Submitted:
26 April 2024
Posted:
26 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Analysis of CO2 Hydrogenation Reaction for Higher Alcohol
2.1. Thermodynamic Analysis
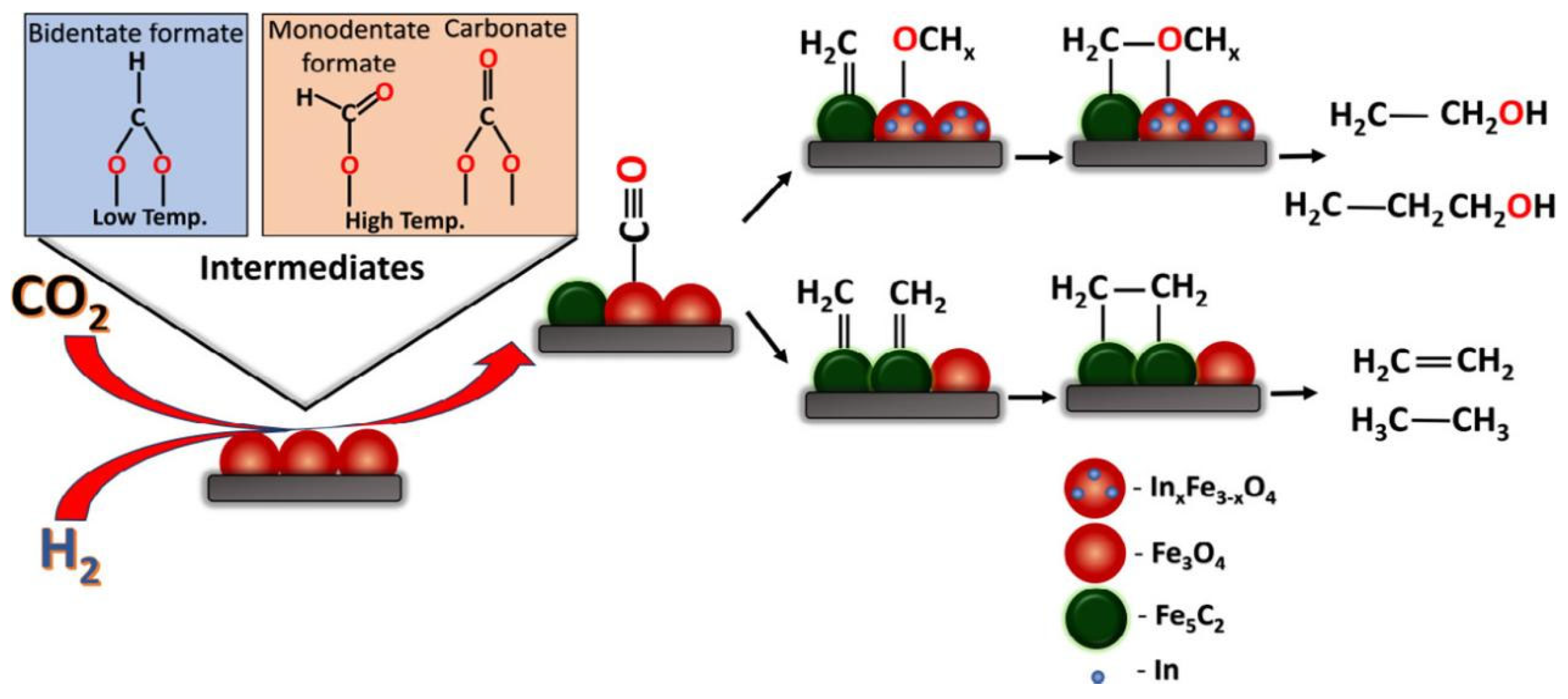
2.2. Reaction Mechanism
3. Catalysts
3.1. Single Metal-Based Catalyst
| Enter | Catalysts | T (℃) |
P (MPa) |
GHSV/ L.g-1h-1 |
H2/ CO2 |
XCO2 (%) |
SCO (%) |
SHC (%) |
SMeOH (%) |
SHA (%) |
STYHA (mmolgcat-1h-1) |
Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rh-TiO2 | 300 | 10 | 6 | 3 | / | / | 69.8 | 21.1 | 9.3 | 0.7 | [40] | |
