Submitted:
27 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Data Selection and Extraction
3. Results
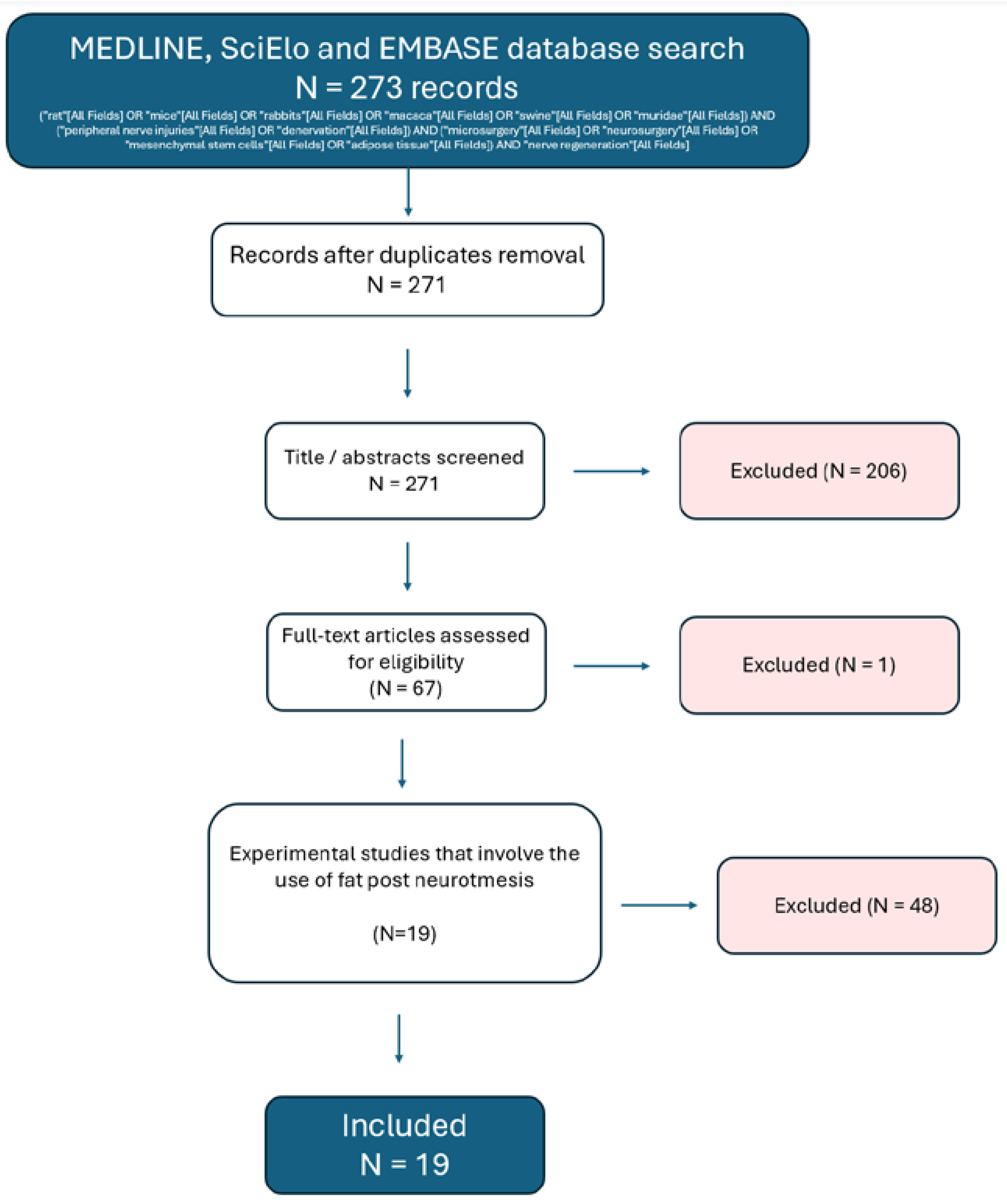
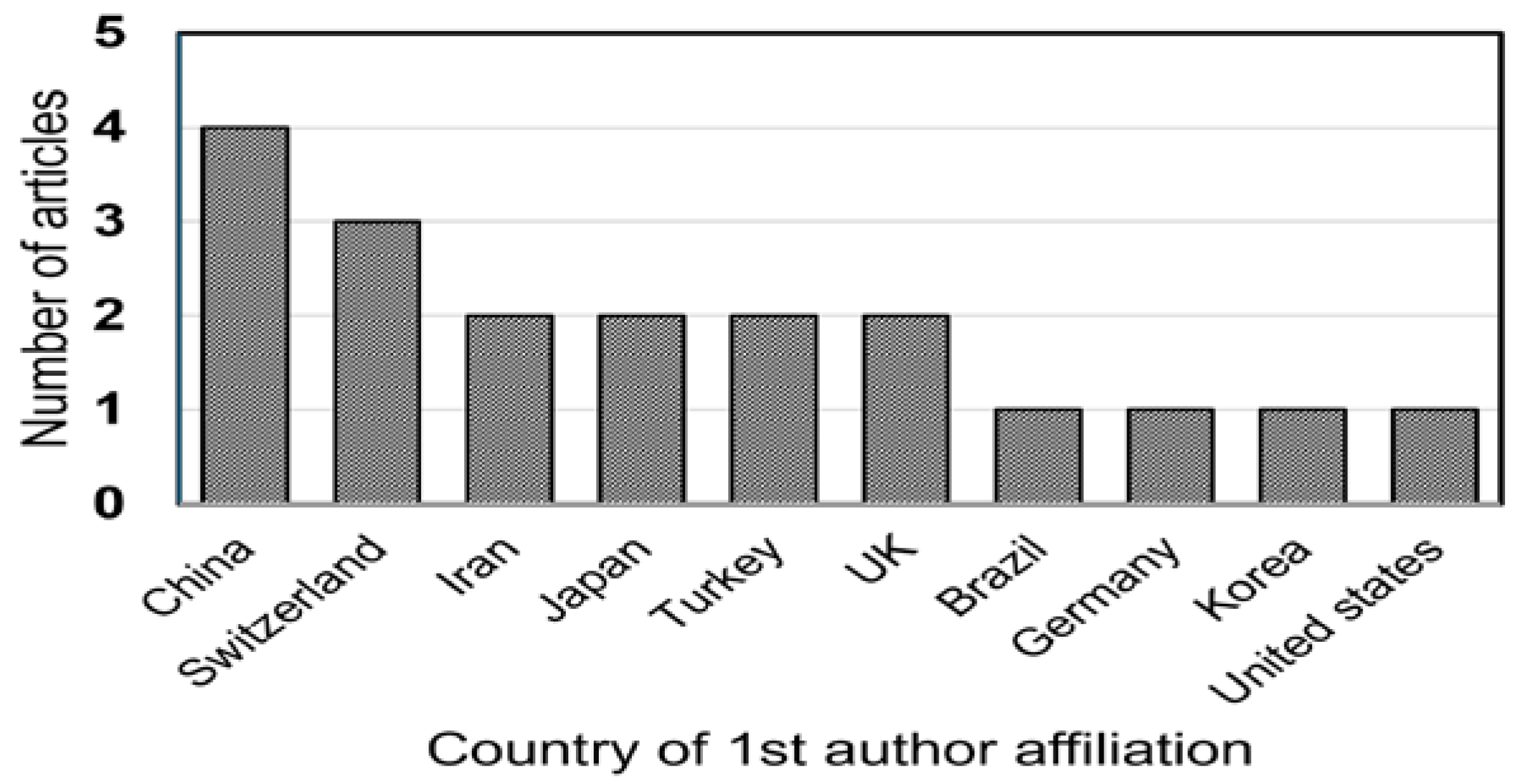
