Introduction
The second most common cause of irreversible blindness in the world is glaucoma [
1], with an elevated intraocular pressure (IOP) the most important risk factor for developing the condition. It has been reported that the speed of the glaucoma progression can be decreased by lowering the IOP [
2]. In order to reduce the IOP, the goal of treatment strategies is to decrease the aqueous humor production or increase the outflow. Prostaglandin analogs, β- adrenoreceptor blockers, carbonic anhydrase inhibitors (CAIs), α
2-adrenergic agonists, and Rho-associated coiled-coil containing protein kinase (Rho kinase, ROCK) inhibitors are the currently available IOP-lowering medications. As insufficient outcomes have been reported after the use of monotherapy treatments, a combination therapy has been subsequently used and found to lead to a successful management of glaucoma [
3]. However, as compared to the administration of unfixed therapy, Barnebey et al.[
4] previously reported that glaucoma patients exhibited both improvement and long-term adherence when using a fixed-combination therapy.
One novel IOP-lowering therapy is the use of ripasudil/brimonidine fixed-combination ophthalmic suspension (RBFC), for which there is a combination of the Rho kinase inhibitor ripasudil and the α
2-adrenergic agonist brimonidine. RBFC which is the second type of fixed-combination IOP-lowering medications without β-adrenoreceptor blockers has been available only in Japan since December 2022. Presently, prostaglandin analogues, β-adrenoreceptor blockers or CAIs are currently the fixed-dose combination that is commonly used to treat glaucoma. However, it is not recommended that a multiple fixed-dose combination with overlapping active ingredients be used to treat patients [
5]. Therefore, as these common agents are not contained in RBFC, this can be used as a novel option for glaucoma medical treatment in combination with currently existing IOP-lowering medications.
The efficacy and safety associated with the switch to BRFC from brimonidine and ripasudil, and brimonidine or ripasudil in glaucoma patients were investigated in the present study.
Materials and Methods
Between December 2022 and January 2024, data were retrospectively collected from patients who were administered RBFC at Hiroshima University Hospital (Hiroshima, Japan), Hirota Eye Clinic (Yamaguchi, Japan), Kusatsu Eye Clinic (Hiroshima, Japan), Nagayama Eye Clinic (Okayama, Japan), Sagara Eye Clinic (Yamaguchi, Japan), Shirai Eye Hospital (Kagawa, Japan), and Suzuki Eye Clinic (Yamaguchi, Japan). This study protocol was approved by the Institutional Review Board of Hiroshima University.
Best-corrected visual acuity (BCVA), refraction, fundus examination using indirect ophthalmoscopy, IOP, slit-lamp examination, gonioscopy examinations, and visual field test were performed in all patients. Patient identification, age, gender, glaucoma disease type, IOP, starting date of the RBFC administration, and current glaucoma therapy data that were recorded during the experiment were used for the analysis. IOPs, which were measured by a Goldmann applanation tonometer, were examined in the morning in all patients, with a single IOP measurement obtained at each visit. When patients chose to stop taking the medication or there were treatment regimen changes, these were all recorded where applicable. To be eligible for enrollment in the study, all subjects had to have an age ≥ 18 years, glaucomatous fundus abnormalities such as thinning of the rim in the optic disc or a defect in the retinal nerve fiber layer corresponding to visual field loss, treatment with at least brimonidine 0.1% (Senju Pharmaceutical Co., Ltd., Osaka, Japan) or ripasudil (Kowa Co., Ltd., Aichi, Japan) or both brimonidine and ripasudil over 3 months. If subjects were: (1) there was a history of glaucoma surgery within one year of the RBFC administration or (2) receiving systemic or local administration of a steroid during the follow-up period, they were excluded from the study. The eye having the higher IOP in the baseline was used for the analysis when both eyes met the inclusion criteria. The right eye was selected for the analysis if both eyes had the same IOP at baseline.
