Submitted:
27 May 2024
Posted:
29 May 2024
You are already at the latest version
Abstract
Keywords:
Background
Methods
Settings
Study Design
Inclusion and Exclusion Criteria and Case Definition
Polymerase Chain Reaction Diagnostics, Culture and Characterisation of the Isolates
Data Collect and Analysis
Definitions of the Variables
Comparison to Other Settings
Ethical Aspects
Results
Baseline Patients’ Characteristics
Clinical Patients’ Characteristics at the Admission in ICU ?
Biological Patients’ Characteristics at the Admission
Outcome
Identification of the Strains
Discussion
Conclusion
References
- Picardeau M. Leptospirosis: Updating the Global Picture of an Emerging Neglected Disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
- Daher EF, Soares DS, de Menezes Fernandes AT, Girao MM, Sidrim PR, Pereira ED, et al. Risk factors for intensive care unit admission in patients with severe leptospirosis: a comparative study according to patients’ severity. BMC Infect Dis. 2016;16:40.
- Lindow JC, Wunder EA, Jr., Popper SJ, Min JN, Mannam P, Srivastava A, et al. Cathelicidin Insufficiency in Patients with Fatal Leptospirosis. PLoS Pathog. 2016;12(11):e1005943.
- Bourhy P, Collet L, Brisse S, Picardeau M. Leptospira mayottensis sp. nov., a pathogenic species of the genus Leptospira isolated from humans. Int J Syst Evol Microbiol. 2014;64(Pt 12):4061-7.
- Centre National de Référence de la Leptospirose, Santé publique France. Rapport annuel d’activité - Année d’exercice 2020-2021. 2022.
- Pagès F, Collet L, Henry S, Margueron T, Achirafi A, Bourhy P, et al. Leptospirose à Mayotte : apports de la surveillance épidémiologique, 2008-2015. Bull Epidemiol Hebdo. 2017;8-9:147-56.
- Subiros M, Brottet E, Solet JL, LeGuen A, Filleul L. Health monitoring during water scarcity in Mayotte, France, 2017. BMC Public Health. 2019;19(1):288.
- Bourhy P, Collet L, Brisse S, Picardeau M. Leptospira mayottensis sp. nov., a pathogenic species of the genus Leptospira isolated from humans. Int J Syst Evol Microbiol. 2014;64(Pt 12):4061-7.
- Desvars A, Michault A, Bourhy P. Leptospirosis in the western Indian Ocean islands: what is known so far? Veterinary research. 2013;44(1):80.
- Desvars A, Naze F, Vourc’h G, Cardinale E, Picardeau M, Michault A, et al. Similarities in Leptospira serogroup and species distribution in animals and humans in the Indian ocean island of Mayotte. Am J Trop Med Hyg. 2012;87(1):134-40.
- Bourhy P, Collet L, Lernout T, Zinini F, Hartskeerl RA, van der Linden H, et al. Human leptospira isolates circulating in Mayotte (Indian Ocean) have unique serological and molecular features. J Clin Microbiol. 2012;50(2):307-11.
- Delmas B, Jabot J, Chanareille P, Ferdynus C, Allyn J, Allou N, et al. Leptospirosis in ICU: A Retrospective Study of 134 Consecutive Admissions. Crit Care Med. 2017.
- Miailhe AF, Mercier E, Maamar A, Lacherade JC, Le Thuaut A, Gaultier A, et al. Severe leptospirosis in non-tropical areas: a nationwide, multicentre, retrospective study in French ICUs. Intensive Care Med. 2019;45(12):1763-73.
- Tubiana S, Mikulski M, Becam J, Lacassin F, Lefèvre P, Gourinat A-C, et al. Risk factors and predictors of severe leptospirosis in New Caledonia. PLoS Negl Trop Dis. 2013;7(1).
- Hochedez P, Theodose R, Olive C, Bourhy P, Hurtrel G, Vignier N, et al. Factors Associated with Severe Leptospirosis, Martinique, 2010-2013. Emerg Infect Dis. 2015;21(12):2221-4.
- Herrmann-Storck C, Saint-Louis M, Foucand T, Lamaury I, Deloumeaux J, Baranton G, et al. Severe leptospirosis in hospitalized patients, Guadeloupe. Emerg Infect Dis. 2010;16(2):331-4.
- Doudier B, Garcia S, Quennee V, Jarno P, Brouqui P. Prognostic factors associated with severe leptospirosis. Clin Microbiol Infect. 2006;12(4):299-300.
- Epelboin L, Le Turnier P, Mosnier E, Schaub R, Fontaine E, Houcke S, et al. Severe leptospirosis in Morocco: comparative data from the Amazonian area. Intensive Care Med. 2017.
- Rajaonarivelo JA, Desmoulin A, Maillard O, Collet L, Baudino F, Jaffar-Bandjee MC, et al. Clinical manifestations of human leptospirosis: bacteria matter. Frontiers in cellular and infection microbiology. 2023;13:1259599.
- Ellinghausen HC, Jr., McCullough WG. Nutrition of Leptospira pomona and growth of 13 other serotypes: fractionation of oleic albumin complex and a medium of bovine albumin and polysorbate 80. Am J Vet Res. 1965;26:45-51.
- Johnson RC, Harris VG. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967;94(1):27-31.
- Guglielmini J, Bourhy P, Schiettekatte O, Zinini F, Brisse S, Picardeau M. Genus-wide Leptospira core genome multilocus sequence typing for strain taxonomy and global surveillance. PLoS Negl Trop Dis. 2019;13(4):e0007374.
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307(23):2526-33.
- Bourhy P, Collet L, Clement S, Huerre M, Ave P, Giry C, et al. Isolation and characterization of new Leptospira genotypes from patients in Mayotte (Indian Ocean). PLoS Negl Trop Dis. 2010;4(6):e724.
- Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
- Klement-Frutos E, Tarantola A, Gourinat AC, Floury L, Goarant C. Age-specific epidemiology of human leptospirosis in New Caledonia, 2006-2016. 2020;15(11):e0242886.
- Institut National des Statistiques et des Etudes Economiques (INSEE). Evolution et structure de la population. Tableau de bord de l’économie française2023.
- Daveluy A, Haramburu F. Consommation de substances psychoactives à la Réunion et à Mayotte, départements français de l’Océan Indien. Thérapie. 2018;73(5):419-27.
- Le Turnier P, Mosnier E, Schaub R, Bourhy P, Jolivet A, Cropet C, et al. Epidemiology of Human Leptospirosis in French Guiana (2007-2014): A Retrospective Study. Am J Trop Med Hyg. 2018.
- Epelboin L, Bourhy P, Le Turnier P, Schaub R, Mosnier E, Berlioz-Arthaud A, et al. La leptospirose en Guyane francaise et sur le bouclier des Guyanes. Etat des connaissances en 2016. Bull Soc Pathol Exot. 2017;110(3):165-79.
- Picardeau M. Diagnosis and epidemiology of leptospirosis. Med Mal Infect. 2013;43(1):1-9.
- Sokolova M, Marshall JC, Benschop J. Risk Factors for Hospitalisation amongst Leptospirosis Patients in New Zealand. Trop Med Infect Dis. 2021;6(4).
- Guégan JF, Epelboin L, Douine M, Le Turnier P, Duron O, Musset L, et al. Emerging infectious diseases and new pandemics: dancing with a ghost! Lessons in inter- and transdisciplinary research in French Guiana, South America. Int J Infect Dis. 2023;133:9-13.
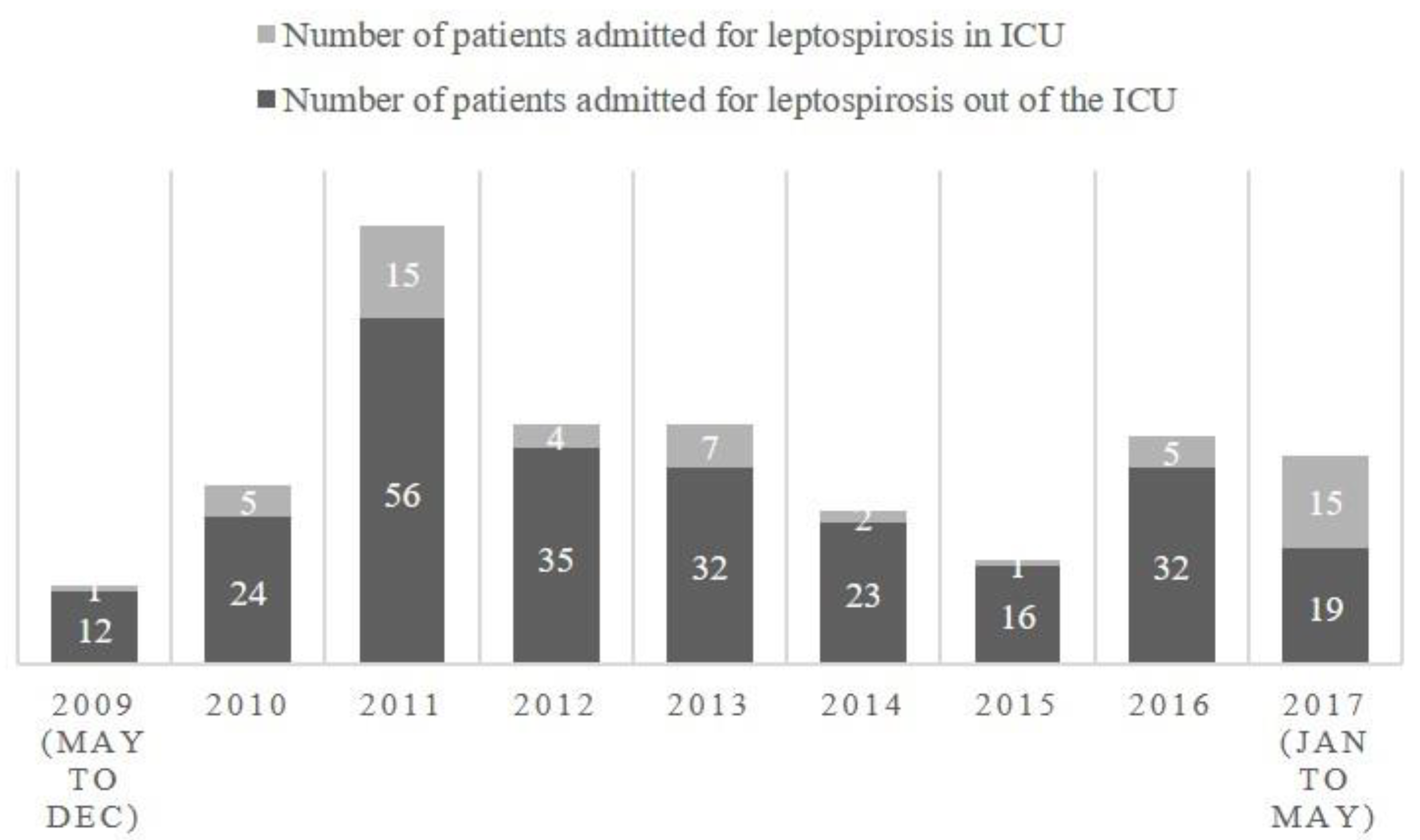
| Year | Number of patients admitted for leptospirosis at the CHM including ICU | Number of patients admitted for leptospirosis in ICU | Percentage of patients admitted in ICU |
| 2009 (may to dec) | 13 | 1 | 8% |
| 2010 | 29 | 5 | 17% |
| 2011 | 71 | 15 | 21% |
| 2012 | 39 | 4 | 10% |
| 2013 | 39 | 7 | 18% |
| 2014 | 25 | 2 | 8% |
| 2015 | 17 | 1 | 6% |
| 2016 | 37 | 5 | 14% |
| 2017 (jan to may) | 34 | 15 | 44% |
| Total | 304 | 55 | 18% |
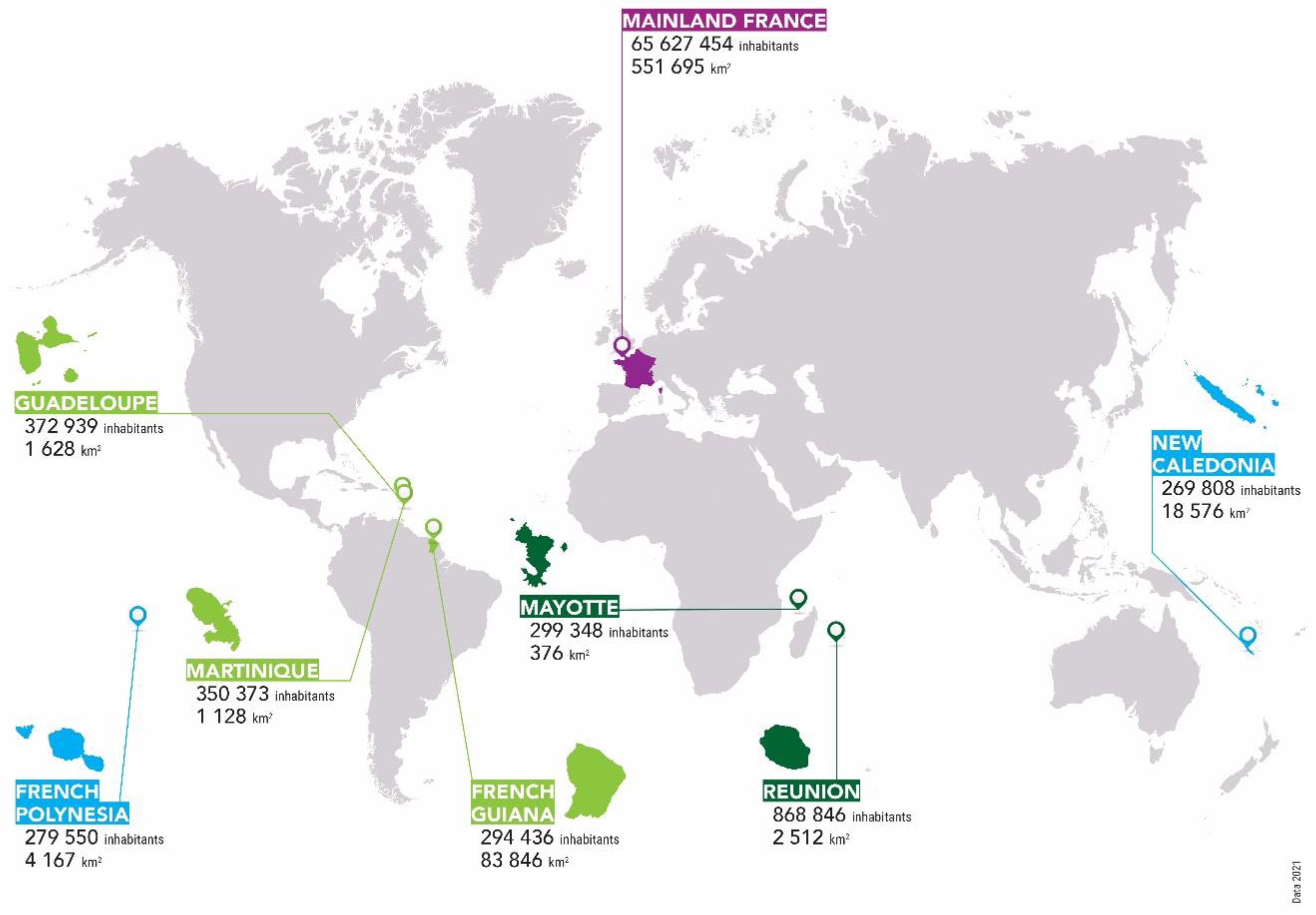
| Setting | Mayotte | La Réunion | p Mayotte vs. Réunion | French Guiana | p Mayotte vs. French Guiana | Guadeloupe | p Mayotte vs. Guadeloupe | Martinique | p Mayotte vs. Martinique | New Caledonia | p Mayotte vs. New-Caledonia | French Polynesia | p Mayotte vs. French Polynesia | Mainland France | p Mayotte vs. Mainland France |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Tantet et al. | Delmas et al., Crity Care Med, 2018 | Epelboin et al. Int Care Med, 2016 | Herrmann-Storck et al. Emerg Inf Dis, 2010 | Hochedez et al. Emerg Inf Dis, 2015 | Tubiana et al., PLoS NTD, 2013 | Doudier et al. Clin Microb Inf 2006 | Miailhe et al., Int Care Med, 2019 | |||||||
| Characteristics of patients | ICU patients | ICU patients | p | dialysis, vasopressor agents, mechanical ventilation and/or death during hospitalization | severe cases = dialysis in case of oliguria, mechanical ventilation or death | Severe leptospirosis = vasoactive drugs or dialysis, or blood transfusion or mechanical ventilation or death | severe leptospirosis : dialysis, or vasoactive drugs, alveolar hemorrhage, blood transfusion, mechanical ventilation or death | Severe leptospirosis (no details) | leptospirosis requiring ICU admission (79 ICU in France) | ||||||
| Microbiological diagnosis | PCR | MAT, ELISA or PCR | PCR, MAT, Elisa IgM | EIA IgM, blood culture, MAT | qPCR | RT-PCR + MAT | MAT or ELISA, PCR, or dark field microscopy. | ||||||||
| Study period | 05/2009-05/2017 | 01/2004-01/2015 | 01/2007-09/2014 | 01/2003-12/2004 | 12/2010-02/2013 | 01/2008-06/2011 | 2 years | 01/2012 - 09/2016 | |||||||
