Submitted:
03 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
1. Highlights
- Passive immunization with a single bNAb offer a transient reduction in HIV-1 viremia – short lived but significant.
- Combination therapy of 2 bNAbs maintain viral suppression for a longer period of time, delay time to rebound after analytical treatment interruption (ATI) in individuals with antibody-sensitive strains.
- Combination therapy enhances the HIV-1 specific T-cell response and change both the size and composition of the intact proviral reservoir.
- Attaining prolonged viral suppression without treatment intervention, whether through ART or bNAb-based immunization, proves challenging due to reservoir diversity and pre-existing or de novo escape mutations. Only a very small subset of individuals have acquired post-treatment viremic control.
- The main barriers of clinical implementation of bNAbs as an alternative treatment to ART are the latent reservoir, viral resistance (pre-existing and de novo mutations) and the elimination half-life of bNAbs.
2. Introduction
2.1. The Difficulties with HIV-1 Treatment, Prevention and Cure
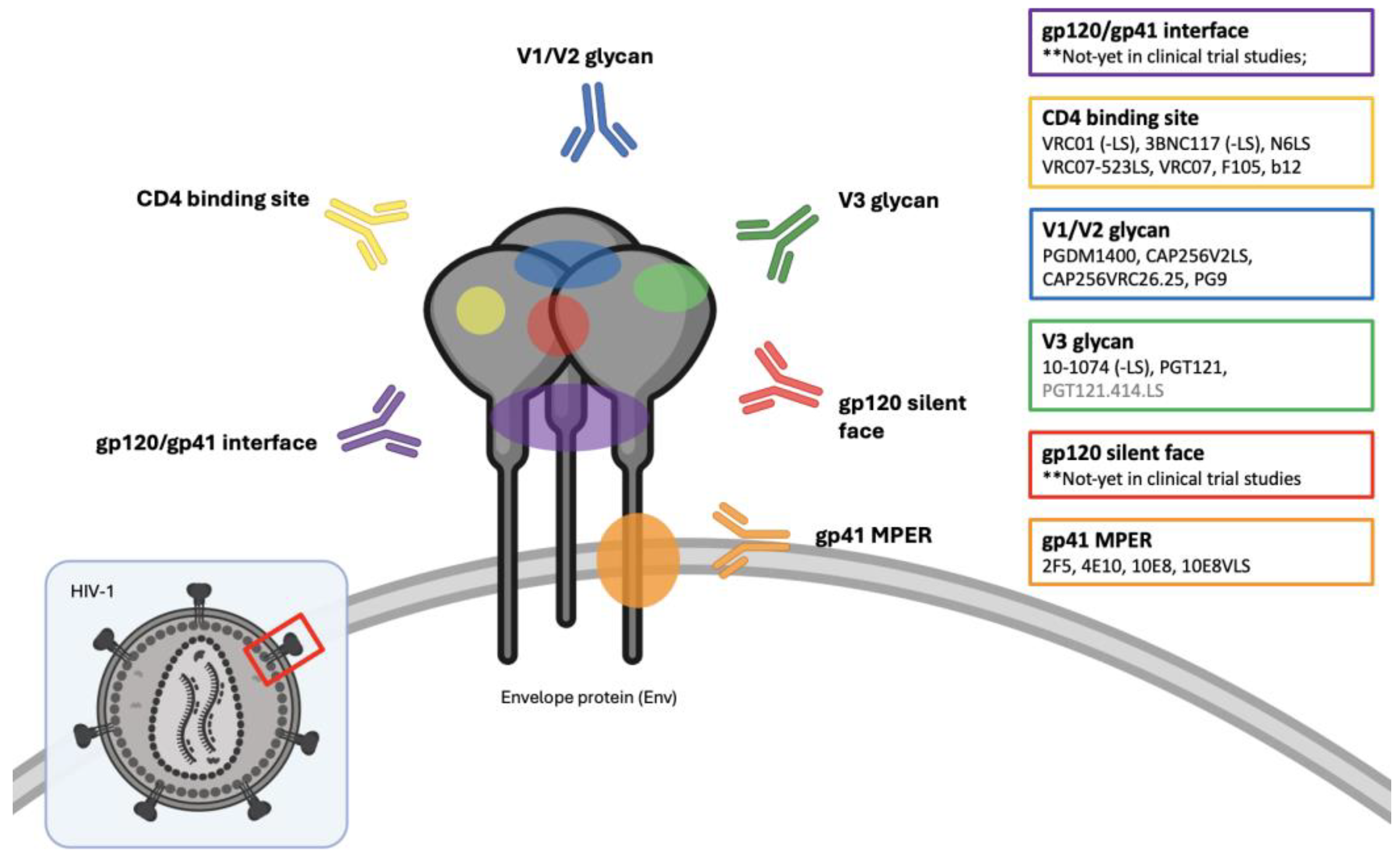
2.2. Broadly Neutralizing Antibodies (bNAbs)
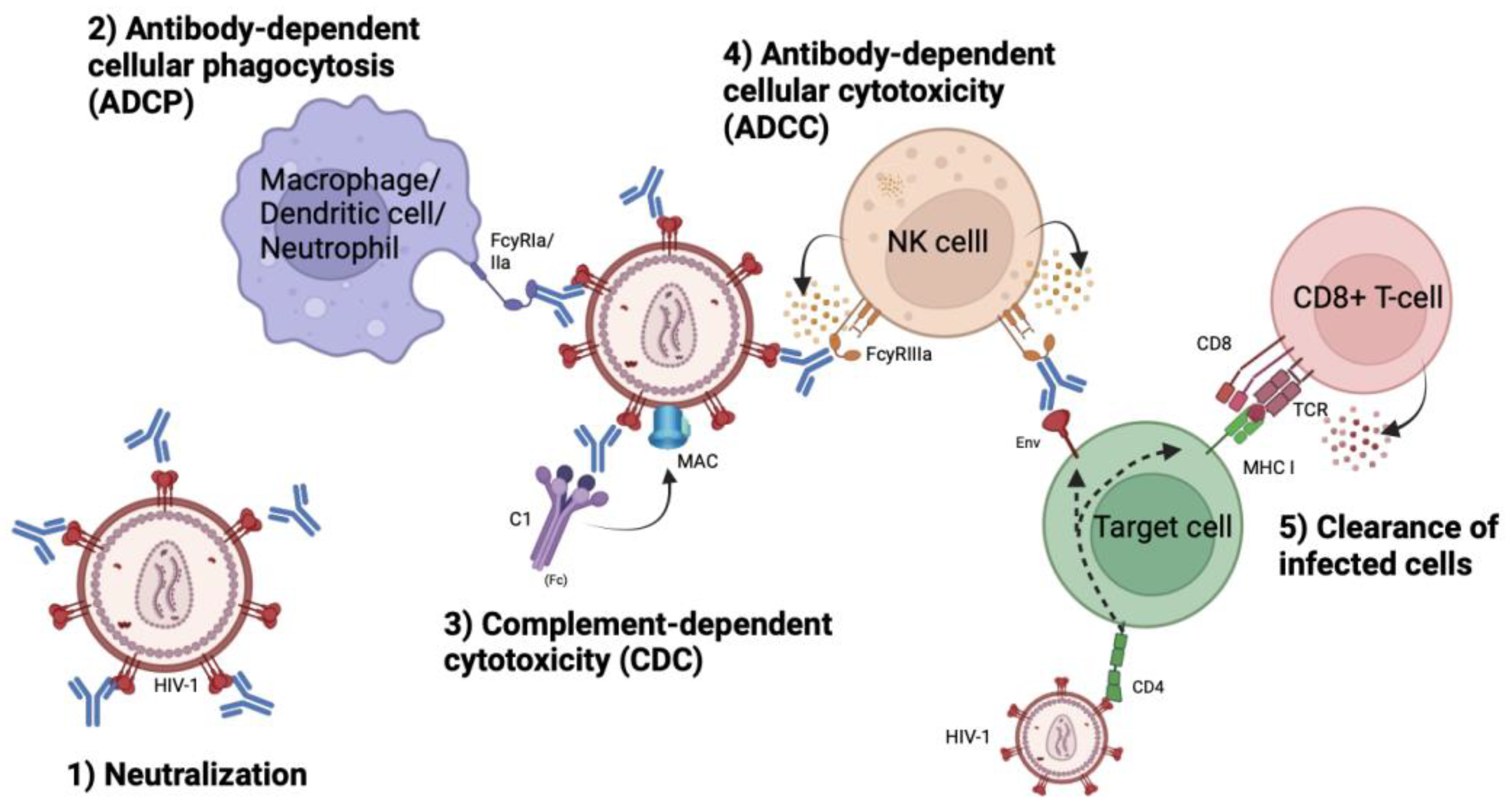
2.3. Mechanism of Action
3. Findings from Clinical Trials Studies
3.1. First Generation bNAbs
| Clinical trial study | Year | bNAb | Study objective | Study population |
Ref |
|---|---|---|---|---|---|
| Cavacini et al. | 1998 | F105 | Safety and pharmacokinetic profile | HIV-1 infected individuals, n = 4 | [53] |
| Armbruster et al.* | 2002 | 2F5 + 2G12 | Safety and pharmacokinetic profile | Asymptomatic, ART-naive HIV-1 infected individuals, n = 7 |
[45] |
| Stiegler et al.* | 2002 | 2F5 + 2G12 | Antiviral effects | Asymptomatic, ART-naive HIV-1 infected individuals, n = 7 |
[44] |
| Armbruster et al. | 2004 | 4E10 / 4E10 + 2F5 + 2G12 |
