Submitted:
20 May 2024
Posted:
21 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Desing
- -
- women aged 18-45 with regular menstrual cycles;
- -
- signed informed consent;
- -
- residing in Moscow.
- -
- endometriosis confirmed by surgical intervention or instrumental visualization methods (ultrasound and/or magnetic resonance imaging of the pelvic organs);
- -
- presence of one or more endometriosis symptoms: dysmenorrhea and/or dyspareunia, and/or chronic pelvic pain, and/or infertility.
- -
- absence of infertility (one or more childbirths);
- -
- regular menstrual cycle;
- -
- absence of severe dysmenorrhea;
- -
- absence of endometriosis confirmed by ultrasound investigation.
- -
- chronic diseases other than endometriosis (diabetes, hypertension, etc.);
- -
- infectious diseases: HIV and viral hepatitis (B and C);
- -
- hormonal medication intake within 3 months prior to study inclusion.
- 0-0.5 minutes: 15% B;
- linear gradient from 10% to 99% B over 15 minutes;
- 99% B for 4 minutes;
- linear gradient from 99% to 15% B over 15 minutes;
- 15% B for 2 minutes.
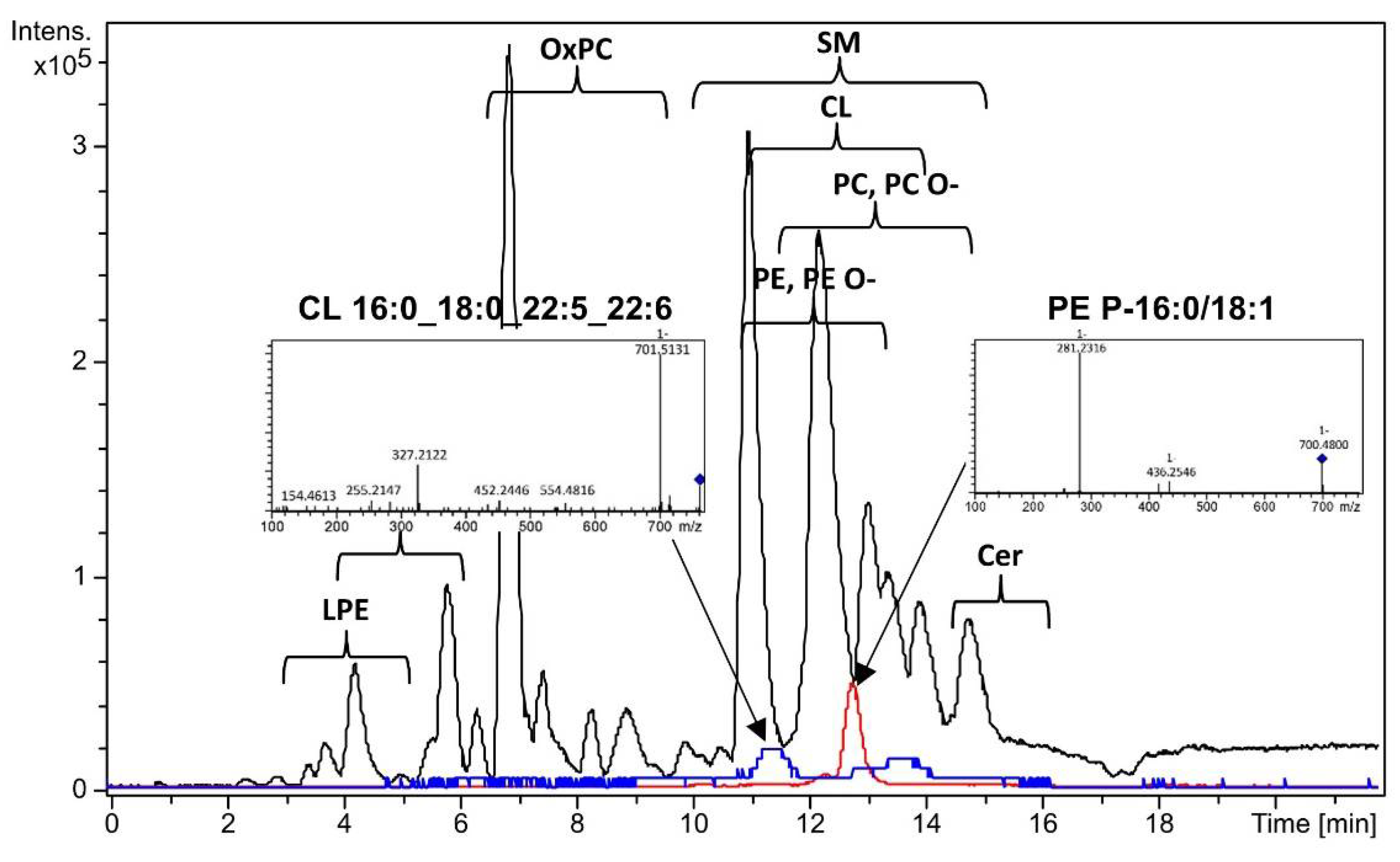
3.1. Dried Menstrual Blood Lipids Profiling
| Lipid | Control | Endometriosis | P | Fold change |
|---|---|---|---|---|
| Cer-NDS d18:0/24:0 | 0.58 (0.31;0.68) | 0.78 (0.53;1.26) | 0.03 | 1.34 |
| Cer-NP t18:0/26:0 | 0.68(0.28;0.91) | 1.19 (0.85;1.83) | 0.049 | 1.76 |
| Cer-NS d18:1/16:0 | 1.30(0.74;2.04) | 2.66 (1.29;3.69) | 0.02 | 2.05 |
| Cer-NS d18:1/24:1 | 0.11(0.10;0.36) | 0.30(0.14;0.52) | 0.007 | 2.87 |
| CerP d18:0/22:0 | 5.08 (3.97;5.70) | 3.52 (2.60;5.22) | 0.046 | 0.69 |
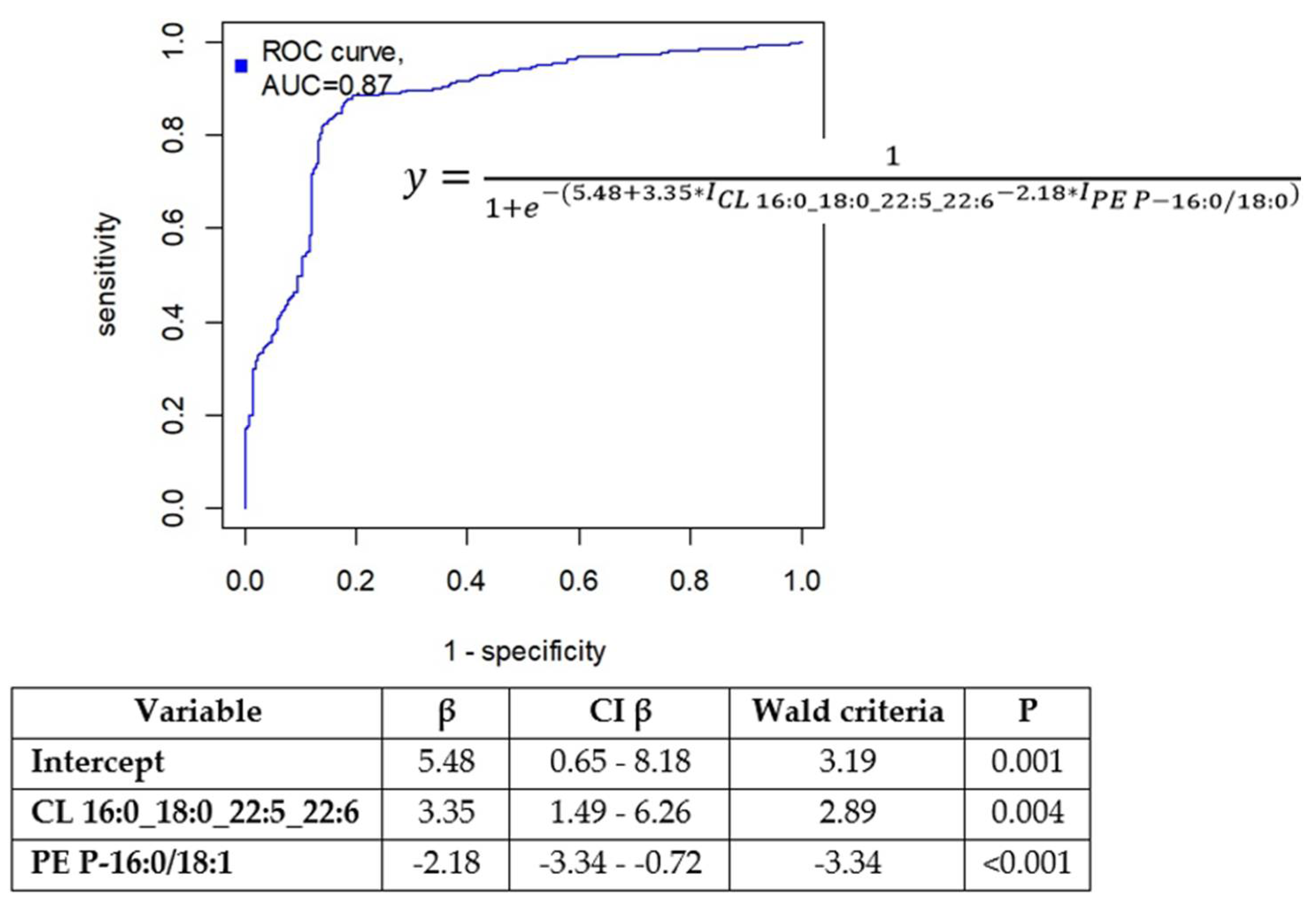
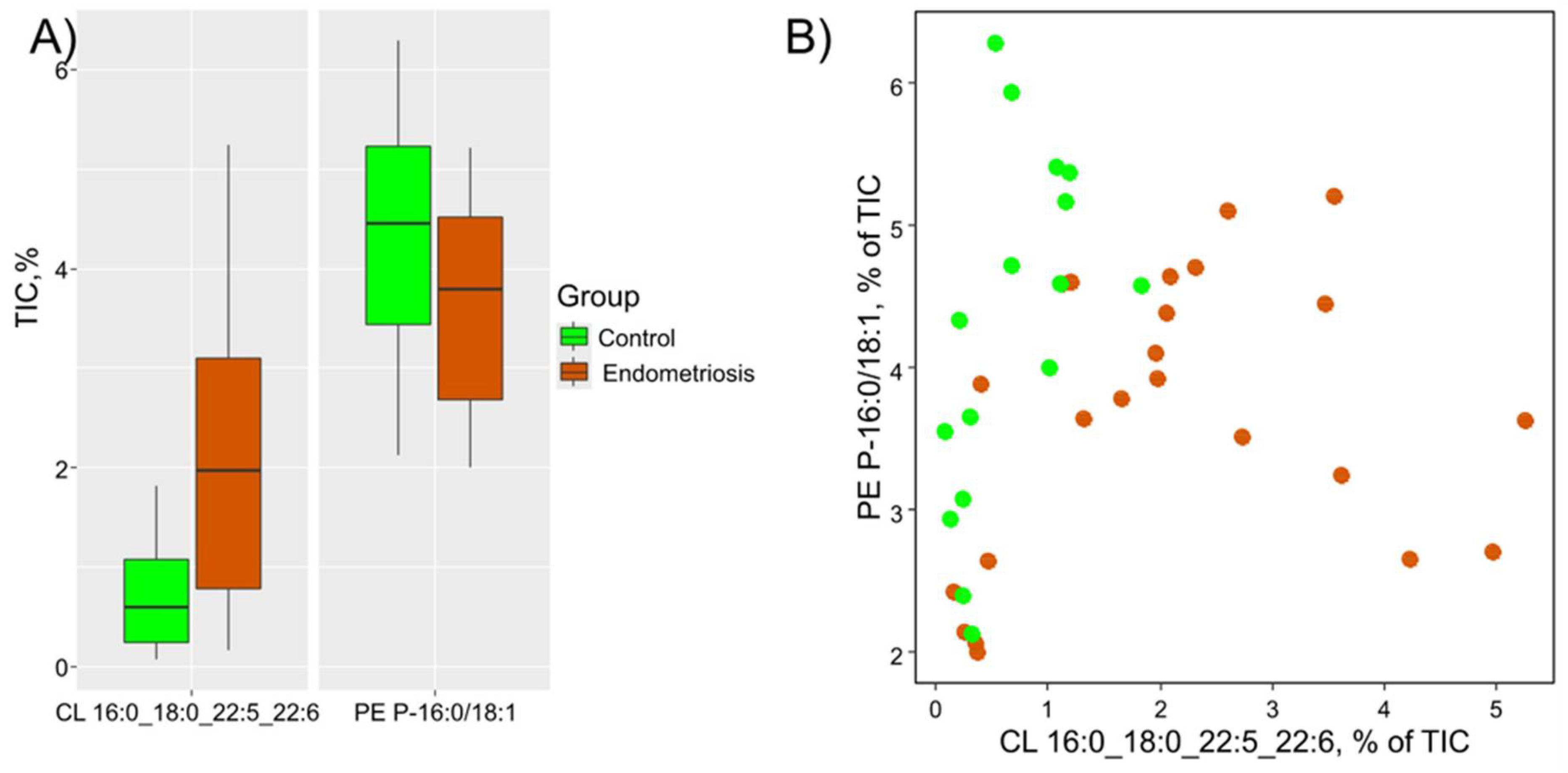
| CL 16:0_18:0_22:5_22:6 | 0.60(0.24;1.08) | 1.97 (0.79;3.09) | 0.001 | 3.31 |
| CL 18:0_18:0_18:1_20:1 | 0.23(0.14;0.28) | 0.32(0.19;0.47) | 0.03 | 1.43 |
| CL 18:1_18:1_18:1_20:3 | 0.81(0.43;1.11) | 1.64 (0.94;2.39) | 0.004 | 2.02 |
| CL 18:1_18:1_18:2_20:4 | 0.25(0.23;0.32) | 0.36(0.32;0.46) | <0.001 | 1.44 |
| CL 18:1_18:1_20:3_20:4 | 0.06(0.04;0.08) | 0.08(0.07;0.13) | 0.005 | 1.33 |
| OxCL 18:1_20:3(OOH)2_20:3(OOH)_20:3(OOH)2 | 0.04(0.03;0.07) | 0.10(0.05;0.18) | 0.03 | 2.38 |
| OxPC 16:0_18:1(OOO) | 0.13(0.09;0.16) | 0.24(0.15;0.37) | 0.009 | 1.95 |
| OxPC 16:0_20:4(OOH) | 0.13(0.07;0.17) | 0.24(0.15;0.30) | 0.001 | 1.90 |
| OxPC 18:1_18:3(OH) | 0.18(0.12;0.28) | 0.27(0.21;0.53) | 0.02 | 1.50 |
| OxPC 20:4_16:1(COOH) | 0.05(0.04;0.08) | 0.12(0.07;0.14) | 0.003 | 2.10 |
| PC 16:0_18:0 | 1.13 (0.78;2.35) | 2.86(1.25;0.34) | 0.02 | 2.52 |
| PC 16:0_18:1 | 10.10(4.54;14.82) | 3.41 (1.70;7.90) | 0.02 | 0.34 |
| PC 18:0_18:1 | 3.38 (2.50;3.90) | 1.98 (1.27;3.33) | 0.007 | 0.59 |
| PC 18:0_18:2 | 5.37 (3.68;6.82) | 2.29 (1.38;4.71) | 0.006 | 0.43 |
| PC 18:1_18:2 | 1.35 (1.06;2.48) | 0.78(0.67;1.30) | 0.03 | 0.58 |
| PE 16:0_18:1 | 0.32(0.28;0.35) | 0.38(0.34;0.41) | 0.004 | 1.18 |
| PE 16:0_18:2 | 0.15(0.10;0.29) | 0.22(0.16;0.29) | 0.04 | 1.48 |
| PE 16:0_20:4 | 0.37(0.30;0.45) | 0.51(0.44;0.57) | 0.005 | 1.40 |
| PE 18:1_18:2 | 0.12(0.08;0.16) | 0.15(0.14;0.17) | 0.01 | 1.34 |
| PG 18:1_18:1 | 0.33(0.22;0.40) | 0.42(0.31;0.58) | 0.04 | 1.29 |
| PC O-16:0/16:0 | 0.42(0.14;0.61) | 0.81(0.45;0.10) | 0.01 | 1.91 |
| PC O-16:0/20:4 | 0.16(0.11;0.18) | 0.22(0.19;0.25) | 0.003 | 1.37 |
| PC P-16:1/22:6 | 0.52(0.34;0.60) | 0.30(0.24;0.37) | 0.02 | 0.58 |
| PC P-18:0/18:1 | 0.46(0.39;0.55) | 0.39(0.34;0.43) | 0.03 | 0.84 |
| PE P-18:0/18:2 | 0.14(0.08;0.37) | 0.44(0.19;0.62) | 0.046 | 3.08 |
| PE P-18:0/20:4 | 2.48 (1.80;2.96) | 3.32 (2.56;3.62) | 0.03 | 1.34 |
