Submitted:
10 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
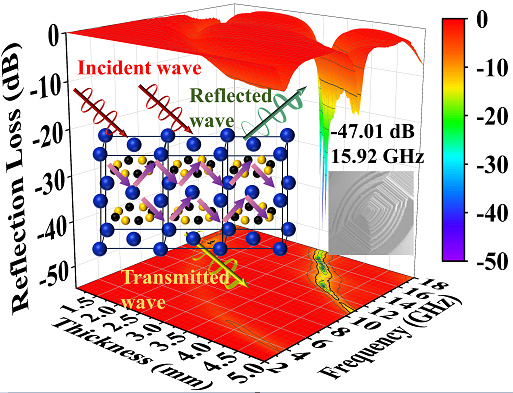
Keywords:
1. Introduction
2. Experimental Procedure
2.1. Materials
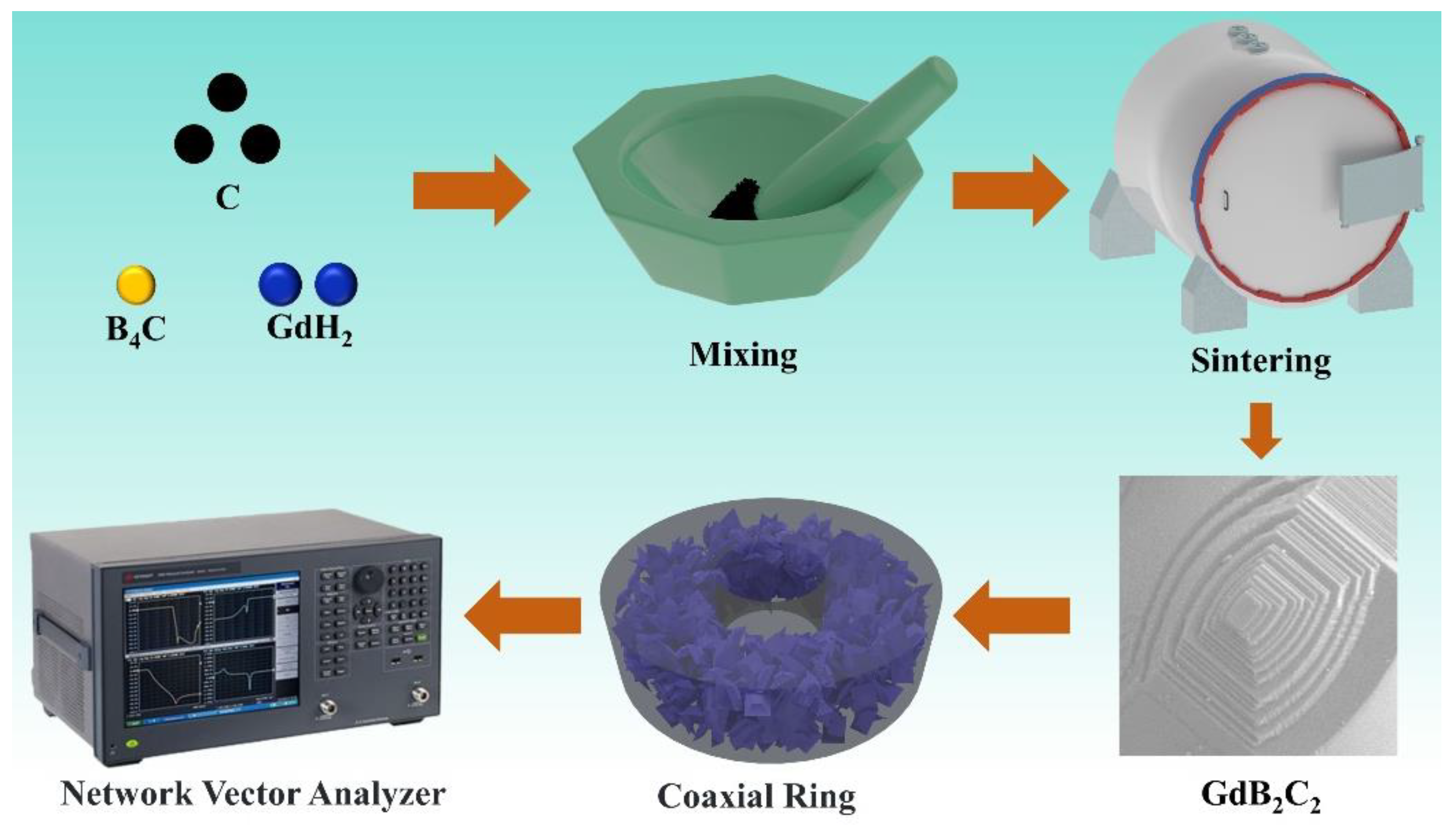
2.2. Fabrication of GdB2C2
2.3. Characterizations
3. Results and Discussion
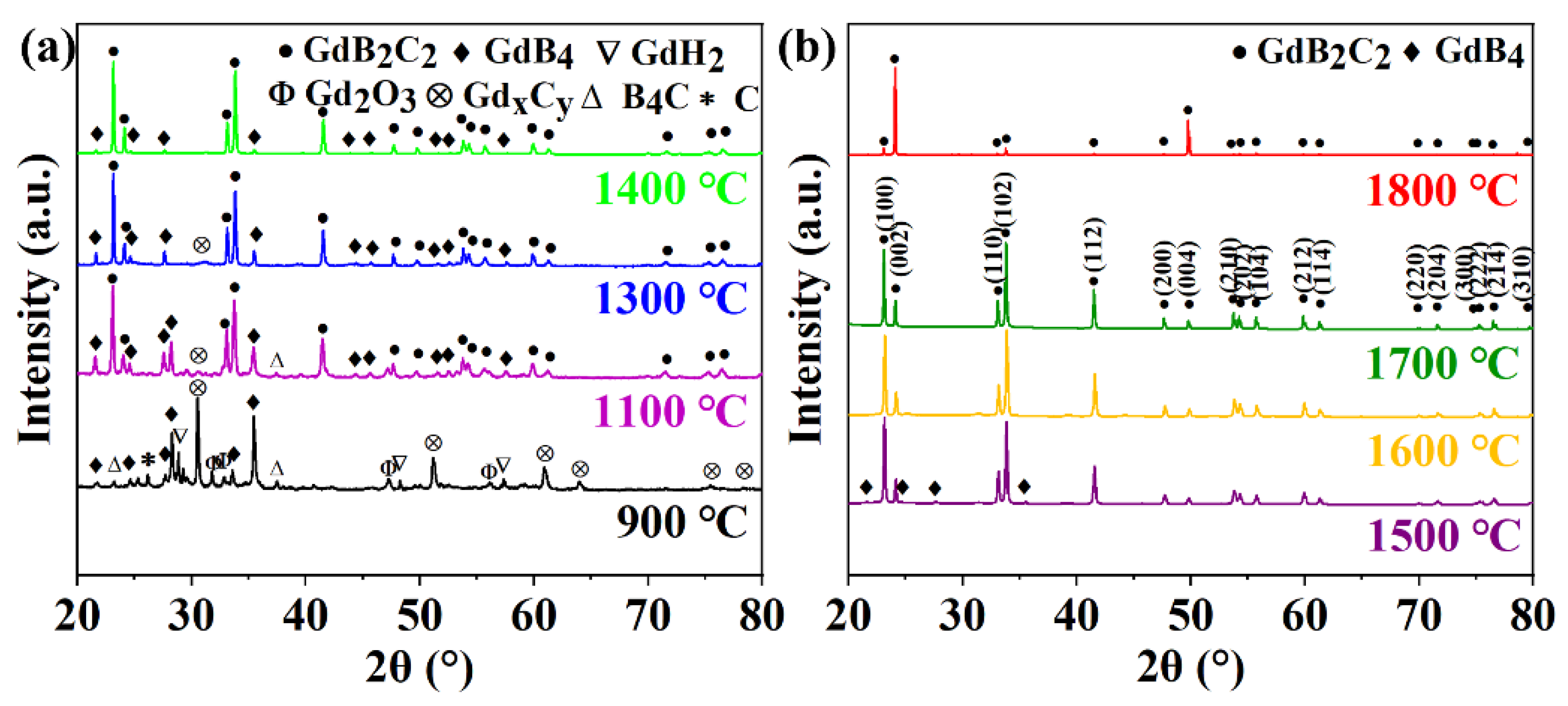
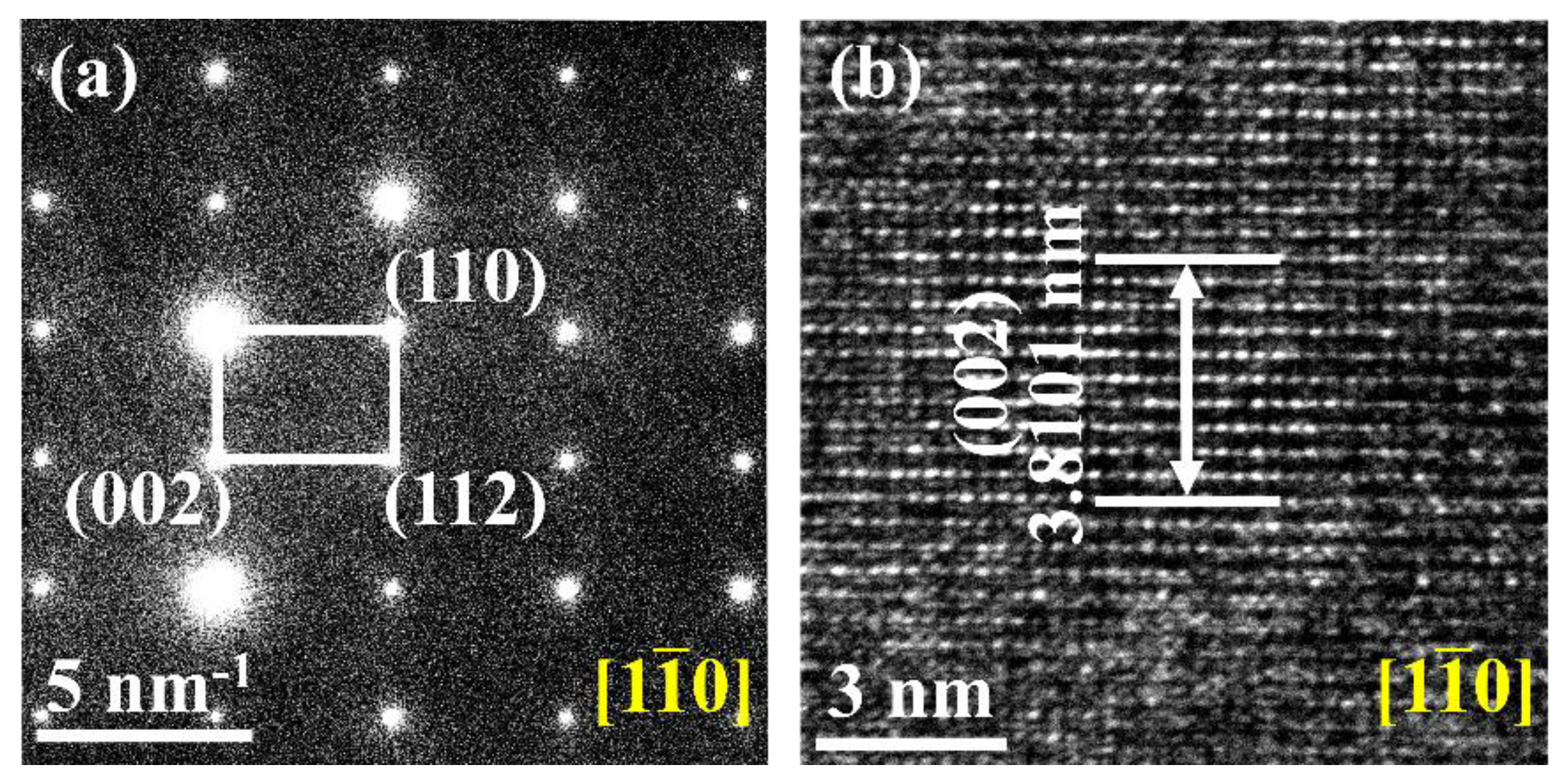
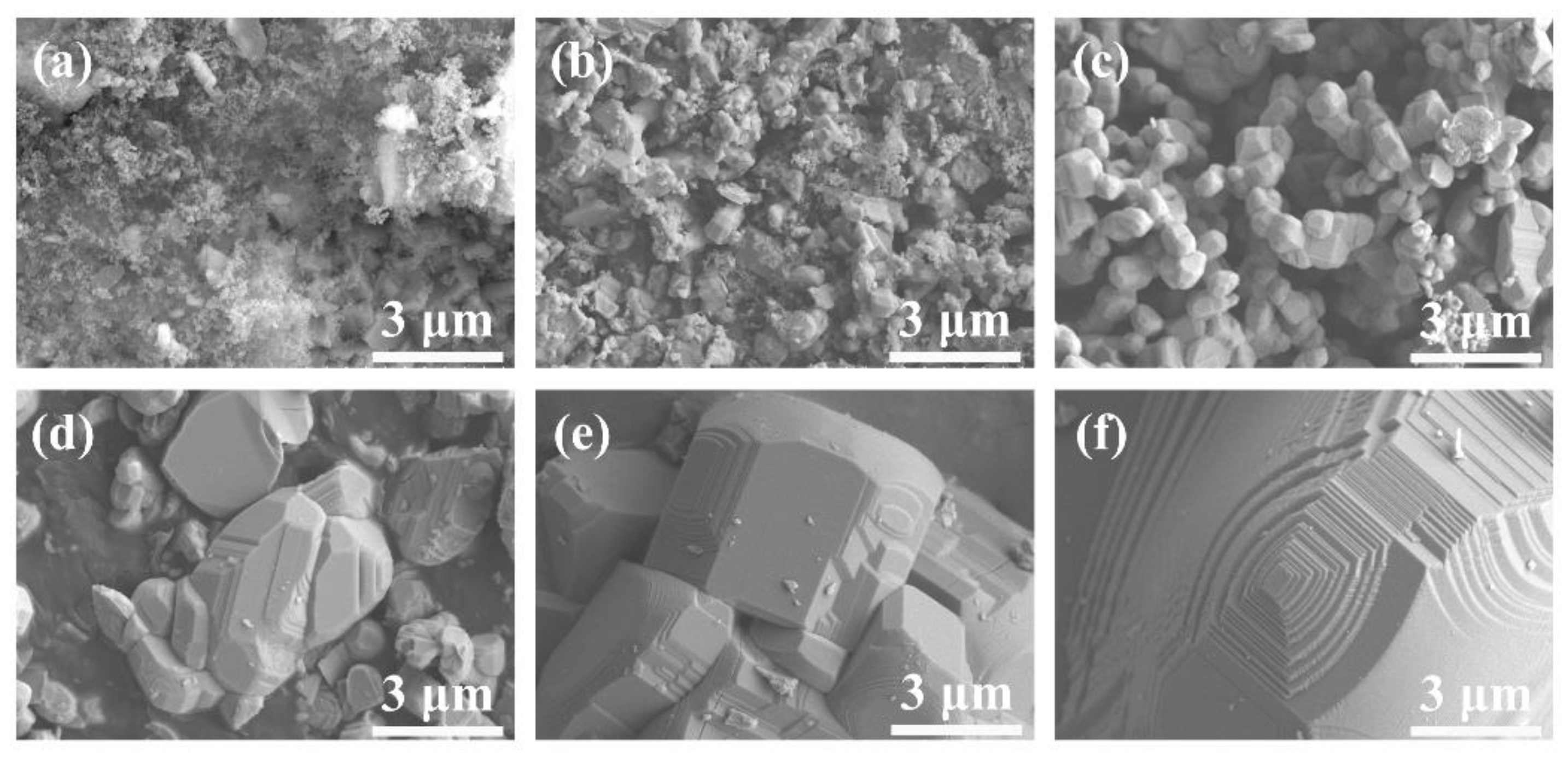
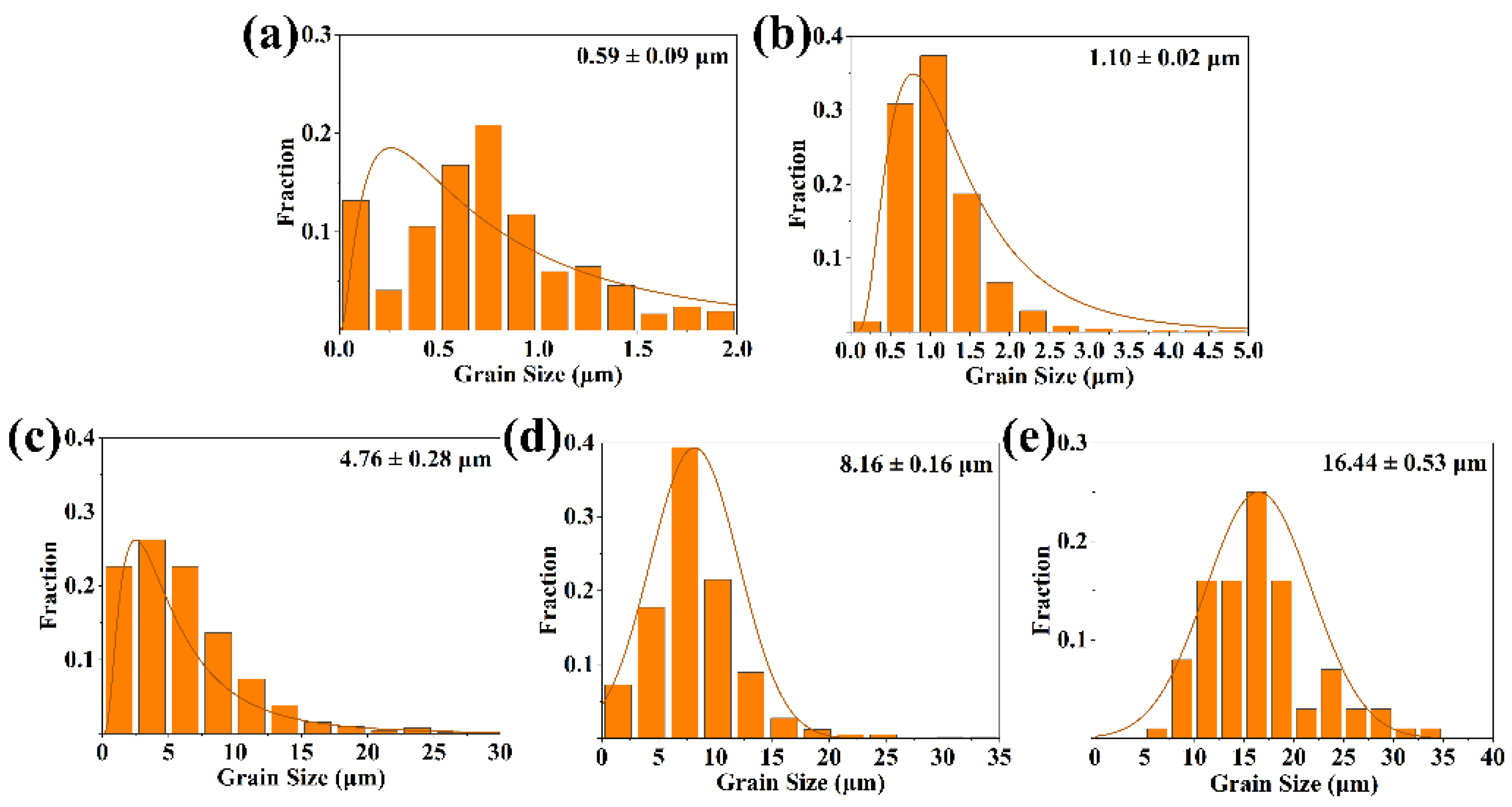
3.1. Microstructure and Phase Composition of GdB2C2
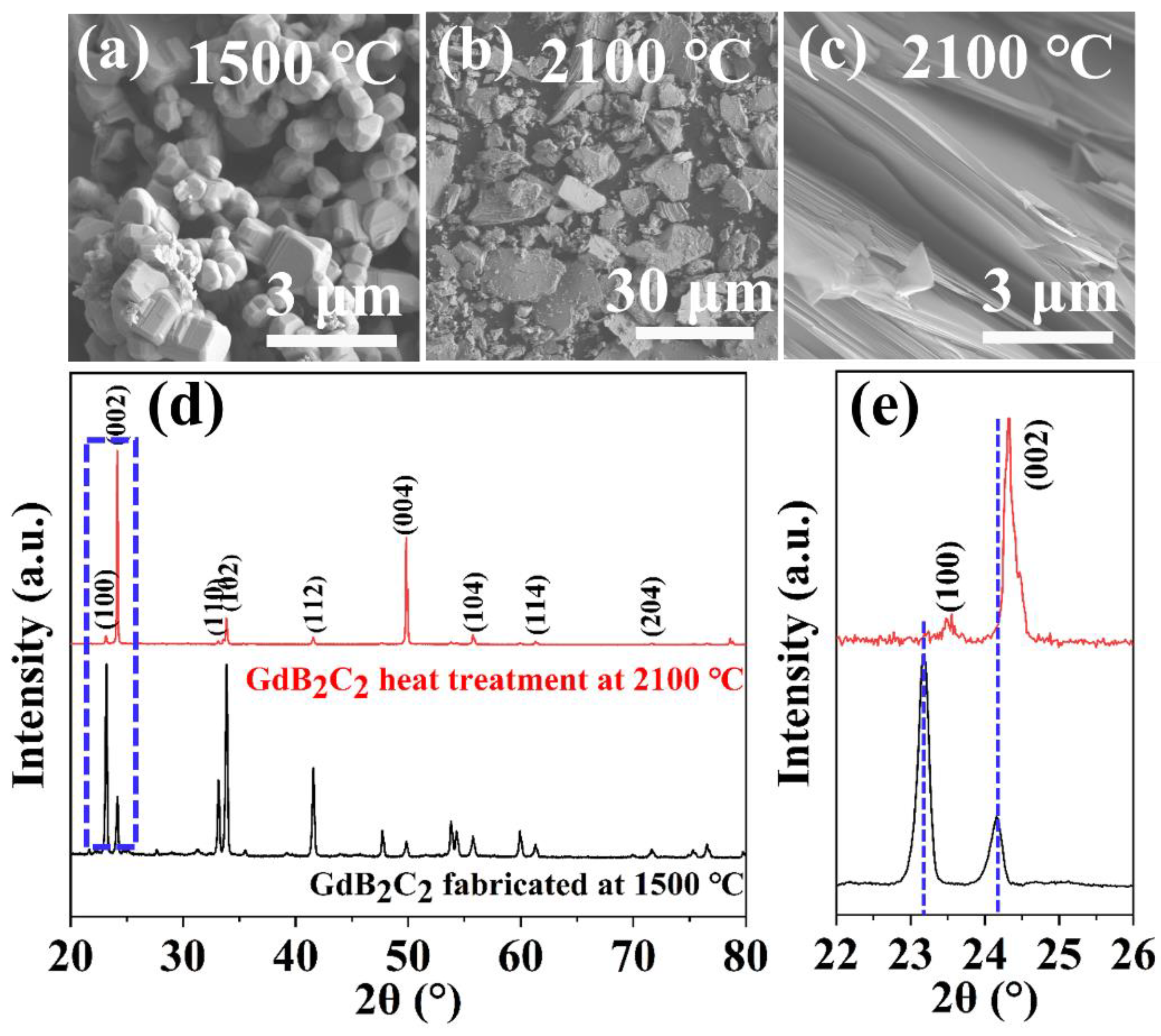
3.2. Ultra-High Temperature Thermal Stability of GdB2C2 at 2100 °C
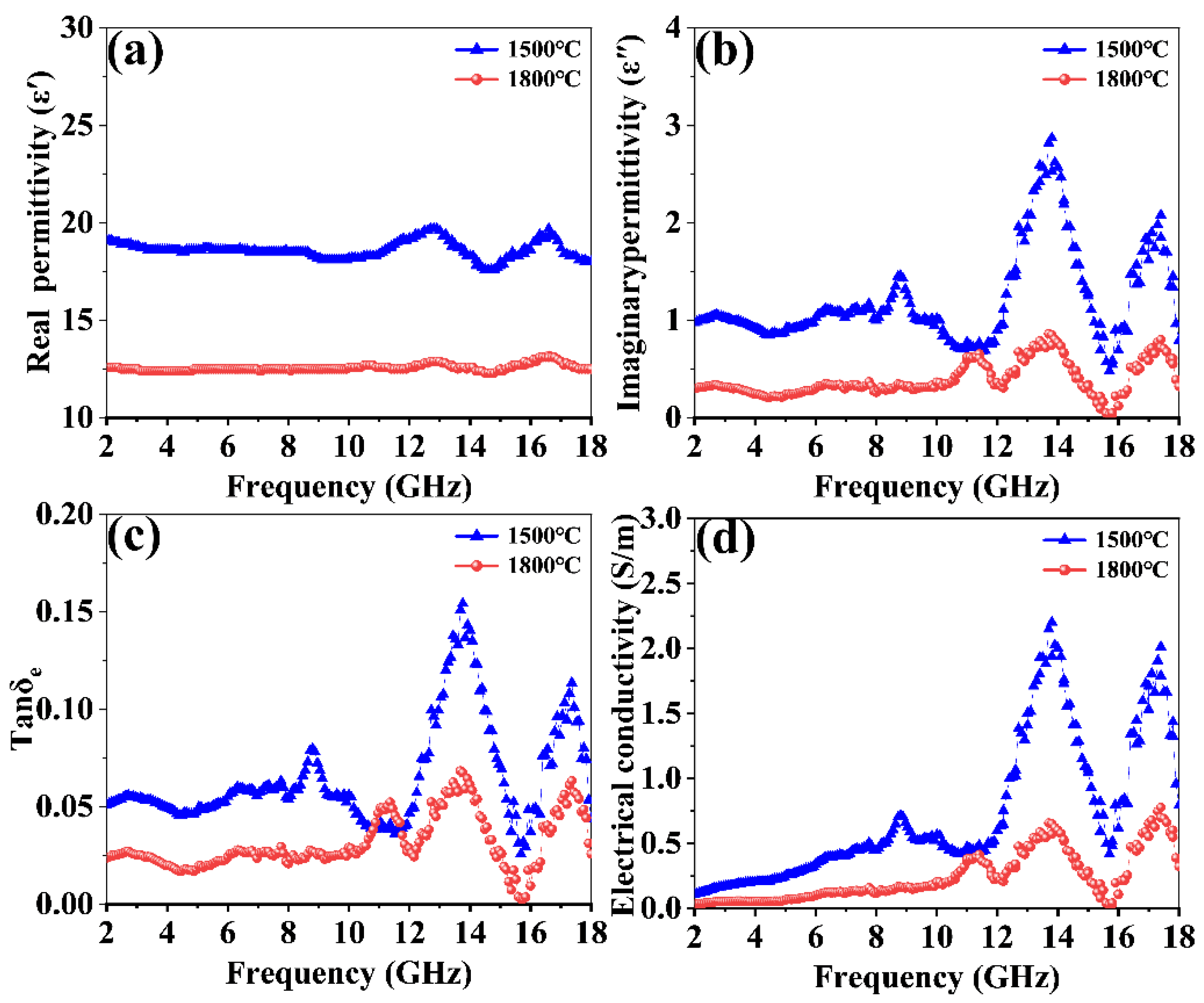
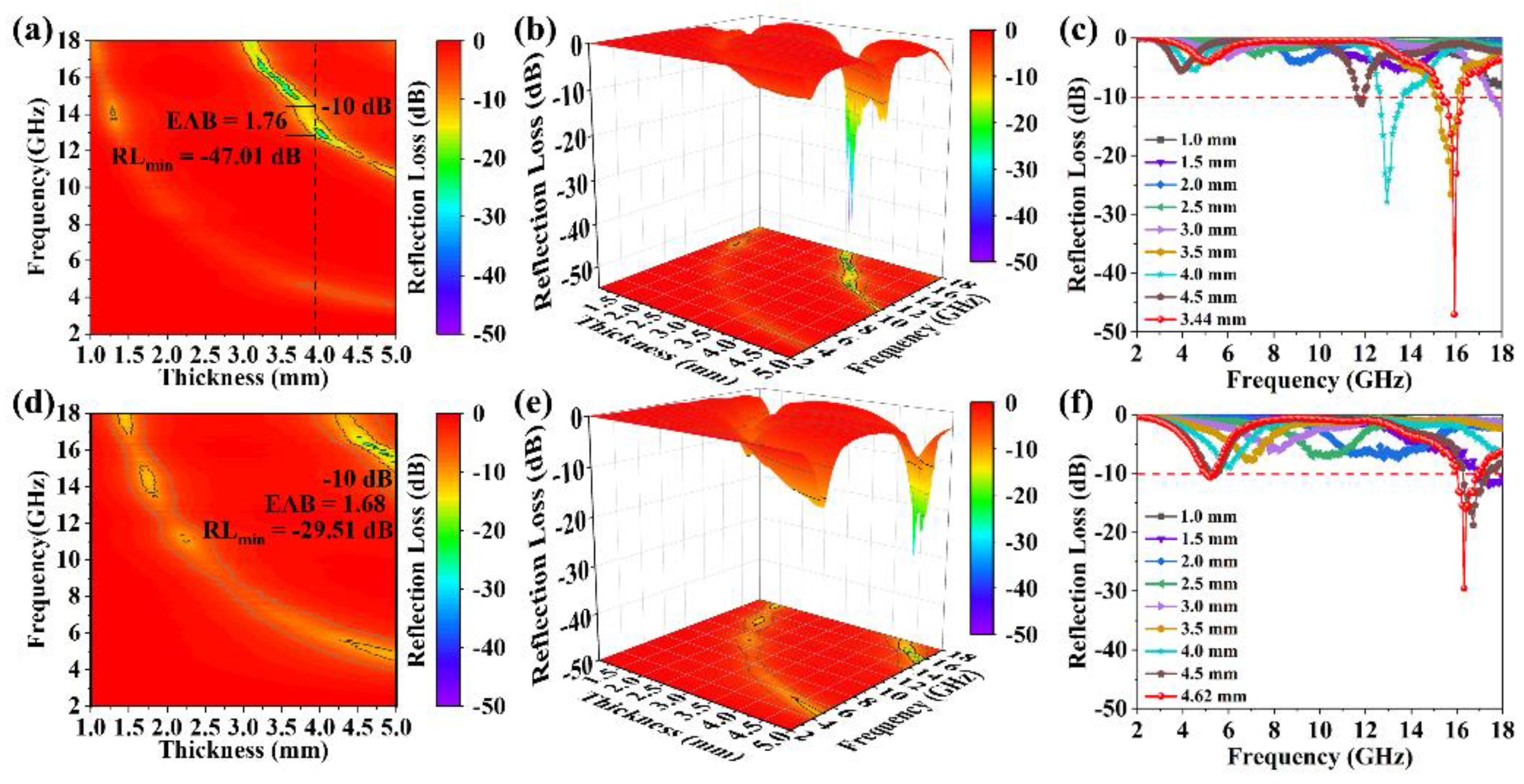
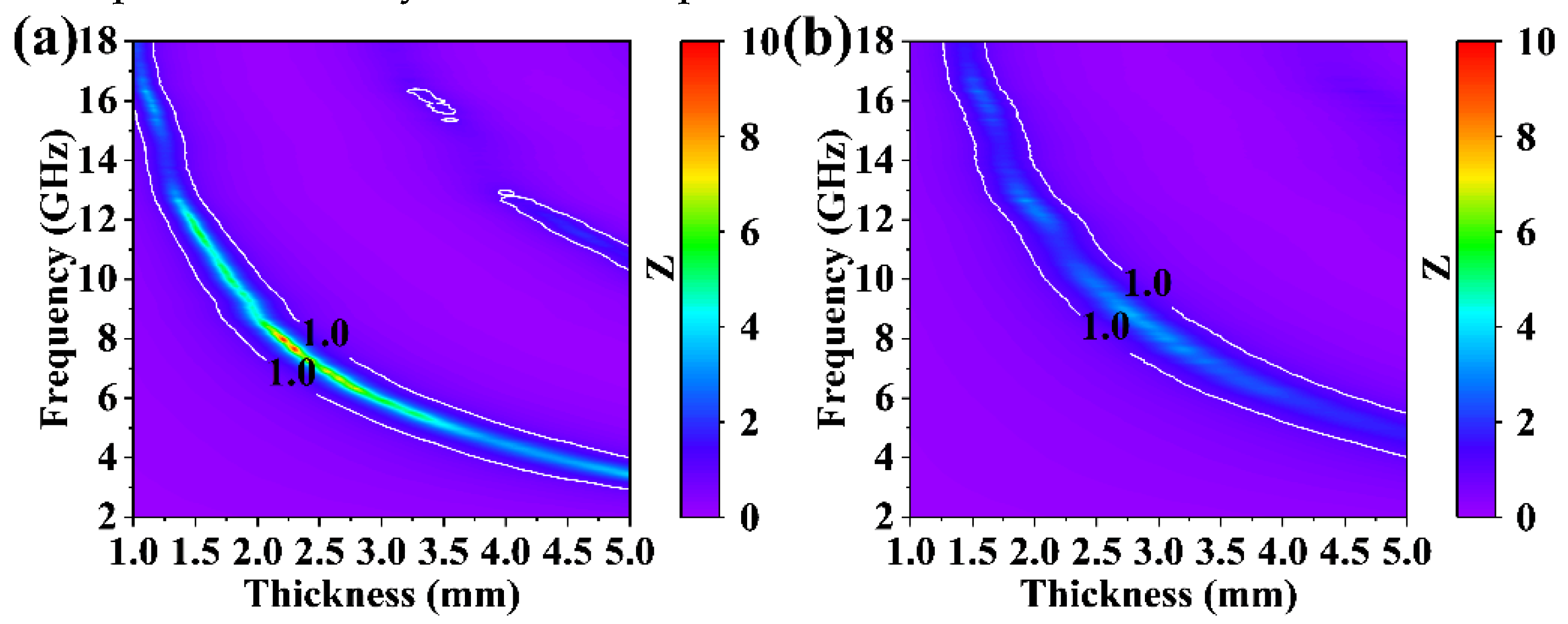
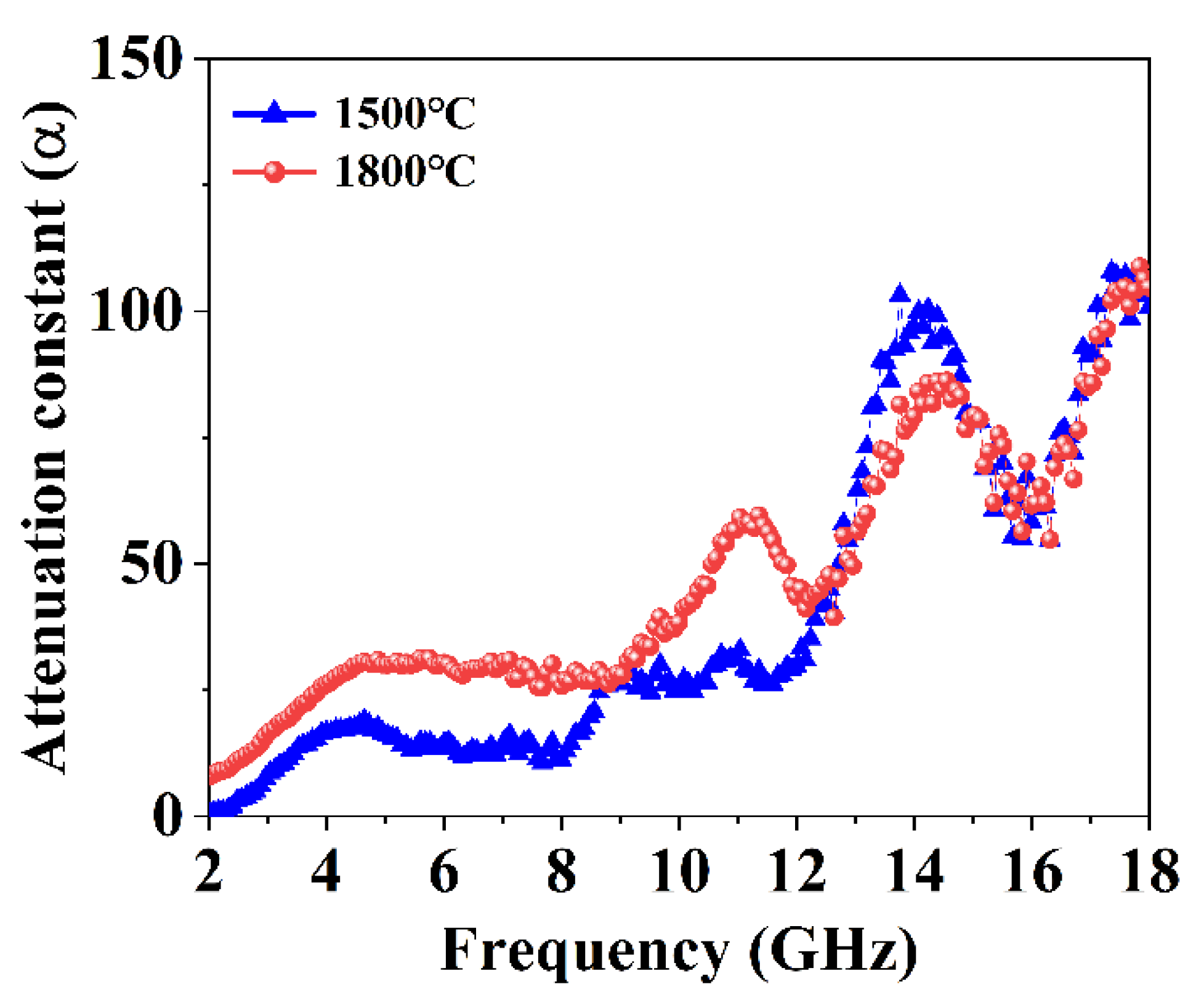
3.3. Electromagnetic Wave Absorption Properties of GdB2C2
4. Conclusions
Acknowledgments
References
- Lun, H.; Zeng, Y.; Xiong, X.; Ye, Z.; Zhang, Z.; Li, X.; Chen, H.; Liu, Y. Oxidation behavior of non-stoichiometric (Zr,Hf,Ti)Cx carbide solid solution powders in air. J. Adv. Ceram. 2021, 10, 741–757. [Google Scholar] [CrossRef]
- Peters, A.B.; Wang, C.; Zhang, D.; Hernandez, A.; Nagle, D.C.; Mueller, T.; Spicer, J.B. Reactive laser synthesis of ultra-high-temperature ceramics HfC, ZrC, TiC, HfN, ZrN, and TiN for additive manufacturing. Ceram. Int. 2023, 49, 11204–11229. [Google Scholar] [CrossRef]
- Yu, Z.; Lv, X.; Lai, S.; Yang, L.; Lei, W.; Luan, X.; Riedel, R. ZrC–ZrB2–SiC ceramic nanocomposites derived from a novel single-source precursor with high ceramic yield. J. Adv. Ceram. 2019, 8, 112–120. [Google Scholar] [CrossRef]
- Zhang, Z.; Sha, J.; Zu, Y.; Dai, J.; Liu, Y. Fabrication and mechanical properties of self-toughening ZrB2–SiC composites from in-situ reaction. J. Adv. Ceram. 2019, 8, 527–536. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.-Z.; Liu, J.; Zhou, Y. Low thermal conductivity and high porosity ZrC and HfC ceramics prepared by in-situ reduction reaction/partial sintering method for ultrahigh temperature applications. J. Mater. Sci. Technol. 2019, 35, 2778–2784. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Zhang, P.; Li, T.; Li, J.; Yang, Y. Ablation resistance of HfC coating reinforced by HfC nanowires in cyclic ablation environment. J. Eur. Ceram. Soc. 2017, 37, 2759–2768. [Google Scholar] [CrossRef]
