Submitted:
29 May 2024
Posted:
30 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
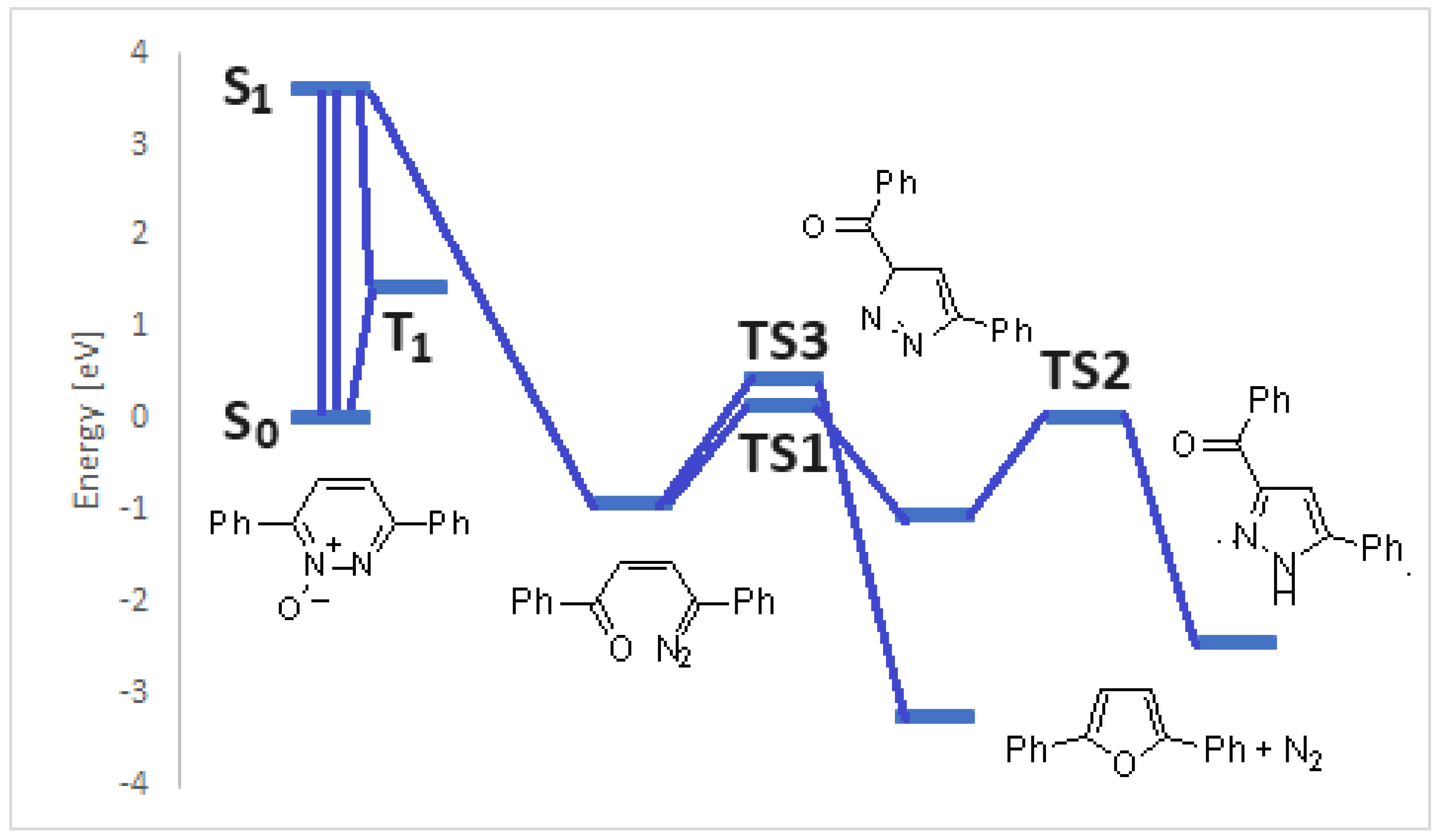
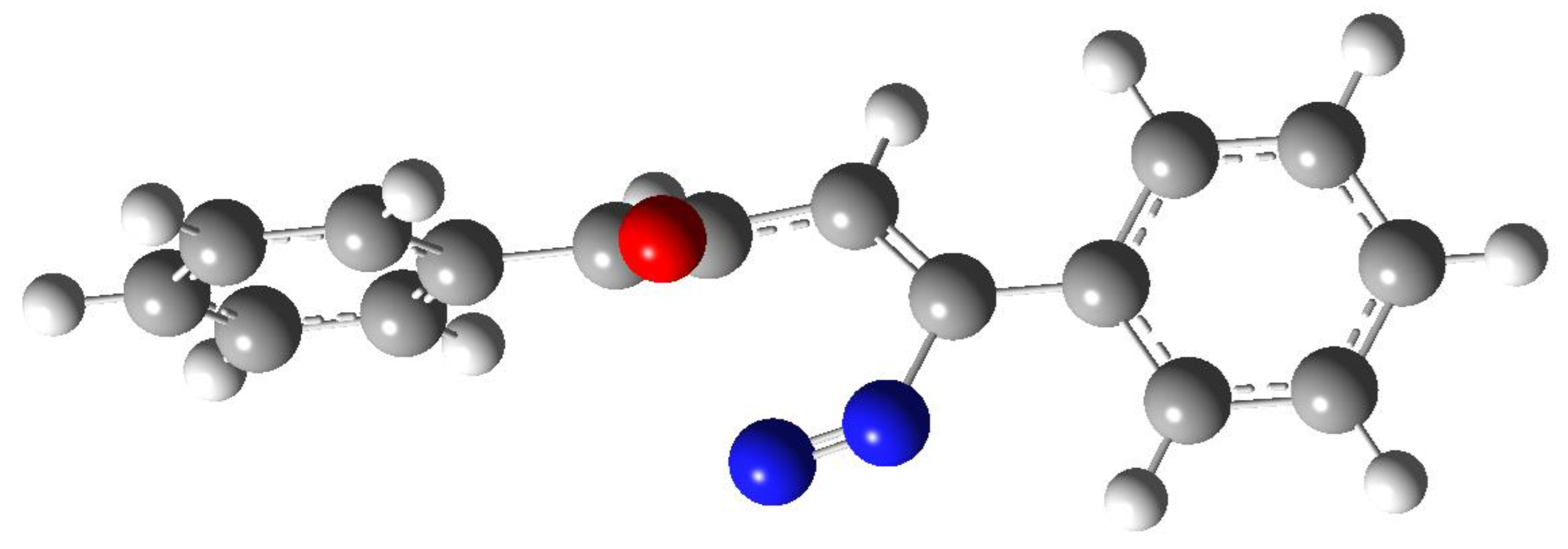
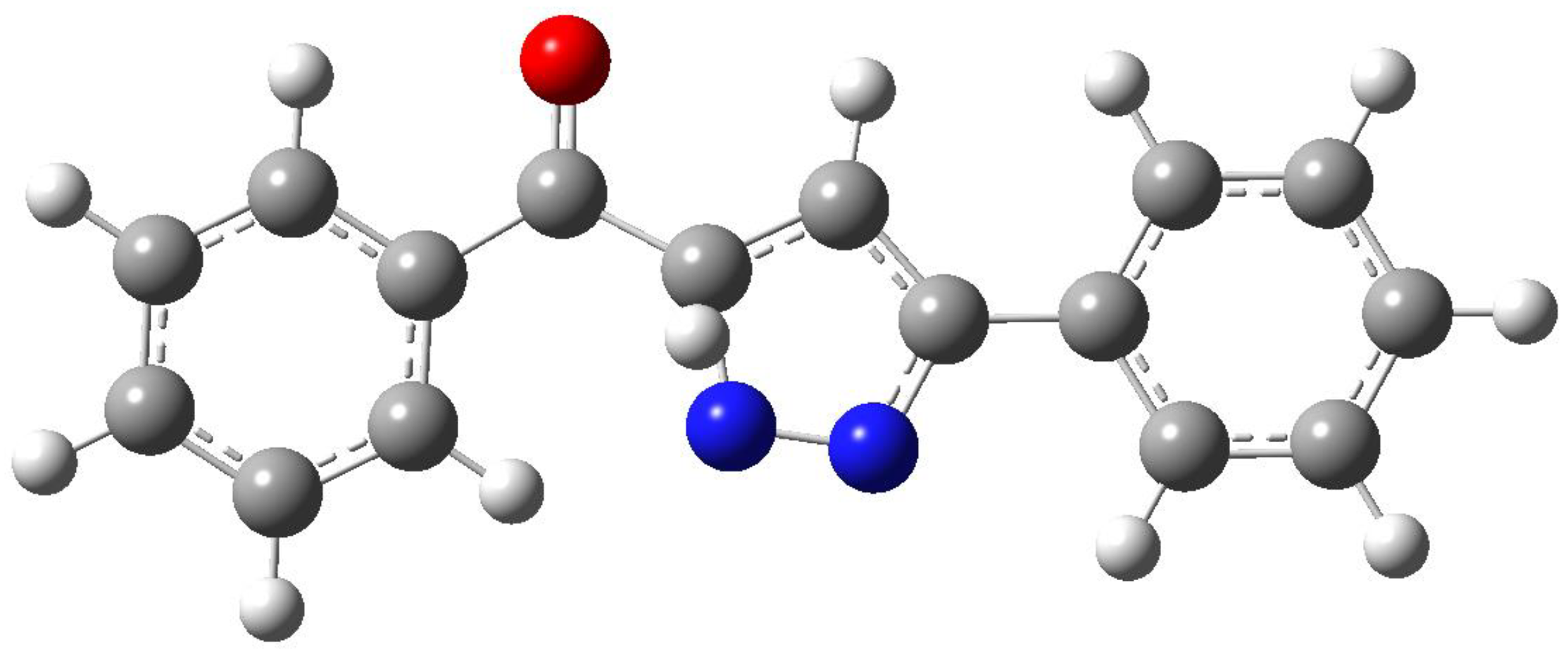
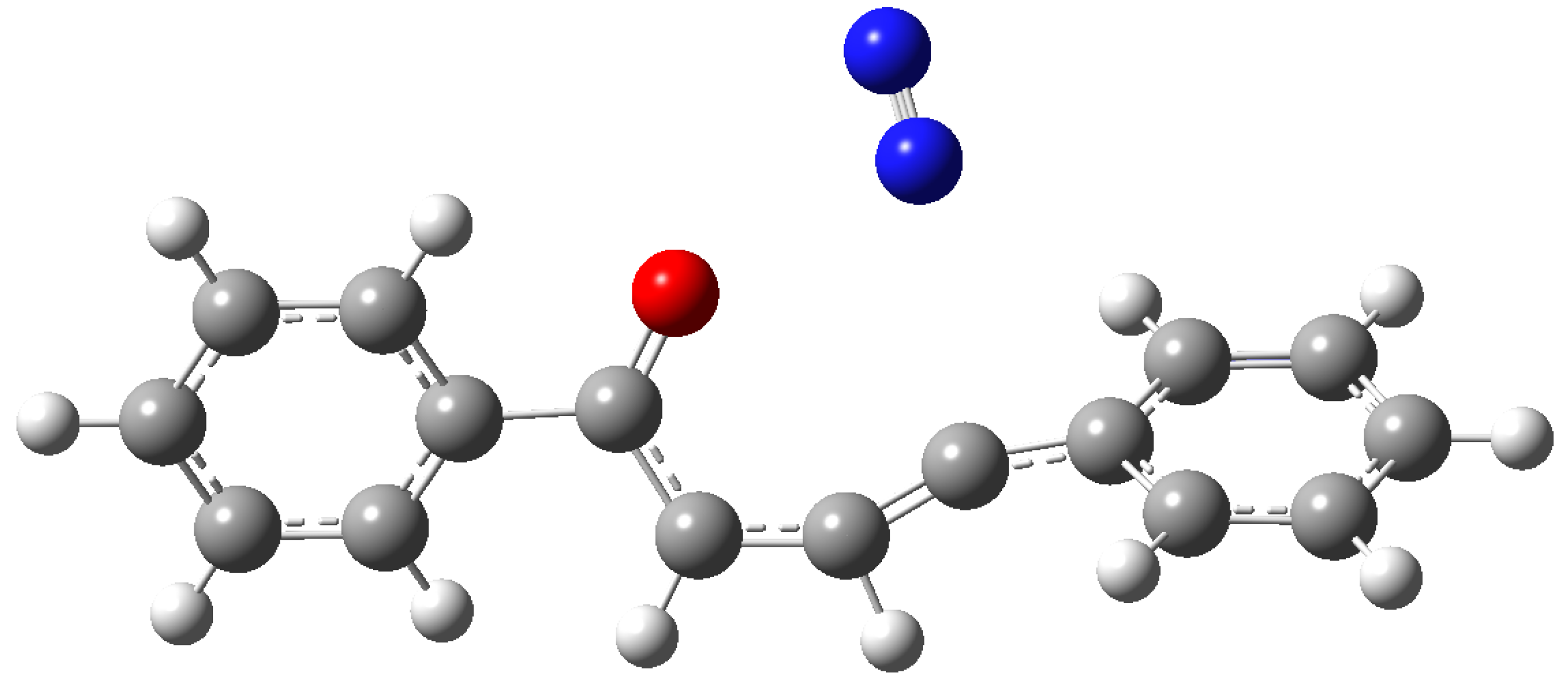
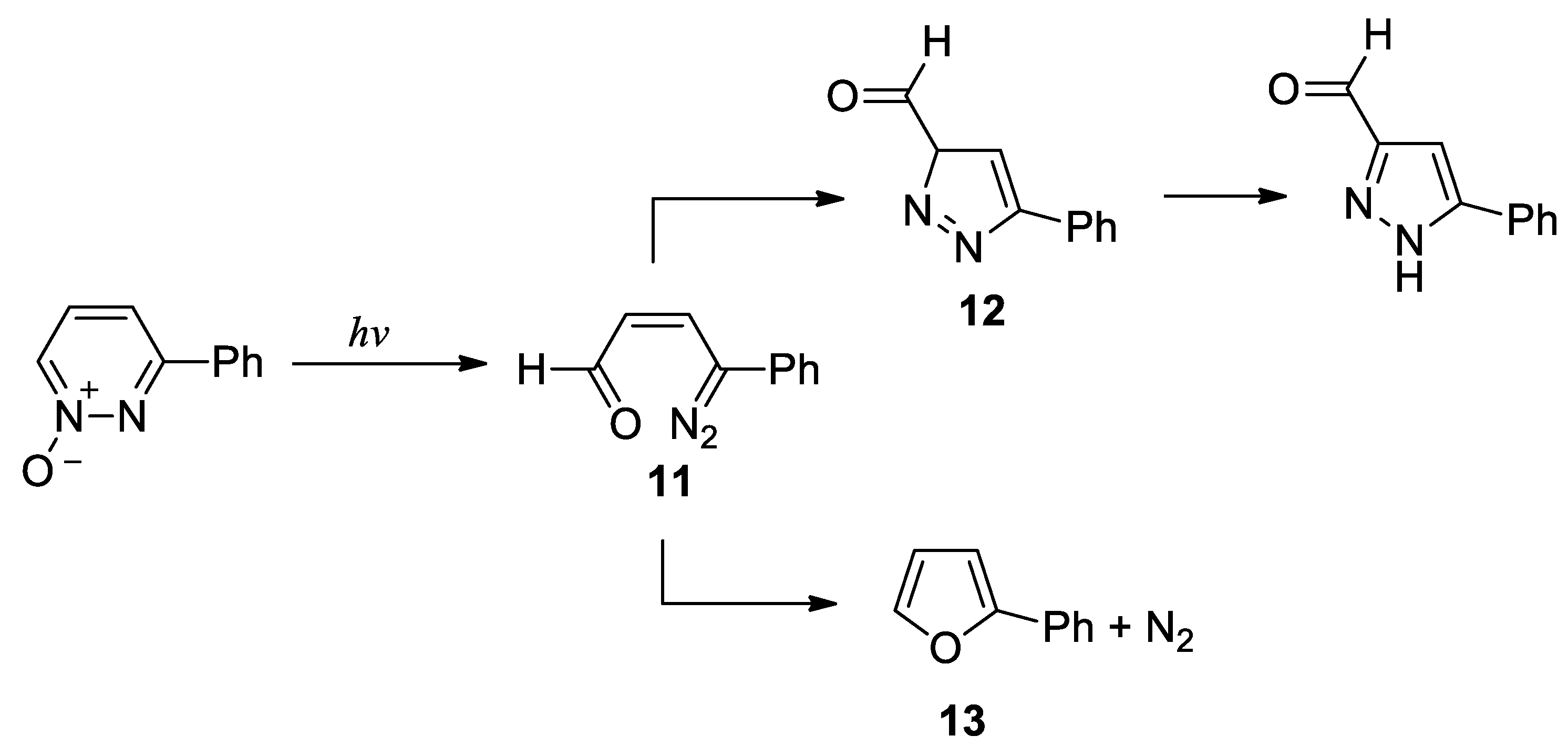
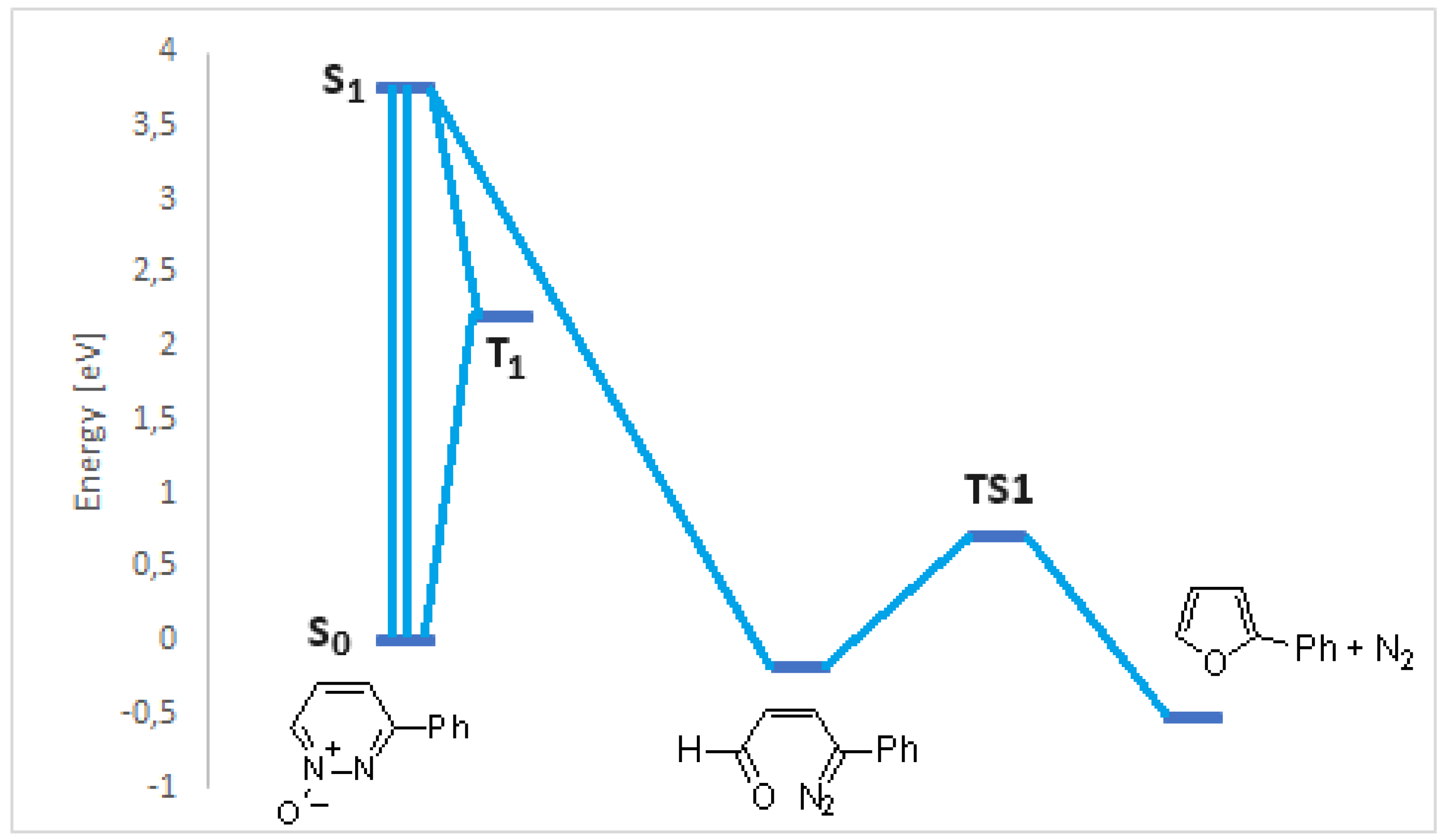
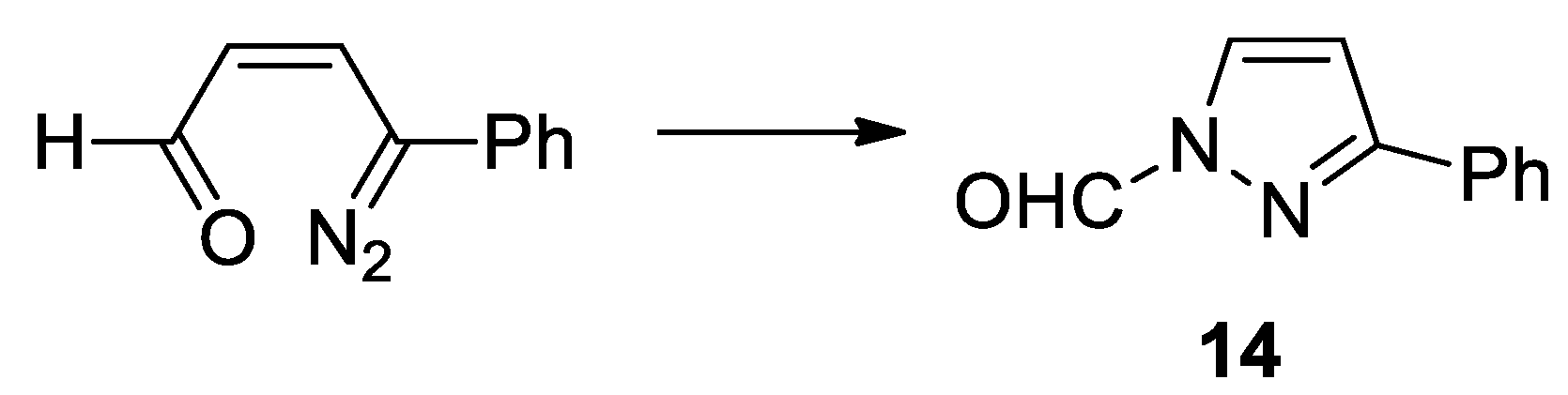
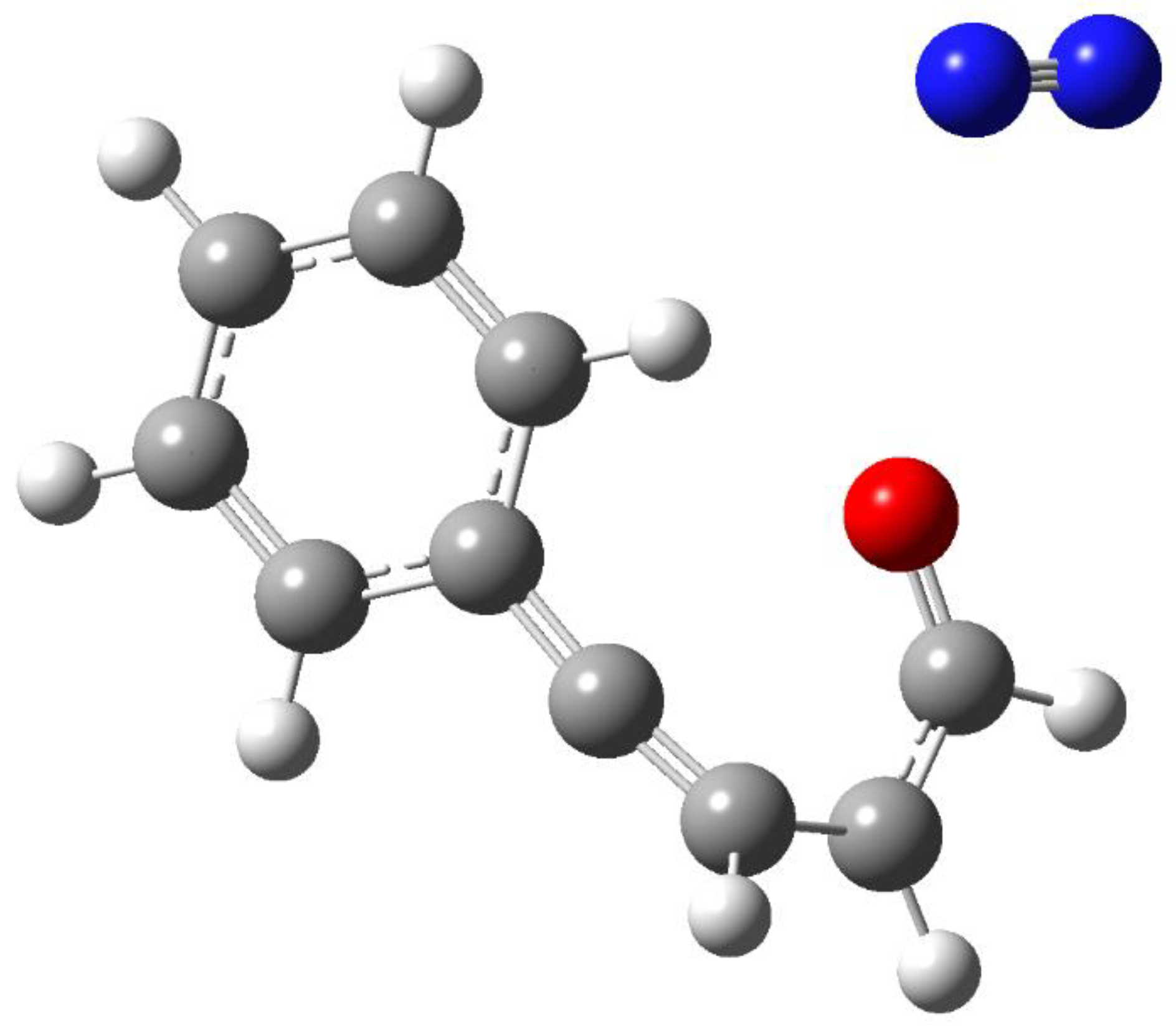
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Braslavsky, S.; Heicklen, J. The gas-phase thermal and photochemical decomposition of heterocyclic compounds containing nitrogen, oxygen, or sulfur. Chem. Rev. 1977, 77, 473–511. [Google Scholar] [CrossRef]
- Lablache-Combier, A. Photorearrangments of nitrogen-containing arenes. In CRC Handbook of Photochemistry and Photobiology; Horspool, W.M., Song, P.-S., Eds.; CRC Press: Boca Raton, NJ, USA, 1995; pp. 1063–1120. [Google Scholar]
- Pavlik, J. WPhotoisomerization of some nitrogen-containing heteroaromatic compounds. In CRC Handbook of Organic Photochemistry and Photobiology; Horspool, W., Lenci, F., Eds.; CRC Press: Boca Raton, NJ, USA, 2004; pp. 97–1. [Google Scholar]
- Pavlik, J. W. Photochemistry of thiazoles, isothiazoles, and 1,2,4-thiadiazoles. In CRC Handbook of organic photochemistry and photobiology, Horspool, W.; Lenci, F., Eds. CRC Press: Boca Raton. 2004, pp. 98–1,98-14.
- Lefebvre, C.; Fortier, L.; Hoffmann, N. Photochemical rearrangements in heterocyclic chemistry. Eur. J. Org. Chem. 2020, 1393–1404. [Google Scholar] [CrossRef]