Examinations of the patients were performed at four visits that occurred over 24 weeks (day 0 and weeks 4, 12 and 24). If patients were treated by brimonidine or ripasudil, or brimonidine and ripasudil, at day 0 (baseline), they were switched to RBFC. All other IOP-lowering medications were continued. IOP measurements by a Goldmann applanation tonometer in the morning, BCVA, fundus examination using indirect ophthalmoscopy, and slit-lamp examination were performed in all patients at each visit.
For conjunctival hyperemia assessment and to determine the hyperemia matching, a 4-point scale using a standard photograph was utilized. This scale included: 0 = no dilatation of vessels was observed, 1 = dilatation of mainly small vessels, 2 = dilatation of small and large vessels and 3 = marked dilatation of small and large vessels [
6]. Furthermore, superficial punctate keratopathy (SPK) were recorded using the slit-lamp microscope based on an A (area) D (density) grading scale [
7].
For continuous variables, the variance equality was assessed through the use of the Anderson-Daring test. Differences between the baseline and every follow-up visit were assessed depending on the results obtained by either Wilcoxon signed-rank test or Student’s t-test. All statistical analyses were performed using JMP software version 17 (SAS Inc., Cary, NC). When the P value was 0.05 or less, this difference was defined as being significance.
Results
After switching 25 patients from brimonidine and ripasudil to RBFC, we first evaluated the associated efficacy and safety. Eleven men and 14 women, with a mean age of 71.2 ± 11.0 years. Among them, 21 had POAG, 1 had exfoliation glaucoma, and three had uveitic glaucoma. The patient characteristics are shown in
