Submitted:
06 June 2024
Posted:
07 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Interactions of the Hypothalamo-Pituitary-Adrenal System with Vasopressin at the Cellular Level
2.1. Genomic and Nongenomic Actions of Steroid Hormones
2.2. Genomic and Non-Genomic Effects of Vasopressin
3. Role of the Hypothalamo-Pituitary- Adrenal System and Vasopressin in Regulation of Energy Balance and Water-Electrolyte Balance at Rest and during Neurogenic Stress
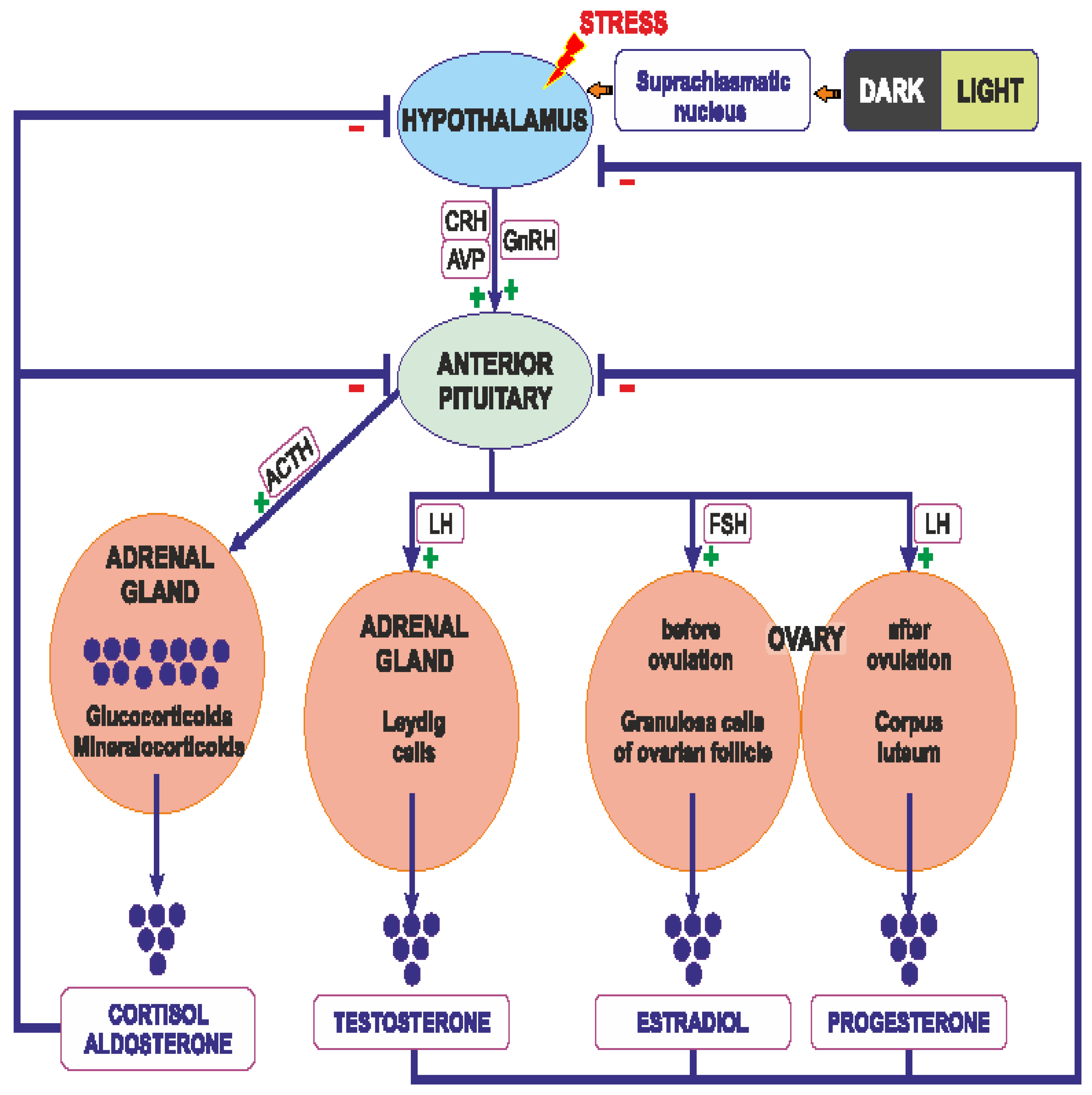
3.1. Regulation of Steroids Secretion
3.2. Interaction of Steroids and AVP in Regulation of Energy Balance
3.3. Interaction of AVP and Steroids in Regulation of Water-Electrolyte Balance
3.4. Interaction of AVP and Steroids in Neurogenic Stress
4. Altered Interactions of Vasopressin with the Hypothalamo-Pituitary-Adrenal System in Cardiovascular and Metabolic Diseases
4.1. Cardiovascular Diseases
4.2. Metabolic Diseases
4.3. Neurogenic Stress with Relevance to Sex Differences
5. Impact of Therapeutic Interventions on Interactions of Vasopressin with the Hypothalamo-Pituitary-Adrenal System in Health and Cardiovascular and Metabolic Diseases
5.1. Steroids and Vasopressin Treatment in Cardiovascular Diseases
5.2. Impact of Anti-Depressive and Neuroleptic Treatments on Vasopressin-HPA Interactions
6. Summary and Conclusions
7. Conclusions
- Vasopressin (AVP) and steroid hormones are frequently released together and closely cooperate in the regulation of blood pressure, metabolism, water-electrolyte balance and behavior.
- Vasopressin interacts with specific components of the hypothalamo-pituitary-adrenal axis in the brain and in several peripheral organs and tissues, including the heart, vessels, kidney and adipose tissue.
- Appropriate interactions of AVP with the HPA axis is essential for efficient regulation of the water-electrolyte balance, blood pressure, and energy balance, and it is justified to consider vasopressin and the hypothalamo-pituitary axis as the highly coordinated functional AVP-HPA system.
- Interactions between AVP and HPA are significantly altered in the cardiovascular, respiratory and metabolic diseases and during inflammation and the neurogenic stress.
- Inappropriate interactions of AVP with steroid hormones may initiate or potentiate cardiovascular complications and metabolic diseases.
- The interplay of vasopressin and steroid hormones is not yet fully recognized and further studies are needed to determine potentially beneficial or harmful consequences of interference with these factors in treatment of specific pathological states.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hilton JG, Scian LF, Westermann SD, Nakano J, Kruesi OR. Vasopressin stimulation of the isolated adrenal glands: nature and mechanism of hydrocortisone secretion. Endocrinology. 1960;67:298-310. [CrossRef]
- Larsen PJ, Vrang N, Møller M, Jessop DS, Lightman SL, Chowdrey HS, Mikkelsen JD. The diurnal expression of genes encoding vasopressin and vasoactive intestinal peptide within the rat suprachiasmatic nucleus is influenced by circulating glucocorticoids. Brain Res Mol Brain Res. 1994;27(2):342-346. [CrossRef]
- Mouri T, Itoi K, Takahashi K, Suda T, Murakami O, Yoshinaga K, Andoh N, Ohtani H, Masuda T, Sasano N. Colocalization of corticotropin-releasing factor and vasopressin in the paraventricular nucleus of the human hypothalamus. Neuroendocrinology. 1993;57(1):34-39.
