Submitted:
17 May 2024
Posted:
20 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
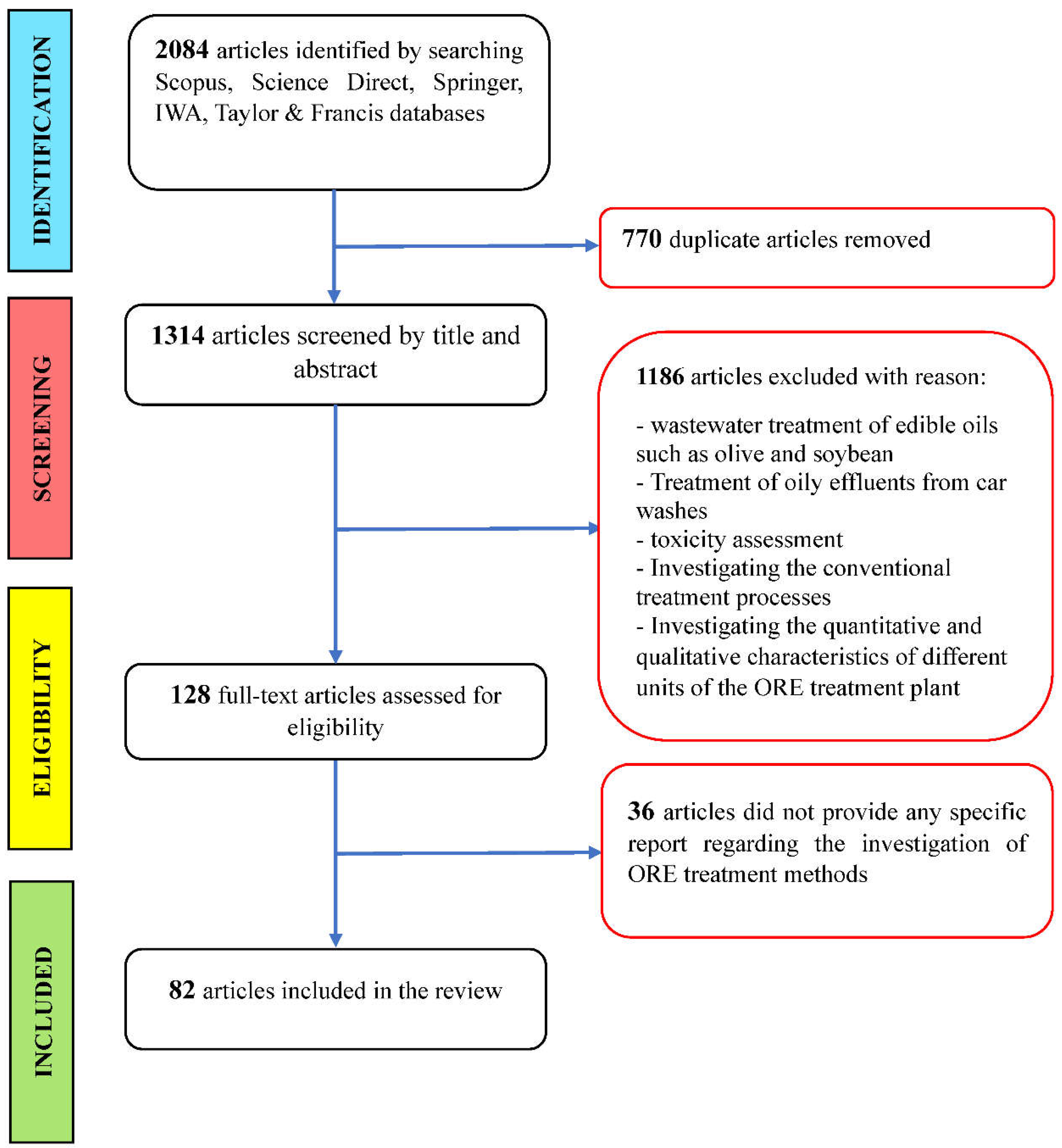
2.1. Search Strategy
2.2. Study Inclusion and Exclusion Criteria
3. Results
3.1. Background Data on the Included Studies in the Reclamation of Oil and Gas Refinery Effluent
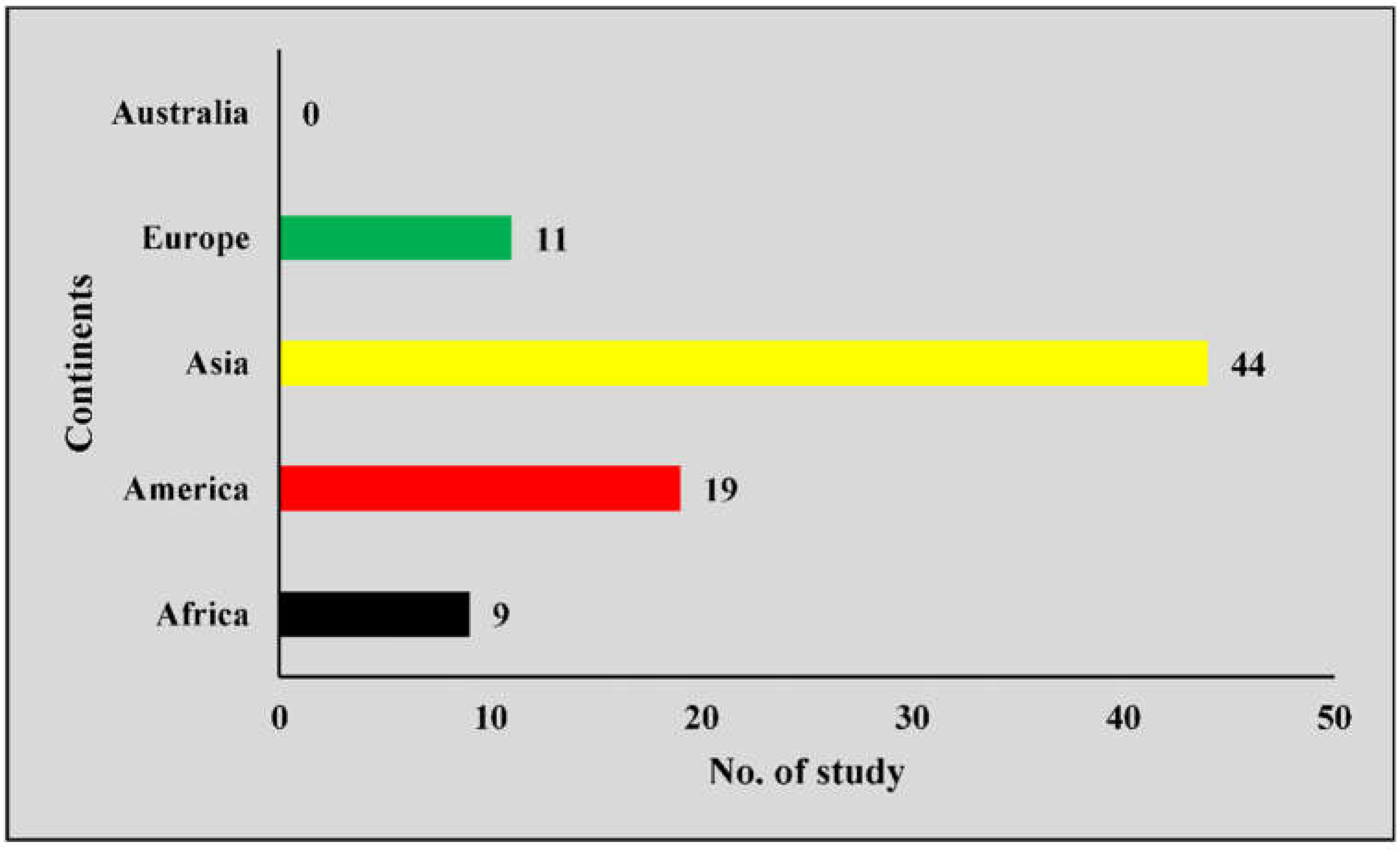
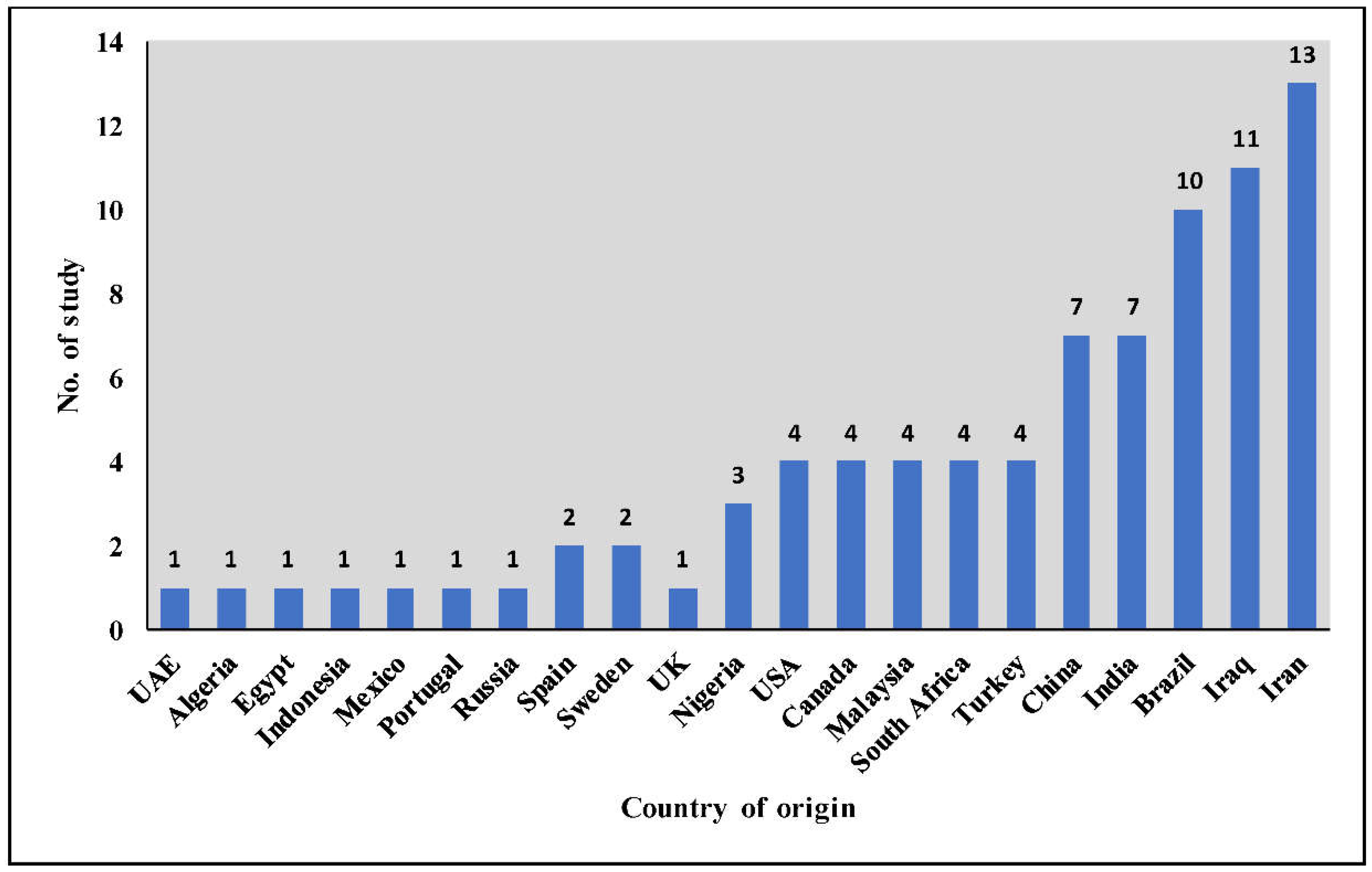
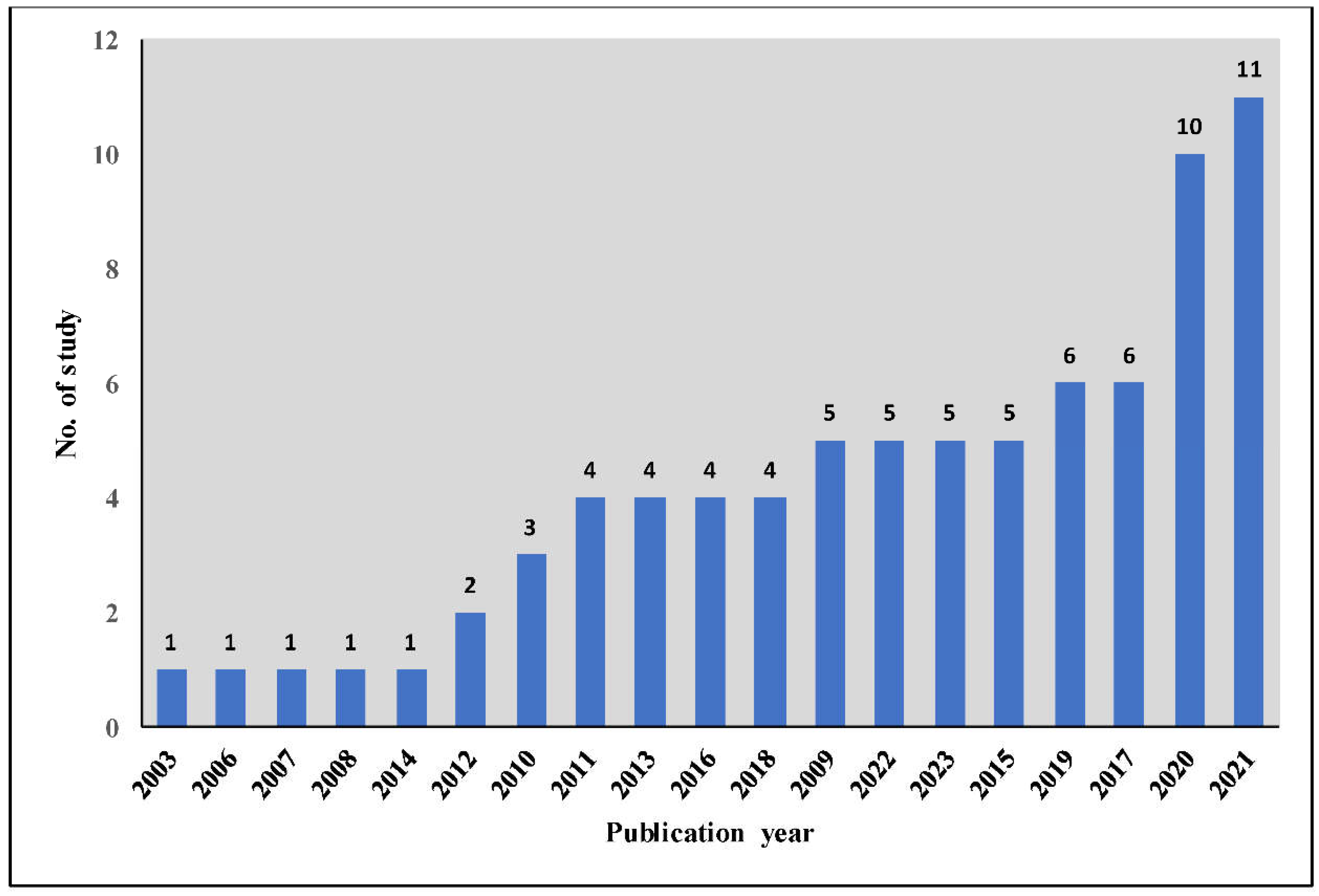
3.2. Scattered Distribution of Literature
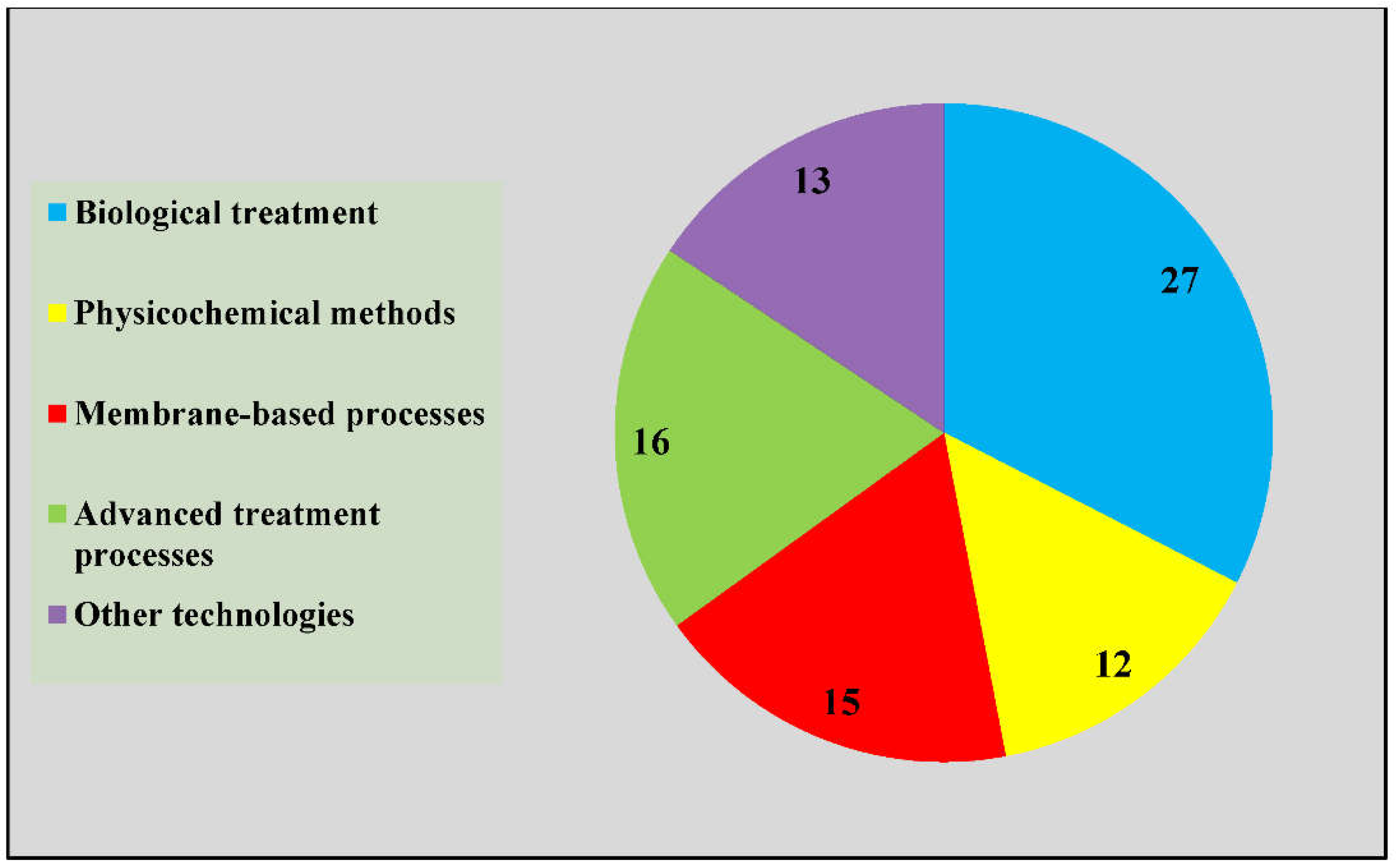
3.3. Oil and Gas Refinery Effluent Treatment Method
4. Discussion
4.1. Water Consumption in an Oil Refinery
4.2. Oil and Gas Refinery Effluent Treatment Methods
4.3. Biological Treatment
4.3.1. Bioremediation and Biosorption
4.3.2. Membrane Bioreactor System
4.3.3. Up-Flow Anaerobic Sludge Blanket (UASB) Reactor
4.3.4. Sequential Batch Reactor (SBR)
4.3.5. Other Biological Processes
4.4. Membrane-Based Processes
4.5. Advanced Treatment Processes
4.5.1. Advanced Oxidation Processes
4.5.2. Electrochemical Processes
4.5.3. Electrochemical Oxidation
4.5.4. Electrofenton Oxidation
4.6. Other Technologies
5. Limitations
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Nomenclature and abbreviations
| AnBR | Anaerobic biofilm reactor | NF | Nanofiltration |
| AOPs | Advanced oxidation processes | NH3-N | Nitrogen content of the ammonia |
| BAC | Biologically activated carbon | O&G | Oil and grease |
| BCF | Bioconcentration factor | OCV | Open-circuit voltage |
| BOD | Biological oxygen demand | OMBR | Osmotic membrane bioreactor |
| CIP | Clean in place | ORE | Oil refinery effluent |
| COD | Chemical oxygen demand | PA | Polyamide |
| EAOPs | Electrochemical advanced oxidation processes | PAHs | Polycyclic aromatic hydrocarbons |
| EC | Electrocoagulation | PBBR | Packed-bed biofilm reactor |
| ECR | Electrocoagulation reactor | PFC | Parallel flow connections |
| EO | Electro-oxidation | PMR | Photocatalytic membrane reactor |
| FO | Forward osmosis | PS | Polysulfone |
| HCs | Hydrocarbons | PTFE | Polytetrafluoroethylene |
