Submitted:
07 May 2024
Posted:
10 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Inborn Errors of Immunity (IEI)
1.2. COVID-19 Infection in People with IEI
1.3. SARS-CoV-2 Variants
2. COVID-19 Vaaccination
2.1. Vaccine Formulations for COVID-19
2.2. The COVID-19 Vaccine-Induced Response in Immunocompetent Individuals
2.3. The COVID-19 Vaccination Response in IEI Patients
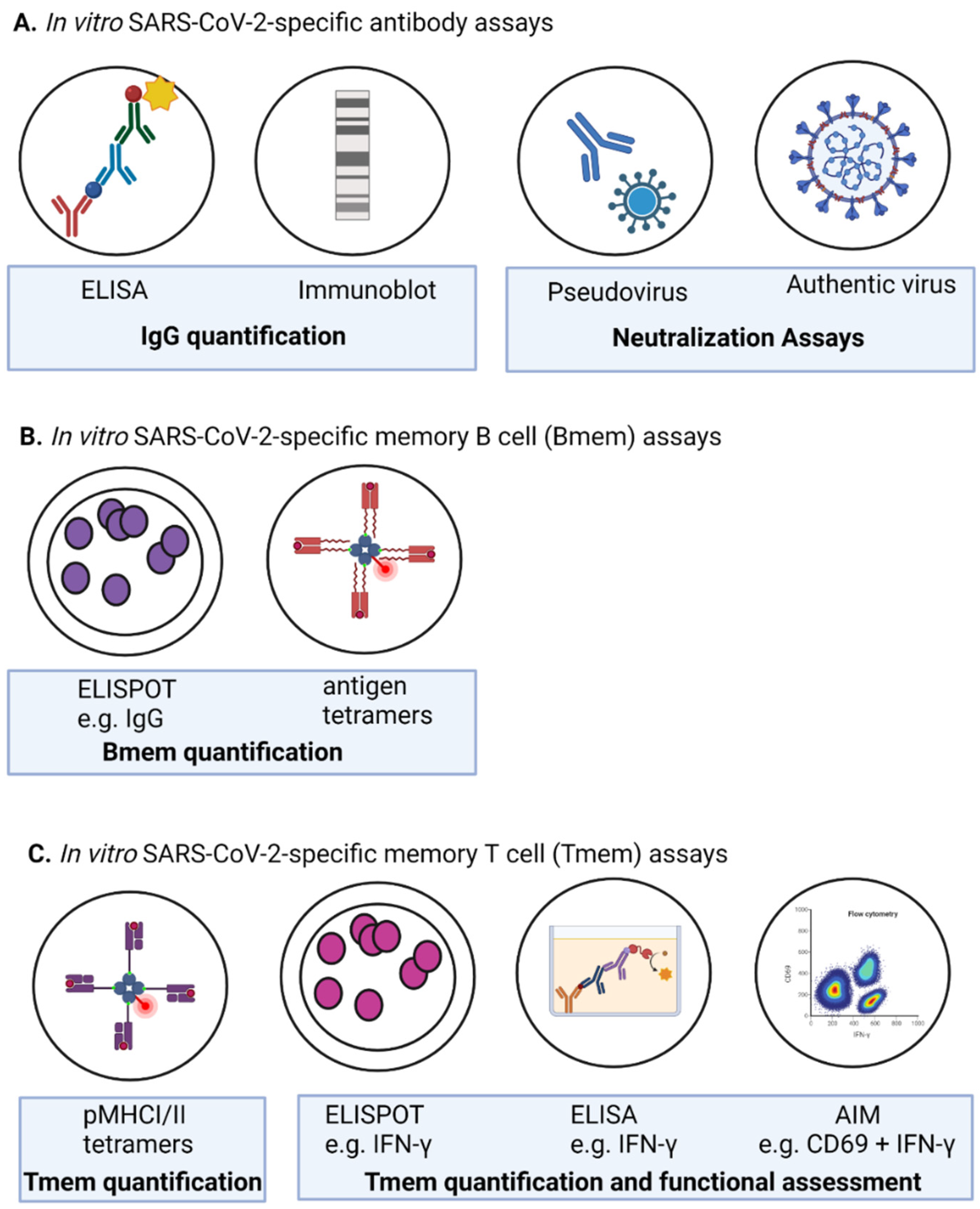
2.3.1. Humoral Response
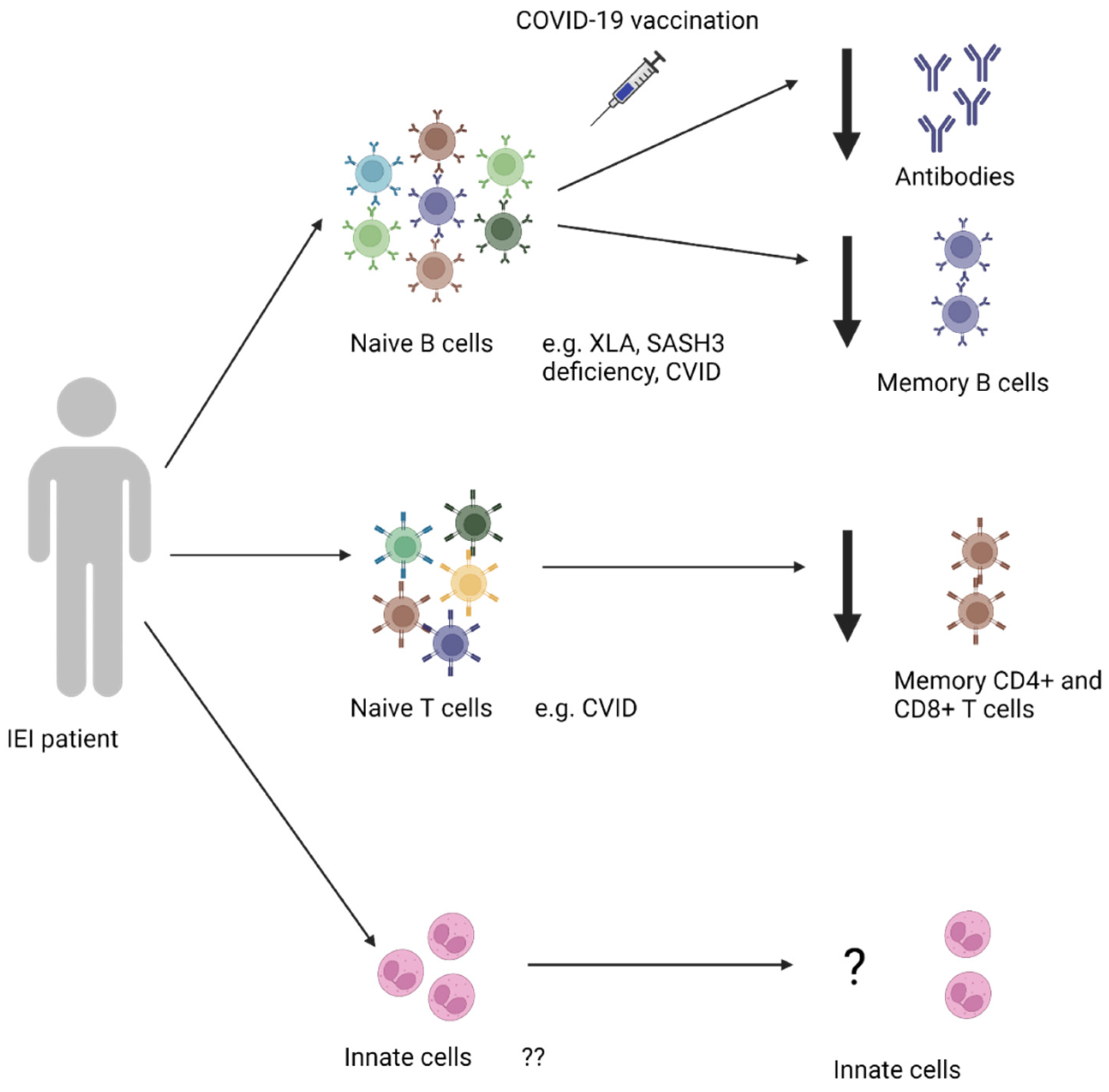
2.3.3. Memory B Cells & Innate Cells
3. Impact of Adjunctive Antibody Therapies on COVID-19 Immunity
3.1. Immunoglobulin Replacement Therapy (IgRT)
3.2. Prophylactic Monoclonal Antibody (mAb) Therapies to Prevent Severe COVID-19 in IEI
3.2.1. Sotrovimab
3.2.2. Evusheld
4. Discussion and Future Directions
Disclaimer
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Organisation, W.H. WHO Coronavirus (COVID-19) Dashboard.
- COVID-19 Data Explorer - Our World in Data. Available online: https://ourworldindata.org/explorers/coronavirus-data-explorer?zoomToSelection=true&time=2020-03-01..latest&facet=none&pickerSort=asc&pickerMetric=location&Metric=Case+fatality+rate&Interval=7-day+rolling+average&Relative+to+Population=true&Color+by+test+positivity=false&country=JPN~USA~GBR~ITA~DEU~FRA~CAN (accessed on 5 January 2023).
- Tangye, S.G. ; COVID Human Genetic Effort consortium Impact of SARS-CoV-2 Infection and COVID-19 on Patients with Inborn Errors of Immunity. J Allergy Clin Immunol 2023, 151, 818–831. [Google Scholar] [CrossRef]
- Chiu, S.S.; Hung Chan, K.; Wing Chu, K.; Kwan, S.W.; Guan, Y.; Man Poon, L.L.; Peiris, J.S.M. Human Coronavirus NL63 Infection and Other Coronavirus Infections in Children Hospitalized with Acute Respiratory Disease in Hong Kong, China. Clinical Infectious Diseases 2005, 40, 1721–1729. [Google Scholar] [CrossRef]
- Hartley, G.E.; Edwards, E.S.J.; O’Hehir, R.E.; van Zelm, M.C. New Insights into Human Immune Memory from SARS-CoV -2 Infection and Vaccination. Allergy 2022, 77, 3553–3566. [Google Scholar] [CrossRef]
- Fryer, H.A.; Hartley, G.E.; Edwards, E.S.J.; O’Hehir, R.E.; van Zelm, M.C. Humoral Immunity and B-Cell Memory in Response to SARS-CoV-2 Infection and Vaccination. Biochemical Society Transactions 2022, 50, 1643–1658. [Google Scholar] [CrossRef]
- Yi, C.; Sun, X.; Ye, J.; Ding, L.; Liu, M.; Yang, Z.; Lu, X.; Zhang, Y.; Ma, L.; Gu, W.; et al. Key Residues of the Receptor Binding Motif in the Spike Protein of SARS-CoV-2 That Interact with ACE2 and Neutralizing Antibodies. Cell Mol Immunol 2020, 17, 621–630. [Google Scholar] [CrossRef]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends in Immunology 2020, 41, 355–359. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and Immunogenicity of the ChAdOx1 nCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. The Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Meyts, I.; Bousfiha, A.; Duff, C.; Singh, S.; Lau, Y.L.; Condino-Neto, A.; Bezrodnik, L.; Ali, A.; Adeli, M.; Drabwell, J. Primary Immunodeficiencies: A Decade of Progress and a Promising Future. Front. Immunol. 2021, 11, 625753. [Google Scholar] [CrossRef]
- Szepanowski, F.; Warnke, C.; Meyer Zu Hörste, G.; Mausberg, A.K.; Hartung, H.-P.; Kleinschnitz, C.; Stettner, M. Secondary Immunodeficiency and Risk of Infection Following Immune Therapies in Neurology. CNS Drugs 2021, 35, 1173–1188. [Google Scholar] [CrossRef]
- Pulvirenti, F.; Cinetto, F.; Milito, C.; Bonanni, L.; Pesce, A.M.; Leodori, G.; Garzi, G.; Miglionico, M.; Tabolli, S.; Quinti, I. Health-Related Quality of Life in Common Variable Immunodeficiency Italian Patients Switched to Remote Assistance During the COVID-19 Pandemic. J Allergy Clin Immunol Pract 2020, 8, 1894–1899.e2. [Google Scholar] [CrossRef] [PubMed]
- Sowers, K.L.; Galantino, M.L. Living with Primary Immunodeficiency Disease during the Covid-19 Pandemic. J Public Health (Berl.) 2022, 30, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Care, A.G.D. of H. and A. About Coronavirus (COVID-19). Available online: https://www.health.gov.au/topics/covid-19/about (accessed on 2 January 2024).
