Submitted:
29 April 2024
Posted:
30 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Examinations, Biochemical Analysis, and Treatments
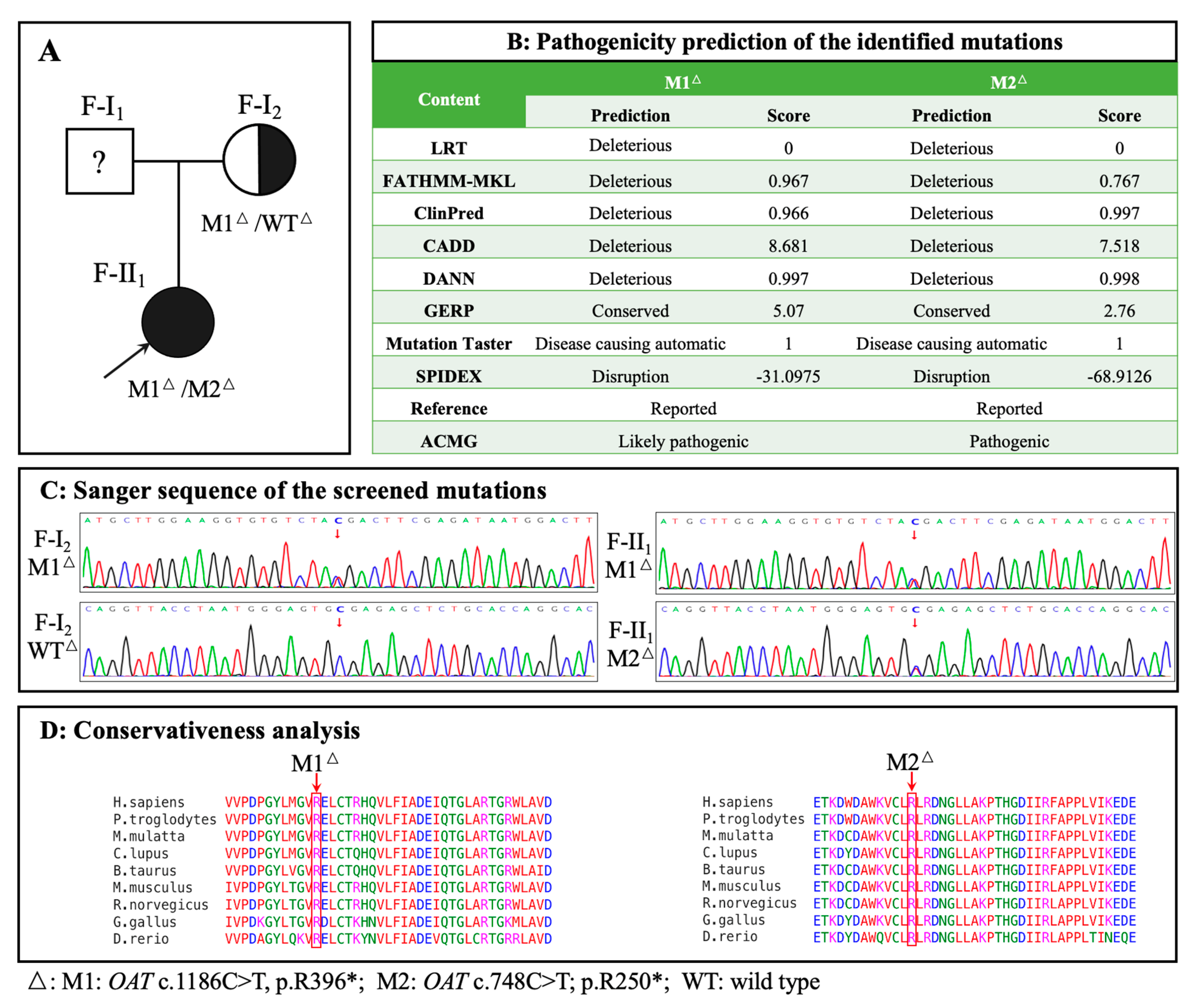
2.3. Genetic Testing and Pathogenicity Assessment
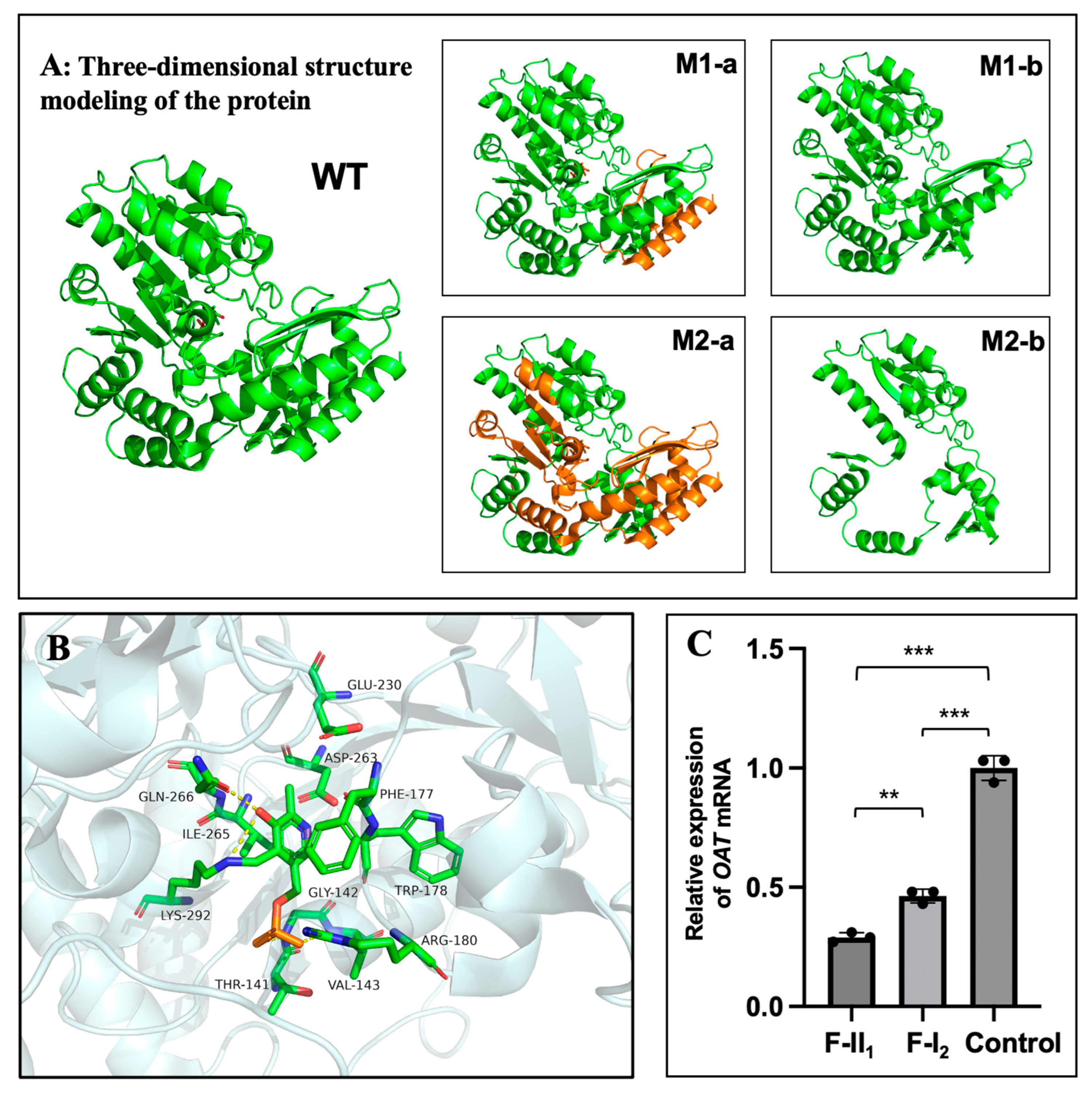
2.4. In Silico Analysis and Protein Structure Modelling
2.5. OAT mRNA Analysis
2.6. Statistical Analyses
3. Results
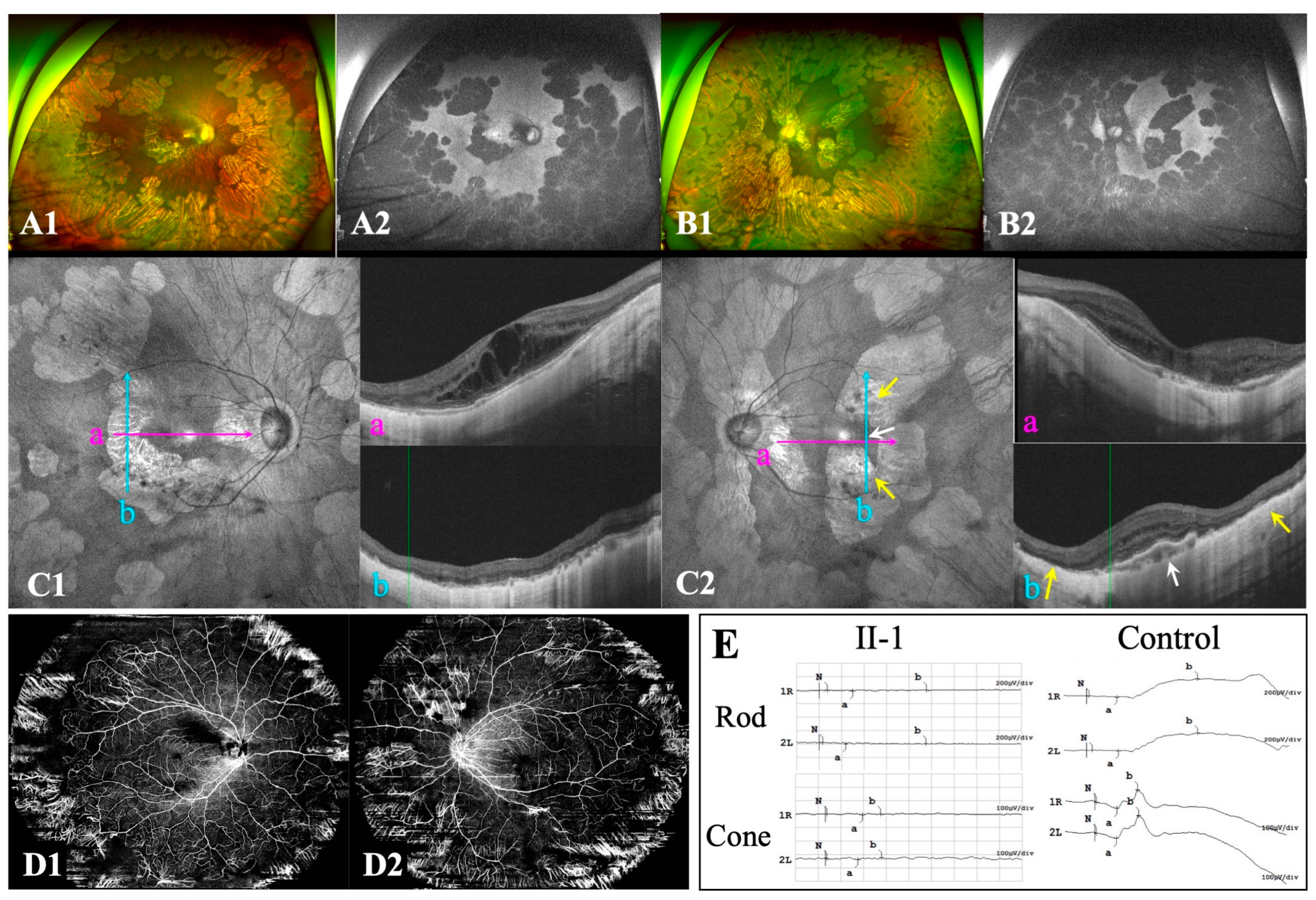
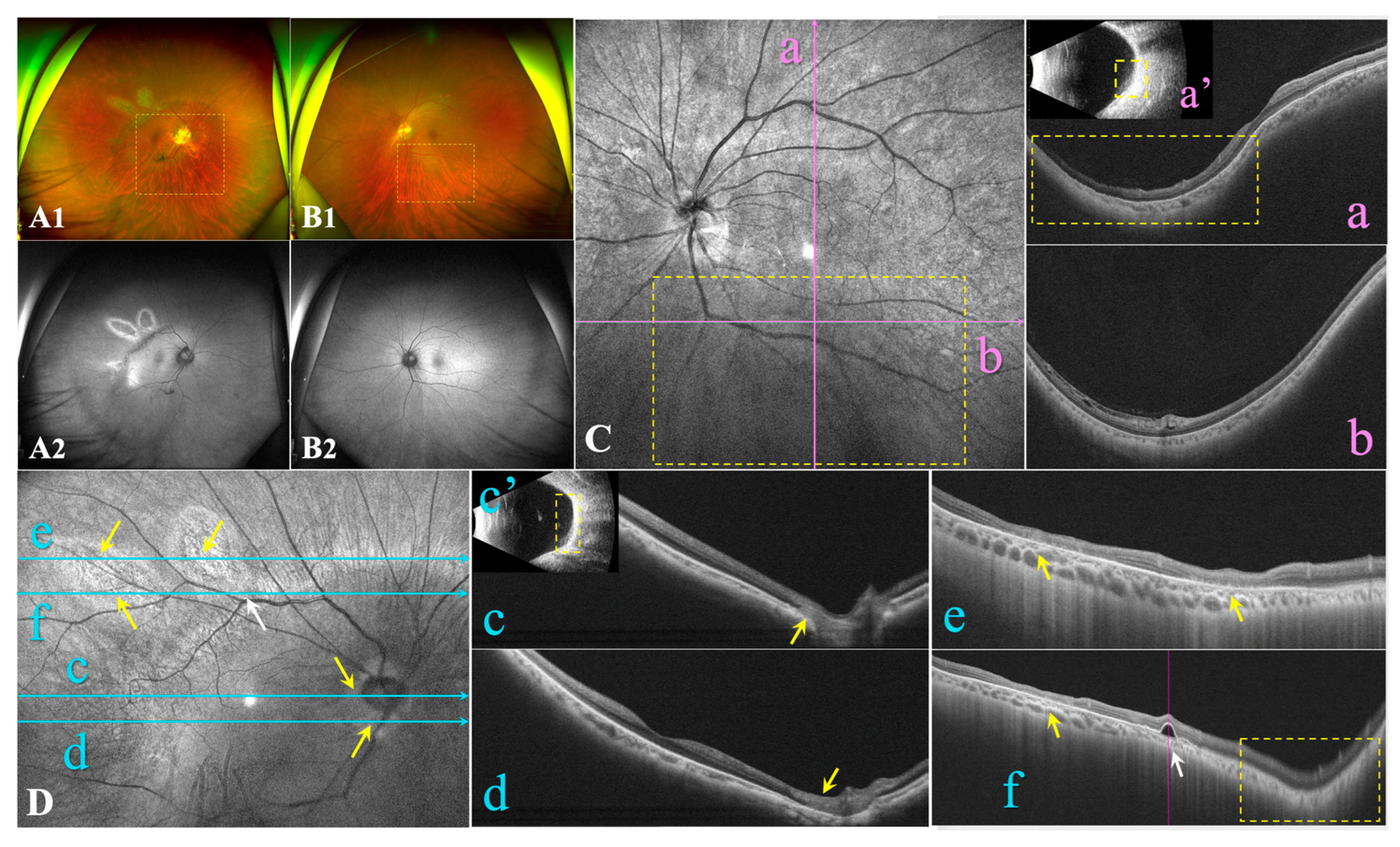
3.1. Ocular Characteristics
3.2. Hematologic Biochemical Findings
3.3. Genetic Findings
3.4. OAT mRNA Detection
3.5. Treatment and Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsang, S.H.; Aycinena, A.R.P.; Sharma, T. Inborn errors of metabolism: Gyrate atrophy. Adv Exp Med Biol 2018, 1085, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Montioli, R.; Bellezza, I.; Desbats, M.A.; Borri Voltattorni, C.; Salviati, L.; Cellini, B. Deficit of human ornithine aminotransferase in gyrate atrophy: Molecular, cellular, and clinical aspects. Biochim Biophys Acta Proteins Proteom 2021, 1869, 140555. [Google Scholar] [CrossRef]
- Ramesh, V.; Benoit, L.A.; Crawford, P.; Harvey, P.T.; Shows, T.B.; Shih, V.E.; Gusella, J.F. The ornithine aminotransferase (OAT) locus: Analysis of RFLPs in gyrate atrophy. Am J Hum Genet 1988, 42, 365–372. [Google Scholar] [PubMed]