- Wilzbach, K. E.; Rausch D., J. Photochemistry of nitrogen heterocycles. Dewar pyridine and its intermediacy in photoreduction and photohydration of pyridine. J. Am. Chem. Soc. 1970, 92, 2178–2179. [Google Scholar] [CrossRef]
- Chapman, O. L.; McIntosh, C. L.; Pacansky, J. Photochemical transformations. XLVIII. Cyclobutadiene. J. Am. Chem. Soc. 1973, 95, 614–617. [Google Scholar] [CrossRef]
- Ratajczak, E.; Sztuba, B. The gas phase photolysis of pentafluoropyridine. J. Photochem. 1980, 13, 233–242. [Google Scholar] [CrossRef]
- Yamazaki, I.; Murao, T.; Yamanaka, T.; Yoshihara, K. Intramolecular electronic relaxation and photoisomerization processes in the isolated azabenzene molecules pyridine, pyrazine and pyrimidine. Farad. Discuss. Chem. Soc. 1983, 75, 395–405. [Google Scholar] [CrossRef]
- Vysotskii, Y. S.; Sivyakova, L. N. Quantum-chemical interpretation of recyclization reactions. 10. Photoisomerization of six-membered heterocycles. Khim. Geterosikl. Soedin. 1986, 22, 357–363. [Google Scholar] [CrossRef]
- Johnstone, D. E.; Sodeau, J. R. Matrix-controlled photochemistry of benzene and pyridine. J. Phys. Chem. 1991, 95, 165–169. [Google Scholar] [CrossRef]
