Submitted:
02 May 2024
Posted:
02 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation
2.2. Fabrication of Ceramic Composites
2.3. Characterization
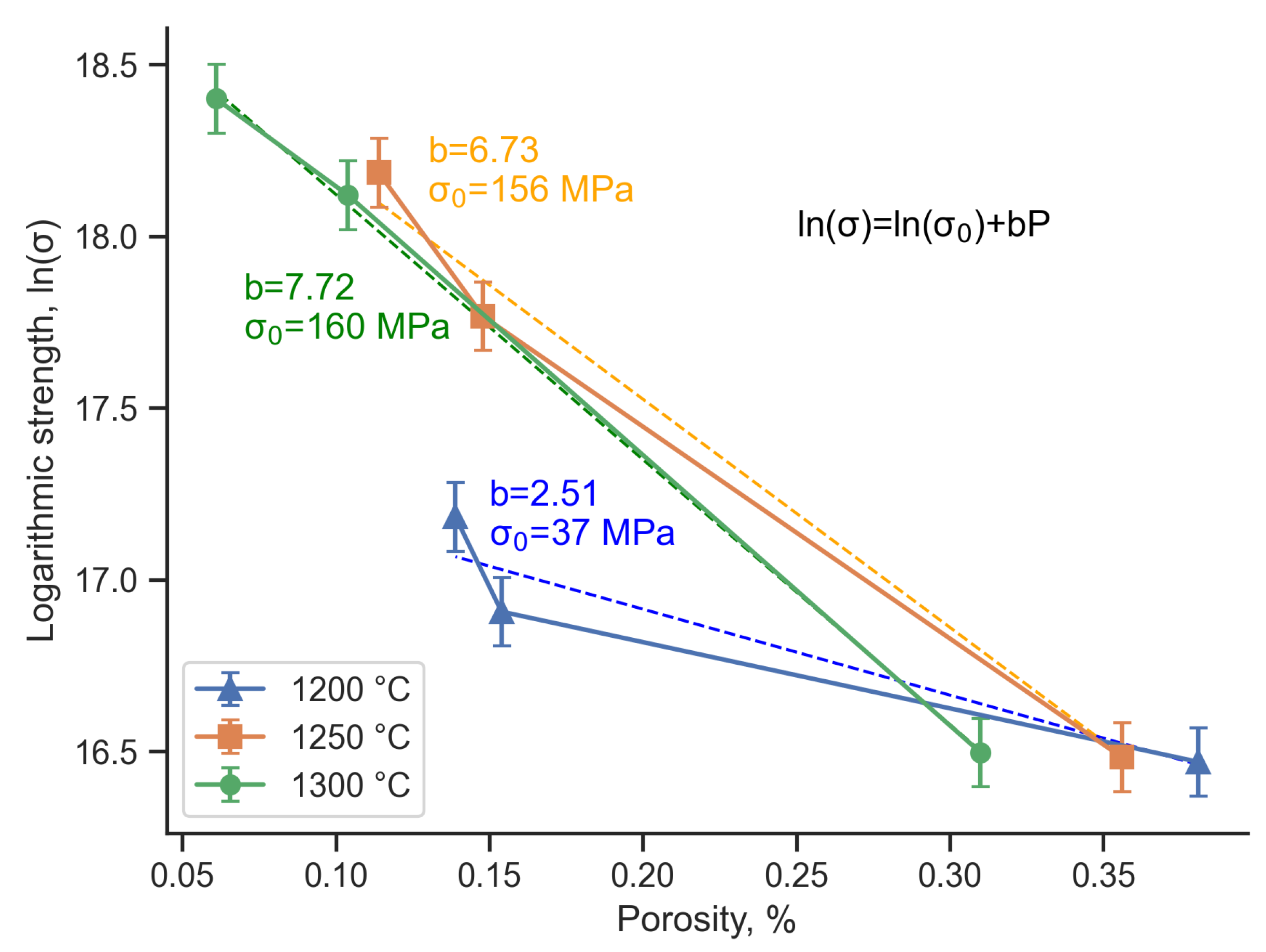
3. Results and Discussion
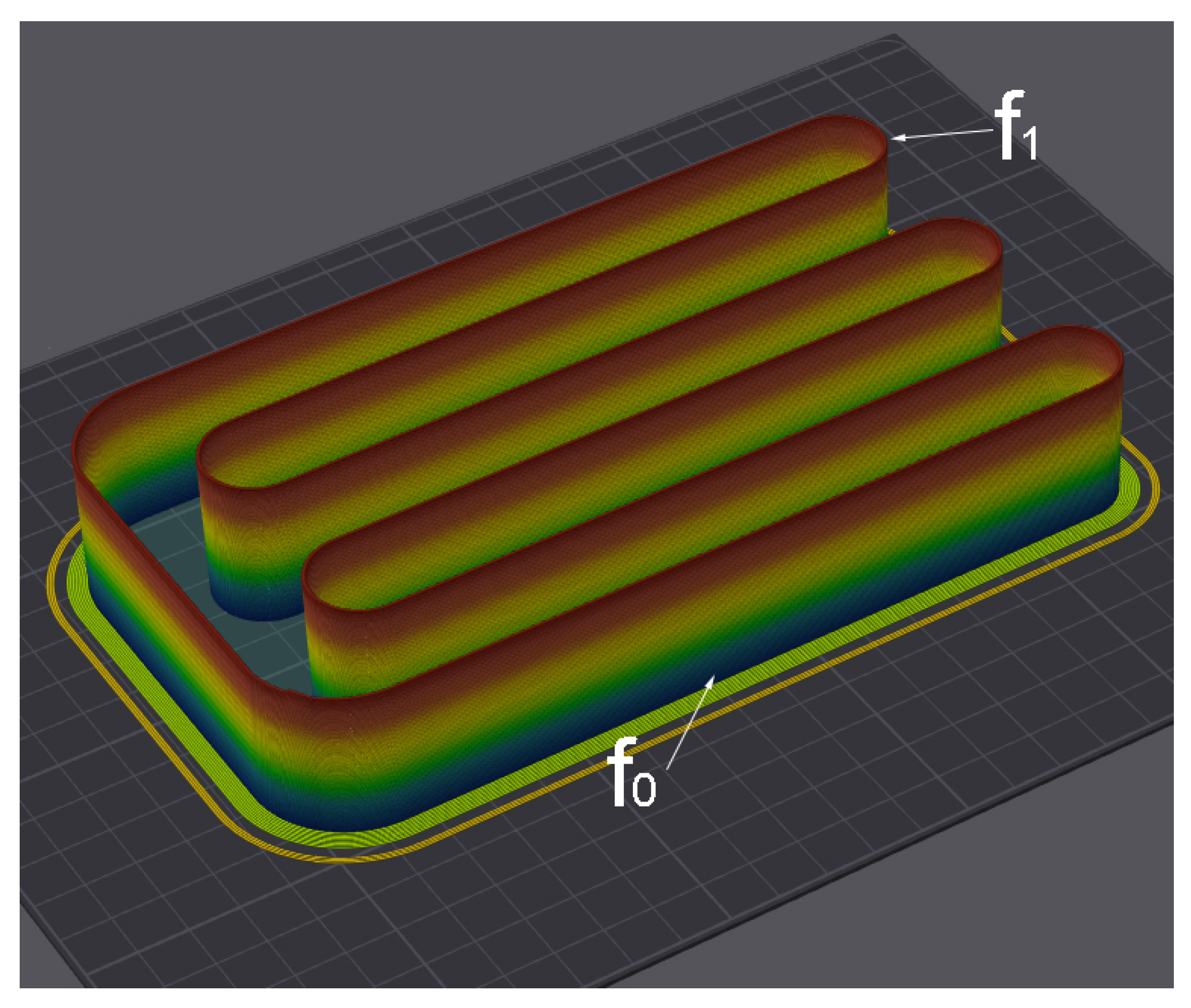
3.1. Parameters of Additive Manufacturing
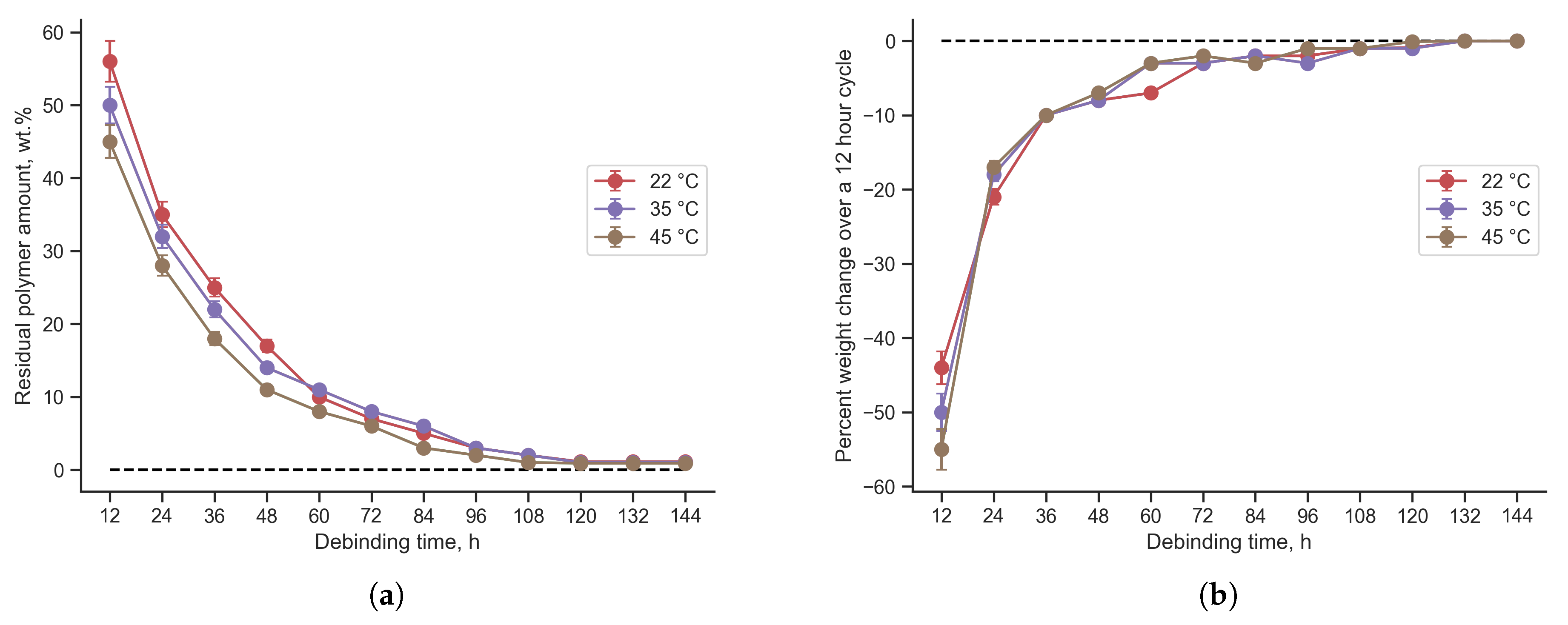
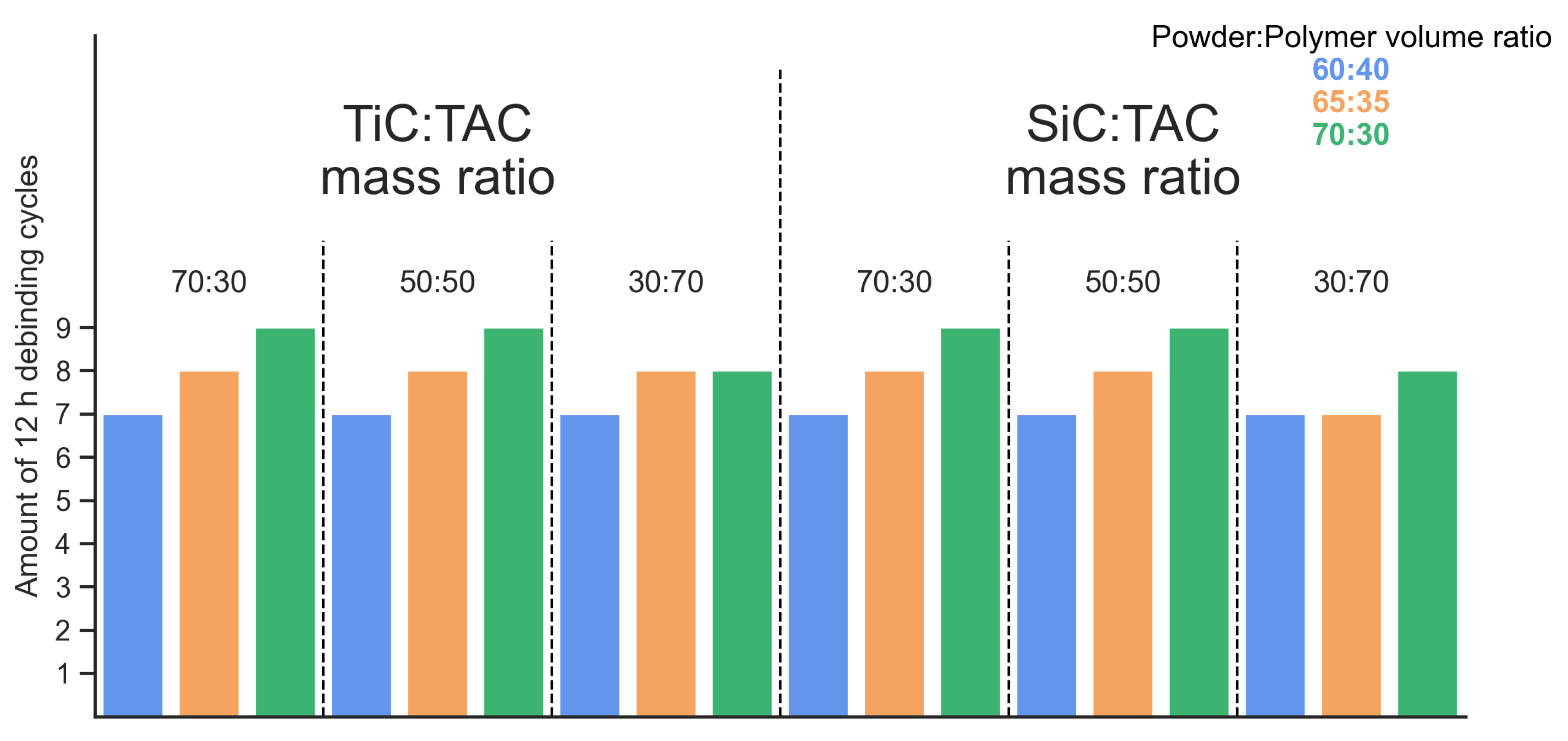
3.2. Debinding
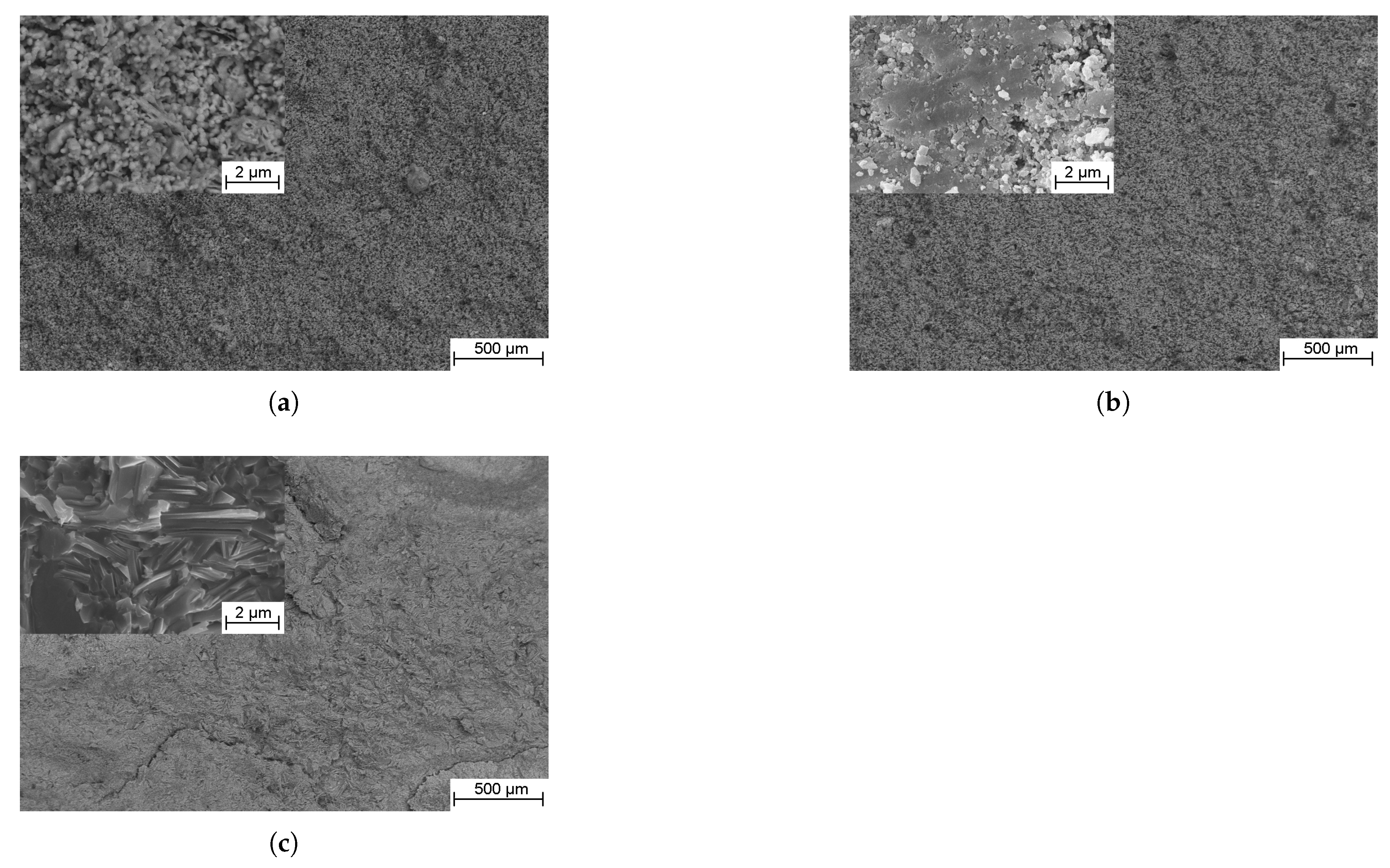
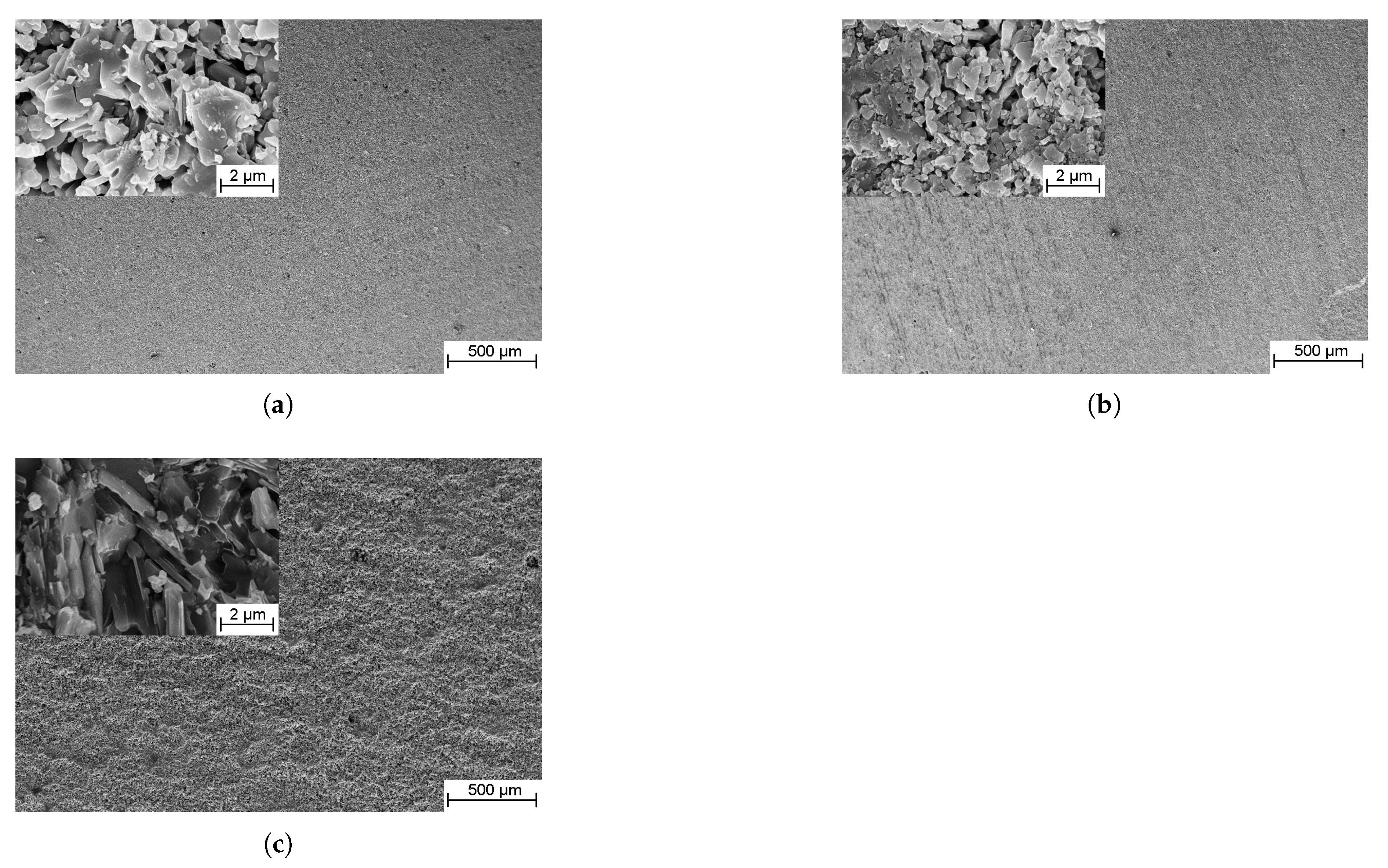