- Otubo A,Kawakami N, Maejima S, Ueda Y, Morris JF, Sakamoto T, Sakamoto H. Vasopressin gene products are colocalised with corticotrophin-releasing factor within neurosecretory vesicles in the external zone of the median eminence of the Japanese macaque monkey (Macaca fuscata). J Neuroendocrinol. 2020;32(8):e12875. [CrossRef]
- Engler D, Pham T, Fullerton MJ, Ooi G, Funder JW, Clarke IJ. Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep. I. Effect of an audiovisual stimulus and insulin-induced hypoglycemia. Neuroendocrinology. 1989;49(4):367-381. [CrossRef]
- Familari M, Smith AI, Smith R, Funder JW. Arginine vasopressin is a much more potent stimulus to ACTH release from ovine anterior pituitary cells than ovine corticotropin-releasing factor. 1. In vitro studies. Neuroendocrinology. 1989;50(2):152-157. [CrossRef]
- Labrie F, Giguere V, Proulx L, Lefevre G. Interactions between CRF, epinephrine, vasopressin and glucocorticoids in the control of ACTH secretion. J Steroid Biochem. 1984;20(1):153-160. [CrossRef]
- Veldhuis HD, de Kloet ER. Vasopressin-related peptides increase the hippocampal corticosterone receptor capacity of diabetes insipidus (Brattleboro) rat. Endocrinology. 1982;110(1):153-157. [CrossRef]
- Papanek PE, Sladek CD, Raff H. Corticosterone inhibition of osmotically stimulated vasopressin from hypothalamic-neurohypophysial explants. Am J Physiol. 1997;272(1 Pt 2):R158-162. [CrossRef]
- Liu X, Wang CA, Chen YZ. Nongenomic effect of glucocorticoid on the release of arginine vasopressin from hypothalamic slices in rats. Neuroendocrinology. 1995;62(6):628-633. [CrossRef]
- Calogero AE, Liapi C, Chrousos GP. Hypothalamic and suprahypothalamic effects of prolonged treatment with dexamethasone in the rat. J Endocrinol Invest. 1991;14(4):277-286. [CrossRef]
- Woodcock EA, Mcleod JK, Johnston CI. Vasopressin stimulates phosphatidylinositol turnover and aldosterone synthesis in rat adrenal glomerulosa cells: comparison with angiotensin II. Endocrinology. 1986;118(6):2432-2436. [CrossRef]
- Saito R, Ishiharada N, Ban Y, Honda K, Takano Y, Kamiya H. Vasopressin V1 receptor in rat hippocampus is regulated by adrenocortical functions. Brain Res. 1994;646(1):170-174. [CrossRef]
- Watters JJ, Poulin P, Dorsa DM. Steroid hormone regulation of vasopressinergic neurotransmission in the central nervous system. Prog Brain Res. 1998;119:247-261. [CrossRef]
- Watters JJ, Wilkinson CW, Dorsa DM. Glucocorticoid regulation of vasopressin V1a receptors in rat forebrain. Brain Res Mol Brain Res. 1996;38(2):276-284. [CrossRef]
- Rabadan-Diehl C, Makara G, Kiss A, Lolait S, Zelena D, Ochedalski T, Aguilera G. Regulation of pituitary V1b vasopressin receptor messenger ribonucleic acid by adrenalectomy and glucocorticoid administration. Endocrinology. 1997;138(12):5189-5194. [CrossRef]
- Hu SB,Tannahill LA, Biswas S, Lightman SL. Release of corticotrophin-releasing factor-41, arginine vasopressin and oxytocin from rat fetal hypothalamic cells in culture: response to activation of intracellular second messengers and to corticosteroids. J Endocrinol. 1992;132(1):57-65. [CrossRef]
- Currie IS, Gillies G, Brooks AN. Modulation of arginine vasopressin secretion from cultured ovine hypothalamic cells by glucocorticoids and opioid peptides. Neuroendocrinology. 1994;60(4):360-367. [CrossRef]
- Kokras N, Hodes GE, Bangasser DA, Dalla Sex differences in the hypothalamic-pituitary-adrenal axis: An obstacle to antidepressant drug development? C.Br J Pharmacol. 2019;176(21):4090-4106. [CrossRef]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R233-R249. [CrossRef]
- Jiang YQ, Kawashima H,Iwasaki Y,Uchida K, Sugimoto K, Itoi K. Differential effects of forced swim-stress on the corticotropin-releasing hormone and vasopressin gene transcription in the parvocellular division of the paraventricular nucleus of rat hypothalamus. Neurosci Lett. 2004;358(3):201-204. [CrossRef]
- Hlavacova N, Bakos J, Jezova D. Eplerenone, a selective mineralocorticoid receptor blocker, exerts anxiolytic effects accompanied by changes in stress hormone release. J Psychopharmacol. 2010;24(5):779-786. [CrossRef]
- de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH, Joëls M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol. 2018;49:124-145. [CrossRef]
- Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102(52):19204-19207. [CrossRef]
- Karst H, den Boon FS, Vervoort N, Adrian M, Kapitein LC, Joëls M. Non-genomic steroid signaling through the mineralocorticoid receptor: Involvement of a membrane-associated receptor? Mol Cell Endocrinol. 2022;541:111501. [CrossRef]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29(2):238-257. [CrossRef]
- Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER, Meijer OC. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl Acad Sci U S A. 2009;106(19):8038-8042. [CrossRef]
- van Weert LTCM, Buurstede JC, Mahfouz A, Braakhuis PSM, Polman JAE, Sips HCM, Roozendaal B, Balog J, de Kloet ER, Datson NA, et al. NeuroD Factors Discriminate Mineralocorticoid From Glucocorticoid Receptor DNA Binding in the Male Rat Brain. Endocrinology. 2017;158(5):1511-1522. [CrossRef]
- Skowron KJ, Booker K, Cheng C, Creed S, David BP, Lazzara PR, Lian A, Siddiqui Z, Speltz TE, Moore TW. Steroid receptor/coactivator binding inhibitors: An update. Mol Cell Endocrinol. 2019 ;493:110471. [CrossRef]
- Stashi E, York B, O’Malley BW. Steroid receptor coactivators: servants and masters for control of systems metabolism. Trends Endocrinol Metab. 2014;25(7):337-347. [CrossRef]
- Grossmann C, Almeida-Prieto B, Nolze A, Alvarez de la Rosa D. Structural and molecular determinants of mineralocorticoid receptor signalling.Br J Pharmacol. 2022;179(13):3103-3118. [CrossRef]
- Vanderhaeghen T, Beyaert R, Libert C. Bidirectional Crosstalk Between Hypoxia Inducible Factors and Glucocorticoid Signalling in Health and Disease. Front Immunol. 2021 ;12:684085. [CrossRef]
- Fadel L, Dacic M, Fonda V, Sokolsky BA, Quagliarini F, Rogatsky I, Uhlenhaut NH. Modulating glucocorticoid receptor actions in physiology and pathology: Insights from coregulators Pharmacol Ther. 2023;251:108531. [CrossRef]
- Vandevyver S, Dejager L, Libert C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr Rev. 2014 ;35(4):671-693. [CrossRef]
- Clayton SA, Jones SW, Kurowska-Stolarska M, Clark AR. The role of microRNAs in glucocorticoid action. J Biol Chem. 2018;293(6):1865-1874. [CrossRef]
- Knutti D, Kaul A, Kralli A. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Mol Cell Biol. 2000;20(7):2411-2422. [CrossRef]
- Meijer OC, Buurstede JC, Schaaf MJM. Corticosteroid Receptors in the Brain: Transcriptional Mechanisms for Specificity and Context-Dependent Effects. Cell Mol Neurobiol. 2019;39(4):539-549. [CrossRef]
- Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132(5):1033-1044. [CrossRef]
- Oakley RH, Cruz-Topete D, He B, Foley JF, Myers PH, Xu X, Gomez-Sanchez CE, Chambon P, Willis MS, Cidlowski JA. Cardiomyocyte glucocorticoid and mineralocorticoid receptors directly and antagonistically regulate heart disease in mice. Sci Signal. 2019;12(577):eaau9685. [CrossRef]
- Nelson CC, Hendy SC, Shukin RJ, Cheng H, Bruchovsky N, Koop BF, Rennie PS. Determinants of DNA sequence specificity of the androgen, progesterone, and glucocorticoid receptors: evidence for differential steroid receptor response elements. Mol Endocrinol. 1999;13(12):2090-2107. [CrossRef]