- Sobolewski, A. L.; Domcke, W. Photophysically relevant potential energy functions of low-lying singlet states of benzene, pyridine and pyrazine: an ab initio study. Chem. Phys. Lett. 1991, 180, 381–386. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, X.; Pulay, P. Geometries, force fields, and vibrational assignments of Dewar benzene and Dewar pyridine. J. Phys. Chem. 1992, 96, 3669–3674. [Google Scholar] [CrossRef]
- Kudoth, S.; Takayanagi, M.; Nakata, M. Dewar pyridine studied by matrix isolation infrared spectroscopy and DFT calculation. J. Photochem. Photobiol. A: Chem. 1999, 123, 25–30. [Google Scholar] [CrossRef]
- Wang, B.; Liu, B.; Wang, Y.; Wang, L. Ultrafast dynamics of pyridine in “channel three” region. Int. J. Mass Spectrom. 2010, 289, 92–97. [Google Scholar] [CrossRef]
- Su, M. -D. Model Study on the Pyridine−Dewar pyridine and some related photoisomerization reactions. J. Phys. Chem. A 2007, 111, 971–975. [Google Scholar] [CrossRef]
- Chachisvilis, M.; Zewail, A. H. Femtosecond dynamics of pyridine in the condensed phase: valence isomerization by conical intersections. J. Phys. Chem. A 1999, 103, 7408–7418. [Google Scholar] [CrossRef]
- Freytag, H.; Neudert, W. Einwirkung ultravioletter Strahlen auf Pyridin. (I. Mitteilung). Ein neuer Nachweis einiger primärer aromatischer Amine und des Pyridins. J. Prakt. Chem. 1932, 135, 15–35. [Google Scholar] [CrossRef]
- Freytag, H.; Hlučka, F. Einwirkung ultravioletter Strahlen auf Pyridin. III. Mitteilung: Über Photopyridinbildung im Spektrum. J. Prakt. Chem. 1933, 136, 288–292. [Google Scholar] [CrossRef]