- Kaiser-Kupfer, M.I.; Valle, D.; Del Valle, L.A. A specific enzyme defect in gyrate atrophy. Am J Ophthalmol 1978, 85, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, S.; Kodama, T.; Ohira, A. Retinal risks of high-dose ornithine supplements: A review. Br J Nutr 2011, 106, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Peltola, K.E.; Näntö-Salonen, K.; Heinonen, O.J.; Jääskeläinen, S.; Heinänen, K.; Simell, O.; Nikoskelainen, E. Ophthalmologic heterogeneity in subjects with gyrate atrophy of choroid and retina harboring the L402P mutation of ornithine aminotransferase. Ophthalmology 2001, 108, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, S.; Gekka, T.; Hayashi, T.; Ida, H.; Ohashi, T.; Eto, Y.; Tsuneoka, H. OAT mutations and clinical features in two Japanese brothers with gyrate atrophy of the choroid and retina. Doc Ophthalmol 2014, 128, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Elnahry, A.G.; Tripathy, K.; Foster, R.E.; Mehanna, C.J.; Vishal, R.; Çavdarlı, C.; Arrigo, A.; Parodi, M.B. Analysis of optical coherence angiography in cystoid macular oedema associated with gyrate atrophy. Eye (Lond) 2021, 35, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, K.; Chawla, R.; Sharma, Y.R.; Gogia, V. Ultrawide field fluorescein angiogram in a family with gyrate atrophy and foveoschisis. Oman J Ophthalmol 2016, 9, 104–106. [Google Scholar] [CrossRef]
- Kaiser-Kupfer, M.I.; de Monasterio, F.; Valle, D.; Walser, M.; Brusilow, S. Visual results of a long-term trial of a low-arginine diet in gyrate atrophy of choroid and retina. Ophthalmology 1981, 88, 307–310. [Google Scholar] [CrossRef]