- Presman DM, Hager GL. More than meets the dimer: What is the quaternary structure of the glucocorticoid receptor?Transcription. 2017;8(1):32-39. [CrossRef]
- Koning ACAM, Buurstede JC, van Weert LTCM, Meijer OC. Glucocorticoid and Mineralocorticoid Receptors in the Brain: A Transcriptional Perspective. J Endocr Soc. 2019;3(10):1917-1930. [CrossRef]
- Sacta MA, Chinenov Y, Rogatsky I. Glucocorticoid Signaling: An Update from a Genomic Perspective. Annu Rev Physiol. 2016;78:155-180. [CrossRef]
- Faulkner JL,Belin de Chantemèle EJ. Mineralocorticoid Receptor and Endothelial Dysfunction in Hypertension. Curr Hypertens Rep. 2019;21(10):78. [CrossRef]
- Kokkinopoulou I, Moutsatsou P. Mitochondrial Glucocorticoid Receptors and Their Actions. Int J Mol Sci. 2021;22(11):6054. [CrossRef]
- Viho EMG, Buurstede JC, Mahfouz A, Koorneef LL, van Weert LTCM, Houtman R, Hunt HJ, Kroon J, Meijer OC. Corticosteroid Action in the Brain: The Potential of Selective Receptor Modulation. Neuroendocrinology. 2019;109(3):266-276. [CrossRef]
- Winnay JN, Xu J, O’Malley BW, Hammer GD. Steroid receptor coactivator-1-deficient mice exhibit altered hypothalamic-pituitary-adrenal axis function. Endocrinology. 2006;147(3):1322-1332. [CrossRef]
- Yi P, Yu X, Wang Z, O’Malley BW. Steroid receptor-coregulator transcriptional complexes: new insights from CryoEM. Essays Biochem. 2021;65(6):857-866. [CrossRef]
- Marchi D, van Eeden FJM. Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic. Cells. 2021 Dec 7;10(12):3441. [CrossRef]
- Syed AP, Greulich F, Ansari SA, Uhlenhaut NH. Anti-inflammatory glucocorticoid action: genomic insights and emerging concepts. Curr Opin Pharmacol. 2020;53:35-44. [CrossRef]
- Kodama T, Shimizu N,Yoshikawa N, Makino Y, Ouchida R, Okamoto K, Hisada T, Nakamura H, Morimoto C, Tanaka H. Role of the glucocorticoid receptor for regulation of hypoxia-dependent gene expression. J Biol Chem. 2003;278(35):33384-33391. [CrossRef]
- Callera GE, Touyz RM, Tostes RC, Yogi A, He Y, Malkinson S, Schiffrin EL. Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension. 2005; 45(4):773-779. [CrossRef]
- Zeyen L, Seternes OM, Mikkola I. Crosstalk between p38 MAPK and GR Signaling. Int J Mol Sci. 2022;23(6):3322. [CrossRef]
- Quatrini L, Ugolini S. New insights into the cell-and tissue-specificity of glucocorticoid actions. Cell Mol Immunol. 2021;18(2):269-278. [CrossRef]
- Davel AP, Anwar IJ, Jaffe IZ. The endothelial mineralocorticoid receptor: mediator of the switch from vascular health to disease. Curr Opin Nephrol Hypertens. 2017;26(2):97-104. [CrossRef]
- Geerling JC, Loewy AD. Aldosterone in the brain. Am J Physiol Renal Physiol. 2009 Sep;297(3):F559-F576. [CrossRef]
- Haque M, Wilson R, Sharma K, Mills NJ, Teruyama R. Localisation of 11β-Hydroxysteroid Dehydrogenase Type 2 in Mineralocorticoid Receptor Expressing Magnocellular Neurosecretory Neurones of the Rat Supraoptic and Paraventricular Nuclei. J Neuroendocrinol.27(11):835-849. [CrossRef]
- Vassiliou AG, Athanasiou N, Vassiliadi DA, Jahaj E, Keskinidou C, Kotanidou A, Dimopoulou I. Glucocorticoid and mineralocorticoid receptor expression in critical illness: A narrative review. World J Crit Care Med. 2021;10(4):102-111. [CrossRef]
- Parker BM, Wertz SL, Pollard CM, Desimine VL, Maning J, McCrink KA, Lymperopoulos A. Novel Insights into the Crosstalk between Mineralocorticoid Receptor and G Protein-Coupled Receptors in Heart Adverse Remodeling and Disease. Int J Mol Sci. 2018;19(12):3764. [CrossRef]
- Perlstein RS, Whitnall MH, Abrams JS, Mougey EH, Neta R. Synergistic roles of interleukin-6, interleukin-1, and tumor necrosis factor in the adrenocorticotropin response to bacterial lipopolysaccharide in vivo. Endocrinology. 1993;132(3):946-952. [CrossRef]
- Terada Y, Ueda S, Hamada K, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Kagawa T, Horino T, Takao T. Aldosterone stimulates nuclear factor-kappa B activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum- and glucocorticoid-inducible protein kinase-1. Clin Exp Nephrol. 2012;16(1):81-88. [CrossRef]
- Maturana A, Lenglet S, Python M, Kuroda S, Rossier MF. Role of the T-type calcium channel CaV3.2 in the chronotropic action of corticosteroids in isolated rat ventricular myocytes. Endocrinology. 2009;150(8):3726-3734. [CrossRef]
- Rossier MF. The Cardiac Mineralocorticoid Receptor (MR): A Therapeutic Target Against Ventricular Arrhythmias. Front Endocrinol (Lausanne). 2021;12:694758. [CrossRef]
- Funder JW. Aldosterone and Mineralocorticoid Receptors-Physiology and Pathophysiology. Int J Mol Sci. 2017;18(5):1032. [CrossRef]
- Igbekele AE, Jia G, Hill MA, Sowers JR, Jia G. Mineralocorticoid Receptor Activation in Vascular Insulin Resistance and Dysfunction. Int J Mol Sci. 2022;23(16):8954. [CrossRef]
- Vanderhaeghen T, Timmermans S, Watts D, Paakinaho V, Eggermont M, Vandewalle J, Wallaeys C, Van Wyngene L, Van Looveren K, Nuyttens L, et al., Reprogramming of glucocorticoid receptor function by hypoxia. EMBO Rep. 2022;23(1):e53083. [CrossRef]
- McEown K, Treit D. Mineralocorticoid receptors in the medial prefrontal cortex and hippocampus mediate rats’ unconditioned fear behaviour. Horm Behav. 2011;60(5):581-588. [CrossRef]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505-2511. [CrossRef]
- Reul JM, de Kloet ER, van Sluijs FJ, Rijnberk A, Rothuizen J. Binding characteristics of mineralocorticoid and glucocorticoid receptors in dog brain and pituitary. Endocrinology. 1990;127(2):907-915. [CrossRef]
- Ahmadpour D, Grange-Messent V. Involvement of Testosterone Signaling in the Integrity of the Neurovascular Unit in the Male: Review of Evidence, Contradictions, and Hypothesis. Neuroendocrinology. 2021;111(5):403-420. [CrossRef]
- Castelli MP, Casti A, Casu A, Frau R, Bortolato M, Spiga S, Ennas MG. Regional distribution of 5α-reductase type 2 in the adult rat brain: an immunohistochemical analysis. Psychoneuroendocrinology. 2013;38(2):281-293. [CrossRef]
- Takahashi K, Hosoya T, Onoe K, Takashima T, Tanaka M, Ishii A, Nakatomi Y, Tazawa S, Takahashi K, Doi H, et al., Association between aromatase in human brains and personality traits. Sci Rep. 2018;8(1):16841. [CrossRef]
- Guennoun R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int J Mol Sci. 2020 ;21(15):5271. [CrossRef]
- Ghoumari AM, Abi Ghanem C, Asbelaoui N, Schumacher M, Hussain R Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination. Int J Mol Sci. 2020;21(9):3163. [CrossRef]
- Hwang WJ, Lee TY, Kim NS, Kwon JS. The Role of Estrogen Receptors and Their Signaling across Psychiatric Disorders. Int J Mol Sci. 2020 Dec 31;22(1):373. [CrossRef]
- Jiao L, Machuki JO, Wu Q, Shi M, Fu L, Adekunle AO, Tao X, Xu C, Hu X, Yin Z, Sun H. Estrogen and calcium handling proteins: new discoveries and mechanisms in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2020;318(4):H820-H829. [CrossRef]
- Trenti A, Tedesco S, Boscaro C, Trevisi L, Bolego C, Cignarella A. Estrogen, Angiogenesis, Immunity and Cell Metabolism: Solving the Puzzle. Int J Mol Sci. 2018;19(3):859. [CrossRef]
- Coolen RL, Cambier JC, Spantidea PI, van Asselt E, Blok BFM. Androgen receptors in areas of the spinal cord and brainstem: A study in adult male cats. J Anat. 2021 Jul;239(1):125-135. [CrossRef]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R233-R249. [CrossRef]