- Freytag, H. Einwirkung ultravioletter Strahlen auf Pyridin. V. Mitteilung: Über den qualitativen Nachweis weiterer primärer aromatischer Amine, über das Verhalten von Pyridinderivaten im UV-Licht und über die Natur des „Photopyridins”︁. J. Prakt. Chem. 1934, 139, 44–62. [Google Scholar] [CrossRef]
- Joussot-Dubien, J.; Houdard, J. Reversible photolysis of pyridine in aqueous solution. Tetrahedron Lett. 1967, 4389–4391. [Google Scholar] [CrossRef]
- Joussot-Dubien, J.; Houdard-Pereyre, J. Photolyse de la pyridine en solution aqueuse. Bull. Soc. Chim. Fr. 1969, 2619–2623. [Google Scholar]
- Destexhe, A.; Smets, J.; Adamowicz, L.; Maes, G. Matrix isolation FT-IR studies and ab initio calculations of hydrogen-bonded complexes of molecules modeling cytosine or isocytosine tautomers. 1. Pyridine and pyrimidine complexes with water in argon matrixes. J. Phys. Chem. 1994, 98, 1506–1514. [Google Scholar] [CrossRef]
- Dkhissi, A.; Adamowicz, L.; Maes, G. Density Functional Theory Study of the Hydrogen-Bonded Pyridine−H2O Complex: A Comparison with RHF and MP2 Methods and with Experimental Data. J. Phys. Chem. A 2000, 104, 2112–2119. [Google Scholar] [CrossRef]
- Reimers, J. R.; Cai, Z. -L. Hydrogen bonding and reactivity of water to azines in their S1 (n,π*) electronic excited states in the gas phase and in solution. Phys. Chem. Chem. Phys. 2012, 14, 8791–8802. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sobolewski, A. L.; Borrelli, R.; Domcke, W. Computational investigation of the photoinduced homolytic dissociation of water in the pyridine–water complex. Phys. Chem. Chem. Phys. 2013, 15, 5957–5966. [Google Scholar] [CrossRef] [PubMed]
