Submitted:
29 April 2024
Posted:
29 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Varicella Vaccines
3. MAV/06 Varicella Vaccines
3.1. Development History
3.1.1. Isolation of MAV Strain
3.1.2. Attenuation of MAV/06 Strain
3.1.3. Genomic characteristics of MAV/06 Strain
- Genotyping
| Strain | Accession Number | Full Length (bp) | Length (bp) | |||||
|---|---|---|---|---|---|---|---|---|
| TRL1 | UL2 | IRL3 | IRS4 | US5 | TRS6 | |||
| Dumas | NC001348 | 124,884 | 88 | 104,836 | 88 | 7,320 | 5,232 | 7,320 |
| pOka* | AB097933 | 125,125 | 88 | 104,798 | 88 | 7,463 | 5,225 | 7,463 |
| vOka* | AB097932 | 125,078 | 88 | 104,822 | 88 | 7,427 | 5,232 | 7,421 |
| VARILRIX | DQ008354 | 124,821 | 88 | 104,761 | 88 | 7,326 | 5,231 | 7,327 |
| VARIVAX | DQ008355 | 124,815 | 88 | 104,758 | 88 | 7,324 | 5,232 | 7,325 |
| MAV/06* | JF306641 | 124,758 | 88 | 104,798 | 88 | 7,276 | 5,232 | 7,276 |
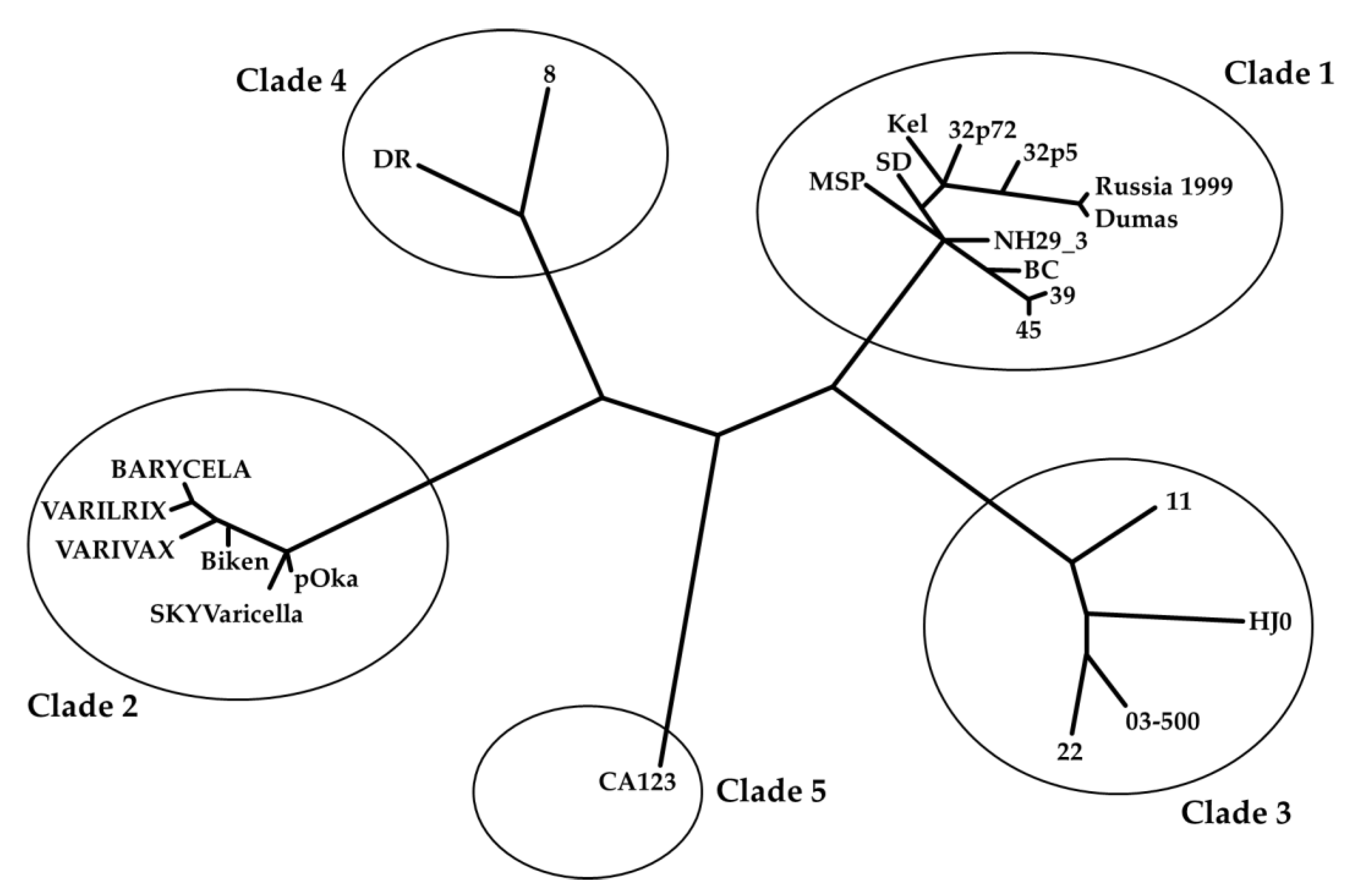
- Clade analysis
3.2. Product Information
3.3. Registration and Distribution
3.3.1. Registration
- Varicella Vaccine-GCC inj. (Suduvax) [44]
| Vaccine | Country | Approved date | Trade Name |
|---|---|---|---|
| Varicella Vaccine-GCC inj. | S.Korea | June, 1993 | Suduvax inj. |
| Peru | March, 2000 | Varicella vaccine KGCC | |
| Dominica | December, 2000 | VACUNA DE VARICELA GCC, 1,400 PFU/0.7mL | |
| Indonesia | September, 2001 | Varicella vaccine KGCC | |
| Philippines | June, 2005 | V-z vax | |
| Egypt | August, 2006 | Varicella vaccine GCC | |
| Thailand | March, 2007 | Varicella vaccine-GCC inj. | |
| Vietnam | October, 2008 | Varicella vaccine-GCC inj. | |
| Colombia | September, 2011 | Vacuna contra la varicella GCC inyeccion | |
| Guatemala | April, 2019 | Vacuna contra la Varicela | |
| BARYCELA inj. | S.Korea | March, 2020 | BARYCELA inj. |
| WHO | February, 2023 | BARYCELA inj. | |
| Pakistan | June, 2023 | BARYCELA inj. |
3.3.2. Distribution
- Varicella Vaccine-GCC inj. (Suduvax)[44]
- BARYCELA inj.[55]
- Over the past 30 years, MAV/06 varicella vaccines have been distributed to over 20 countries worldwide, totaling approximately 30 million doses. Through the Pan American Health Organization (PAHO) bidding, the vaccines have been stably supplied to countries in Central and South America. Throughout this process, MAV/06 varicella vaccines have been confirmed as the most cost-effective option, offering the most rational pricing in terms of cost [56,57,58] (Table 6). On average, the price of the MAV/06 vaccine supplied to the PAHO was 20% lower than that of the Oka vaccines during 2021-2023 according to the PAHO.
3.4. Safety, Immunogenicity and Effectiveness
3.4.1. Safety
- Evidence from clinical trials
- 1.
- Safety and immunogenicity of live attenuated varicella vaccine (MAV/06 strain) in adults and children (1994) [59]