| 2 | RhLi-SiO2 | 240 | 5 | 6 | 3 | 7.0 | 15.7 | 63.5 | 5.2 | 15.5 | 0.36 | [42] | |
| 4 | 1Pd2Ce@Si16 | 250 | 3 | 3 | / | 5.9 | / | / | / | 98.7 | 11.6 | [47] | |
| 3 | Mo/SiO2 | 500 | 1.6 | / | 1 | / | / | 46.6 | 22.1 | 2.8 | / | [23] | |
| 4 | Cu/Mo2C | 200 | 4 | / | 3 | 4.6 | 8.6 | 15.7 | 63 | 14 | / | [48] | |
| 5 | Co/Mo2C | 200 | 4 | / | 3 | 4.8 | 9.5 | 17.1 | 46 | 25 | / | [48] | |
| 6 | Fe/Mo2C | 200 | 4 | / | 3 | 3.9 | 6.8 | 18.6 | 58 | 16 | / | [48] | |
| 7 | Na-Co/SiO2 | 220 | 1.9 | 3 | 3 | 15.7 | / | 99.3 | / | 0.7 | / | [50] | |
| 8 | Co3O4 | 200 | 2 | 6 | 3 | 28.9 | 0 | 53.6 | / | 19.2 | 1.6 | [51] | |