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.-X.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; Zhang, X.; Fu, Q.; Zhang, G.-J. Advances in ultra-high temperature ceramics, composites, and coatings. J. Adv. Ceram. 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Chen, H.; Han, L.; Yin, X.; Fu, Q.; Sun, J. Ultra-high temperature performance of carbon fiber composite reinforced by HfC nanowires: A promising lightweight composites for aerospace engineering. Compos B Eng. 2023, 250, 110453. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, X.; Wang, Y.; Ai, S. Study on mechanical properties and thermal conductivity of 3D short carbon fiber reinforced ultra-high temperature ceramic matrix composites: A novel material performance evaluation model. Comp. Mater. Sci. 2024, 237, 112880. [Google Scholar] [CrossRef]
- Xia, Y.; Gao, W.; Gao, C. A Review on Graphene-Based Electromagnetic Functional Materials: Electromagnetic Wave Shielding and Absorption. Adv. Funct. Mater. 2022, 32, 2204591. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Li, Y.; Gou, Y.; Zhou, X. In-situ synthesis of ternary layered Y3Si2C2 ceramic on silicon carbide fiber for enhanced electromagnetic wave absorption. Ceram Int. 2022, 48, 1908–1915. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Lu, X.; Zhu, W.; Xu, H.; Xue, J.; Ye, F.; Liu, Y.; Fan, X.; Cheng, L. A sheath-core shaped ZrO2-SiC/SiO2 fiber felt with continuously distributed SiC for broad-band electromagnetic absorption. Chem Eng J. 2021, 419, 129414. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Long, L.; Luo, H.; Wang, Y. High-temperature electromagnetic wave absorption properties of Cf/SiCNFs/Si3N4 composites. J Am Ceram Soc. 2020, 103, 6822–6832. [Google Scholar] [CrossRef]
- Chen, C.; Xi, J.; Zhou, E.; Peng, L.; Chen, Z.; Gao, C. Porous Graphene Microflowers for High-Performance Microwave Absorption. Nano-Micro Lett. 2017, 10, 26. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N.-H. Carbon materials and their composites for electromagnetic interference shielding effectiveness in X-band. Carbon. 2019, 152, 159–187. [Google Scholar] [CrossRef]