| HF | Hollow fiber | RO | Reverse osmosis |
| HPC | Heterotrophic plate count | RSM | Response surface methodology |
| HRT | Hydraulic retention time | SFC | Serial flow connections |
| IX | Ion exchange | SS | Suspended solids |
| IXMB | Mixed bed ion exchange | TDS | Total dissolved solids |
| MBR | Membrane bioreactors | TiO2 | Titanium dioxide |
| MD | Membrane desalination | TOC | Total organic carbon |
| MDC | Microbial desalination cell | TPH | Total petroleum hydrocarbon |
| MF | Microfiltration | UASB | Up-flow anaerobic sludge blanket |
| MFC | Microbial fuel cells | UF | Ultrafiltration |
| MOX | Multi-oxidant disinfectant | UWR | unconventional water resources |
References
- Abass, O.K.; Fang, F.; Zhuo, M.; Zhang, K. Integrated interrogation of causes of membrane fouling in a pilot-scale anoxic-oxic membrane bioreactor treating oil refinery wastewater. Sci. Total. Environ. 2018, 642, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.N.R.; Ang, W.L.; Teow, Y.H.; Mohammad, A.W.; Hilal, N. Nanofiltration membrane processes for water recycling, reuse and product recovery within various industries: A review. J. Water Process. Eng. 2021, 45, 102478. [Google Scholar] [CrossRef]
- Akhidime, I. D. (2009). Aspects of Expanded Bed Nitrification Including Treatment of Oil Refinery Wastewaters. Manchester Metropolitan University.
- Al-Nidawi, W. (2022). Evaluation of refinery wastewater treatment plant and studying possibility to reuse the effluent as a makeup for cooling tower system. Altınbaş Üniversitesi/Lisansüstü Eğitim Enstitüsü.
- Al Hashemi, W.; Maraqa, M.; Rao, M.; Hossain, M. Characterization and removal of phenolic compounds from condensate-oil refinery wastewater. Desalination Water Treat. 2014, 54, 660–671. [Google Scholar] [CrossRef]
- Alexandre, V.; de Castro, T.; de Araújo, L.; Santiago, V.; Freire, D.; Cammarota, M. Minimizing solid wastes in an activated sludge system treating oil refinery wastewater. Chem. Eng. Process. - Process. Intensif. 2015, 103, 53–62. [Google Scholar] [CrossRef]