- Cunningham RL, Lumia AR, McGinnis MY. Androgen receptors, sex behavior, and aggression. Neuroendocrinology. 2012;96(2):131-140. [CrossRef]
- Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology. 2007;148(5):2066-2074. [CrossRef]
- Davey RA, Grossmann M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev. 2016;37(1):3-15.
- Thomas P. Membrane Androgen Receptors Unrelated to Nuclear Steroid Receptors. Endocrinology. 2019;160(4):772-781. [CrossRef]
- Venkatesh VS, Grossmann M, Zajac JD, Davey RA. The role of the androgen receptor in the pathogenesis of obesity and its utility as a target for obesity treatments. Obes Rev. 2022;23(6):e13429. [CrossRef]
- Lucas-Herald AK, Touyz RM. Androgens and Androgen Receptors as Determinants of Vascular Sex Differences Across the Lifespan. Can J Cardiol. 2022;38(12):1854-1864. [CrossRef]
- Arterburn JB, Prossnitz ER. G Protein-Coupled Estrogen Receptor GPER: Molecular Pharmacology and Therapeutic Applications. Annu Rev Pharmacol Toxicol. 2023;63:295-320. [CrossRef]
- Chakraborty B, Byemerwa J, Krebs T, Lim F, Chang CY, McDonnell DP. Estrogen Receptor Signaling in the Immune System. Endocr Rev. 2023 ;44(1):117-141. [CrossRef]
- Gregorio KCR, Laurindo CP, Machado UF. Estrogen and Glycemic Homeostasis: The Fundamental Role of Nuclear Estrogen Receptors ESR1/ESR2 in Glucose Transporter GLUT4 Regulation. Cells. 2021 Jan 7;10(1):99. [CrossRef]
- Rzemieniec J, Castiglioni L, Gelosa P, Muluhie M, Mercuriali B, Sironi L. Nuclear Receptors in Myocardial and Cerebral Ischemia-Mechanisms of Action and Therapeutic Strategies. Int J Mol Sci. 2021;22(22):12326. [CrossRef]
- Guajardo-Correa E, Silva-Agüero JF, Calle X, Chiong M, Henríquez M, García-Rivas G, Latorre M, Parra V. Estrogen signaling as a bridge between the nucleus and mitochondria in cardiovascular diseases. Front Cell Dev Biol. 2022 ;10:968373. [CrossRef]
- Wang H, Zhao Z, Lin M, Groban L Activation of GPR30 inhibits cardiac fibroblast proliferation. Mol Cell Biochem. 2015;405(1-2):135-148. [CrossRef]
- Kurmann L, Okoniewski M, Dubey RK. Estradiol Inhibits Human Brain Vascular Pericyte Migration Activity: A Functional and Transcriptomic Analysis. Cells. 2021;10(9):2314. [CrossRef]
- Machuki JO, Zhang HY, Harding SE, Sun H. Molecular pathways of oestrogen receptors and β-adrenergic receptors in cardiac cells: Recognition of their similarities, interactions and therapeutic value. Acta Physiol (Oxf). 2018;222(2):e12978. [CrossRef]
- da Silva JS, Montagnoli TL, Rocha BS, Tacco MLCA, Marinho SCP, Zapata-Sudo G. Estrogen Receptors: Therapeutic Perspectives for the Treatment of Cardiac Dysfunction after Myocardial Infarction. Int J Mol Sci. 2021 Jan 7;22(2):525. [CrossRef]
- Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135-170. [CrossRef]
- Tran QK Reciprocality Between Estrogen Biology and Calcium Signaling in the Cardiovascular System. Front Endocrinol (Lausanne). 2020;11:568203. [CrossRef]
- Menazza S, Murphy E. The Expanding Complexity of Estrogen Receptor Signaling in the Cardiovascular System. Circ Res. 2016;118(6):994-1007. [CrossRef]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535-1565. [CrossRef]
- Sun J, Aponte AM,Menazza S, Gucek M, Steenbergen C, Murphy E. Additive cardioprotection by pharmacological postconditioning with hydrogen sulfide and nitric oxide donors in mouse heart: S-sulfhydration vs. S-nitrosylation. Cardiovasc Res. 2016;110(1):96-106. [CrossRef]
- Zimmerman MA, Budish RA, Kashyap S, Lindsey SH. GPER-novel membrane oestrogen receptor. Clin Sci (Lond). 2016;130(12):1005-1016. [CrossRef]
- Klinge CM. Estrogenic control of mitochondrial function. Redox Biol. 2020 Apr;31:101435. [CrossRef]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507-525. [CrossRef]
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715-726. [CrossRef]
- Aickareth J, Hawwar M, Sanchez N, Gnanasekaran R, Zhang J. Membrane Progesterone Receptors (mPRs/PAQRs) Are Going beyond Its Initial Definitions. Membranes (Basel). 2023 Feb 22;13(3):260. [CrossRef]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193(2):311-321. [CrossRef]
- Shah NM, Imami N, Johnson MR. Progesterone Modulation of Pregnancy-Related Immune Responses. Front Immunol. 2018;9:1293. [CrossRef]
- Singh M, Su C, Ng S. Non-genomic mechanisms of progesteroneaction in the brain. Front Neurosci. 2013 Sep 19;7:159. [CrossRef]
- Vítků J, Hampl R. Steroid Conjugates and Their Physiological Role. Physiol Res. 2023 ;72(S4):S317-S322. [CrossRef]
- Pinna G. Allopregnanolone (1938-2019): A trajectory of 80 years of outstanding scientific achievements. Neurobiol Stress. 2020;13:100246. [CrossRef]
- Brann DW, Lu Y, Wang J, Zhang Q, Thakkar R, Sareddy GR, Pratap UP, Tekmal RR, Vadlamudi RK. Brain-derived estrogen and neural function. Neurosci Biobehav Rev. 2022;132:793-817. [CrossRef]
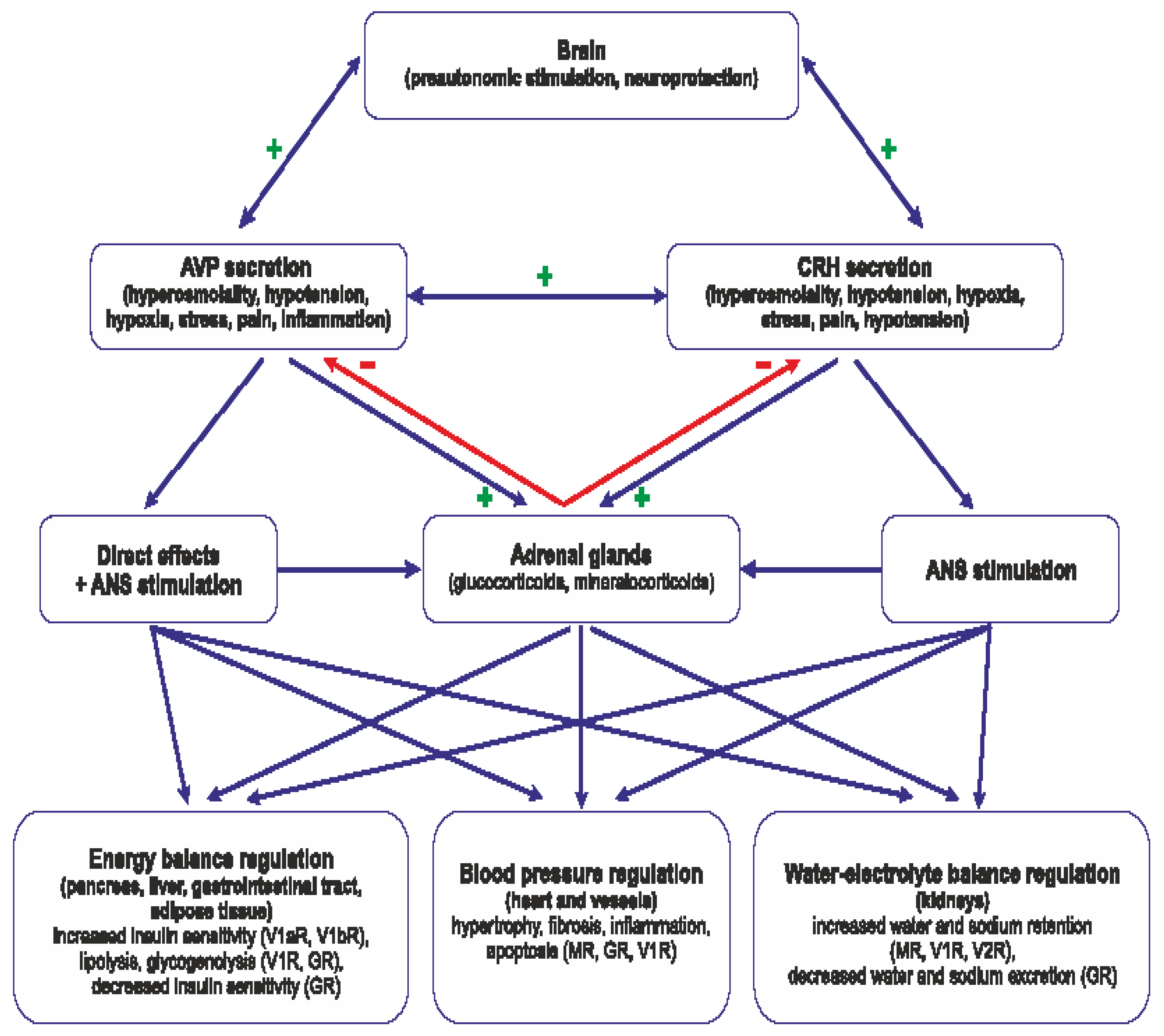
- Buijs RM, Hermes MH, Kalsbeek A. The suprachiasmatic nucleus-paraventricular nucleus interactions: a bridge to the neuroendocrine and autonomic nervous system. Prog Brain Res. 1998;119:365-382. [CrossRef]
- Szczepanska-Sadowska E, Czarzasta K, Cudnoch-Jedrzejewska A. Dysregulation of the Renin-Angiotensin System and the Vasopressinergic System Interactions in Cardiovascular Disorders. Curr Hypertens Rep. 2018;20(3):19. [CrossRef]
- Szczepanska-Sadowska E, Zera T, Sosnowski P, Cudnoch-Jedrzejewska A, Puszko A, Misicka A. Vasopressin and Related Peptides; Potential Value in Diagnosis, Prognosis and Treatment of Clinical Disorders. Curr Drug Metab. 2017;18(4):306-345. [CrossRef]
- Amico JA, Finn FM, Haldar J. Oxytocin and vasopressin are present in human and rat pancreas. Am J Med Sci. 1988;296(5):303-307. [CrossRef]
- Hupf H, Grimm D, Riegger GA, Schunkert H. Evidence for a vasopressin system in the rat heart. Circ Res. 1999;84(3):365-370. [CrossRef]
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001;81(3):1197-1267. [CrossRef]
- Sparapani S, Millet-Boureima C, Oliver J, Mu K, Hadavi P, Kalostian T, Ali N, Avelar CM, Bardies M, Barrow B, et al., The Biology of Vasopressin. Biomedicines. 2021;9(1):89. [CrossRef]
- Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112-119. [CrossRef]
- Rechlin T, Hochholzer W, Stelzi C, Laule K, Freidank H, Morgenthaler NG, et al. Incremental value of Copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol.2009;54:60–68. [CrossRef]
- Roffi M, Patrono C. CardioPulse: ‘Ten Commandments’ of 2015 European Society of Cardiology Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation (NSTE-ACS). Eur Heart J. 2016;37(3):208.
- Aikins AO, Nguyen DH, Paundralingga O, Farmer GE, Shimoura CG, Brock C, Cunningham JT. Cardiovascular Neuroendocrinology: Emerging Role for Neurohypophyseal Hormones in Pathophysiology. Endocrinology. 202;162(8):bqab082. [CrossRef]
- Costello HM, Krilis G, Grenier C, Severs D, Czopek A, Ivy JR, Nixon M, Holmes MC, Livingstone DEW, Hoorn EJ et al. High salt intake activates the hypothalamic-pituitary-adrenal axis, amplifies the stress response, and alters tissue glucocorticoid exposure in mice. Cardiovasc Res. 2023;119(8):1740-1750. [CrossRef]
- Goldstein DS. The extended autonomic system, dyshomeostasis, and COVID-19. Clin Auton Res. 2020;30(4):299-315. [CrossRef]
- Iwasaki Y, Oiso Y, Saito H, Majzoub JA. Positive and negative regulation of the rat vasopressin gene promoter. Endocrinology. 1997;138(12):5266-5274. [CrossRef]