Table 1.
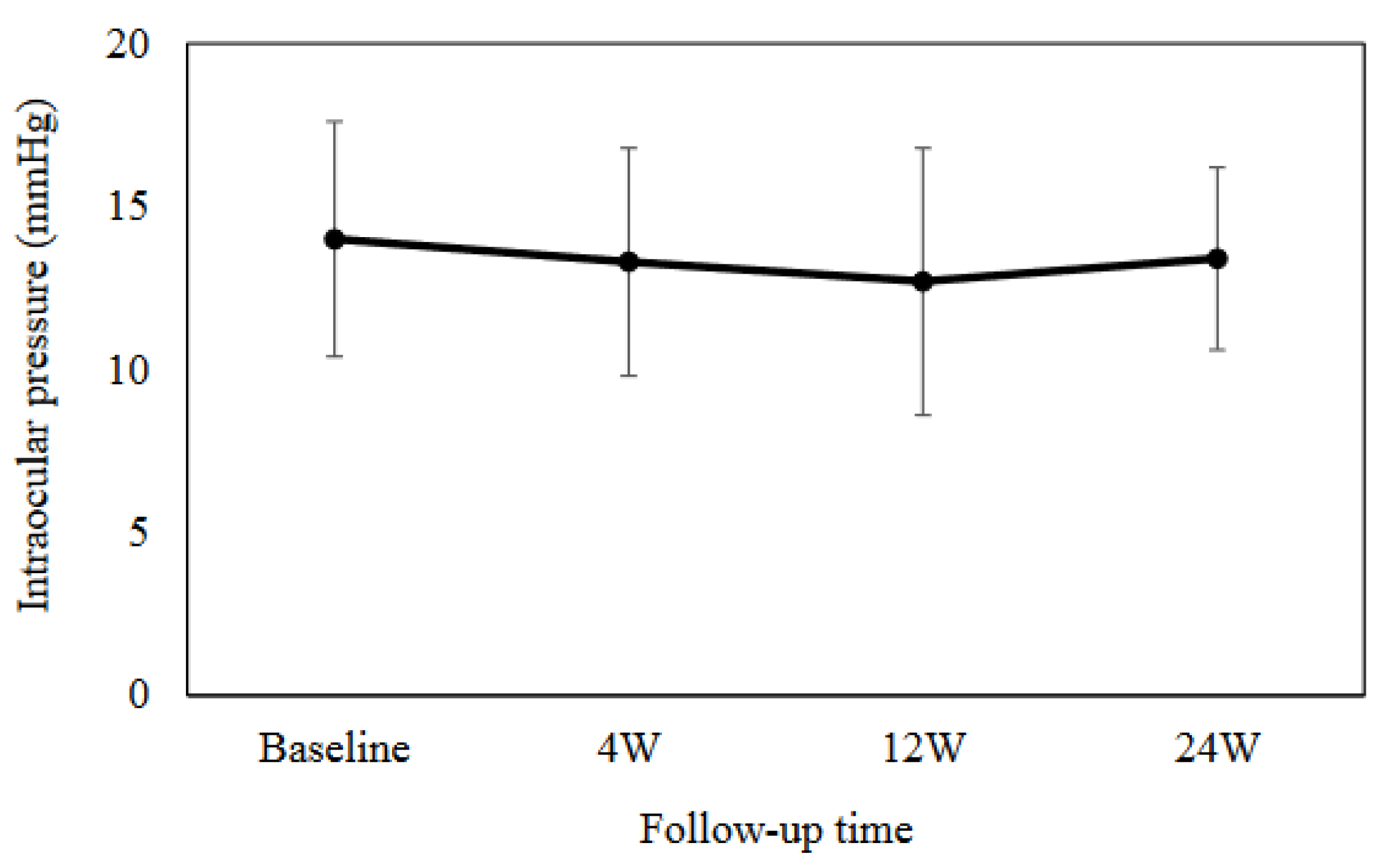
The mean IOP was as follows: 14.0 ± 3.6 mmHg at baseline, 13.3 ± 3.5 mmHg (
P = 0.18) at 4 weeks, 12.7 ± 4.1 mmHg (
P = 0.05) at 12 weeks, and 13.4 ± 2.8 mmHg (
P = 0.93) at 24 weeks, with no significant change from baseline (Fig. 1). As shown in
Table 2, the AD grading scale or the conjunctival hyperemia scores were used to assess the degree of SPK or conjunctival hyperemia, respectively. At all of the evaluated time points, there were no significant differences. However, due to blepharitis (1 patient) and foreign body sensation (1 patient), the RBFC was discontinued in two patients.
Figure 1.
Mean intraocular pressure at baseline and at 4, 12 and 24 weeks. There was no significant difference observed in the mean intraocular pressure as compared to the baseline.
Figure 1.
Mean intraocular pressure at baseline and at 4, 12 and 24 weeks. There was no significant difference observed in the mean intraocular pressure as compared to the baseline.
Second, after switching 45 patients from brimonidine or ripasudil to RBFC, we also evaluated the associated efficacy and safety. Twenty men and 25 women, with a mean age of 69.1 ± 9.8 years. Among them, 31 had POAG, 10 had exfoliation glaucoma, and three had uveitic glaucoma.
Table 3 shows the patient demographic data.
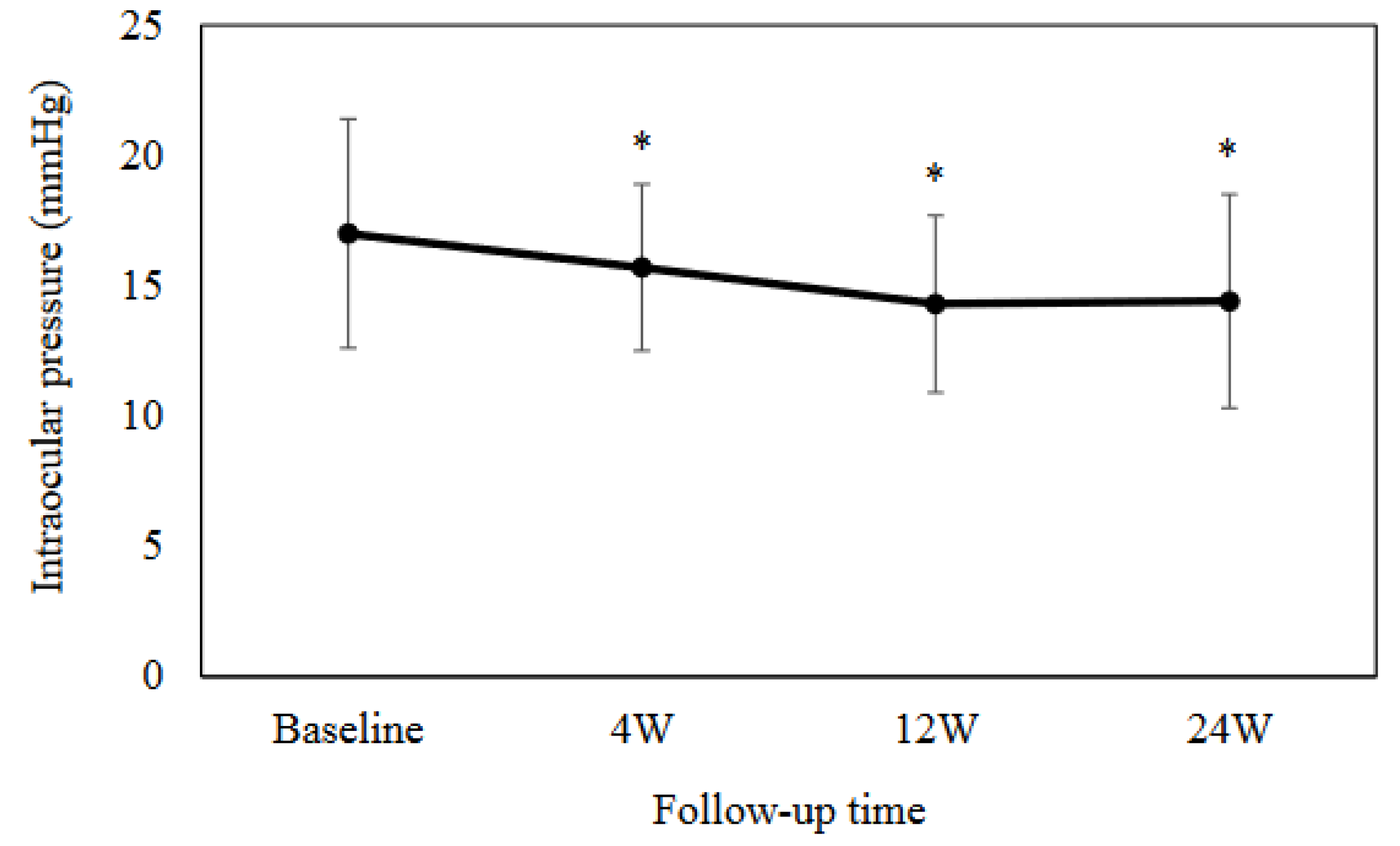
The mean IOP was as follows: 17.0 ± 4.4 mmHg at baseline, 15.7 ± 3.2 mmHg (
P = 0.0003) at 4 weeks, 14.3 ± 3.4 mmHg (
P < 0.0001) at 12 weeks, and 14.4 ± 4.1 mmHg (
P = 0.005) at 24 weeks (Fig. 2). Results between the baseline and each visit indicated that there were significant differences at each time point. As shown in