- McInnes, R.R.; Arshinoff, S.A.; Bell, L.; McCulloch, C. Treatment of gyrate atrophy of the choroid and retina with low arginine diet. Trans Am Ophthalmol Soc 1980, 78, 226–242. [Google Scholar] [PubMed]
- Valle, D.; Walser, M.; Brusilow, S.W.; Kaiser-Kupfer, M. Gyrate atrophy of the choroid and retina: Amino acid metabolism and correction of hyperornithinemia with an arginine-deficient diet. J Clin Invest 1980, 65, 371–378. [Google Scholar] [CrossRef]
- Hames, A.; Khan, S.; Gilliland, C.; Goldman, L.; Lo, H.W.; Magda, K.; Keathley, J. Carriers of autosomal recessive conditions: Are they really “unaffected? ” J Med Genet 2023, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R.; et al. The genetic landscape and epidemiology of phenylketonuria. Am J Hum Genet 2020, 107, 234–250. [Google Scholar] [CrossRef] [PubMed]
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat Rev Dis Primers 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Polgreen, P.M.; Comellas, A.P. Clinical phenotypes of cystic fibrosis carriers. Annu Rev Med 2022, 73, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, M.; ter Rahe, B.; van Aarem, A.; Huygen, P.; Admiraal, R.; Bleeker-Wagemakers, E.; Pinckers, A.; Kimberling, W.; Cremers, C. Clinical findings in obligate carriers of type I Usher syndrome. Am J Med Genet 1995, 59, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Matsui, K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology 2014, 121, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Curtin, B.J. The posterior staphyloma of pathologic myopia. Trans Am Ophthalmol Soc 1977, 75, 67–86. [Google Scholar]