- Song, W.; Wang, J.; Fan, L.; Li, Y.; Wang, C.; Cao, M. Interfacial Engineering of Carbon Nanofiber–Graphene–Carbon Nanofiber Heterojunctions in Flexible Lightweight Electromagnetic Shielding Networks. ACS Appl Mater Interfaces. 2014, 6, 10516–10523. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Zhao, K.; Nie, A.; Alharthi, S.; Amin, M.A.; El-Bahy, Z.M.; Li, H.; Chen, L.; Xu, B.B.; Ma, Y.; Li, T. Research progress on electromagnetic wave absorption based on magnetic metal oxides and their composites. Adv. Compos. Hybrid Mater. 2023, 6, 120. [Google Scholar] [CrossRef]
- Yang, Q.-x.; Yu, L.-j.; Dong, Y.-b.; Fu, Y.-q.; Zhu, Y.-f. Preparation and microwave absorption properties of magnetic functional porous biomass carbon composites. Carbon. 2020, 158, 931. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, B.; Xiang, H.; Dai, F.-Z.; Liu, Y.; Zhang, R.; Zhou, Y. High-entropy spinel ferrites MFe2O4 (M = Mg, Mn, Fe, Co, Ni, Cu, Zn) with tunable electromagnetic properties and strong microwave absorption. J. Adv. Ceram. 2022, 11, 754–768. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, J. A Perspective for Developing Polymer-Based Electromagnetic Interference Shielding Composites. Nano-Micro Lett. 2022, 14, 89. [Google Scholar] [CrossRef]
- Qin, S.; Cao, Y.; Zhang, J.; Ren, Y.; Sun, C.; Zhang, S.; Zhang, L.; Hu, W.; Yu, M.; Yang, H. Polymer dispersed ionic liquid electrolytes with high ionic conductivity for ultrastable solid-state lithium batteries. Carbon Energy. 2023, 5, e316. [Google Scholar] [CrossRef]
- Long, L.; Xu, J.; Luo, H.; Xiao, P.; Zhou, W.; Li, Y. Dielectric response and electromagnetic wave absorption of novel macroporous short carbon fibers/mullite composites. J Am Ceram Soc. 2020, 103, 6869–6880. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science. 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Zhu, X.; Qiu, H.; Chen, P.; Chen, G.; Min, W. Graphitic carbon nitride (g-C3N4) in situ polymerization to synthesize MOF-Co@CNTs as efficient electromagnetic microwave absorption materials. Carbon. 2021, 176, 530–539. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of graphene materials by electrochemical exfoliation: Recent progress and future potential. Carbon Energy. 2019, 1, 173–199. [Google Scholar] [CrossRef]