Safety and pharmacokinetic profile | Asymptomatic, ART-naive HIV-1 infected individuals, n = 8 |
[46] |
| Joos et al.** | 2006 | 4E10 + 2F5 + 2G12 | Safety and pharmacokinetic profile | Chronic (8) and acutely (6) HIV-1 infected individuals undergoing ATI, n = 14 | [47] |
| Trkola et al.** | 2005 | 4E10 + 2F5 + 2G12 | Efficacy | Chronic (8) and acutely (6) HIV-1 infected individuals undergoing ATI, n = 14 | [48] |
| Mehandru et al. | 2007 | 4E10 + 2F5 + 2G12 | Efficacy | Acutely HIV-1 infected individuals undergoing ATI, n = 10 | [49] |
3.2. Second Generation bNAbs
3.3. Monotherapy with a Single bNAb
3.4. Combination Therapy with ≥ 2 bNAbs
| Clinical trial study | Year | bNAb | Study objective | Study population | Ref |
|---|---|---|---|---|---|
| NCT02825797 (Bar-on et al.) |
2018 | 3BNC117 + 10-1074 |
Safety, pharmacokinetics and antiviral activity | Viremic HIV-1 infected individuals with antibody-sensitivity and have been on/off ART, n = 7 |
[77] |
| NCT028 (Mendoza et al.) |
2018 | 3BNC117 + 10-1074 |
Antiviral activity incl. neutralization efficacy and latent reservoir |
ART-treated and virally suppressed HIV-1 infected indivi- duals with antibody-sensitivity undergoing ATI, n = 11 |
[79] |
| NCT02824536 (Cohen et al.) |
2019 | 3BNC117 + 10-1074 |
Safety and pharmacokinetics | HIV-1 negative individuals, n = 24 |
[71] |
| NCT02825797 (Niessl et al.) |
2020 | 3BNC117 + 10-1074 |
Antiviral activity including Gag-specific CD8+ and CD4+ T-cell responses |
Aviremic HIV-1 infected individuals undergoing ATI, n = 9 |
[86] |
| NCT03526848 (Geabler et al.) |
2022 | 3BNC117 + 10-1074 + ART |
Antiviral activity incl. neutralization efficacy and reservoir size |
HIV-1 infected individuals in the presence or absence of ART, n = 26 |
[80] |
| NCT03571204 (Sneller et al.) |
2022 | 3BNC117 + 10-1074 |