- Bergen, A.A.; Buijs, M.J.; Ten Asbroek, A.L.; Balfoort, B.M.; Boon, C.J.; Dutch GACR “Bird’s Eye View” Consortium; Brands, M.M.; Wanders, R.J.; van Karnebeek, C.D.; Houtkooper, R.H. Vision on gyrate atrophy: Why treat the eye? EMBO Mol Med 2024, 16, 4–7. [Google Scholar] [CrossRef]
- Ju, Y.; Zhang, L.; Gao, F.; Zong, Y.; Chen, T.; Ruan, L.; Chang, Q.; Zhang, T.; Huang, X. Genetic characteristics and clinical manifestations of foveal hypoplasia in familial exudative vitreoretinopathy. Am J Ophthalmol 2024, 262, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Liao, K.; Zhao, Y.; Long, Z.; Zhu, S.; Wu, J.; Xiao, M.; Zhou, J.; Zhang, S.; Li, L.; et al. A novel system for the detection of spontaneous abortion-causing aneuploidy and its erroneous chromosome origins through the combination of low-pass copy number variation sequencing and NGS-based STR tests. J Clin Med 2023, 12, 1809. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ohnaka, M.; Okuda-Ashitaka, E.; Kaneko, S.; Ando, A.; Maeda, M.; Furuta, K.; Suzuki, M.; Takahashi, K.; Ito, S. Induction of arginase II mRNA by nitric oxide using an in vitro model of gyrate atrophy of choroid and retina. Invest Ophthalmol Vis Sci 2011, 52, 1493–1500. [Google Scholar] [CrossRef]
- Sergouniotis, P.I.; Davidson, A.E.; Lenassi, E.; Devery, S.R.; Moore, A.T.; Webster, A.R. Retinal structure, function, and molecular pathologic features in gyrate atrophy. Ophthalmology 2012, 119, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Tauqeer, Z.; Yonekawa, Y. Familial