- Lee S, Rivier C. Hypophysiotropic role and hypothalamic gene expression of corticotropin-releasing factor and vasopressin in rats injected with interleukin-1 beta systemically or into the brain ventricles. J Neuroendocrinol. 1994 ;6(2):217-224. [CrossRef]
- Grinevich V, Ma XM, Jirikowski G, Verbalis J, Aguilera G. Lipopolysaccharide endotoxin potentiates the effect of osmotic stimulation on vasopressin synthesis and secretion in the rat hypothalamus. J Neuroendocrinol. 2003;15(2):141-149. [CrossRef]
- Pardy K, Murphy D, Carter D, Hui KM. The influence of interleukin-2 on vasopressin and oxytocin gene expression in the rodent hypothalamus. J Neuroimmunol. 1993;42(2):131-138. [CrossRef]
- Zelazowski P, Patchev VK, Zelazowska EB, Chrousos GP, Gold PW, Sternberg EM. Release of hypothalamic corticotropin-releasing hormone and arginine-vasopressin by interleukin 1 beta and alpha MSH: studies in rats with different susceptibility to inflammatory disease. Brain Res. 1993;631(1):22-26. [CrossRef]
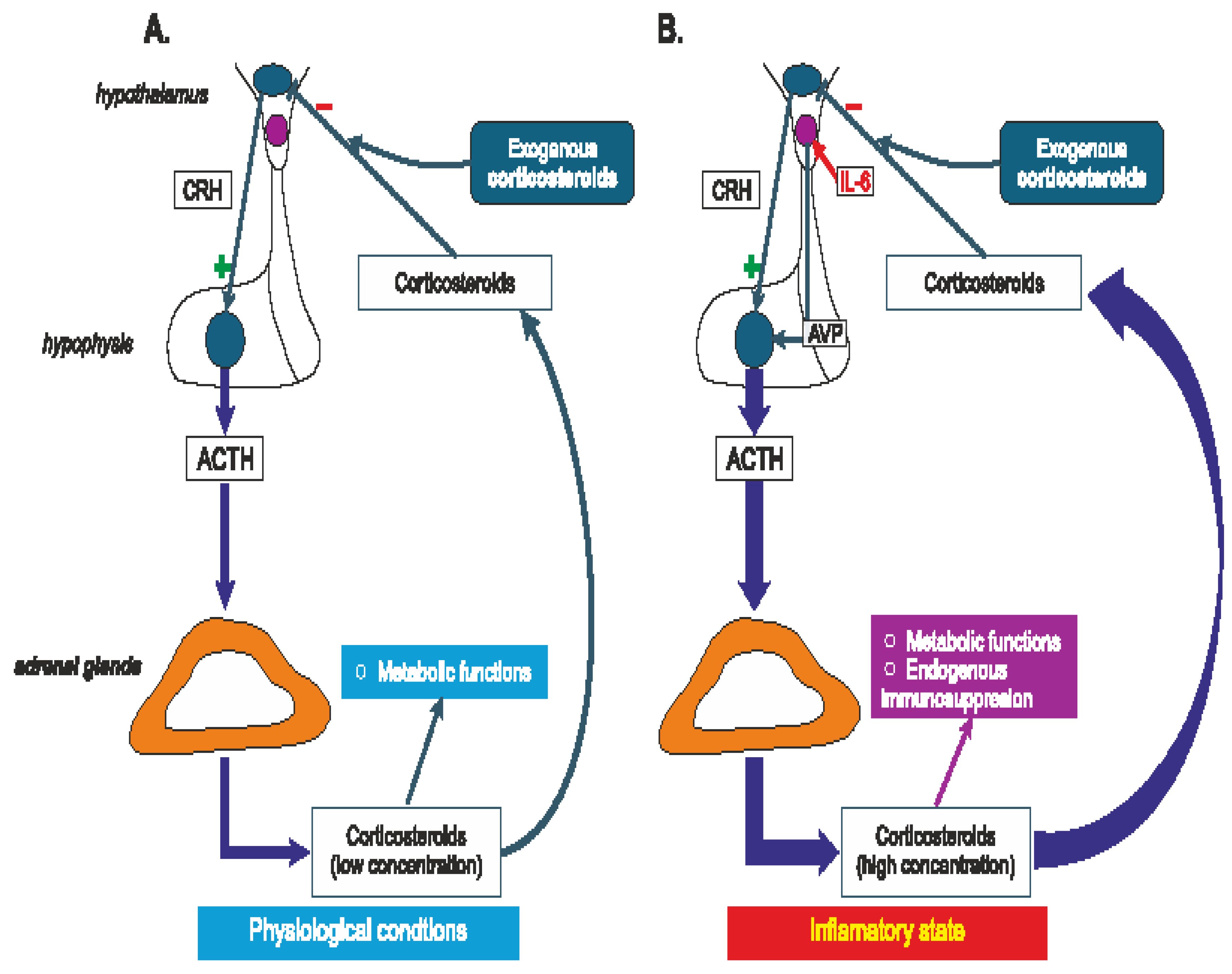
- Antoni FA. Magnocellular Vasopressin and the Mechanism of “Glucocorticoid Escape”.Front Endocrinol (Lausanne). 2019 Jun 26;10:422. [CrossRef]
- Luo X, Kiss A, Makara G, Lolait SJ, Aguilera G Stress-specific regulation of corticotropin releasing hormone receptor expression in the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Neuroendocrinol. 1994;6(6):689-696. [CrossRef]
- Sawchenko PE. Adrenalectomy-induced enhancement of CRF and vasopressin immunoreactivity in parvocellular neurosecretory neurons: anatomic, peptide, and steroid specificity. J Neurosci. 1987r;7(4):1093-1106. [CrossRef]
- Kovács KJ, Földes A, Sawchenko PE. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci. 2000;20(10):3843-3852. [CrossRef]
- Kageyama K, Suda T. Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus. Endocr J. 2009;56(3):335-344. [CrossRef]
- Yoshida M. Gene regulation system of vasopressin and corticotropin-releasing hormone.Gene Regul Syst Bio. 2008;2:71-88. [CrossRef]
- Mills NJ, Sharma K, Haque M, Moore M, Teruyama R. Aldosterone Mediated Regulation of Epithelial Sodium Channel (ENaC) Subunits in the Rat Hypothalamus. Neuroscience. 2018;390:278-292. [CrossRef]
- Geerling JC, Loewy AD. Aldosterone in the brain. Am J Physiol Renal Physiol. 2009; 297(3):F559-F576. [CrossRef]
- Viau V, Chu A, Soriano L, Dallman MF. Independent and overlapping effects of corticosterone and testosterone on corticotropin-releasing hormone and arginine vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus and stress-induced adrenocorticotropic hormone release. J Neurosci. 1999;19(15):6684-6693. [CrossRef]
- Herman JP,Tasker JG. Paraventricular Hypothalamic Mechanisms of Chronic Stress Adaptation. Front Endocrinol (Lausanne). 2016;7:137. [CrossRef]
- Sheng JA, Tan SML, Hale TM, Handa RJ. Androgens and Their Role in Regulating Sex Differences in the Hypothalamic/Pituitary/Adrenal Axis Stress Response and Stress-Related Behaviors. Androg Clin Res Ther. 2021;2(1):261-274. [CrossRef]
- Aguilera G,Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012 ;33(1):67-84. [CrossRef]
- Bous J, Fouillen A, Orcel H, Granier S, Bron P, Mouillac B. Structures of the arginine-vasopressin and oxytocin receptor signaling complexes. Vitam Horm. 2023;123:67-107. [CrossRef]
- Holmes CL, Landry DW, Granton JT. Science Review: Vasopressin and the cardiovascular system part 2 - clinical physiology. Crit Care. 2004;8(1):15-23. [CrossRef]
- Thai BS, Chia LY, Nguyen ATN, Qin C, Ritchie RH, Hutchinson DS, Kompa A, White PJ, May LT. Targeting G protein-coupled receptors for heart failure treatment. Br J Pharmacol. 2023. Online ahead of print. [CrossRef]
- Morello JP,Bichet DG. Nephrogenic diabetes insipidus. Annu Rev Physiol. 2001;63:607-630. [CrossRef]
- Zang L, Gong Y, Li Y, Dou J, Lyu Z, Su X, Zhang Y, Mu Y. A Novel Missense Mutation of Arginine Vasopressin Receptor 2 in a Chinese Family with Congenital Nephrogenic Diabetes Insipidus: X-Chromosome Inactivation in Female CNDI Patients with Heterozygote 814A>G Mutation. Biomed Res Int. 2022;2022:7073158. [CrossRef]
- Dekan Z, Kremsmayr T, Keov P, Godin M, Teakle N, Dürrauer L, Xiang H, Gharib D, Bergmayr C, Hellinger R et al. Nature-inspired dimerization as a strategy to modulate neuropeptide pharmacology exemplified with vasopressin and oxytocin. Chem Sci. 2021;12(11):4057-4062. [CrossRef]
- Murat B, Devost D, Andrés M, Mion J, Boulay V, Corbani M, Zingg HH, Guillon G. V1b and CRHR1 receptor heterodimerization mediates synergistic biological actions of vasopressin and CRH. Mol Endocrinol. 2012;26(3):502-520. [CrossRef]
- Patchev VK, Almeida OF. Corticosteroid regulation of gene expression and binding characteristics of vasopressin receptors in the rat brain. Eur J Neurosci. 1995;7(7):1579-1583. [CrossRef]
- Wasilewski MA, Grisanti LA, Song J, Carter RL, Repas AA, Myers VD, Gao E, Koch WJ, Cheung JY, Feldman AM et al., Vasopressin type 1A receptor deletion enhances cardiac contractility, β-adrenergic receptor sensitivity and acute cardiac injury-induced dysfunction. Clin Sci (Lond). 2016 ;130(22):2017-2027. [CrossRef]
- Tsuchiya M, Tsuchiya K, Maruyama R, Takemura G, Minatoguchi S, Fujiwara H. Vasopressin inhibits sarcolemmal ATP-sensitive K+ channels via V1 receptors activation in the guinea pig heart. Circ J. 2002;66(3):277-282. [CrossRef]
- Hantash BM, Thomas AP, Reeves JP. Regulation of the cardiac L-type calcium channel in L6 cells by arginine-vasopressin. Biochem J. 2006;400(3):411-419. [CrossRef]
- Tilley DG, Zhu W, Myers VD, Barr LA, Gao E, Li X, Song J, Carter RL, Makarewich CA, Yu D et al., β-adrenergic receptor-mediated cardiac contractility is inhibited via vasopressin type 1A-receptor-dependent signaling. Circulation. 2014;130(20):1800-1811. [CrossRef]
- Zhu W, Tilley DG, Myers VD, Coleman RC, Feldman AM. Arginine vasopressin enhances cell survival via a G protein-coupled receptor kinase 2/β-arrestin1/extracellular-regulated kinase 1/2-dependent pathway in H9c2 cells. Mol Pharmacol. 2013:227-235. [CrossRef]
- Zhu W, Tilley DG, Myers VD, Tsai EJ, Feldman AM. Increased vasopressin 1A receptor expression in failing human hearts. J Am Coll Cardiol. 2014;63(4):375-376. [CrossRef]
- Xu F, Sun S, Wang X, Ni E, Zhao L, Zhu W. GRK2 Mediates Arginine Vasopressin-Induced Interleukin-6 Production via Nuclear Factor-kappaB Signaling Neonatal Rat Cardiac Fibroblast.Mol Pharmacol. 2017;92(3):278-284. [CrossRef]
- Bucher M, Hobbhahn J, Taeger K, Kurtz A. Cytokine-mediated downregulation of vasopressin V(1A) receptors during acute endotoxemia in rats. Am J Physiol Regul Integr Comp Physiol. 2002;282(4):R979-984. [CrossRef]
- Gray M, Innala L, Viau V. Central vasopressin V1A receptor blockade impedes hypothalamic-pituitary-adrenal habituation to repeated restraint stress exposure in adult male rats. Neuropsychopharmacology. 2012;37(12):2712-2719. [CrossRef]
- Roper J, O’Carroll AM, Young W 3rd, Lolait S. The vasopressin Avpr1b receptor: molecular and pharmacological studies. Stress. 2011:98-115. [CrossRef]
- Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS 3rd, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci U S A. 1995;92(15):6783-6787. [CrossRef]