- Esteves-López, N.; Coussan, S.; Dedonder-Lardeux, C.; Jouvet, C. Photoinduced water splitting in pyridine water clusters. Phys. Chem. Chem. Phys. 2016, 18, 25637–25644. [Google Scholar] [CrossRef] [PubMed]
- Esteves-López, N.; Coussan, S. UV photochemistry of pyridine-water and pyridine-ammonia complexes trapped in cryogenic matrices. J. Mol. Struct. 2018, 1172, 65–73. [Google Scholar] [CrossRef]
- Kellogg, R. M.; van Bergen, T. J.; Wynberg, H. Photochemical ring contraction, reduction, and solvent addition in pyridines. Tetrahedron Lett. 1969, 5211–5214. [Google Scholar] [CrossRef]
- Van Bergen, T. J.; Kellogg, R. M. Photochemistry of 3,5-dicarboalkoxypyridines. Reduction and rearrangement. J. Am. Chem. Soc. 1972, 94, 8451–8471. [Google Scholar] [CrossRef]
- Caplain, S.; Catteau, J. P.; Lablache-Combier, A. Liquid-phase photochemistry of pyridine and 2- and 4-picoline. J. Chem. Soc. D, Chem. Commun, 1475. [Google Scholar] [CrossRef]
- Barlow, M. G.; Dingwall, J. G.; Haszeldine, R. N. Valence-bond isomers of heterocyclic compounds: isomers of pentakis(pentafluoroethyl)pyridine. J. Chem. Soc. D, Chem. Commun. [CrossRef]
- Mathias, E.; Heicklen, J. The gas phase photolysis of pyridine. Mol. Photochem. 1972, 4, 483–500. [Google Scholar]
