1. Introduction
Single or few layers 2D graphene sheets have excellent mechanical and optical properties. They are formed by carbon atoms arranged to form a honeycomb structure, defined by carbon atoms in a sp
2 hybridization, as described in a general public magazine in 2008 [
1]. The word graphene has a beginning in graph- to recall carbons species. Its –ene end is associated to its polycyclic aromatic hydrocarbon’s nature [
2]. The electrons inside the sigma bonds between the carbon atoms are stable. Extraordinary optical properties are due to the electrons inside the atomic 2p
z orbitals of the carbon atoms. These atomic orbitals are overlapped and extended valence bonding π and conducting anti-bounding π* molecular orbitals are formed. Intrabands and interbands electronic transitions in the spectral range UV, visible and Far Infrared are observed.
The introduction of graphene in photocatalysis was reviewed in 2016 [
3]. Different methods for its exfoliation and to obtain 2D graphene particles of several dimensions and thicknesses were reviewed in ref. [
4]. The UV-visible-NIR absorption spectra of reduced graphene particles is complex mainly because of electrons interactions. The first detailed general description of electrons interactions has been published in 1952 [
5]. Localized surface plasmons have then been described in 2007 by Maier, S.A. [
6]. Initial studies were devoted to metallic particles but possible application also to reduced graphene have been reported in 2014 [
7,
8,
9]. With added copper chloride, RGo particles are associated with strong absorption, that can be called plasmons. Furthermore, if the graphene particles are suspended in a solvent, they have indirect semi-conducting properties and a band gap can be detected between their valence and their conduction bands. [
10,
11]. These bands positions in energy are strongly affected by the size and the shape of the graphene nanoparticles, as well as by their O-containing defects (OH, peroxide and carboxylic acid groups) and structural defects (C-vacancies). We have looked to plasmons and also to band-gap value in our samples prepared with CuCl
2 3H
2O and reduced graphene obtained by ultra-sounds and dispersed in ethanol, the four questions which were now arising being:
1) is the added Copper chloride hydrated salt necessary to observe plasmons?
2) can we adjust plasmons positions and intensities by a simple dilution?
3) can we evidence the plasmons coupling with C-C vibrations?
4) can we eliminate s the plasmons to study photocatalytic properties that can be attributed to our Copper species and reduced graphene particles.
We will answer to these four questions, using a simple UV-visible-NIR spectra. In addition, small negative peaks are observed in the UV range and suggest that some emission of light, fluorescence also takes place, and can be due to copper and to graphene sheets. To clarify that point, and check that the fluorescence was due to the graphene sheets in themselves, measurements of fluorescence before the addition of copper have been made. Fluorescence results are then compared with published ones [
12]. Two methods aimed at suppressing the plasmons, dilution or use of special cells with a 0.2 cm optical cell are compared. When plasmons are suppressed, band-gap measurements of C-species can be made using classical method [
13] and the methods developed in our laboratory [
13,
14,
15,
16,
17]. Obtained optical characterizations, band gap values, are compared to published ones [
18,
19]. Catalytic tests about two dyes, eosin and bromophenol blue decomposition, are finally introduced to follow the subject of dye degradation under visible light irradiation over copper.
We have acquired a commercial graphite containing 3D graphite particles and stable 2D nanoscrolls. Nanoscrolls are described and their practical use is details by several authors [
20,
21,
22]. We have reported that ultra-sound technique can be used to exfoliate them and the 3D graphite GR particles to obtain flat particles and to use them after as supports for Zn-ferrite nanoparticles to eliminate antibiotic traces of an antibiotic, Amoxycillin [
23]. In the most common preparation method using ultra-sounds, in water, oxidized GO sheets are obtained using the Hummer’s method. GO is then reduced to give RGo, and three distinct three distinct reducers have been used, hydrazine, ascorbic acid and natural extracts of Amaranthus hybrids [
24]. Here, ethanol was used as solvent and also as a reducer [
23]. The RGo symbol was kept to describe our reduced graphene containing samples, having a black color, even if no intermediate oxidized step was necessary to prepare them. Ultra-sound treatments to prepare graphene particles suspended in a solvent are common. Some of the reported data concern samples prepared indirectly, using ultrasounds in thermostatic beakers introduced in a water bath or a horn introduced inside the solution that can be water [
25], ethanol [
26] or water with strong mineral acids [
27]. As made in refs 10 to 12, we have used a horn directly introduced inside the liquid solvent. More details about the three publications (power, frequencies, times of the ultrasound treatment are summarized in Supplementary Information).
Copper is an interesting metal, cheap and abundant. Solid catalysts containing isolated copper ions dispersed on porous nanocrystals of zeolite FAU or of Reduced graphene oxide for instance are interesting for oxidation reactions of oxygen and also for the degradation of pollutants [
19,
29]. The complexation of transition metal ions in the surface of graphene has been recently reviewed [
30] and the grafting of copper yielding to Cu(I) isolated sites has been studied for instance in refs. [
31,
32]. Liquid Cu/Zn on graphene have also been formed by CVD [
33]. To avoid graphene agglomeration, neutral surfactants and negatively charged species can be added. But simple addition of organics is affecting the physical-chemical properties of the graphene sheets. Here, similar solids are obtained by a simple contact of suspended graphene nanoparticles with mineral species Cu (Cl)
2 3H
2O after an ultra-sound treatment aimed at eliminating the nanoscrolls of graphene and a centrifugation aimed at eliminating graphite 3D particles. Solids characterizations are performed by XRD, SEM and TEM micrographs, associated with Selected Area Electrons Diffraction. We will focus on the description of the plasmons intensities and positions as a function of the Cu/RGo dilution in ethanol. In addition, small negative peaks are observed in the UV range will suggest that some emission of light, fluorescence also takes place, and can be due to copper and to graphene sheets. To clarify that point, and check that the fluorescence was due to the graphene sheets in themselves, measurements of fluorescence before the addition of copper have been made. Fluorescence results are then compared with published ones [
14]. Two methods aimed at suppressing the plasmons, dilution or use of special cells with a 0.2 cm optical cell are compared. We will see that plasmons and vibrations of the C-species are coupled. When plasmons are suppressed, band-gap measurements of C-species can be made using classical method [
15] and the methods developed with our students [
16,
17,
18] and with students of another group [
19]. Obtained optical characterizations, band gap values, are compared to published ones [
12,
34]. Catalytic tests about two dyes, eosin and bromophenol blue decomposition, are finally introduced to follow the subject of dye degradation under visible light irradiation over Copper/ RGo.