Table 4, the degree of the SPK or conjunctival hyperemia was no significant differences at any of the evaluated time points. RBFC was discontinued in 4 patients due to blepharitis (3 patients) and itching (1 patient).
Figure 2.
Mean intraocular pressure at baseline and at 4, 12 and 24 weeks. There was a significant difference observed in the mean intraocular pressure as compared to the baseline. *P < 0.001.
Figure 2.
Mean intraocular pressure at baseline and at 4, 12 and 24 weeks. There was a significant difference observed in the mean intraocular pressure as compared to the baseline. *P < 0.001.
Discussion
After switching to RBFC from brimonidine and ripasudil, IOP values were sustained throughout the 24-week observation period. However, after switching to RBFC from brimonidine or ripasudil, there was a significant decrease in the IOP.
Inoue et al.[
8] recently investigated changes in the IOP and found that as compared to the baseline value of 15.1 ± 3.3 mmHg, at 3 months it was 15.9 ± 3.6 mmHg, while it was 14.6 ± 3.3 mmHg at 6 months after switching. These changes from baseline were not significant. Our findings support the results of this previous study. In patients with primary open-angle glaucoma or ocular hypertension, the IOP-lowering effect of RBFC was reported to be superior to that of brimonidine or ripasudil alone over 8 weeks [
10]. Changes in the IOP after switching from ripasudil to RBFC were -2.6 mmHg [
10]. Furthermore, changes in the IOP were -3.4 mmHg after switching from brimonidine to RBFC [
9]. In present study, after switching from brimonidine or ripasudil to RBFC, we found the changes in the IOP to be -2.6 mmHg.