- Caplain, S.; Lablache-Combier, A. Gas-phase photochemistry of picolines and lutidines. J. Chem. Soc. D, Chem. Commun. 1248. [Google Scholar] [CrossRef]
- Roebke, W. Gas-phase photolysis of 2-picoline. J. Phys. Chem. 1970, 74, 4198–4203. [Google Scholar] [CrossRef]
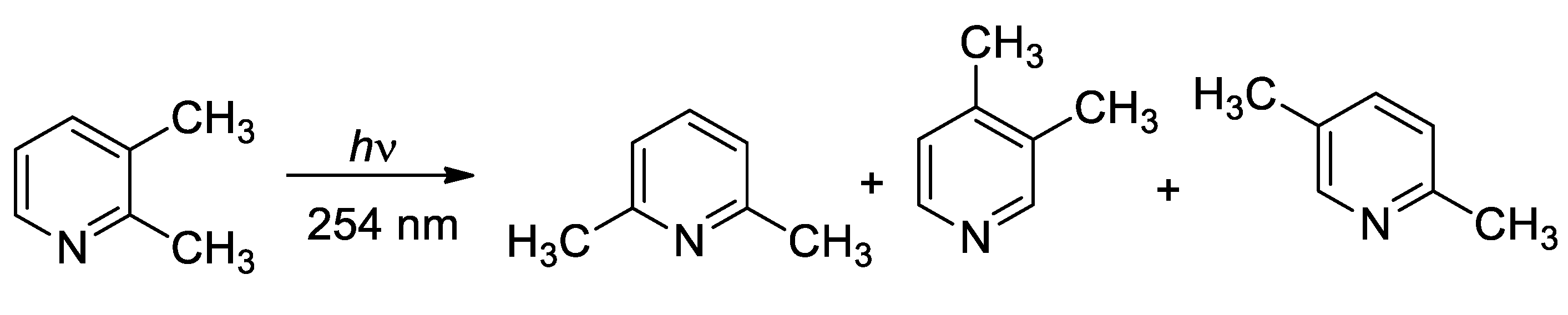
- Pavlik J., W.; Kebede, N.; Thompson, M.; Colin Day, A.; Barltrop, J. A. Vapor-phase photochemistry of dimethylpyridines. J. Am. Chem. Soc. 1999, 121, 5666–5673. [Google Scholar] [CrossRef]
- Pavlik, J. W.; Laohhasurayotin, S. The photochemistry of 3,4,5-trideuteriopyridine. Tetrahedron Lett. 2003, 44, 8109–8111. [Google Scholar] [CrossRef]
- Ogata, Y.; Takagi, K. Photoisomerization of 2-pyridylacetonitrile to anthranilonitrile. J. Am. Chem. Soc. 1974, 96, 5933–5934. [Google Scholar] [CrossRef]
- D’Auria, M. On the photochemical reaction of pyridinium salts with nucleophiles, Photochem. Photobiol. Sci. 2021, 20, 923–926. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M. The photoreaction of pyrilium cation with water: a DFT study. Lett. Org. Chem. 2022, 19, 739–742. [Google Scholar] [CrossRef]
- D’Auria, M. A theoretical study on the photochemical isomerization of 2,6-dimethylpyrazine. Organics 2022, 3, 95–101. [Google Scholar] [CrossRef]
- Gaussian 09, Revision A.1, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
- Parr, R. G.; Yang, W. Density Functional Theory of Atoms and Molecules, Oxford University Press: Oxford, UK, 1989.