2. Materials and Methods
2.1. Materials
Initial commercial graphite flakes, GR, are 99% Carbon, 100 Mesh, Natural from Sigma Aldrich n° 808091, CAS 7782-42-5. We used absolute alcohol CAS 64-17-5 and CuCl2 2.5 H2O, CAS 10125-13-0 from the same society. All these reagents lack of intrinsic toxicity. We focused here on the work made with the catalysts in a liquid state, its dilution in ethanol. Graphene suspensions contained reduced graphene RGo and are easily recognized because of their black color. The ultrasounds treatment was applied directly inside centrifugation vials, to warrant the absence of unwanted contamination due to metallic species and ascertain that the containers always have the same shape and volume. The vials have a conical basic shape and a height of 115 cm. They contain between 40 and 45 mL of ethanol.
2.2. Methods
Scanning electron microscopy (SEM) imaging was performed with a Hitachi SU-70 FESEM. Transmission electron micrographs were registered on a JEOL JEM 2011 UHR (LaB6) microscope operating at 200 kV and equipped with an ORIUS Gatan Camera. For the observations, the liquids were deposited on 3 mm copper grids coated with an amorphous carbon film and let to dry.
Ultrasonic irradiations were generated by a Vibracell VCX 500 apparatus from Bioblock (500 W power, used at 40%, with a frequency of 20 kHz). The horn of this apparatus of 13 mm in diameter and was made with an alloy of Ti-6Al-4V, warranted for an overall volume of solution of 50 ml. The probe was penetrating by 6 cm inside the used liquid inside the vial. We have used either constant irradiations for 5, 6, 7 min or for 2 hours with and without pulses of 15s and stops of 15s. Experimental conditions, the influence of the ultra sounds power, several sonication times and of the initial graphite concentration GR were detailed for instance by Navik et al. [
26]. The used initial concentration of graphite was equal to 5 mg/ ml, the sonication was realized in sonication bath with a power of 1.08 kW. The cited publication was devoted to graphene stabilization by an organic molecule, curcumin, stable for a graphene concentration of 1.44 mg/mL, but it was also containing complementary data concerning the detection of graphene sheets in ethanol with several initial GR concentrations. There is a main difference between these published results and ours, since the used ultra sound horn is penetrating deeply in solution.
In our liquors, to avoid solids precipitation, we have used 6 min or 2h of ultrasound treatment, and we have used concentrations of initial graphite GR introduced in concentrations lower than 0.5 mg/ml. After the ultrasonic treatment, a centrifugation was applied at 3000 and/or 5000 rotations by min, rpm, 12 min. Bulk 3D graphite was recovered at the bottom of the flask. It contains a lot of 3D graphite (demonstrated by SAXS measurements,
Figure S1) and was eliminated. The transparent black liquor was then used directly to dissolve the salt, copper chloride trihydrate. The solution was changing of color spontaneously and became green in less than 2 min. We were surprised to observe that the green color of the solution was extremely stable, remaining constant even after several months of storage in air, whereas the species that we have detected that can be green are Cu (I) cations and are known to be instable so that only the redox potential of the couple Cu (II) Cu (metal) is given in books. To explain their stability, a positive role of ethanol to protect them is proposed.
The presence of the graphene sheets in suspension in ethanol was studied first by WAXS Wide Angle Scattering. WAXS measurements were made on a Bruker type D8 Advance within the range 2
θ from 0.3 to 90 with a copper 1.54186 Å, with a mixture of Kα
1 and Kα
2, with a ratio of 1 for 2. A Bragg Brentano set-up was used to obtain graphics in intensities versus 2
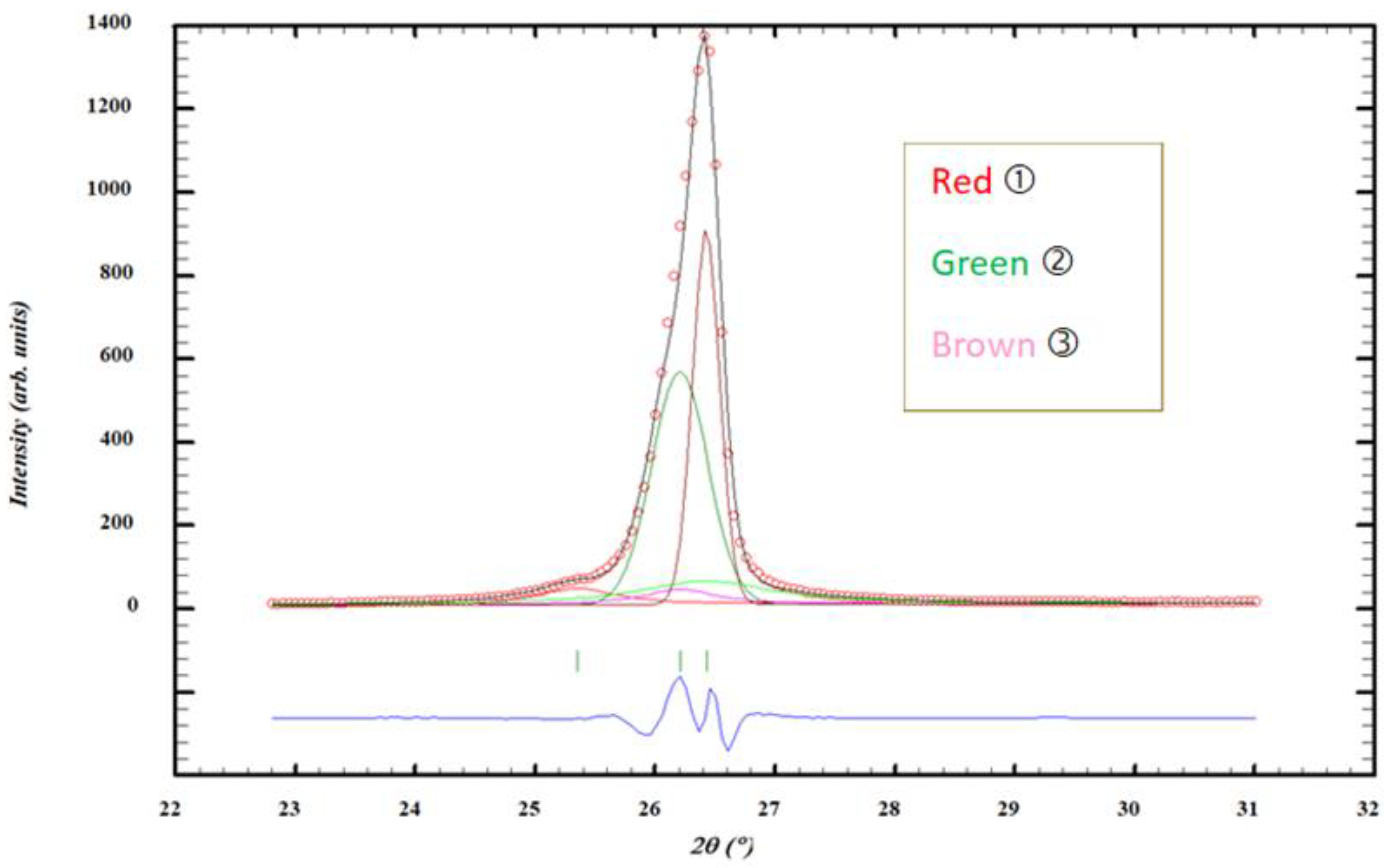
θ. Simulations were made using the Fullprof program. With this program, precise values of peaks positions and intensities (integrated units) were obtained. Within the range 23-31°, three observed peaks were labelled (1), (2) and (3). (1) is a small peak on the left part. Peaks (2) and (3) are more than 5 times more intense and are on the right part. Two additional peaks attributed to X-ray diffusion are presented in
Figure S2 about the shape of the diffraction peaks (2) and (3) were calculated, based on two parameters H = FWHM, the Full-Width at Half Maximum and ETA, η, that was a linear combination of a Lorentzian L and a Gaussian G line-shapes with the same FWHM.