- AlJaberi, F.Y. Removal of TOC from oily wastewater by electrocoagulation technology. IOP Conf. Series: Mater. Sci. Eng. 2020, 928, 022024. [Google Scholar] [CrossRef]
- AlJaberi, F.Y.; Abdulmajeed, B.A.; Hassan, A.A.; Ghadban, M.L. Assessment of an Electrocoagulation Reactor for the Removal of Oil Content and Turbidity from Real Oily Wastewater Using Response Surface Method. Recent Innov. Chem. Eng. (Formerly Recent Patents Chem. Eng. 2020, 13, 55–71. [Google Scholar] [CrossRef]
- AlJaberi, F.Y.; Ahmed, S.A.; Makki, H.F. Electrocoagulation treatment of high saline oily wastewater: evaluation and optimization. Heliyon 2020, 6, e03988. [Google Scholar] [CrossRef]
- Alkhazraji, H. A. J. , & Alatabe, M. J. A. (2021). Oil recovery from oilfield produced water using zinc oxide nano particle as catalyst in batch and continuous system. Journal of Ecological Engineering, 22(8).
- Alkmim, A.R.; da Costa, P.R.; Moser, P.B.; Neta, L.S.F.; Santiago, V.M.J.; Cerqueira, A.C.; Reis, B.G.; Amaral, M.C.S. Potential use of membrane bioreactor to treat petroleum refinery effluent: comprehension of dynamic of organic matter removal, fouling characteristics and membrane lifetime. Bioprocess Biosyst. Eng. 2017, 40, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Arabzadeh, M.; Eslamidoost, Z.; Rajabi, S.; Hashemi, H.; Aboulfotoh, A.; Rosti, F.; Nazari, F.; Borj, B.P.; Hajivand, M. Wastewater quality index (WWQI) as an indicator for the assessment of sanitary effluents from the oil and gas industries for reliable and sustainable water reuse. Groundw. Sustain. Dev. 2023, 23. [Google Scholar] [CrossRef]
- Ashwaniy, V.; Perumalsamy, M.; Pandian, S. Enhancing the synergistic interaction of microalgae and bacteria for the reduction of organic compounds in petroleum refinery effluent. Environ. Technol. Innov. 2020, 19, 100926. [Google Scholar] [CrossRef]
- Atiyah, A.S.; Al-Samawi, A.A.A.; Hassan, A.A. (2020). Photovoltaic cell electro-Fenton oxidation for treatment oily wastewater. AIP Conference Proceedings, 2235(1).