- Becke, A. D. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Peng, C.; Schlegel, H. B. Combining Synchronous Transit and Quasi-Newton Methods to Find Transition State. Israel J. Chem. 1993, 33, 449–454. [Google Scholar] [CrossRef]
- Peng, C.; Ayala, P. Y.; Schlegel, H. B.; Frisch, M. J. Using Redundant Internal Coordinates to Optimize Equilibrium Geometries and Transition States. J. Comp. Chem. 1996, 17, 49–56. [Google Scholar] [CrossRef]
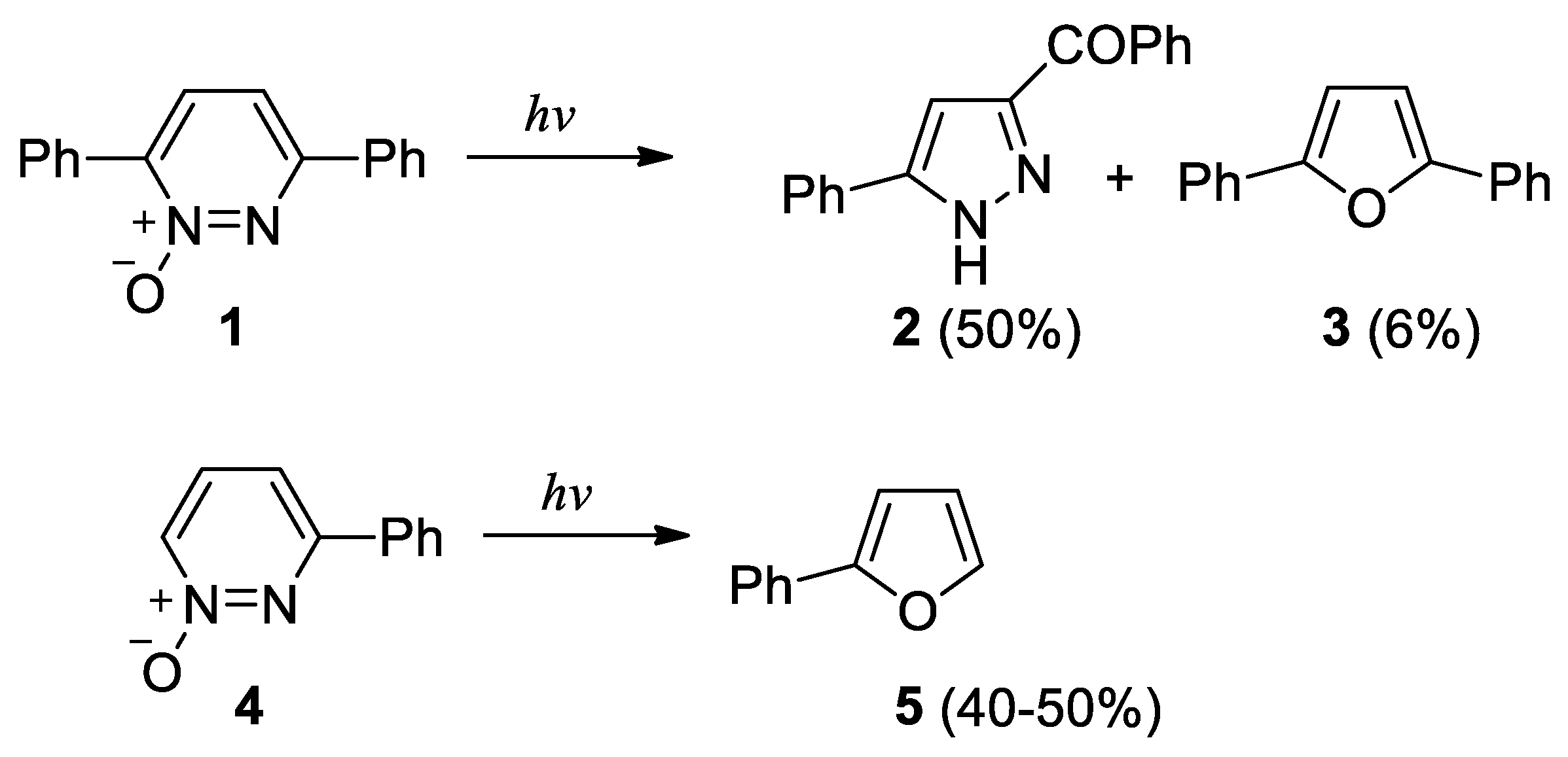
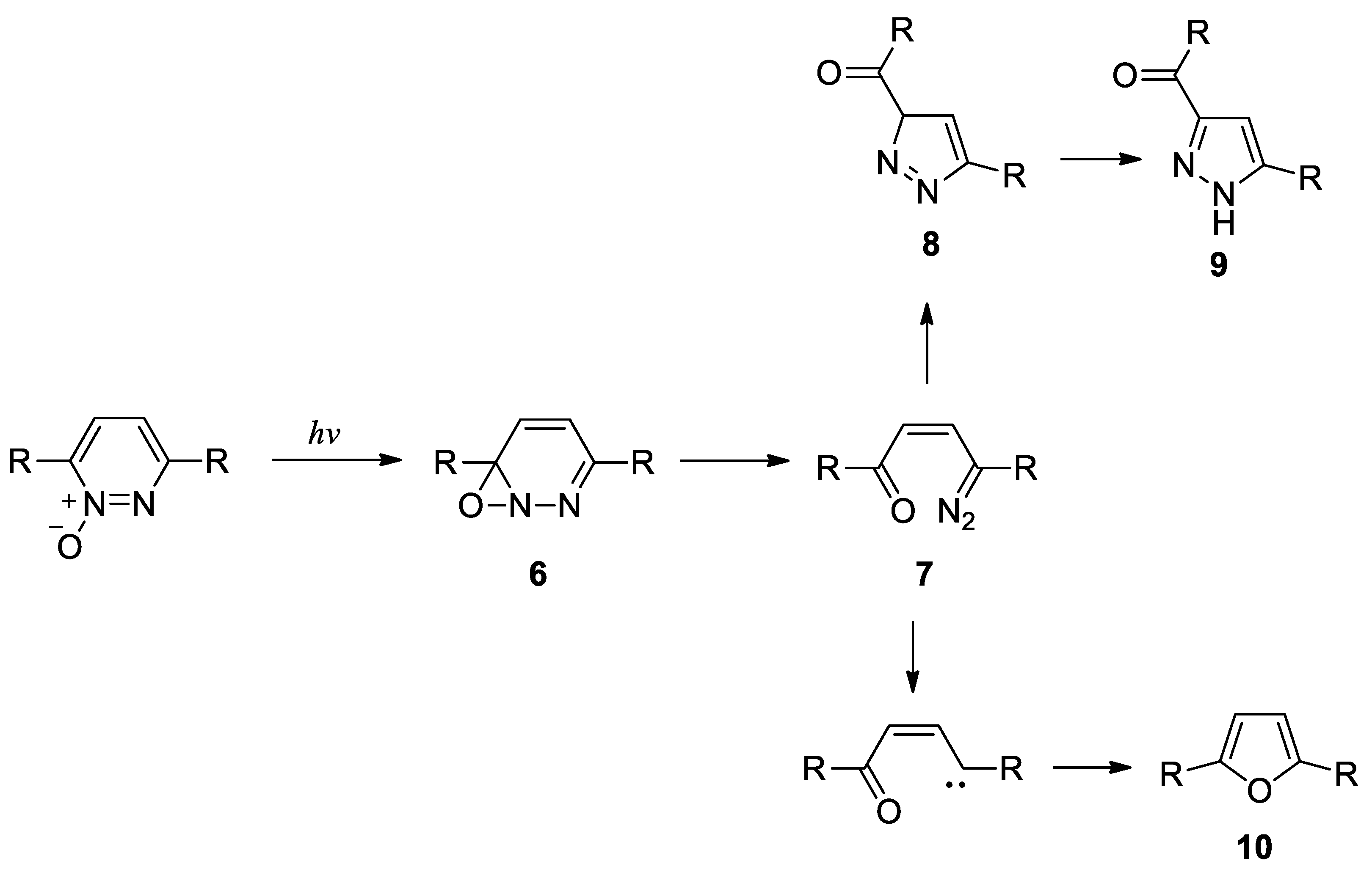
- Kumler, P. L.; Buchardt, O. The photolysis of 3,6-diphenylpyridazine N-oxide. Detection of a transient diazo compound. J. Am. Chem. Soc. 1968, 90, 3640–3641. [Google Scholar] [CrossRef]
- Buchardt, O. The photolysis of 1,4-diphenylphthalazine N-oxide to 1,3-diphenylisobenzofuran. Tetrahedron Lett. 1968, 1911–1912. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Arai, H.; Igeta, H. Photochemistry. III. The photolysis of 3,4,5,6-tetraphenylpyridazine N.oxides. Tetrahedron Lett. 2582. [Google Scholar]