3.3. Structure, Phase Composition and Mechanical Properties
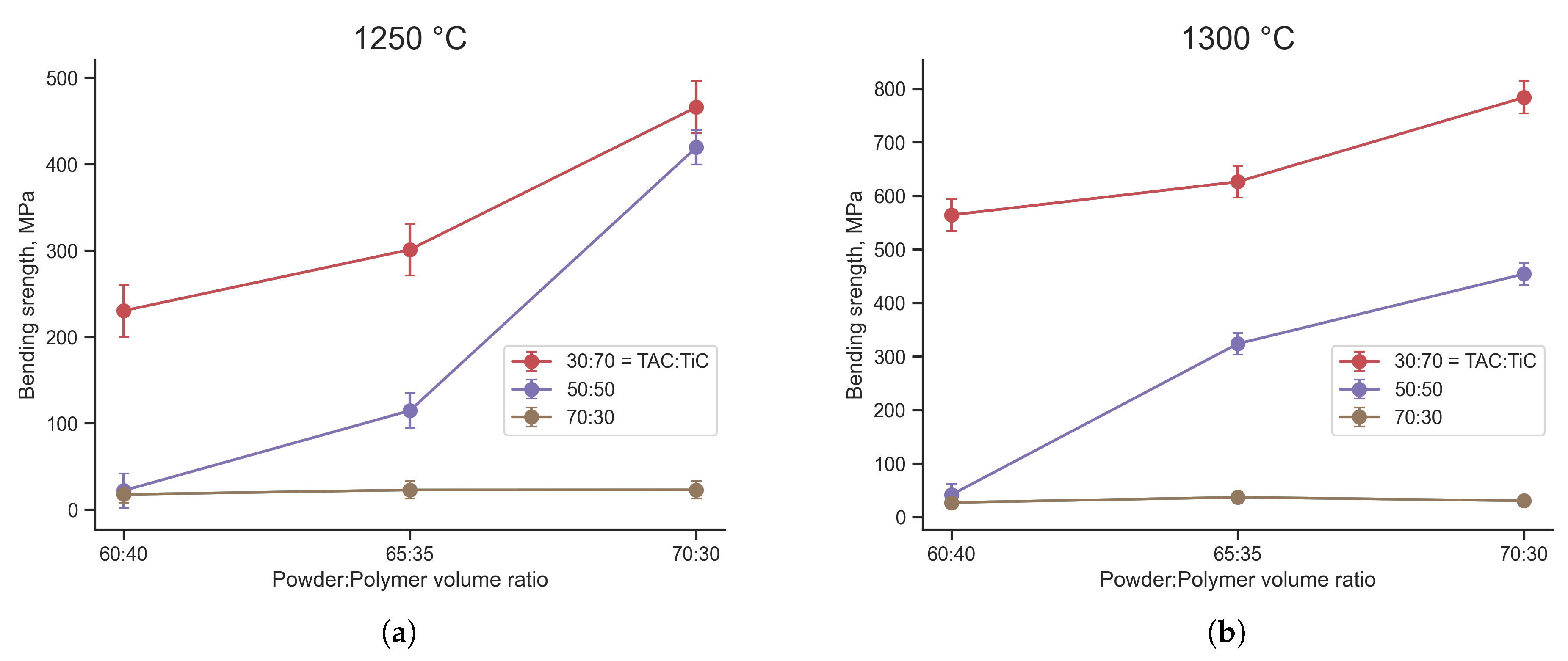
3.3.1. TiC-Containing Composites
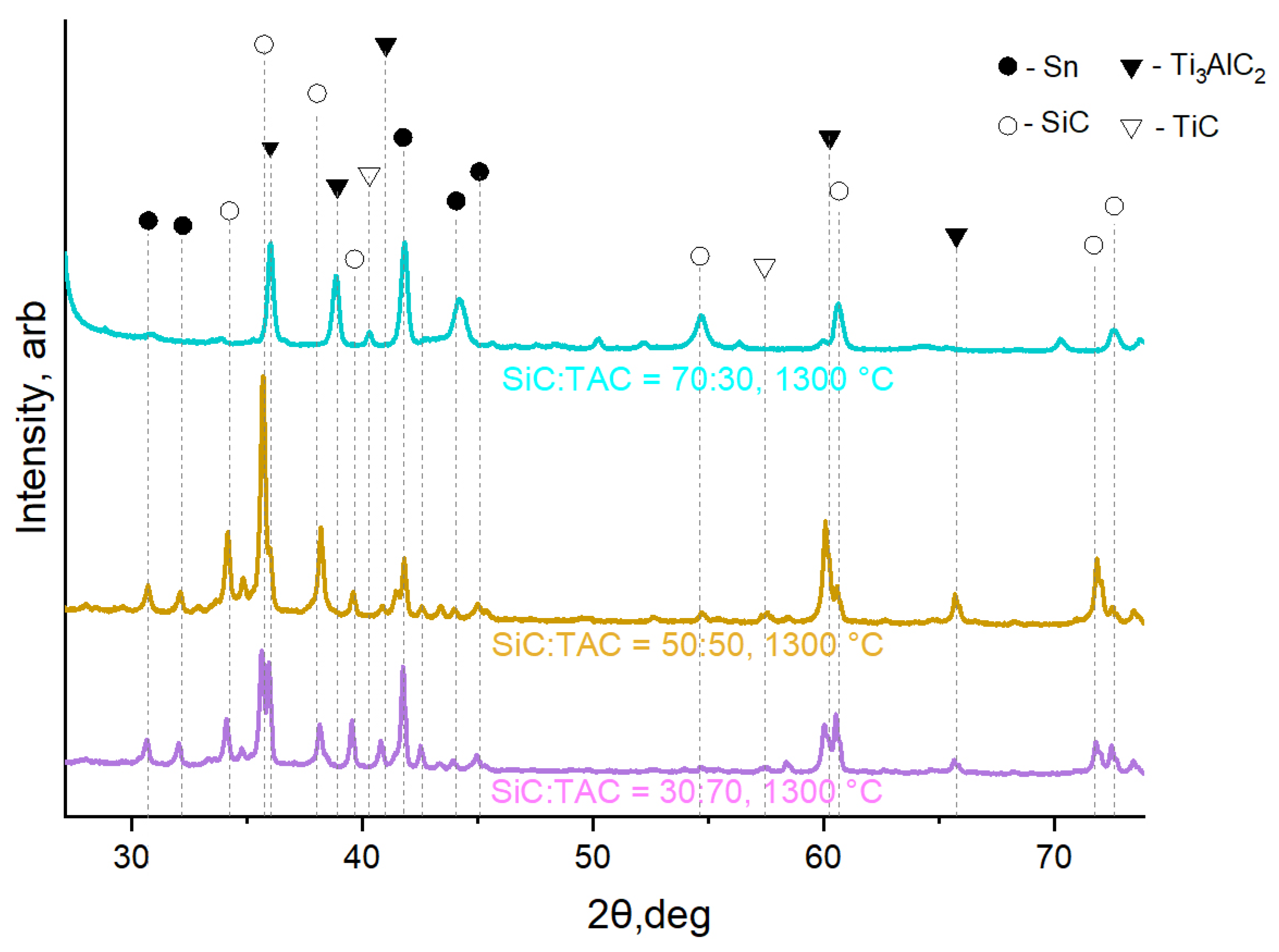
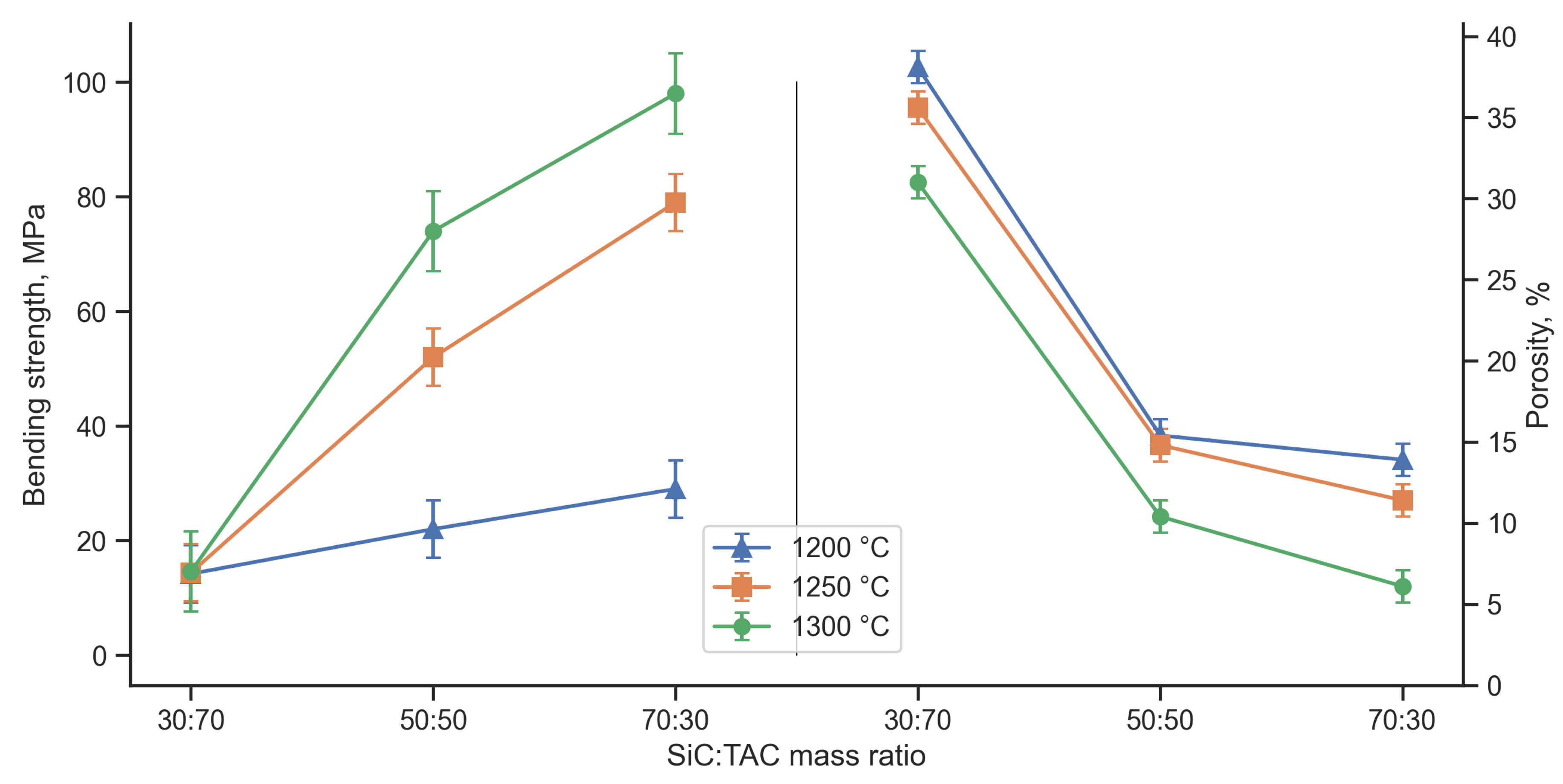
3.3.2. SiC-Containing Composites
4. Conclusions
- -
- FGF-samples of TiC:TAC and SiC:TAC composites were succesfully printed without any macroscopic printing defects. Minimum debinding time for samples with mm cross-section that is enough to remove 99% of polymer