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 2022, 42, 1473–1507. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.S.J.; Bosco, J.J.; Ojaimi, S.; O’Hehir, R.E.; van Zelm, M.C. Beyond Monogenetic Rare Variants: Tackling the Low Rate of Genetic Diagnoses in Predominantly Antibody Deficiency. Cell Mol Immunol 2021, 18, 588–603. [Google Scholar] [CrossRef]
- Quinn, J.; Modell, V.; Orange, J.S.; Modell, F. Growth in Diagnosis and Treatment of Primary Immunodeficiency within the Global Jeffrey Modell Centers Network. Allergy Asthma Clin Immunol 2022, 18, 19. [Google Scholar] [CrossRef]
- Hartley, G.E.; Edwards, E.S.J.; Bosco, J.J.; Ojaimi, S.; Stirling, R.G.; Cameron, P.U.; Flanagan, K.; Plebanski, M.; Hogarth, P.M.; O’Hehir, R.E.; et al. Influenza-Specific IgG1+ Memory B-Cell Numbers Increase upon Booster Vaccination in Healthy Adults but Not in Patients with Predominantly Antibody Deficiency. Clin Transl Immunology 2020, 9, e1199. [Google Scholar] [CrossRef]
- Edwards, E.S.J.; Bosco, J.J.; Aui, P.M.; Stirling, R.G.; Cameron, P.U.; Chatelier, J.; Hore-Lacy, F.; O’Hehir, R.E.; van Zelm, M.C. Predominantly Antibody-Deficient Patients With Non-Infectious Complications Have Reduced Naive B, Treg, Th17, and Tfh17 Cells. Front Immunol 2019, 10, 2593. [Google Scholar] [CrossRef] [PubMed]
- Durandy, A.; Kracker, S.; Fischer, A. Primary Antibody Deficiencies. Nat Rev Immunol 2013, 13, 519–533. [Google Scholar] [CrossRef]
- Lucas, M.; Lee, M.; Lortan, J.; Lopez-Granados, E.; Misbah, S.; Chapel, H. Infection Outcomes in Patients with Common Variable Immunodeficiency Disorders: Relationship to Immunoglobulin Therapy over 22 Years. Journal of Allergy and Clinical Immunology 2010, 125, 1354–1360.e4. [Google Scholar] [CrossRef]
- Hartley, G.E.; Edwards, E.S.J.; Aui, P.M.; Varese, N.; Stojanovic, S.; McMahon, J.; Peleg, A.Y.; Boo, I.; Drummer, H.E.; Hogarth, P.M.; et al. Rapid Generation of Durable B Cell Memory to SARS-CoV-2 Spike and Nucleocapsid Proteins in COVID-19 and Convalescence. Sci. Immunol. 2020, 5, eabf8891. [Google Scholar] [CrossRef]
- Puel, A.; Bastard, P.; Bustamante, J.; Casanova, J.-L. Human Autoantibodies Underlying Infectious Diseases. Journal of Experimental Medicine 2022, 219, e20211387. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Boisson, B.; Onodi, F.; Matuozzo, D.; Moncada-Velez, M.; Maglorius Renkilaraj, M.R.L.; Zhang, P.; Meertens, L.; Bolze, A.; Materna, M.; et al. X-Linked Recessive TLR7 Deficiency in ~1% of Men under 60 Years Old with Life-Threatening COVID-19. Sci. Immunol. 2021, 6, eabl4348. [Google Scholar] [CrossRef]
- Mantovani, S.; Daga, S.; Fallerini, C.; Baldassarri, M.; Benetti, E.; Picchiotti, N.; Fava, F.; Gallì, A.; Zibellini, S.; Bruttini, M.; et al. Rare Variants in Toll-like Receptor 7 Results in Functional Impairment and Downregulation of Cytokine-Mediated Signaling in COVID-19 Patients. Genes Immun 2022, 23, 51–56. [Google Scholar] [CrossRef]
- Fallerini, C.; Daga, S.; Mantovani, S.; Benetti, E.; Picchiotti, N.; Francisci, D.; Paciosi, F.; Schiaroli, E.; Baldassarri, M.; Fava, F.; et al. Association of Toll-like Receptor 7 Variants with Life-Threatening COVID-19 Disease in Males: Findings from a Nested Case-Control Study. eLife 2021, 10, e67569. [Google Scholar] [CrossRef]
- Solanich, X.; Vargas-Parra, G.; Van Der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; Antolí, A.; Del Valle, J.; Rocamora-Blanch, G.; Setién, F.; Esteller, M.; et al. Genetic Screening for TLR7 Variants in Young and Previously Healthy Men With Severe COVID-19. Front. Immunol. 2021, 12, 719115. [Google Scholar] [CrossRef] [PubMed]
- Van Der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; Van Den Heuvel, G.; Mantere, T.; Kersten, S.; Van Deuren, R.C.; Steehouwer, M.; Van Reijmersdal, S.V.; Jaeger, M.; et al. Presence of Genetic Variants Among Young Men With Severe COVID-19. JAMA 2020, 324, 663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn Errors of Type I IFN Immunity in Patients with Life-Threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- Abolhassani, H.; Landegren, N.; Bastard, P.; Materna, M.; Modaresi, M.; Du, L.; Aranda-Guillén, M.; Sardh, F.; Zuo, F.; Zhang, P.; et al. Inherited IFNAR1 Deficiency in a Child with Both Critical COVID-19 Pneumonia and Multisystem Inflammatory Syndrome. J Clin Immunol 2022, 42, 471–483. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Rezaei, N.; Khazaei, M.; Shirkani, A. A Case of Autosomal Recessive Interferon Alpha/Beta Receptor Alpha Chain (IFNAR1) Deficiency with Severe COVID-19. J Clin Immunol 2022, 42, 19–24. [Google Scholar] [CrossRef]
- Schmidt, A.; Peters, S.; Knaus, A.; Sabir, H.; Hamsen, F.; Maj, C.; Fazaal, J.; Sivalingam, S.; Savchenko, O.; Mantri, A.; et al. TBK1 and TNFRSF13B Mutations and an Autoinflammatory Disease in a Child with Lethal COVID-19. npj Genom. Med. 2021, 6, 55. [Google Scholar] [CrossRef]
- Duncan, C.J.A.; Skouboe, M.K.; Howarth, S.; Hollensen, A.K.; Chen, R.; Børresen, M.L.; Thompson, B.J.; Stremenova Spegarova, J.; Hatton, C.F.; Stæger, F.F.; et al. Life-Threatening Viral Disease in a Novel Form of Autosomal Recessive IFNAR2 Deficiency in the Arctic. Journal of Experimental Medicine 2022, 219, e20212427. [Google Scholar] [CrossRef] [PubMed]
- Platanias, L.C. Mechanisms of Type-I- and Type-II-Interferon-Mediated Signalling. Nat Rev Immunol 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Zhang, Q.; Matuozzo, D.; Le Pen, J.; Lee, D.; Moens, L.; Asano, T.; Bohlen, J.; Liu, Z.; Moncada-Velez, M.; Kendir-Demirkol, Y.; et al. Recessive Inborn Errors of Type I IFN Immunity in Children with COVID-19 Pneumonia. Journal of Experimental Medicine 2022, 219, e20220131. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.A.; Habiballah, S.B.; LaBere, B.; Day-Lewis, M.; Elkins, M.; Al-Musa, A.; Chu, A.; Jones, J.; Fried, A.J.; McDonald, D.; et al. Rethinking Immunological Risk: A Retrospective Cohort Study of Severe SARS-Cov-2 Infections in Individuals With Congenital Immunodeficiencies. The Journal of Allergy and Clinical Immunology: In Practice 2023, 11, 3391–3399.e3. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 24 January 2023).
- Parra-Lucares, A.; Segura, P.; Rojas, V.; Pumarino, C.; Saint-Pierre, G.; Toro, L. Emergence of SARS-CoV-2 Variants in the World: How Could This Happen? Life 2022, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Van Egeren, D.; Novokhodko, A.; Stoddard, M.; Tran, U.; Zetter, B.; Rogers, M.S.; Joseph-McCarthy, D.; Chakravarty, A. Controlling Long-Term SARS-CoV-2 Infections Can Slow Viral Evolution and Reduce the Risk of Treatment Failure. Sci Rep 2021, 11, 22630. [Google Scholar] [CrossRef]
- Wilkinson, S.A.J.; Richter, A.; Casey, A.; Osman, H.; Mirza, J.D.; Stockton, J.; Quick, J.; Ratcliffe, L.; Sparks, N.; Cumley, N.; et al. Recurrent SARS-CoV-2 Mutations in Immunodeficient Patients. Virus Evolution 2022, 8, veac050. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Minor, N.R.; Haddock, L.A.; Maddox, R.; Moreno, G.K.; Braun, K.M.; Baker, D.A.; Riemersa, K.K.; Prasad, A.; Alman, K.J.; et al. Evolution of a Globally Unique SARS-CoV-2 Spike E484T Monoclonal Antibody Escape Mutation in a Persistently Infected, Immunocompromised Individual; Infectious Diseases (except HIV/AIDS), 2022.
- Scherer, E.M.; Babiker, A.; Adelman, M.W.; Allman, B.; Key, A.; Kleinhenz, J.M.; Langsjoen, R.M.; Nguyen, P.-V.; Onyechi, I.; Sherman, J.D.; et al. SARS-CoV-2 Evolution and Immune Escape in Immunocompromised Patients. New England Journal of Medicine 2022, 386, 2436–2438. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.; Czudnochowski, N.; Rosen, L.E.; Zepeda, S.K.; Bowen, J.E.; Walls, A.C.; Hauser, K.; Joshi, A.; Stewart, C.; Dillen, J.R.; et al. Structural Basis of SARS-CoV-2 Omicron Immune Evasion and Receptor Engagement. Science 2022, 375, 864–868. [Google Scholar] [CrossRef]
- Enhancing Response to Omicron SARS-CoV-2 Variant. Available online: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states (accessed on 3 July 2023).
- Panja, A.; Roy, J.; Mazumder, A.; Choudhury, S.M. Divergent Mutations of Delta and Omicron Variants: Key Players behind Differential Viral Attributes across the COVID-19 Waves. VirusDis. 2023. [Google Scholar] [CrossRef]
- Ao, D.; He, X.; Hong, W.; Wei, X. The Rapid Rise of SARS-CoV-2 Omicron Subvariants with Immune Evasion Properties: XBB.1.5 and BQ.1.1 Subvariants. MedComm 2023, 4, e239. [Google Scholar] [CrossRef] [PubMed]
- Commissioner, O. of the FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 (accessed on 31 January 2024).
- Commissioner, O. of the FDA Approves First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 31 January 2024).