| Number of patients | 55 | 134 | 12 | 24 | 12 (among 23 admitted in ICU) | 71 | 71 | 160 | |||||||
| n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | ||||||||
| Demographic caracteristics | |||||||||||||||
| Male gender | 45/55 (82) | 125 (93) | 0.32 | 11/12 (92) | 0.41 | 18 (75) | 0.55 | NA | NA | 45/71 (63.4) | 0.029 | NA | NA | 146 (91) | 0.43 |
| Male to female sex ratio | 4.5 | 13.8 | 11,0 | 3.0 | NA | 1.7 | NA | 10.4 | |||||||
| Age (years, median, IQR 25%–75%,mean ± standard deviation) | 44 (33-55) / 43,7 ± 14.9 | 40 (30-52) | - | 46.8 ± 17.2 | 0.26 | 52.7 ± 6.5 | 0.003 | 49 (37–57) | - | 42.6 ± 17.8 | 0.64 | NA | NA | 54 (38–65) | - |
| Age>60 years | 6/55 (11) | NA | NA | 3/12 (25) | 0.35 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Age category<3030-59≥60 | 13 (24) 35 (64) 7 (13) |
- | NA | 2 (17) 7 (58) 3 (25) |
- | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Medical history | |||||||||||||||
| Liver disease | 1/54 (2) | 1 (1) | 0.49 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 4 (3) | 1 |
| Cancer or immune deficiency | 0/55 (0) | 0 (0) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 2 (1) | 1 |
| Diabetes mellitus | 7/55 (13) | 14 (10) | 0.62 | NA | NA | 3/22 (134) | 1 | NA | NA | 8/70 (11.4) | 1 | NA | NA | 9 (6) | 0.13 |
| Chronic hypertension | 6/55 (11) | 10 (7) | 0.57 | NA | NA | 9/22 (41) | 0.18 | NA | NA | 8/70 (11.4) | 1 | NA | NA | NA | NA |
| Respiratory insufficiency | 0/55 (0) | 0 (0) | 1 | NA | NA | NA | NA | NA | NA | 2/70 (2.9) | 0.5 | NA | NA | 0 (0) | 1 |
| Chronic kidney disease | 0/55 (0) | 0 (0) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 (0) | 1 |
| Cardiovascular disease | 0/55 (0) | 4 (3) | 0.32 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 (0) | 1 |
| Chronic alcoholism | 3/55 (5) | 34 (25) | 0.17 | NA | NA | 11/22 (50) | <0.001 | NA | NA | 18/70 (25.0) | 0.003 | NA | NA | 29 (18.2) | 0.027 |
| Current cigarette smoking | 2/55 (4) | 45 (34) | <0.001 | NA | NA | NA | NA | NA | NA | 37/70 (52.9) | 0.035 | NA | NA | 49 (31.2) | <0.001 |
| Underlying comorbidities | 14/55 (25) | 47 (35) | 0.23 | 3/11 (27) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Risk factors | |||||||||||||||
| Exposure to occupational risk‡ | 24/38 (64) | NA | NA | 8/9 (89) | 0.24 | 12/20 (60) | 1 | NA | NA | NA | NA | NA | NA | NA | NA |
| Activity at risk for contamination | 25/54 (46) | NA | NA | 6/12 (50) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | 98 (65) | 0.058 |
| Contact with water at risk for contamination | 4/54 (7) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 101 (68) | 0.047 |
| Contact with rodents | 29/54 (54) | NA | NA | 1/2 (50) | 1 | 3/9 (33) | 0.30 | NA | NA | NA | NA | NA | NA | 44 (31) | <0.001 |
| Contact with animals | 28/54 (52) | NA | NA | 1/2 (50) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | 79 (56) | 0.88 |
| Durations | |||||||||||||||
| Time between first symptoms and biological diagnosis (days) | 3 (2-5) | NA | NA | 4 (4–5.75) | - | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Time between first symptoms and biological diagnosis >5 days | 27/53 (51) | NA | NA | 3/10 (30) | 0.31 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Time between beginning of symptoms and hospital admission (days) | 3 (2-5) | 5 (4–6) | - | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Time between first symptoms and hospital admission > 3 days | 25/53 (47) | NA | NA | NA | NA | NA | NA | NA | NA | 37/70 (52.9) | 0.59 | NA | NA | NA | NA |
