phase between the imaging points. Following this, the necessary SBTI camera was procured, and the implementation commenced. However, several obstacles were encountered during this phase. Initially, images were collected by different individuals (nursing staff) without adhering to the study protocol guidelines consequently. As a result, not all collected images could be used for the study and had to be excluded. Specifically, six patients were excluded due to the following reasons: inadequate distance (n=2) or incomplete capture of the region of interest (n=1). Additionally, patients were excluded if all required images from T1 to T4 were not available (n=3), leading to a loss of follow-up. To address these issues, a team of two surgeons was involved in conducting SBTI in the operating room.
3.2. Patient Specifications
The dataset encompasses a total of 11 (4m; 7f) patients of varying ages [64:93;77,37yrs] who underwent reconstructive surgery for facial, head and neck defects [1x1cm:6x6cm] following diagnoses such as squamous cell carcinoma (SCC, n=3), basal cell carcinoma (BCC, n=6), malignant melanoma (MM, n=1), or actinic keratosis (n=1). Considering perioperative risk profiles, 3 patients had diabetes mellitus and 6 patients had hypertension. A total of two were smokers with 30 and 40 pack years, respectively. Surgical techniques employed were diverse, including procedures such as Rieger-flaps (n=5), Esser-flap (n=1), bilobed-flaps (n=1), two island-flaps, one transposition-flap and one Hatchet-flap. In total, we included n=3 axial pattern and n=8 random pattern flaps. Surgery dates spanned from August 2023 to February 2024. Intraoperative assessments consistently indicated good perfusion, with a Doppler signal present in n=1 cases. One post-operative dehiscence was recorded which was treated by secondary intention, with revision surgery not occasionally necessary.
3.3. Descriptive Analysis of SBTI
As depicted in the two subsequent tables, the assessment of imaging data by both surgeons demonstrates consistent outcomes, albeit exhibiting a greater variability from time points T2-T4 in SBTI imaging. (see 1 and Error! Reference source not found.)
Table 1.
descriptive data from Surgeon No.1 showing the clinical and SBTI assessments in T1-T4.
Table 1.
descriptive data from Surgeon No.1 showing the clinical and SBTI assessments in T1-T4.
| |
Clinical Assessment T1 |
Clinical Assessment T2 |
Clinical Assessment T3 |
Clinical Assessment T4 |
SBTI Perfusion T1 |
SBTI Perfusion T2 |
SBTI Perfusion T3 |
SBTI Perfusion T4 |
| Valid |
|
11 |
|
11 |
|
11 |
|
11 |
|
11 |
|
11 |
|
11 |
|
11 |
|
| Median |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
| Mean |
|
1.000 |
|
1.182 |
|
1.273 |
|
1.273 |
|
1.000 |
|
1.727 |
|
1.727 |
|
1.273 |
|
| Std. Deviation |
|
0.000 |
|
0.405 |
|
0.467 |
|
0.647 |
|
0.000 |
|
0.905 |
|
0.905 |
|
0.647 |
|
| Variance |
|
0.000 |
|
0.164 |
|
0.218 |
|
0.418 |
|
0.000 |
|
0.818 |
|
0.818 |
|
0.418 |
|
| Minimum |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
| Maximum |
|
1.000 |
|
2.000 |
|
2.000 |
|
3.000 |
|
1.000 |
|
3.000 |
|
3.000 |
|
3.000 |
|
Table 2.
descriptive data from Surgeon No.2 showing the clinical and SBTI assessments in T1-T4.
Table 2.
descriptive data from Surgeon No.2 showing the clinical and SBTI assessments in T1-T4.