is 84 hours. The optimal sintering temperature to form the best mechanical properties for both TiC:TAC and SiC:TAC composites is 1300 °C.
- -
- Both SiC:TAC and TiC:TAC samples are porous and the more TAC is in the sample the more porous it is. Thus, TAC content, sintering temperature, and polymer content in the feedstock are the main parameters that regulates the porosity of FGF samples.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.X.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; others. Advances in ultra-high temperature ceramics, composites, and coatings. Journal of Advanced Ceramics 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Sun, J.; Ye, D.; Zou, J.; Chen, X.; Wang, Y.; Yuan, J.; Liang, H.; Qu, H.; Binner, J.; Bai, J. A review on additive manufacturing of ceramic matrix composites. Journal of Materials Science & Technology 2023, 138, 1–16. [Google Scholar]
- Qinglong, A.; Jie, C.; Weiwei, M.; Ming, C. Machining of SiC ceramic matrix composites: A review. Chinese Journal of Aeronautics 2021, 34, 540–567. [Google Scholar]
- Wei, S.; Xu, B.Q.; Bin, Y.; Sun, H.Y.; Song, J.X.; Wan, H.L.; Dai, Y.N. Preparation of TiC powders by carbothermal reduction method in vacuum. Transactions of Nonferrous Metals Society of China 2011, 21, 185–190. [Google Scholar]
- Zhang, H.; Li, F.; Jia, Q.; Ye, G. Preparation of titanium carbide powders by sol–gel and microwave carbothermal reduction methods at low temperature. Journal of Sol-Gel Science and Technology 2008, 46, 217–222. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Delbari, S.A.; Asl, M.S.; Van Le, Q.; Shokouhimehr, M.; Namini, A.S.; Mohammadi, M. Microstructure–property correlation in nano-diamond and TiN added TiC-based ceramics. Ceramics International 2021, 47, 449–460. [Google Scholar] [CrossRef]
- Ishizaki, K.; Komarneni, S.; Nanko, M. Porous Materials: Process technology and applications; Vol. 4, Springer science & business media, 2013.
- Choyke, W.; Pensl, G. Physical properties of SiC. Mrs Bulletin 1997, 22, 25–29. [Google Scholar] [CrossRef]
- Snead, L.L.; Nozawa, T.; Katoh, Y.; Byun, T.S.; Kondo, S.; Petti, D.A. Handbook of SiC properties for fuel performance modeling. Journal of nuclear materials 2007, 371, 329–377. [Google Scholar] [CrossRef]
- Ishizaki, K.; Komarneni, S.; Nanko, M. Porous Materials: Process technology and applications; Vol. 4, Springer science & business media, 2013.