| Time between first symptoms and initiation of antibiotic treatment (days) | 3 (2-5) | 5 (4–6) | - | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 5 (4–6) | - |
| Time between first symptoms and antibiotic treatment > 2 days | 35/53 (66) | NA | NA | NA | NA | NA | NA | NA | NA | 51/69 (73.9) | 0.42 | NA | NA | NA | NA |
| Time between first symptoms and antibiotic treatment >10 days | 2/53 (4) | NA | NA | NA | NA | 7/22 (31.8) | 0.089 | NA | NA | NA | NA | NA | NA | NA | NA |
| Time between first symptoms and occurrence of severity criteria (days) | 3 (2-5) | NA | NA | NA | NA | 3 (3-4) | - | 3 (3-4) | - | NA | NA | NA | NA | NA | NA |
| Time between first symptoms and and ICU admission (days) | 3.5 (2-5) | 5 (4-6) | - | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 5 (4–6) | - |
| Antibiotic treatment duration (days) | 7 (7-8) | 7 (7–9.5) | - | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 10 (8–12) | - |
| Length of hospital stay (days) | 9 (6-13) | 12 (8–16) | - | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 11 (8–20) | - |
| Length of ICU stay (days) | 5 (3-9) | 6 (4-9) | - | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 5 (2–10) | - |
| Time between admission and death (days) | 1 (1-5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 3 (2–20). | - |
| Setting | Mayotte | La Réunion | p Mayotte vs. Réunion | French Guiana | p Mayotte vs. French Guiana | Guadeloupe | p Mayotte vs. Guadeloupe | Martinique | p Mayotte vs. Martinique | New Caledonia | p Mayotte vs. New-Caledonia | French Polynesia | p Mayotte vs. French Polynesia | Mainland France | p Mayotte vs. Mainland France |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Tantet et al. | Delmas et al., Crity Care Med, 2018 | Epelboin et al. Int Care Med, 2016 | Herrmann-Storck et al. Emerg Inf Dis, 2010 | Hochedez et al. Emerg Inf Dis, 2015 | Tubiana et al., PLoS NTD, 2013 | Doudier et al. Clin Microb Inf 2006 | Miailhe et al., Int Care Med, 2019 | |||||||
| Number of patients | 55 | 134 | 12 | 24 | 12 (among 23 admitted in ICU) | 71 | 71 | 160 | |||||||
| n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N () or median, IQR 25–75 and/or mean ± standard deviation) | ||||||||
| Signs and symptoms, n (%) | |||||||||||||||
| Fever reported by patient | 36/55 (65) | 116 (86) | 0.002 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 135 (84) | 0.74 |
| Temperature >37,7 °C | 38/55 (69) | NA | NA | 7/12 (58) | 0 | 10/23 (44) | 0.043 | NA | NA | NA | NA | NA | NA | NA | NA |
| Temperature >38 °C | 36/55 (65) | NA | NA | 6/12 (50) | 0.51 | NA | NA | 9/12 (75) | 0.73 | 31/67 (46) | 0.044 | NA | NA | NA | NA |
| Temperature ≥ 38.5 °C | 28/55 (51) | NA | NA | 6/12 (50) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Hypothermia (<36.5°C) | 5/55 (9) | NA | NA | 2/12 (17) | 0.60 | 7/23 (30) | 0.033 | NA | NA | NA | NA | NA | NA | NA | NA |
| Hypotension, SBP <100 mm Hg | 30/55 (55) | NA | NA | 4/12 (33) | 0.22 | 7/20 (35) | 0.45 | NA | NA | NA | NA | NA | NA | NA | NA |
| Hypotension, SBP <90 mm Hg | 20/55 (36) | NA | NA | 3/12 (25) | 0.52 | NA | NA | 5 (42) | 1 | NA | NA | NA | NA | NA | NA |
| Myalgia | 36 (65) | 108 (80) | 0.038 | NA | NA | 12/13 (92) | 0.16 | NA | NA | 68/71 (96) | 0.085 | NA | NA | 95 (59) | 0.52 |
| Arthralgia | 17 (31) | 42 (31) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 35 (22) | 0.20 |
| Jaundice | 32 (58) | 108 (80) | 0.0019 | 3/12 (25) | 0.055 | 9/12 (75) | 0.089 | 9 (75) | 0.34 | 61/71 (86) | 0.11 | NA | NA | 74 (46) | 0.16 |
| Oligoanuria | 36 (65) | 69 (51) | 0.11 | 5/8 (63) | 1 | 10/23 (44) | 0.083 | ‡<500 mL urine/day 5 (41.7) | 0.19 | 66/71 (93) | <0.001 | NA | NA | NA | NA |