| 9 | Cu@Na-Beta | 300 | 1.3 | 12 | 1 | 7.9 | 30.5 | / | / | ~100 | 17.1 | [53] | |
| 10 | FeNaS-0.6 | 320 | 3 | 8 | / | 32.0 | 20.7 | / | / | 12.8 | 78.5a | [59] | |
| 11 | Na-ZnFe@C | 320 | 5 | 0.9 | 3 | 38.4 | 7.6 | 43.2 | 1.8 | 20.3 | 158.1a | [60] | |
3.2. Bi- and Multi-Metallic Catalysts
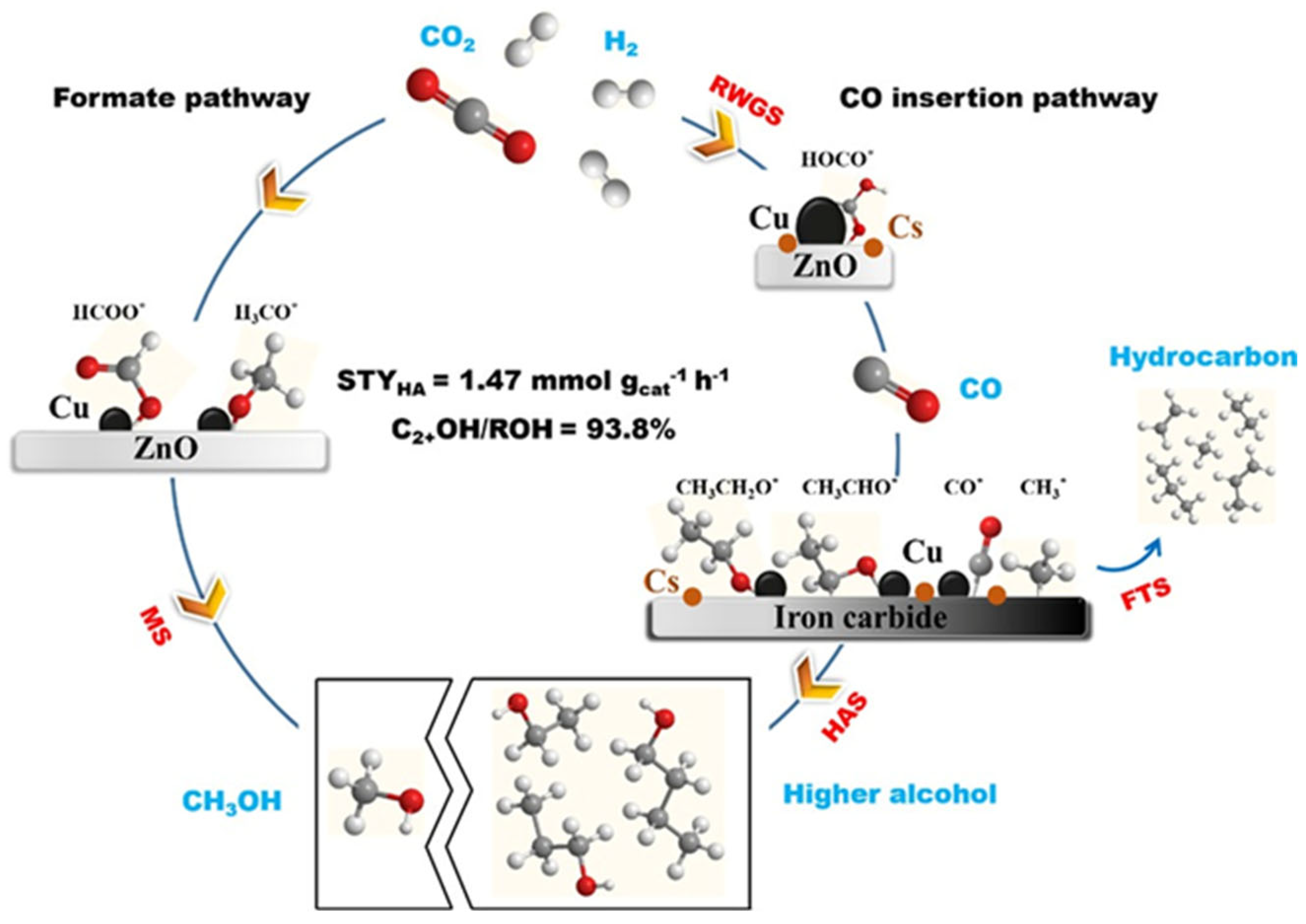
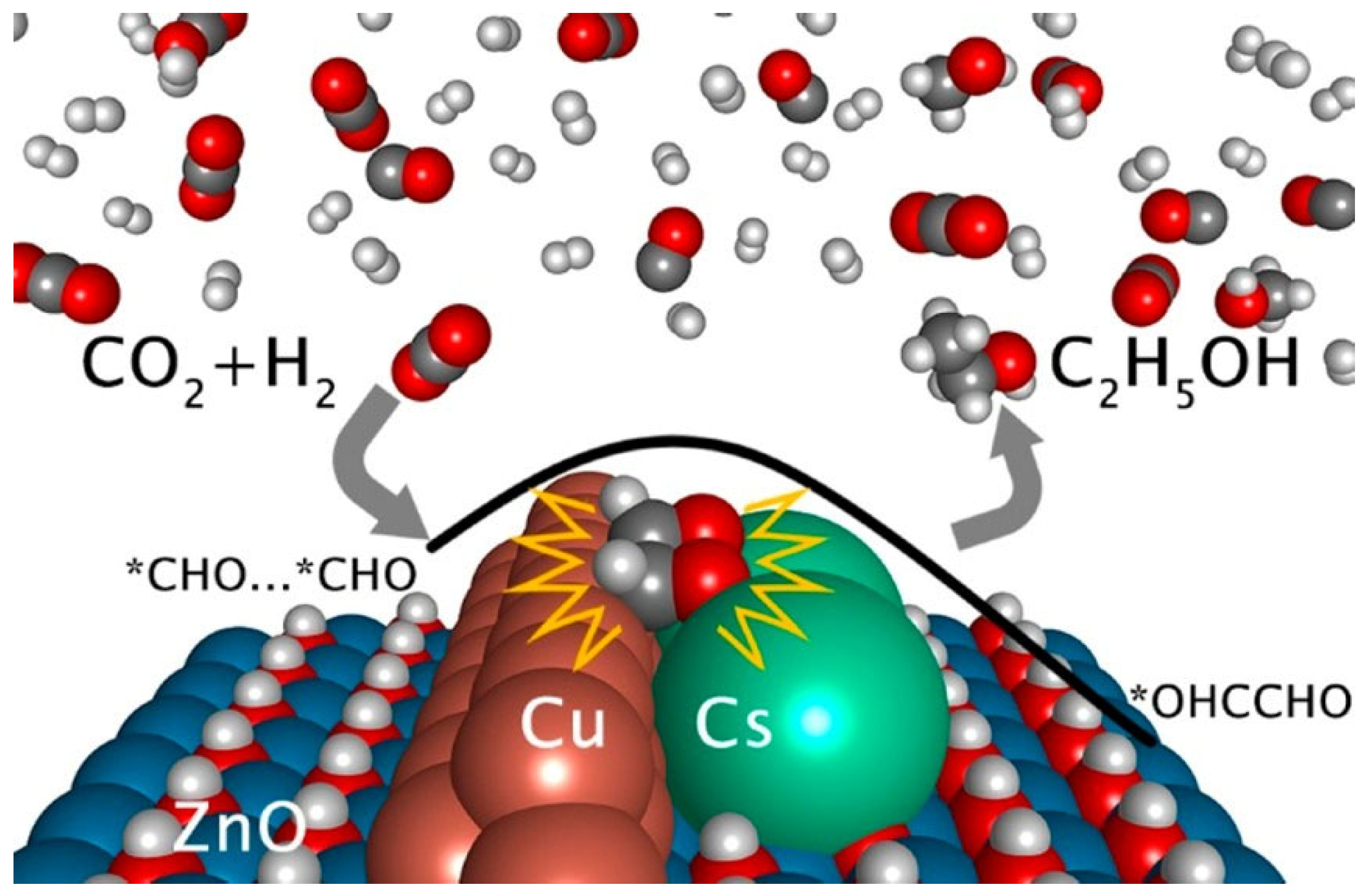
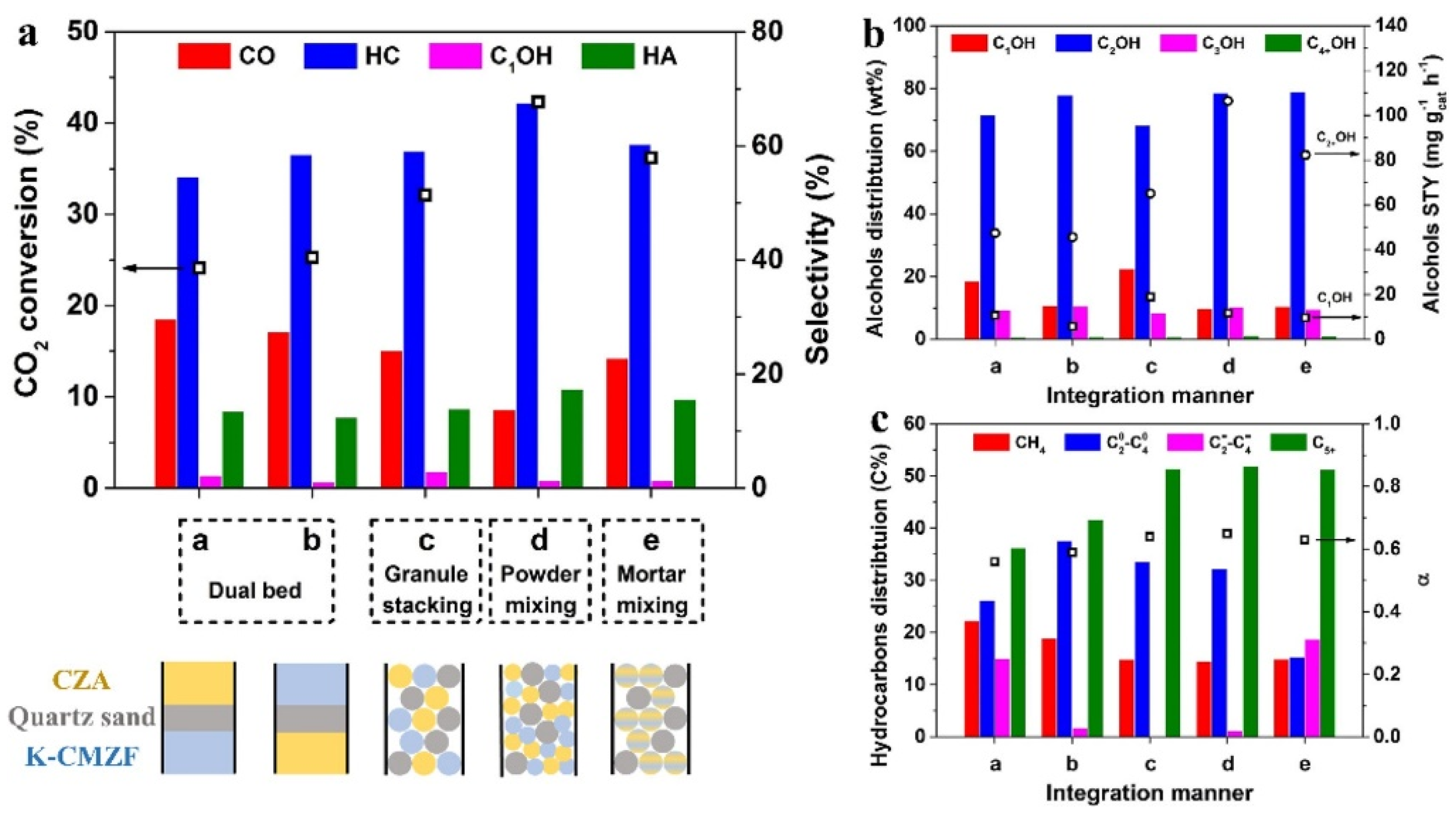
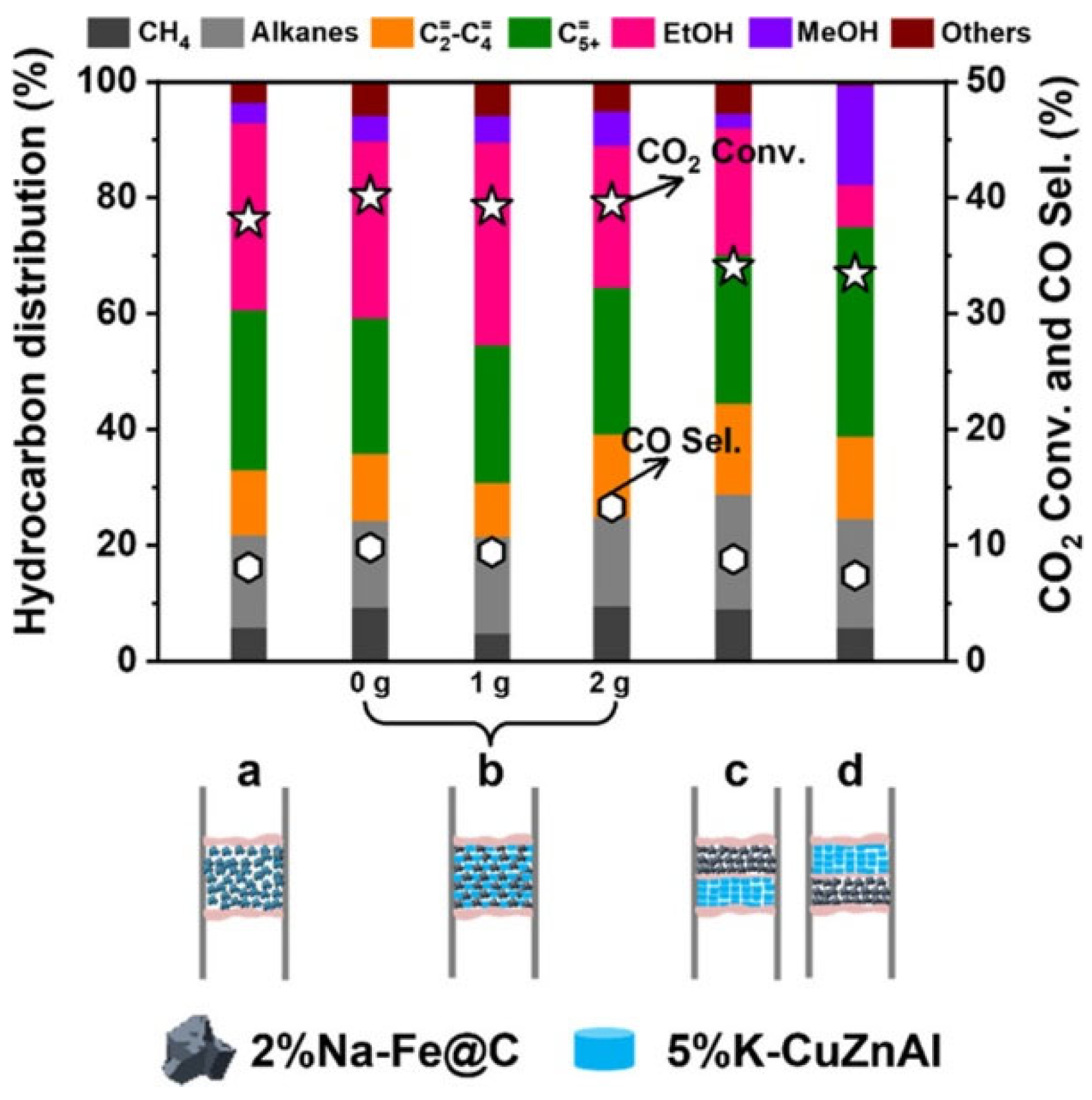
3.3. Tandem Catalyst
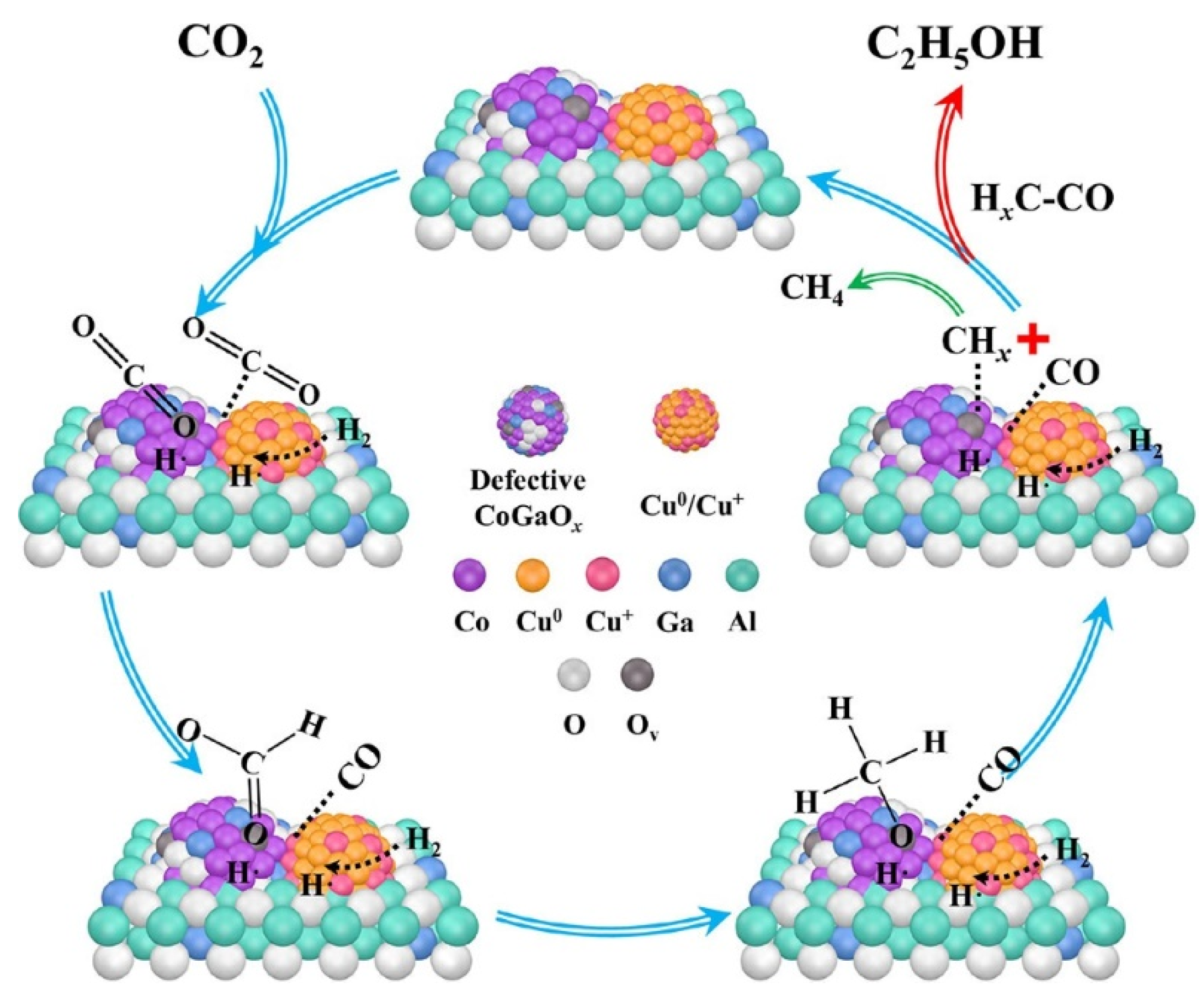