- Augulyte, L.; Kliaugaite, D.; Racys, V.; Jankunaite, D.; Zaliauskiene, A.; Bergqvist, P.-A.; Andersson, P.L. Multivariate analysis of a biologically activated carbon (BAC) system and its efficiency for removing PAHs and aliphatic hydrocarbons from wastewater polluted with petroleum products. J. Hazard. Mater. 2009, 170, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.; Olin, T.J.; Bricka, R.; Adrian, D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Barzegar, G.; Wu, J.; Ghanbari, F. Enhanced treatment of greywater using electrocoagulation/ozonation: Investigation of process parameters. Process. Saf. Environ. Prot. 2018, 121, 125–132. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Roy, A.; Sarkar, S.; Ghosh, S.; Majumdar, S.; Chakraborty, S.; Mandal, S.; Mukhopadhyay, A.; Bandyopadhyay, S. Combination technology of ceramic microfiltration and reverse osmosis for tannery wastewater recovery. Water Resour. Ind. 2013, 3, 48–62. [Google Scholar] [CrossRef]
- Cerqueira, A.C.; Lopes, T.; Santiago, V.; Vallero, M.; Trovati, J.; Arntsen, B.; Syed, W. Design and Performance of the First Full Scale Membrane Bioreactor Plant Treating Oil Refinery Effluent in Brazil. Proc. Water Environ. Fed. 2013, 2013, 3573–3584. [Google Scholar] [CrossRef]
- Cesaro, A.; Naddeo, V.; Belgiorno, V. Wastewater Treatment by Combination of Advanced Oxidation Processes and Conventional Biological Systems. J. Bioremediation Biodegradation 2013, 4. [Google Scholar] [CrossRef]
- Corrêa, A. X. R. , Tiepo, E. N., Somensi, C. A., Sperb, R. M., & Radetski, C. M. (2010). Use of ozone-photocatalytic oxidation (O 3/UV/TiO 2) and biological remediation for treatment of produced water from petroleum refineries. Journal of Environmental Engineering, 136(1), 40–45.
- de Oliveira, C. P. M. , Viana, M. M., Silva, G. R., Lima, L. S. F., de Paula, E. C., & Amaral, M. C. S. (2020). Potential use of green TiO2 and recycled membrane in a photocatalytic membrane reactor for oil refinery wastewater polishing. Journal of Cleaner Production 257, 120526.
- De Souza, M. P. , Pickering, I. J., Walla, M., & Terry, N. (2002). Selenium assimilation and volatilization from selenocyanate-treated Indian mustard and muskgrass. Plant Physiology, 128(2), 625–633.
- Delnavaz, M. , & Bos’ hagh, M. A. (2021). Photocatalytic treatment of real oil refinery wastewater using TiO2/Ag-doped nanoparticles. Sharif Journal of Civil Engineering, 37(2.1), 121–129.
- Demir-Duz, H.; Aktürk, A.; Ayyildiz, O.; Álvarez, M.; Contreras, S. Reuse and recycle solutions in refineries by ozone-based advanced oxidation processes: A statistical approach. J. Environ. Manag. 2020, 263, 110346. [Google Scholar] [CrossRef]
- Dong, H.; Dong, H.; Zhang, Z.; Sun, S.; Wang, W.; Ke, M.; Song, Z.; Zhang, Z.; Wang, J.; Wu, W.-M. Microbial community dynamics in an anaerobic biofilm reactor treating heavy oil refinery wastewater. RSC Adv. 2016, 6, 107442–107451. [Google Scholar] [CrossRef]
- EO, E. , Rathilal, S., Ishwarlall, S., & Tetteh, K. (2020). Removal of Cl−, SO4 2− and CO3 2− Salts from Oil Refinery Effluent Using Forward Osmosis. Proceedings of the 18th JOHANNESBURG International Conference on Science, Engineering, Technology & Waste Management (SETWM-20), Johannesburg, South Africa, 16–17.
- Estrada-Arriaga, E.B.; Zepeda-Aviles, J.A.; García-Sánchez, L. Post-treatment of real oil refinery effluent with high concentrations of phenols using photo-ferrioxalate and Fenton’s reactions with membrane process step. Chem. Eng. J. 2016, 285, 508–516. [Google Scholar] [CrossRef]
- Ezugbe, E. O. (2021). Desalination of a local oil refinery effluent to meet discharge limits.
- Ezugbe, E.O.; Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D. Assessment of Forward Osmosis in PRO Mode during Desalination of a Local Oil Refinery Effluent. Membranes 2021, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Foureaux, A.F.S.; Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.d.S.; Amaral, M.C.S. A sustainable solution for fresh-water demand in mining sectors: Process water reclamation from POX effluent by membrane distillation. Sep. Purif. Technol. 2020, 256, 117797. [Google Scholar] [CrossRef]
- Gasim, H. A. , Kutty, S. R. M., Isa, M. H., & Isa, M. P. M. (2012). Treatment of petroleum refinery wastewater by using UASB reactors. International Journal of Environmental and Ecological Engineering 6(2), 58–61.
- Gosling, S.N.; Arnell, N.W. A global assessment of the impact of climate change on water scarcity. Clim. Chang. 2016, 134, 371–385. [Google Scholar] [CrossRef]
- Hashemi., F. H. H. Hashemi., F. H. H. (2023). A Practical Integrative Method For Technical Assembly, Lca, and Lcc Analysis of Advanced Processes in Recovery Oil Plant Unit Effluent Reclamation. Available at SSRN. [CrossRef]
- Hashemi, F.; Hashemi, H.; Abbasi, A.; Schreiber, M.E. Life cycle and economic assessments of petroleum refineries wastewater recycling using membrane, resin and on site disinfection (UF-IXMB-MOX) processes. Process. Saf. Environ. Prot. 2022, 162, 419–425. [Google Scholar] [CrossRef]
- Hashemi, F. , Hashemi, H., Dehghani, M., & Hoseini, M. (2018). Removal of heavy metals from oil refinery effluent by micellar-enhanced ultrafiltration (MEUF). Journal of Health Sciences & Surveillance System, 6(3), 123–129.
- Hashemi, F.; Hashemi, H.; Shahbazi, M.; Dehghani, M.; Hoseini, M.; Shafeie, A. Reclamation of real oil refinery effluent as makeup water in cooling towers using ultrafiltration, ion exchange and multioxidant disinfectant. Water Resour. Ind. 2020, 23, 100123. [Google Scholar] [CrossRef]
- Hassan, A. A. , Naeem, H. T., & Hadi, R. T. (2018). Degradation of oily waste water in aqueous phase using solar (ZnO, TiO2 and Al2O3) catalysts. Pakistan Journal of Biotechnology 15(4), 927–934.
- Hesas, R. H. , Baei, M. S., Rostami, H., Gardy, J., & Hassanpour, A. (2019). An investigation on the capability of magnetically separable Fe3O4/mordenite zeolite for refinery oily wastewater purification. Journal of Environmental Management, 241, 525–534.
- Ibrahim, D. S. (2013). Treatment of Petroleum Refinery Effluent Using Electrochemical Techniques. PH. D. thesis.
- Imam, A.; Kanaujia, P.K.; Ray, A.; Suman, S.K. Removal of Petroleum Contaminants Through Bioremediation with Integrated Concepts of Resource Recovery: A Review. Indian J. Microbiol. 2021, 61, 250–261. [Google Scholar] [CrossRef]
- Ismail, Z.; Beddri, A.M. Potential of Water Hyacinth as a Removal Agent for Heavy Metals from Petroleum Refinery Effluents. Water, Air, Soil Pollut. 2008, 199, 57–65. [Google Scholar] [CrossRef]
- Issaoui, M.; Jellali, S.; Zorpas, A.A.; Dutournie, P. Membrane technology for sustainable water resources management: Challenges and future projections. Sustain. Chem. Pharm. 2022, 25, 100590. [Google Scholar] [CrossRef]
- Jalayer, M. , Gougol, M., & Alizadehfard, M. R. (2022). MEMBRANE DISTILLATION AS AN ENVIRONMENTALLY FRIENDLY DESALINATION SYSTEM FOR PETROLEUM REFINERY’S WASTEWATER REUSE–A TECHNICAL AND ENVIRONMENTAL CASE STUDY. Conference: IDA 2022 Sydney.