- McIntyre, P.B.; Aggarwal, R.; Jani, I.; Jawad, J.; Kochhar, S.; MacDonald, N.; Madhi, S.A.; Mohsni, E.; Mulholland, K.; Neuzil, K.M.; et al. COVID-19 Vaccine Strategies Must Focus on Severe Disease and Global Equity. The Lancet 2022, 399, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Chirico, F.; Teixeira Da Silva, J.; Tsigaris, P.; Sharun, K. Safety & Effectiveness of COVID-19 Vaccines: A Narrative Review. Indian J Med Res 2022, 155, 91. [Google Scholar] [CrossRef] [PubMed]
- Commissioner, O. of the Coronavirus (COVID-19) Update: FDA Limits Use of Janssen COVID-19 Vaccine to Certain Individuals. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-janssen-covid-19-vaccine-certain-individuals (accessed on 15 February 2023).
- Hartley, G.E.; Fryer, H.A.; Gill, P.A.; Boo, I.; Bornheimer, S.J.; Hogarth, P.M.; Drummer, H.E.; O’Hehir, R.E.; Edwards, E.S.J.; Van Zelm, M.C. Third Dose COVID-19 mRNA Vaccine Enhances IgG4 Isotype Switching and Recognition of Omicron Subvariants by Memory B Cells after mRNA but Not Adenovirus Priming; Immunology, 2023.
- Hartley, G.E.; Edwards, E.S.J.; Varese, N.; Boo, I.; Aui, P.M.; Bornheimer, S.J.; Hogarth, P.M.; Drummer, H.E.; O’Hehir, R.E.; van Zelm, M.C. The Second COVID -19 mRNA Vaccine Dose Enhances the Capacity of Spike-specific Memory B Cells to Bind Omicron BA.2. Allergy 2022, all.15624. [Google Scholar] [CrossRef]
- Fryer, H.A.; Hartley, G.E.; Edwards, E.S.J.; Varese, N.; Boo, I.; Bornheimer, S.J.; Hogarth, P.M.; Drummer, H.E.; O’Hehir, R.E.; Van Zelm, M.C. COVID-19 Adenoviral Vector Vaccination Elicits a Robust Memory B Cell Response with the Capacity to Recognize Omicron BA.2 and BA.5 Variants. J Clin Immunol 2023. [Google Scholar] [CrossRef] [PubMed]
- Ikegame, S.; Siddiquey, M.N.A.; Hung, C.-T.; Haas, G.; Brambilla, L.; Oguntuyo, K.Y.; Kowdle, S.; Chiu, H.-P.; Stevens, C.S.; Vilardo, A.E.; et al. Neutralizing Activity of Sputnik V Vaccine Sera against SARS-CoV-2 Variants. Nat Commun 2021, 12, 4598. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat Med 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Marta, R.A.; Nakamura, G.E.K.; De Matos Aquino, B.; Bignardi, P.R. COVID-19 Vaccines: Update of the Vaccines in Use and under Development. Vacunas 2022, 23, S88–S102. [Google Scholar] [CrossRef] [PubMed]
- Comirnaty® COVID-19 Vaccines (COVID-19 Vaccines) | Pfizer Medical Information - Australia. Available online: https://www.pfizermedicalinformation.com.au/en-au/pfizer-biontech-covid-19-vaccine (accessed on 3 July 2023).
- Statement on the Antigen Composition of COVID-19 Vaccines. Available online: https://www.who.int/news/item/13-12-2023-statement-on-the-antigen-composition-of-covid-19-vaccines (accessed on 31 January 2024).
- Care, A.G.D. of H. and A. ATAGI Recommendations on Use of the Moderna and Pfizer Monovalent Omicron XBB.1.5 COVID-19 Vaccines. Available online: https://www.health.gov.au/news/atagi-recommendations-on-use-of-the-moderna-and-pfizer-monovalent-omicron-xbb15-covid-19-vaccines (accessed on 31 January 2024).
- Edwards, E.; Quinn, K. Why Is a Third COVID-19 Vaccine Dose Important for People Who Are Immunocompromised? Available online: http://theconversation.com/why-is-a-third-covid-19-vaccine-dose-important-for-people-who-are-immunocompromised-166569 (accessed on 3 July 2023).
- Gernez, Y.; Murugesan, K.; Cortales, C.R.; Banaei, N.; Hoyte, L.; Pinsky, B.A.; Lewis, D.B.; Pham, M.N. Immunogenicity of a Third COVID-19 Messenger RNA Vaccine Dose in Primary Immunodeficiency Disorder Patients with Functional B-Cell Defects. The Journal of Allergy and Clinical Immunology: In Practice 2022, 10, 1385–1388.e2. [Google Scholar] [CrossRef]
- Care, A.G.D. of H. and A. ATAGI Recommendations on the Use of a Third Primary Dose of COVID-19 Vaccine in Individuals Who Are Severely Immunocompromised. Available online: https://www.health.gov.au/resources/publications/atagi-recommendations-on-the-use-of-a-third-primary-dose-of-covid-19-vaccine-in-individuals-who-are-severely-immunocompromised?language=en (accessed on 3 July 2023).
- Commissioner, O. of the Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised (accessed on 4 July 2023).
- Joint Committee on Vaccination and Immunisation (JCVI) Advice on Third Primary Dose Vaccination. Available online: https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination (accessed on 3 July 2023).
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The Efficacy and Effectiveness of the COVID-19 Vaccines in Reducing Infection, Severity, Hospitalization, and Mortality: A Systematic Review. Human Vaccines & Immunotherapeutics 2022, 18, 2027160. [Google Scholar] [CrossRef]
- DeSantis, S.M.; Yaseen, A.; Hao, T.; León-Novelo, L.; Talebi, Y.; Valerio-Shewmaker, M.A.; Pinzon Gomez, C.L.; Messiah, S.E.; Kohl, H.W.; Kelder, S.H.; et al. Incidence and Predictors of Breakthrough and Severe Breakthrough Infections of SARS-CoV-2 After Primary Series Vaccination in Adults: A Population-Based Survey of 22 575 Participants. The Journal of Infectious Diseases 2023, 227, 1164–1172. [Google Scholar] [CrossRef]
- Arroyo-Sánchez, D.; Cabrera-Marante, O.; Laguna-Goya, R.; Almendro-Vázquez, P.; Carretero, O.; Gil-Etayo, F.J.; Suàrez-Fernández, P.; Pérez-Romero, P.; Rodríguez de Frías, E.; Serrano, A.; et al. Immunogenicity of Anti-SARS-CoV-2 Vaccines in Common Variable Immunodeficiency. J Clin Immunol 2022, 42, 240–252. [Google Scholar] [CrossRef]
- Hagin, D.; Freund, T.; Navon, M.; Halperin, T.; Adir, D.; Marom, R.; Levi, I.; Benor, S.; Alcalay, Y.; Freund, N.T. Immunogenicity of Pfizer-BioNTech COVID-19 Vaccine in Patients with Inborn Errors of Immunity. J Allergy Clin Immunol 2021, 148, 739–749. [Google Scholar] [CrossRef]
- van Leeuwen, L.P.M.; GeurtsvanKessel, C.H.; Ellerbroek, P.M.; de Bree, G.J.; Potjewijd, J.; Rutgers, A.; Jolink, H.; van de Veerdonk, F.; van Gorp, E.C.M.; de Wilt, F.; et al. Immunogenicity of the mRNA-1273 COVID-19 Vaccine in Adult Patients with Inborn Errors of Immunity. J Allergy Clin Immunol 2022, 149, 1949–1957. [Google Scholar] [CrossRef]
- Fernandez Salinas, A.; Piano Mortari, E.; Terreri, S.; Milito, C.; Zaffina, S.; Perno, C.F.; Locatelli, F.; Quinti, I.; Carsetti, R. Impaired Memory B-Cell Response to the Pfizer-BioNTech COVID-19 Vaccine in Patients with Common Variable Immunodeficiency. J Allergy Clin Immunol 2022, 149, 76–77. [Google Scholar] [CrossRef]
- Shields, A.M.; Faustini, S.E.; Hill, H.J.; Al-Taei, S.; Tanner, C.; Ashford, F.; Workman, S.; Moreira, F.; Verma, N.; Wagg, H.; et al. Increased Seroprevalence and Improved Antibody Responses Following Third Primary SARS-CoV-2 Immunisation: An Update From the COV-AD Study. Front Immunol 2022, 13, 912571. [Google Scholar] [CrossRef]
- Zendt, M.; Bustos Carrillo, F.A.; Kelly, S.; Saturday, T.; DeGrange, M.; Ginigeme, A.; Wu, L.; Callier, V.; Ortega-Villa, A.; Faust, M.; et al. Characterization of the Antispike IgG Immune Response to COVID-19 Vaccines in People with a Wide Variety of Immunodeficiencies. Science Advances 2023, 9, eadh3150. [Google Scholar] [CrossRef]
- Hlongwa, L.; Peter, J.; Mayne, E. Value of Diagnostic Vaccination in Diagnosis of Humoral Inborn Errors of Immunity. Human Immunology 2023, 84, 337–341. [Google Scholar] [CrossRef]
- Bergman, P.; Wullimann, D.; Gao, Y.; Wahren Borgström, E.; Norlin, A.-C.; Lind Enoksson, S.; Aleman, S.; Ljunggren, H.-G.; Buggert, M.; Smith, C.I.E. Elevated CD21low B Cell Frequency Is a Marker of Poor Immunity to Pfizer-BioNTech BNT162b2 mRNA Vaccine Against SARS-CoV-2 in Patients with Common Variable Immunodeficiency. J Clin Immunol 2022, 42, 716–727. [Google Scholar] [CrossRef]
- Amodio, D.; Ruggiero, A.; Sgrulletti, M.; Pighi, C.; Cotugno, N.; Medri, C.; Morrocchi, E.; Colagrossi, L.; Russo, C.; Zaffina, S.; et al. Humoral and Cellular Response Following Vaccination With the BNT162b2 mRNA COVID-19 Vaccine in Patients Affected by Primary Immunodeficiencies. Front Immunol 2021, 12, 727850. [Google Scholar] [CrossRef]
- Bitzenhofer, M.; Suter-Riniker, F.; Moor, M.B.; Sidler, D.; Horn, M.P.; Gschwend, A.; Staehelin, C.; Rauch, A.; Helbling, A.; Jörg, L. Humoral Response to mRNA Vaccines against SARS-CoV-2 in Patients with Humoral Immunodeficiency Disease. PLoS One 2022, 17, e0268780. [Google Scholar] [CrossRef]
- Barmettler, S.; DiGiacomo, D.V.; Yang, N.J.; Lam, T.; Naranbhai, V.; Dighe, A.S.; Burke, K.E.; Blumenthal, K.G.; Ling, M.; Hesterberg, P.E.; et al. Response to Severe Acute Respiratory Syndrome Coronavirus 2 Initial Series and Additional Dose Vaccine in Patients With Predominant Antibody Deficiency. J Allergy Clin Immunol Pract 2022, 10, 1622–1634.e4. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; Chen, P.; Söderdahl, G.; Österborg, A.; Smith, C.I.E.; Wullimann, D.; et al. Safety and Efficacy of the mRNA BNT162b2 Vaccine against SARS-CoV-2 in Five Groups of Immunocompromised Patients and Healthy Controls in a Prospective Open-Label Clinical Trial. EBioMedicine 2021, 74, 103705. [Google Scholar] [CrossRef]
- Salinas, A.F.; Mortari, E.P.; Terreri, S.; Quintarelli, C.; Pulvirenti, F.; Di Cecca, S.; Guercio, M.; Milito, C.; Bonanni, L.; Auria, S.; et al. SARS-CoV-2 Vaccine Induced Atypical Immune Responses in Antibody Defects: Everybody Does Their Best. J Clin Immunol 2021, 41, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Abo-Helo, N.; Muhammad, E.; Ghaben-Amara, S.; Cohen, S. Specific Antibody Response of 14 Patients with Common Variable Immunodeficiency to 3 BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccinations. Ann Allergy Asthma Immunol 2022, 129, 108–109. [Google Scholar] [CrossRef]