Safety, pharmacokinetics and antiviral activity including reservoir size | HIV-1 infected individuals, who initiated ART during early/acute infection, undergoing ATI (n = 14), ART-naïve HIV-1 infected individuals with viraemic control (n = 5), n = 19 | [81] |
| PACTR201808919297 244 (Mahomed et al.) |
2022 | VRC07-523LS + PGT121 |
Safety and pharmacokinetics | HIV-1 negative women, n = 45 |
[83] |
| PACTR202003767867 253 (Mahomed et al.) |
2023 | VRC07-523LS + CAP256V2- LS |
Safety, pharmacokinetics and antiviral activity | HIV-1 negative women, n = 42 |
[84] |
| NCT03205917 (Julg et al.) |
2022 | VRC07-523LS + PGDM1400 + PGT121 |
Safety, pharmacokinetics and antiviral activity | HIV-1 negative individuals (n = 24) and viremic, ART-naïve HIV-1 infected individuals (n = 5), n = 29 |
[78] |
| NCT03928821 (Sobieszczyk et al.) |
2023 | VRC07-523LS + PGDM1400 + PGT121 + 10-1074 |
Safety, pharmacokinetics and antiviral activity | HIV-1 negative individuals who were randomly assigned to either dual- (n = 18) or triple-bNAb therapy (n = 9), n = 27 |
[82] |
| NCT03565315 (Awan et al.) |
2024 | VRC07-523LS + 10E8VLS |
Safety and pharmacokinetics | HIV-1 negative individuals, n = 8 |
[85] |
3.5. Combination Therapy with bNAb(s) and Immunostimulatory Agents
3.6. Elimination Half-Time
| Broadly neutralizing antibody | Elimination T1/2 (days) | Reference |
|---|---|---|
| VRC01 | 17 | [66] |
| VRC01-LS | 71 | [63] |
| VRC07-523LS | 29-66 | [65,82,83,84] |
| 3BNC117 | 16 | [71] |
| 3BNC117-LS | 62* | [97] |
| 3BNC07-523-LS | ND | - |
| 10-1074 | 23 - 27 | [71,82] |
| 10-1074-LS | 80* | [97] |
| PGT121 | 20 - 32 | [82,83] |
| PGDM1400 | 20-25 11* |
[78,82] [78] |
| 10E8VLS | 8 | [85] |
| N6LS | > 30 | [55] |
| CAP256V2LS | 46 | [84] |
3.7. Post-Treatment Viral Control
3.8. HIV-1 Prevention
4. Challenges, Considerations & Improvements
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS, Global HIV & AIDS statistics — Fact sheet. 2023.