A value of η close to zero is indicating a Gaussian line shape. Intermediates values between 0 and 1 indicated a pseudo-Voigt shape with given % of G(x) and L(x). Numerical correct expressions of the integration of L(x) and G(x) functions between as a function of x = 2θ, with corresponding to the peak maximum, can be written:
Two very small diffraction peaks associated with X-ray diffusion are located at the same positions than peaks (2) and (3) and are labelled peaks (4) and (5) (
Figure 1).
UV-visible-NIR spectra were measured on a Varian 4000 spectrometer within the range 200-1400 nm. They were measured in the UV (200 to 400 nm), the visible (400 to 800 nm) and the NIR ranges inside two kinds of quartz cell, one in quartz with an optical path of 1 cm and a second with an optical path (internal) of 2 mm. Fluorescence measurements were made with a Horiba Jobin Yvon spectrometer Fluorolog®FL3-22. We have tried two kinds of fluorescence measurements. We have tried two kinds of florescence measurements, the first one with diluted solutions in ethanol and the second one directly with the concentrated initial solutions obtained by applying ultrasounds 6 min and without centrifugation.
The amount of graphene dispersed in absolute ethanol was determined by comparing the weight of an Eppendorf of 4 mL filled with several volumes of dilution (see details in the text) before and after ethanol evaporation.
Better results in fluorescence were obtained on the non-diluted solutions and for an angle between excitation light and detector set at 90° to avoid detector saturation.
Conclusions and Perspectives
Ultra-sound treatments of ethanol dispersions of a commercial graphite (GR) were very efficient to obtain reduced and “flat” graphene sheets that we have characterized by XRD, SEM, TEM. UV-visible-NIR results were complemented by fluorescence spectroscopy. For ultrasounds at a power of 500 W (used at 40% and with a frequency of 20 MHz) treatments were made with a horn directly introduced inside the solution and were performed either in a constant method or using pulses of 15s, stops of 15s, for overall times of 5- 7 min and of 2 h.
Thanks to the systematic use of centrifugation vials having the same volume (45 mL), of solvent, the same amount of commercial graphite GR, and similar centrifugation conditions, we can conclude that the graphene sheets dimension was directly affected by the time of the used ultra-sound treatment. The main dimension of the graphene particles was decreasing from larger than a micron (ultrasounds of 6 min) to smaller of 100 nm (after ultrasounds 2h). Even after 2h of ultra-sound treatment, the C-particles remained stacked, more than 5 to 25 sheets were detected.
Interesting plasmons (collective excitation of electrons, considered as a gaseous plasma) were observed after centrifugation and addition of CuCl2 3H20 and both their intensities (absorption that can be larger than 10) and their positions were affected by dilutions. The existence of a coupling between the plasmons and the vibrations of the C-lattice was demonstrated. At a high dilution to remove the plasmons, the prepared Cl/Cu/graphene solutions were very strong oxidants as demonstrated by catalytic tests performed with a dye (eosin and blue of bromophenol in water and in ethanol). In our experimental conditions, the visible signal of the dye was indeed eliminated in a few seconds with both small and large RGo particles.
In the future, more concentrated solutions will be used to preserve plasmons. A more complete study including several solvents of varied polarity and using atomic force microscopy to study, molecular interactions between Copper, RGo and added organic molecules and to study process dynamic will be proposed.
Figure 1.
Commercial sample, GR. Possible decomposition of an XRD peak into five components , and , and small added and peaks, above a not visible straight line using the program Fullprof within 2θ in the range 23 – 31°. The first very broad peak on the left is not attributed yet. and labels are used for the two intense peaks, on the right part, both having Gaussian shapes. I (Observed) in black with red spots, I (Calculated) for three peaks labelled (1) in red (2) in green and (3) in brown, and in blue: difference between I (Observed) and I (Calculated). Pink and green very are small peaks.
Figure 1.
Commercial sample, GR. Possible decomposition of an XRD peak into five components , and , and small added and peaks, above a not visible straight line using the program Fullprof within 2θ in the range 23 – 31°. The first very broad peak on the left is not attributed yet. and labels are used for the two intense peaks, on the right part, both having Gaussian shapes. I (Observed) in black with red spots, I (Calculated) for three peaks labelled (1) in red (2) in green and (3) in brown, and in blue: difference between I (Observed) and I (Calculated). Pink and green very are small peaks.
Figure 2.
SEM images of the nanoscrolls already published [
1]. Replaced by the second SEM and the images collected with an optical microscope (x 25000, scale barre 2 μm).
Figure 2.
SEM images of the nanoscrolls already published [
1]. Replaced by the second SEM and the images collected with an optical microscope (x 25000, scale barre 2 μm).