- 2.
- Immunogenicity and safety of live attenuated vaccine (MAV/06 strain) on healthy children and immunocompromised children (1995) [60]
- 3.
- Immunogenicity and safety of a new live attenuated varicella vaccine (MAV/06 strain) among healthy Filipino children of ages 9 months to 17 years (2001) [61]
- 4.
- 5.
- 6.
- 7.
- Evidence from post-marketing real world uses
| Vaccine | Report version | Reporting Period | Distribution (vials) | Overall reported ADRs1 cases | Conclusion |
|---|---|---|---|---|---|
| Varicella Vaccine-GCC inj. | PSUR v1.0 | January 2005-June 2013 | 6,512,915 | 3 | There were no new safety issues associated with Varicella Vaccine-GCC inj. during this PSUR reporting period |
| PSUR v2.1 | June 2013- June 2016 | 5,070,608 | 30 | There were no new safety issues associated with Varicella Vaccine-GCC inj. during this PSUR reporting period | |
| PBRER v3.0 | June 2016-June 2019 | 11,475,897 | 77 | No new relevant safety findings which would necessitate an analysis and a change in the current reference safety information | |
| PBRER v4.0 | June 2019-June 2022 | 6,165,350 | 10 | No new relevant safety findings which would necessitate an analysis and a change in the current reference safety information | |
| Total | 29,224,770 | 120 | - | ||
| BARYCELA inj. | PBRER v1.0 | March 2020-September 2020 | - * | - | No new relevant safety findings which would necessitate an analysis and a change in the current reference safety information |
| PBRER v2.0 | September 2020 -March 2021 | - * | - | No new relevant safety findings which would necessitate an analysis and a change in the current reference safety information | |
| PBRER v3.0 | March 2021-September 2021 | - * | - | No new relevant safety findings which would necessitate an analysis and a change in the current reference safety information | |
| PBRER v4.0 | September 2021 -March 2022 | 52,070 | - | No new relevant safety findings which would necessitate an analysis and a change in the current reference safety information | |
| PBRER v5.0 | March 2022-March 2023 | 182,980 | 1 | No new relevant safety findings which would necessitate an analysis and a change in the current reference safety information | |
| PBRER v6.0 | March 2023-March 2024 | 293,310 | 2 | No new relevant safety findings which would necessitate an analysis and a change in the current reference safety information | |
| Total | 528,360 | 3 | - | ||
- Strain Interchangeability
- Antibiotic-Free
3.4.2. Immunogenicity
- Results of clinical trials
- Safety and immunogenicity of live attenuated varicella vaccine (MAV/06 strain) in children (1994) [59]
- 2.
- Immunogenicity and safety of live attenuated vaccine (MAV/06 strain) on healthy children and immunocompromised children (1995) [60]
- 3.