| SM d18:0/18:0 | 0.24(0.17;0.30) | 0.20(0.14;0.23) | 0.01 | 0.83 |
| SM d18:1/24:0 | 4.05 (3.44;4.76) | 5.29 (3.98;6.08) | 0.02 | 1.31 |
| SM d20:0/18:2 | 0.27(0.18;0.36) | 0.36(0.31;0.61) | 0.003 | 1.32 |
| SM d22:6/20:2 | 0.09(0.04;0.12) | 0.12(0.08;0.15) | 0.047 | 1.37 |
| TG 16:1_18:1_18:2 | 1.72 (1.48;2.03) | 2.51 (1.66;2.91) | 0.03 | 1.46 |
| TG 16:1_18:2_18:2 | 0.28(0.19;0.33) | 0.38(0.29;0.52) | 0.01 | 1.37 |
| TG 18:1_18:2_18:2 | 1.48 (0.93;1.78) | 1.86 (1.36;2.73) | 0.03 | 1.26 |
| TG 18:1_18:2_18:3 | 0.58(0.39;1.21) | 1.10(0.07;1.61) | 0.01 | 1.92 |
| TG 18:1_22:3_8:0 | 2.18 (1.81;2.36) | 2.53 (2.20;2.95) | 0.02 | 1.16 |
3.2. Diagnostic Model Creation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; Deleire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Schliep, K.C.; Mumford, S.L.; Peterson, C.M.; Chen, Z.; Johnstone, E.B.; Sharp, H.T.; Stanford, J.B.; Hammoud, A.O.; Sun, L.; Buck Louis, G.M. Pain typology and incident endometriosis. Hum. Reprod. 2015, 30, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Moss, K.M.; Doust, J.; Homer, H.; Rowlands, I.J.; Hockey, R.; Mishra, G.D. Delayed diagnosis of endometriosis disadvantages women in ART: A retrospective population linked data study. Hum. Reprod. 2021, 36, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Oskotsky, T.T.; Falako, S.; Opoku-Anane, J.; Sirota, M. Endometriosis in the era of precision medicine and impact on sexual and reproductive health across the lifespan and in diverse populations. FASEB J. 2023, 37, e23130. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Filho, D.P.; de Oliveira, L.J.; do Amaral, V.F. Accuracy of laparoscopy for assessing patients with endometriosis. Sao Paulo Med. J. 2008, 126, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P.; Hummelshoj, L. Consensus on current management of endometriosis. Hum. Reprod. 2013, 28, 1552–1568. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Hull, M.; Fraser, I.; Miller, L.; Bossuyt, P.; Johnson, N.; Nisenblat, V. Endometrial biomarkers for the non-invasive diagnosis of endometriosis (Review). Cochrane Database Syst. Rev. 2016, 2016, CD012165. [Google Scholar] [CrossRef] [PubMed]
- Ametzazurra, A.; Matorras, R.; García-Velasco, J.A.; Prieto, B.; Simón, L.; Martínez, A.; Nagore, D. Endometrial fluid is a specific and non-invasive biological sample for protein biomarker identification in endometriosis. Hum. Reprod. 2009, 24, 954–965. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.J.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2016, CD012179. [Google Scholar] [CrossRef]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, K.; Bourdon, M.; Bartkowski, R.; Verbanck, M.; Chapron, C.; Marcellin, L.; Batteux, F.; Santulli, P.; Doridot, L. Menstrual Blood Donation for Endometriosis Research: A Cross-Sectional Survey on Women’s Willingness and Potential Barriers. Reprod. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Young, S.; Cruz, G.; Blumenthal, P.D. Screening for High-Risk Human Papillomavirus Using Passive, Self-Collected Menstrual Blood. Obstet. Gynecol. 2022, 140, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, B.; Prinz, M.; Siegel, D. Proteomic analysis of menstrual blood. Mol. Cell. Proteomics 2012, 11, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, Y.; Yan, L.; Zhang, Y.; Li, Y.; Zhu, Q.; Xia, W.; Ge, S.; Zhang, J. DIA-based analysis of the menstrual blood proteome identifies association between CXCL5 and IL1RN and endometriosis. J. Proteomics 2023, 289, 104995. [Google Scholar] [CrossRef] [PubMed]