- Eisinger, R.W. and A.S. Fauci, Ending the HIV/AIDS Pandemic(1). Emerg Infect Dis, 2018. 24(3): p. 413-416. [CrossRef]
- Serrão, R., et al., Non-AIDS-related comorbidities in people living with HIV-1 aged 50 years and older: The AGING POSITIVE study. Int J Infect Dis, 2019. 79: p. 94-100. [CrossRef]
- Kontomanolis, E.N., et al., The social stigma of HIV-AIDS: society's role. HIV AIDS (Auckl), 2017. 9: p. 111-118. [CrossRef]
- Chawla, A., et al., A Review of Long-Term Toxicity of Antiretroviral Treatment Regimens and Implications for an Aging Population. Infect Dis Ther, 2018. 7(2): p. 183-195. [CrossRef]
- Pennings, P.S., HIV Drug Resistance: Problems and Perspectives. Infect Dis Rep, 2013. 5(Suppl 1): p. e5. [CrossRef]
- Klein, F., et al., HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature, 2012. 492(7427): p. 118-22. [CrossRef]
- Shingai, M., et al., Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature, 2013. 503(7475): p. 277-80. [CrossRef]
- Barouch, D.H., et al., Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature, 2013. 503(7475): p. 224-8. [CrossRef]
- Cummins, N.W. and A.D. Badley, Can HIV Be Cured and Should We Try? Mayo Clin Proc, 2015. 90(6): p. 705-9. [CrossRef]
- Yeo, J.Y., et al., The Determination of HIV-1 RT Mutation Rate, Its Possible Allosteric Effects, and Its Implications on Drug Resistance. Viruses, 2020. 12(3). [CrossRef]
- Coffin, J.M., HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science, 1995. 267(5197): p. 483-9. [CrossRef]
- Jin, X., et al., The transmission of drug-resistant strains of HIV in heterosexual populations based on genetic sequences. PLoS One, 2021. 16(12): p. e0259023. [CrossRef]
- Mokgethi, P.T., et al., High prevalence of pre-treatment and acquired HIV-1 drug resistance mutations among non-citizens living with HIV in Botswana. Front Microbiol, 2024. 15: p. 1338191. [CrossRef]
- Zhu, P., et al., Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature, 2006. 441(7095): p. 847-52. [CrossRef]
- Burton, D.R. and L. Hangartner, Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu Rev Immunol, 2016. 34: p. 635-59. [CrossRef]
- Schriek, A.I., et al., Next-generation bNAbs for HIV-1 cure strategies. Antiviral Research, 2024. 222: p. 105788. [CrossRef]
- Bandera, A., et al., Phylogenies in ART: HIV reservoirs, HIV latency and drug resistance. Curr Opin Pharmacol, 2019. 48: p. 24-32. [CrossRef]
- Guo, C., et al., Transmitted Drug Resistance in Antiretroviral Therapy-Naive Persons With Acute/Early/Primary HIV Infection: A Systematic Review and Meta-Analysis. Front Pharmacol, 2021. 12: p. 718763. [CrossRef]
- Nachega, J.B., et al., HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets, 2011. 11(2): p. 167-74. [CrossRef]
- Klein, F., et al., Antibodies in HIV-1 vaccine development and therapy. Science, 2013. 341(6151): p. 1199-204. [CrossRef]
- Miner, M.D., L. Corey, and D. Montefiori, Broadly neutralizing monoclonal antibodies for HIV prevention. J Int AIDS Soc, 2021. 24 Suppl 7(Suppl 7): p. e25829. [CrossRef]
- Ward, A.B. and I.A. Wilson, Insights into the trimeric HIV-1 envelope glycoprotein structure. Trends Biochem Sci, 2015. 40(2): p. 101-7. [CrossRef]
- Wei, X., et al., Antibody neutralization and escape by HIV-1. Nature, 2003. 422(6929): p. 307-12. [CrossRef]
- Kumar, S., S. Singh, and K. Luthra, An Overview of Human Anti-HIV-1 Neutralizing Antibodies against Diverse Epitopes of HIV-1. ACS Omega, 2023. 8(8): p. 7252-7261. [CrossRef]
- Walker, L.M., et al., Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature, 2011. 477(7365): p. 466-70. [CrossRef]
- Caskey, M., F. Klein, and M.C. Nussenzweig, Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat Med, 2019. 25(4): p. 547-553. [CrossRef]
- Paneerselvam, N., A. Khan, and B.R. Lawson, Broadly neutralizing antibodies targeting HIV: Progress and challenges. Clin Immunol, 2023. 257: p. 109809. [CrossRef]
- Irani, V., et al., Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Molecular Immunology, 2015. 67(2, Part A): p. 171-182. [CrossRef]
- Bruel, T., et al., Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun, 2016. 7: p. 10844. [CrossRef]
- Walker, L.M., et al., Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science, 2009. 326(5950): p. 285-9. [CrossRef]
- Moore, P.L., et al., Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol, 2006. 80(5): p. 2515-28. [CrossRef]
- Pejchal, R., et al., A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science, 2011. 334(6059): p. 1097-103. [CrossRef]
- Day, C.L., et al., PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature, 2006. 443(7109): p. 350-4. [CrossRef]
- Rosignoli, G., et al., Programmed death (PD)-1 molecule and its ligand PD-L1 distribution among memory CD4 and CD8 T cell subsets in human immunodeficiency virus-1-infected individuals. Clin Exp Immunol, 2009. 157(1): p. 90-7. [CrossRef]
- Graham, B.S. and D.M. Ambrosino, History of passive antibody administration for prevention and treatment of infectious diseases. Curr Opin HIV AIDS, 2015. 10(3): p. 129-34. [CrossRef]
- Keller, M.A. and E.R. Stiehm, Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev, 2000. 13(4): p. 602-14. [CrossRef]
- Delgado, M. and J.A. Garcia-Sanz, Therapeutic Monoclonal Antibodies against Cancer: Present and Future. Cells, 2023. 12(24). [CrossRef]