- Immunogenicity and safety of a new live attenuated varicella vaccine (MAV/06 strain) among healthy Filipino children of ages 9 months to 17 years (2001) [61]
- 4.
- 5.
- 6.
- 7.
- 8.
- Results of seroprevalence study
- Cell-Mediated Immunity (CMI)
- Cross-reactive humoral immunity
3.4.3. Effectiveness
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Depledge, D.P.; Sadaoka, T.; Ouwendijk, W.J.D. Molecular Aspects of Varicella-Zoster Virus Latency. Viruses 2018, 10. [CrossRef]
- Gershon, A.A.; Silverstein, S.J. Varicella-Zoster Virus. In Clinical Virology: Third Edition; 2022.
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular Mechanisms of Varicella Zoster Virus Pathogenesis. Nat Rev Microbiol 2014, 12. [CrossRef]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella Zoster Virus Infection. Nat Rev Dis Primers 2015, 1. [CrossRef]
- Plotkin, S.; Orenstein, W.; Offit, P.; Kathryn, M.E. Plotkin’s Vaccines ; 7th Edition.; 2018; pp. 1145-1180.
- Varicella and Herpes Zoster Vaccines: WHO Position Paper, June 2014 - Recommendations. Vaccine 2016, 34.
- Barrett-Muir, W.; Scott, F.T.; Aaby, P.; John, J.; Matondo, P.; Chaudhry, Q.L.; Siqueira, M.; Poulsen, A.; Yaminishi, K.; Breuer, J. Genetic Variation of Varicella-Zoster Virus: Evidence for Geographical Separation of Strains. In Proceedings of the Journal of Medical Virology; 2003; Vol. 70. [CrossRef]
- Breuer, J.; Grose, C.; Norberg, P.; Tipples, G.; Schmid, D.S. A Proposal for a Common Nomenclature for Viral Clades That Form the Species Varicella-Zoster Virus: Summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry, 24-25 July 2008. Journal of General Virology 2010, 91. [CrossRef]
- Garcés-Ayala, F.; Rodríguez-Castillo, A.; Ortiz-Alcántara, J.M.; Gonzalez-Durán, E.; Segura-Candelas, J.M.; Pérez-Agüeros, S.I.; Escobar-Escamilla, N.; Méndez-Tenorio, A.; Diaz-Quiñonez, J.A.; Ramirez-González, J.E. Full-Genome Sequence of a Novel Varicella-Zoster Virus Clade Isolated in Mexico. Genome Announc 2015, 3. [CrossRef]
- Chen, J.J.; Zhu, Z.; Gershon, A.A.; Gershon, M.D. Mannose 6-Phosphate Receptor Dependence of Varicella Zoster Virus Infection in Vitro and in the Epidermis during Varicella and Zoster. Cell 2004, 119. [CrossRef]
- Sengupta, N.; Breuer, J. A Global Perspective of the Epidemiology and Burden of Varicella-Zoster Virus. Curr Pediatr Rev 2010, 5. [CrossRef]
- Finger, R.; Hughes, J.P.; Meade, B.J.; Pelletier, A.R.; Palmer, C.T. Age-Specific Incidence of Chickenpox. Public Health Reports 1994, 109.
- Lolekha, S.; Tanthiphabha, W.; Sornchai, P.; Kosuwan, P.; Sutra, S.; Warachit, B.; Chup-Upprakarn, S.; Hutagalung, Y.; Weil, J.; Bock, H.L. Effect of Climatic Factors and Population Density on Varicella Zoster Virus Epidemiology within a Tropical Country. American Journal of Tropical Medicine and Hygiene 2001, 64. [CrossRef]
- Marin, M.; Leung, J.; Gershon, A.A. Transmission of Vaccine-Strain Varicella-Zoster Virus: A Systematic Review. Pediatrics 2019, 144. [CrossRef]
- Ramilo, O. Bacterial Complications of Primary Varicella in Children. Clinical Infectious Diseases 1996, 23. [CrossRef]
- Marin, M.; Seward, J.F.; Gershon, A.A. 25 Years of Varicella Vaccination in the United States. Journal of Infectious Diseases 2022, 226, S375–S379. [CrossRef]
- Lee, Y.H.; Choe, Y.J.; Lee, J.; Kim, E.; Lee, J.Y.; Hong, K.; Yoon, Y.; Kim, Y.K. Global Varicella Vaccination Programs. Clin Exp Pediatr 2022, 65, 555–562. [CrossRef]
- Marin, M.; Lopez, A.S.; Melgar, M.; Dooling, K.; Curns, A.T.; Leung, J. Decline in Severe Varicella Disease during the United States Varicella Vaccination Program: Hospitalizations and Deaths, 1990–2019. Journal of Infectious Diseases 2022, 226. [CrossRef]
- Varela, F.H.; Pinto, L.A.; Scotta, M.C. Global Impact of Varicella Vaccination Programs. Hum Vaccin Immunother 2019, 15, 645–657. [CrossRef]
- Marijam, A.; Safonova, E.; Scherbakov, M.; Shpeer, E.; Van Oorschot, D.; Rudakova, A.; Tatochenko, V.; Briko, N. Cost Effectiveness and Budget Impact of Universal Varicella Vaccination in Russia. Hum Vaccin Immunother 2022, 18. [CrossRef]