exudative vitreoretinopathy: Pathophysiology, diagnosis, and management. Asia Pac J Ophthalmol (Phila) 2018, 7, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Snead, M.P.; Yates, J.R. Clinical and molecular genetics of Stickler syndrome. J Med Genet 1999, 36, 353–359. [Google Scholar] [CrossRef]
- Araújo, J.R.; Tavares-Ferreira, J.; Estrela-Silva, S.; Rocha, P.; Brandão, E.; Faria, P.A.; Falcão-Reis, F.; Rocha-Sousa, A. Wagner syndrome: Anatomic, functional and genetic characterization of a Portuguese family. Graefes Arch Clin Exp Ophthalmol 2018, 256, 163–171. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Jonas, J.B. Posterior staphyloma in pathologic myopia. Prog Retin Eye Res 2019, 70, 99–109. [Google Scholar] [CrossRef]
- Komori, S.; Ueno, S.; Ito, Y.; Sayo, A.; Meinert, M.; Kominami, T.; Inooka, D.; Kitagawa, M.; Nishida, K.; Takahashi, K.; et al. Steeper macular curvature in eyes with non-highly myopic retinitis pigmentosa. Invest Ophthalmol Vis Sci 2019, 60, 3135–3141. [Google Scholar] [CrossRef] [PubMed]
- El Matri, L.; Falfoul, Y.; El Matri, K.; El Euch, I.; Ghali, H.; Habibi, I.; Hassairi, A.; Chaker, N.; Schorderet, D.; Chebil, A. Posterior staphylomas in non-highly myopic eyes with retinitis pigmentosa. Int Ophthalmol 2020, 40, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.C.; Comellas, A.P.; Hornick, D.B.; Stoltz, D.A.; Cavanaugh, J.E.; Gerke, A.K.; Welsh, M.J.; Zabner, J.; Polgreen, P.M. Cystic fibrosis carriers are at increased risk for a wide range of cystic fibrosis-related