- Hafeez, U., H.K. Gan, and A.M. Scott, Monoclonal antibodies as immunomodulatory therapy against cancer and autoimmune diseases. Curr Opin Pharmacol, 2018. 41: p. 114-121. [CrossRef]
- Mascola, J.R., et al., Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol, 1999. 73(5): p. 4009-18. [CrossRef]
- Shibata, R., et al., Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med, 1999. 5(2): p. 204-10. [CrossRef]
- Baba, T.W., et al., Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med, 2000. 6(2): p. 200-6. [CrossRef]
- Hessell, A.J., et al., Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog, 2009. 5(5): p. e1000433. [CrossRef]
- Stiegler, G., et al., Antiviral activity of the neutralizing antibodies 2F5 and 2G12 in asymptomatic HIV-1-infected humans: a phase I evaluation. Aids, 2002. 16(15): p. 2019-25. [CrossRef]
- Armbruster, C., et al., A phase I trial with two human monoclonal antibodies (hMAb 2F5, 2G12) against HIV-1. Aids, 2002. 16(2): p. 227-33. [CrossRef]
- Armbruster, C., et al., Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J Antimicrob Chemother, 2004. 54(5): p. 915-20. [CrossRef]
- Joos, B., et al., Long-term multiple-dose pharmacokinetics of human monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 envelope gp120 (MAb 2G12) and gp41 (MAbs 4E10 and 2F5). Antimicrob Agents Chemother, 2006. 50(5): p. 1773-9. [CrossRef]
- Trkola, A., et al., Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med, 2005. 11(6): p. 615-22. [CrossRef]
- Mehandru, S., et al., Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol, 2007. 81(20): p. 11016-31. [CrossRef]
- Mehandru, S., et al., Neutralization profiles of newly transmitted human immunodeficiency virus type 1 by monoclonal antibodies 2G12, 2F5, and 4E10. J Virol, 2004. 78(24): p. 14039-42. [CrossRef]
- Haynes, B.F., et al., Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science, 2005. 308(5730): p. 1906-8. [CrossRef]
- Yang, G., et al., Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med, 2013. 210(2): p. 241-56. [CrossRef]
- Cavacini, L.A., et al., Phase I study of a human monoclonal antibody directed against the CD4-binding site of HIV type 1 glycoprotein 120. AIDS Res Hum Retroviruses, 1998. 14(7): p. 545-50. [CrossRef]
- Frattari, G.S., M. Caskey, and O.S. Søgaard, Broadly neutralizing antibodies for HIV treatment and cure approaches. Current Opinion in HIV and AIDS, 2023. 18(4): p. 157-163. [CrossRef]
- Widge, A.T., A PHASE I DOSE-ESCALATION TRIAL OF HUMAN MONOCLONAL ANTIBODY N6LS IN HEALTHY ADULTS. March 8-11, 2020: CROI conference - poster.
- NCT04871113, A Study to Evaluate the Antiviral Effect, Safety and Tolerability of GSK3810109A in Viremic Human Immunodeficiency Virus (HIV)-1 Infected Adults. 2023: Clinicaltrials.gov.
- Ledgerwood, J.E., et al., Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol, 2015. 182(3): p. 289-301. [CrossRef]
- Lynch, R.M., et al., Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med, 2015. 7(319): p. 319ra206. [CrossRef]
- Bar, K.J., et al., Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med, 2016. 375(21): p. 2037-2050. [CrossRef]
- Mayer, K.H., et al., Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial. PLoS Med, 2017. 14(11): p. e1002435. [CrossRef]
- Riddler, S.A., et al., Randomized Clinical Trial to Assess the Impact of the Broadly Neutralizing HIV-1 Monoclonal Antibody VRC01 on HIV-1 Persistence in Individuals on Effective ART. Open Forum Infect Dis, 2018. 5(10): p. ofy242. [CrossRef]
- Crowell, T.A., et al., Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet HIV, 2019. 6(5): p. e297-e306. [CrossRef]
- Gaudinski, M.R., et al., Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med, 2018. 15(1): p. e1002493. [CrossRef]
- Chen, G., Safety and virologic effect of the HIV-1 broadly neutralizing antibodies, VRC01LS or VRC07-523LS, administered to HIV-infected adults in a phase 1 clinical trial. 2021: Clinicaltrials.gov.
- Gaudinski, M.R., et al., Safety and pharmacokinetics of broadly neutralising human monoclonal antibody VRC07-523LS in healthy adults: a phase 1 dose-escalation clinical trial. Lancet HIV, 2019. 6(10): p. e667-e679. [CrossRef]
- Caskey, M., et al., Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature, 2015. 522(7557): p. 487-91. [CrossRef]
- Scheid, J.F., et al., HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature, 2016. 535(7613): p. 556-60. [CrossRef]
- Cohen, Y.Z., et al., Relationship between latent and rebound viruses in a clinical trial of anti-HIV-1 antibody 3BNC117. J Exp Med, 2018. 215(9): p. 2311-2324. [CrossRef]
- Caskey, M., et al., Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med, 2017. 23(2): p. 185-191. [CrossRef]
- Stephenson, K.E., et al., Safety, pharmacokinetics and antiviral activity of PGT121, a broadly neutralizing monoclonal antibody against HIV-1: a randomized, placebo-controlled, phase 1 clinical trial. Nat Med, 2021. 27(10): p. 1718-1724. [CrossRef]
- Cohen, Y.Z., et al., Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10-1074 in healthy adults: A randomized, phase 1 study. PLoS One, 2019. 14(8): p. e0219142. [CrossRef]
- Andriesen, J., Evaluating the Safety, Pharmacokinetics, and Anti-Viral Activity of VRC01 and VRC01LS in the Serum and Mucosa of Healthy, HIV-Uninfected Adults. 2019, Fred Hutchinson Cancer Research Center: Clinicaltrials.gov.