- Erra, L.; Uriarte, I.; Colado, A.; Paolini, M.V.; Seminario, G.; Fernández, J.B.; Tau, L.; Bernatowiez, J.; Moreira, I.; Vishnopolska, S.; et al. COVID-19 Vaccination Responses with Different Vaccine Platforms in Patients with Inborn Errors of Immunity. J. Clin. Immunol. 2022, 43, 271–285. [Google Scholar] [CrossRef]
- Nielsen, B.U.; Drabe, C.H.; Barnkob, M.B.; Johansen, I.S.; Hansen, A.K.K.; Nilsson, A.C.; Rasmussen, L.D. Antibody Response Following the Third and Fourth SARS-CoV-2 Vaccine Dose in Individuals with Common Variable Immunodeficiency. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Antolí, A.; Rocamora-Blanch, G.; Framil, M.; Mas-Bosch, V.; Navarro, S.; Bermudez, C.; Martinez-Yelamos, S.; Dopico, E.; Calatayud, L.; Garcia-Muñoz, N.; et al. Evaluation of Humoral and Cellular Immune Responses to the SARS-CoV-2 Vaccine in Patients With Common Variable Immunodeficiency Phenotype and Patient Receiving B-Cell Depletion Therapy. Front Immunol 2022, 13, 895209. [Google Scholar] [CrossRef]
- Pham, M.N.; Murugesan, K.; Banaei, N.; Pinsky, B.A.; Tang, M.; Hoyte, E.; Lewis, D.B.; Gernez, Y. Immunogenicity and Tolerability of COVID-19 Messenger RNA Vaccines in Primary Immunodeficiency Patients with Functional B-Cell Defects. J Allergy Clin Immunol 2022, 149, 907–911. [Google Scholar] [CrossRef]
- Lin, F.J.; Doss, A.M.A.; Davis-Adams, H.G.; Adams, L.J.; Hanson, C.H.; VanBlargan, L.A.; Liang, C.-Y.; Chen, R.E.; Monroy, J.M.; Wedner, H.J.; et al. SARS-CoV-2 Booster Vaccination Rescues Attenuated IgG1 Memory B Cell Response in Primary Antibody Deficiency Patients. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Abo-Helo, N.; Muhammad, E.; Ghaben-Amara, S.; Panasoff, J.; Cohen, S. Specific Antibody Response of Patients with Common Variable Immunodeficiency to BNT162b2 Coronavirus Disease 2019 Vaccination. Ann Allergy Asthma Immunol 2021, 127, 501–503. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Jalil, M.; Rowane, M.; Rajan, J.; Hostoffer, R. Successful Anti-SARS-CoV-2 Spike Protein Antibody Response to Vaccination in MAGT1 Deficiency. Allergy�Rhinol (Providence) 2021, 12, 215265672110562. [Google Scholar] [CrossRef]
- Pulvirenti, F.; Di Cecca, S.; Sinibaldi, M.; Piano Mortari, E.; Terreri, S.; Albano, C.; Guercio, M.; Sculco, E.; Milito, C.; Ferrari, S.; et al. T-Cell Defects Associated to Lack of Spike-Specific Antibodies after BNT162b2 Full Immunization Followed by a Booster Dose in Patients with Common Variable Immune Deficiencies. Cells 2022, 11, 1918. [Google Scholar] [CrossRef]
- Sheward, D.J.; Kim, C.; Ehling, R.A.; Pankow, A.; Dopico, X.C.; Martin, D.; Reddy, S.; Dillner, J.; Karlsson Hedestam, G.B.; Albert, J.; et al. Variable Loss of Antibody Potency against SARS-CoV-2 B.1.1.529 (Omicron); Immunology, 2021.
- Leung, D.; Mu, X.; Duque, J.S.R.; Cheng, S.M.S.; Wang, M.; Zhang, W.; Zhang, Y.; Tam, I.Y.S.; Lee, T.S.S.; Lam, J.H.Y.; et al. Safety and Immunogenicity of 3 Doses of BNT162b2 and CoronaVac in Children and Adults with Inborn Errors of Immunity. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Illes, P.; Csomos, K.; Ujhazi, B.; Dasso, J.; Nagy, B.; Alas, E.; Gordon, S.; Timothy, P.; Miranda, M.A.; Sullivan, S.; et al. The Humoral Immune Response To SARS-CoV-2 Infection And/or Immunization in Immunocompromised Versus Immunocompetent Individuals. J. Allergy Clin. Immunol. 2022, 149, AB21. [Google Scholar] [CrossRef]
- Obeid, M.; Suffiotti, M.; Pellaton, C.; Bouchaab, H.; Cairoli, A.; Salvadé, V.; Stevenel, C.; Hottinger, R.; Pythoud, C.; Coutechier, L.; et al. Humoral Responses Against Variants of Concern by COVID-19 mRNA Vaccines in Immunocompromised Patients. JAMA Oncology 2022, 8, e220446. [Google Scholar] [CrossRef] [PubMed]
- Lucane, Z.; Slisere, B.; Ozola, L.; Rots, D.; Papirte, S.; Vilne, B.; Gailite, L.; Kurjane, N. Long-Term Immunological Memory of SARS-CoV-2 Is Present in Patients with Primary Antibody Deficiencies for up to a Year after Vaccination. Vaccines 2023, 11, 354. [Google Scholar] [CrossRef]
- Pulvirenti, F.; Fernandez Salinas, A.; Milito, C.; Terreri, S.; Piano Mortari, E.; Quintarelli, C.; Di Cecca, S.; Lagnese, G.; Punziano, A.; Guercio, M.; et al. B Cell Response Induced by SARS-CoV-2 Infection Is Boosted by the BNT162b2 Vaccine in Primary Antibody Deficiencies. Cells 2021, 10, 2915. [Google Scholar] [CrossRef]
- Shields, A.M.; Faustini, S.E.; Hill, H.J.; Al-Taei, S.; Tanner, C.; Ashford, F.; Workman, S.; Moreira, F.; Verma, N.; Wagg, H.; et al. SARS-CoV-2 Vaccine Responses in Individuals with Antibody Deficiency: Findings from the COV-AD Study. J Clin Immunol 2022. [Google Scholar] [CrossRef]
- Oyaert, M.; De Scheerder, M.-A.; Van Herrewege, S.; Laureys, G.; Van Assche, S.; Cambron, M.; Naesens, L.; Hoste, L.; Claes, K.; Haerynck, F.; et al. Evaluation of Humoral and Cellular Responses in SARS-CoV-2 mRNA Vaccinated Immunocompromised Patients. Front Immunol 2022, 13, 858399. [Google Scholar] [CrossRef]
- Sauerwein, K.M.T.; Geier, C.B.; Stemberger, R.F.; Akyaman, H.; Illes, P.; Fischer, M.B.; Eibl, M.M.; Walter, J.E.; Wolf, H.M. Antigen-Specific CD4+ T-Cell Activation in Primary Antibody Deficiency After BNT162b2 mRNA COVID-19 Vaccination. Front Immunol 2022, 13, 827048. [Google Scholar] [CrossRef]
- Murray, C.E.; O’Brien, C.; Alamin, S.; Phelan, S.H.; Argue, R.; Kiersey, R.; Gardiner, M.; Naughton, A.; Keogh, E.; Holmes, P.; et al. Cellular and Humoral Immunogenicity of the COVID-19 Vaccine and COVID-19 Disease Severity in Individuals with Immunodeficiency. Front Immunol 2023, 14, 1131604. [Google Scholar] [CrossRef]
- Hurme, A.; Jalkanen, P.; Marttila-Vaara, M.; Heroum, J.; Jokinen, H.; Vara, S.; Liedes, O.; Lempainen, J.; Melin, M.; Julkunen, I.; et al. T Cell Immunity Following COVID-19 Vaccination in Adult Patients with Primary Antibody Deficiency – a 22-Month Follow-Up. Front Immunol 2023, 14, 1146500. [Google Scholar] [CrossRef]
- Piano Mortari, E.; Pulvirenti, F.; Marcellini, V.; Terreri, S.; Salinas, A.F.; Ferrari, S.; Di Napoli, G.; Guadagnolo, D.; Sculco, E.; Albano, C.; et al. Functional CVIDs Phenotype Clusters Identified by the Integration of Immune Parameters after BNT162b2 Boosters. Front Immunol 2023, 14, 1194225. [Google Scholar] [CrossRef] [PubMed]
- La Civita, E.; Zannella, C.; Brusa, S.; Romano, P.; Schettino, E.; Salemi, F.; Carrano, R.; Gentile, L.; Punziano, A.; Lagnese, G.; et al. BNT162b2 Elicited an Efficient Cell-Mediated Response against SARS-CoV-2 in Kidney Transplant Recipients and Common Variable Immunodeficiency Patients. Viruses 2023, 15, 1659. [Google Scholar] [CrossRef]
- Steiner, S.; Schwarz, T.; Corman, V.M.; Jeworowski, L.M.; Bauer, S.; Drosten, C.; Scheibenbogen, C.; Hanitsch, L.G. Impaired B Cell Recall Memory and Reduced Antibody Avidity but Robust T Cell Response in CVID Patients After COVID-19 Vaccination. J Clin Immunol 2023, 43, 869–881. [Google Scholar] [CrossRef]
- Mizera, D.; Dziedzic, R.; Drynda, A.; Gradzikiewicz, A.; Jakieła, B.; Celińska-Löwenhoff, M.; Padjas, A.; Matyja-Bednarczyk, A.; Zaręba, L.; Bazan-Socha, S. Cellular Immune Response to SARS-CoV-2 in Patients with Primary Antibody Deficiencies. Front Immunol 2023, 14, 1275892. [Google Scholar] [CrossRef]
- Nourizadeh, M.; Feizabadi, E.; Mirmoghtadaei, M.; Mohammadi, A.; Fazlollahi, M.R.; Moradi, L.; Pourpak, Z. Antibody Production after COVID-19 Vaccination in Patients with Inborn Errors of Immunity. Iranian Journal of Immunology 2023, 20, 400–409. [Google Scholar] [CrossRef]
- Yam-Puc, J.C.; Hosseini, Z.; Horner, E.C.; Gerber, P.P.; Beristain-Covarrubias, N.; Hughes, R.; Lulla, A.; Rust, M.; Boston, R.; Ali, M.; et al. Age-Associated B Cells Predict Impaired Humoral Immunity after COVID-19 Vaccination in Patients Receiving Immune Checkpoint Blockade. Nat Commun 2023, 14, 3292. [Google Scholar] [CrossRef]
- Tandon, M.; DiGiacomo, D.V.; Zhou, B.; Hesterberg, P.; Rosenberg, C.E.; Barmettler, S.; Farmer, J.R. Response to SARS-CoV-2 Initial Series and Additional Dose Vaccine in Pediatric Patients with Predominantly Antibody Deficiency. Front Immunol 2023, 14, 1217718. [Google Scholar] [CrossRef]
- Shin, J.J.; Par-Young, J.; Unlu, S.; McNamara, A.; Park, H.-J.; Shin, M.S.; Gee, R.J.; Doyle, H.; Afinogenova, Y.; Zidan, E.; et al. Defining Clinical and Immunological Predictors of Poor Immune Responses to COVID-19 mRNA Vaccines in Patients with Primary Antibody Deficiency. J Clin Immunol 2022. [Google Scholar] [CrossRef]
- Quinti, I.; Locatelli, F.; Carsetti, R. The Immune Response to SARS-CoV-2 Vaccination: Insights Learned From Adult Patients With Common Variable Immune Deficiency. Front Immunol 2021, 12, 815404. [Google Scholar] [CrossRef]
- Bloomfield, M.; Parackova, Z.; Hanzlikova, J.; Lastovicka, J.; Sediva, A. Immunogenicity and Safety of COVID-19 mRNA Vaccine in STAT1 GOF Patients. J Clin Immunol 2022, 42, 266–269. [Google Scholar] [CrossRef]
- Timothy, P.; Illes, P.; Ujhazi, B.; Gordon, S.; Nagy, B.; Alas, E.; Sullivan, S.; Miranda, M.A.; Duff, C.; Farmer, J.; et al. Effects Of The COVID-19 Pandemic On A Group Of Patients With Pathogenic Variant of Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4) in a Tertiary Center in Florida. J. Allergy Clin. Immunol. 2022, 149, AB28. [Google Scholar] [CrossRef]