| Hepatosplenomegaly | 4/55 (7) | 25 (18) | 0.077 | NA | NA | 7/11 (64) | <0.001 | NA | NA | NA | NA | NA | NA | NA | NA |
| Abdominal pain | 34/55 (62) | 49 (36) | 0.002 | 10/12 (83) | 0.20 | 14/18 (78) | 0.26 | 5 (42) | 0.22 | NA | NA | NA | NA | 41 (26) | <0.001 |
| Digestive disorders (diarrhea, vomiting) | 16/55 (29) | NA | NA | NA | NA | 12/15 (80) | 0.74 | NA | NA | NA | NA | NA | NA | NA | NA |
| Nausea/vomiting | 14/55 (25) | 65 (48) | 0.0036 | 5/12 (42) | 0.30 | NA | NA | 5 (42) | 0.30 | NA | NA | NA | NA | 50 (31) | 0.50 |
| Diarrhea | 10/55 (18) | NA | NA | 8/12 (67) | 0.0017 | NA | NA | 3 (25) | 0.69 | NA | NA | NA | NA | NA | NA |
| Gastrointestinal bleeding | 1 (2) | 8 (6) | 0.45 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Hemoptysis | 1 (2) | 42 (31) | <0.001 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 11 (7) | 0.30 |
| Dyspnea | 17 (31) | 37 (27) | 0.72 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 42 (26) | 0.60 |
| Cough | 4 (7) | 47 (35) | <0.001 | NA | NA | NA | NA | 3 (25) | 0.10 | NA | NA | NA | NA | 35 (22) | 0.015 |
| Chest pain | 8/55 (15) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 6 (4) | 0.0095 |
| Abnormalities at chest auscultation | 16/54 (30) | NA | NA | NA | NA | 8/17 (47) | 0.24 | 4 (33) | 0.74 | NA | NA | NA | NA | 43 (27) | 0.73 |
| Chest radiologic abnormalities | 24/52 (46) | NA | NA | NA | NA | 6/16 (38) | 0.58 | NA | NA | NA | NA | NA | NA | NA | NA |
| Alveolar infiltrate | 10/52 (19) | NA | NA | NA | NA | 5/16 (31) | 0.32 | NA | NA | NA | NA | NA | NA | NA | NA |
| Headache | 9/47 (19) | NA | NA | 5/12 (42) | 0.13 | 5/7 (71) | 0.10 | NA | NA | 61/70 (87) | 0.037 | NA | NA | 47 (29) | 0.19 |
| Confusion | 11/47 (23) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 11 (7) | 0.0027 |
| Meningeal syndrome | 0 (0) | 4 (3) | 0.32 | NA | NA | 2/12 (17) | 0.030 | NA | NA | NA | NA | NA | NA | NA | NA |
| Specific organ involvment | |||||||||||||||
| Pulmonary involvement | 23/53 (43) | NA | NA | 6/12 (50) | 0.75 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Intra-alveolar hemorrhage | 4/55 (7) | 53 (40) | <0.001 | NA | NA | NA | NA | NA | NA | 39/70 (56) | <0.001 | NA | NA | 23 (14) | 0.24 |
| ARDS | 9/55 (16) | 28 (21) | 0.55 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 58 (36) | 0.0067 |
| Macrophage activation syndrome | 0/55 (0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 5 (3) | 0.33 |
| Meningitis | 1/55 (2) | 2 (1) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 4 (2) | 1 |
| Encephalitis | 1/55 (2) | 4 (3) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Myocarditis | 8/55 (15) | 30 (22) | 0.32 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 4 (2) | 0.0025 |
| Pericarditis | 1/55 (2) | 3 (2) | 1 | NA | NA | 2/24 (8) | 0.22 | NA | NA | NA | NA | NA | NA | NA | NA |
| Cardiac arrest | 4/55 (7) | 3 (2) | 20 | NA | NA | 2/24 (8) | 1 | NA | NA | NA | NA | NA | NA | NA | NA |
| Cardiogenic shock | 10/55 (18) | 11 (8) | 0.072 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Sepsis | 17/55 (31) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 40 (22) | 0.38 |
| Organ failure | |||||||||||||||
| Circulatory failure | 32/55 (58) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 33 (21) | <0.001 |
| Acute kidney failure | 48/55 (87) | 127 (95) | 0.12 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 24 (15) | <0.001 |
| Acute respiratory failure | 6/53 (11) | 76 (57) | <0.001 | 5/12 (42) | 0.024 | NA | NA | NA | NA | NA | NA | NA | NA | 14 (9) | 0.59 |