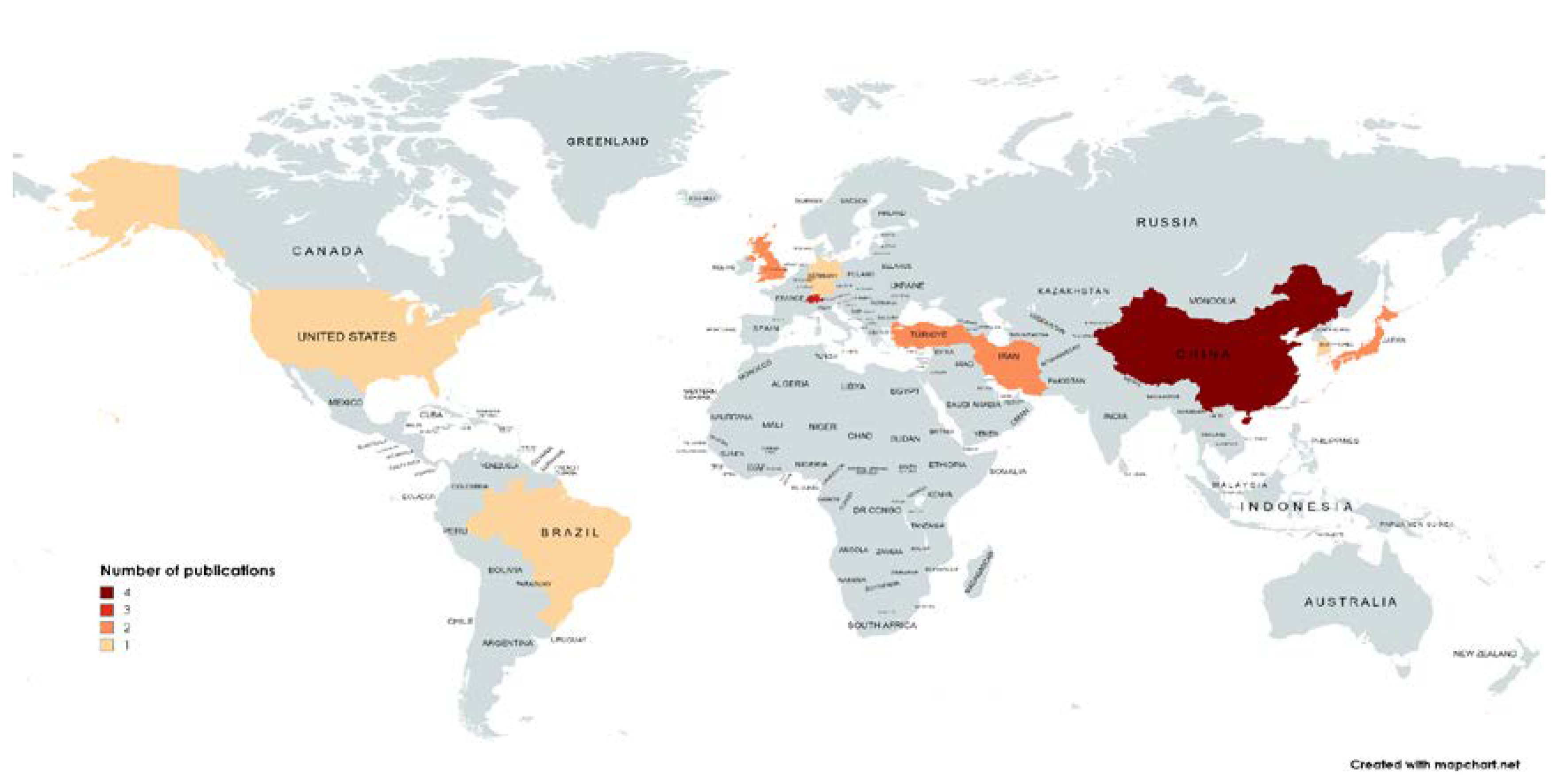
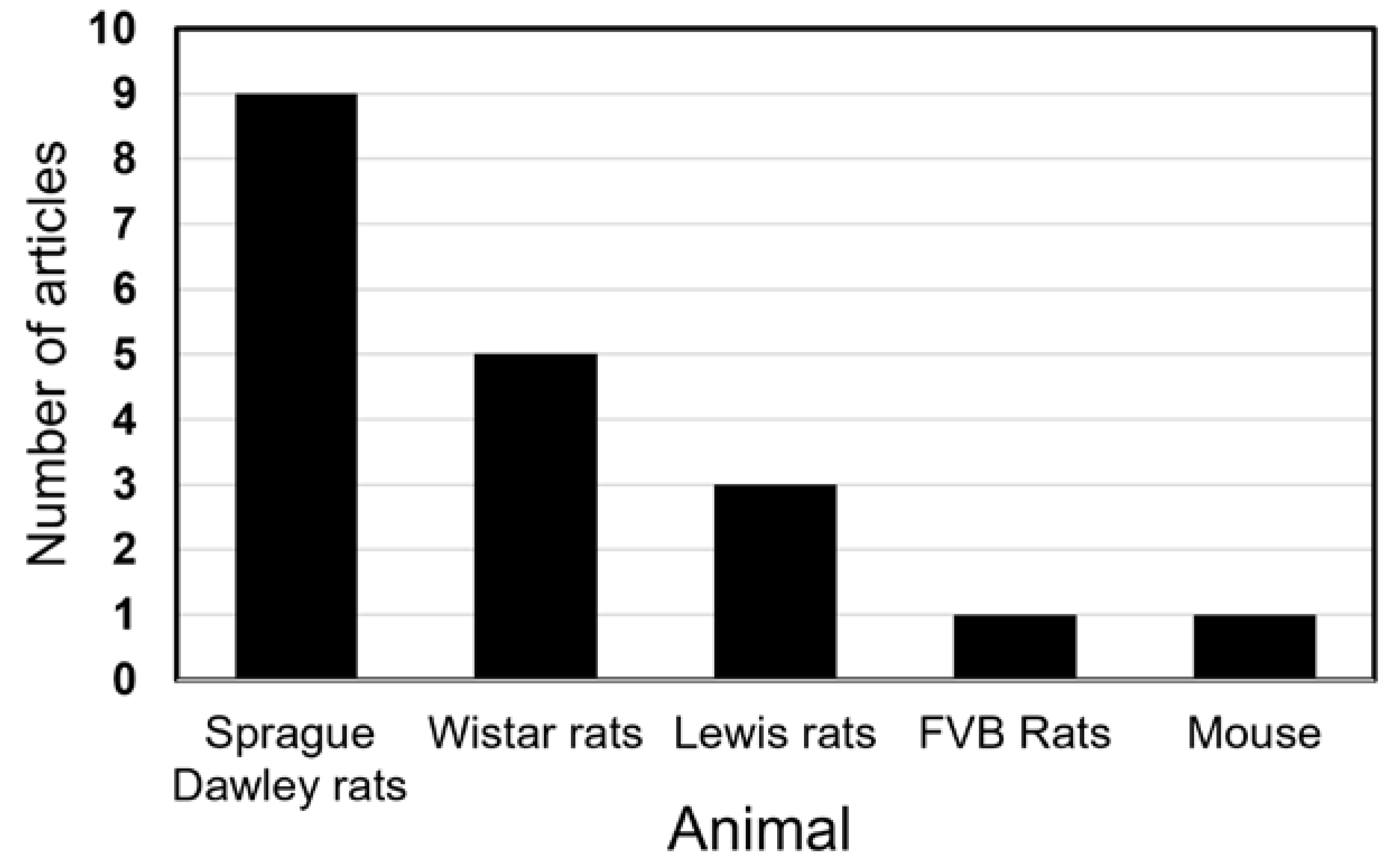
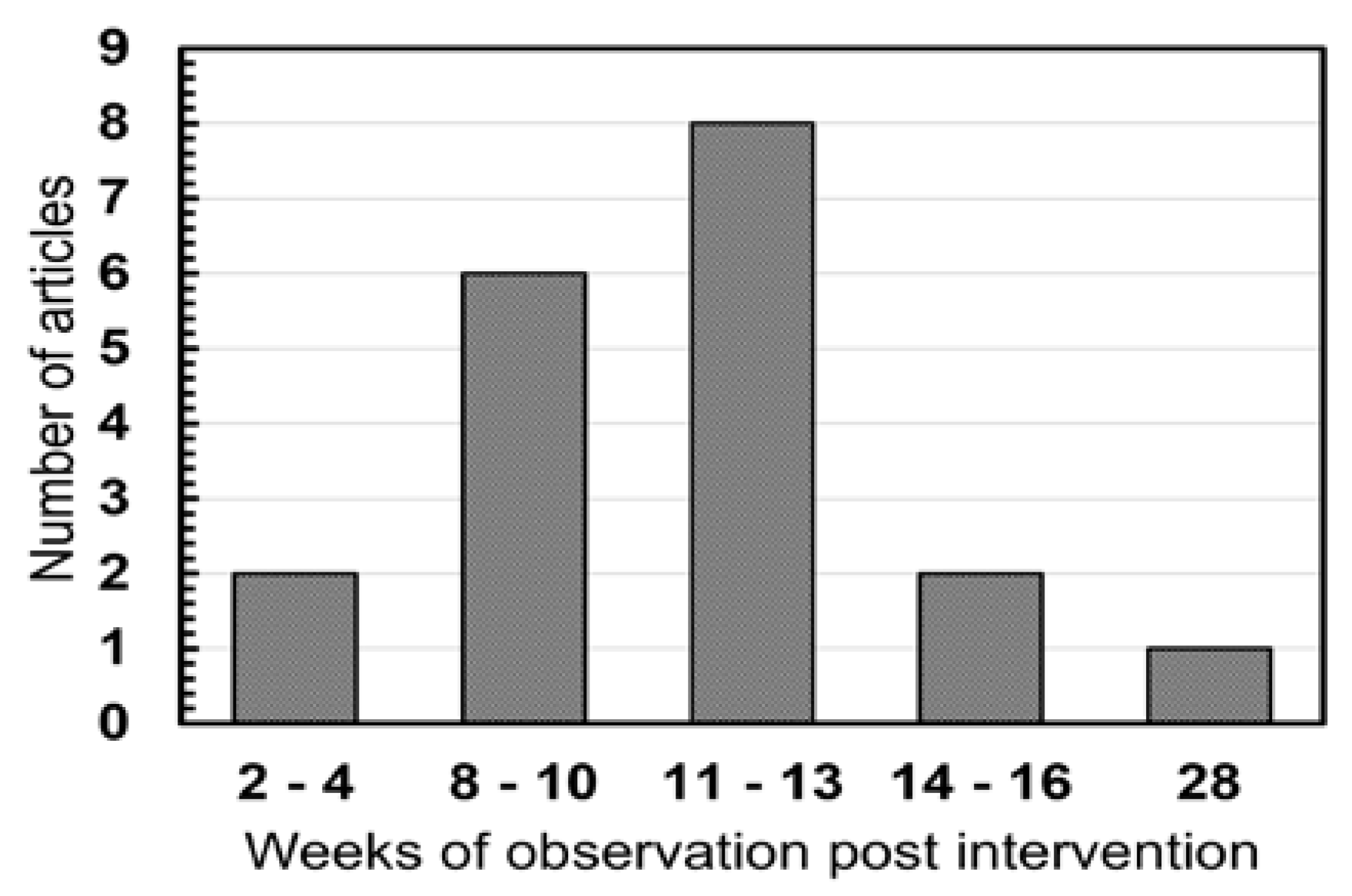
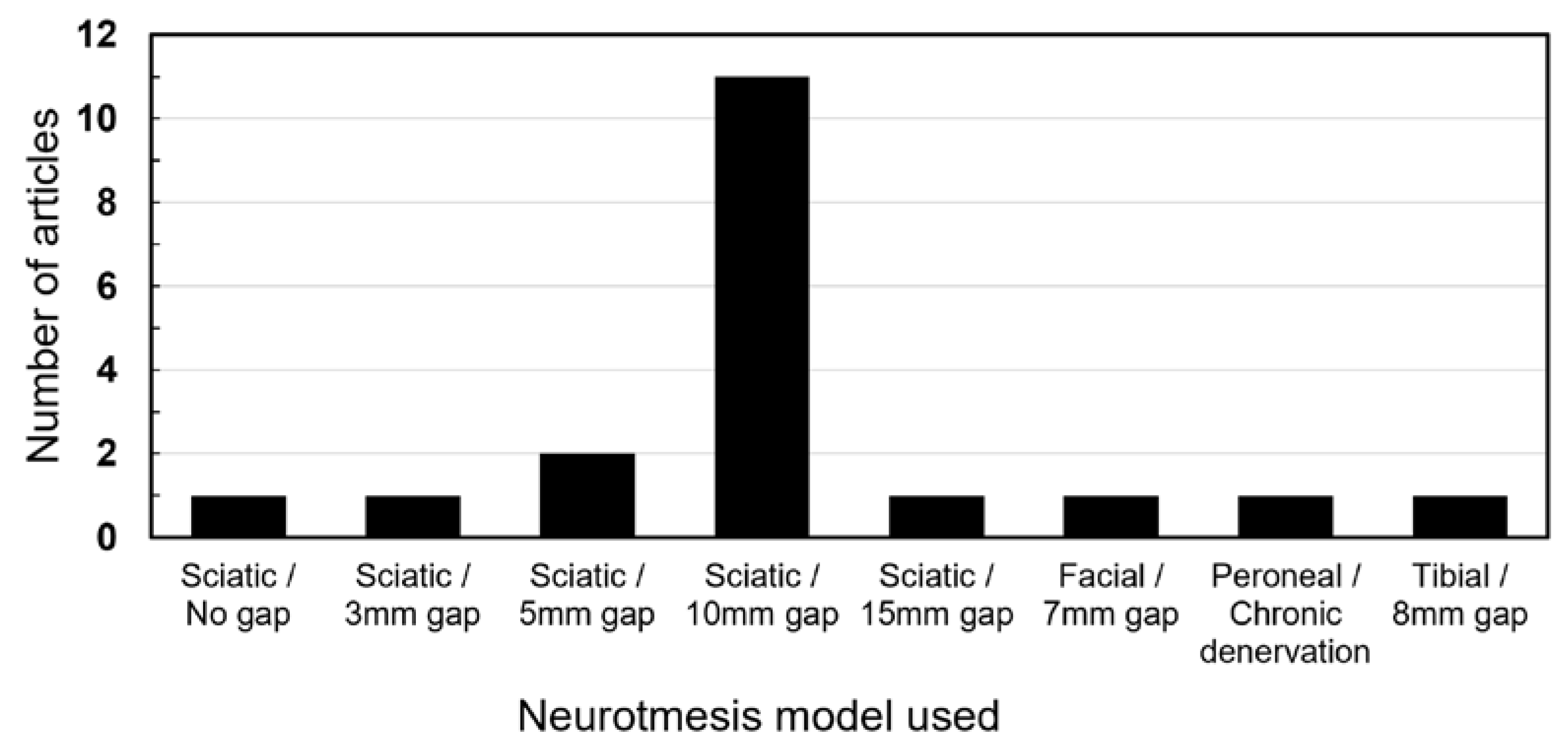