- Jamal, M.T.; Pugazhendi, A. Degradation of petroleum hydrocarbons and treatment of refinery wastewater under saline condition by a halophilic bacterial consortium enriched from marine environment (Red Sea), Jeddah, Saudi Arabia. 3 Biotech 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jasim, M.A.; AlJaberi, F.Y. Investigation of oil content removal performance in real oily wastewater treatment by electrocoagulation technology: RSM design approach. Results Eng. 2023, 18. [Google Scholar] [CrossRef]
- Jasim, M.A.; AlJaberi, F.Y. Removal of COD from real oily wastewater by electrocoagulation using a new configuration of electrodes. Environ. Monit. Assess. 2023, 195, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jasim, M.A.; AlJaberi, F.Y.; Salman, A.D.; Alardhi, S.M.; Le, P.-C.; Kulcsar, G.; Jakab, M. Studying the effect of reactor design on the electrocoagulation treatment performance of oily wastewater. Heliyon 2023, 9, e17794. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S. , Naidu, G., Leiknes, T., & Vigneswaran, S. (2017). Membrane biofouling: Biofouling assessment and reduction strategies in seawater reverse osmosis desalination. Elsevier BV.
- Jiad, M.M.; Abbar, A.H. Treatment of petroleum refinery wastewater by electrofenton process using a low cost porous graphite air-diffusion cathode with a novel design. Chem. Eng. Res. Des. 2023, 193, 207–221. [Google Scholar] [CrossRef]
- Judd, S. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2015, 305, 37–45. [Google Scholar] [CrossRef]
- Kadivarian, M.; Dadkhah, A.A.; Esfahany, M.N. Oily wastewater treatment by a continuous flow microbial fuel cell and packages of cells with serial and parallel flow connections. Bioelectrochemistry 2020, 134, 107535. [Google Scholar] [CrossRef] [PubMed]
- Karimidastenaei, Z.; Avellan, T.; Sadegh, M.; Klove, B.; Haghighi, A.T. Unconventional water resources: Global opportunities and challenges. Sci. Total. Environ. 2022, 827, 154429. [Google Scholar] [CrossRef] [PubMed]
- Keane, D.A.; McGuigan, K.G.; Ibáñez, P.F.; Polo-López, M.I.; Byrne, J.A.; Dunlop, P.S.M.; O'Shea, K.; Dionysiou, D.D.; Pillai, S.C. Solar photocatalysis for water disinfection: materials and reactor design. Catal. Sci. Technol. 2014, 4, 1211–1226. [Google Scholar] [CrossRef]
- Keyvan Hosseini, P. (2022). EXPERIMENTAL INVESTIGATION OF A PILOT-SCALE MEMBRANE FILTRATION SYSTEM FOR OILY WASTEWATER TREATMENT.
- Khodakarami, M.; Bagheri, M. Recent advances in synthesis and application of polymer nanocomposites for water and wastewater treatment. J. Clean. Prod. 2021, 296, 126404. [Google Scholar] [CrossRef]
- Kummu, M.; Ward, P.J.; de Moel, H.; Varis, O. Is physical water scarcity a new phenomenon? Global assessment of water shortage over the last two millennia. Environ. Res. Lett. 2010, 5, 034006. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; da Costa, P.R.; Alkmin, A.R.; Neta, L.S.d.F.; Cerqueira, A.C.; Amaral, M.C.S. Chemical cleaning procedures on permeability recovery and lifespan of MBR membranes treating petroleum refinery wastewater: From bench- to pilot-scale applications. J. Water Process. Eng. 2021, 44, 102411. [Google Scholar] [CrossRef]
- Liu, B. , Chen, B., & Zhang, B. (2017a). Oily wastewater treatment by nano-TiO 2-induced photocatalysis: Seeking more efficient and feasible solutions. IEEE Nanotechnology Magazine, 11(3), 4–15.
- Liu, B. , Chen, B., & Zhang, B. (2017b). Oily wastewater treatment by nano-TiO2-induced photocatalysis: seeking more efficient and feasible solutions. IEEE Nanotechnol Mag 11 (3): 4–15.
- Liu, B. , Zheng, J. S., Chen, B., & Zhang, B. Y. (2013). A preliminary study on nano-catalyst enhanced heterogeneous photodegradation of polycyclic aromatic hydrocarbons (PAHs) in produced water. Proceedings of the 36th AMOP Technical Seminar on Environmental Contamination and Response, 4–6.
- Lopez, D. C. , Lee, J. R., Hu, L. H., Clark, J., & Reddy, S. (2006). High-Purity Water from Wastewater…A “Rare” Opportunity. WEFTEC 2006. WEFTEC 2006, 126–138. [Google Scholar]
- Macedonio, F. , Drioli, E., Gusev, A. A., Bardow, A., Semiat, R., & Kurihara, M. (2012). Efficient technologies for worldwide clean water supply. Chemical Engineering and Processing: Process Intensification, 51, 2–17.
- Mallick, S.K.; Chakraborty, S. Treatment of synthetic refinery wastewater in anoxic–aerobic sequential moving bed reactors and sulphur recovery. J. Environ. Sci. Heal. Part A 2017, 52, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A. (2022). Cobalt Ferrite Nano-composites for the oxidation of Phenols in oil refinery wastewater.
- Mohammadi, L.; Rahdar, A.; Bazrafshan, E.; Dahmardeh, H.; Susan, M.; Kyzas, G.Z. Petroleum Hydrocarbon Removal from Wastewaters: A Review. Process. 2020, 8, 447. [Google Scholar] [CrossRef]
- Mondal, B.; Srivastava, V.C.; Kushwaha, J.P.; Bhatnagar, R.; Singh, S.; Mall, I.D. Parametric and multiple response optimization for the electrochemical treatment of textile printing dye-bath effluent. Sep. Purif. Technol. 2013, 109, 135–143. [Google Scholar] [CrossRef]
- Moser, P.B.; Bretas, C.; Paula, E.C.; Faria, C.; Ricci, B.C.; Cerqueira, A.C.F.; Amaral, M.C. Comparison of hybrid ultrafiltration-osmotic membrane bioreactor and conventional membrane bioreactor for oil refinery effluent treatment. Chem. Eng. J. 2019, 378, 121952. [Google Scholar] [CrossRef]
- Moser, P. B. , Ricci, B. C., Reis, B. G., Neta, L. S. F., Cerqueira, A. C., & Amaral, M. C. S. (2018). Effect of MBR-H2O2/UV Hybrid pre-treatment on nanofiltration performance for the treatment of petroleum refinery wastewater. Separation and Purification Technology, 192, 176–184.
- Musa, N. M. , & Suleiman, A. (2015). Bioremediation of petroleum refinery wastewater effluent via augmented native microbes. Journal of Emerging Trends in Engineering and Applied Sciences 6(1), 1–6.
- Nadjafi, M.; Reyhani, A.; Al Arni, S. Feasibility of Treatment of Refinery Wastewater by a Pilot Scale MF/UF and UF/RO System for Reuse at Boilers and Cooling Towers. J. Water Chem. Technol. 2018, 40, 167–176. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mousavi, S.M.; Shojaosadati, S.A. Biodegradation potential of hydrocarbons in petroleum refinery effluents using a continuous anaerobic-aerobic hybrid system. Korean J. Chem. Eng. 2015, 32, 874–881. [Google Scholar] [CrossRef]
- Ni, L.; Li, Y.; Zhang, C.; Li, L.; Zhang, W.; Wang, D. Novel floating photocatalysts based on polyurethane composite foams modified with silver/titanium dioxide/graphene ternary nanoparticles for the visible-light-mediated remediation of diesel-polluted surface water. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Norouzbahari, S.; Roostaazad, R.; Hesampour, M. Crude oil desalter effluent treatment by a hybrid UF/RO membrane separation process. Desalination 2009, 238, 174–182. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Osuoha, J.O.; Abbey, B.W.; Egwim, E.C.; Nwaichi, E.O. Production and Characterization of Tyrosinase Enzyme for Enhanced Treatment of Organic Pollutants in Petroleum Refinery Effluent. SPE Nigeria Annual International Conference and Exhibition. LOCATION OF CONFERENCE, NigeriaDATE OF CONFERENCE;
- Parikh, P. S. , & Mazumder, S. (2015). Capacity of Azolla pinnata var. imbricata to absorb heavy metals and fluorides from the wastewater of oil and petroleum refining industry at Vadodara. International Journal of Allied Practice, Research and Review 2, 37–43.
- Passos, F.; Hernández-Mariné, M.; García, J.; Ferrer, I. Long-term anaerobic digestion of microalgae grown in HRAP for wastewater treatment. Effect of microwave pretreatment. Water Res. 2014, 49, 351–359. [Google Scholar] [CrossRef]
- Pintar, A.; Batista, J.; Levec, J. Integrated ion exchange/catalytic process for efficient removal of nitrates from drinking water. Chem. Eng. Sci. 2001, 56, 1551–1559. [Google Scholar] [CrossRef]
- Rajasulochana, P. , Dhamotharan, R. ( 5(4), 17–22.