- Taslicukur, Z.; Balaban, C.; Kuskonmaz, N. Production of ceramic foam filters for molten metal filtration using expanded polystyrene. Journal of the European Ceramic Society 2007, 27, 637–640. [Google Scholar] [CrossRef]
- Sun, B.; Fan, T.; Zhang, D. Porous TiC ceramics derived from wood template. Journal of Porous Materials 2002, 9, 275–277. [Google Scholar] [CrossRef]
- Gringoz, A.; Glandut, N.; Valette, S. Electrochemical hydrogen storage in TiC0. 6, not in TiC0. 9. Electrochemistry Communications 2009, 11, 2044–2047. [Google Scholar] [CrossRef]
- Sang, K.; Lü, Z.; Jin, Z. A study of the SiC-L composite ceramics for self-lubrication. Wear 2002, 253, 1188–1193. [Google Scholar] [CrossRef]
- Gao, F.; Lu, J.; Liu, W. Tribological behavior of carbide-derived carbon coating on SiC polycrystal against SAE52100 steel in moderately humid air. Tribology Letters 2007, 27, 339–345. [Google Scholar] [CrossRef]
- Barsoum, M.W. The MN+ 1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Progress in solid state chemistry 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Barsoum, M.W. The MN+ 1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Progress in solid state chemistry 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Li, S.B.; Zhai, H.X.; Bei, G.P.; Zhou, Y.; Zhang, Z.L. Synthesis and microstructure of Ti3AlC2 by mechanically activated sintering of elemental powders. Ceramics international 2007, 33, 169–173. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y. Stability and selective oxidation of aluminum in nano-laminate Ti3AlC2 upon heating in argon. Chemistry of materials 2003, 15, 3716–3720. [Google Scholar] [CrossRef]
- Pang, W.K.; Low, I.M.; Sun, Z.M. In situ high-temperature diffraction study of the thermal dissociation of Ti3AlC2 in vacuum. Journal of the American Ceramic Society 2010, 93, 2871–2876. [Google Scholar] [CrossRef]
- Ruan, M.M.; Feng, X.M.; Ai, T.T.; Yu, N.; Hua, K. Microstructure and mechanical properties of TiC/Ti3AlC2 in situ composites prepared by hot pressing method. Materials Science Forum. Trans Tech Publ, 2015, Vol. 816, pp. 200–204.
- Chen, W.; Chen, J.; Zhu, M.; Zheng, J.; Ma, N.n.; Liu, X.; Chen, Z.; Huang, Z. Fabrication of SiC ceramics with invariable value resistivity in the range of 20–400℃ using MAX phase-Ti3AlC2 additives. Journal of the European Ceramic Society 2021, 41, 6248–6254. [Google Scholar] [CrossRef]
- Mingxing, A.; Hongxiang, Z.; Yang, Z.; Zhaoyun, T.; Zhenying, H.; Zhili, Z.; Shibo, L. Synthesis of Ti3AlC2 powders using Sn as an additive. Journal of the American Ceramic Society 2006, 89, 1114–1117. [Google Scholar] [CrossRef]
- Vasanthakumar, K.; Bakshi, S.R. Effect of C/Ti ratio on densification, microstructure and mechanical properties of TiCx prepared by reactive spark plasma sintering. Ceramics International 2018, 44, 484–494. [Google Scholar] [CrossRef]
- Panigrahi, B.B.; Gracio, J.J.; Chu, M.C.; Cho, S.J.; Reddy, N.S. Powder Synthesis, Sintering Kinetics, and Nickel-Activated Pressureless Sintering of Ti3AlC2. International Journal of Applied Ceramic Technology 2010, 7, 752–759. [Google Scholar] [CrossRef]
- Omori, M.; Takei, H. Pressureless sintering of SiC. Journal of the American Ceramic Society 1982, 65, c92–c92. [Google Scholar] [CrossRef]
- Raju, K.; Yoon, D.H. Sintering additives for SiC based on the reactivity: A review. Ceramics International 2016, 42, 17947–17962. [Google Scholar] [CrossRef]
- TANAKA, H. Sintering of silicon carbide and theory of sintering. Journal of the Ceramic Society of Japan 2002, 110, 877–883. [Google Scholar] [CrossRef]
- Chi, W.; Jiang, D.; Huang, Z.; Tan, S. Sintering behavior of porous SiC ceramics. Ceramics International 2004, 30, 869–874. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Z.; Wang, X.; Chen, S. Ab initio geometry optimization and ground state properties of layered ternary carbides Ti3MC2 (M= Al, Si and Ge). Journal of Physics: Condensed Matter 2001, 13, 10001. [Google Scholar]
- Zhang, H.; Wang, S. First-principles study of Ti3AC2 (A= Si, Al)(0 0 1) surfaces. Acta Materialia 2007, 55, 4645–4655. [Google Scholar] [CrossRef]
- Kleykamp, H. Gibbs energy of formation of SiC: A contribution to the thermodynamic stability of the modifications. Berichte der Bunsengesellschaft für physikalische Chemie 1998, 102, 1231–1234. [Google Scholar] [CrossRef]
- Zhu, C.C.; Zhu, J.; Wu, H.; Lin, H. Synthesis of Ti 3 AlC 2 by SHS and thermodynamic calculation based on first principles. Rare Metals 2015, 34, 107–110. [Google Scholar] [CrossRef]
- Rice, R. Evaluation and extension of physical property-porosity models based on minimum solid area. Journal of materials science 1996, 31, 102–118. [Google Scholar] [CrossRef]
- Rice, R. Comparison of physical property-porosity behaviour with minimum solid area models. Journal of Materials Science 1996, 31, 1509–1528. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Li, J. Factors that influence the flexural strength of SiC-based porous ceramics used for hot gas filter support. Journal of the European Ceramic Society 2011, 31, 825–831. [Google Scholar] [CrossRef]
- Salvo, M.; Rizzo, S.; Casalegno, V.; Handrick, K.; Ferraris, M. Shear and bending strength of SiC/SiC joined by a modified commercial adhesive. International Journal of Applied Ceramic Technology 2012, 9, 778–785. [Google Scholar] [CrossRef]
- Vargas-Gonzalez, L.; Speyer, R.F.; Campbell, J. Flexural strength, fracture toughness, and hardness of silicon carbide and boron carbide armor ceramics. International Journal of Applied Ceramic Technology 2010, 7, 643–651. [Google Scholar] [CrossRef]
- Gulden, T. Mechanical Properties of Polycrystalline β-SiC. Journal of the American Ceramic Society 1969, 52, 585–590. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