| Central nervous system failure | 12/55 (22) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 9 (6) | 0.0012 |
| Acute liver failure | 25/55 (45) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 8 (5) | <0.001 |
| Multiorgan failure | 12/55 (22) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 30 (19) | 0.69 |
| Hemorrhagic syndrome | 2 (4) | 72 (54) | 0.033 | 2/12 (17) | 0.14 | 5/24 (21) | 0.024 | 1 (8.3) | 0.45 | NA | NA | NA | NA | 11 (7) | 0.51 |
| Supportive care | |||||||||||||||
| Shock treated with vaso-active drugs | 31/55 (56) | NA | NA | 10/12 (83.3) | 0.11 | NA | NA | 9/12 (75) | 0.33 | 62/70 (89) | <0.001 | NA | NA | 92 (57) | 1 |
| Pulmonary involvement needing mechanical ventilation | 15/55 (27) | 41 (31) | 0.73 | 9/12 (75.0) | <0.001 | NA | NA | 8/12 (67) | 0.017 | 29/71 (41) | 0.13 | NA | NA | 58 (36) | <0.001 |
| Non-invasive ventilation | 10/55 (18) | 13 (10) | 0.14 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 32 (20) | 0.85 |
| ExtraCorporeal Membrane Oxygenation | 0/55 (0) | 5 | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 3 (2) | 0.57 |
| Use of neuromuscular blocking agent (curare) | 10/55 (18) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 33 (20) | 0.85 |
| Prone position | 1/55 (2) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 9 (6) | <0.001 |
| Bleeding requiring blood transfusion | 6/55 (11) | NA | 1/11 (9) | 1 | NA | NA | NA | 8/12 (67) | <0.001 | 23/71 (32) | 0.0053 | NA | NA | NA | NA |
| Acute renal failure with dialysis | 31/55 (56) | 75 (56) | 1 | 7/12 (58) | 1 | NA | NA | 7/12 (58) | 1 | 23/71 (32) | 0.011 | NA | NA | 56 (35) | 0.0067 |
| Outcome | |||||||||||||||
| Diagnosis of leptospirosis suspected at admission | 43/55 (78) | NA | NA | 2/12 (17) | <0.001 | NA | NA | NA | NA | NA | NA | NA | NA | 8 (5) | <0.001 |
| Simplified Acute Physiology Score (SAPS II) | 41 (25.5-50.5) | 38 (27-50) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 40 (28–58) | ||
| Case-fatality rate | 3/55 (5) | 8/134 (6) | 1 | 3/12 (25) | 0.065 | 6/24 (25) | 0.020 | 0/12 (0) | 1 | 10/71 (14) | 0.15 | 5/71 (7) | 1 | 14/160 (9) | 0.57 |
| Setting | Mayotte | La Réunion | p Mayotte vs. Réunion | French Guiana | p Mayotte vs. French Guiana | Guadeloupe | p Mayotte vs. Guadeloupe | Martinique | p Mayotte vs. Martinique | New Caledonia | p Mayotte vs. New-Caledonia | French Polynesia | p Mayotte vs. French Polynesia | Mainland France |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Tantet et al. | Delmas et al., Crity Care Med, 2018 | Epelboin et al. Int Care Med. 2016 | Herrmann-Storck et al. Emerg Inf Dis. 2010 | Hochedez et al. Emerg Inf Dis, 2015 | Tubiana et al., PLoS NTD, 2013 | Doudier et al. Clin Microb Inf 2006 | Miailhe et al., Int Care Med, 2019 | ||||||
| Number of patients | 55 | 134 | 12 | 24 | 12 (among 23 admitted in ICU) | 71 | 71 | 160 | ||||||
| n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median. IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median. IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | n/N (%) or median, IQR 25%–75% and/or mean ± standard deviation) | |||||||
| L. interrogans serovar Icterohemorrhagiae | 0/21 | 17/18 (94) | <0.001 | 5/9 (56) | <0.001 | 6/8 (75) | <0.001 | 11/12 (92) | <0.001 | 54/66 (82) | <0.001 | NA | NA | NA |
| pH | 7.40 (7.36-7.44) | 7.42 (7.38-7.45) | - | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Leukocyte count ( G/L) | 11.9 (8-17) / 13,2 ± 6,6 | 12 (8-16) | - | 12.1 ± 10.1 | 0.64 | NA | NA | 10.3 (9.1-11.4) | NA | NA | NA | NA | NA | 10.2 (7–14) |