- Ramachandran, S.K.; Sathishkumar, P. Membrane-based techniques for pollutants removal: An outlook on recent advancements. Curr. Opin. Environ. Sci. Heal. 2023, 36. [Google Scholar] [CrossRef]
- Rastegar, S.; Mousavi, S.; Shojaosadati, S.; Sheibani, S. Optimization of petroleum refinery effluent treatment in a UASB reactor using response surface methodology. J. Hazard. Mater. 2011, 197, 26–32. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Rastegar, S.O.; Shojaosadati, S.A.; Sheibani, S. Kinetic constants determination of petroleum refinery effluent treatment in a UASB reactor using RSM. Environ. Eng. Manag. J. 2017, 16, 121–130. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.; Levchuk, I.; Salcedo, I.; Acevedo-Merino, A.; Manzano, M. Post-treatment of refinery wastewater effluent using a combination of AOPs (H2O2 photolysis and catalytic wet peroxide oxidation) for possible water reuse. Comparison of low and medium pressure lamp performance. Water Res. 2016, 91, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Salahi, A.; Badrnezhad, R.; Abbasi, M.; Mohammadi, T.; Rekabdar, F. Oily wastewater treatment using a hybrid UF/RO system. Desalination Water Treat. 2011, 28, 75–82. [Google Scholar] [CrossRef]
- Santos, A.V.; Lin, A.R.A.; Amaral, M.C.S.; Oliveira, S.M.A.C. Improving control of membrane fouling on membrane bioreactors: A data-driven approach. Chem. Eng. J. 2021, 426, 131291. [Google Scholar] [CrossRef]
- Sarathy, B.P.; Hoy, P.M.; Duff, S.J. Removal of Oxygen Demand and Acute Toxicity during Batch Biological Treatment of a Petroleum Refinery Effluent. Water Qual. Res. J. 2002, 37, 399–411. [Google Scholar] [CrossRef]
- Sarhan Jawad, S. , & H Abbar, A. (2019). Treatment of petroleum refinery wastewater by electrochemical oxidation using graphite anodes. AL-Qadisiyah Journal for Engineering Sciences, 12(3), 144–150.
- Sarmadi, M.; Foroughi, M.; Saleh, H.N.; Sanaei, D.; Zarei, A.A.; Ghahrchi, M.; Bazrafshan, E. Efficient technologies for carwash wastewater treatment: a systematic review. Environ. Sci. Pollut. Res. 2020, 27, 34823–34839. [Google Scholar] [CrossRef] [PubMed]
- Sherhan, B. Y. , Abbas, A. D., Alsalhy, Q. F., Rashad, A. A., Rashad, Z. W., Shawkat, A. A., Abbas, T. K., & Kareem, N. A. A. (2016). Produced water treatment using ultrafiltration and nanofiltration membranes. Al-Khwarizmi Engineering Journal, 12(3), 10–18.
- Souza, R.; Ruotolo, L. Electrochemical treatment of oil refinery effluent using boron-doped diamond anodes. J. Environ. Chem. Eng. 2013, 1, 544–551. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Xie, H.; Liu, G. Removal of pollutants and accumulation of high-value cell inclusions in heavy oil refinery wastewater treatment system using Rhodopseudomonas and Pseudomonas:Effects of light intensity. Chem. Eng. J. 2021, 430, 132586. [Google Scholar] [CrossRef]
- Syarizan, M. (2004). Removal of Phenol and Benzene Using Photo Fenton Reagent.
- Tetteh, E. K. , Obotey Ezugbe, E., Rathilal, S., & Asante-Sackey, D. (2020). Removal of COD and SO42− from oil refinery wastewater using a photo-catalytic system—comparing tio2 and zeolite efficiencies. Water, 12(1), 214.
- Umejuru, E.C.; Mashifana, T.; Kandjou, V.; Amani-Beni, M.; Sadeghifar, H.; Fayazi, M.; Karimi-Maleh, H.; Sithole, N.T. Application of zeolite based nanocomposites for wastewater remediation: Evaluating newer and environmentally benign approaches. Environ. Res. 2023, 231, 116073. [Google Scholar] [CrossRef] [PubMed]
- Urgun-Demirtas, M.; Benda, P.L.; Gillenwater, P.S.; Negri, M.C.; Xiong, H.; Snyder, S.W. Achieving very low mercury levels in refinery wastewater by membrane filtration. J. Hazard. Mater. 2012, 215-216, 98–107. [Google Scholar] [CrossRef]
- Urgun-Demirtas, M.; Negri, M.C.; Gillenwater, P.S.; Nnanna, A.A.; Yu, J. Meeting world's most stringent Hg criterion: A pilot-study for the treatment of oil refinery wastewater using an ultrafiltration membrane process. J. Environ. Manag. 2013, 117, 65–75. [Google Scholar] [CrossRef]
- Vahid, B.; Khataee, A. Photoassisted electrochemical recirculation system with boron-doped diamond anode and carbon nanotubes containing cathode for degradation of a model azo dye. Electrochimica Acta 2012, 88, 614–620. [Google Scholar] [CrossRef]
- Valverde, J.L.; De Lucas, A.; Carmona, M.; Pérez, J.P.; González, M.; Rodríguez, J.F. Minimizing the environmental impact of the regeneration process of an ion exchange bed charged with transition metals. Sep. Purif. Technol. 2006, 49, 167–173. [Google Scholar] [CrossRef]
- Wuyep, P. A. , Chuma, A.G., Awodi, S., & Nok, A. J. (2007). Biosorption of Cr, Mn, Fe, Ni, Cu and Pb metals from petroleum refinery effluent by calcium alginate immobilized mycelia of Polyporus squamosus. Scientific Research and Essay. 2(7), 217–221.
- Yadav, D.; Das, R.K.; Saxena, S.; Shukla, S. OGF nanocomposite foam for enhanced recyclability and oil-recovery. J. Clean. Prod. 2023, 411. [Google Scholar] [CrossRef]
- Yadav, S.; Kamsonlian, S. A review of electrochemical methods for treatment of wastewater. Mater. Today: Proc. 2023, 78, 36–39. [Google Scholar] [CrossRef]
- Yan, L.; Ma, H.; Wang, B.; Mao, W.; Chen, Y. Advanced purification of petroleum refinery wastewater by catalytic vacuum distillation. J. Hazard. Mater. 2010, 178, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Ma, H.; Wang, B.; Wang, Y.; Chen, Y. Electrochemical treatment of petroleum refinery wastewater with three-dimensional multi-phase electrode. Desalination 2011, 276, 397–402. [Google Scholar] [CrossRef]
- Yavuz, Y.; Koparal, A.S.; Öğütveren. B. Phenol Degradation in a Bipolar Trickle Tower Reactor Using Boron-Doped Diamond Electrode. J. Environ. Eng. 2008, 134, 24–31. [Google Scholar] [CrossRef]
- Yavuz, Y.; Koparal, A.S.; Öğütveren. B. Treatment of petroleum refinery wastewater by electrochemical methods. Desalination 2010, 258, 201–205. [Google Scholar] [CrossRef]
- Yazdandoost, F.; Noruzi, M.M.; Yazdani, S.A. Sustainability assessment approaches based on water-energy Nexus: Fictions and nonfictions about non-conventional water resources. Sci. Total. Environ. 2020, 758, 143703. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Hajiaghaei-Keshteli, M.; Bokhari, A.; Sundaramurthy, S.; Panneerselvam, B.; Rezakhani, Y. Wastewater treatment with nanomaterials for the future: A state-of-the-art review. Environ. Res. 2023, 216. [Google Scholar] [CrossRef] [PubMed]
| Scenario | Purpose | Main results and conclusions | Country & Reference | Year | |
|---|---|---|---|---|---|
| MBR-PMR with TiO2 | Removal of recalcitrant organic compounds | PMR with green TiO2 and recycled membrane with high efficiency and stability in removing organic matter. | Brazil, (de Oliveira et al., 2020) | 2020 | |
| MBR on full-scale | Water supply required for Greenfields | MBR reduces the concentration of NH3-N to less than 0.5 ppm and reduces the potential for nitrification. | Brazil, (Cerqueira et al., 2013) | 2013 | |
| Sequencing batch reactor system | Removal of phenolic compounds | High effectiveness in removing total phenols around 98%. | UAE, (W. Al Hashemi et al., 2015) | 2014 | |
| Anaerobic biofilm reactor (AnBR) | Removal of organic compounds | The significant relationship between system efficiency and bacterial diversity. The vital role of Acinetobacter and Pseudomonas bacteria in hydrocarbon degradation. Removal of COD by 80% after 11 days from the system launch. |
China, (Dong et al., 2016) | 2016 | |
| MBR on a pilot scale | Removal of organic compounds | MBR has high efficiency in removing COD, NH3-N, turbidity, color, phenol, and toxicity and subsequently meets standards for disposal and reuse of non-potable water. | Brazil, (Alkmim et al., 2017) | 2017 | |