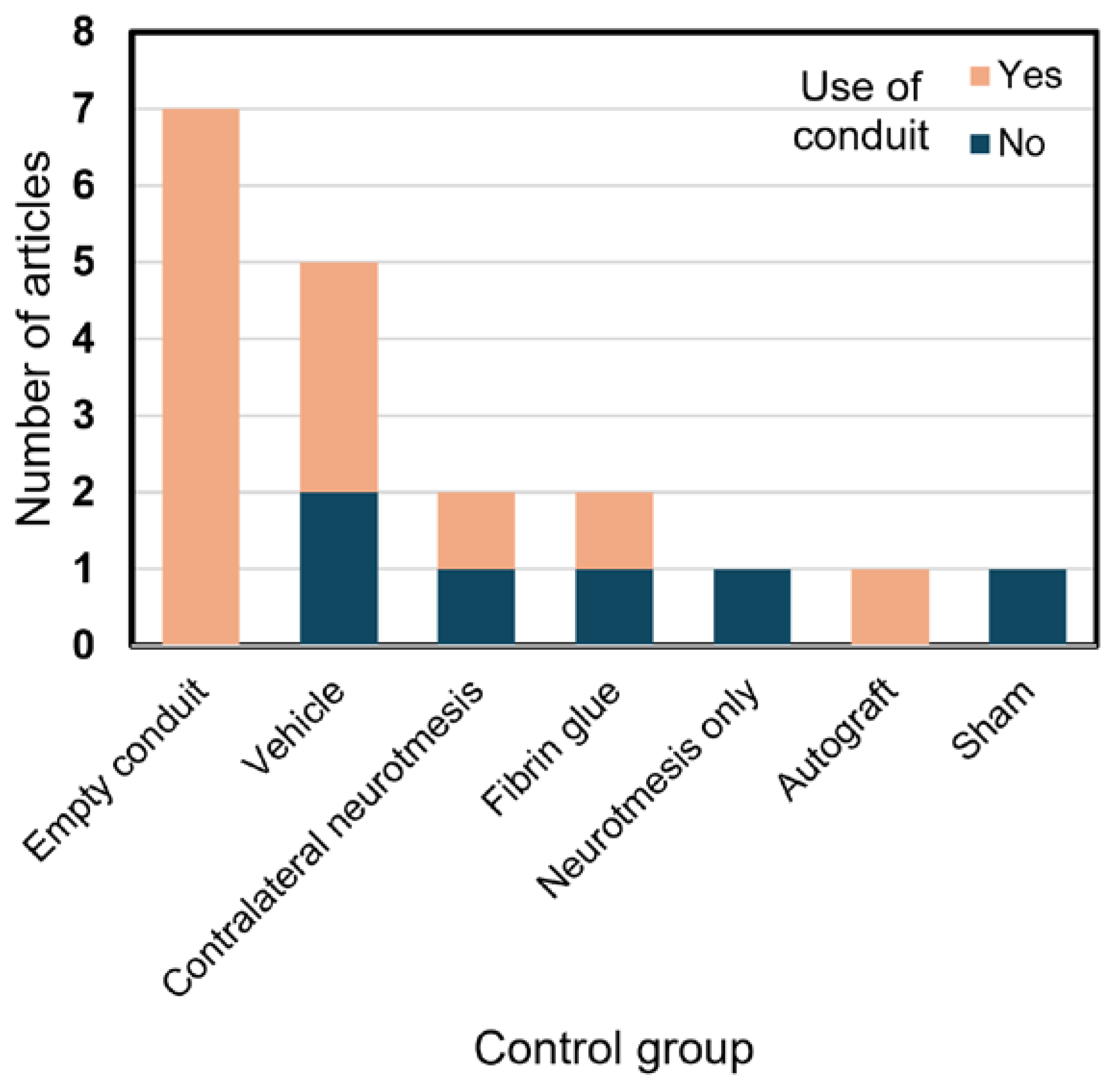
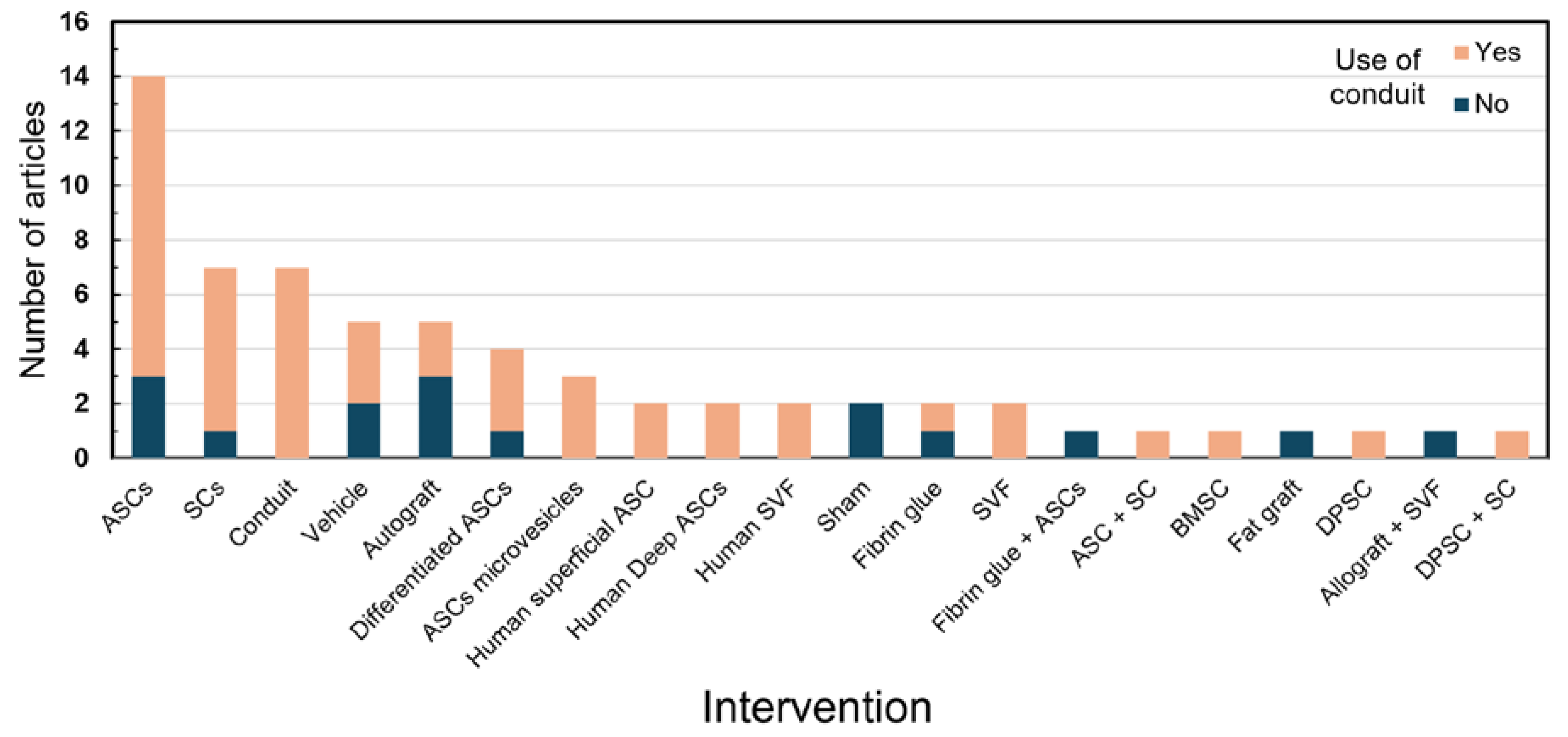
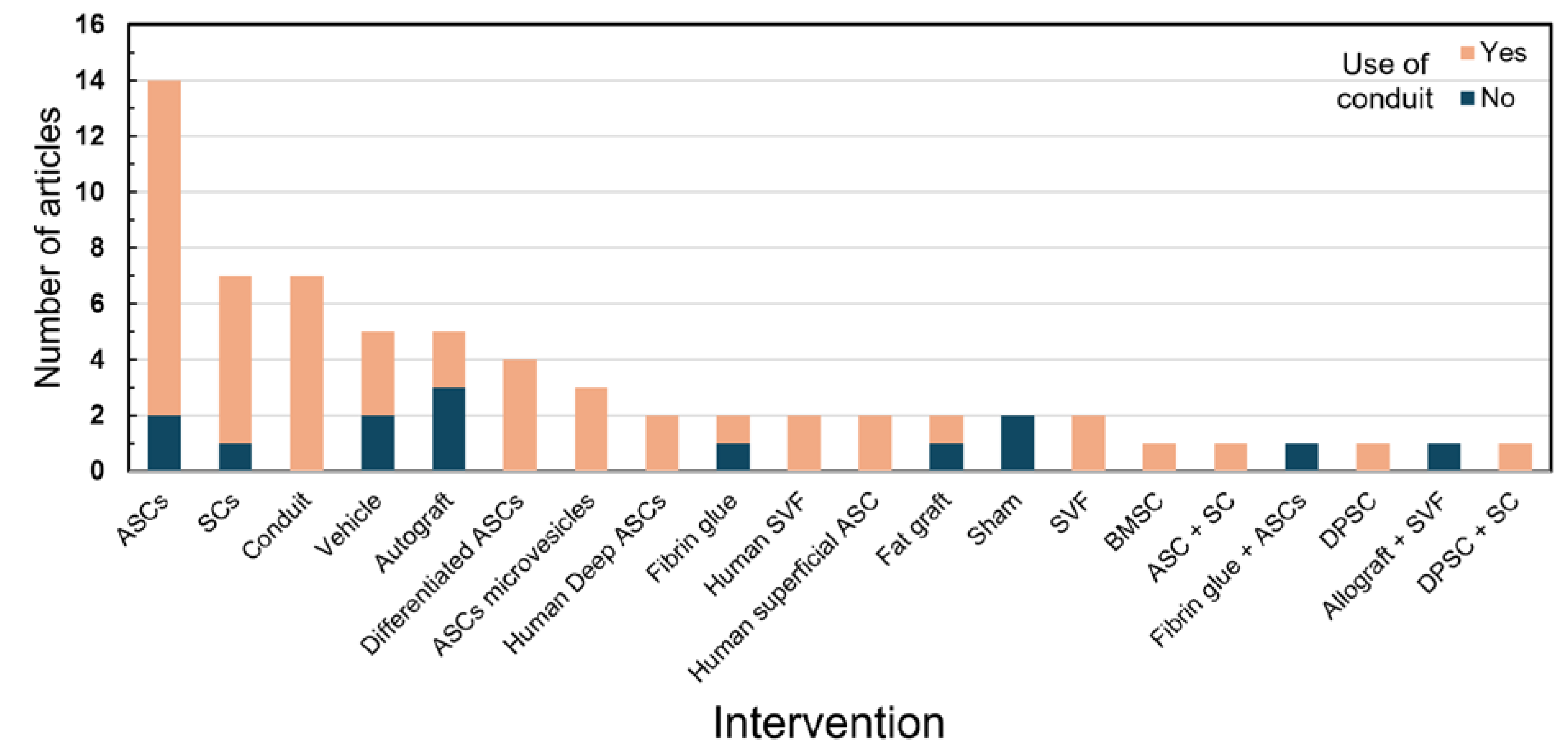
4. Discussion
4.1. General Aspects
4.2. Fat Grafting and Stem Cells
4.3. Trauma Mechanism
4.4. Functional Analysis
4.5. Histological Analysis
4.6. Other Analysis
4.7. Strengths and Limitations
5. Conclusion
Acknowledgements
Formatting of funding sources
Declarations of interest
References
- England JD, Asbury AK. Peripheral neuropathy. The Lancet. 2004 Jun;363(9427):2151–61.
- Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. Journal of Neurology, Neurosurgery & Psychiatry [Internet]. 1997 Apr 1;62(4):310–8. Available from:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1074084/pdf/jnnpsyc00004-0006.pdf.
- Robinson LR. Traumatic injury to peripheral nerves. Muscle & Nerve. 2022 Sep 7;66(6):661–70.
- Scholz TD, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar KZ, et al. Peripheral Nerve Injuries: An International Survey of Current Treatments and Future Perspectives. 2009 Mar 19;25(06):339–44.
- Hems T. Nerve injury: Classification, clinical assessment, investigation, and management. [cited 2022 Mar 25]; Available from: https://series.publisso.de/de/publisso_gold/export/chapter/43/pdf/lhhs000030.pdf.
- Robinson LR. Electrodiagnosis and Rehabilitation of Peripheral Nerve Injuries. The Japanese Journal of Rehabilitation Medicine. 2004;41(Supplement):S71–1.
- Walsh S, _ _, Midha R. Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurgical Focus. 2009 Feb;26(2):E2.
- Leberfinger AN, Ravnic DJ, Payne R, Rizk E, Koduru SV, Hazard SW. Adipose-Derived Stem Cells in Peripheral Nerve Regeneration. Current Surgery Reports. 2017 Feb;5(2).
- Nichols CM, Myckatyn TM, Rickman SR, Fox IK, Hadlock T, Mackinnon SE. Choosing the correct functional assay: A comprehensive assessment of functional tests in the rat. Behavioural Brain Research. 2005 Sep;163(2):143–58.
- Erba P, Mantovani C, Kalbermatten DF, Gerhard Pierer, Giorgio Terenghi, Kingham PJ. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. Journal of Plastic Reconstructive and Aesthetic Surgery. 2010 Dec 1;63(12):e811–7.
- Gentile P, Calabrese C, De Angelis B, Pizzicannella J, Kothari A, Garcovich S. Impact of the Different Preparation Methods to Obtain Human Adipose-Derived Stromal Vascular Fraction Cells (AD-SVFs) and Human Adipose-Derived Mesenchymal Stem Cells (AD-MSCs): Enzymatic Digestion Versus Mechanical Centrifugation. International Journal of Molecular Sciences. 2019 Nov 2;20(21):5471.
- Verpaele A, Tonnard P. Discussion: Nanofat Cell Aggregates: A Nearly Constitutive Stromal Cell Inoculum for Regenerative Site-Specific Therapies. Plastic and reconstructive surgery/PSEF CD journals. 2019 Nov 1;144(5):1089–90.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. A declaração PRISMA 2020: diretriz atualizada para relatar revisões sistemáticas. Revista Panamericana de Salud Pública [Internet]. 2022 Dec 30;46:1. Available from: https://iris.paho.org/bitstream/handle/10665.2/56882/v46e1122022.pdf?sequence=5.
- Reid AJ, Sun M, Wiberg M, Downes S, Terenghi G, Kingham PJ. Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience. 2011 Dec;199:515–22.
- di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011 May;181:278–91.
- Tong X. Transplantation of adipose-derived stem cells for peripheral nerve repair. International Journal of Molecular Medicine. 2011 Jun 17.
- Tomita K, Madura T, Mantovani C, Terenghi G. Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. Journal of Neuroscience Research. 2012 Mar 15;90(7):1392–402.
- Hea Gu J, Hwa Ji Y, Dhong ES, Hwee Kim D, Yoon ES. Transplantation of Adipose Derived Stem Cells for Peripheral Nerve Regeneration in Sciatic Nerve Defects of the Rat. Current Stem Cell Research & Therapy. 2012 Aug 1;7(5):347–55.
- Dai LG, Huang GS, Hsu SH. Sciatic Nerve Regeneration by Cocultured Schwann Cells and Stem Cells on Microporous Nerve Conduits. Cell Transplantation. 2013 Nov;22(11):2029–39.
- Raisi A, Azizi S, Delirezh N, Heshmatian B, Farshid AA, Amini K. The mesenchymal stem cell–derived microvesicles enhance sciatic nerve regeneration in rat. Journal of Trauma and Acute Care Surgery. 2014 Apr;76(4):991–7.
- Kappos EA, Engels PE, Tremp M, Meyer zu Schwabedissen M, di Summa P, Fischmann A, et al. Peripheral Nerve Repair: Multimodal Comparison of the Long-Term Regenerative Potential of Adipose Tissue-Derived Cells in a Biodegradable Conduit. Stem Cells and Development [Internet]. 2015 Sep 15 [cited 2024 Apr 22];24(18):2127–41. Available from: https://pubmed.ncbi.nlm.nih.gov/26134465/.
- Mohammadi R, Moein Mehrtash, Moeid Mehrtash, Seyedeh-Sepideh Sajjadi. Nonexpanded Adipose Stromal Vascular Fraction Local Therapy on Peripheral Nerve Regeneration Using Allografts. Journal of Investigative Surgery. 2015 Dec 18;29(3):149–56.
- Reichenberger MA, Mueller W, Hartmann J, Diehm Y, Lass U, Koellensperger E, et al. ADSCs in a fibrin matrix enhance nerve regeneration after epineural suturing in a rat model. Microsurgery. 2015 Dec 30;36(6):491–500.
- Tremp M, Meyer Zu Schwabedissen M, Kappos EA, Engels PE, Fischmann A, Scherberich A, et al. The regeneration potential after human and autologous stem cell transplantation in a rat sciatic nerve injury model can be monitored by MRI. Cell Transplantation [Internet]. 2015 [cited 2024 Apr 22];24(2):203–11. Available from: https://pubmed.ncbi.nlm.nih.gov/24380629/.