- Squire, J.D.; Joshi, A.Y. Response to mRNA COVID-19 Vaccination in Three XLA Patients. Vaccine 2022. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive Immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Zimmerman, O.; Altman Doss, A.M.; Kaplonek, P.; Liang, C.-Y.; VanBlargan, L.A.; Chen, R.E.; Monroy, J.M.; Wedner, H.J.; Kulczycki, A.; Mantia, T.L.; et al. mRNA Vaccine Boosting Enhances Antibody Responses against SARS-CoV-2 Omicron Variant in Individuals with Antibody Deficiency Syndromes. Cell Reports Medicine 2022, 3, 100653. [Google Scholar] [CrossRef]
- Abella, E.; Trigueros, M.; Pradenas, E.; Muñoz-Lopez, F.; Garcia-Pallarols, F.; Ben Azaiz Ben Lahsen, R.; Trinité, B.; Urrea, V.; Marfil, S.; Rovirosa, C.; et al. Efficacy of SARS-CoV-2 Vaccination in Patients with Monoclonal Gammopathies: A Cross Sectional Study. Life Sci Alliance 2022, 5, e202201479. [Google Scholar] [CrossRef]
- Van Leeuwen, L.P.M.; Grobben, M.; GeurtsvanKessel, C.H.; Ellerbroek, P.M.; De Bree, G.J.; Potjewijd, J.; Rutgers, A.; Jolink, H.; Van De Veerdonk, F.L.; Van Gils, M.J.; et al. Immune Responses 6 Months After mRNA-1273 COVID-19 Vaccination and the Effect of a Third Vaccination in Patients with Inborn Errors of Immunity. J Clin Immunol 2023, 43, 1104–1117. [Google Scholar] [CrossRef]
- Karabiber, E.; Atik, Ö.; Tepetam, F.M.; Ergan, B.; İlki, A.; Karakoç Aydıner, E.; Özen, A.; Özyer, F.; Barış, S. Clinical and Immunological Outcomes of SARS-CoV-2 Infection in Patients with Inborn Errors of Immunity Receiving Different Brands and Doses of COVID-19 Vaccines. Tuberk Toraks 2023, 71, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Goda, V.; Kriván, G.; Kulcsár, A.; Gönczi, M.; Tasnády, S.; Matula, Z.; Nagy, G.; Bekő, G.; Horváth, M.; Uher, F.; et al. Specific Antibody and the T-Cell Response Elicited by BNT162b2 Boosting After Two ChAdOx1 nCoV-19 in Common Variable Immunodeficiency. Front Immunol 2022, 13, 907125. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agrawal, S.; Sandoval, A.; Su, H.; Tran, M.; Demirdag, Y. SARS-CoV-2-Specific and Functional Cytotoxic CD8 Cells in Primary Antibody Deficiency: Natural Infection and Response to Vaccine. J Clin Immunol 2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Jacob-Dolan, C.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; et al. Vaccines Elicit Highly Conserved Cellular Immunity to SARS-CoV-2 Omicron. Nature 2022, 603, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Barrios, Y.; Franco, A.; Alava-Cruz, C.; Cuesta-Martin, R.; Camara, C.; Matheu, V. Easy Approach to Detect Cell Immunity to COVID Vaccines in Common Variable Immunodeficiency Patients. Allergol Immunopathol (Madr) 2022, 50, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, C.; Wullimann, D.; Niessl, J.; Rivera-Ballesteros, O.; Chen, P.; Lange, J.; Cuapio, A.; Blennow, O.; Hansson, L.; et al. Immunodeficiency Syndromes Differentially Impact the Functional Profile of SARS-CoV-2-Specific T Cells Elicited by mRNA Vaccination. Immunity 2022, S1074-7613(22)00338-7. [Google Scholar] [CrossRef] [PubMed]
- Göschl, L.; Mrak, D.; Grabmeier-Pfistershammer, K.; Stiasny, K.; Haslacher, H.; Schneider, L.; Deimel, T.; Kartnig, F.; Tobudic, S.; Aletaha, D.; et al. Reactogenicity and Immunogenicity of the Second COVID-19 Vaccination in Patients with Inborn Errors of Immunity or Mannan-Binding Lectin Deficiency. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Schwarz, T.; Corman, V.M.; Gebert, L.; Kleinschmidt, M.C.; Wald, A.; Gläser, S.; Kruse, J.M.; Zickler, D.; Peric, A.; et al. SARS-CoV-2 T Cell Response in Severe and Fatal COVID-19 in Primary Antibody Deficiency Patients Without Specific Humoral Immunity. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, R.; Woon, S.-T.; Jordan, A.; Longhurst, H.; Leung, E.; Steele, R.; Lehnert, K.; Snell, R.; Brooks, A.E.S. Perspective: Diagnostic Laboratories Should Urgently Develop T Cell Assays for SARS-CoV-2 Infection. Expert Review of Clinical Immunology 2021, 17, 421–430. [Google Scholar] [CrossRef]
- Ameratunga, R.; Woon, S.-T.; Steele, R.; Lehnert, K.; Leung, E.; Edwards, E.S.J.; Brooks, A.E.S. Common Variable Immunodeficiency Disorders as a Model for Assessing COVID-19 Vaccine Responses in Immunocompromised Patients. Front Immunol 2021, 12, 798389. [Google Scholar] [CrossRef]
- Labrador-Horrillo, M.; Franco-Jarava, C.; Garcia-Prat, M.; Parra-Martínez, A.; Antolín, M.; Salgado-Perandrés, S.; Aguiló-Cucurull, A.; Martinez-Gallo, M.; Colobran, R. Case Report: X-Linked SASH3 Deficiency Presenting as a Common Variable Immunodeficiency. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Durkee-Shock, J.; Jensen-Wachspress, M.; Kankate, V.; Lang, H.; Lazarski, C.A.; Keswani, A.; Webber, K.; Montgomery-Recht, K.; Walkiewicz, M.; et al. Robust Antibody and T Cell Responses to SARS-CoV-2 in Patients with Antibody Deficiency. 2021. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Butler, D.K.; Otter, A.D.; Menacho, K.; Fontana, M.; Smit, A.; Sackville-West, J.E.; Cutino-Moguel, T.; et al. Prior SARS-CoV-2 Infection Rescues B and T Cell Responses to Variants after First Vaccine Dose. Science 2021, 372, 1418–1423. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-Reactive T Cells in Healthy Donors and Patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef]
- Barouch, D.H.; Stephenson, K.E.; Sadoff, J.; Yu, J.; Chang, A.; Gebre, M.; McMahan, K.; Liu, J.; Chandrashekar, A.; Patel, S.; et al. Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination. N Engl J Med 2021, 385, 951–953. [Google Scholar] [CrossRef]
- Sanchez, D.; Radigan, L.; Cunningham-Rundles, C. Assessing SARS-CoV-2 Antigen Specific T-Cell Responses After mRNA Vaccination and/or Omicron Variant COVID-19 Infection in Patients with Primary Humoral Immunodeficiencies. Journal of Allergy and Clinical Immunology 2023, 151, AB195. [Google Scholar] [CrossRef]
- Cuapio, A.; Boulouis, C.; Filipovic, I.; Wullimann, D.; Kammann, T.; Parrot, T.; Chen, P.; Akber, M.; Gao, Y.; Hammer, Q.; et al. NK Cell Frequencies, Function and Correlates to Vaccine Outcome in BNT162b2 mRNA Anti-SARS-CoV-2 Vaccinated Healthy and Immunocompromised Individuals. Mol Med 2022, 28, 20. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.; Hossny, E.; Weiler, C.; Ballow, M.; Berger, M.; Bonilla, F.; Buckley, R.; Chinen, J.; Elgamal, Y.; Mazer, B. Use of Intravenous Immunoglobulin in Human Disease: A Review of Evidence by Members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. Journal of Allergy and Clinical Immunology 2006, 117, S525–S553. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Díez, J.M.; Gajardo, R. Anti-SARS-CoV-2 Antibodies in Healthy Donor Plasma Pools and IVIG Products. The Lancet Infectious Diseases 2021, 21, 765–766. [Google Scholar] [CrossRef]
- Volk, A.; Covini-Souris, C.; Kuehnel, D.; De Mey, C.; Römisch, J.; Schmidt, T. SARS-CoV-2 Neutralization in Convalescent Plasma and Commercial Lots of Plasma-Derived Immunoglobulin. BioDrugs 2022, 36, 41–53. [Google Scholar] [CrossRef]
- Ahn, T.S.; Han, B.; Krogstad, P.; Bun, C.; Kohn, L.A.; Garcia-Lloret, M.I.; Damoiseaux, R.; Butte, M.J. Commercial Immunoglobulin Products Contain Cross-Reactive but Not Neutralizing Antibodies against SARS-CoV-2. Journal of Allergy and Clinical Immunology 2021, 147, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Bitzogli, K.; Alexopoulos, H. Anti-SARS-CoV-2 Antibodies Within IVIg Preparations: Cross-Reactivities With Seasonal Coronaviruses, Natural Autoimmunity, and Therapeutic Implications. Front. Immunol. 2021, 12, 627285. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.-M.; Romero, C.; Gajardo, R. Currently Available Intravenous Immunoglobulin Contains Antibodies Reacting against Severe Acute Respiratory Syndrome Coronavirus 2 Antigens. Immunotherapy 2020, 12, 571–576. [Google Scholar] [CrossRef]
- Manian, D.V.; Jensen, C.; Theel, E.S.; Mills, J.R.; Joshi, A. Non-Neutralizing Antibodies and Limitations of Serologic Testing for Severe Acute Respiratory Syndrome Coronavirus 2 in Patients Receiving Immunoglobulin Replacement Products. Annals of Allergy, Asthma & Immunology 2021, 126, 206–207. [Google Scholar] [CrossRef]
- Schwaiger, J.; Karbiener, M.; Aberham, C.; Farcet, M.R.; Kreil, T.R. No SARS-CoV-2 Neutralization by Intravenous Immunoglobulins Produced From Plasma Collected Before the 2020 Pandemic. The Journal of Infectious Diseases 2020, 222, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Farcet, M.R.; Karbiener, M.; Schwaiger, J.; Ilk, R.; Kreil, T.R. Rapidly Increasing Severe Acute Respiratory Syndrome Coronavirus 2 Neutralization by Intravenous Immunoglobulins Produced From Plasma Collected During the 2020 Pandemic. The Journal of Infectious Diseases 2022, 226, 1357–1361. [Google Scholar] [CrossRef]
- Romero, C.; Díez, J.-M.; Gajardo, R. Anti-SARS-CoV-2 Antibodies in Healthy Donor Plasma Pools and IVIG Products—an Update. The Lancet Infectious Diseases 2022, 22, 19. [Google Scholar] [CrossRef]
- Farcet, M.R.; Karbiener, M.; Knotzer, S.; Schwaiger, J.; Kreil, T.R. Omicron Severe Acute Respiratory Syndrome Coronavirus 2 Neutralization by Immunoglobulin Preparations Manufactured From Plasma Collected in the United States and Europe. The Journal of Infectious Diseases 2022, 226, 1396–1400. [Google Scholar] [CrossRef]
- Lindahl, H.; Klingström, J.; Da Silva Rodrigues, R.; Christ, W.; Chen, P.; Ljunggren, H.-G.; Buggert, M.; Aleman, S.; Smith, C.I.E.; Bergman, P. Neutralizing SARS-CoV-2 Antibodies in Commercial Immunoglobulin Products Give Patients with X-Linked Agammaglobulinemia Limited Passive Immunity to the Omicron Variant. J Clin Immunol 2022, 42, 1130–1136. [Google Scholar] [CrossRef]