- Samare-Najaf, M.; Razavinasab, S.A.; Samareh, A.; Jamali, N. Omics-based novel strategies in the diagnosis of endometriosis. Crit. Rev. Clin. Lab. Sci. 2023, 25, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fiscus, J.; Fraison, É.; Renault, L.; Salle, B.; Panthu, B.; Labrune, E. Metabolic signature of follicular fluid to understand infertility-related diseases: a narrative review. Reprod. Biomed. Online 2023, 48, 103762. [Google Scholar] [CrossRef] [PubMed]
- Angioni, S.; Congiu, F.; Vitale, S.G.; D’Alterio, M.N.; Noto, A.; Monni, G.; Santoru, M.L.; Fanos, V.; Murgia, F.; Atzori, L. Gas Chromatography–Mass Spectrometry (GC–MS) Metabolites Analysis in Endometriosis Patients: A Prospective Observational Translational Study. J. Clin. Med. 2023, 12, 922. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, N.E.; Girling, J.E.; Boughton, B.; Holdsworth-Carson, S.J. Is there a role for small molecule metabolite biomarkers in the development of a diagnostic test for endometriosis? Syst. Biol. Reprod. Med. 2022, 68, 89–112. [Google Scholar] [CrossRef]
- Yang, H.; Lau, W.B.; Lau, B.; Xuan, Y.; Zhou, S.; Zhao, L.; Luo, Z.; Lin, Q.; Ren, N.; Zhao, X.; et al. A mass spectrometric insight into the origins of bening gynecological sudorder. Mass Spectrom. Rev. 2017, 36, 450–470. [Google Scholar] [CrossRef]
- Tokarz, J.; Adamski, J.; Rižner, T.L. Metabolomics for diagnosis and prognosis of uterine diseases? A systematic review. J. Pers. Med. 2020, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Morales-Romero, B.; González de Aledo-Castillo, J.M.; Fernández Sierra, C.; Martínez Carreira, C.; Zaragoza Bonet, C.; Fernández Bonifacio, R.; Caro Miró, M.A.; Argudo-Ramírez, A.; López Galera, R.M.; García-Villoria, J. Plasma C24:0- and C26:0-lysophosphatidylcholines are reliable biomarkers for the diagnosis of peroxisomal β-oxidation disorders. J. Lipid Res. 2024, 65, 100516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, J.; Jiang, Y.; Ju, Y.; He, J.; Yu, K.; Kan, G.; Zhang, H. Determination of amino acid metabolic diseases from dried blood spots with a rapid extraction method coupled with nanoelectrospray ionization mass spectrometry. Talanta 2024, 272, 125768. [Google Scholar] [CrossRef]
- Koelmel, J.P.; Kroeger, N.M.; Ulmer, C.Z.; Bowden, J.A.; Patterson, R.E.; Cochran, J.A.; Beecher, C.W.W.; Garrett, T.J.; Yost, R.A. LipidMatch: An automated workflow for rule-based lipid identification using untargeted high-resolution tandem mass spectrometry data. BMC Bioinformatics 2017, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Tokareva, A.O.; Chagovets, V. V.; Kononikhin, A.S.; Starodubtseva, N.L.; Nikolaev, E.N.; Frankevich, V.E. Comparison of the effectiveness of variable selection method for creating a diagnostic panel of biomarkers for mass spectrometric lipidome analysis. J. Mass Spectrom. 2021, 56, e4702. [Google Scholar] [CrossRef] [PubMed]
- Starodubtseva, N.L.; Tokareva, A.O.; Rodionov, V. V; Brzhozovskiy, A.G.; Bugrova, A.E.; Chagovets, V. V; Kometova, V. V; Kukaev, E.N.; Soares, N.C.; Kovalev, G.I.; et al. Integrating Proteomics and Lipidomics for Evaluating the Risk of Breast Cancer Progression : A Pilot Study. Biomedicines 2023, 11, 1786. [Google Scholar] [CrossRef]
- Kamburov, A.; Stelzl, U.; Lehrach, H.; Herwig, R. The ConsensusPathDB interaction database: 2013 Update. Nucleic Acids Res. 2013, 41, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J.J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K. ESHRE PAGES The members of the Endometriosis Guideline Core Group, Hum. Reprod. 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Han, Z.; Li, J.; Yi, X.; Zhang, T.; Liao, D.; You, J.; Ai, J. Diagnostic accuracy of interleukin-6 in multiple diseases: An umbrella review of meta-analyses. Heliyon 2024, 10, e27769. [Google Scholar] [CrossRef] [PubMed]