- Hasani, A.; Teklagne, M.A.; Do, H.H.; Hong, S.H.; Van Le, Q.; Ahn, S.H.; Kim, S.Y. Graphene-based catalysts for electrochemical carbon dioxide reduction. Carbon Energy. 2020, 2, 158–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Chen, G. Carbon and carbon composites for thermoelectric applications. Carbon Energy. 2020, 2, 408–436. [Google Scholar] [CrossRef]
- Li, M.; Song, X.; Xue, J.; Ye, F.; Yin, L.; Cheng, L.; Fan, X. Construction of Hollow Carbon Nanofibers with Graphene Nanorods as Nano-Antennas for Lower-Frequency Microwave Absorption. ACS Appl Mater Interfaces. 2023, 15, 31720–31728. [Google Scholar] [CrossRef]
- Li, M.; Fan, X.; Xu, H.; Ye, F.; Xue, J.; Li, X.; Cheng, L. Controllable synthesis of mesoporous carbon hollow microsphere twined by CNT for enhanced microwave absorption performance. J. Mater. Sci. Technol. 2020, 59, 164–172. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, H.; Lv, H.; Yang, L.; Liang, G.; Zhang, J.; Lai, Y.; Cheng, H.-W.; Che, R. Recent progress in carbon-based materials and loss mechanisms for electromagnetic wave absorption. Carbon. 2024, 219, 118834. [Google Scholar] [CrossRef]
- Xia, Q.; Han, Z.; Zhang, Z.; Huang, Z.; Wang, X.; Chang, J.; Chen, Q.; Chen, M. High temperature microwave absorbing materials. J Mater Chem C. 2023, 11, 4552–4569. [Google Scholar] [CrossRef]
- Zhou, A.; Liu, Y.; Li, S.; Wang, X.; Ying, G.; Xia, Q.; Zhang, P. From structural ceramics to 2D materials with multi-applications: A review on the development from MAX phases to MXenes. J. Adv. Ceram. 2021, 10, 1194–1242. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Q.; Wu, D.; Jiang, Y.; Liu, Z.; Chen, B.; Zhu, M.; Schmidt, O.G. Flexible MXene films for batteries and beyond. Carbon Energy. 2022, 4, 598–620. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, J.; Zhu, Y.; He, K.; Chen, H.; Hoh, H.Y.; Batmunkh, M.; Benedetti, T.M.; Zhang, Q.; Su, C.; Zhang, S.; Zhong, Y.L. Green and scalable electrochemical routes for cost-effective mass production of MXenes for supercapacitor electrodes. Carbon Energy. 2023, 5, e295. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.; Liu, K. Rational modulation of emerging MXene materials for zinc-ion storage. Carbon Energy. 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Reckeweg, O.; DiSalvo, F.J. Different Structural Models of YB2C2 and GdB2C2 on the Basis of Single-Crystal X-Ray Data. Z.Naturforsch.(B). 2014, 69, 289–293. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, L.; Chen, K.; Huang, Q.; Zhou, X. High-entropy rare-earth diborodicarbide: A novel class of high-entropy (Y0.25Yb0.25Dy0.25Er0.25)B2C2 ceramics. J. Adv. Ceram. 2023, 12, 1430–1440. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Ohoyama, K.; Yamauchi, H.; Indoh, K.; Onodera, H. Neutron-diffraction study of the magnetic structure of GdB2C2. Appl Phys A. 2002, 74, s877–s879. [Google Scholar] [CrossRef]
- Roy, L.E.; Hughbanks, T. A Tight-Binding Method for Predicting Magnetic Ordering in Gd-Containing Solids: Application to GdB2C2. J. Phys. Chem. B. 2006, 110, 20290–20296. [Google Scholar] [CrossRef]
- Sakai, T.; Adachi, G.-Y.; Shiokawa, J. Electrical properties of rare earth diborodicarbides (RB2C2-type layer compounds). J Less-Common Met. 1982, 84, 107–114. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, T. Mechanical and thermodynamic properties of YB2C2 under pressure. Physica B. 2017, 525, 154–158. [Google Scholar] [CrossRef]
- Li, Y.; Tian, L.; Bao, Y.; Chen, J.; Xu, J.; Zhao, G. YB2C2: The first damage tolerant ceramic with melting point over 2500 °C. J Eur Ceram Soc. 2023, 43, 3830–3835. [Google Scholar] [CrossRef]
- Wu, N.; Zhao, B.; Chen, X.; Hou, C.; Huang, M.; Alhadhrami, A.; Mersal, G.A.M.; Ibrahim, M.M.; Tian, J. Dielectric properties and electromagnetic simulation of molybdenum disulfide and ferric oxide-modified Ti3C2TX MXene hetero-structure for potential microwave absorption. Adv Compos Hybrid Mater. 2022, 5, 1548–1556. [Google Scholar] [CrossRef]
- Mudasar, M.; Xu, Z.H.; Lian, S.Y.; Li, X.; Wang, J.; Cheng, X. Featuring heterogeneous composite of W-type hexagonal ferrite with 2D vanadium carbide MXene for wideband microwave absorption. J. Mater. Res. Technol. 2024, 28, 2699–2713. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Q.; Song, L.; Lu, H.; Xu, H.; Shao, G.; Wang, H.; Zhang, R.; Wang, C.; Fan, B. Enhancing electromagnetic wave absorption with core-shell structured SiO2@MXene@MoS2 nanospheres. Carbon Energy. 2024, e502. [Google Scholar] [CrossRef]