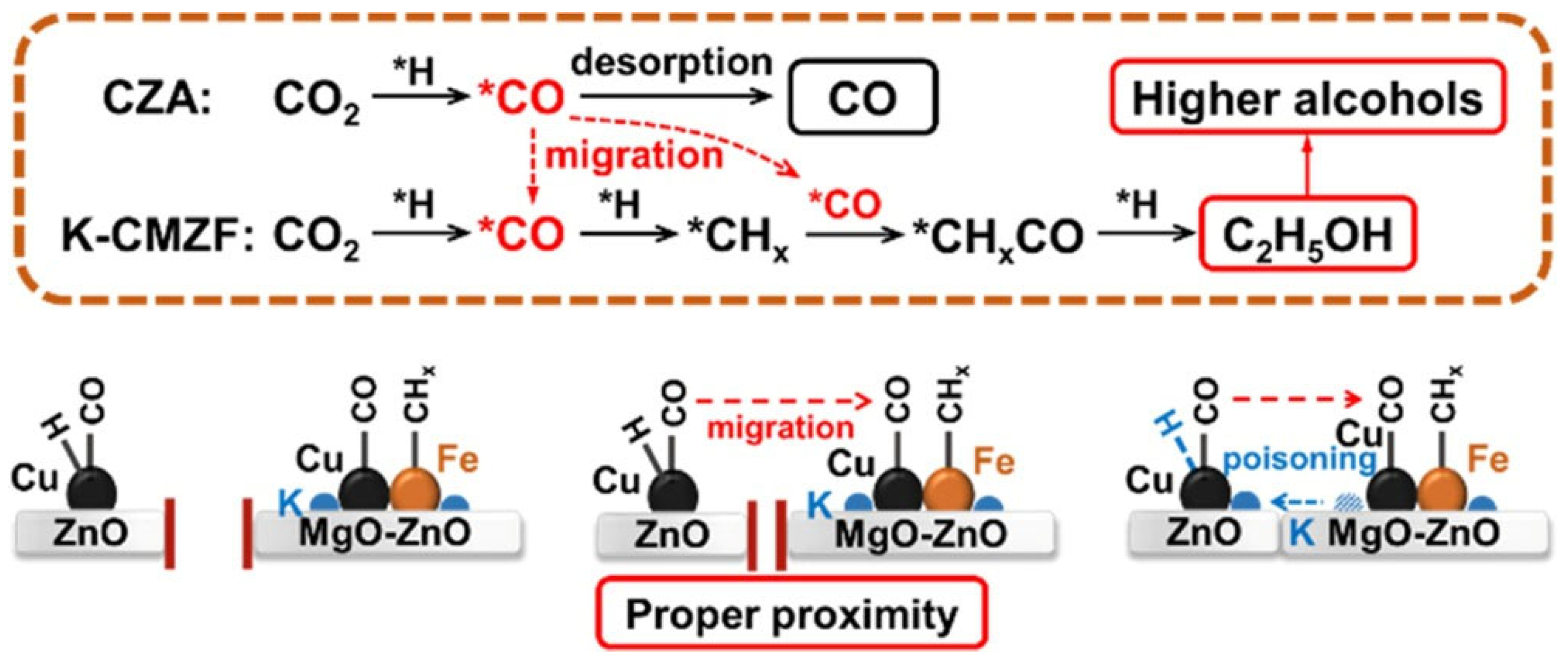
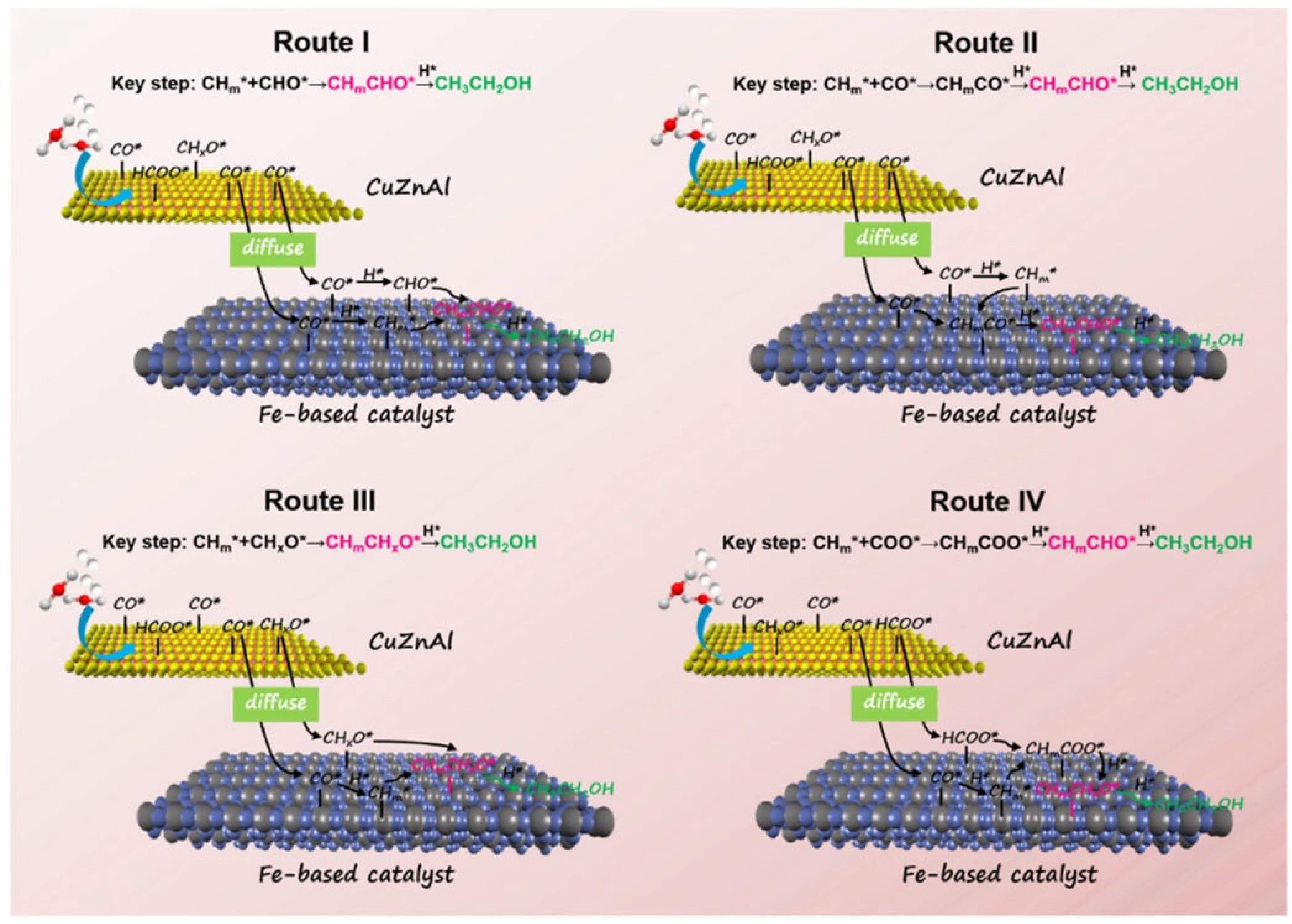
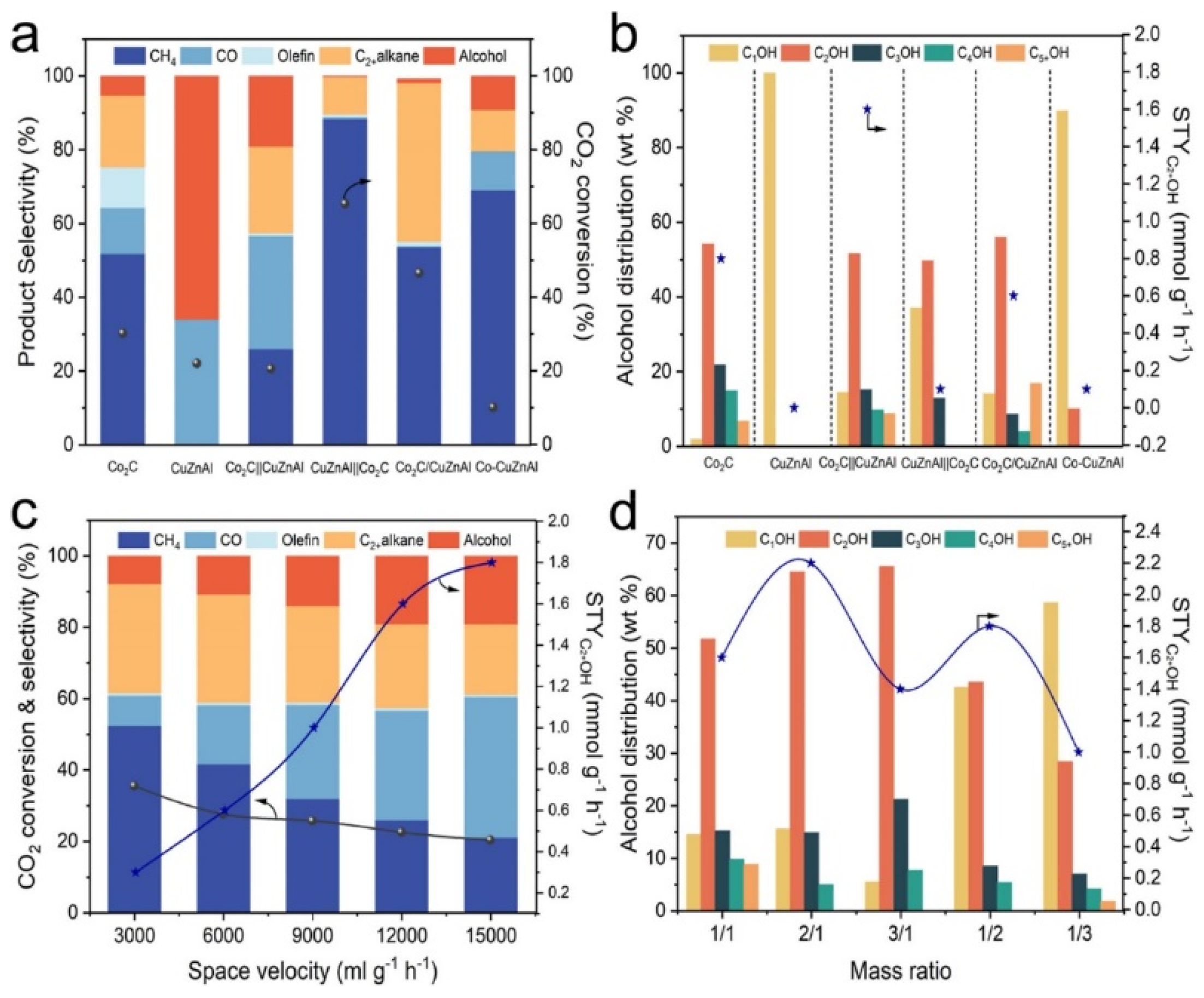
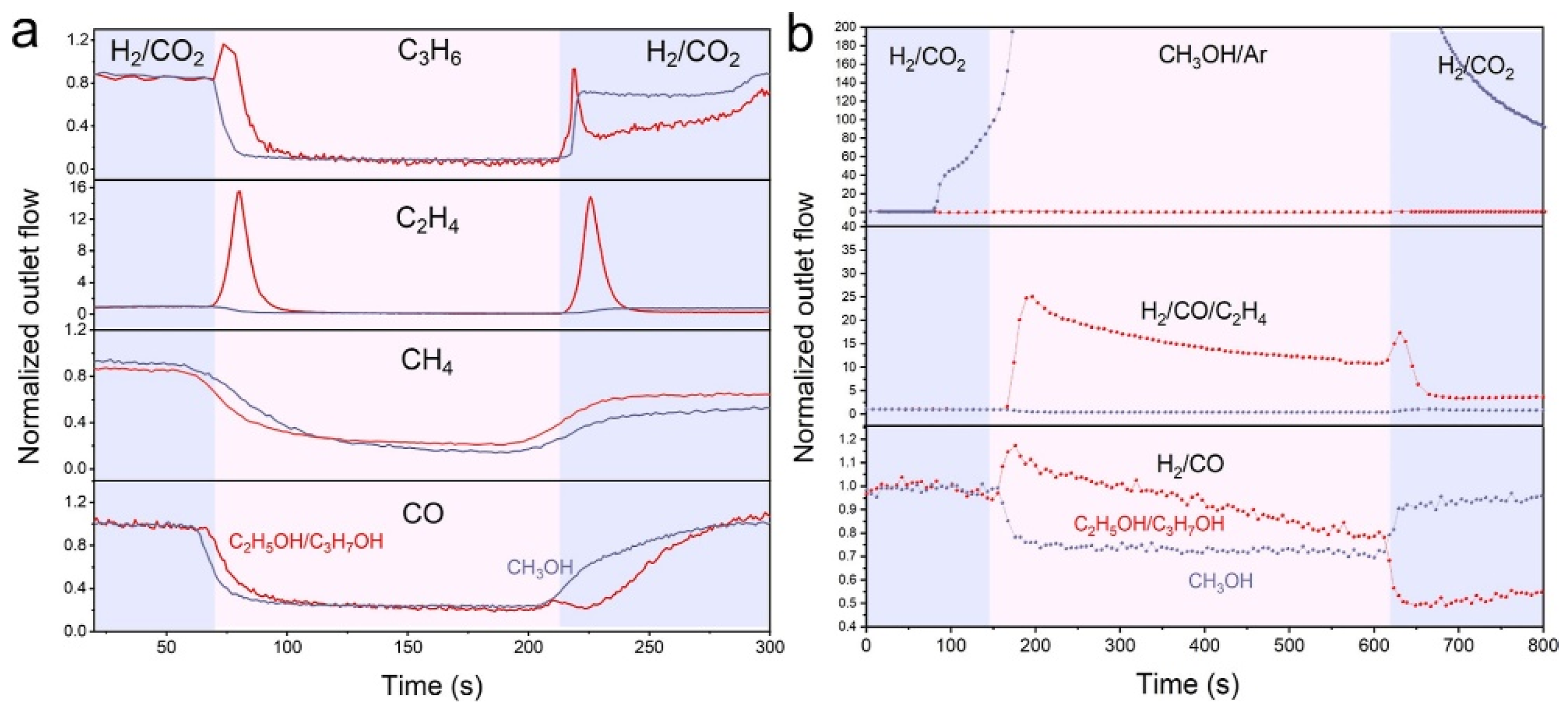
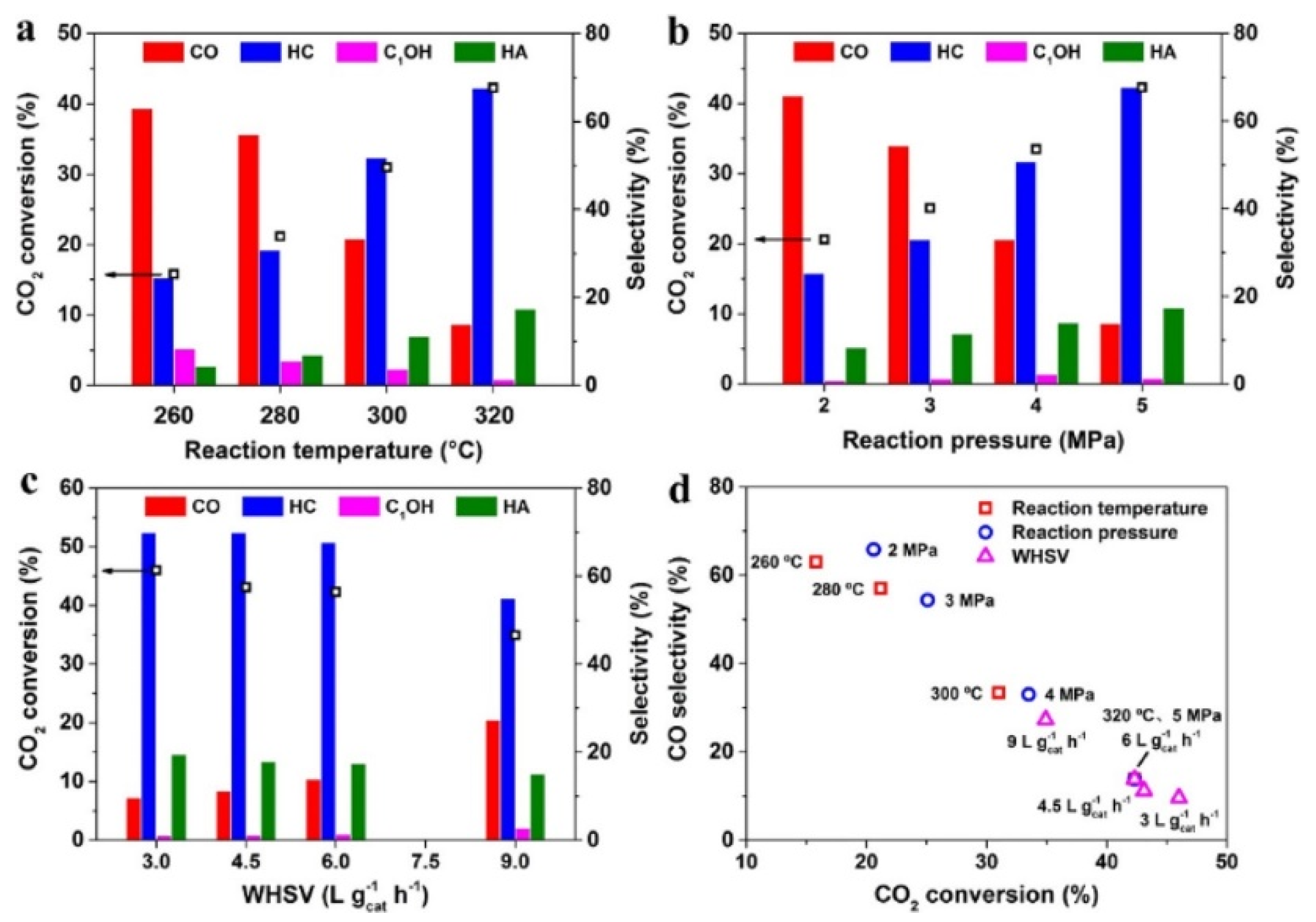
3.4. The Regulation Mechanism of CO2 Hydrogenation to Higher Alcohols on Tandem Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [CrossRef]
- Lacis, A. A.; Schmidt, G. A.; Rind, D.; Ruedy, R. A., Atmospheric CO2: Principal Control Knob Governing Earth's Temperature. Science 2010, 330, (6002), 356-359.
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [CrossRef]