- Brulport, A.; Bourdon, M.; Vaiman, D.; Drouet, C.; Pocate-Cheriet, K.; Bouzid, K.; Marcellin, L.; Santulli, P.; Abo, C.; Jeljeli, M.; et al. An integrated multi-tissue approach for endometriosis candidate biomarkers: a systematic review. Reprod. Biol. Endocrinol. 2024, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.A.; Lamichhane, S.; Dickens, A.; McGlinchey, A.; Ribeiro, H.C.; Sen, P.; Wei, F.; Hyötyläinen, T.; Orešič, M. Systems biology approaches to study lipidomes in health and disease. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2021, 1866, 158857. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, L.; Yan, F.; Wang, X. Clinical lipidomics: a new way to diagnose human diseases. Clin. Transl. Med. 2018, 7, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.N.; Torres-Reverón, A.; Appleyard, C.B. Metabolomics in endometriosis: challenges and perspectives for future studies. Reprod. Fertil. 2021, 2, R35–R50. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.P.A.F.; Montani, D.A.; Setti, A.S.; Turco, E.G.L.; Oliveira-Silva, D.; Borges, E. Metabolomic profile as a noninvasive adjunct tool for the diagnosis of Grades III and IV endometriosis-related infertility. Mol. Reprod. Dev. 2019, 86, 1044–1052. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Guan, L.; Zhang, H.; Sun, J.; Gong, X.; Li, D.; Chen, P.; Ma, Z.; Liang, X.; et al. Discovery of phosphatidic acid, phosphatidylcholine, and phosphatidylserine as biomarkers for early diagnosis of endometriosis. Front. Physiol. 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, F.; Ferrando, M.; Díaz-Gimeno, P.; Quintana, F.; Fernández, G.; Castells, I.; Simón, C. Lipidomic profiling of endometrial fluid in women with ovarian endometriosis. Biol. Reprod. 2017, 96, 772–779. [Google Scholar] [CrossRef]
- Sasamoto, N.; Zeleznik, O.A.; Vitonis, A.F.; Missmer, S.A.; Laufer, M.R.; Avila-Pacheco, J.; Clish, C.B.; Terry, K.L. Presurgical blood metabolites and risk of postsurgical pelvic pain in young patients with endometriosis. Fertil. Steril. 2022, 117, 1235–1245. [Google Scholar] [CrossRef]
- Starodubtseva, N.; Chagovets, V.; Borisova, A.; Salimova, D.; Aleksandrova, N.; Chingin, K.; Chen, H.; Frankevich, V. Identification of potential endometriosis biomarkers in peritoneal fluid and blood plasma via shotgun lipidomics. Clin. Mass Spectrom. 2019, 13, 21–26. [Google Scholar] [CrossRef]
- Andrieu, T.; Chicca, A.; Pellegata, D.; Bersinger, N.A.; Imboden, S.; Nirgianakis, K.; Gertsch, J.; Mueller, M.D. Association of endocannabinoids with pain in endometriosis. Pain 2022, 163, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.A.; Shih, A.; Renteira, S.M.; Seckin, T.; Blau, B.; Simpfendorfer, K.; Lee, A.; Metz, C.N.; Gregersen, P.K. Analysis of menstrual effluent: Diagnostic potential for endometriosis. Mol. Med. 2018, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.J.; Adelson, R.P.; Vashistha, H.; Khalili, H.; Nayyar, A.; Puran, R.; Herrera, R.; Chatterjee, P.K.; Lee, A.T.; Truskinovsky, A.M.; et al. Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis. BMC Med. 2022, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, J.; Xiang, C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res. Ther. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Zailani, N.N.B.; Ho, P.C.L. Dried Blood Spots—A Platform for Therapeutic Drug Monitoring (TDM) and Drug/Disease Response Monitoring (DRM). Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 467–494. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, A.K.; Bastani, N.E.; Ellingjord-Dale, M.; Gundersen, T.E.; Blomhoff, R.; Ursin, G. Feasibility of self-sampled dried blood spot and saliva samples sent by mail in a population-based study. BMC Cancer 2015, 15, 265. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.J.; Mangwiro, Y.; Wake, M.; Saffery, R.; Greaves, R.F. Multi-omics analysis from archival neonatal dried blood spots: Limitations and opportunities. Clin. Chem. Lab. Med. 2022, 60, 1318–1341. [Google Scholar] [CrossRef] [PubMed]
- Malsagova, K.; Kopylov, A.; Stepanov, A.; Butkova, T.; Izotov, A.; Kaysheva, A. Dried blood spot in laboratory: Directions and prospects. Diagnostics 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Chagovets, V. V.; Wang, Z.; Kononikhin, A.S.; Starodubtseva, N.L.; Borisova, A.; Salimova, D.; Popov, I.A.; Kozachenko, A. V.; Chingin, K.; Chen, H.; et al. Endometriosis foci differentiation by rapid lipid profiling using tissue spray ionization and high resolution mass spectrometry. Sci. Rep. 2017, 7, 2546. [Google Scholar] [CrossRef]
- Vouk, K.; Hevir, N.; Ribič-Pucelj, M.; Haarpaintner, G.; Scherb, H.; Osredkar, J.; Möller, G.; Prehn, C.; Rižner, T.L.; Adamski, J. Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Hum. Reprod. 2012, 27, 2955–2965. [Google Scholar] [CrossRef]
- Vouk, K.; Ribič-Pucelj, M.; Adamski, J.; Rižner, T.L. Altered levels of acylcarnitines, phosphatidylcholines, and sphingomyelins in peritoneal fluid from ovarian endometriosis patients. J. Steroid Biochem. Mol. Biol. 2016, 159, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef]
- Zoeller, R.A.; Lake, A.C.; Nagan, N.; Gaposchkin, D.P.; Legner, M.A.; Lieberthal, W. Plasmalogens as endogenous antioxidants: Somatic cell mutants reveal the importance of the vinyl ether. Biochem. J. 1999, 338, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Broniec, A.; Klosinski, R.; Pawlak, A.; Wrona-Krol, M.; Thompson, D.; Sarna, T. Interaction of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic. Biol. Med. 2011, 50, 892–898. [Google Scholar] [CrossRef]
- Feider, C.L.; Woody, S.; Ledet, S.; Zhang, J.; Sebastian, K.; Breen, M.T.; Eberlin, L.S. Molecular Imaging of Endometriosis Tissues using Desorption Electrospray Ionization Mass Spectrometry. Sci. Rep. 2019, 9, 15690. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Zhang, L.; Zhang, L. Association between endometriosis and metabolic syndrome: a cross-sectional study based on the National Health and Nutrition Examination Survey data. Gynecol. Endocrinol. 2023, 39, 2254844. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Forman, J.P.; Missmer, S.A. Association between Endometriosis and Hypercholesterolemia or Hypertension. Hypertension 2017, 70, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T. V.; Markina, Y. V.; Bogatyreva, A.I.; Tolstik, T. V.; Varaeva, Y.R.; Starodubova, A. V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Farland, L. V.; Degnan, W.J.; Harris, H.R.; Tobias, D.K.; Missmer, S.A. A prospective study of endometriosis and risk of type 2 diabetes. Diabetologia 2021, 64, 552–560. [Google Scholar] [CrossRef]