- O’Carroll AM, Howell GM, Roberts EM, Lolait SJ. Vasopressin potentiates corticotropin-releasing hormone-induced insulin release from mouse pancreatic beta-cells. J Endocrinol. 2008;197(2):231-239. [CrossRef]
- Khan S, Raghuram V, Chen L, Chou CL, Yang CR, Khundmiri SJ, Knepper MA. Vasopressin V2 receptor, tolvaptan, and ERK1/2 phosphorylation in the renal collecting duct. Am J Physiol Renal Physiol. 2024;326(1):F57-F68. [CrossRef]
- Schrier RW. Molecular mechanisms of clinical concentrating and diluting disorders. Prog Brain Res. 2008;170:539-550. [CrossRef]
- Lightman SL, Birnie MT, Conway-Campbell BL. Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr Rev. 2020 Jun 1;41(3):bnaa002. [CrossRef]
- Yates FE, Russell SM, Dallman MF, Hodge GA, McCann SM, Dhariwal AP. Potentiation by vasopressin of corticotropin release induced by corticotropin-releasing factor. Endocrinology. 1971;88(1):3-15. [CrossRef]
- Joseph DN, Whirledge S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int J Mol Sci. 2017 Oct 24;18(10):2224. [CrossRef]
- Robertson-Dixon I, Murphy MJ, Crewther SG, Riddell N. The Influence of Light Wavelength on Human HPA Axis Rhythms: A Systematic Review. Life (Basel). 2023 Sep 26;13(10):1968. [CrossRef]
- Otsuka H, Abe M, Kobayashi H. The Effect of Aldosterone on Cardiorenal and Metabolic Systems. Int J Mol Sci. 2023 Mar 11;24(6):5370. [CrossRef]
- Sztechman D, Czarzasta K, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, Zera T. Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. J Physiol Pharmacol. 2018 Dec;69(6). [CrossRef]
- Albert KM, Newhouse PA. Estrogen, Stress, and Depression: Cognitive and Biological Interactions. Annu Rev Clin Psychol. 2019;15:399-423. [CrossRef]
- Patel S, Homaei A, Raju AB, Meher BR. Estrogen: The necessary evil for human health, and ways to tame it. Biomed Pharmacother. 2018;102:403-411. [CrossRef]
- Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001 Sep;45(3 Suppl):S116-24. [CrossRef]
- Naamneh Elzenaty R, du Toit T, Flück CE. Basics of androgen synthesis and action. Best Pract Res Clin Endocrinol Metab. 2022 Jul;36(4):101665. [CrossRef]
- Fanelli F, Baronio F, Ortolano R, Mezzullo M, Cassio A, Pagotto U, Balsamo A. Normative Basal Values of Hormones and Proteins of Gonadal and Adrenal Functions from Birth to Adulthood. Sex Dev. 2018;12(1-3):50-94. [CrossRef]
- Kulle AE, Riepe FG, Melchior D, Hiort O, Holterhus PM. A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: age- and sex-specific reference data. J Clin Endocrinol Metab. 2010;95(5):2399-2409. [CrossRef]
- Zirkin BR, Papadopoulos V. Leydig cells: formation, function, and regulation. Biol Reprod. 2018;99(1):101-111. [CrossRef]
- Midzak A, Akula N, Lecanu L, Papadopoulos V. Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. J Biol Chem. 2011;286(11):9875-9887. [CrossRef]
- Beattie MC, Adekola L, Papadopoulos V, Chen H, Zirkin BR. Leydig cell aging and hypogonadism. Exp Gerontol. 2015;68:87-91. [CrossRef]
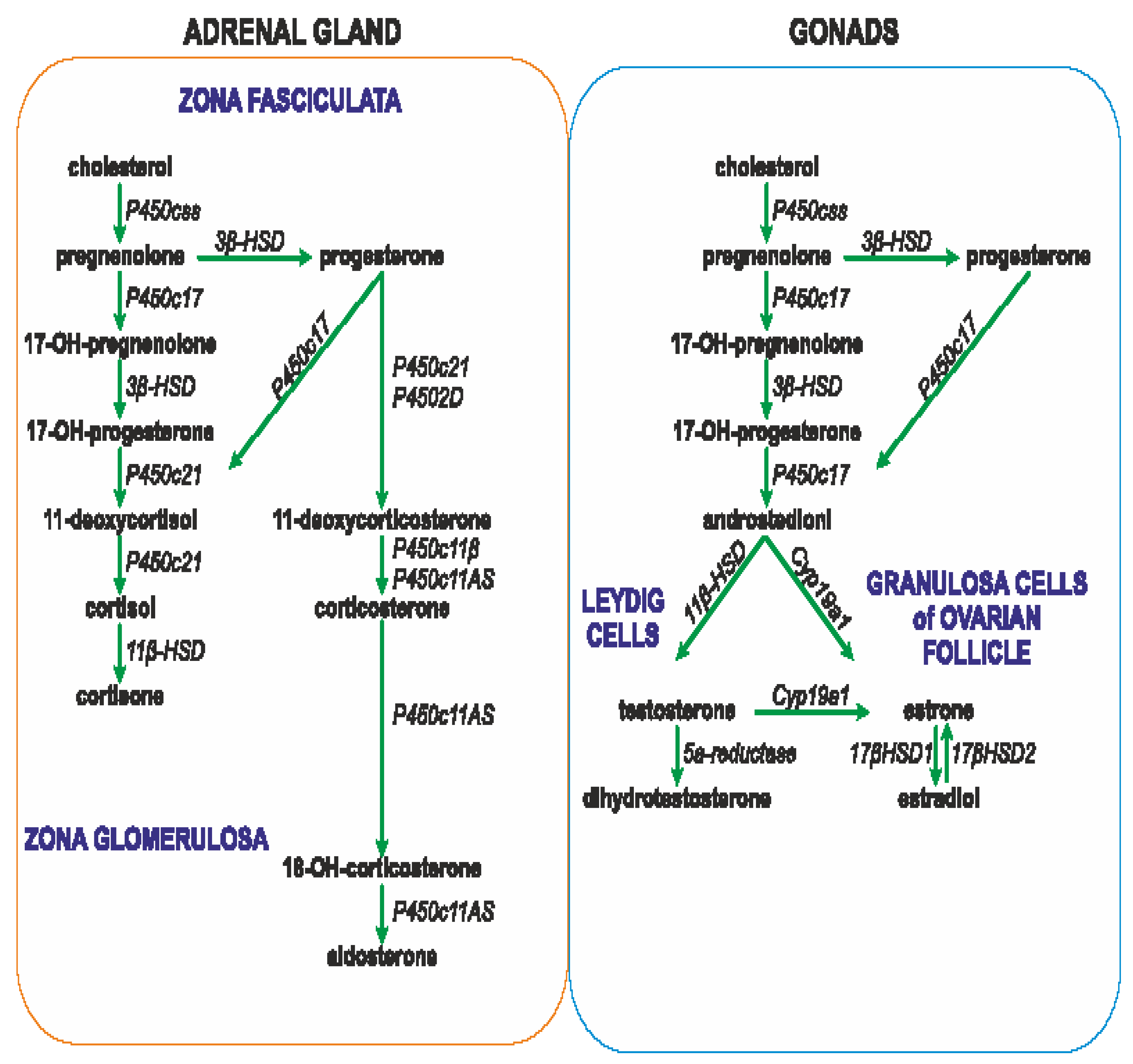
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25(6):947-970. [CrossRef]
- Wang Y, Chen F, Ye L, Zirkin B, Chen H. Steroidogenesis in Leydig cells: effects of aging and environmental factors. Reproduction. 2017;154(4):R111-R122. [CrossRef]
- Kuo T, McQueen A, Chen TC, Wang JC. Regulation of Glucose Homeostasis by Glucocorticoids. Adv Exp Med Biol. 2015;872:99-126. [CrossRef]
- Yoshimura M, Conway-Campbell B, Ueta Y. Arginine vasopressin: Direct and indirect action on metabolism. Peptides. 2021 Aug;142:170555. [CrossRef]
- Nakata M, Gantulga D, Santoso P, Zhang B, Masuda C, Mori M, Okada T, Yada T. Paraventricular NUCB2/Nesfatin-1 Supports Oxytocin and Vasopressin Neurons to Control Feeding Behavior and Fluid Balance in Male Mice. Endocrinology. 2016;157(6):2322-2332. [CrossRef]
- Mohan S, Flatt PR, Irwin N, Moffett RC. Weight-reducing, lipid-lowering and antidiabetic activities of a novel arginine vasopressin analogue acting at the V1a and V1b receptors in high-fat-fed mice. Diabetes Obes Metab. 2021;23(10):2215-2225. [CrossRef]
- Haam J, Halmos KC, Di S, Tasker JG. Nutritional state-dependent ghrelin activation of vasopressin neurons via retrograde trans-neuronal-glial stimulation of excitatory GABA circuits. J Neurosci. 2014;34(18):6201-6213. [CrossRef]
- Iwama S, Sugimura Y, Murase T, Hiroi M, Goto M, Hayashi M, Arima H, Oiso Y. Central adiponectin functions to inhibit arginine vasopressin release in conscious rats. J Neuroendocrinol. 2009;21(9):753-759. [CrossRef]
- Küchler S, Perwitz N, Schick RR, Klein J, Westphal S. Arginine-vasopressin directly promotes a thermogenic and pro-inflammatory adipokine expression profile in brown adipocytes. Regul Pept. 2010;164(2-3):126-132. [CrossRef]
- Rofe AM, Williamson DH. Metabolic effects of vasopressin infusion in the starved rat. Reversal of ketonaemia. Biochem J. 1983;212(1):231-239. [CrossRef]
- VAUGHAN M. EFFECT OF PITRESSIN ON LIPOLYSIS AND ON PHOSPHORYLASE ACTIVITY IN RAT ADIPOSE TISSUE. Am J Physiol. 1964;207:1166-1168. [CrossRef]
- Hiroyama M, Aoyagi T, Fujiwara Y, Oshikawa S, Sanbe A, Endo F, Tanoue A. Hyperammonaemia in V1a vasopressin receptor knockout mice caused by the promoted proteolysis and reduced intrahepatic blood volume. J Physiol. 2007;581(Pt 3):1183-1192. [CrossRef]
- Hiroyama M, Fujiwara Y, Nakamura K, Aoyagi T, Mizutani R, Sanbe A, Tasaki R, Tanoue A. Altered lipid metabolism in vasopressin V1B receptor-deficient mice. Eur J Pharmacol. 2009;602(2-3):455-461. [CrossRef]
- Velho G, El Boustany R, Lefèvre G, Mohammedi K, Fumeron F, Potier L, Bankir L, Bouby N, Hadjadj S, Marre M, Roussel R. Plasma Copeptin, Kidney Outcomes, Ischemic Heart Disease, and All-Cause Mortality in People With Long-standing Type 1 Diabetes. Diabetes Care. 2016;39(12):2288-2295. [CrossRef]
- Vanhaecke T, Perrier ET, Melander O. A Journey through the Early Evidence Linking Hydration to Metabolic Health. Ann Nutr Metab. 2020;76 Suppl 1:4-9. [CrossRef]
- Roussel R, El Boustany R, Bouby N, Potier L, Fumeron F, Mohammedi K, Balkau B, Tichet J, Bankir L, Marre M, Velho G. Plasma Copeptin, AVP Gene Variants, and Incidence of Type 2 Diabetes in a Cohort From the Community. J Clin Endocrinol Metab. 2016 Jun;101(6):2432-9. Enhörning S, Leosdottir M, Wallström P, Gullberg B, Berglund G, Wirfält E, Melander O. Relation between human vasopressin 1a gene variance, fat intake, and diabetes. Am J Clin Nutr. 2009;89(1):400-406. [CrossRef]
- Enhörning S, Leosdottir M, Wallström P, Gullberg B, Berglund G, Wirfält E, Melander O. Relation between human vasopressin 1a gene variance, fat intake, and diabetes. Am J Clin Nutr. 2009;89(1):400-406. [CrossRef]
- Enhörning S, Sjögren M, Hedblad B, Nilsson PM, Struck J, Melander O. Genetic vasopressin 1b receptor variance in overweight and diabetes mellitus. Eur J Endocrinol. 2016 ;174(1):69-75. [CrossRef]
- Hew-Butler T, Smith-Hale V, Pollard-McGrandy A, VanSumeren M. Of Mice and Men-The Physiology, Psychology, and Pathology of Overhydration. Nutrients. 2019 Jul 7;11(7):1539. [CrossRef]
- Szczepanska-Sadowska E, Wsol A, Cudnoch-Jedrzejewska A, Żera T. Complementary Role of Oxytocin and Vasopressin in Cardiovascular Regulation. Int J Mol Sci. 2021 Oct 24;22(21):11465. [CrossRef]
- Bichet DG. Regulation of Thirst and Vasopressin Release. Annu Rev Physiol. 2019; 81:359-373. [CrossRef]
- Szczepanska-Sadowska E. Neuromodulation of Cardiac Ischemic Pain: Role of the Autonomic Nervous System and Vasopressin. J Integr Neurosci. 2024;23(3):49. [CrossRef]