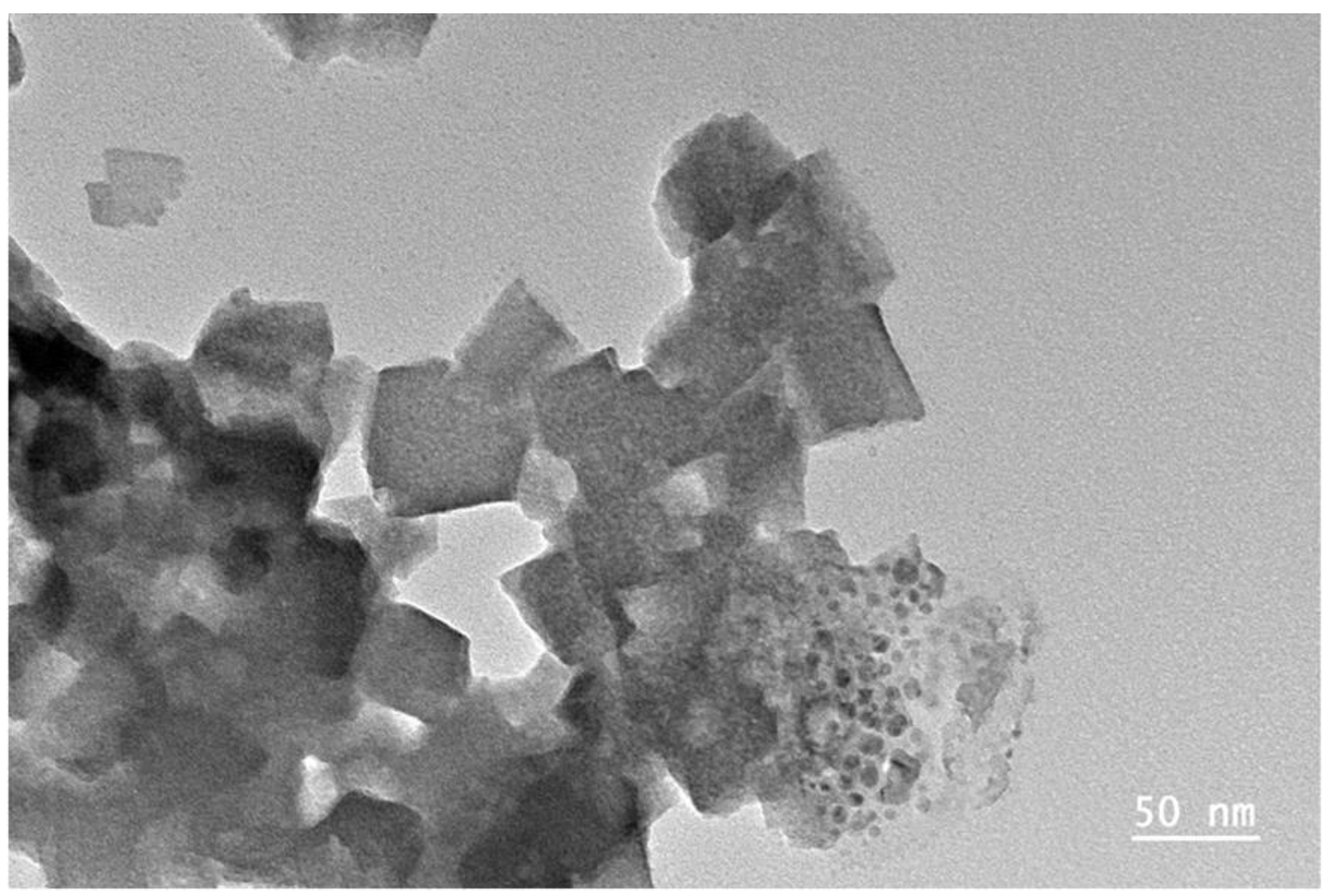
Figure 3.
Graphene sheets inside a Cu/graphene sample after 2 h of ultrasounds – mixture of graphene with several thicknesses, flats. From 2 to 20 layers, their sizes can be larger than 20 nm. (x60000, scaling barre 50 nm).
Figure 3.
Graphene sheets inside a Cu/graphene sample after 2 h of ultrasounds – mixture of graphene with several thicknesses, flats. From 2 to 20 layers, their sizes can be larger than 20 nm. (x60000, scaling barre 50 nm).
Figure 4.
Selected area electron diffraction (a) SAED on a graphene particle and (b) TEM micrograph on a sample prepared with 2 hours of ultrasounds.
Figure 4.
Selected area electron diffraction (a) SAED on a graphene particle and (b) TEM micrograph on a sample prepared with 2 hours of ultrasounds.
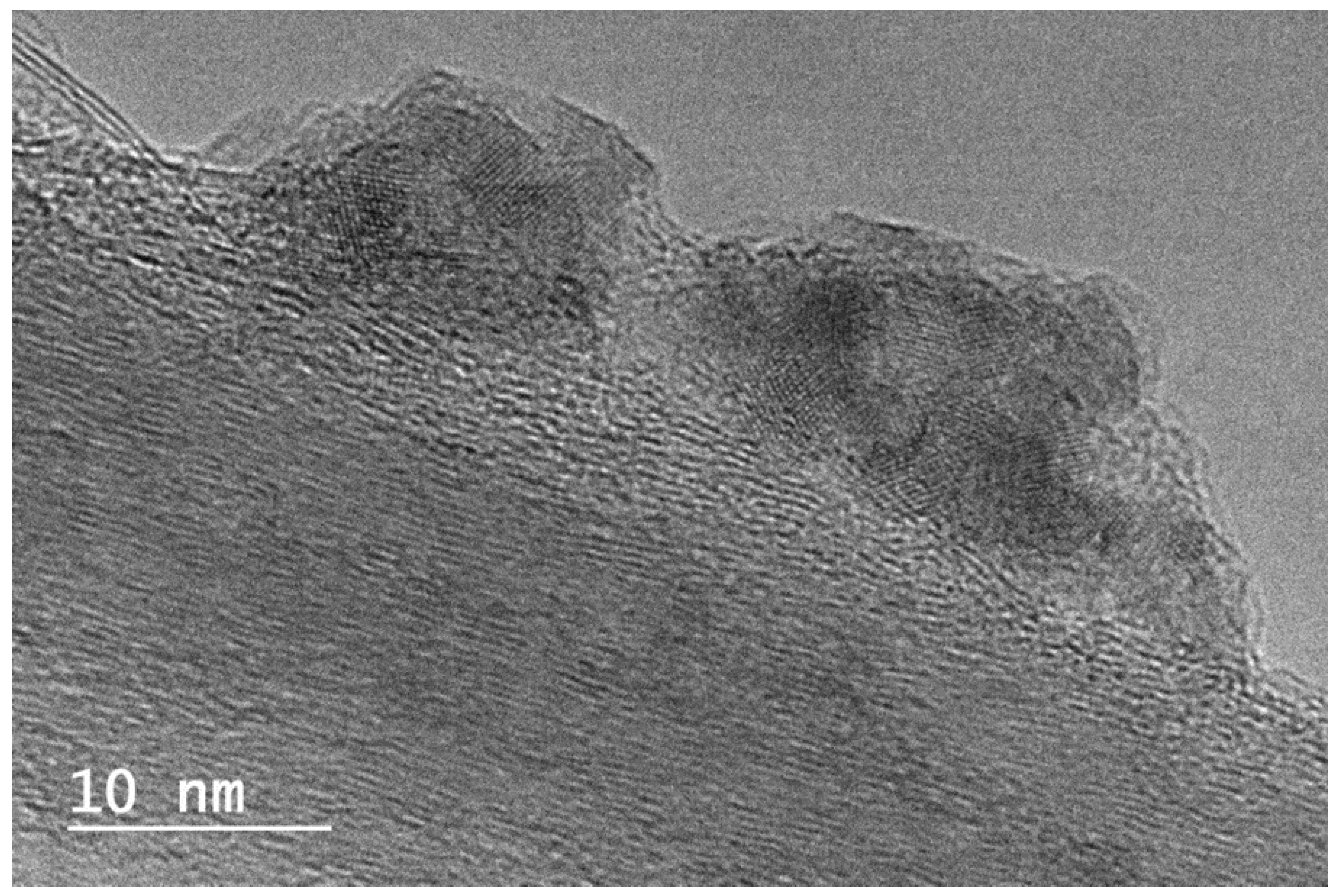
Figure 5.