- Sekulovski, N.; Whorton, A.E.; Shi, M.; Hayashi, K.; MacLean, J.A. Insulin signaling is an essential regulator of endometrial proliferation and implantation in mice. FASEB J. 2021, 35, e21440. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Kong, W.; Suo, H.; Shen, X.; Newton, M.A.; Burkett, W.C.; Zhao, Z.; John, C.; Sun, W.; Zhang, X.; et al. Oleic Acid Exhibits Anti-Proliferative and Anti-Invasive Activities via the PTEN/AKT/mTOR Pathway in Endometrial Cancer. Cancers (Basel). 2023, 15, 5407. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Wang, Q.; Bi, E.; Ma, X.; Liu, L.; Yang, M.; Qian, J.; Yi, Q. Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2020, 80, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Stables, M.J.; Gilroy, D.W. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 2011, 50, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bozza, P.T.; Viola, J.P.B. Lipid droplets in inflammation and cancer. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rakhila, H.; Bourcier, N.; Akoum, A.; Pouliot, M. Abnormal Expression of Prostaglandins E2 and F2 α Receptors and Transporters in Patients with Endometriosis. Biomed Res. Int. 2015, 2015, 808146. [Google Scholar] [CrossRef]
- Mandelboum, S.; Manber, Z.; Elroy-Stein, O.; Elkon, R. Recurrent functional misinterpretation of RNA-seq data caused by sample-specific gene length bias. PLoS Biol. 2019, 17, e3000481. [Google Scholar] [CrossRef]
- Von Moltke, J.; Trinidad, N.J.; Moayeri, M.; Kintzer, A.F.; Wang, S.B.; Van Rooijen, N.; Brown, C.R.; Krantz, B.A.; Leppla, S.H.; Gronert, K.; et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 2012, 490, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.Z.; Yang, H.L.; Ha, S.Y.; Chang, K.K.; Mei, J.; Zhou, W.J.; Qiu, X.M.; Wang, X.Q.; Zhu, R.; Li, D.J.; et al. Cyclooxygenase-2 in endometriosis. Int. J. Biol. Sci. 2019, 15, 2783–2797. [Google Scholar] [CrossRef]
- Levin, G.; Duffin, K.L.; Obukowicz, M.G.; Hummert, S.L.; Fujiwara, H.; Needleman, P.; Raz, A. Differential metabolism of dihomo-γ-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: Implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem. J. 2002, 365, 489–496. [Google Scholar] [CrossRef]
- Wang, X.; Lin, H.; Gu, Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012, 11, 25. [Google Scholar] [CrossRef]
- Mustonen, A.M.; Nieminen, P. Dihomo-γ-Linolenic Acid (20:3n-6)—Metabolism, Derivatives, and Potential Significance in Chronic Inflammation. Int. J. Mol. Sci. 2023, 24, 2116. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef]
- Ren, M.; Phoon, C.K.L.; Schlame, M. Metabolism and function of mitochondrial cardiolipin. Prog. Lipid Res. 2014, 55, 1–16. [Google Scholar] [CrossRef]
- May, K.E.; Conduit-Hulbert, S.A.; Villar, J.; Kirtley, S.; Kennedy, S.H.; Becker, C.M. Peripheral biomarkers of endometriosis: A systematic review. Hum. Reprod. Update 2010, 16, 651–674. [Google Scholar] [CrossRef]
- Ye, C.; Chen, P.; Xu, B.; Jin, Y.; Pan, Y.; Wu, T.; Du, Y.; Mao, J.; Wu, R. Abnormal expression of fission and fusion genes and the morphology of mitochondria in eutopic and ectopic endometrium. Eur. J. Med. Res. 2023, 28, 209. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Lu, S.; Wang, X.; Shi, X.; Mao, P. Autophagy-dependent ferroptosis is involved in the development of endometriosis. Gynecol. Endocrinol. 2023, 39, 2242962. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, X.; Guo, J.; Wang, D.; Li, X.; Cheng, X.; Wang, X. CHCHD2 Regulates Mitochondrial Function and Apoptosis of Ectopic Endometrial Stromal Cells in the Pathogenesis of Endometriosis. Reprod. Sci. 2022, 29, 2152–2164. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: Molecular and pharmacological aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef]