- Cale, E.M., et al., Neutralizing antibody VRC01 failed to select for HIV-1 mutations upon viral rebound. J Clin Invest, 2020. 130(6): p. 3299-3304. [CrossRef]
- Corey, L., et al., Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. N Engl J Med, 2021. 384(11): p. 1003-1014. [CrossRef]
- Schoofs, T., et al., HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science, 2016. 352(6288): p. 997-1001. [CrossRef]
- Wang, C.Y., et al., Effect of Anti-CD4 Antibody UB-421 on HIV-1 Rebound after Treatment Interruption. N Engl J Med, 2019. 380(16): p. 1535-1545. [CrossRef]
- Bar-On, Y., et al., Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med, 2018. 24(11): p. 1701-1707. [CrossRef]
- Julg, B., et al., Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial. Nat Med, 2022. 28(6): p. 1288-1296. [CrossRef]
- Mendoza, P., et al., Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature, 2018. 561(7724): p. 479-484. [CrossRef]
- Gaebler, C., et al., Prolonged viral suppression with anti-HIV-1 antibody therapy. Nature, 2022. 606(7913): p. 368-374. [CrossRef]
- Sneller, M.C., et al., Combination anti-HIV antibodies provide sustained virological suppression. Nature, 2022. 606(7913): p. 375-381. [CrossRef]
- Sobieszczyk, M.E., et al., Safety, tolerability, pharmacokinetics, and immunological activity of dual-combinations and triple-combinations of anti-HIV monoclonal antibodies PGT121, PGDM1400, 10-1074, and VRC07-523LS administered intravenously to HIV-uninfected adults: a phase 1 randomised trial. Lancet HIV, 2023. 10(10): p. e653-e662. [CrossRef]
- Mahomed, S., et al., Safety and Pharmacokinetics of Monoclonal Antibodies VRC07-523LS and PGT121 Administered Subcutaneously for Human Immunodeficiency Virus Prevention. J Infect Dis, 2022. 226(3): p. 510-520. [CrossRef]
- Mahomed, S., et al., Safety and pharmacokinetics of escalating doses of neutralising monoclonal antibody CAP256V2LS administered with and without VRC07-523LS in HIV-negative women in South Africa (CAPRISA 012B): a phase 1, dose-escalation, randomised controlled trial. Lancet HIV, 2023. 10(4): p. e230-e243. [CrossRef]
- Awan, S.F., et al., Phase 1 trial evaluating safety and pharmacokinetics of HIV-1 broadly neutralizing mAbs 10E8VLS and VRC07-523LS. JCI Insight, 2024. 9(7). [CrossRef]
- Niessl, J., et al., Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat Med, 2020. 26(2): p. 222-227. [CrossRef]
- Betts, M.R., et al., HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood, 2006. 107(12): p. 4781-9. [CrossRef]
- Borducchi, E.N., et al., Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature, 2018. 563(7731): p. 360-364. [CrossRef]
- Moldt, B., et al., HIV envelope antibodies and TLR7 agonist partially prevent viral rebound in chronically SHIV-infected monkeys. PLoS Pathog, 2022. 18(4): p. e1010467. [CrossRef]
- Hsu, D.C., et al., TLR7 agonist, N6-LS and PGT121 delayed viral rebound in SHIV-infected macaques after antiretroviral therapy interruption. PLoS Pathog, 2021. 17(2): p. e1009339. [CrossRef]
- Gruell, H., et al., Effect of 3BNC117 and romidepsin on the HIV-1 reservoir in people taking suppressive antiretroviral therapy (ROADMAP): a randomised, open-label, phase 2A trial. Lancet Microbe, 2022. 3(3): p. e203-e214. [CrossRef]
- Gay, C.L., et al., Stable Latent HIV Infection and Low-level Viremia Despite Treatment With the Broadly Neutralizing Antibody VRC07-523LS and the Latency Reversal Agent Vorinostat. J Infect Dis, 2022. 225(5): p. 856-861. [CrossRef]
- Gunst, J.D., et al., Early intervention with 3BNC117 and romidepsin at antiretroviral treatment initiation in people with HIV-1: a phase 1b/2a, randomized trial. Nat Med, 2022. 28(11): p. 2424-2435. [CrossRef]
- Gunst, J.D., et al., Impact of a TLR9 agonist and broadly neutralizing antibodies on HIV-1 persistence: the randomized phase 2a TITAN trial. Nat Med, 2023. 29(10): p. 2547-2558. [CrossRef]
- Tebas, P., BEAT2: Peg-INF-a2b + 3BNC117 and 10-1074 keeps HIV at < 20 c/uL during a 26-week ATI. 2022: CROI conference 2022 (poster).
- Lowenthal, E.D., et al., Acceptability and tolerability of long-acting injectable cabotegravir or rilpivirine in the first cohort of virologically suppressed adolescents living with HIV (IMPAACT 2017/MOCHA): a secondary analysis of a phase 1/2, multicentre, open-label, non-comparative dose-finding study. Lancet HIV, 2024. 11(4): p. e222-e232. [CrossRef]
- al., M.C.e., PHASE I STUDY OF LONG-ACTING 3BNC117 AND 10-1074 IN VIREMIC ADULTS LIVING WITH HIV. 2022: Croiconference.org.