- Raphael, A.; Shamriz, O.; Lev, A.; Simon, A.J.; Tal, Y.; Somech, R.; Eisenberg, R.; Toker, O. SARS-CoV-2 Spike Antibody Concentration in Gamma Globulin Products from High-Prevalence COVID-19 Countries Are Transmitted to X-Linked Agammaglobulinemia Patients. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Hirsiger, J.R.; Weigang, S.; Walz, A.-C.; Fuchs, J.; Daly, M.-L.; Eggimann, S.; Hausmann, O.; Schwemmle, M.; Kochs, G.; Panning, M.; et al. Passive Immunization against COVID-19 by Anti-SARS-CoV-2 Spike IgG in Commercially Available Immunoglobulin Preparations in Severe Antibody Deficiency. J. Allergy Clin. Immunol. Pract. 2022, 10, 2452–2455.e3. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Rider, N.L.; Pyles, R.B.; Judy, B.; Xie, X.; Shi, P.-Y.; Ksiazek, T.G. The Arrival of SARS-CoV-2–Neutralizing Antibodies in a Currently Available Commercial Immunoglobulin. Journal of Allergy and Clinical Immunology 2022, 149, 1958–1959. [Google Scholar] [CrossRef] [PubMed]
- Stinca, S.; Barnes, T.W.; Vogel, P.; Meyers, W.; Schulte-Pelkum, J.; Filchtinski, D.; Steller, L.; Hauser, T.; Manni, S.; Gardiner, D.F.; et al. Modelling the Concentration of Anti-SARS-CoV-2 Immunoglobulin G in Intravenous Immunoglobulin Product Batches. PLoS ONE 2021, 16, e0259731. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Gada, S. SARS-CoV-2 Antibody Profile of Immune Globulin Preparations. Journal of Allergy and Clinical Immunology 2022, 149, AB65. [Google Scholar] [CrossRef]
- Lindahl, H.; Chen, P.; Åberg, M.; Ljunggren, H.-G.; Buggert, M.; Aleman, S.; Smith, C.I.E.; Bergman, P. SARS-CoV-2 Antibodies in Commercial Immunoglobulin Products Show Markedly Reduced Cross-Reactivities Against Omicron Variants. J Clin Immunol 2023, 43, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Upasani, V.; Townsend, K.; Wu, M.Y.; Carr, E.J.; Hobbs, A.; Dowgier, G.; Ragno, M.; Herman, L.S.; Sharma, S.; Shah, D.; et al. Commercial Immunoglobulin Products Contain Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein. Clinical Infectious Diseases 2023, 77, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Cousins, K.; Sano, K.; Lam, B.; Röltgen, K.; Bhavsar, D.; Singh, G.; McRae, O.; Jeong, S.; Aboelregal, N.; Ho, H.; et al. Detection of SARS-CoV-2 Antibodies in Immunoglobulin Products. The Journal of Allergy and Clinical Immunology: In Practice 2023, 11, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Karbiener, M.; Farcet, M.R.; Schwaiger, J.; Powers, N.; Lenart, J.; Stewart, J.M.; Tallman, H.; Kreil, T.R. Highly Potent SARS-CoV-2 Neutralization by Intravenous Immunoglobulins Manufactured from Post-COVID-19 and COVID-19-Vaccinated Plasma Donations. The Journal of Infectious Diseases 2021, jiab482. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Bertoglio, F.; Steinke, S.; Heine, P.A.; Ynga-Durand, M.A.; Maass, H.; Sammartino, J.C.; Cassaniti, I.; Zuo, F.; Du, L.; et al. Human Serum from SARS-CoV-2-Vaccinated and COVID-19 Patients Shows Reduced Binding to the RBD of SARS-CoV-2 Omicron Variant. BMC Med 2022, 20, 102. [Google Scholar] [CrossRef]
- Cox, M.; Peacock, T.P.; Harvey, W.T.; Hughes, J.; Wright, D.W.; COVID-19 Genomics UK (COG-UK) Consortium; Willett, B.J.; Thomson, E.; Gupta, R.K.; Peacock, S.J.; et al. SARS-CoV-2 Variant Evasion of Monoclonal Antibodies Based on in Vitro Studies. Nat Rev Microbiol 2023, 21, 112–124. [Google Scholar] [CrossRef]
- Commissioner, O. of the Coronavirus (COVID-19) Update: FDA Authorizes Additional Monoclonal Antibody for Treatment of COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-monoclonal-antibody-treatment-covid-19 (accessed on 25 April 2023).
- Totschnig, D.; Augustin, M.; Niculescu, I.; Laferl, H.; Jansen-Skoupy, S.; Lehmann, C.; Wenisch, C.; Zoufaly, A. SARS-CoV-2 Pre-Exposure Prophylaxis with Sotrovimab and Tixagevimab/Cilgavimab in Immunocompromised Patients—A Single-Center Experience. Viruses 2022, 14, 2278. [Google Scholar] [CrossRef]
- Martin-Blondel, G.; Marcelin, A.-G.; Soulié, C.; Kaisaridi, S.; Lusivika-Nzinga, C.; Dorival, C.; Nailler, L.; Boston, A.; Melenotte, C.; Gaube, G.; et al. Outcome of Very High-Risk Patients Treated by Sotrovimab for Mild-to-Moderate COVID-19 Omicron, a Prospective Cohort Study (the ANRS 0003S COCOPREV Study). J Infect 2022, 84, e101–e104. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Rodrigues Falci, D.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Effect of Sotrovimab on Hospitalization or Death Among High-Risk Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2022, 327, 1236–1246. [Google Scholar] [CrossRef]
- Stadler, E.; Chai, K.L.; Schlub, T.E.; Cromer, D.; Polizzotto, M.N.; Kent, S.J.; Beecher, C.; White, H.; Turner, T.; Skoetz, N.; et al. Determinants of Passive Antibody Efficacy in SARS-CoV-2 Infection; Infectious Diseases (except HIV/AIDS), 2022.
- Zheng, B.; Green, A.C.A.; Tazare, J.; Curtis, H.J.; Fisher, L.; Nab, L.; Schultze, A.; Mahalingasivam, V.; Parker, E.P.K.; Hulme, W.J.; et al. Comparative Effectiveness of Sotrovimab and Molnupiravir for Prevention of Severe Covid-19 Outcomes in Patients in the Community: Observational Cohort Study with the OpenSAFELY Platform. BMJ 2022, e071932. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Yarwood, M.J.; Levick, B.; Gibbons, D.C.; Drysdale, M.; Kerr, W.; Watkins, J.D.; Young, S.; Pierce, B.F.; Lloyd, E.J.; et al. Characteristics and Outcomes of Patients with COVID-19 at High-Risk of Disease Progression Receiving Sotrovimab, Oral Antivirals or No Treatment in England; Infectious Diseases (except HIV/AIDS), 2022.
- Zheng, B.; Tazare, J.; Nab, L.; Mehrkar, A.; MacKenna, B.; Goldacre, B.; Douglas, I.J.; Tomlinson, L.A. Comparative Effectiveness of Paxlovid versus Sotrovimab and Molnupiravir for Preventing Severe COVID-19 Outcomes in Non-Hospitalised Patients: Observational Cohort Study Using the OpenSAFELY Platform; Epidemiology, 2023.
- Harman, K.; Nash, S.G.; Webster, H.H.; Groves, N.; Hardstaff, J.; Bridgen, J.; Blomquist, P.B.; Hope, R.; Ashano, E.; Myers, R.; et al. Comparison of the Risk of Hospitalisation among BA.1 and BA.2 COVID-19 Cases Treated with Sotrovimab in the Community in England. Influenza and Other Respiratory Viruses 2023, 17, e13150. [Google Scholar] [CrossRef]
- Cheng, M.M.; Reyes, C.; Satram, S.; Birch, H.; Gibbons, D.C.; Drysdale, M.; Bell, C.F.; Suyundikov, A.; Ding, X.; Maher, M.C.; et al. Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19 During SARS-CoV-2 Delta and Omicron Waves in the USA. Infect Dis Ther 2023, 12, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Martin-Blondel, G.; Marcelin, A.-G.; Soulié, C.; Kaisaridi, S.; Lusivika-Nzinga, C.; Dorival, C.; Nailler, L.; Boston, A.; Melenotte, C.; Cabié, A.; et al. Sotrovimab to Prevent Severe COVID-19 in High-Risk Patients Infected with Omicron BA.2. J Infect 2022, 85, e104–e108. [Google Scholar] [CrossRef]
- Zaqout, A.; Almaslamani, M.A.; Chemaitelly, H.; Hashim, S.A.; Ittaman, A.; Alimam, A.; Rustom, F.; Daghfal, J.; Abukhattab, M.; AlMukdad, S.; et al. Effectiveness of the Neutralizing Antibody Sotrovimab among High-Risk Patients with Mild-to-Moderate SARS-CoV-2 in Qatar. Int J Infect Dis 2022, 124, 96–103. [Google Scholar] [CrossRef]
- Case, J.B.; Mackin, S.; Errico, J.M.; Chong, Z.; Madden, E.A.; Whitener, B.; Guarino, B.; Schmid, M.A.; Rosenthal, K.; Ren, K.; et al. Resilience of S309 and AZD7442 Monoclonal Antibody Treatments against Infection by SARS-CoV-2 Omicron Lineage Strains. Nat Commun 2022, 13, 3824. [Google Scholar] [CrossRef]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Halfmann, P.; Watanabe, S.; Maeda, K.; et al. Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2. N Engl J Med 2022, 386, 1475–1477. [Google Scholar] [CrossRef]
- Takashita, E.; Yamayoshi, S.; Halfmann, P.; Wilson, N.; Ries, H.; Richardson, A.; Bobholz, M.; Vuyk, W.; Maddox, R.; Baker, D.A.; et al. In Vitro Efficacy of Antiviral Agents against Omicron Subvariant BA.4.6. N Engl J Med 2022, 387, 2094–2097. [Google Scholar] [CrossRef]
- Takashita, E.; Yamayoshi, S.; Simon, V.; van Bakel, H.; Sordillo, E.M.; Pekosz, A.; Fukushi, S.; Suzuki, T.; Maeda, K.; Halfmann, P.; et al. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N Engl J Med 2022, 387, 468–470. [Google Scholar] [CrossRef]
- Imai, M.; Ito, M.; Kiso, M.; Yamayoshi, S.; Uraki, R.; Fukushi, S.; Watanabe, S.; Suzuki, T.; Maeda, K.; Sakai-Tagawa, Y.; et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N Engl J Med 2023, 388, 89–91. [Google Scholar] [CrossRef]
- Park, Y.-J.; Pinto, D.; Walls, A.C.; Liu, Z.; De Marco, A.; Benigni, F.; Zatta, F.; Silacci-Fregni, C.; Bassi, J.; Sprouse, K.R.; et al. Imprinted Antibody Responses against SARS-CoV-2 Omicron Sublineages. Science 2022, 378, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Carr, E.J.; Harvey, R.; Mears, H.V.; Kjaer, S.; Townsley, H.; Hobbs, A.; Ragno, M.; Herman, L.S.; Adams, L.; et al. WHO’s Therapeutics and COVID-19 Living Guideline on mAbs Needs to Be Reassessed. The Lancet 2022, S0140673622019389. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jian, F.; Wang, J.; Yu, Y.; Song, W.; Yisimayi, A.; Wang, J.; An, R.; Chen, X.; Zhang, N.; et al. Imprinted SARS-CoV-2 Humoral Immunity Induces Convergent Omicron RBD Evolution. Nature 2022. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Song, W.; Wang, L.; Jian, F.; Chen, X.; Gao, F.; Shen, Z.; Wang, Y.; Wang, X.; Cao, Y. ACE2 Binding and Antibody Evasion in Enhanced Transmissibility of XBB.1.5. The Lancet Infectious Diseases 2023, 23, 278–280. [Google Scholar] [CrossRef]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming Antibody Evasion Properties of Rising SARS-CoV-2 BQ and XBB Subvariants. Cell 2023, 186, 279–286.e8. [Google Scholar] [CrossRef]