- Zhang, Z.; Wang, T.; Blunt, M.J.; Anthony, E.J.; Park, A.-H.A.; Hughes, R.W.; Webley, P.A.; Yan, J. Advances in carbon capture, utilization and storage. Appl. Energy 2020, 278, 115627. [CrossRef]
- Corma, A. Preface to Special Issue of ChemSusChem on Green Carbon Science: CO2 Capture and Conversion. ChemSusChem 2020, 13, 6054–6055. [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [CrossRef]
- Tapia, J. F. D.; Lee, J. Y.; Ooi, R. E. H.; Foo, D. C. Y.; Tan, R. R., A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consump. 2018, 13, 1-15.
- Ni, Y.; Chen, Z.; Fu, Y.; Liu, Y.; Zhu, W.; Liu, Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 1–7. [CrossRef]
- Gao, P.; Li, S.; Bu, X.; Dang, S.; Liu, Z.; Wang, H.; Zhong, L.; Qiu, M.; Yang, C.; Cai, J.; et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024. [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing Catal. Today 2006, 115, 2–32. [CrossRef]
- Sancho-Sanz, I.; Korili, S.; Gil, A. Catalytic valorization of CO2 by hydrogenation: current status and future trends. Catal. Rev. 2021, 65, 698–772. [CrossRef]
- Mandal, S.C.; Das, A.; Roy, D.; Das, S.; Nair, A.S.; Pathak, B. Developments of the heterogeneous and homogeneous CO2 hydrogenation to value-added C2+-based hydrocarbons and oxygenated products. Co-ord. Chem. Rev. 2022, 471, 214737. [CrossRef]
- Ateka, A.; Rodriguez-Vega, P.; Ereña, J.; Aguayo, A.; Bilbao, J. A review on the valorization of CO2. Focusing on the thermodynamics and catalyst design studies of the direct synthesis of dimethyl ether. Fuel Process. Technol. 2022, 233. [CrossRef]
- Wang, L. X.; Wang, L.; Xiao, F. S., Tuning product selectivity in CO2 hydrogenation over metal-based catalysts. Chem Sci 2021, 12, (44), 14660-14673.