Details about copper oxide nanoparticles, external and covered by carbon layers. FFT made on external oxide particles (x500000, scaling barre 10 nm).
Figure 5.
Details about copper oxide nanoparticles, external and covered by carbon layers. FFT made on external oxide particles (x500000, scaling barre 10 nm).
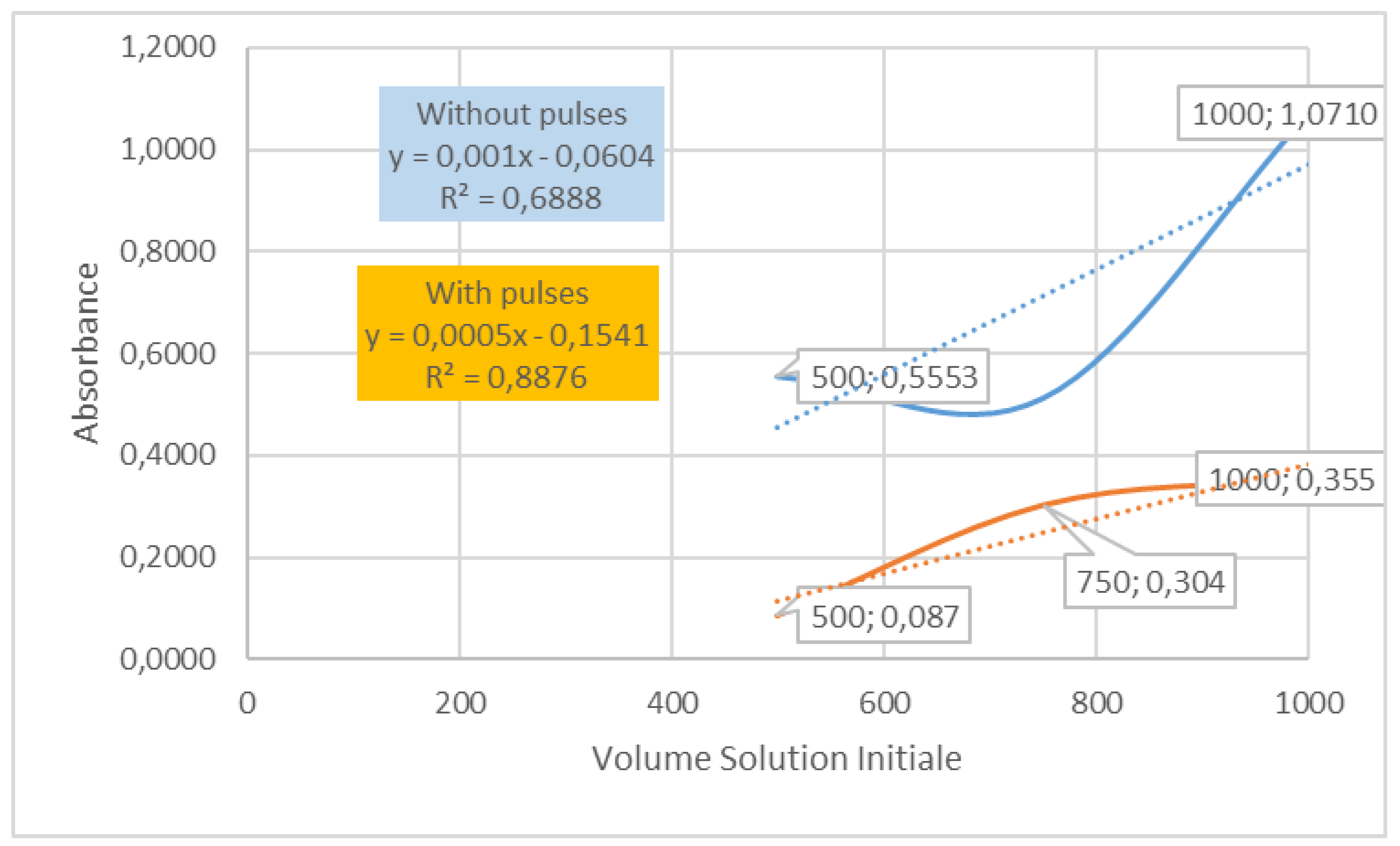
Figure 6.
UV visible results, absorbance collected on dispersed graphene diluted in absolute ethanol: 1000, 750 and 500 μl diluted inside 3.00, 3.25 and 3.50 ml (cm3) of ethanol to obtain a total volume of 4 ml, studied in a one cm cell (in quartz) after ultrasounds 6 min, with and without pulses of 15s and stops of 15s and centrifugation at 5000 rpm 12 min.
Figure 6.
UV visible results, absorbance collected on dispersed graphene diluted in absolute ethanol: 1000, 750 and 500 μl diluted inside 3.00, 3.25 and 3.50 ml (cm3) of ethanol to obtain a total volume of 4 ml, studied in a one cm cell (in quartz) after ultrasounds 6 min, with and without pulses of 15s and stops of 15s and centrifugation at 5000 rpm 12 min.