- Lee, Y.H.; Tan, C.W.; Venkatratnam, A.; Tan, C.S.; Cui, L.; Loh, S.F.; Griffith, L.; Tannenbaum, S.R.; Chan, J.K.Y. Dysregulated sphingolipid metabolism in endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, E1913–E1921. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yang, J.X.; Allen, J.C.; Tan, C.S.; Chern, B.S.M.; Tan, T.Y.; Tan, H.H.; Mattar, C.N.Z.; Chan, J.K.Y. Elevated peritoneal fluid ceramides in human endometriosis-associated infertility and their effects on mouse oocyte maturation. Fertil. Steril. 2018, 110, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, G.; Wang, L.; Meng, L.; Ren, J.; Shang, L.; Li, D.; Bao, Y. Dysregulation of sphingolipid metabolism in pain. Front. Pharmacol. 2024, 15, 1337150. [Google Scholar] [CrossRef]
- Gonzalez, P.A.; Simcox, J.; Raff, H.; Wade, G.; Von Bank, H.; Weisman, S.; Hainsworth, K. Lipid signatures of chronic pain in female adolescents with and without obesity. Lipids Health Dis. 2022, 21, 80. [Google Scholar] [CrossRef]
- Chrobak, A.; Sieradzka, U.; Sozański, R.; Chełmońska-Soyta, A.; Gabryś, M.; Jerzak, M. Ectopic and eutopic stromal endometriotic cells have a damaged ceramide signaling pathway to apoptosis. Fertil. Steril. 2009, 92, 1834–1843. [Google Scholar] [CrossRef]
- Santulli, P.; Marcellin, L.; Noël, J.C.; Borghese, B.; Fayt, I.; Vaiman, D.; Chapron, C.; Méhats, C. Sphingosine pathway deregulation in endometriotic tissues. Fertil. Steril. 2012, 97, 904–911e5. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.Y.; Bae, Y.S. Functional roles of sphingolipids in immunity and their implication in disease. Exp. Mol. Med. 2023, 55, 1110–1130. [Google Scholar] [CrossRef] [PubMed]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.L.; Li, Y.K.; Chen, D.K.; He, J.F.; Yao, N. Functions of Sphingolipids in Pathogenesis During Host–Pathogen Interactions. Front. Microbiol. 2021, 12, 701041. [Google Scholar] [CrossRef]
- MacEyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Malan, T.P.; Porreca, F. Lipid mediators regulating pain sensitivity. Prostaglandins Other Lipid Mediat. 2005, 77, 123–130. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Yamashita, A.; Matsuda, M.; Kawai, K.; Sawa, T.; Amaya, F. NLRP2 inflammasome in dorsal root ganglion as a novel molecular platform that produces inflammatory pain hypersensitivity. Pain 2019, 160, 2149–2160. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Kajimoto, T.; Okada, T.; Yu, H.; Goparaju, S.K.; Jahangeer, S.; Nakamura, S. Involvement of Sphingosine-1-Phosphate in Glutamate Secretion in Hippocampal Neurons. Mol. Cell. Biol. 2007, 27, 3429–3440. [Google Scholar] [CrossRef]
- Weth-Malsch, D.; Langeslag, M.; Beroukas, D.; Zangrandi, L.; Kastenberger, I.; Quarta, S.; Malsch, P.; Kalpachidou, T.; Schwarzer, C.; Proia, R.L.; et al. Ablation of sphingosine 1-phosphate receptor subtype 3 impairs hippocampal neuron excitability in vitro and spatial working memory in vivo. Front. Cell. Neurosci. 2016, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Vincent, K.; Brawn, J.; Zondervan, K.T.; Becker, C.M. Peripheral changes in endometriosis-associated pain. Hum. Reprod. Update 2014, 20, 717–736. [Google Scholar] [CrossRef]
- Jamjoum, R.; Majumder, S.; Issleny, B.; Stiban, J. Mysterious sphingolipids: metabolic interrelationships at the center of pathophysiology. Front. Physiol. 2023, 14, 1229108. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.F.P.; Samadder, A.N.; Agarwal, A.; Fernandes, L.F.C.; Abrão, M.S. Oxidative stress biomarkers in patients with endometriosis: Systematic review. Arch. Gynecol. Obstet. 2012, 286, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Mier-Cabrera, J.; Jiménez-Zamudio, L.; García-Latorre, E.; Cruz-Orozco, O.; Hernández-Guerrero, C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG An Int. J. Obstet. Gynaecol. 2011, 118, 6–16. [Google Scholar] [CrossRef]