| |
Clinical Assessment T1_1 |
Clinical Assessment T2_1 |
Clinical Assessment T3_1 |
Clinical Assessment T4_1 |
SBTI Perfusion T1_1 |
SBTI Perfusion T2_1 |
SBTI Perfusion T3_1 |
SBTI Perfusion T4_1 |
| Valid |
|
11 |
|
11 |
|
11 |
|
11 |
|
11 |
|
11 |
|
11 |
|
11 |
|
| Median |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
| Mean |
|
1.000 |
|
1.091 |
|
1.273 |
|
1.182 |
|
1.000 |
|
1.545 |
|
1.455 |
|
1.273 |
|
| Std. Deviation |
|
0.000 |
|
0.302 |
|
0.467 |
|
0.405 |
|
0.000 |
|
0.688 |
|
0.688 |
|
0.647 |
|
| Variance |
|
0.000 |
|
0.091 |
|
0.218 |
|
0.164 |
|
0.000 |
|
0.473 |
|
0.473 |
|
0.418 |
|
| Minimum |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
| Maximum |
|
1.000 |
|
2.000 |
|
2.000 |
|
2.000 |
|
1.000 |
|
3.000 |
|
3.000 |
|
3.000 |
|
3.4. Outcome
The pedicled skin flaps examined all showed a preserved, uniform thermal image signature peri- and postoperatively for time points T1-4 in both surgeons’ evaluations. This corresponded to a good postoperative result regarding the vitality of the tissue transfer in the clinical assessments 24 hours and one week postoperatively. A single postoperative dehiscence occurred in a transposition flap, which exhibited a diminished thermal signature in the periphery intraoperatively. Both evaluating surgeons consistently rated it as “3” from T2-T4 in SBTI evaluations, while clinical assessments yielded a “2,” indicating a more favourable outcome. In small flaps approximately one centimetre in length, thermal imaging appeared to have limited ability to accurately distinguish from the surrounding tissues.
4. Discussion
Despite progressive medical and surgical as well as technical developments, no technique has yet been found that permits a uniform, simple valid and cost-effective postoperative flap assessment. As a result, direct clinical assessment remains the gold standard. [
5,
6] The use of thermal imaging to assess perfusion is not a new concept, in the identification of perforators in free and pedicled perforator flaps[
16,
17] and perfusion monitoring in free and pedicled flaps.[
13] Multiple studies focus on the assessment of DIEP flaps[
1] and lower limb reconstruction with propeller flaps[
16] but, to our knowledge, no study was yet conducted using SBTI in head-/face-/ and neck pedicled skin flaps. Further, we missed focus on implementation protocols and there obstacles in other studies.
In our study, we aimed to establish an implementable algorithm for SBTI use in the perioperative setting and evaluate its accuracy in assessing cutaneous perfusion in pedicled flaps. The implementation of our SBTI protocol encountered initial challenges, including inconsistent image collection and exclusion of patients due to protocol deviations. However, with the involvement of a dedicated surgical team, these challenges were addressed, highlighting the importance of standardized protocols and interdisciplinary collaboration in adopting new technologies.
Our patient cohort consisted of individuals undergoing reconstructive surgery for various diagnoses, with diverse surgical techniques employed. Despite this heterogeneity, intraoperative assessments consistently indicated good perfusion, supporting the efficacy of our SBTI protocol in evaluating flap viability. Statistical analysis revealed consistent outcomes in both clinical and SBTI assessments, with SBTI demonstrating a greater variability at certain time points. However, overall, pedicled skin flaps showed preserved thermal signatures peri- and postoperatively, correlating with positive clinical outcomes.
The defined acquisition (T1-T4) of a thermal image signature as a surrogate marker for preserved tissue perfusion by SBTI proved to be simple, inexpensive, noninvasive, and efficient in the present sentinel study. Thermal imaging stands out among the assessment techniques due to its non-invasive nature and affordability, particularly when implemented via smartphone-based devices. It offers the advantage of intraoperative detection of vascular insults and provides valuable postoperative monitoring in pedicled flaps. However, its utility is constrained by its limitation to surface temperature changes, and its specificity may vary depending on the circumstances. The evaluation of flaps of the nose and the ears showed continuous cold areas, therefore an assessment with thermal imaging can bring inaccurate results.
5. Conclusions
The concordance of the obtained thermal image signature with a positive clinical outcome suggests that the presented method is suitable for perioperative prognostic statements on postoperative flap vitality in pedicled skin flaps and for monitoring those during surgery. This visualization method is an indirect indicator for perfusion and can be used for residents in training to establish a learning curve, reduce reaction time in flap failure and as a consequence, maintain quality in surgical care. Due to the visualization of temperature differences, the method seems to work better in larger flaps than in small ones. Further studies are necessary for the implementation of this method in clinical practice to support the surgeon’s intraoperative decision-making in the future.
Authorship statement
Lukas S. Fiedler, MD MBA, Adrian Lukas, Prof. Burkard M. Lippert, MD, and Tobias Meyer, MD, collectively contributed to the conception, design, data acquisition, analysis, and interpretation of this study. Lukas S. Fiedler, MD MBA, and Adrian Lukas drafted and critically revised the manuscript. All authors approved the final version for publication and agree to be accountable for its content.
Funding and Conflict of Interests
The authors declare any potential conflicts of interest related to this research. This encompasses financial interests, such as patent or stock ownership, board memberships, advisory roles, and consultancy or speaker fees from companies.