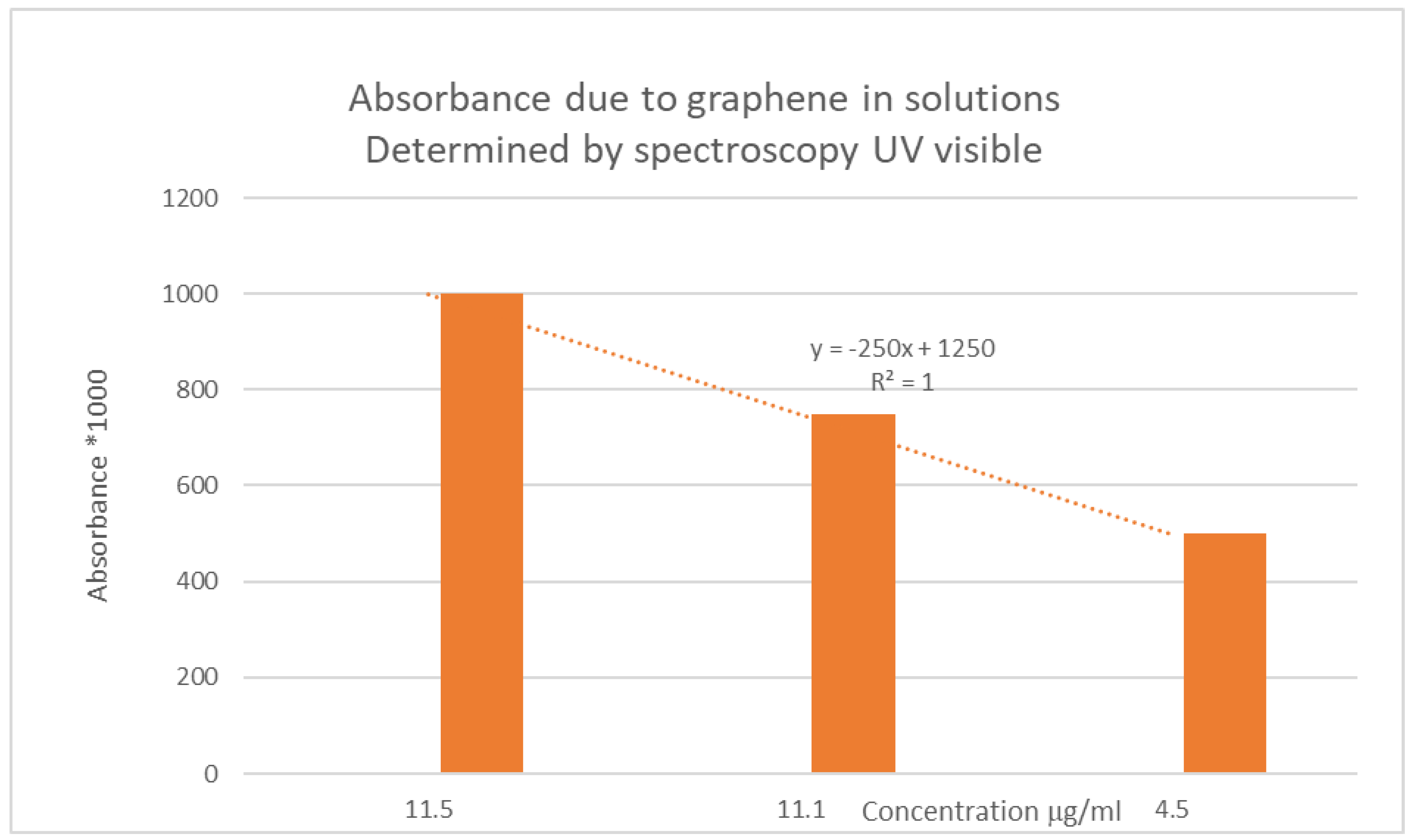
Figure 7.
Absorbance measured as a function of the composition of the studied solution expressed in μg by ml.
Figure 7.
Absorbance measured as a function of the composition of the studied solution expressed in μg by ml.
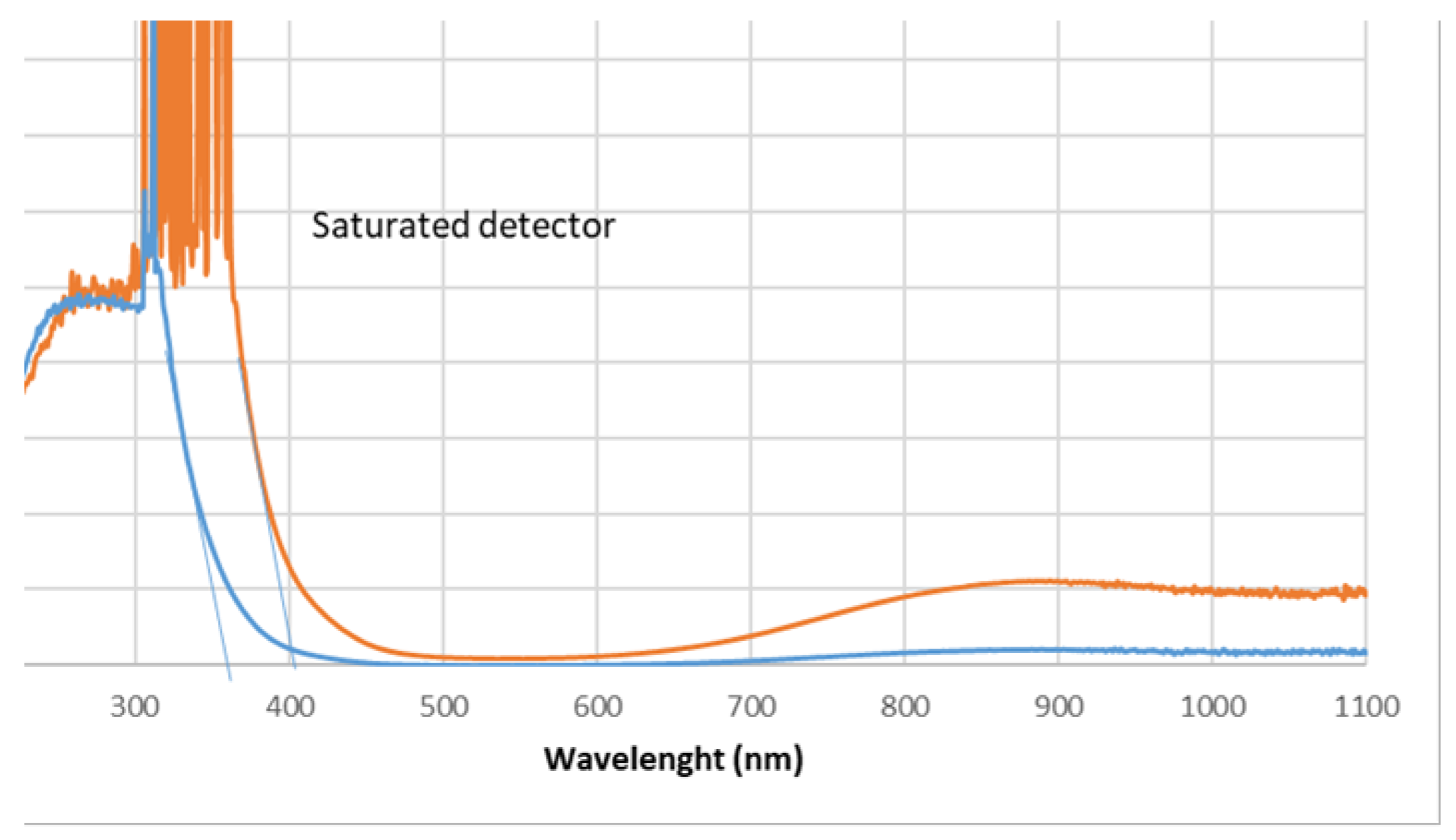
Figure 8.