| >15 | 17/55 (31) | NA | NA | 1/12 (8) | 0.16 | NA | NA | NA | NA | 18/71 (25) | 0.55 | NA | NA | NA |
| >10 | 38/55 (69) | NA | NA | 6/12 (50) | 0.31 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| PMN count (G/L) | 9.7 (6.8-14.0) / 11,0 ± 6,4 | NA | NA | 10.3 ± 9.4 | 0.75 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| > 7.5 | 33/50 (66) | NA | NA | 7/12 (58) | 0.73 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| > 12 | 17/50 (34) | NA | NA | 2/12 (16) | 0.31 | 10/23 (44) | 0.45 | NA | NA | NA | NA | NA | NA | NA |
| Platelet count (G/L) | 49 (27-79) / 72,0 ± 109 | 45 (26-84) | - | 146.8 ± 142.4 | 0.046 | NA | NA | 70.5 (32.5-115) | NA | NA | NA | NA | NA | 40 (26–76) |
| < 20 | 9/55 (16) | 17 (13) | 0.49 | 0/12 (0) | 0.20 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| <50 | 28/55 (51) | NA | NA | 3/12 (25) | 0.12 | 8/23 (35) | 0.15 | NA | NA | 31/71 (44) | 0.47 | NA | NA | 89 (57%) |
| <92 | 43/55 (78) | NA | NA | 6/12 (50) | 0.07 | NA | NA | 7/12 (58.3) | 0.16 | NA | NA | NA | NA | NA |
| < 100 | 47/55 (85) | NA | NA | 7/12 (58) | 0.046 | NA | NA | NA | NA | NA | NA | NA | NA | 138 (89%) |
| < 150 | 50/55 (91) | 124 (93) | 0.77 | 7/12 (58) | 0.012 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Hemoglobin (g/dL) | 10.7 (8.8-12.2) / 10,5 ± 2,9 | 11.7 (10.3-12.8) | - | 11.9 ± 3.0 | 0.14 | 11.1 ± 2.0 (23) | 0.36 | 12.2 (11.6. 13) | NA | NA | NA | NA | NA | NA |
| ≤ 8 | 11/54 (20) | NA | NA | 1/12 (8) | 0.44 | NA | NA | NA | NA | 7/71 (10) | 0.12 | NA | NA | NA |
| < 10 | 21/54 (39) | NA | NA | 2/12 (16) | 0.19 | 5/23 (22) | 0.19 | NA | NA | NA | NA | NA | NA | NA |
| < 12 | 37/54 (69) | NA | NA | 6/12 (50) | 0.31 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| < 12,2 | 40/54 (74) | NA | NA | 6/12 (50) | 0.16 | NA | NA | 6/12 (50) | 0.16 | NA | NA | NA | NA | NA |
| Creatinine (µmol/L) | 277 (169-439) / 386,3 ± 416,9 | 308 (184-521) | - | 339.2 ± 256.4 | 0.71 | 246.4 ± 220 (21) | 0.13 | 169.5 (132.5-217.5) | NA | NA | NA | NA | NA | 323 (191–483) |
| > 110 | 48/55 (87) | 127 (95) | 0.12 | 11/12 (92) | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| > 132 | 45/55 (82) | NA | NA | 10/12 (83) | 1 | 11/21 (52) | 0.18 | NA | NA | NA | NA | NA | NA | NA |
| > 154 | 42/55 (76) | NA | NA | 9/12 (75) | 1 | NA | NA | 7/12 (58) | 0.28 | NA | NA | NA | NA | NA |
| > 200 | 36/55 (65) | NA | NA | 8/12 (67) | 1 | NA | NA | NA | NA | 36/67 (54) | 0.20 | NA | NA | NA |
| Urea (mmol/L) | 18.3 (10.0-28.9) / 23,0 ± 20,2 | 16 (10–24) | 13.9 ± 9.1 | 0.13 | 14.3 ± 12.7 (23) | 0.23 | 10.1 (8-18.5) | NA | NA | NA | NA | NA | NA | |
| > 9.3 | 45/55 (82) | NA | NA | 8/12 (67) | 0.26 | NA | NA | 4/8 (50) | 0.065 | NA | NA | NA | NA | NA |
| > 15 | 32/55 (58) | NA | NA | 4/12 (33) | 0.20 | NA | NA | NA | NA | NA | NA | NA | NA | 97 (61%) |
| ASAT (IU/L) | 124 (70-266) / 419,9 ± 1260 | 148 (89-234) | - | 138.0 ± 145.0 | 0.44 | NA | NA | 73.5 (59-126.5) | NA | NA | NA | NA | NA | 112 (64–181) |
| >102 | 35/55 (64) | NA | NA | 4/11 (36) | 0.11 | 17/23 (74) | 0.44 | NA | NA | NA | NA | NA | NA | NA |
| > 150 | 25/55 (45) | NA | NA | 3/11 (27) | 0.33 | NA | NA | NA | NA | 18/66 (27) | 0.056 | NA | NA | NA |
| ALAT (IU/L) | 69 (37-125) / 151,5 ± 326,2 | 77 (52-107) | - | 116.8 ± 224.4 | 0.73 | NA | NA | NA | NA | NA | NA | NA | NA | 81 (50–128) |
| >119 | 15/55 (27) | NA | NA | 3/12 (25) | 1 | 6/23 (26) | 1 | NA | NA | NA | NA | NA | NA | NA |