- He X, Ao Q, Wei Y, Song J. Transplantation of miRNA-34a overexpressing adipose-derived stem cell enhances rat nerve regeneration. Wound Repair and Regeneration: Official Publication of the Wound Healing Society [and] the European Tissue Repair Society [Internet]. 2016 May 1 [cited 2024 Apr 22];24(3):542–50. Available from: https://pubmed.ncbi.nlm.nih.gov/26899299/.
- Sowa Y, Kishida T, Imura T, Numajiri T, Nishino K, Tabata Y, et al. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo without Differentiation into Schwann-Like Lineage. Plastic and Reconstructive Surgery [Internet]. 2016 Feb 1 [cited 2024 Apr 22];137(2):318e330e. Available from: https://pubmed.ncbi.nlm.nih.gov/26818322/.
- Irkoren S. Repairing peripheral nerve defects by vein grafts filled with adipose tissue derived stromal vascular fraction: an experimental study in rats. Turkish Journal of Trauma and Emergency Surgery. 2015.
- Shimizu M, Matsumine H, Osaki H, Ueta Y, Tsunoda S, Kamei W, et al. Adipose-derived stem cells and the stromal vascular fraction in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Wound Repair and Regeneration. 2018 Oct 25;26(6):446–55.
- Chen J, Ren S, Duscher D, Kang Y, Liu Y, Wang C, et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. Journal of Cellular Physiology. 2019 May 23;234(12):23097–110.
- Durço DFPA, Pestana FM, Oliveira JT, Ramalho B dos S, Souza LM, Cardoso FS, et al. Grafts of human adipose-derived stem cells into a biodegradable poly (acid lactic) conduit enhances sciatic nerve regeneration. Brain Research. 2020 Nov;1747:147026.
- Schilling BK, Baker JS, Komatsu C, Turer DM, Bengur FB, Nerone WV, et al. Intramuscular Nanofat Injection Promotes Inflammation-Induced Gastrocnemius Regeneration in a Syngeneic Rat Sciatic Nerve Injury Model. Plastic and Reconstructive Surgery [Internet]. 2023 Jun 1 [cited 2024 Apr 22];151(6):947e958e. Available from: https://pubmed.ncbi.nlm.nih.gov/36728782/.
- Kastamoni M, Yavaş SE, Ozgenel GY, Ersoy S. The effects of fat graft and platelet-rich fibrin combination after epineurectomy in rats. Revista da Associação Médica Brasileira [Internet]. 2023 Mar 3 [cited 2024 Apr 16];69:272–8. Available from: https://www.scielo.br/j/ramb/a/qJvqDHfjC6vzNg3xKZryQNS/?lang=en#.
- Woolston C. What China’s leading position in natural sciences means for global research. Nature [Internet]. 2023 Aug 9 [cited 2023 Sep 13];620(7973):S2–5. Available from: https://www.nature.com/articles/d41586-023-02159-7#:~:text=China%20is%20already%20one%20of.
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Raff M, editor. Molecular Biology of the Cell. 2002 Dec;13(12):4279–95.
- Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X, et al. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomedicine & Pharmacotherapy [Internet]. 2019 Jun 1;114:108765. Available from: https://www.sciencedirect.com/science/article/pii/S0753332219307346.
- Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells – a review. Biotechnology Advances. 2018 Jul;36(4):1111–26.
- Kokai LE, Rubin JP, Marra KG. The Potential of Adipose-Derived Adult Stem Cells as a Source of Neuronal Progenitor Cells. Plastic and Reconstructive Surgery. 2005 Oct;116(5):1453–60.
- Li X, Zhao S, Wang L. Therapeutic effect of adipose-derived stem cell transplantation on optic nerve injury in rats. Molecular Medicine Reports. 2017 Nov 20;
- Ahmet Gokce, Peak TC, Abdel-Mageed AB, Hellstrom WJ. Adipose Tissue-Derived Stem Cells for the Treatment of Erectile Dysfunction. Current urology reports. 2016 Jan 13;17(2).
- Kim YJ, Jeong JH. Clinical Application of Adipose Stem Cells in Plastic Surgery. Journal of Korean Medical Science. 2014;29(4):462.
- Bin Bin Wang, Guo C, Sheng Qiao Sun, Xing Nan Zhang, Li Z, Wei Jie Li, et al. Comparison of the Nerve Regeneration Capacity and Characteristics between Sciatic Nerve Crush and Transection Injury Models in Rats. PubMed. 2023 Feb 20;36(2):160–73.
- Martins RS, Siqueira MG, Silva CF da, Plese JPP. Correlation between parameters of electrophysiological, histomorphometric and sciatic functional index evaluations after rat sciatic nerve repair. Arquivos de Neuro-Psiquiatria. 2006 Sep;64(3b):750–6.
- de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Experimental Neurology. 1982 Sep;77(3):634–43.
- Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. Journal of Plastic, Reconstructive & Aesthetic Surgery [Internet]. 2012 May 1 [cited 2022 Aug 23];65(5):657–64. Available from: https://www.jprasurg.com/article/S1748-6815(11)00652-8/fulltext.
- Shen N, Zhu J. Application of sciatic functional index in nerve functional assessment. Microsurgery. 1995;16(8):552–5.
- Shenaq JM, Shenaq SM, Spira M. Reliability of sciatic function index in assessing nerve regeneration across a 1 cm gap. Microsurgery. 1989;10(3):214–9.
- Moattari M, Kouchesfehani HM, Kaka G, Sadraie SH, Naghdi M, Mansouri K. Chitosan-film associated with mesenchymal stem cells enhanced regeneration of peripheral nerves: A rat sciatic nerve model. Journal of Chemical Neuroanatomy. 2018 Mar;88:46–54.
- Guo Q, Liu C, Hai B, Ma T, Zhang W, Tan J, et al. Chitosan conduits filled with simvastatin/Pluronic F-127 hydrogel promote peripheral nerve regeneration in rats. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2017 Apr 3;106(2):787–99.
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of Neurotrauma [Internet]. 1995 Feb 1;12(1):1–21. Available from: https://pubmed.ncbi.nlm.nih.gov/7783230/.
- Bervar M. An alternative video footprint analysis to assess functional loss following injury to the rat sciatic nerve. Acta Chirurgiae Plasticae [Internet]. 2002 [cited 2024 Apr 22];44(3):86–9. Available from: https://pubmed.ncbi.nlm.nih.gov/12514995/.
- Bain JR, Mackinnon SE, Hunter DA. Functional Evaluation of Complete Sciatic, Peroneal, and Posterior Tibial Nerve Lesions in the Rat. Plastic and Reconstructive Surgery. 1989 Jan;83(1):129–36.