| Biocathode microbial desalination cell (interaction of microalgae and bacteria) | Removing the organic compounds of ORE coupled with seawater desalination and bioelectricity production | Reduction of 70% COD, 81% BOD, 67% phosphorous, 61% sulfide, 67% TDS and 62% TSS. Save 1.245 kWh/m3 of power by microbial desalination cell (MDC) |
India, (Ashwaniy et al., 2020) | 2020 | |
| Biological treatment using Tyrosinase Enzyme produced from different microbial strains | The degradation of toxic organic pollutants | Significant removal of 95% phenol and 89% PAHs in effluent. | Nigeria, (Osuoha et al., 2019) | 2019 | |
| UASB-PBBR | Biodegradation of recalcitrant organic compounds (COD & PAHs) | COD removal efficiency in the UASB and PBBR over 118 days was 68.48% and 38.28%, respectively. Complete removal of PAHs. |
Iran, (Nasirpour et al., 2015) | 2015 | |
| Anoxic–aerobic sequential moving bed reactors | Removal of hydrocarbon, phenol, sulfide, and ammonia-nitrogen | The optimum HRT of 16 h for complete removals of phenol, TPH, COD, and NH3-N | India, (Mallick & Chakraborty, 2017) | 2017 | |
| Scenario | Purpose | Main results and conclusions | Country & Reference | Year | |
| Submerged ultrafiltration system using hollow fiber (HF) polytetrafluoroethylene (PTFE) membranes | The removal of total petroleum hydrocarbon (TPH) | The removal efficiency of TPH was found to be more than 91%. Different fractions of petroleum and PAH compounds were reduced. | Iran, (Keyvan Hosseini, 2022) | 2023 | |
| Continuous flow microbial fuel cell (MFC) and packages of cells with serial and parallel flow connections | COD removal and electricity generation | At HRT 45 h, COD removal increased to 87% by increasing HRT. Open-circuit voltage (OCV) produced was 760 mV in parallel flow connections (PFC). COD removal in SFC (89%) and PFC (42%). | Iran, (Kadivarian et al., 2020) | 2020 | |
| Bioremediation (using Azolla pinnata var. imbricata) | Absorb Heavy Metals and Fluorides | A significant difference between the initial and final concentrations of metal ions and fluoride after using the Azolla plant. bioconcentration factor (BCF) of fluoride, zinc, cadmium, and iron ≤ 1 and BCF of lead, chromium, hexavalent chromium, and copper ≅ 1. | India, (Parikh & Mazumder, 2015) | 2015 | |
| Bioremediation: A Review | Removal of Petroleum Contaminants | Degradation of complex petroleum chemical pollutants into simpler forms using bioremediation (through microbes, plants, or biocatalysts (via enzymatic pathways), biosorbents (use of microbial biomass), or the use of biological products (natural fibers, composite biologicals). | India, (Imam et al., 2021) | 2021 | |
| The use of Biosurfactants | Minimizing solid wastes | 50 mg/l of rhamnolipid reduces sludge disposal by 52%, removes COD by 81-97%. | Brazil, (Alexandre et al., 2016) | 2015 | |
| anoxic-oxic MBR on pilot scale | Removal of organic compounds | COD removal of 97.15 ± 1.85%, while oil and grease removal at 96.6 ± 2.6% | China, (Abass et al., 2018) | 2018 | |
| UASB | Removal of organic compounds | In four organic volumetric loading rates of 0.58, 0.89, 1.21, and 2.34 kg/m3 d, COD removal was 78, 82, 83, and 81% respectively. | Malaysia, (Gasim et al., 2012) | 2012 | |
| Bioremediation (Photosynthetic bacteria) using effects of light intensity | Removal of pollutants and accumulation of high-value cell inclusions | 500 lx was the optimal intensity for 62.66% SCOD and 91.54% NH4+-N removal. 4000 lx was the optimal light intensity for the carotenoid, bacteriochlorophyll, and biomass production | China, (Sun et al., 2022) | 2021 | |
| Scenario | Purpose | Main results and conclusions | Country & Reference | Year | |
| UASB reactor using RSM | Removal of organic compounds | the effluent COD was 120 mg/L, the VSS effluent was 0.4 mg/L and the biogas rate was 0.025 L biogas/L feed. | Iran, (Rastegar et al., 2017) | 2017 | |
| MBR | Removal of organic compounds | The use of oxalic acid at pH 2.5 followed by the use of NaOCl (5000ppm) increased the permeability of the membrane up to 92.7%. | Brazil, (Lebron et al., 2021) | 2021 | |
| Phytoremediation (using Brassica juncea) muskgrass (a macroalga, Chara canescens) | Removal of Selenium | Decomposition of all accumulated SeCN(-) into other forms of SeCN | USA, (M. P. De Souza et al., 2002) | 2002 | |
| Expanded Bed Nitrification | Nitrification | Biofilms incubated in ORE achieved higher ammonia removal than those incubated in the synthetic wastewater (SWW). | UK, (Akhidime, 2009) | 2009 | |
| BAC | removing PAHs and aliphatic hydrocarbons | Removal of PAH by 97% under condition contact time (24 h), temperature (24 °C), and moderate oxygen concentration (6–7 mg O2 L−1) | Sweden, (Augulyte et al., 2009) | 2009 | |
| UASB reactor | Removal of COD | 76.3% COD removal efficiency and a 0.25 L biogas/L feed d biogas production rate | Iran, (Rastegar et al., 2011) | 2011 | |
| Bioremediation | Removal of COD & BOD using Scenedesmus obliquus |
Bioremediation is an effective technology in the reduction of pollutants like inorganic and organic compounds | India, (Rajasulochana et al., 2009) | 2009 | |
| Batch biological reactor | Removal of COD, BOD, and Acute Toxicity | removal of 93% of BOD, 77% of COD, and 27.8% EC50 | Canada, (Sarathy et al., 2002) | 2002 | |
| Biosorption | Removal of Cr, Mn, Fe, Ni, Cu, and Pb metals | Maximum uptake of cationic metal ions at pH 4-6 by immobilized P. squamosus with fungal biomass | Nigeria, (Wuyep et al., 2007) | 2007 | |
| Phytoremediation (using water hyacinth) | Removal of heavy metals | To overcome this limitation, factors such as pH, temperature, amount of water hyacinth, effluent flow and retention time, metal concentrations, and size of lagoon need also to be considered. | Malaysia, (Ismail & Beddri, 2009) | 2008 | |
| Scenario | Purpose | Main results and conclusions | Country & Reference | Year |
|---|---|---|---|---|
| UF-IX/MOX | Supply of makeup water for cooling towers | In the optimum pressure of 1 bar, removal efficiency of COD (57%), TDS (80%), Turbidity (94%), SiO2 (67%), Oil (88%), and HPC (99%) was achieved. | Iran, (Hashemi et al., 2020) | 2020 |
| Comparison of hybrid UF-OMBR and MBR | oil refinery effluent treatment | The high removal efficiency for UF in UF-OMBR [COD removal (99.6)] compared to UF in conventional MBR [COD removal (66.8)] | Brazil, (Moser et al., 2019) | 2019 |
| FO using NaCl as the draw solute | Desalination | SO42- rejection of 100%, CO32- rejection of 95.66 ± 0.32%, and flux recovery of 95% after CIP. | South Africa, (Ezugbe et al., 2021) | 2021 |
| UF process | Removal of turbidity and mercury to meet the discharge standard | Removal of mercury less than 1.3 ppt and turbidity to less than 0.16 NTU. | USA, (Urgun-Demirtas et al., 2013) | 2013 |
| Comparison of FO, RO, FO-RO Hybrid | Desalination of ORE to achieve effluent discharge standards | For FO (permeation flux: 3.64 ± 0.13 L/m2 h, Cl-: 35.5, SO42-: 100%, CO32-: 94.59 ± 0.32 and flux recovery of 86%. For RO (permeation flux: 2.29 ± 0.24 L/m2h, Cl- rejection: 90.5%, SO42-: 95.1%, CO32-:97.3 ± 0.4 and flux recovery: 62.52%. The FO-RO hybrid process proved unsuccessful | South Africa, (EO et al., 2020; Ezugbe, 2021) | 2021 |
| Membrane desalination | Effluent desalination | In optimum conditions, final treated effluent by MD, the maximum amount of conductivity, COD, and chloride were 5.6 μS/cm, 4 mg/L, and less than 7 mg/L respectively. | Iran, (Jalayer et al., 2022) | 2022 |
| Membrane process | possibility to reuse the effluent as a makeup water | UF was more efficient in reaching the makeup water. | Turkey, (Al-Nidawi, 2022) | 2022 |