- Sáez-Cirión, A. and G. Pancino, HIV controllers: a genetically determined or inducible phenotype? Immunol Rev, 2013. 254(1): p. 281-94. [CrossRef]
- Casado, C., et al., Permanent control of HIV-1 pathogenesis in exceptional elite controllers: a model of spontaneous cure. Sci Rep, 2020. 10(1): p. 1902. [CrossRef]
- Auvert, B., et al., Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med, 2005. 2(11): p. e298. [CrossRef]
- Scott-Sheldon, L.A., et al., Efficacy of behavioral interventions to increase condom use and reduce sexually transmitted infections: a meta-analysis, 1991 to 2010. J Acquir Immune Defic Syndr, 2011. 58(5): p. 489-98. [CrossRef]
- LeMessurier, J., et al., Risk of sexual transmission of human immunodeficiency virus with antiretroviral therapy, suppressed viral load and condom use: a systematic review. Cmaj, 2018. 190(46): p. E1350-e1360. [CrossRef]
- E, O.M., et al., Oral pre-exposure prophylaxis (PrEP) to prevent HIV: a systematic review and meta-analysis of clinical effectiveness, safety, adherence and risk compensation in all populations. BMJ Open, 2022. 12(5): p. e048478. [CrossRef]
- Spinner, C.D., et al., HIV pre-exposure prophylaxis (PrEP): a review of current knowledge of oral systemic HIV PrEP in humans. Infection, 2016. 44(2): p. 151-8. [CrossRef]
- UNAIDS, Barriers to PrEP must be removed. 2019.
- Hessell, A.J., et al., Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol, 2010. 84(3): p. 1302-13. [CrossRef]
- Gautam, R., et al., A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature, 2016. 533(7601): p. 105-109. [CrossRef]
- Stephenson, K.E., et al., Vaccines and Broadly Neutralizing Antibodies for HIV-1 Prevention. Annu Rev Immunol, 2020. 38: p. 673-703. [CrossRef]
- WHO, WHO preferred product characteristics for monoclonal antibodies for HIV prevention. 2022: p. 25-26.
- Clinicaltrials.gov. Phase II Trial of ART + Dual bNAbs vs. ART + Placebo During Primary HIV-1 Infection-impact on Post-ART Control (RHIVIERA-02). 2024; Available from: https://classic.clinicaltrials.gov/ct2/show/NCT05300035.
- Lee, J.H. and S. Crotty, HIV vaccinology: 2021 update. Semin Immunol, 2021. 51: p. 101470. [CrossRef]
- Crowell, T.A., et al., Impact of antiretroviral therapy during acute or early HIV infection on virologic and immunologic outcomes: results from a multinational clinical trial. Aids, 2024. [CrossRef]
- (pih.org), P.I.H. ‘A Moral Failure’: Global Vaccine Inequity Hits Africa Hardest. 2022; Available from: https://www.pih.org/article/moral-failure-global-vaccine-inequity-hits-africa-hardest.
- Wei, C.R., S. Kamande, and G.C. Lang’at, Vaccine inequity: a threat to Africa’s recovery from COVID-19. Tropical Medicine and Health, 2023. 51(1): p. 69. [CrossRef]
- Casazza, J.P., et al., Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nat Med, 2022. 28(5): p. 1022-1030. [CrossRef]
- Priddy, F.H., et al., Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV, 2019. 6(4): p. e230-e239. [CrossRef]
- Huang, Y., et al., Engineered Bispecific Antibodies with Exquisite HIV-1-Neutralizing Activity. Cell, 2016. 165(7): p. 1621-1631. [CrossRef]
- Xu, L., et al., Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science, 2017. 358(6359): p. 85-90. [CrossRef]
- Steinhardt, J.J., et al., Rational design of a trispecific antibody targeting the HIV-1 Env with elevated anti-viral activity. Nat Commun, 2018. 9(1): p. 877. [CrossRef]
- Moshoette, T., et al., Engineering and characterising a novel, highly potent bispecific antibody iMab-CAP256 that targets HIV-1. Retrovirology, 2019. 16(1): p. 31. [CrossRef]
- Vaisman-Mentesh, A., et al., The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front Immunol, 2020. 11: p. 1951. [CrossRef]
- Visseaux, B., et al., Hiv-2 molecular epidemiology. Infect Genet Evol, 2016. 46: p. 233-240. [CrossRef]
- Esbjörnsson, J., et al., HIV-2 as a model to identify a functional HIV cure. AIDS Res Ther, 2019. 16(1): p. 24. [CrossRef]
| Clinical trial study | Year | bNAb | Study objective | Study population | Ref |
|---|---|---|---|---|---|
| (Widge et al.) **CROI 2020 |
2020 | N6LS | Safety and pharmacokinetics | HIV-1 negative individuals, n = 23 |
[55] |
| NCT04871113 | 2023 | N6LS | Safety, pharmacokinetics and antiviral activity | ART-naïve HIV-1 infected individuals, n = 63 | [56] |