- Bonanni, P.; Breuer, J.; Gershon, A.; Gershon, M.; Hryniewicz, W.; Papaevangelou, V.; Rentier, B.; Rümke, H.; Sadzot-Delvaux, C.; Senterre, J.; et al. Varicella Vaccination in Europe - Taking the Practical Approach. BMC Med 2009, 7. [CrossRef]
- Chacon-Cruz, E.; Meroc, E.; Costa-Clemens, S.A.; Clemens, R.; Verstraeten, T. Economic Evaluation of Universal Varicella Vaccination in Mexico. Pediatric Infectious Disease Journal 2022, 41. [CrossRef]
- Feng, H.; Zhang, H.; Ma, C.; Zhang, H.; Yin, D.; Fang, H. National and Provincial Burden of Varicella Disease and Cost-Effectiveness of Childhood Varicella Vaccination in China from 2019 to 2049: A Modelling Analysis. Lancet Reg Health West Pac 2023, 32. [CrossRef]
- Chui, K.S.; Wu, H.L.; You, J.H.S. Cost-Effectiveness Analysis of Varicella Vaccine as Post-Exposure Prophylaxis in Hong Kong. Scand J Infect Dis 2014, 46. [CrossRef]
- Azzari, C.; Baldo, V.; Giuffrida, S.; Gani, R.; O’brien, E.; Alimenti, C.; Daniels, V.J.; Wolfson, L.J. The Cost-Effectiveness of Universal Varicella Vaccination in Italy: A Model-Based Assessment of Vaccination Strategies. ClinicoEconomics and Outcomes Research 2020, 12. [CrossRef]
- Wolff, E.; Widgren, K.; Tomba, G.S.; Roth, A.; Lep, T.; Andersson, S. Cost-Effectiveness of Varicella and Herpes Zoster Vaccination in Sweden: An Economic Evaluation Using a Dynamic Transmission Model. PLoS One 2021, 16. [CrossRef]
- Scuffham, P.; Devlin, N.; Eberhart-Phillips, J.; Wilson-Salt, R. The Cost-Effectiveness of Introducing a Varicella Vaccine to the New Zealand Immunisation Schedule. Soc Sci Med 1999, 49. [CrossRef]
- Pawaskar, M.; Burgess, C.; Pillsbury, M.; Wisløff, T.; Flem, E. Clinical and Economic Impact of Universal Varicella Vaccination in Norway: A Modeling Study. PLoS One 2021, 16. [CrossRef]
- Hussey, H.; Abdullahi, L.; Collins, J.; Muloiwa, R.; Hussey, G.; Kagina, B. Varicella Zoster Virus-Associated Morbidity and Mortality in Africa - A Systematic Review. BMC Infect Dis 2017, 17. [CrossRef]
- Ali, H.A.; Hartner, A.M.; Echeverria-Londono, S.; Roth, J.; Li, X.; Abbas, K.; Portnoy, A.; Vynnycky, E.; Woodruff, K.; Ferguson, N.M.; et al. Vaccine Equity in Low and Middle Income Countries: A Systematic Review and Meta-Analysis. Int J Equity Health 2022, 21. [CrossRef]
- Moon, J.Y.; Seo, J.; Lee, J.; Park, D. Assessment of Attenuation of Varicella-Zoster Virus Vaccines Based on Genomic Comparison. J Med Virol 2023, 95. [CrossRef]
- Argaw, T.; Cohen, J.I.; Klutch, M.; Lekstrom, K.; Yoshikawa, T.; Asano, Y.; Krause, P.R. Nucleotide Sequences That Distinguish Oka Vaccine from Parental Oka and Other Varicella-Zoster Virus Isolates. Journal of Infectious Diseases 2000, 181. [CrossRef]
- Gomi, Y.; Sunamachi, H.; Mori, Y.; Nagaike, K.; Takahashi, M.; Yamanishi, K. Comparison of the Complete DNA Sequences of the Oka Varicella Vaccine and Its Parental Virus. J Virol 2002, 76. [CrossRef]
- Kim, J.; Jung, G.; Kim, Y.; Ji, G.; Kim, H.; Wang, W.; Park, H.; Park, S.; Kim, G.; Kwon, S.; et al. Sequencing and Characterization of Varicella-Zoster Virus Vaccine Strain SuduVax. Virol J 2011, 8. [CrossRef]
- Depledge, D.P.; Yamanishi, K.; Gomi, Y.; Gershon, A.A.; Breuer, J. Deep Sequencing of Distinct Preparations of the Live Attenuated Varicella-Zoster Virus Vaccine Reveals a Conserved Core of Attenuating Single-Nucleotide Polymorphisms. J Virol 2016, 90. [CrossRef]
- Hao, B.; Chen, Z.; Zeng, G.; Huang, L.; Luan, C.; Xie, Z.; Chen, J.; Bao, M.; Tian, X.; Xu, B.; et al. Efficacy, Safety and Immunogenicity of Live Attenuated Varicella Vaccine in Healthy Children in China: Double-Blind, Randomized, Placebo-Controlled Clinical Trial. Clinical Microbiology and Infection 2019, 25. [CrossRef]
- Hwang KK; Chun BH; Park HS; Park SY; Kim KY; Moon HM Marker Test for Attenuation of Varicella-Zoster Viruses Isolated in Korea. J of Kor Soc of Virology 1992, 22, 105–109.