- Li, M.; Zhu, W.; Li, X.; Xu, H.; Fan, X.; Wu, H.; Ye, F.; Xue, J.; Li, X.; Cheng, L.; Zhang, L. Ti3C2Tx/MoS2 Self-Rolling Rod-Based Foam Boosts Interfacial Polarization for Electromagnetic Wave Absorption. Adv Sci. 2022, 9, 2201118. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yao, D.; Liang, H.; Xia, Y.; Zuo, K.; Yin, J.; Zeng, Y.-P. Improved thermal conductivity of β-Si3N4 ceramics through the modification of the liquid phase by using GdH2 as a sintering additive. Ceram Int. 2021, 47, 5631–5638. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, X.; Yu, R.; Stucky, G.D. Electromagnetic microwave absorption theory and recent achievements in microwave absorbers. Carbon. 2020, 168, 606–623. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, L.; Wu, H. Dielectric Loss Mechanism in Electromagnetic Wave Absorbing Materials. Adv Sci. 2022, 9, 2105553. [Google Scholar] [CrossRef]
- Du, J.; Li, T.; Xu, Z.; Tang, J.; Qi, Q.; Meng, F. Structure–Activity Relationship in Microstructure Design for Electromagnetic Wave Absorption Applications. Small Struct. 2023, 4, 2300152. [Google Scholar] [CrossRef]
- Debye, P. Polar molecules, the chemical catalog company. Inc., New York. 1929, 89.
- Li, J.; Xu, T.; Bai, H.; Shen, Z.; Huang, Y.; Xing, W.; Zhou, Z. Structural Modifications and Electromagnetic Property Regulations of Ti3AlC2 MAX for Enhancing Microwave Absorption through the Strategy of Fe Doping. Adv Mater Interfaces. 2022, 9, 2101510. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, H.-W.; Jin, C.; Yang, B.; Xu, C.; Pei, K.; Zhang, H.; Yang, Z.; Che, R. Dimensional Design and Core–Shell Engineering of Nanomaterials for Electromagnetic Wave Absorption. Adv. Mater. 2022, 34, 2107538. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, W.; Zeng, G.; Chen, H.; Luo, H.; Fan, X.; Li, Y. Rational design of multi-shell hollow carbon submicrospheres for high-performance microwave absorbers. Carbon. 2021, 175, 233–242. [Google Scholar] [CrossRef]
- Tong, Y.; He, M.; Zhou, Y.; Nie, S.; Zhong, X.; Fan, L.; Huang, T.; Liao, Q.; Wang, Y. Three-Dimensional Hierarchical Architecture of the TiO2/Ti3C2Tx/RGO Ternary Composite Aerogel for Enhanced Electromagnetic Wave Absorption. ACS Sustainable Chem Eng. 2018, 6, 8212–8222. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.; Hou, Z.; Song, W.; Zhang, L.; Lu, M.; Jin, H.; Fang, X.; Wang, W.; Yuan, J. Temperature dependent microwave attenuation behavior for carbon-nanotube/silica composites. Carbon. 2013, 65, 124–139. [Google Scholar] [CrossRef]
- Duan, Y.; Guan, H. Microwave absorbing materials. Jenny Stanford Publishing: 2016.