The preservative used in ripasudil and RBFC is 0.002% benzalkonium chloride (BAC). In contrast, the preservative in brimonidine does not contain BAC, but instead uses 0.005% sodium chloride (NaClO
2). A previous study found that there was toxicity on human corneal epithelial cell sheets associated with BAC concentrations that were higher than 0.01% [
10]. After corneal epithelial cells underwent a 30-minute exposure to 0.002-0.1% NaClO
2, another study found that the survival rate was 80% or more [
11]. These results suggest that there is relatively little corneal epithelial damage after exposure to 0.002% BAC or 0.005% NaClO
2. Therefore, after switching to RBFC, there was no significant difference in the AD grading scale.
Blepharitis was the most commonly reported adverse event in the present study, with blepharitis also previously having been reported in patients treated with brimonidine [
12,
13] or ripasudil [
14,
15]. A significant risk factor for the onset of blepharitis following ripasudil administration is a history of an allergic reaction to other IOP-lowering medication [
15,
16]. In the present study, 4 eyes out of the 70 patients (5.7%) were found to have blepharitis, with most of these events reported to be mild in severity. In comparison, at 52 weeks, the incidence of blepharitis was found to be 17% [
18]. A shorter observational period (24 weeks vs. 52 weeks) could be one possible explanation as to why the incidence of blepharitis was lower. In fact, the highest blepharitis incidence occurred between weeks 24-36 [
17].
Glaucoma treatments using multidrug regimens often result in adherence issues due to the inconvenience of the treatments. Therefore, there are some merits to switching to fixed-dose combination therapies. In addition, it has been reported there was improved convenience and patient satisfaction after changing from brinzolamide and brimonidine to brinzolamide/brimonidine fixed-combination ophthalmic suspension (BBFC), due to decrease the number of IOP-lowering medications [
18]. Furthermore, patients were more aware of the effectiveness of the treatment after switching from brinzolamide or brimonidine to BBFC, due to the decrease in the IOP [
19]. Thus, there should be an improved adherence due to a reduction in the medication burden after implementing fixed-dose combination therapies.
The present study had some limitations. First, only a short observation period was utilized in this study. In order to obtain clearer, more definitive results, a longer observation period will be required. Second, this study only analyzed a small number of samples and retrospective study. Thus, large-scale and prospective studies will need to be conducted in the future to obtain more rigorous and definitive evidence.
In conclusion, sustained IOP values were obtained after switching from brimonidine and ripasudil to RBFC throughout a 24-week evaluation period. In contrast, switching to RBFC from brimonidine or ripasudil resulted in a significant decrease in the IOP. Furthermore, the present results show not only that the use of RBFC was safe, but also that there was a low incidence of adverse drug reactions or treatment discontinuation in these patients.
Author Contributions
Conceptualization, K.H.; Methodology, K.H.; Software, H.O.; Validation, K.H. and Y.K.; Formal Analysis, H.O.; Investigation, H.O.; Resources, T.B.; Data Curation, T.B., A.H., M.N., K.S., T.S., and H.M.; Writing – Original Draft Preparation, K.H.; Writing – Review & Editing, Y.K.; Visualization, H.O..; Supervision, K.H.; Project Administration, K.H.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Hiroshima University (protocol code E2023-0173, 24 November, 2023)
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets and materials used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bourne, R.R.A. , Stevens, G.A., White, R.A., Smith, J.L., Flaxman, S.R., Price, H., Jonas, J.B., Keeffe, J., Leasher, J., Naidoo, K., Pesudovs, K., Resnikoff, S., Taylor, H.R.; Vision Loss Expert Group. Causes of vision loss worldwide, 1990-2010: a systemic analysis. Lancet. Glob. Health. 2013, 1, e339–e349. [Google Scholar]
- Heijl, A., Leske. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. In Arch. Ophthalmol; 2002; Volume 120, pp. 1268–1279. [Google Scholar]
- Li, F., Huang, W., Zhang, X. Efficacy and safety of different regiments for primary open-angle glaucoma or ocular hypertension: a systemic review and network meta-analysis. Acta. Ophthalmol. 2018, 96, e277–e284.