- Park SY; Hwang KK; Choi MK; Ryu YW; Paik SB; Kim KH Propagation of Varicella-Zoster Virus Isolated in Korea. J kor Soc Virol 1991, 21, 1–9.
- WHO Prequalified Vaccines. Available online: URL (https://extranet.who.int/prequal/vaccines/prequalified-vaccines).
- Hayakawa, Y.; Torigoe, S.; Shiraki, K.; Yamanishi, K.; Takahashi, M. Biologic and Biophysical Markers of a Live Varicella Vaccine Strain (Oka): Identification of Clinical Isolates from Vaccine Recipients. Journal of Infectious Diseases 1984, 149. [CrossRef]
- Schmid, D.S. Varicella-Zoster Virus Vaccine: Molecular Genetics. Curr Top Microbiol Immunol 2010, 342. [CrossRef]
- Loparev, V.N.; Rubtcova, E.; Seward, J.F.; Levin, M.J.; Schmid, D.S. DNA Sequence Variability in Isolates Recovered from Patients with Postvaccination Rash or Herpes Zoster Caused by Oka Varicella Vaccine. Journal of Infectious Diseases 2007, 195. [CrossRef]
- Jeon, J.S.; Won, Y.H.; Kim, I.K.; Ahn, J.H.; Shin, O.S.; Kim, J.H.; Lee, C.H. Analysis of Single Nucleotide Polymorphism among Varicella-Zoster Virus and Identification of Vaccine-Specific Sites. Virology 2016, 496. [CrossRef]
- Ko EB.; Park HI.; Park HM.; Shin SK. Periodic Benefit Risk Evaluation Report for: Varicella Vaccine-GCC Inj._ver4.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2022.
- Information approved by the Ministry of Food and Drug Safety of the Republic of Korea for BARYCELA. Available online: URL (https://nedrug.mfds.go.kr/searchDrug).
- Park JW.; Lee JS.; Park HM.; Shin SK. Periodic Benefit Risk Evaluation Report for: BARYCELA Inj._ver5.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2023.
- BARYCELA package insert. Available online: URL (https://extranet.who.int/prequal/sites/default/files/vwa_ vaccine/pq_381_varicella__GC_PI-2023.pdf).
- Information approved by the Ministry of Food and Drug Safety of the Republic of Korea for SKYvaricella. Available online: URL (https://nedrug.mfds.go.kr/searchDrug).
- VARIVAX package insert. Available online: URL (https://www.fda.gov/media/76008/download?Attachment).
- VARILRIX product monograph. Available online: URL (https://ca.gsk.com/Media/6263/Varilrix.Pdf).
- SKYvaricella package insert. Available online: URL (https://extranet.who.int/prequal/sites/default/files/vwa_ vaccine/pq_347_varicella_1dose_SK_PI-2019.pdf).
- Information approved by the Ministry of Food and Drug Safety of the Republic of Korea for Varicella Vaccine-GCC. Available online: URL (https://nedrug.mfds.go.kr/searchDrug).
- Varicella Vaccine-GCC package insert. Available online: URL (https://www.globalgreencross.com/eng/index.do).
- Drug Regulatory Authority of Pakistan. (T.F. Complex, Mauve Area, G-9/4 Islamabad, Pakistan). Minutes of 329th Meeting of Registration Board Held on 6th to 8th June, 2023_Pakistan;. Unpublished work, 2023.
- Park HI.; Kim JA.; Park HM.; Shin SK. Periodic Benefit Risk Evaluation Report for: BARYCELA Inj._ver6.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2024.
- Expanded Program of Immunization Vaccine Prices for year 2023. Available online: URL (https://www.paho.org /en/documents/paho-revolving-fund-vaccine-prices-2023).
- Expanded Program of Immunization Vaccine Prices for year 2022. Available online: URL (https://www.paho.org /en/documents/paho-revolving-fund-vaccine-prices-2022).
- Expanded Program of Immunization Vaccine Prices for year 2021. Available online: URL (https://www.paho.org /en/documents/paho-revolving-fund-vaccine-prices-2021).
- Sohn, Y.M.; Park, C.Y.; Hwang, K.K.; Woo, G.J.; Park, S.Y. Safety and Immunogenicity of Live Attenuated Varicella Virus Vaccine (MAV/06 Strain). Kor Pediatr Soc 1994, 37, 1405–1413.
- Immunogenicity and Safety of Live Attenuated Vaccine(MAV/06srtain) on Healthy Children and Immunocompromised Children. Available online: URL (https://www.e-cep.org/journal/ view.php?number=1995380604).
- Gonzales, MA.L.M.; Gonzaga, E.M. Final Report_Immunogenicity and Safety of a New Live Attenuated Varicella Vaccine (MAV/06 Strain) among Healthy Filipino Children of Ages 9 Months to 17 Years; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2001.