- Kim, S.; Jo, S.; Gueon, K.; Choi, K.; Kim, J.; Churn, K. Complex permeability and permittivity and microwave absorption of ferrite-rubber composite at X-band frequencies. IEEE Trans Magn. 1991, 27, 5462–5464. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Zheng, Y.; Guo, C.; Yang, M.; Li, Z.; Wu, H.; Qiu, H.; Yan, H.; Qi, S. Preparation of Polyaniline@MoS2@Fe3O4 Nanowires with a Wide Band and Small Thickness toward Enhancement in Microwave Absorption. ACS Appl Nano Mater. 2018, 1, 5865–5875. [Google Scholar] [CrossRef]
- Song, L.; Wu, C.; Zhi, Q.; Zhang, F.; Song, B.; Guan, L.; Chen, Y.; Wang, H.; Zhang, R.; Fan, B. Multifunctional SiC aerogel reinforced with nanofibers and nanowires for high-efficiency electromagnetic wave absorption. Chem Eng J. 2023, 467, 143518. [Google Scholar] [CrossRef]
- Qin, G.; Li, Y.; Zhou, W.; Xu, H.; Hu, F.; Zhou, X. In Situ Grown 1D/2D Structure of Dy3Si2C2 on SiCw for Enhanced Electromagnetic Wave Absorption. Materials. 2023, 16, 3455. [Google Scholar] [CrossRef]
- Feng, W.; Luo, H.; Wang, Y.; Zeng, S.; Tan, Y.; Deng, L.; Zhou, X.; Zhang, H.; Peng, S. Mxenes Derived Laminated and Magnetic Composites with Excellent Microwave Absorbing Performance. Sci Rep. 2019, 9, 3957. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; Bai, T.; Liu, H.; Zheng, Y.; Liu, C.; Shen, C. Electrostatic self-assembled NiFe2O4/Ti3C2Tx MXene nanocomposites for efficient electromagnetic wave absorption at ultralow loading level. Adv Compos Hybrid Mater. 2021, 4, 602–613. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Li, X.; Anasori, B.; Zhang, L.; Cheng, L.; Gogotsi, Y. Laminated and Two-Dimensional Carbon-Supported Microwave Absorbers Derived from MXenes. ACS Appl Mater Interfaces. 2017, 9, 20038–20045. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yin, X.; Wu, H.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. Ti3C2 MXenes with Modified Surface for High-Performance Electromagnetic Absorption and Shielding in the X-Band. ACS Appl Mater Interfaces. 2016, 8, 21011–21019. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, S.; Deng, L.; Shan, D.; Cao, C.; Luo, H.; Yan, S. Tunable electromagnetic and enhanced microwave absorption properties in CoFe2O4 decorated Ti3C2 MXene composites. Appl Surf Sci. 2020, 504, 144210. [Google Scholar] [CrossRef]
- Hu, F.; Wang, X.; Niu, H.; Zhang, S.; Fan, B.; Zhang, R. Synthesis and electromagnetic wave absorption of novel Mo2TiC2Tx MXene with diverse etching methods. J Mater Sci. 2022, 57, 7849–7862. [Google Scholar] [CrossRef]