- Research, C. for D.E. and FDA Updates Sotrovimab Emergency Use Authorization. FDA 2022. [Google Scholar]
- Loo, Y.-M.; McTamney, P.M.; Arends, R.H.; Abram, M.E.; Aksyuk, A.A.; Diallo, S.; Flores, D.J.; Kelly, E.J.; Ren, K.; Roque, R.; et al. The SARS-CoV-2 Monoclonal Antibody Combination, AZD7442, Is Protective in Nonhuman Primates and Has an Extended Half-Life in Humans. Sci. Transl. Med. 2022, 14, eabl8124. [Google Scholar] [CrossRef]
- Stiasny, K.; Weseslindtner, L.; Heinzel, A.; Camp, J.V.; Oberbauer, R.; Reindl-Schwaighofer, R. SARS-CoV-2 Omicron BA.1/BA.2 Neutralization up to 8 Weeks After PrEP With Sotrovimab or Cilgavimab/Tixagevimab. Transpl Int 2022, 35, 10906. [Google Scholar] [CrossRef] [PubMed]
- Bruel, T.; Hadjadj, J.; Maes, P.; Planas, D.; Seve, A.; Staropoli, I.; Guivel-Benhassine, F.; Porrot, F.; Bolland, W.-H.; Nguyen, Y.; et al. Serum Neutralization of SARS-CoV-2 Omicron Sublineages BA.1 and BA.2 in Patients Receiving Monoclonal Antibodies. Nat Med 2022, 28, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Velay, A.; Gautier-Vargas, G.; Olagne, J.; Obrecht, A.; Cognard, N.; Heibel, F.; Braun-Parvez, L.; Keller, N.; Martzloff, J.; et al. Breakthrough COVID-19 Cases despite Prophylaxis with 150 Mg of Tixagevimab and 150 Mg of Cilgavimab in Kidney Transplant Recipients. American Journal of Transplantation 2022, 22, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Research, C. for D.E. and FDA Announces Evusheld Is Not Currently Authorized for Emergency Use in the U.S. FDA 2023. [Google Scholar]
- AstraZeneca A Phase I/III Randomized, Double Blind Study to Evaluate the Safety and Neutralizing Activity of AZD5156/AZD3152 for Pre Exposure Prophylaxis of COVID 19 in Participants With Conditions Causing Immune Impairment; clinicaltrials.gov, 2023.
- First Participant Dosed in SUPERNOVA Phase I/III Trial Evaluating AZD5156, a next-Generation Long-Acting Antibody Combination, for Prevention of COVID-19. Available online: https://www.astrazeneca-us.com/media/statements/2022/first-participant-dosed-in-supernova-phase-I-III-trial-evaluating-azd5156-a-next-generation-long-acting-antibody-combination-for-prevention-of-covid-19.html (accessed on 26 April 2023).
- Delmonte, O.M.; Bergerson, J.R.E.; Burbelo, P.D.; Durkee-Shock, J.R.; Dobbs, K.; Bosticardo, M.; Keller, M.D.; McDermott, D.H.; Rao, V.K.; Dimitrova, D.; et al. Antibody Responses to the SARS-CoV-2 Vaccine in Individuals with Various Inborn Errors of Immunity. J Allergy Clin Immunol 2021, 148, 1192–1197. [Google Scholar] [CrossRef]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated Transmissibility and Impact of SARS-CoV-2 Lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef]
- SARS-CoV-2 Variants of Concern as of 9 March 2023. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 20 March 2023).
- Davies, N.G.; Jarvis, C.I.; Group, C.C.-19 W.; Edmunds, W.J.; Jewell, N.P.; Diaz-Ordaz, K.; Keogh, R.H. Increased Mortality in Community-Tested Cases of SARS-CoV-2 Lineage B.1.1.7. Nature 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 Variant of Concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Gazy, I.; Jackson, L.; Hwa, S.-H.; Tegally, H.; Lustig, G.; Giandhari, J.; Pillay, S.; Wilkinson, E.; Naidoo, Y.; et al. Escape of SARS-CoV-2 501Y.V2 from Neutralization by Convalescent Plasma. Nature 2021, 593, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced Sensitivity of SARS-CoV-2 Variant Delta to Antibody Neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D. da S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and Epidemiology of the P.1 SARS-CoV-2 Lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Zhou, D.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Antibody Evasion by the P.1 Strain of SARS-CoV-2. Cell 2021, 184, 2939–2954.e9. [Google Scholar] [CrossRef]
- Shiehzadegan, S.; Alaghemand, N.; Fox, M.; Venketaraman, V. Analysis of the Delta Variant B.1.617.2 COVID-19. Clinics and Practice 2021, 11, 778–784. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. ; Public Health Scotland and the EAVE II Collaborators SARS-CoV-2 Delta VOC in Scotland: Demographics, Risk of Hospital Admission, and Vaccine Effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Stowe, J.; Andrews, N.; Kirsebom, F.; Ramsay, M.; Bernal, J.L. Effectiveness of COVID-19 Vaccines against Omicron and Delta Hospitalisation, a Test Negative Case-Control Study. Nat Commun 2022, 13, 5736. [Google Scholar] [CrossRef]
- Nextstrain / Ncov / Open / Global / 6m. Available online: https://nextstrain.org/ncov/open/global/6m (accessed on 20 March 2023).
- Fan, Y.; Li, X.; Zhang, L.; Wan, S.; Zhang, L.; Zhou, F. SARS-CoV-2 Omicron Variant: Recent Progress and Future Perspectives. Sig Transduct Target Ther 2022, 7, 141. [Google Scholar] [CrossRef]
- He, X.; Hong, W.; Pan, X.; Lu, G.; Wei, X. SARS-CoV-2 Omicron Variant: Characteristics and Prevention. MedComm 2021, 2, 838–845. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Ho, J.C.W.; Cheung, M.-C.; Ng, K.-C.; Ching, R.H.H.; Lai, K.-L.; Kam, T.T.; Gu, H.; Sit, K.-Y.; Hsin, M.K.Y.; et al. SARS-CoV-2 Omicron Variant Replication in Human Bronchus and Lung Ex Vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef]
- Rössler, A.; Knabl, L.; von Laer, D.; Kimpel, J. Neutralization Profile after Recovery from SARS-CoV-2 Omicron Infection. N Engl J Med 2022, 386, 1764–1766. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N Engl J Med 2022, 386, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 Escape Antibodies Elicited by Omicron Infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Outbreak.Info SARS-CoV-2 Data Explorer. Available online: https://outbreak.info/ (accessed on 20 March 2023).
- Zappa, M.; Verdecchia, P.; Angeli, F. Severe Acute Respiratory Syndrome Coronavirus 2 Evolution: How Mutations Affect XBB.1.5 Variant. European Journal of Internal Medicine 2023, 112, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Uriu, K.; Ito, J.; Zahradnik, J.; Fujita, S.; Kosugi, Y.; Schreiber, G.; Sato, K. Enhanced Transmissibility, Infectivity, and Immune Resistance of the SARS-CoV-2 Omicron XBB.1.5 Variant. The Lancet Infectious Diseases 2023, 23, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Faraone, J.N.; Evans, J.P.; Zheng, Y.-M.; Carlin, C.; Anghelina, M.; Stevens, P.; Fernandez, S.; Jones, D.; Panchal, A.; et al. Extraordinary Evasion of Neutralizing Antibody Response by Omicron XBB.1.5, CH.1.1 and CA.3.1 Variants; Microbiology, 2023.
- Luoma, E.; Rohrer, R.; Parton, H.; Hughes, S.; Omoregie, E.; Taki, F.; Wang, J.C.; Akther, S.; Amin, H.; Chang, C.; et al. Notes from the Field: Epidemiologic Characteristics of SARS-CoV-2 Recombinant Variant XBB.1.5 — New York City, November 1, 2022–January 4, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 212–214. [Google Scholar] [CrossRef] [PubMed]
- 215. COVID XBB 1.16, Nicknamed as Arcturus, Is Spreading Faster than Its Ancestral Variants: Know More. The Times of India.
- SARS-CoV-2 Variants of Concern as of 17 May 2023. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 29 May 2023).
- Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 17 March 2023).
- Giardino, G.; Milito, C.; Lougaris, V.; Punziano, A.; Carrabba, M.; Cinetto, F.; Scarpa, R.; Dellepiane, R.M.; Ricci, S.; Rivalta, B.; et al. The Impact of SARS-CoV-2 Infection in Patients with Inborn Errors of Immunity: The Experience of the Italian Primary Immunodeficiencies Network (IPINet). J. Clin. Immunol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Hodl, I.; Forstner, P.; Hatzl, S.; Sareban, N.; Moritz, M.; Fessler, J.; Dreo, B.; Uhl, B.; Url, C.; et al. CD19+IgD+CD27- Naïve B Cells as Predictors of Humoral Response to COVID 19 mRNA Vaccination in Immunocompromised Patients. Front. Immunol. 2021, 12, 803742. [Google Scholar] [CrossRef]
- Sokal, A.; Bastard, P.; Chappert, P.; Barba-Spaeth, G.; Fourati, S.; Vanderberghe, A.; Lagouge-Roussey, P.; Meyts, I.; Gervais, A.; Bouvier-Alias, M.; et al. Human Type I IFN Deficiency Does Not Impair B Cell Response to SARS-CoV-2 mRNA Vaccination. J Exp Med 2022, 220, e20220258. [Google Scholar] [CrossRef]
- Evusheld (Tixagevimab and Cilgavimab) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/evusheld-tixagevimab-cilgavimab-4000251 (accessed on 10 August 2023).
- Zhang, J.; Zhang, H.; Sun, L. Therapeutic Antibodies for COVID-19: Is a New Age of IgM, IgA and Bispecific Antibodies Coming? mAbs 2022, 14, 2031483. [Google Scholar] [CrossRef]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently Neutralizing and Protective Human Antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Park, Y.-J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-Neutralization of SARS-CoV-2 by a Human Monoclonal SARS-CoV Antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Tuccori, M.; Ferraro, S.; Convertino, I.; Cappello, E.; Valdiserra, G.; Blandizzi, C.; Maggi, F.; Focosi, D. Anti-SARS-CoV-2 Neutralizing Monoclonal Antibodies: Clinical Pipeline. MAbs 2020, 12, 1854149. [Google Scholar] [CrossRef] [PubMed]
- Administration (TGA), T.G. Evusheld. Available online: https://www.tga.gov.au/resources/auspmd/evusheld (accessed on 10 August 2023).