- Thézénas, M.L.; De Leo, B.; Laux-Biehlmann, A.; Bafligil, C.; Elger, B.; Tapmeier, T.; Morten, K.; Rahmioglu, N.; Dakin, S.G.; Charles, P.; et al. Amine oxidase 3 is a novel pro-inflammatory marker of oxidative stress in peritoneal endometriosis lesions. Sci. Rep. 2020, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H.; Imanaka, S. Current Understanding of and Future Directions for Endometriosis-Related Infertility Research with a Focus on Ferroptosis. Diagnostics 2023, 13, 1926. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Igarashi, S.; Kato, N.; Tanaka, T. Aberrant expression of glutathione peroxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil. Steril. 2000, 74, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Polak, G.; Barczyński, B.; Kwaśniewski, W.; Bednarek, W.; Wertel, I.; Derewianka-Polak, M.; Kotarski, J. Low-density lipoproteins oxidation and endometriosis. Mediators Inflamm. 2013, 2013, 12–15. [Google Scholar] [CrossRef]
- Murphy, A.A.; Santanam, N.; Parthasarathy, S. Endometriosis: A disease of oxidative stress? Semin. Reprod. Endocrinol. 1998, 16, 263–273. [Google Scholar] [CrossRef]
- Nasiri, N.; Moini, A.; Eftekhari-Yazdi, P.; Karimian, L.; Salman-Yazdi, R.; Arabipoor, A. Oxidative Stress Statues in Serum and Follicular Fluid of Women with Endometriosis Citation: Nasiri N, Moini A, Eftekhari-Yazdi P, Karimian L, Salman-Yazdi R, Arabipoor A. Oxidative stress statues in serum and follicular fluid of women with endometriosis. CELL JOURNAL(Yakhteh) Cell Journal(Yakhteh) Cell J 2017, 18, 582–587. [Google Scholar]
- Murphy, A.A.; Palinski, W.; Rankin, S.; Morales, A.J.; Parthasarathy, S. Macrophage scavenger receptor(s) and oxidatively modified proteins in endometriosis. Fertil. Steril. 1998, 69, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.A.; Palinski, W.; Rankin, S.; Morales, A.J.; Parthasarathy, S. Evidence for oxidatively modified lipid-protein complexes in endometrium and endometriosis. Fertil. Steril. 1998, 69, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, M.; Koźlik, J.; Skrzypczak, J.; Mikołajczyk, M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil. Steril. 2003, 79, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.H.; Huang, H.C.; Hsieh, R.H.; Chen, S.C.; Tsai, M.C.; Tzeng, C.R. Oxidative damage and mitochondrial DNA mutations with endometriosis. Ann. N. Y. Acad. Sci. 2005, 1042, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Mier-Cabrera, J.; Genera-García, M.; De la Jara-Díaz, J.; Perichart-Perera, O.; Vadillo-Ortega, F.; Hernández-Guerrero, C. Effect of vitamins C and E supplementation on peripheral oxidative stress markers and pregnancy rate in women with endometriosis. Int. J. Gynecol. Obstet. 2008, 100, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 2008, 14, 32–40. [Google Scholar] [CrossRef]
- Kajihara, H.; Yamada, Y.; Kanayama, S.; Furukawa, N.; Noguchi, T.; Haruta, S.; Yoshida, S.; Sado, T.; Oi, H.; Kobayashi, H. New insights into the pathophysiology of endometriosis: From chronic inflammation to danger signal. Gynecol. Endocrinol. 2011, 27, 73–79. [Google Scholar] [CrossRef]
- Clower, L.; Fleshman, T.; Geldenhuys, W.J.; Santanam, N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules 2022, 12, 1055. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