- Barnebey, H.S., Robin, A.L. Adherence to fixed-combination versus unfixed travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Am. J. Ophthalmol. 2017, 176, 61–69.
- Kiuchi, Y., Inoue, T., Shoji, N., Nakamura, M., Tanito, M.; Glaucoma Guideline Preparation Committee, Japan Glaucoma Society. The Japan Glaucoma Society guidelines for glaucoma 5th edition. Jpn. J. Ophthalmol. 2023, 67, 189–254.
- Nakano, T. , Yoshikawa, K., Kimura, T., Suzumura, H., Nanno, M., Noro, T. Efficacy and safety of tafluprost in normal-tension glaucoma with intraocular pressure of 16 mmHg or less. Jpn J Ophthalmol. 2011, 55, 605–613. [Google Scholar]
- Miyata, K., Amano, S., Sawa, M., Nishida, T. A novel grading method for superficial punctate keratopathy magnitude and its correlation with corneal epithelial permeability. Arch. Ophthalmol. 2003, 121, 1537–1539.
- Inoue, K., Shiokawa, M., Kunimatsu-Sanuki, S., Tomita, G., Ishida, K. Switching to brimonidine/ripasudil from brimonidine + ripasudil. Clin. Ophthalmol. 2024, 18, 423–430.
- Tanihara, H., Yamamoto, T., Aihara, M., Kawakita, K., Kojima, S., Kanazawa, M., Nojima, T., Suganami, H.; K-232 Clinical Study Group. Ripasudil-brimonidine fixed-dose combination vs ripasudil or brimonidine: two phase 3 randomized clinical trial. Am. J. Ophthalmol. 2023, 248, 35–44.
- Nakagawa, S., Usui, T., Yokota, S., Omichi, S., Kimakura, M., Mori, Y., Miyata, K., Aihara, M., Amano, S., Araie, M. Toxicity evaluation of antiglaucoma drugs using stratified human cultivated corneal epithelial sheets. Invest. Ophthalmol. Vis. Sci. 2021, 53, 5154–5160.
- Ingram, P.R., Pitt, A.R., Wilson, C.G., Olejnik, O., Spickett, C.M. A comparison of the effects of ocular preservatives on mammalian and microbial ATP and glutathione levels. Free. Radic. Res. 2004, 38, 739–750.
- LeBlanc, R.P. Twelve-month results of an ongoing randomized trial comparing brimonidine tartrate 0.2% and timolol 0.5% given twice daily in patients with glaucoma or ocular hypertension. Ophthalmology. 1998, 105, 1960–1967.
- Katz, L.J. Brimonidine tartrate 0.2% twice daily vs timolol 0.5% twice daily: 1-year results in glaucoma patients. Brimonidine Study Group. Am. J. Ophthalmol. 1999, 127, 20–26.
- Tanihara, H., Inoue, T., Yamamoto, T., Kuwayama, Y., Abe, H., Fukushima, A., Suganami, H., Araie, M. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta. Ophthalmol. 2016, 94, e26–e34.
- Tanihara, H., Kakuda, T., Sano, T., Kanno, T., Kurihara, Y. Long-term intraocular pressure-lowering effects and adverse events of ripasudil in patients with glaucoma or ocular hypertension over 24 months. Adv. Ther. 2022, 39, 1659–1677.
- Saito, H., Kagami, S., Mishima, K., Mataki, N., Fukushima, A., Araie, M. Long-term side effects including blepharitis leading to discontinuous of ripasudil. J. Glaucoma. 2019, 28, 289–293.