- Coronavirus Disease (COVID-19) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 10 July 2023).
- CoVariants: Per Country. Available online: https://covariants.org/per-country (accessed on 10 July 2023).
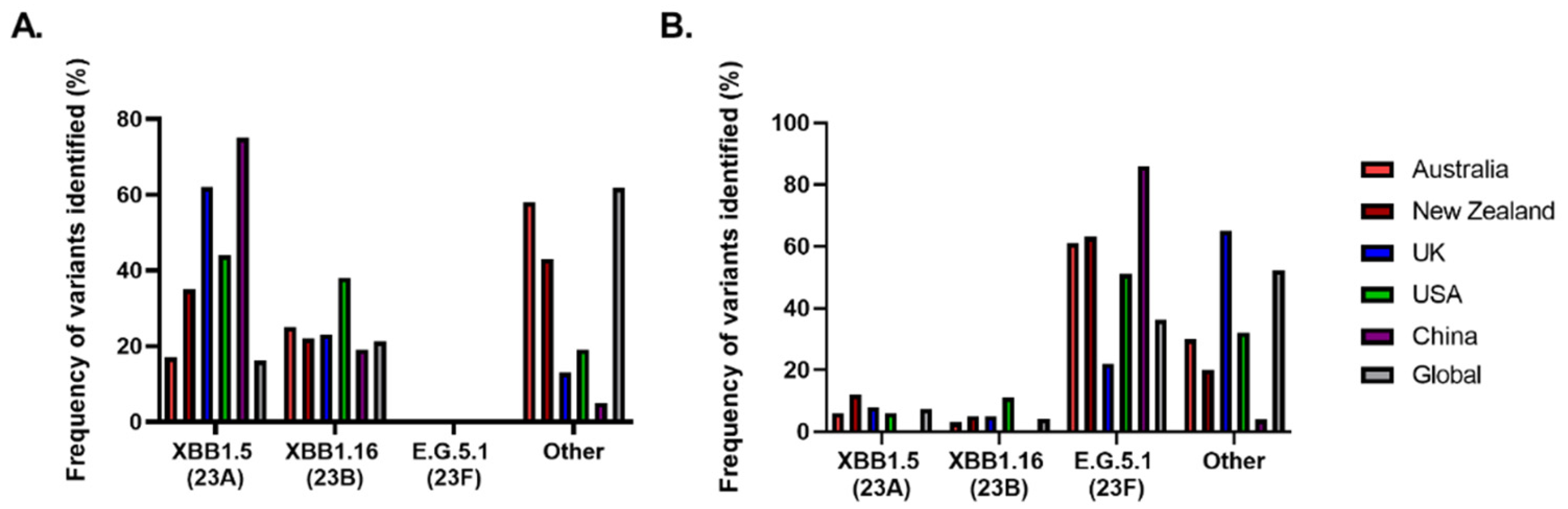
| WHO label | Earliest documented samples | Date designated as a variant by WHO | Pango lineage (Nextstrain Clade) |
Sublineage | RBD mutations compared to Wuhan (AA #319-541) | Other proteins harboring mutations | Rationale for designation as a variant of interest |
|---|---|---|---|---|---|---|---|
| Alpha | UK, September 2020 | December 18 2020 – March 9 2022 | B.1.1.7 (20I) |
- | N501Y [190] | N, ORF1ab, ORF8 [191] | Increased transmissibility, immune evasion and disease severity [190,192]. |
| Beta | South Africa, September 2020 | December 18 2020 - March 9 2022 | B.1.351 (20H) |
- | K417N, E484, N501Y [193] | E, N, ORF1ab, ORF3a, ORF8 [191] | Increased transmissibility, immune evasion and disease severity [190,193,194,195]. |
| Gamma | Brazil November 2020 | January 11 2021 – March 9 2022 | P.1 (20J) |
- | K417T, E484, N501Y [196] | N, ORF1ab, ORF3a, ORF8 [191] | Increased transmissibility, immune evasion and disease severity [190,197,198]. |
| Delta | India, October 2020 | May 11 2021 – June 7 2022 | B.1.617.2 (21I/21J) |
- | L452R, T478K [199] | M, N, ORF1ab, ORF3a, ORF7a [191] | Increased transmissibility, immune evasion and disease severity [200,201,202]. |
| Omicron | Multiple countries incl. South Africa, November 2021 | November 26 2021 – March 2023 | B.1.1.529 (21K) |
BA.1 | G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H [191,203,204,205] | E, M, N, ORF1ab, ORF3a [191] | High transmissibility and immune evasion ability, reduced disease severity [206,207,208]. |
| South Africa, November 2021 | ? – March 2023 | (21L) | BA.2 | G339D, S371L, S373P, S375F, T376A, D405N, R408S, K417N, N440K, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H and reversion to Wuhan G446 and G496 [191,204]. | E, M, N, ORF1ab, ORF3a, ORF6 [191] | Enhanced transmissibility and immune evasion ability, reduced disease severity [209]. | |
| South Africa, January and February 2022 respectively | ? – March 2023 | (22A/22B) | BA.4/5 | BA.2 variants plus L452R, F486V and reversion to Wuhan R493Q revertant [210] | E, M, N, ORF1ab, ORF3a (+ORF6 for BA4 and ORF7a, ORF8 for BA5 respectively) [191] | Impact on transmissibility and disease severity unclear, increased immune evasion capacity [209]. | |
| February 2022 | October 2022 | (22E) | BQ.1.1. (descendent of BA.5) |
BA.5 variants plus R346T, K444T, N460K | N, ORF1ab, ORF9b [191] | Unknown. | |
| United States, October 2022 | January 2023 | (23A) | XBB.1.5 |
G339H, R346T, L368I, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, V445P, G446P, N460K, S447N, T478K, E484A, F490P, F490S, Q498R, N501Y, Y505H [211] | E, M, N, ORF1ab, ORF3a, ORF6, ORF8, nsp1 [211] | Enhanced transmissibility, and impact on immunity. Disease severity similar to baseline [179,212,213,214]. | |
| Unknown, January 2023 | April 2023 | (23B) | XBB.1.16 |
BA.2.10.1 and BA.2.75 plus K478R, F486P [215] | E, M, N, ORF1ab, ORF3a, ORF6, ORF8, nsp1 [211] | No evidence of impact on transmissibility, immunity or disease severity [216]. | |
| Unknown, February 2023 |
August 2023 | (23F) | E.G.5.1 | G339H, R346T, L368I, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, V445P, F456L, G446P, N460K, S447N, T478K, E484A, S486P, F490S, Q498R, N501Y, Y505H [217] | ? | No evidence of impact on transmissibility, immunity or disease severity [216]. | |
| Unknown, July 2023 | November 2023 | (23I) | BA.2.86 | G339D, S371L, S373P, S375F, T376A, D405N, R408S, K417N, N440K, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H and reversion to Wuhan G446 and G496 [191,204] | Some evidence that the variant evades antibodies. No evidence of changes in clinical severity [38]. | ||
| Unknown, August 2023 | February 2024 | (not assigned) | JN.1 | G339D, S371L, S373P, S375F, T376A, D405N, R408S, K417N, N440K, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H and reversion to Wuhan G446 and G496, L5455S [38,191,204] | ORF1a, ORF7b [38] | Some evidence that the variant evades antibodies. No evidence of changes in clinical severity [38]. |
| Assay | References | |
|---|---|---|
| Studies investigating | ||
| IEI patient responses to COVID-19 vaccination | Quantity and quality IgG antibodies in IgRT | |
| SARS-CoV-2 binding IgG Antibodies | ||
| SARS-CoV-2 IgG II Quant Assay | [84] | - |
| Luciferase immunoprecipitation | [189] | - |
| ELISA | [79,80,81,83,89,91,94,99,101,102,104,105,106,109,110,111,112,113,114,115,116,117] | [137,138,139,140,141,142,149,150] |
| Abbott/Phadia ELiA SARS-CoV-2-Sp1 IgG assay | - | [147,151] |
| Roche Elecsys immunoassay | [77,79,80,101,125,219] | [154] |
| DiaSorin IgG immunoassay | [77,78,85,99,120,219] | - |
| Microblot assay | [112] | - |
| Abbot nucleoprotein assay | [78] | - |
| His-tagged spike and RBD binding probes | [87] | - |
| Abbott IgG assay | [82,88] | - |
| Luminex Ab binding | [95,149] | - |
| Extracted cell free DNA sequenced by DNBSEQ | [89] | - |
| Abbott Quant chemiluminescent microparticle immunoassay | - | [156] |
| Abbot AdviseDx SARS-CoV-2 IgG II assay | - | |
| V-PLEX SARS-CoV-2 10-plex IgG | [153] | |
| SARS-CoV-2 neutralizing antibodies | ||
| SARS-CoV-2 Neutralization Antibody Detection Kit | [100,110,149] | - |
| Pseudovirus Neutralization assay | [71,79,83,87,89,92,117,120,133] | [139,143,144,145,146,150,156] |
| ELISA | [69,70,86,93] | - |
| Live neutralization assays using Vero cells | [73,98,125] | [147] |
| B-cell measurement | [81] | - |
| Luminex based assays | [95] | [154] |
| SARS-CoV-2-specific B cells | ||
| SARS-CoV-2-specific tetramers | [70,81,87,97,220] | - |
| SARS-CoV-2-specific T-cells | ||
| AIMS (cell surface + intracellular cytokines) | [76,83,91,100,110,124] | - |
| Intracellular cytokine staining | [89,93,121,133] | - |
| Interferon-y ELISA | [85,86,98,99,123] | - |
| Interferon-y ELISPOT | [70,73,76,81,83,89,97,120,125,134] | - |
| Whole blood IGRA | [71,96,112] | - |
| Tetramers/multimers pMHCI |
[121] |
|
| pMHCII | [87] | |
| Monoclonal | Polyclonal | ||
| Example | Evusheld (AZD7442) | Xevudy (sotrovimab) | IgRT (IVIg or SCIg) |
| Company | AstraZeneca | VUR Biotechnology GlaxoSmithKline | Various |
| Pre or Post Exposure Prophylaxis | Pre | Post | N/A |
| Antibody Isotype | Hu IgG1κ | Hu IgG1κ | Various |
| SARS-CoV-2 Target | RBD | RBD | Broad range including NCP and RBD |
| Origin/Platform | Isolated from B cells of convalescent patients | Natural antibody from convalescent patient with SARS-CoV-2 | Purified antibodies from plasma of >1000 donors/product batch |
| Half life | ~6months | LS mutation of Fc increases half life | ~28 days |
| Doses required | 1* | 1 | ongoing |
| Dosage | 150mg of tixagevimab + 150mg cilgavimab |
500mg | Dependent on factors including weight and IgG trough levels |
| Frequency of redosing | Every 6 months | N/A | Every week to every month depending on IgG trough levels |
| Route of Administration | IM | IV | IV or SC |
| Approved recipients Age COVID-19 exposure Clinical disease |
>12 years weighing at least 40kg Not infected and no recent exposure Moderate to severe immunocompromised state |
>12 years weighing at least 40kg COVID-19+ PCR Moderate to severe immunocompromised state |
Dependent on established clinical guidelines |
| Neutralization Omicron BA.1 BA.2 |
344-fold decrease 9-fold decrease |
Unknown Unknown |
Unknown Unknown |
| Emergency Authorization US FDA Australian TGA |
12/21-1/23 11/21-1/22 |
05/21-3/22 08/21-3/22 |
N/A |
| Emergency approval for use in | Australia, UK and USA | Australia, UK and EU | N/A |
| References | [221,222,223] | [52,222,224,225] | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