Influence of dilution by ethanol on UV visible spectra of “green” liquors of Cu/Graphene/Ethanol:.
Figure 8.
Influence of dilution by ethanol on UV visible spectra of “green” liquors of Cu/Graphene/Ethanol:.
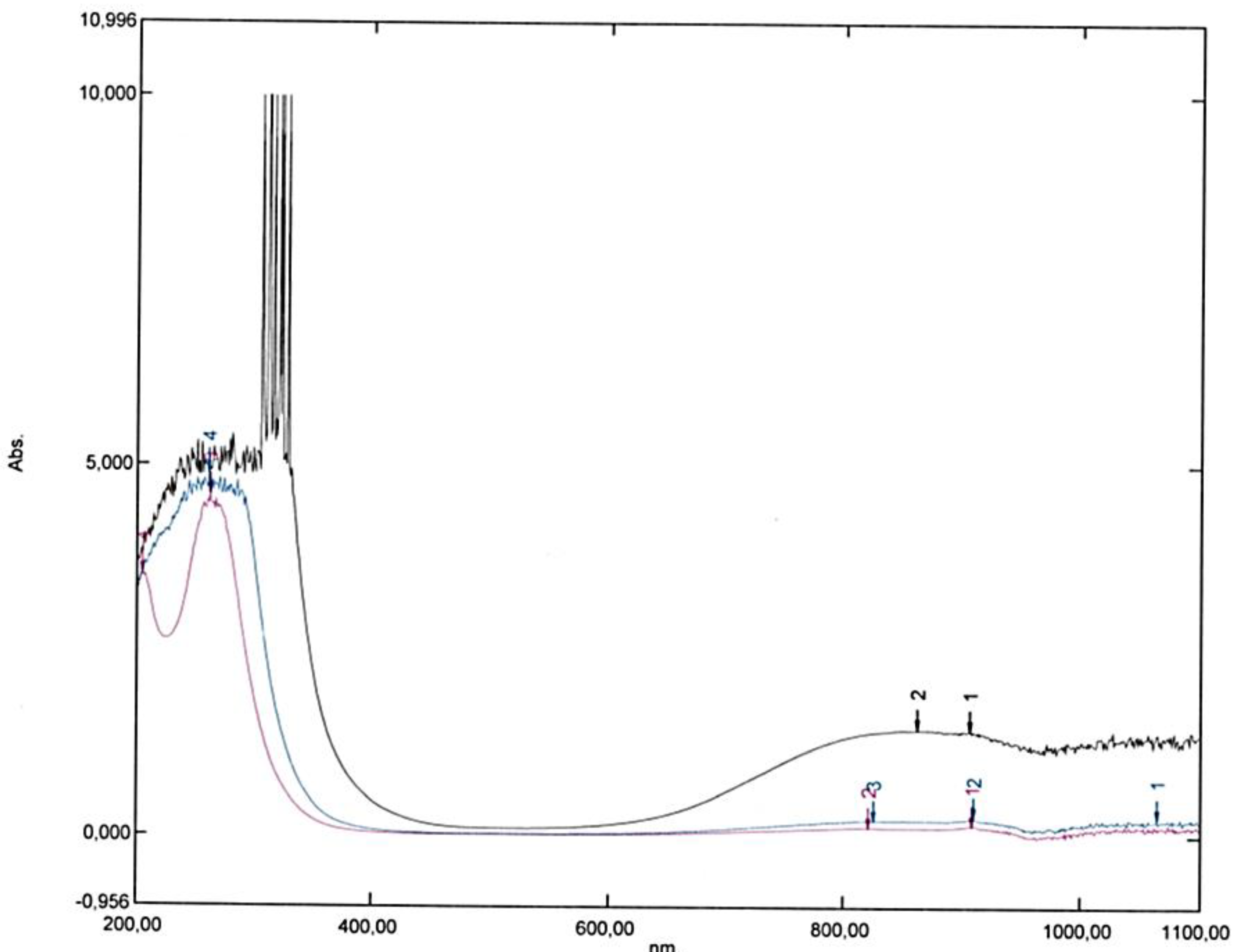
Figure 9.
Influence of the optical path of UV visible cells on plasmons coupling with the others vibrations: C-C vibrations on the left (from above to below 400 nm) and [Cu Ethanol2(H2O)4]2+ complex (864 nm instead of 800 nm for the hexa-aqua complex), orange in a cell of 1 cm and blue in a cell of 0.2 cm (internal).
Figure 9.
Influence of the optical path of UV visible cells on plasmons coupling with the others vibrations: C-C vibrations on the left (from above to below 400 nm) and [Cu Ethanol2(H2O)4]2+ complex (864 nm instead of 800 nm for the hexa-aqua complex), orange in a cell of 1 cm and blue in a cell of 0.2 cm (internal).
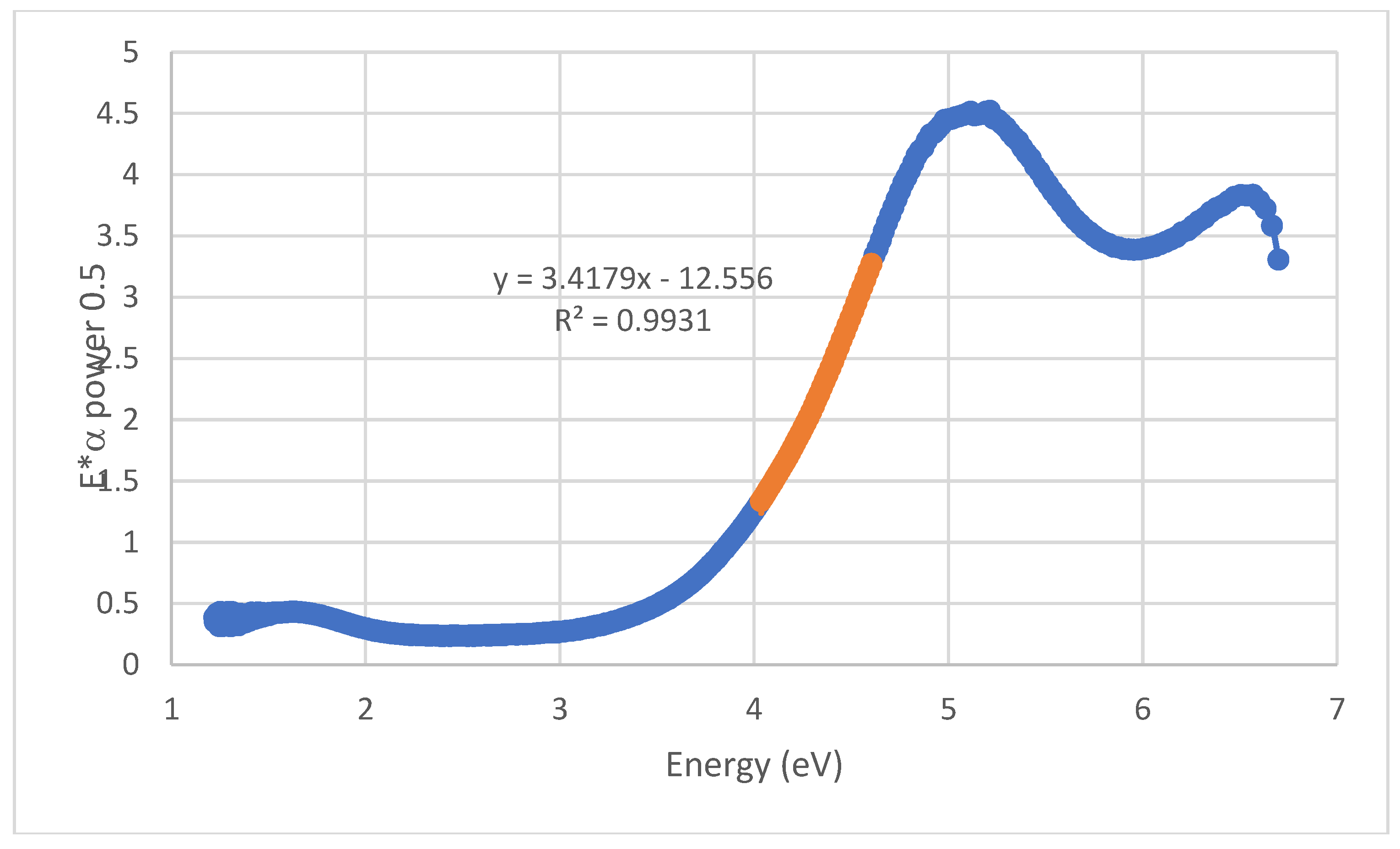
Figure 10.