- Tsuchiya, T.; Arai, H.; Igeta, H. Photolysis of pyridazine N-oxides; formation of cyclopropenyl ketones. J. Chem. Soc. Chem. Commun. 1972, 550–551. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Arai, H.; Tonami, T.; Igeta, H. Photochemistry. VII. Photolyses of polyphenyl pyridazine N-oxides. Chem. Pharm. Bull. 1972, 20, 300–303. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Arai, H.; Igeta, H. Photochemistry – IX. Formation of cyclopropenyl ketones and furans from pyridazine N-oxides by irradiation. Tetrahedron 1973, 29, 2747–2751. [Google Scholar] [CrossRef]
- Tomer, K. B.; Harrit, N.; Rosenthal, I.; Buchardt, O.; Kumler, P. L.; Creed, D. Photochemical behavior of aromatic 1,2-diazine N-oxides. J. Am. Chem. Soc. 1973, 95, 7402–7406. [Google Scholar] [CrossRef]
- Portillo, M.; Maxwell, M. A.; Frederich J., H. Synthesis of nitrogen heterocycles via photochemical ring opening of pyridazine N-oxides. Org. Lett. 2016, 18, 5142–5145. [Google Scholar] [CrossRef]
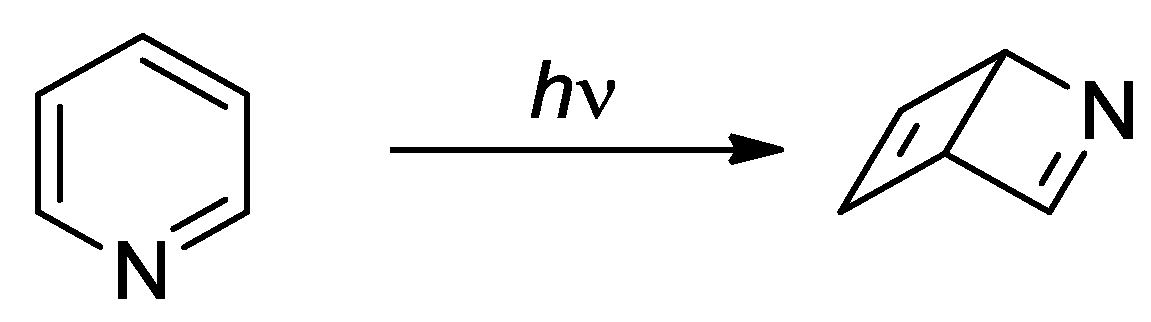
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