- Li, J.; Xu, T.; Bai, H.; Shen, Z.; Huang, Y.; Xing, W.; Zhou, Z. Structural Modifications and Electromagnetic Property Regulations of Ti3AlC2 MAX for Enhancing Microwave Absorption through the Strategy of Fe Doping. Adv. Mater. Interfaces. 2022, 9, 2101510. [Google Scholar] [CrossRef]
- Li, X.; Wen, C.; Yang, L.; Zhang, R.; Li, X.; Li, Y.; Che, R. MXene/FeCo films with distinct and tunable electromagnetic wave absorption by morphology control and magnetic anisotropy. Carbon. 2021, 175, 509–518. [Google Scholar] [CrossRef]
- Tong, Y.; He, M.; Zhou, Y.; Nie, S.; Zhong, X.; Fan, L.; Huang, T.; Liao, Q.; Wang, Y. Three-Dimensional Hierarchical Architecture of the TiO2/Ti3C2Tx/RGO Ternary Composite Aerogel for Enhanced Electromagnetic Wave Absorption. ACS Sustain. Chem. Eng. 2018, 6, 8212–8222. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, J.; Zhang, L.; Lu, H.; Guo, Y.; Ma, X.; Zhang, M.; Yin, J.; Dai, L.; Jian, X.; Yin, L.; Xie, J.; Liang, D.; Deng, L. High antioxidant lamellar structure Cr2AlC: Dielectric and microwave absorption properties in X band. J Alloy Compd. 2021, 860, 157896. [Google Scholar] [CrossRef]
- Zhao, K.-Y.; Luo, C.-L.; Sun, C.; Huang, M.-L.; Wang, M. Construction of heterogeneous interfaces on Ti3AlC2 micro-particles via surface dotting liquid metal to enhance electromagnetic wave absorption performance. Compos Pt A-Appl Sci Manuf. 2023, 173, 107640. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, C.; Su, X.; Xu, J.; He, X. Electromagnetic and microwave absorption properties of Ti3SiC2 powders decorated with Ag particles. J Alloy Compd. 2020, 820, 153154. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, J.; Wang, H.; Chen, M.; Fang, D.; Wang, Z.; Li, Z. Dielectric and microwave absorption properties of resin-matrix composite coating filled with multi-wall carbon nanotubes and Ti3SiC2 particles. J Mater Sci-Mater Electron. 2020, 31, 15852–15858. [Google Scholar] [CrossRef]
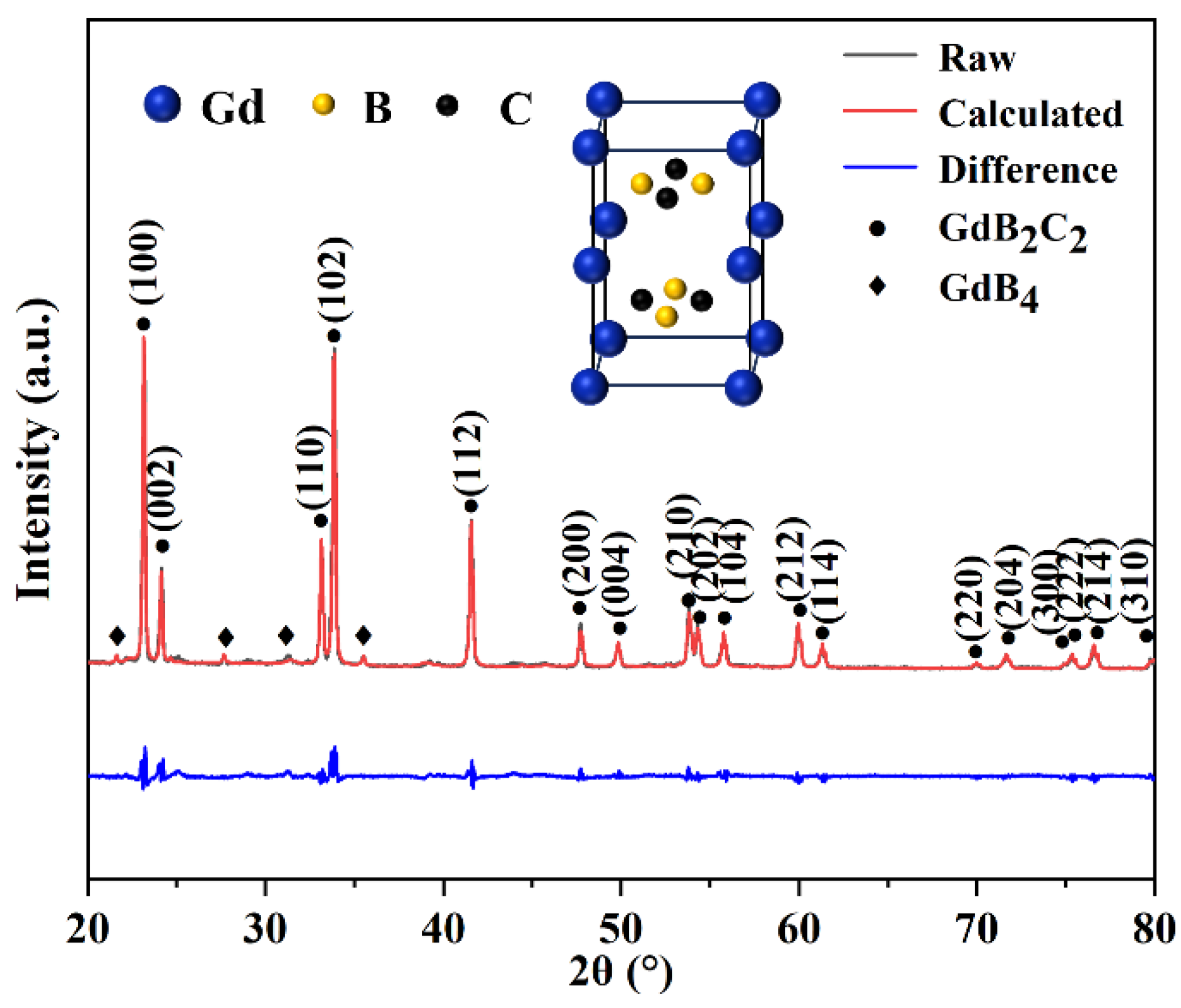
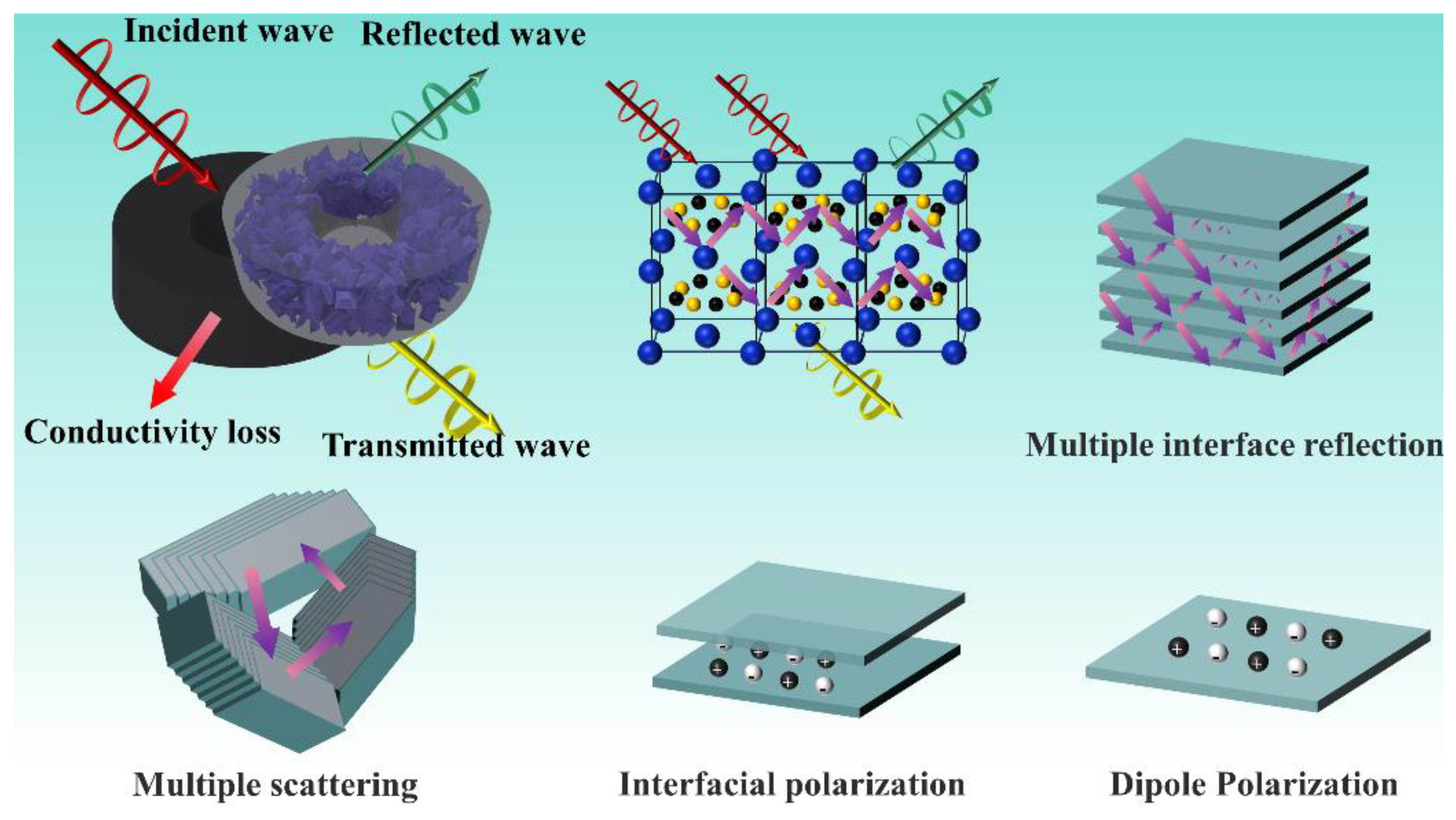
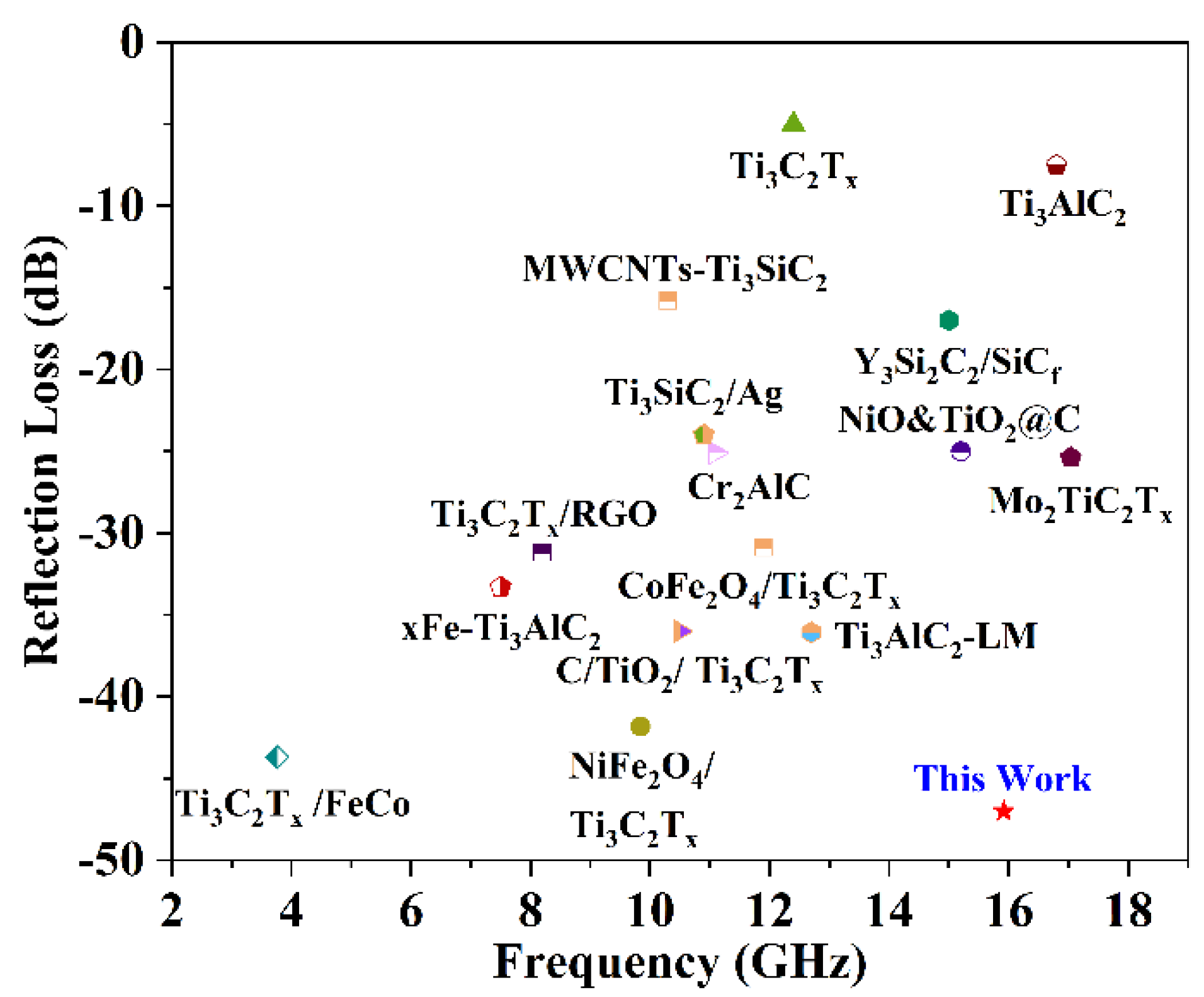
|
Holding temperature (°C) |
Rwp | Experimental |
GdB2C2 (wt%) |
GdB4 (wt%) |
|
| a (Å) | c (Å) | ||||
| 1400 | 8.4 | 3.78 | 7.28 | 95.47 | 4.53 |
| 1500 | 9.4 | 3.78 | 7.27 | 96.38 | 3.62 |
| 1600 | 8.4 | 3.79 | 7.27 | 99.24 | 0.76 |
| 1700 | 8.3 | 3.79 | 7.27 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