| NCT01993706 (Ledgerwood et al.) |
2015 | VRC01 | Safety and pharmacokinetics | HIV-1 negative individuals, n = 28 |
[57] |
| NCT01950325 (Lynch et al.) |
2015 | VRC01 | Safety and antiviral activity incl. viral suppression and cell-associated reservoir | ART-treated (aviremic) and -naïve (viremic) chronically HIV-1 infected individuals, n = 27 |
[58] |
| NCT02463227 NCT02471326 (Bar et al.) |
2016 | VRC01 | Safety, pharmacokinetics and antiviral activity incl. viral suppression and time to rebound | Aviremic chronically HIV-1 infected individuals undergoing ATI, n = 24 | [59] |
| NCT02165267 (Mayer et al.) |
2017 | VRC01 | Safety, pharmacokinetics | At low-risk HIV-1 negative individuals, n = 88 | [60] |
| NCT02411539 (Riddler et al.) |
2018 | VRC01 | Safety, pharmacokinetics and antiviral activity incl. viral suppression and cell-associated reservoir | ART-treated chronically HIV-1 infected individuals, n = 40 | [61] |
| NCT02664415 (Crowell et al.) |
2019 | VRC01 | Safety and antiviral activity | Acutely ART-treated and virally suppressed HIV-1 infected individuals undergoing ATI, n = 23 | [62] |
| NCT02797171 (*not published) |
2019 | VRC01 |
Safety, pharmacokinetics and antiviral activity | HIV-1 negative individuals n = 80 |
[72] |
| (Cale et al.) | 2020 | VRC01 | Antiviral activity incl. viral rebound | ART-treated, durably suppressed HIV-1 infected individuals undergoing ATI, n = 18 | [73] |
| NCT02716675 NCT02568215 (Corey et al.) |
2021 | VRC01 | Prevention efficacy | At-risk HIV-1 negative cis-gender men, transgender individuals (n = 2699) and women from sub-Saharan Africa (n = 1924) | [74] |
| NCT02599896 (Gaudinski et al.) |
2018 | VRC01-LS | Safety and pharmacokinetics | HIV-1 negative individuals n = 37 |
[63] |
| NCT02840474 (Chen et al.) |
2021 | VRC01-LS /VRC07-523LS |
Safety, pharmacokinetics and antiviral activity | Viremic HIV-1 infected individuals, n = 16 | [64] |
| NCT03015181 (Gaudinski et al.) |
2019 | VRC07-523LS | Safety and pharmacokinetics | HIV-1 negative individuals, n = 26 |
[65] |
| NCT02018510 (Caskey et al.) |
2015 | 3BNC117 | Safety and pharmacokinetics | Asymptomatic, ART-naive HIV-1 infected individuals, n = 7 |
[66] |
| NCT02446847 (Scheid et al.) |
2016 | 3BNC117 | Safety and antiviral activity incl. delay in viral rebound | ART-treated HIV-1 infected individuals with 3BNC117-sensitivity undergoing ATI, n = 13 | [67] |
| NCT02018510 (Schoofs et al.) |
2016 | 3BNC117 | Antiviral activity incl. neutralization efficacy | Viremic HIV-1 infected individuals with 3BNC117-sensitive strains (8/9), n = 9 | [75] |
| NCT02588586 (Cohen et al.) |
2018 | 3BNC117 | Safety, pharmacokinetics and antiviral activity | ART-treated HIV-1 infected individuals undergoing ATI, n = 15 |
[68] |
| NCT02511990 (Caskey et al.) |
2017 | 10-1074 | Safety, pharmacokinetics and antiviral activity | Viremic HIV-1 infected individuals, n = 33 | [69] |
| NCT02960581 (Stephenson et al.) |
2021 | PGT121 | Safety, pharmacokinetics and antiviral activity | Viremic and ART-naïve HIV-1 infected individuals, n = 48 ART-treated HIV-1 infected individuals, n = 12 |
[70] |
| Clinical trial study | Year | bNAb | Study objective | Study population | Ref |
|---|---|---|---|---|---|
| NCT02850016 (Gruell et al.) |
2022 | 3BNC117 + romidepsin |
Safety, efficacy on plasma viremia and latent reservoir | ART-treated HIV-1 infected individuals , n = 20 | [91] |
| NCT03803605 (Gay et al.) |
2022 | VRC07-523-LS + vorinostat + ART |
Safety and effects on viral reservoir |
ART-treated HIV-1 infected individuals, n = 8 | [92] |
| NCT03041012 (Gunst et al.) |
2022 | 3BNC117 + romidepsin + ART |
Safety, efficacy on plasma viremia and latent reservoir | Early diagnosed HIV-1 individuals initiating ART, n = 55 |
[93] |
| NCT03588715 (Tebas et al.) **CROI 2023 |
2022 | 3BNC117 + 10-1074 + pegylated-interferon Alpha2b |
Safety and effects on viral replication and rebound | ART-treated HIV-1 infected individuals undergoing ATI, n = 14 |
[95] |
| NCT03837756 (Gunst et al.) |
2023 | 3BNC117 + 10-1074 + lefitolimod |
Safety, tolerability and time to loss of virological control after ATI |
HIV-1 infected individuals on ART undergoing ATI at week 3, n = 43 | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