Associated TAUC plot for an indirect semi-conductor, obtained after 6 min of ultra-sounds treatment with pulses. The value of the band gap is calculated by the extrapolation of the straight line in orange, by dividing its origin (its common point with the Ox axis) by its slope. Here, a value of 3.67 eV is obtained.
Figure 10.
Associated TAUC plot for an indirect semi-conductor, obtained after 6 min of ultra-sounds treatment with pulses. The value of the band gap is calculated by the extrapolation of the straight line in orange, by dividing its origin (its common point with the Ox axis) by its slope. Here, a value of 3.67 eV is obtained.
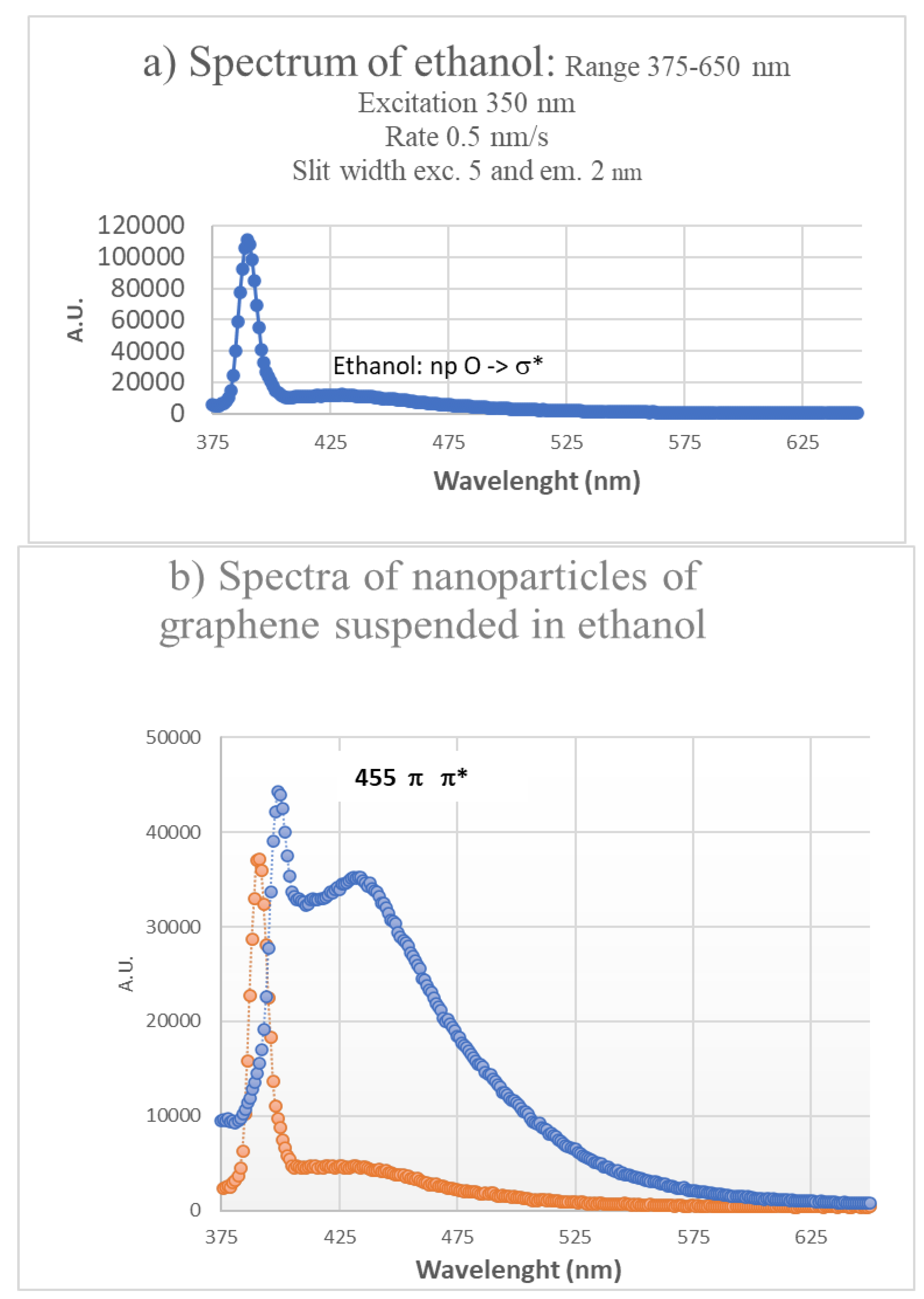
Figure 11.
Fluorescence spectra of ethanol and of two solutions of graphene suspended in ethanol prepared respectively with 6 min and with 2h of the ultrasound treatment (excitation at 358 nm). No centrifugation.
Figure 11.
Fluorescence spectra of ethanol and of two solutions of graphene suspended in ethanol prepared respectively with 6 min and with 2h of the ultrasound treatment (excitation at 358 nm). No centrifugation.