- Danziger J, Zeidel ML. Osmotic homeostasis. Clin J Am Soc Nephrol. 2015;10(5):852-862. [CrossRef]
- Zimmerman CA, Lin YC, Leib DE, Guo L, Huey EL, Daly GE, Chen Y, Knight ZA. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature. 2016;537(7622):680-684. [CrossRef]
- Zaelzer C, Hua P, Prager-Khoutorsky M, Ciura S, Voisin DL, Liedtke W, Bourque CW. ΔN-TRPV1: A Molecular Co-detector of Body Temperature and Osmotic Stress. Cell Rep. 2015;13(1):23-30. [CrossRef]
- Saker P, Farrell MJ, Adib FR, Egan GF, McKinley MJ, Denton DA. Regional brain responses associated with drinking water during thirst and after its satiation. Proc Natl Acad Sci U S A. 2014;111(14):5379-5384. [CrossRef]
- Cheung PW, Bouley R, Brown D. Targeting the Trafficking of Kidney Water Channels for Therapeutic Benefit. Annu Rev Pharmacol Toxicol. 2020;60:175-194. [CrossRef]
- Bankir L, Bichet DG, Bouby N. Vasopressin V2 receptors, ENaC, and sodium reabsorption: a risk factor for hypertension? Am J Physiol Renal Physiol. 2010;299(5):F917-F928. [CrossRef]
- Fenton RA. Essential role of vasopressin-regulated urea transport processes in the mammalian kidney. Pflugers Arch. 2009;458(1):169-177. [CrossRef]
- Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res. 2001;51(3):372-390. [CrossRef]
- Wang W, Li C, Summer SN, Falk S, Cadnapaphornchai MA, Chen YC, Schrier RW. Molecular analysis of impaired urinary diluting capacity in glucocorticoid deficiency. Am J Physiol Renal Physiol. 2006;290(5):F1135-1142. [CrossRef]
- Zhu X, Huang Y, Li S, Ge N, Li T, Wang Y, Liu K, Liu C. Glucocorticoids Reverse Diluted Hyponatremia Through Inhibiting Arginine Vasopressin Pathway in Heart Failure Rats. J Am Heart Assoc. 2020;9(10):e014950. [CrossRef]
- Frenkel A, Abuhasira R, Bichovsky Y, Bukhin A, Novack V, Brotfain E, Zlotnik A, Klein M. Examination of the association of steroids with fluid accumulation in critically ill patients, considering the possibility of biases. Sci Rep. 2021;11(1):5557. [CrossRef]
- Kenyon CJ, Saccoccio NA, Morris DJ. Aldosterone effects on water and electrolyte metabolism. J Endocrinol. 1984 Jan;100(1):93-100. [CrossRef]
- Johnston JG, Welch AK, Cain BD, Sayeski PP, Gumz ML, Wingo CS. Aldosterone: Renal Action and Physiological Effects. Compr Physiol. 2023;13(2):4409-4491. [CrossRef]
- Patel S, Rauf A, Khan H, Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317-325. [CrossRef]
- Sladek CD, Somponpun SJ. Estrogen receptors: their roles in regulation of vasopressin release for maintenance of fluid and electrolyte homeostasis. Front Neuroendocrinol. 2008;29(1):114-127. [CrossRef]
- Wang YX, Crofton JT, Liu H, Sato K, Brooks DP, Share L. Estradiol attenuates the antidiuretic action of vasopressin in ovariectomized rats. Am J Physiol. 1995;268(4 Pt 2):R951-R957. [CrossRef]
- Somponpun SJ, Johnson AK, Beltz T, Sladek CD. Estrogen receptor-alpha expression in osmosensitive elements of the lamina terminalis: regulation by hypertonicity. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R661-R669. [CrossRef]
- Somponpun SJ. Neuroendocrine regulation of fluid and electrolyte balance by ovarian steroids: contributions from central oestrogen receptors. J Neuroendocrinol. 2007;19(10):809-818. [CrossRef]
- Voisin DL, Simonian SX, Herbison AE. Identification of estrogen receptor-containing neurons projecting to the rat supraoptic nucleus. Neuroscience. 1997;78(1):215-228. [CrossRef]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863-870. [CrossRef]
- Li X, Kuang W, Qiu Z, Zhou Z. G protein-coupled estrogen receptor: a promising therapeutic target for aldosterone-induced hypertension. Front Endocrinol (Lausanne). 2023 Aug 17;14:1226458. [CrossRef]
- Rodriguez-Giustiniani P, Rodriguez-Sanchez N, Galloway SDR. Fluid and electrolyte balance considerations for female athletes. Eur J Sport Sci. 2022;22(5):697-708. [CrossRef]
- Swenson KL, Sladek CD. Gonadal steroid modulation of vasopressin secretion in response to osmotic stimulation. Endocrinology. 1997;138(5):2089-2097. [CrossRef]
- Siegenthaler J, Walti C, Urwyler SA, Schuetz P, Christ-Crain M. Copeptin concentrations during psychological stress: the PsyCo study. Eur J Endocrinol. 2014;171(6):737-742. [CrossRef]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Aging Res Rev.2005; 4; 141-194. [CrossRef]
- Milutinović-Smiljanić S, Šarenac O, Lozić-Djurić M, Murphy D, Japundžić-Žigon N. Evidence for involvement of central vasopressin V1b and V2 receptors in stress-induced baroreflex desensitization. Br J Pharmacol. 2013;169(4):900-908. [CrossRef]
- Grassi D, Lagunas N, Calmarza-Font I, Diz-Chaves Y, Garcia-Segura LM, Panzica GC. Chronic unpredictable stress and long-term ovariectomy affect arginine-vasopressin expression in the paraventricular nucleus of adult female mice. Brain Res. 2014 Nov 7;1588:55-62. [CrossRef]
- Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15(9):525-534. [CrossRef]
- Borrow AP, Bales NJ, Stover SA, Handa RJ. Chronic Variable Stress Induces Sex-Specific Alterations in Social Behavior and Neuropeptide Expression in the Mouse. Endocrinology. 2018;159(7):2803-2814. [CrossRef]
- Das S, Komnenov D, Newhouse L, Rishi AK, Rossi NF. Paraventricular Nucleus V1a Receptor Knockdown Blunts Neurocardiovascular Responses to Acute Stress in Male Rats after Chronic Mild Unpredictable Stress. Physiol Behav. 2022 Sep 1;253:113867. [CrossRef]
- Powell-Roach KL, Yao Y, Jhun EH, He Y, Suarez ML, Ezenwa MO, Molokie RE, Wang ZJ, Wilkie DJ. Vasopressin SNP pain factors and stress in sickle cell disease PLoS One. 2019 Nov 11;14(11):e0224886. [CrossRef]
- Komnenov D, Quaal H, Rossi NF. V1a and V1b vasopressin receptors within the paraventricular nucleus contribute to hypertension in male rats exposed to chronic mild unpredictable stress. Am J Physiol Regul Integr Comp Physiol. 2021;320(3):R213-R225. [CrossRef]
- Pietranera L, Saravia F, Roig P, Lima A, De Nicola AF. Mineralocorticoid treatment upregulates the hypothalamic vasopressinergic system of spontaneously hypertensive rats. Neuroendocrinology. 2004;80(2):100-110. [CrossRef]
- Matsuguchi H, Schmid PG. Acute interaction of vasopressin and neurogenic mechanisms in DOC-salt hypertension. Am J Physiol. 1982;242(1):H37-H43. [CrossRef]
- Ferrario CM, Mohara O, Ueno Y, Brosnihan KB. Hemodynamic and neurohormonal changes in the development of DOC hypertension in the dog. Am J Med Sci. 1988;295(4):352-359. [CrossRef]
- Rademaker MT, Charles CJ, Nicholls MG, Richards AM. Interactions of enhanced urocortin 2 and mineralocorticoid receptor antagonism in experimental heart failure. Circ Heart Fail. 2013;6(4):825-832. [CrossRef]
- Pasquali R, Gagliardi L, Vicennati V, Gambineri A, Colitta D, Ceroni L, Casimirri F. ACTH and cortisol response to combined corticotropin releasing hormone-arginine vasopressin stimulation in obese males and its relationship to body weight, fat distribution and parameters of the metabolic syndrome. Int J Obes Relat Metab Disord. 1999;23(4):419-424. [CrossRef]
- Schinke C, Hesse S, Rullmann M, Becker GA, Luthardt J, Zientek F, Patt M, Stoppe M, Schmidt E, Meyer K, Meyer PM, Orthgieß J, Blüher M, Kratzsch J, Ding YS, Then Bergh F, Sabri O. Central noradrenaline transporter availability is linked with HPA axis responsiveness and copeptin in human obesity and non-obese controls. Stress. 2019;22(1):93-102.