- Lim DS.; Clinical Study Report_MG1111_P1 _ver1.0; 2015; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2015.
- Dose-escalation Phase 1 to Evaluate the Safety and Efficacy of MG1111 in Healthy Adults. Available online: URL (https://clinicaltrials.gov/study/NCT02367638?cond=mg1111&rank=2).
- A Study of MG1111 in Healthy Children. Available online: URL (https://clinicaltrials.gov/study/ NCT03375502?cond=mg1111&rank=1).
- Kang JH.; Clinical Study Report_MG1111_P2,3_Stage1,2; 2018; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2018.
- Choi, U.Y.; Kim, K.H.; Lee, J.; Eun, B.W.; Kim, D.H.; Ma, S.H.; Kim, C.S.; Lapphra, K.; Tangsathapornpong, A.; Kosalaraksa, P.; et al. Immunogenicity and Safety Profiles of a New MAV/06 Strain Varicella Vaccine in Healthy Children: A Multinational, Multicenter, Randomized, Double-Blinded, Active-Controlled Phase III Study. Vaccine 2021, 39. [CrossRef]
- An Open-label, Bridging Study of BARYCELA Inj. in Healthy Vietnamese Children Aged Between 12 Months to 12 Years. Available online: URL (https://clinicaltrials.gov/study/NCT05664152?cond=mg1111&rank=3).
- Le Minh Giang.; Clinical Study Report_Bridging Study for Marketing Authorization in Vietnam_ver2.0; 2024; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2024.
- ICH guideline E2C (R2) on periodic benefit-risk evaluation report (PBRER). Available online: URL (https://www. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use-guideline-e2c-r2-periodic-benefit-risk-evaluation-report-step-5_en.pdf).
- Yoo DJ.; Kim JA.; Ko EB.; Park HM.; Kim C. Periodic Benefit Risk Evaluation Report for: BARYCELA Inj._ver1.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2020.
- Ko EB.; Lee MJ.; Park HM.; Kim C. Periodic Benefit Risk Evaluation Report for: BARYCELA Inj._ver2.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2021.
- Jeong JE.; Lee JS.; Park HM.; Shin SK. Periodic Benefit Risk Evaluation Report for: BARYCELA Inj._ver3.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2021.
- Jeong JE.; Kim JA.; Park HM.; Shin SK. Periodic Benefit Risk Evaluation Report for: BARYCELA Inj._ver4.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2022.
- Beth S.; Lee SY.; Kim SH.; Lee CH. Periodic Safety Update Report for: Varicella Vaccine-GCC Inj._ver1.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2014.
- Lee JS.; Park HM. Periodic Safety Update Report for: Varicella Vaccine-GCC Inj._ver2.1; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2018.
- Yoo DJ.; Kim YA.; Park HM.; Kim C. Periodic Benefit Risk Evaluation Report for: Varicella Vaccine-GCC Inj._ver3.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2019.
- Yoshikawa, T.; Ando, Y.; Nakagawa, T.; Gomi, Y. Safety Profile of the Varicella Vaccine (Oka Vaccine Strain) Based on Reported Cases from 2005 to 2015 in Japan. Vaccine 2016, 34. [CrossRef]
- Galea, S.A.; Sweet, A.; Beninger, P.; Steinberg, S.P.; LaRussa, P.S.; Gershon, A.A.; Sharrar, R.G. The Safety Profile of Varicella Vaccine: A 10-Year Review. In Proceedings of the Journal of Infectious Diseases; 2008; Vol. 197.
- Sharrar, R.G.; LaRussa, P.; Galea, S.A.; Steinberg, S.P.; Sweet, A.R.; Keatley, R.M.; Wells, M.E.; Stephenson, W.P.; Gershon, A.A. The Postmarketing Safety Profile of Varicella Vaccine. Vaccine 2000, 19. [CrossRef]
- Kang, H.M.; Kim, G.; Choe, Y.J. Safety of Interchanging the Live Attenuated MAV/06 Strain and OKA Strain Varicella Vaccines in Children. Vaccines (Basel) 2023, 11. [CrossRef]
- Mahler, V.; Junker, A.C. Anaphylaxis to Additives in Vaccines. Allergo J Int 2022, 31.
- Kim, S.H.; Lee, H.J.; Park, S.E.; Oh, S.H.; Lee, S.Y.; Choi, E.H. Seroprevalence Rate after One Dose of Varicella Vaccine in Infants. Journal of Infection 2010, 61. [CrossRef]
- Gershon, A.A. The Immunological Basis for Immunization Series : Module 10: Varicella-Zoster Virus; Immunization, Vaccines and Biologicals, WHO: 20 Avenue Appia, 1211 Geneva 27, Switzerland, 2008; ISBN 9789241596770.
- Gonzaga, E.M. Final Report_A 5-Year Follow-up Immunogenicity Study For a Live Attenuated Varicella Vaccine (MAV/06 Strain) Among Healthy Filipino Children Immunized at Ages 9 Months to 17 Years from May 2000 to April 2001_ver1.0; GC Biopharma, Yongin-si, Gyeonggi-do, Republic of Korea. Unpublished Report, 2006.