| Nanofiltration membrane processes | water recycling, reuse, and product recovery: A review | NF was more efficient in ORE reclamation, recycling, reuse, and recovery applications due to its capability to separate the divalent/polyvalent ions while allowing permeation for monovalent ions and small molecules. | Malaysia, (Ahmad et al., 2022) | 2022 |
| Micellar-enhanced ultrafiltration (MEUF) | Removal of heavy metals | Ni, Pb, Cd, and Cr decreased by 96%, 95%, 92%, and 86%, respectively | Iran, (Hashemi et al., 2018) | 2018 |
| MF-RO | Removal of pollutants in petroleum effluents | MF-RO in the reclamation of ORE to supply water to steam boilers was efficient. | USA, (Lopez et al., 2006) | 2006 |
| Scenario | Purpose | Main results and conclusions | Country & Reference | Year |
| UF-NF | Removal of turbidity, COD, and Oil content, SO4-2, and NO3 | Removal of turbidity by 95%, COD (160 mg/l), Oil content (26.8 mg/l), SO4-2 (110 mg/l), and NO3 (48.4 mg/l) were agreed with the permissible limits of WHO. The Cl-1 (8900 mg/l) component was not within the allowable limits. This method is seen to be not sufficient to remove the salinity of the produced water. | Iraq, (Sherhan et al., 2016) | 2016 |
| UF (PS membrane)-RO (PA membrane) | Desalter effluent treatment | The UF membrane as an effective pretreatment removed more than 75% of the oil content, and RO removed more than 95% of TDS | Iran, (Norouzbahari et al., 2009) | 2009 |
| Membrane desalination | Removal of mercury | MF, UF, NF, and RO membranes were efficient in achieving the Hg discharge criterion (<1.3 ng/L). P≥34.5 bar had a significant effect on NF and RO flux and permeate quality. | USA, (Urgun-Demirtas et al., 2012) | 2012 |
| Hybrid UF/RO membrane using polyacrylonitrile and polyamide membranes | Removal of oil and grease content, TOC, COD, TDS and turbidity | The hybrid UF/RO system reduced 100%, 98%, 98%, 95%, and 100% in Oil and G content, TOC, COD, TDS, and turbidity, respectively. | Iran, (Salahi et al., 2011) | 2011 |
| Scenario | Purpose | Main results and conclusions | Country & Reference | Year |
|---|---|---|---|---|
| Electrochemical oxidation using three-dimensional multi-phase electrode | Removal of COD, salinity, and phenol | Under optimum conditions (pH: 6.5; v:12V): Removal of COD by 92.8%, and salinity (84 μS cm−1) | China, (Yan et al., 2011) | 2011 |
| Electrochemical oxidation methods: using a boron-doped diamond anode, ruthenium mixed metal oxide (Ru-MMO) electrode, electro-Fenton, and electrocoagulation | Removal of COD, and phenol | Complete phenol and COD removal in almost all electrochemical methods, except electrocoagulation. The most efficient method: the electro-Fenton process followed by the electrochemical oxidation using a boron-doped diamond anode |
Turkey, (Yavuz et al., 2010) | 2010 |
| Electrochemical oxidation using graphite anodes | Removal of COD, and phenol | Under best conditions (current density 12 mA cm-2, pH 7, and NaCl: 2 gl-1, and treatment time of 60 min): COD removal by 100% and phenol removal by 99.12%. | Iraq, (Sarhan Jawad & H Abbar, 2019) | 2019 |
| Batch ozone-photocatalytic oxidation (O3/UV/TiO2), and biological remediation by macroalgae | Removal of phenol, sulfide, COD, O&G, and ammonia | the physicochemical results showed that a combination of (O3/UV/TiO2) for 10 min followed by macroalgae depuration seems to be a good option for cost-effective treatment of produced water streams. | Brazil, (Corrêa et al., 2010) | 2010 |
| Combination of AOPs (H2O2 photolysis and catalytic wet peroxide oxidation) | Removal of pollutants in petroleum effluents | H2O2/UVC process with LP lamp: removal of phenolic compounds, TOC, and COD was 100%, 52.3%, and 84.3%, respectively. Complete elimination of phenolic compounds, 47.6% of TOC, and 91% of COD was achieved during the H2O2/UVC process with an MP lamp. |
Spain, (Rueda-Márquez et al., 2016) | 2016 |
| Electrocoagulation: RSM design approach | Removal of turbidity, TOC, COD, TDS, and Oil content | Removal of turbidity by 84.5%, COD by 82%, TDS by 20%, and Oil content by 99%. | Iraq, (Jasim et al., 2023; Jasim & AlJaberi, 2023a, 2023b) | 2023 |
| Electrocoagulation Reactor Using Response Surface Method | Removal of TOC, Oil Content, and Turbidity | Removal of turbidity by 84.43%, TOC by 84%, and Oil content by 86%. | Iraq, (AlJaberi, 2020a; AlJaberi et al., 2020) | 2020 |
| Ozone-Based Advanced Oxidation Processes | Reuse and Recycle Solutions | ↑ H2O2 amount to 80 mg/L, ↓ to 37.5 min →decreasing the energy and reagent consumption costs by 37%, reaching a final TOC under 4 mg/L. | Spain, (Demir-Duz et al., 2020) | 2020 |
| Scenario | Purpose | Main results and conclusions | Country & Reference | Year |
| Electrocoagulation (EC) and electrochemical oxidation (EO) techniques | Removal of COD | EC (aluminum and mild steel were used as the anode): COD removal by 87% EO (ruthenium oxide-coated titanium (RuO2/Ti) was used as the anode): COD removal by 92% |
India, (Ibrahim, 2013) | 2013 |
| Electrochemical: using boron-doped diamond anodes | Organic compounds removal | The anode could be successfully used to treat effluents containing organic compounds. The anode (which was deposited onto a niobium substrate) was not stable and showed intense pitting corrosion after 300 h of use. |
Brazil, (R. B. A. Souza & Ruotolo, 2013) | 2013 |
| Scenario | Purpose | Main results and conclusions | Country & Reference | Year |
|---|---|---|---|---|
| Electrofenton process: using a porous graphite air-diffusion cathode | COD removal | COD removal efficiency: 94% with lowering specific energy consumption of 3.75 kWh/kg COD | Iraq, (Jiad & Abbar, 2023) | 2023 |
| Photo-catalytic system (TiO2 and zeolite) | Removal of COD and SO42- | Removal efficiency: 92% for zeolite and 91% for TiO2, TiO2 exhibited more efficiency in terms of mixing rate and reaction time requirements. | South Africa, (Tetteh et al., 2020) | 2020 |
| TiO2/Ag photocatalyst fixed on lightweight concrete plates | Removal and degradation of organic pollutants | COD removal under sunlight for 8 hours: 51.8% COD removal using UV-A lamps: 76.3% |
Iran, (Delnavaz & Bos’ hagh, 2021) | 2021 |
| Photo-ferrioxalate and Fenton’s reactions with UF step | Removal of pollutants | Removal of COD, phenol, sulfides, TSS, turbidity, and color, were 94%, <0.5 mg/L, <0.2 mg/L, <1 mg/L, 2 NTU, and 254 Pt-Co, respectively. | Mexico, (Estrada-Arriaga et al., 2016) | 2015 |
| Photovoltaic cell electro-Fenton oxidation | Removal of organic compounds | More than 98% removal of organic content and 39.67 kWh/m3 for the consumption of energy. | Iraq, (Atiyah et al., 2020) | 2020 |
| Nano-TiO2-Induced Photocatalysis | Removal of TPH | The use of solar light with doped TiO2 can replace UV light, which has a much higher energy consumption. Light-emitting diode light can also be an option because of its higher electron-photon conversion rate. | Canada, (Liu et al., 2017a) | 2017 |
| Zinc Oxide Nano Particle as Catalyst in Batch and Continuous Systems | Removal of Oil content | Removal efficiency of the Oil content of the ZnO/UV was 80% at 20 mL/min and irradiation time 120 min. | Iraq, (Alkhazraji & Alatabe, 2021) | 2021 |
| Photo Fenton Reagent | Removal of Phenol and Benzene | The optimum ratio of Fenton Reagent is Fe: H202=l:25, at a COD reduction of 53.8%. The optimum temperature for operating a photo-Fenton reaction is 40°C, at a COD reduction of 68%. | Malaysia, (Syarizan, 2004) | 2004 |
| A semiconductor (ZnO, TiO2, and AL2O3) in the presence of solar as source of energy | Removal of oil content | Removal of oil content by ZnO, TiO2, and AL2O3 were 95.2 % and 92.11%, 80.7%, respectively. | Iraq, (Hassan et al., 2018) | 2018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