| ASAT and/or ALAT > 10 N | 30/55 (55) | NA | NA | 2/12 (17) | 0.025 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Total bilirubin (µmol/L) | 107 (24-286) / 196,1 ± 215,7 | 152 (46-293) | - | 101.7 ± 163.5 | 0.16 | 258.4 ± 199 (18) | 0.055 | 56.5 (35.5-103) | NA | NA | NA | NA | NA | 80 (33–186) |
| > 20 | 43/55 (78) | 120 (90) | 0.061 | 7/12 (58) | 0.16 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| > 49 | 34/55 (62) | NA | NA | 6/12 (50) | 0.52 | NA | NA | 7/12 (58) | 1 | NA | NA | NA | NA | NA |
| > 50 | 34/55 (62) | NA | NA | 6/12 (50) | 0.52 | NA | NA | NA | NA | 38/66 (58) | 0.71 | NA | NA | NA |
| > 119 | 27/55 (49) | NA | NA | 3/12 (25) | 0.20 | 13/18 (72) | 0.11 | NA | NA | NA | NA | NA | NA | NA |
| > 150 | 26/55 (47) | NA | NA | 2/12 (16) | 0.06 | NA | NA | NA | NA | NA | NA | NA | NA | 50 (32%) |
| Prothrombin ratio (%) | 69 (53-87) | 82 (72-90) | - | NA | NA | NA | NA | 66.5 (56-74.5) | NA | NA | NA | NA | NA | NA |
| < 68 | 26/54 (48) | NA | NA | NA | NA | NA | NA | 7/12 (58) | 0.75 | NA | NA | NA | NA | NA |
| < 70 | 29/54 (54) | NA | NA | NA | NA | 5/21 (24) | 0.022 | NA | NA | 21/57 (37) | 0.088 | NA | NA | NA |
| CRP (mg/L) | 293 (228-388) / 299 ± 140,3 | 220 (155-313) | - | 263.4 ± 126.4 | 0.42 | NA | NA | 338.5 (197.5-464.5) | NA | NA | NA | NA | NA | 237 (166–301) |
| > 150 | 47/55 (85) | NA | NA | 9/12 (75) | 0.40 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| > 282 | 32/55 (58) | NA | NA | 7/12 (58) | 1 | NA | NA | 7/12 (58) | 1 | NA | NA | NA | NA | NA |
| Kalemia (mmol/L) | 3.6 (3.3-4.1) | 3.5 (3.1–3.5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| < 3,5 | 19/54 (35) | 62 (46) | 0.009 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lactate (mmol/L) | 3.6 (2-6) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1.7 (1.1–2.6) |
| > 2.5 | 24/41 (59) | 16 (18) | <0.001 | NA | NA | NA | NA | NA | NA | 30/50 (60) | 1 | NA | NA | NA |
| CPK (IU/L) (median IQR 25-75) | 232 (65-646) | 2085 (1010–4875) | NA | NA | NA | NA | 953 (204-1332) | NA | NA | NA | NA | NA | 94 (55–192) | |
| > 443 | 13/41 (32) | NA | NA | NA | NA | NA | NA | 5/9 (56) | 1 | NA | NA | NA | NA | NA |
| >1000 | 8/41 (20) | NA | NA | NA | NA | 5/18 (28) | 0.50 | NA | NA | NA | NA | NA | NA | NA |
| LDH (IU/L) (median IQR 25-75) | 950 (462-6123) / 2294,6 ± 1744,9 | NA | NA | 576.0 ± 579.6 | 0.0013 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| > 500 | 24/34 (71) | NA | NA | 3/7 (43) | 0.20 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| > 800 | 16/34 (47) | NA | NA | 1/7 (14) | 0.21 | 3/17 (18) | 0.065 | NA | NA | NA | NA | NA | NA | NA |
| Lipase (IU/L) | 164 (69-543) | 54 (28–150) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| >60 | 25/31 (81) | NA | NA | NA | NA | 2/8 (25) | 0.0056 | NA | NA | NA | NA | NA | NA | NA |
| Base excess < –5 mmol/L | 26/54 (48) | 18 (16) | <0.001 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ID reference (BIGSdb) | Species | Serogroup | Number CGs of cgMLST in BIGSdb | Genus human strains in data base BIGdb (n=91) | Genus human strains in this study (n=19) |
| 148 | borgpetersenii | Mini | 78 | 37 (41%) | 13 (68%) |
| 150 | borgpetersenii | Pomona | 80 | 5 (5%) | 0 |
| 729 | borgpetersenii | Unknown | 145 | 1 (1%) | 0 |
| 159 | interrogans | Pyrogenes | 81 | 13 (14%) | 4 (21%) |
| 174 | kirschneri | Grippotyphosa | 85 | 8 (9%) | 0 |
| 112 | kirschneri | Mini | 63 | 5 (5%) | 1 (5%) |
| 113 | kirschneri | Unknown | 64 | 3 (3%) | 0 |
| 175 | kirschneri | Mini | 83 | 1 (1%) | 0 |
| 172 | kirschneri | Mini | 84 | 1 (1%) | 0 |
| 178 | mayottensis | Unknown | 82 | 16 (18%) | 0 |
| 149 | mayottensis | Mini | 79 | 1 (1%) | 1 (5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