- Pollari E, Prior R, Robberecht W, Van Damme P, Van Den Bosch L. In Vivo Electrophysiological Measurement of Compound Muscle Action Potential from the Forelimbs in Mouse Models of Motor Neuron Degeneration. Journal of Visualized Experiments. 2018 Jun 15;(136).
- Mohammadi R, Sanaei N, Ahsan S, Masoumi-Verki M, Khadir F, Mokarizadeh A. Stromal vascular fraction combined with silicone rubber chamber improves sciatic nerve regeneration in diabetes. Chinese Journal of Traumatology = Zhonghua Chuang Shang Za Zhi [Internet]. 2015 [cited 2024 Apr 22];18(4):212–8. Available from: https://pubmed.ncbi.nlm.nih.gov/26764542/.
- Ao Q, Fung CK, Yat-Ping Tsui A, Cai S, Zuo HC, Chan YS, et al. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011 Jan;32(3):787–96.
- Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, et al. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)–collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Research. 2000 Jun;868(2):315–28.
- Bin Bin Wang, Guo C, Sheng Qiao Sun, Xing Nan Zhang, Li Z, Wei Jie Li, et al. Comparison of the Nerve Regeneration Capacity and Characteristics between Sciatic Nerve Crush and Transection Injury Models in Rats. PubMed. 2023 Feb 20;36(2):160–73.
- Wang G, Lu G, Ao Q, Gong Y, Zhang X. Preparation of cross-linked carboxymethyl chitosan for repairing sciatic nerve injury in rats. Biotechnology Letters [Internet]. 2010 Jan 1 [cited 2024 Apr 22];32(1):59–66. Available from: https://pubmed.ncbi.nlm.nih.gov/19760120/.
- Ghnenis AB, Czaikowski RE, Zhang ZJ, Bushman JS. Toluidine Blue Staining of Resin-Embedded Sections for Evaluation of Peripheral Nerve Morphology. Journal of Visualized Experiments. 2018 Jul 3;(137).
- Sridharan G, Shankar A. Toluidine blue: A review of its chemistry and clinical utility. Journal of Oral and Maxillofacial Pathology [Internet]. 2012;16(2):251. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3424943/.
- Scipio FD, Raimondo S, Tos P, Geuna S. A simple protocol for paraffin-embedded myelin sheath staining with osmium tetroxide for light microscope observation. Microscopy Research and Technique. 2008 Jul;71(7):497–502.
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nature Reviews Neuroscience [Internet]. 2005 Sep 1;6(9):671–82. Available from: https://www.nature.com/articles/nrn1746.
- Jessen KR, Mirsky R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Frontiers in Cellular Neuroscience [Internet]. 2019 Feb 11 [cited 2019 Sep 26];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6378273/.
- Harboe M, Torvund-Jensen J, Kjaer-Sorensen K, Laursen LS. Ephrin-A1-EphA4 signaling negatively regulates myelination in the central nervous system. Glia. 2018 Jan 19;66(5):934–50.
- Wang Y, Zheng Z, Hu D. Inhibition of EphA4 expression promotes Schwann cell migration and peripheral nerve regeneration. Neuroscience Letters. 2013 Aug;548:201–5.
- Chen R, Yang X, Zhang B, Wang S, Bao S, Gu Y, et al. EphA4 Negatively Regulates Myelination by Inhibiting Schwann Cell Differentiation in the Peripheral Nervous System. Frontiers in Neuroscience. 2019 Nov 13;13.
- Wang Y, Sun B, Shibata B, Guo F. Transmission electron microscopic analysis of myelination in the murine central nervous system. STAR Protocols. 2022 Jun;3(2):101304.
- Yu Hwa Nam, Park S, Yum Y, Jeong S, Hyo Eun Park, Ho Jin Kim, et al. Preclinical Efficacy of Peripheral Nerve Regeneration by Schwann Cell-like Cells Differentiated from Human Tonsil-Derived Mesenchymal Stem Cells in C22 Mice. Biomedicines. 2023 Dec 17;11(12):3334–4.
- Rui B, Guo S, Zeng B, Wang J, Chen X. An implantable electrical stimulator used for peripheral nerve rehabilitation in rats. Experimental and Therapeutic Medicine. 2013 May 13;6(1):22–8.
- Ge J, Zhu S, Yang Y, Liu Z, Hu X, Huang L, et al. Experimental immunological demyelination enhances regeneration in autograft-repaired long peripheral nerve gaps. Scientific Reports [Internet]. 2016 Dec 23 [cited 2024 Apr 22];6(1):39828. Available from: https://www.nature.com/articles/srep39828.
- Sulong AF, Hassan NH, Hwei NM, Lokanathan Y, Naicker AS, Abdullah S, et al. Collagen-coated polylactic-glycolic acid (PLGA) seeded with neural-differentiated human mesenchymal stem cells as a potential nerve conduit. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University [Internet]. 2014 [cited 2024 Apr 22];23(3):353–62. Available from: https://pubmed.ncbi.nlm.nih.gov/24979505/.
- Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, et al. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience [Internet]. 2011 Aug 31 [cited 2020 Jun 15];31(35):12533–42. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21880915.
- McKinnon KM. Flow Cytometry: an Overview. Current Protocols in Immunology [Internet]. 2018 Feb 21;120(1):5.1.1–11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5939936/.
- Hayashi A, Moradzadeh A, Hunter D, Kawamura D, Puppala V, Tung T, et al. Retrograde Labeling in Peripheral Nerve Research: It Is Not All Black and White. Journal of Reconstructive Microsurgery. 2007 Oct;23(7):381–9.
- Catapano J, Willand MP, Zhang JJ, Scholl D, Gordon T, Borschel GH. Retrograde labeling of regenerating motor and sensory neurons using silicone caps. Journal of neuroscience methods. 2016 Feb 1;259:122–8.
- Chen L, Leng C, Ru Q, Xiong Q, Zhou M, Wu Y. Retrograde Labeling of Different Distribution Features of DRG P2X2 and P2X3 Receptors in a Neuropathic Pain Rat Model. BioMed Research International [Internet]. 2020 Jul 22;2020:9861459. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7396081/.
- Pettersson J, Kalbermatten DF, McGrath A, Novikova LN. Biodegradable fibrin conduit promotes long-term regeneration after peripheral nerve injury in adult rats. Journal of Plastic Reconstructive and Aesthetic Surgery. 2010 Nov 1;63(11):1893–9.
- Rodrı́guezFJ, Verdú E, Ceballos D, Navarro X. Nerve Guides Seeded with Autologous Schwann Cells Improve Nerve Regeneration. Experimental Neurology. 2000 Feb;161(2):571–84.
- Vleggeert-Lankamp CLAM. The role of evaluation methods in the assessment of peripheral nerve regeneration through synthetic conduits: a systematic review. Journal of Neurosurgery. 2007 Dec;107(6):1168–89.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