- Choi, U.Y.; Huh, D.H.; Kim, J.H.; Kang, J.H. Seropositivity of Varicella Zoster Virus in Vaccinated Korean Children and MAV Vaccine Group. Hum Vaccin Immunother 2016, 12. [CrossRef]
- Michalik, D.E.; Steinberg, S.P.; LaRussa, P.S.; Edwards, K.M.; Wright, P.F.; Arvin, A.M.; Gans, H.A.; Gershon, A.A. Primary Vaccine Failure after 1 Dose of Varicella Vaccine in Healthy Children. Journal of Infectious Diseases 2008, 197. [CrossRef]
- Shin, D.; Shin, Y.; Kim, E.; Nam, H.; Nan, H.; Lee, J. Immunological Characteristics of MAV/06 Strain of Varicella-Zoster Virus Vaccine in an Animal Model. BMC Immunol 2022, 23. [CrossRef]
- Quinlivan, M.; Hawrami, K.; Barrett-Muir, W.; Aaby, P.; Arvin, A.; Chow, V.T.; John, T.J.; Matondo, P.; Peiris, M.; Poulsen, A.; et al. The Molecular Epidemiology of Varicella-Zoster Virus: Evidence for Geographic Segregation. Journal of Infectious Diseases 2002, 186. [CrossRef]
- Sauerbrei, A.; Stefanski, J.; Gruhn, B.; Wutzler, P. Immune Response of Varicella Vaccinees to Different Varicella-Zoster Virus Genotypes. Vaccine 2011, 29. [CrossRef]
- Hwang, J.Y.; Kim, Y.; Lee, K.M.; Shin, O.S.; Gim, J.A.; Shin, Y.; Park, H. Cross-Reactive Humoral Immunity of Clade 2 Oka and MAV/06 Strain-Based Varicella Vaccines against Different Clades of Varicella–Zoster Virus. Hum Vaccin Immunother 2023, 19. [CrossRef]
- Choi, J.K.; Park, S.H.; Park, S.; Choi, S.M.; Kim, S.H.; Lee, D.G.; Yoo, J.H.; Choi, J.H.; Kang, J.H. Trends in Varicella and Herpes Zoster Epidemiology before and after the Implementation of Universal One-Dose Varicella Vaccination over One Decade in South Korea, 2003–2015. Hum Vaccin Immunother 2019, 15. [CrossRef]
- Jung, J.; Ko, Y.J.; Kim, Y.E.; Huh, K.; Park, B.J.; Yoon, S.J. Epidemiological Impact of the Korean National Immunization Program on Varicella Incidence. J Korean Med Sci 2019, 34. [CrossRef]
- Choi, B.K.; Shin, J.H.; Lee, J.E.; Koh, S.B. Letter to the Editor: Effectiveness of the Varicella Vaccine in Korea: Unresolved Issues. J Korean Med Sci 2021, 36. [CrossRef]
- Cho, H.; Sun, E.; Jeon, S.; Ann, S.; Choi, B. Trends in the Incidence of Varicella Cases with Complications among Korean Children during 2010-2020 after the Universal One-Dose Varicella Vaccination Program. In Proceedings of the 38th International Conference for Pharmacoepidemiology, GC Bioharma, Copenhagen, Denmark, 2022; 1016.
- Choi EH. Effectivenss of Varicella Immunization in Korea; 2017; J Korean Med Sci 2021, published.
- Hong, K.; Sohn, S.; Choe, Y.J.; Rhie, K.; Lee, J.K.; Han, M.S.; Chun, B.C.; Choi, E.H. Waning Effectiveness of One-Dose Universal Varicella Vaccination in South Korea, 2011–2018: A Propensity Score Matched National Population Cohort. J Korean Med Sci 2021, 36. [CrossRef]
- Zhu, S.; Zeng, F.; Xia, L.; He, H.; Zhang, J. Incidence Rate of Breakthrough Varicella Observed in Healthy Children after 1 or 2 Doses of Varicella Vaccine: Results from a Meta-Analysis. Am J Infect Control 2018, 46. [CrossRef]
- Clements, D.A.; Moreira, S.P.; Coplan, P.M.; Bland, C.L.; Walter, E.B. Postlicensure Study of Varicella Vaccine Effectiveness in a Day-Care Setting. Pediatric Infectious Disease Journal 1999, 18. [CrossRef]
- Black, S.; Ray, P.; Shinefield, H.; Saddier, P.; Nikas, A. Lack of Association between Age at Varicella Vaccination and Risk of Breakthrough Varicella, within the Northern California Kaiser Permanente Medical Care Program. In Proceedings of the Journal of Infectious Diseases; 2008; Vol. 197.