Previous Publication
The contents of this paper were presented under the title “SBTI for evaluation of regional skin flaps in the face, head and neck” at the “Crossing Borders” 95. German annual ENT Congress organized by the German Society of Otorhinolaryngology, Head and Neck Surgery in Essen, Germany on May 15th.
Conflicts of Interest
Lukas S. Fiedler, MD MBA, Adrian Lukas, Prof. Burkard M. Lippert, MD, and Tobias Meyer, MD, declared that they have no conflicts of interest related to this manuscript. No financial or personal relationships with other people or organizations could potentially influence their objectivity in conducting or reporting the research described in the manuscript. All potential sources of conflict of interest have been disclosed by the journal’s guidelines.
References
- Nischwitz: S.P., et al., Thermal, Hyperspectral, and Laser Doppler Imaging: Non-Invasive Tools for Detection of The Deep Inferior Epigastric Artery Perforators-A Prospective Comparison Study. J Pers Med, 2021. 11(10). [CrossRef]
- Simman, R., Wound closure and the reconstructive ladder in plastic surgery. J Am Col Certif Wound Spec, 2009. 1(1): p. 6-11. [CrossRef]
- Veldhuizen, I.J., et al., Nasal skin reconstruction: Time to rethink the reconstructive ladder? J Plast Reconstr Aesthet Surg, 2022. 75(3): p. 1239-1245.
-
1 Soft Tissue Biomechanics and Physiology, in Principles of Facial Reconstruction: A Subunit Approach to Cutaneous Repair, W.F. Larrabee Jr, D.A. Sherris, and J. Teixeira, Editors. 2021, Thieme Medical Publishers, Inc.
- Whitaker, I.S., et al., Postoperative monitoring of free flaps in autologous breast reconstruction: a multicenter comparison of 398 flaps using clinical monitoring, microdialysis, and the implantable Doppler probe. J Reconstr Microsurg, 2010. 26(6): p. 409-16. [CrossRef]
- Bootz, F., Postoperative Überwachung (Monitoring), in Expertise Lappenplastiken und Transplantate im Kopf-Hals-Bereich, S.H. Lang, F. Bootz, and S. Remmert, Editors. 2018, Georg Thieme Verlag KG.
- Spiegel, J.H. and J.K. Polat, Microvascular flap reconstruction by otolaryngologists: prevalence, postoperative care, and monitoring techniques. Laryngoscope, 2007. 117(3): p. 485-90. [CrossRef]
- Hallock, G.G., The use of smartphone thermography to more safely unmask and preserve circulation to keystone advancement flaps in the lower extremity. Injury, 2020. 51 Suppl 4: p. S121-s125. [CrossRef]
- Nischwitz, S.P., H. Luze, and L.P. Kamolz, Thermal imaging via FLIR One—A promising tool in clinical burn care and research. Burns, 2020. 46(4): p. 988-989.
- Xue, E.Y., et al., Use of FLIR ONE Smartphone Thermography in Burn Wound Assessment. Ann Plast Surg, 2018. 80(4 Suppl 4): p. S236-s238. [CrossRef]
- Meyer, A., et al., Thermal imaging for microvascular free tissue transfer monitoring: Feasibility study using a low cost, commercially available mobile phone imaging system. Head Neck, 2020. 42(10): p. 2941-2947. [CrossRef]
- Luze, H., et al., Assessment of Mastectomy Skin Flaps for Immediate Reconstruction with Implants via Thermal Imaging-A Suitable, Personalized Approach? J Pers Med, 2022. 12(5).
- Rabbani, M.J., A.Z. Bhatti, and A. Shahzad, Flap Monitoring using Thermal Imaging Camera: A Contactless Method. J Coll Physicians Surg Pak, 2021. 30(6): p. 703-706. [CrossRef]
- Tattersall, G.J., Infrared thermography: A non-invasive window into thermal physiology. Comp Biochem Physiol A Mol Integr Physiol, 2016. 202: p. 78-98. [CrossRef]
- Luximon, A., et al., Theory and applications of InfraRed and thermal image analysis in ergonomics research. Frontiers in Computer Science, 2022. 4. [CrossRef]
- Hallock, G.G., Smartphone Thermal Imaging Can Enable the Safer Use of Propeller Flaps. Semin Plast Surg, 2020. 34(3): p. 161-164. [CrossRef]
- Ismail, S., et al., Smartphone Thermal Imaging for Preoperative Perforator Mapping in Perforator Based Flaps. Cureus, 2024. 16(1): p. e51755. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).