conditions. Proc Natl Acad Sci U S A 2020, 117, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Keathley, J.; Garneau, V.; Zavala-Mora, D.; Heister, R.R.; Gauthier, E.; Morin-Bernier, J.; Green, R.; Vohl, M.C. A systematic review and recommendations around frameworks for evaluating scientific validity in nutritional genomics. Front Nutr 2021, 8, 789215. [Google Scholar] [CrossRef]
- Karousis, E.D.; Mühlemann, O. Nonsense-mediated mRNA decay begins where translation ends. Cold Spring Harb Perspect Biol 2019, 11, a032862. [Google Scholar] [CrossRef]
| II-1 | I-2 | ||||
| Age (years) | 19 | 54 | |||
| Chief complaint | Decreased vision bilaterally | High myopia and fluttering dark shadows in the right eye | |||
| Ophthalmic parameters |
OD | OS | OD | OS | |
| IOP (mmHg) | 12.6 | 12.4 | 14.5 | 13.6 | |
| AL (mm) | 29.40 | 29.40 | 26.45 | 24.52 | |
| SE→BCVA | -17.00D→20/66 | -18.25D→20/66 | -9.00D→20/28 | -2.75D→20/28 | |
| Ocular manifestations | Punctate posterior subcapsular cataract | / | Nuclear cataract | Nuclear cataract | |
| Bilateral high myopia Bilateral PS (Type 1) Bilateral sharply demarcated circular areas of peripheral chorioretinal atrophy Bilateral macular atrophy |
High myopia PS (Type 1) Focal circular areas of retinal atrophy / |
Mild myopia Inferior PS (Type 5) / / |
|||
| Haematological testing (μM) | Serum value | Serum value | Reference | ||
| Ornithine | 257.92↑ | 102.08↑ | 10-100 | ||
| Creatine | 94.83↓ | 121.75 | 95-1000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