- Choi, B.K.; Cho, H.; Shin, Y.; Lee, E.K. Letter to the Editor: Effectiveness of the Varicella Vaccine Among Korean Children: Suggestions for Future Research. J Korean Med Sci 2022, 37. [CrossRef]
| Strain | Trade Name | Manufacturer | Country | WHO prequalification |
|---|---|---|---|---|
| Oka/Biken | OKAVAX | Biken | Japan | - |
| Oka/Merck | VARIVAX | MSD | USA | Yes |
| Oka/RIT | VARILRIX | GSK | Belgium | - |
| Oka | VARI-L | Changchun Keygen Biological Products | China | - |
| Oka | Varicella Vaccine, Live | Sinovac | China | Yes |
| Oka/SK | SKYVaricella | SK Bioscience | S.Korea | Yes |
| MAV/06 | Varicella Vaccine-GCC inj. (Suduvax) | GC Biopharma Corp. | S.Korea | - |
| MAV/06 | BARYCELA | GC Biopharma Corp. | S.Korea | Yes |
| Isolate | Clinical Symptom | Ages(months)/Sex |
|---|---|---|
| MAV 1/1 | Varicella | 22/Male |
| MAV/2 | Varicella | 16/Male |
| MAV/3 | Varicella | 20/Male |
| MAV/4 | Varicella | 45/Female |
| MAV/5 | Varicella | 28/Female |
| MAV/6 | Varicella | 33/Male |
| MAV/7 | Varicella | 23/Male |
| Cell | Passage Number | Remark |
|---|---|---|
| HEL1 | 11 | LuMa2 cell |
| GEL3 | 13 | - |
| HEL | 8 | LuMa cell |
| HEL | 4 | MRC-54 cell |
| HEL | 24 | LuMa cell |
| Strain | 2021 | 2022 | 2023 | Average |
|---|---|---|---|---|
| Oka | 16.93 | 16.19 | 16.93 | 16.68 |
| MAV/06 | 13.80 | 12.50 | 13.80 | 13.37 |
| Clinical trial (year) | Cohort | Number of subjects by cohort | Country |
|---|---|---|---|
| 1994* [59] | 300 PFU1 500 PFU 1,000 PFU 1,500 PFU 2,000 PFU |
6 children 7 children 19 adults, 13 children 6 children 11 adults |
S.Korea |
| 1995* [60] | Healthy children Immunocompromised children |
177 children 22 children (6 leukemia, 10 solid tumors, 6 nephrotic syndromes) |
S.Korea |
| 2001* [61] | 9-12 months 1-12 years 13-17 years |
102 children 172 children 101 children |
Philippines |
| 2015** [62,63] | 2,000 PFU 8,000 PFU 25,000 PFU |
10 adults 10 adults 10 adults |
S.Korea |
| 2017** [64,65] | 2,000 PFU 8,000 PFU 25,000 PFU |
75 children 75 children 76 children |
Thailand |
| 2018** [64,65,66] | MG11112 | 258 children | S.Korea/Thailand |
| 2024** [67,68] | 12-24 months 2-12 years |
124 children 126 children |
Vietnam |
| Strain | Oka | MAV/06 | |||
|---|---|---|---|---|---|
| Vaccine | VARIVAX1 | VARIVAX2 | OKAVAX3 | Varicella Vaccine-GCC inj. | BARYCELA inj. |
| Total number of vials distributed | 16,100,000 | 55,700,000 | 9,467,000 | 29,224,770 | 528,360 |
| Number of reported adverse events | 7,963 | 16,683 | 351 | 120 | 3 |
| Estimated incidence rates (/100,000 doses) | 49.46 | 29.95 | 3.71 | 0.41 | 0.57 |
| Clinical trial (year) | Cohort | SCR1 No, of subjects achieved/ No. of total subjects (%) | FAMA GMT2 (95% CI) |
|---|---|---|---|
| 1994* [59] | 300 PFU3 500 PFU 1,000 PFU 1,500 PFU |
6/6(100) 7/7(100) 13/13(100) 6/6(100) |
72.0 116.2 83.3 160.9 |
| 1995* [60] | Healthy children Immunocompromised children |
161/161(100) 18/18(100) |
173.7 111.4 |
| 2001* [61] | 9-12 months 1-12 years 13-17 years |
100/100(100) 99/100(99) 99/99(100) |
741.7 227.6 500.9 |
| 2006* [74,84] | 9-12 months 1-12 years 13-17 years |
49/49(100) 62/62(100) 51/52(98.1) |
20.6 (15.3, 25.8) 26.4 (19.2, 33.7) 27.0 (18.7, 35.3) |
| 2015** [62,63] | 2,000 PFU 8,000 PFU 25,000 PFU |
- - - |
78.8 (44.3, 140.0) 90.5 (53.0, 154.6) 68.6 (41.9, 112.3) |
| 2017** [64,65] | 2,000 PFU 8,000 PFU 25,000 PFU |
43/43(100) 41/41(100) 57/57(100) |
85.5 (63.7, 114.9) 109.9 (88.6, 136,5) 65.6 (51.4, 83.6) |
| 2018** [64,65,66] | BARYCELA VARIVAX |
234/239(97.9) 237/239(99.2) |
74.2 (65.0, 84.8) 112.7 (99.1, 128.1) |
| 2024** [67,68] | 12-24 months 2-12 years |
- - |
69.9 (63.7, 76.7)*** 126.5 (95.0, 168.5)*** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