- Tanihara, H., Yamamoto, T., Aihara, M., Koizumi, N., Fukushima, A., Kawakita, K., Kojima, S., Nakamura, T., Suganami, H.; K-232 Clinical Study Group. Graefes. Arch. Clin. Exp. Ophthalmol. Online ahead of print.
- Onoe, H., Hirooka, K., Nagayama, M., Hirota, A., Mochizuki, H., Sagara, T., Suzuki, K., Okumichi, H., Kiuchi, Y. The efficacy, safety, and satisfaction associated with switching from brinzolamide 1% and brimonidine 0.1% to a fixed combination of brinzolamide 1% and brimonidine 0.1% in glaucoma patients. J Clin Med. 2021, 10, 5228.
- Onoe, H., Hirooka, K., Nagayama, M., Mochizuki, H., Hirota, A., Suzuki, K., Sagara, T., Kiuchi, Y. The efficacy, safety, and satisfaction associated with switching from brinzolamide or brimonidine to brinzolamide/brimonidine in open-angle glaucoma patients. J Pers Med. 2022, 28, 12–2057.
Table 1.
Baseline patient who were treated with brimonidine and ripasudil characteristics.
Table 1.
Baseline patient who were treated with brimonidine and ripasudil characteristics.
| Age (years) |
71.2 ± 11.0 |
| Gender (M/F) |
11/14 |
| Baseline IOP (mmHg) |
14.0 ± 3.6 |
| Type of glaucoma |
|
| POAG |
21 |
| Exfoliation glaucoma |
1 |
| Uveitic glaucoma |
3 |
| IOP-lowering medications |
|
| (except brimonidine and ripasudil) |
|
| PGA |
13 |
| PGA/β-blocker |
6 |
| CAI |
2 |
| β-blocker/CAI |
2 |
| PGA+β-blocker/CAI |
2 |
| M: male, F: female, IOP: intraocular pressure, POAG: primary open-angle glaucoma |
| PGA: prostaglandin analogue, CAI: carbonic anhydrase inhibitor |
Table 2.
SPK and hyperemia score.
Table 2.
SPK and hyperemia score.
| |
SPK Score |
*P value |
Hyperemia score |
*P value |
| Baseline |
1.38 ± 1.19 |
|
0.53 ± 0.64 |
|
| 4W |
0.89 ± 1.45 |
0.38 |
0.60 ± 0.84 |
0.63 |
| 12W |
1.27 ± 1.01 |
0.28 |
0.46 ± 0.66 |
0.63 |
| 24W |
1.43 ± 0.98 |
>0.99 |
0.43 ± 0.55 |
>0.99 |
Table 3.
Baseline patient who were treated with brimonidine or ripasudil characteristics.
Table 3.
Baseline patient who were treated with brimonidine or ripasudil characteristics.
| Age (years) |
69.1 ± 9.8 |
| Gender (M/F) |
20/25 |
| Baseline IOP (mmHg) |
17.0 ± 4.4 |
| Type of glaucoma |
|
| POAG |
32 |
| Exfoliation glaucoma |
10 |
| Uveitic glaucoma |
3 |
| IOP-lowering medications |
|
| (except brimonidine and ripasudil) |
|
| PGA |
16 |
| PGA/β-blocker |
13 |
| β-blocker/CAI |
8 |
| PGA+β-blocker/CAI |
2 |
| none |
6 |
| M: male, F: female, IOP: intraocular pressure, POAG: primary open-angle glaucoma |
| PGA: prostaglandin analogue, CAI: carbonic anhydrase inhibitor |
Table 4.
SPK and hyperemia score.
Table 4.
SPK and hyperemia score.
| |
SPK Score |
*P value |
Hyperemia score |
*P value |
| Baseline |
0.63 ± 1.13 |
|
0.39 ± 0.58 |
|
| 4W |
0.50 ± 1.04 |
0.07 |
0.57 ± 0.79 |
0.10 |
| 12W |
0.57 ± 1.07 |
0.68 |
0.45 ± 0.65 |
0.53 |
| 24W |
0.61 ± 0.90 |
0.73 |
0.39 ± 0.56 |
0.53 |
| SPK: superficial punctate keratopathy |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).