- Canivell S, Mohaupt M, Ackermann D, Pruijm M, Guessous I, Ehret G, Escher G, Pechère-Bertschi A, Vogt B, Devuyst O, Burnier M, Martin PY, Ponte B, Bochud M. Copeptin and insulin resistance: effect modification by age and 11 β-HSD2 activity in a population-based study. J Endocrinol Invest. 2018;41(7):799-808. [CrossRef]
- Nye EJ, Bornstein SR, Grice JE, Tauchnitz R, Hockings GI, Strakosch CR, Jackson RV, Torpy DJ. Interactions between the stimulated hypothalamic-pituitary-adrenal axis and leptin in humans. J Neuroendocrinol. 2000;12(2):141-145. [CrossRef]
- Kacheva S, Kolk K, Morgenthaler NG, Brabant G, Karges W. Gender-specific co-activation of arginine vasopressin and the hypothalamic-pituitary-adrenal axis during stress. Clin Endocrinol (Oxf). 2015;82(4):570-576. [CrossRef]
- Zelena D, Mergl Z, Makara GB. The role of vasopressin in diabetes mellitus-induced hypothalamo-pituitary-adrenal axis activation: studies in Brattleboro rats. Brain Res Bull. 2006;69(1):48-56. [CrossRef]
- Balapattabi K, Little JT, Bachelor ME, Cunningham RL, Cunningham JT. Sex Differences in the Regulation of Vasopressin and Oxytocin Secretion in Bile Duct-Ligated Rats. Neuroendocrinology. 2021;111(3):237-248. [CrossRef]
- Coiro V, Volpi R, Capretti L, Bacchi-Modena A, Cigarini C, Bianconi L, Rossi G, Gramellini D, Chiodera P. Arginine vasopressin secretion in non-obese women with polycystic ovary syndrome. Acta Endocrinol (Copenh). 1989;121(6):784-790. [CrossRef]
- Pavlidi P, Kokras N, Dalla C. Sex Differences in Depression and Anxiety. Curr Top Behav Neurosci. 2023;62:103-132. [CrossRef]
- Woodward E, Rangel-Barajas C, Ringland A, Logrip ML, Coutellier L. Sex-Specific Timelines for Adaptations of Prefrontal Parvalbumin Neurons in Response to Stress and Changes in Anxiety- and Depressive-Like Behaviors. eNeuro. 2023 Mar 2;10(3):ENEURO.0300-22.2023. [CrossRef]
- Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol Biochem Behav. 1999;64(4):739-751. [CrossRef]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14(6):506-513. [CrossRef]
- Teo CH, Wong ACH, Sivakumaran RN, Parhar I, Soga T. Gender Differences in Cortisol and Cortisol Receptors in Depression: A Narrative Review. Int J Mol Sci. 2023 Apr 12;24(8):7129. [CrossRef]
- Rosinger ZJ, Jacobskind JS, De Guzman RM, Justice NJ, Zuloaga DG. A sexually dimorphic distribution of corticotropin-releasing factor receptor 1 in the paraventricular hypothalamus. Neuroscience. 2019;409:195-203. [CrossRef]
- Cox KH, Quinnies KM, Eschendroeder A, Didrick PM, Eugster EA, Rissman EF. Number of X-chromosome genes influences social behavior and vasopressin gene expression in mice. Psychoneuroendocrinology. 2015;51:271-81. [CrossRef]
- Cohen JE, Holsen LM, Ironside M, Moser AD, Duda JM, Null KE, Perlo S, Richards CE, Nascimento NF, Du F, Zuo C, Misra M, Pizzagalli DA, Goldstein JM. Neural response to stress differs by sex in young adulthood. Psychiatry Res Neuroimaging. 2023 Jul;332:111646. [CrossRef]
- Zoorob RJ, Cender D. A different look at corticosteroids. Am Fam Physician. 1998;58(2):443-450.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073. [CrossRef]
- Zanardo V, Golin R, Chiozza ML, Faggian D. Dexamethasone does not affect vasopressin release in bronchopulmonary dysplasia. Pediatr Nephrol. 2000;15(3-4):241-244. [CrossRef]
- Gordijn MS, Gemke RJ, van Dalen EC, Rotteveel J, Kaspers GJ. Hypothalamic-pituitary-adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia. Cochrane Database Syst Rev. 2012 May 16;(5):CD008727. Update in: Cochrane Database Syst Rev. 2015;(8):CD008727. [CrossRef]
- Liu RY, Unmehopa UA, Zhou JN, Swaab DF. Glucocorticoids suppress vasopressin gene expression in human suprachiasmatic nucleus. J Steroid Biochem Mol Biol. 2006;98(4-5):248-253. [CrossRef]
- Antoni FA. Magnocellular Vasopressin and the Mechanism of “Glucocorticoid Escape”. Front Endocrinol (Lausanne). 2019 Jun 26;10:422. [CrossRef]
- Escudero DS, Fantinelli JC, Martínez VR, González Arbeláez LF, Amarillo ME, Pérez NG, Díaz RG. Hydrocortisone cardioprotection in ischaemia/reperfusion injury involves antioxidant mechanisms. Eur J Clin Invest. 2024 Jan 31:e14172. Epub ahead of print. [CrossRef]
- Giugliano GR, Giugliano RP, Gibson CM, Kuntz RE. Meta-analysis of corticosteroid treatment in acute myocardial infarction. Am J Cardiol. 2003;91(9):1055-1059. [CrossRef]
- Tol MM, Shekar K, Barnett AG, McGree J, McWhinney BC, Ziegenfuss M, Ungerer JP, Fraser JF. A preliminary investigation into adrenal responsiveness and outcomes in patients with cardiogenic shock after acute myocardial infarction. J Crit Care. 2014 Jun;29(3):470.e1-6. [CrossRef]
- Torgersen C, Luckner G, Schröder DC, Schmittinger CA, Rex C, Ulmer H, Dünser MW. Concomitant arginine-vasopressin and hydrocortisone therapy in severe septic shock: association with mortality. Intensive Care Med. 2011;37(9):1432-1437. [CrossRef]
- Penn J, Douglas W, Curran J, Chaudhuri D, Dionne JC, Fernando SM, Granton D, Mathew R, Rochwerg B. Efficacy and safety of corticosteroids in cardiac arrest: a systematic review, meta-analysis and trial sequential analysis of randomized control trials. Crit Care. 2023 Jan 11;27(1):12. [CrossRef]
- Andersen LW, Isbye D, Kjærgaard J, Kristensen CM, Darling S, Zwisler ST, Fisker S, Schmidt JC, Kirkegaard H, Grejs AM, et al. Effect of Vasopressin and Methylprednisolone vs Placebo on Return of Spontaneous Circulation in Patients With In-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2021;326(16):1586-1594. [CrossRef]
- Scott LV, Dinan TG. Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci1998;62(22):1985-1998;. [CrossRef]
- Scott LV, Dinan TG. Vasopressin as a target for antidepressant development: an assessment of the available evidence. J Affect Disord. 2002;72(2):113-124. [CrossRef]
- Simon NG, Guillon C, Fabio K, Heindel ND, Lu SF, Miller M, Ferris CF, Brownstein MJ, Garripa C, Koppel GA. Vasopressin antagonists as anxiolytics and antidepressants: recent developments. Recent Pat CNS Drug Discov. 2008;3(2):77-93. [CrossRef]
- Poretti MB, Sawant RS, Rask-Andersen M, de Cuneo MF, Schiöth HB, Perez MF, Carlini VP. Reduced vasopressin receptors activation mediates the anti-depressant effects of fluoxetine and venlafaxine in bulbectomy model of depression. Psychopharmacology (Berl). 2016 ;233(6):1077-1086. [CrossRef]
- Stewart LQ, Roper JA, Young WS 3rd, O’Carroll AM, Lolait SJ. The role of the arginine vasopressin Avp1b receptor in the acute neuroendocrine action of antidepressants. Psychoneuroendocrinology. 2008;33(4):405-415. [CrossRef]
- Kiss A, Bundzikova J, Pirnik Z, Mikkelsen JD. Different antipsychotics elicit different effects on magnocellular oxytocinergic and vasopressinergic neurons as revealed by Fos immunohistochemistry. J Neurosci Res. 2010;88(3):677-685.
- Florkowski CM, Crozier IG, Nightingale S, Evans MJ, Ellis MJ, Joyce P, Donald RA. Plasma cortisol, PRL, ACTH, AVP and corticotrophin releasing hormone responses to direct current cardioversion and electroconvulsive therapy. Clin Endocrinol (Oxf). 1996;44(2):163-168. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