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C.-J. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2020, 44, 101413. [CrossRef]
- Chen, G. B.; Waterhouse, G. I. N.; Shi, R.; Zhao, J. Q.; Li, Z. H.; Wu, L. Z.; Tung, C. H.; Zhang, T. R., From Solar Energy to Fuels: Recent Advances in Light-Driven C1 Chemistry. Angew. Chem.-Int. Edit. 2019, 58, (49), 17528-17551.
- Dalena, F.; Senatore, A.; Basile, M.; Knani, S.; Basile, A.; Iulianelli, A. Advances in Methanol Production and Utilization, with Particular Emphasis toward Hydrogen Generation via Membrane Reactor Technology. Membranes 2018, 8, 98. [CrossRef]
- Wang, W.; Wang, S. P.; Ma, X. B.; Gong, J. L., Recent advances in catalytic hydrogenation of carbon dioxide. Chem Soc Rev 2011, 40, (7), 3703-3727.
- Christensen, E.; Yanowitz, J.; Ratcliff, M.; McCormick, R.L. Renewable Oxygenate Blending Effects on Gasoline Properties. Energy Fuels 2011, 25, 4723–4733. [CrossRef]
- Angelici, C.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Chemocatalytic Conversion of Ethanol into Butadiene and Other Bulk Chemicals. ChemSusChem 2013, 6, 1595–1614. [CrossRef]
- Bai, F.; Anderson, W.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [CrossRef]
- Tatsumi, T.; Muramatsu, A.; Tominaga, H. O., Alcohol synthesis from CO2/H2 on silica-supported molybdenum catalysts. Chem Let 1985, (5), 593-594.
- Li, Z. Qu, Y. Wang, J. Liu, H. Li, M. Miao, S. Li, C., Highly selective conversion of carbon dioxide to aromatics over tandem catalysts. Joule 2019, (3), 570−583.
- Wei, J. Yao, R. Han, Y. Ge, Q. Sun, J., Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons. Chem SocvRev 2021, 50, (19), 10764-10805.
- Kang, J.; He, S.; Zhou, W.; Shen, Z.; Li, Y.; Chen, M.; Zhang, Q.; Wang, Y. Single-pass transformation of syngas into ethanol with high selectivity by triple tandem catalysis. Nat. Commun. 2020, 11, 1–11. [CrossRef]
- Nie, X. W.; Li, W. H.; Jiang, X.; Guo, X. W.; Song, C. S., Recent advances in catalytic CO2 hydrogenation to alcohols and hydrocarbons. Adv Catal 2019, 65, 121-233.
- Yi, Q.; Li, W. Y.; Feng, J.; Xie, K. C., Carbon cycle in advanced coal chemical engineering. Chem Soc Rev 2015, 44, (15), 5409-5445.
- Yi, X. H., CO2 Hydrogenation for Ethanol Production: A Thermodynamic Analysis. Science PG 2017, 5, (6), 145-152.
- Jia, C.; Gao, J.; Dai, Y.; Zhang, J.; Yang, Y. The thermodynamics analysis and experimental validation for complicated systems in CO 2 hydrogenation process. J. Energy Chem. 2016, 25, 1027–1037. [CrossRef]
- Hitoshi, K.; Kiyomi, O.; Kazuhiro, S.; Hironori, A., CO2 hydrogenation to ethanol over promoted Rh/SiO2 catalysts. Catal Today. 1996, 28, 261-266.
- Xu, D.; Ding, M.; Hong, X.; Liu, G.; Tsang, S. C. E., Selective C2+ Alcohol Synthesis from Direct CO2 Hydrogenation over a Cs-Promoted Cu-Fe-Zn Catalyst. Acs Catal 2020, 10, (9), 5250-5260.
- He, Z.; Qian, Q.; Ma, J.; Meng, Q.; Zhou, H.; Song, J.; Liu, Z.; Han, B., Water-Enhanced Synthesis of Higher Alcohols from CO2 Hydrogenation over a Pt/Co3O4 Catalyst under Milder Conditions. Angew. Chem.-Int. Edit. 2016, 55, (2), 737-741.
- Wang, X.; Ramirez, P. J.; Liao, W.; Rodriguez, J. A.; Liu, P., Cesium-Induced Active Sites for C-C Coupling and Ethanol Synthesis from CO2 Hydrogenation on Cu/ZnO(000(1)over-bar) Surfaces. J Am Chem Soc 2021, 143, (33), 13103-13112.
- Aresta, M.; Dibenedetto, A.; Angelini, A., Catalysis for the Valorization of Exhaust Carbon: from CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, (3), 1709-1742.
- Gupta, M.; Smith, M.L.; Spivey, J.J. Heterogeneous Catalytic Conversion of Dry Syngas to Ethanol and Higher Alcohols on Cu-Based Catalysts. ACS Catal. 2011, 1, 641–656. [CrossRef]
- Xu, D.; Wang, Y.; Ding, M.; Hong, X.; Liu, G.; Tsang, S.C.E. Advances in higher alcohol synthesis from CO2 hydrogenation. Chem 2021, 7, 849–881. [CrossRef]
- Zeng, F.; Mebrahtu, C.; Xi, X.; Liao, L.; Ren, J.; Xie, J.; Heeres, H.J.; Palkovits, R. Catalysts design for higher alcohols synthesis by CO2 hydrogenation: Trends and future perspectives. Appl. Catal. B Environ. 2021, 291, 120073. [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 1–15. [CrossRef]
- Sheerin, E.; Reddy, G. K.; Smirniotis, P., Evaluation of Rh/CexTi1-xO2 catalysts for synthesis of oxygenates from syngas using XPS and TPR techniques. Catal Today 2016, 263, 75-83.
- Inoue, T.; Iizuka, T.; Tanabe, K. Hydrogenation of carbon dioxide and carbon monoxide over supported rhodium catalysts under 10 bar pressure. Appl. Catal. 1989, 46, 1–9. [CrossRef]
- Bando, K.K.; Soga, K.; Kunimori, K.; Arakawa, H. Effect of Li additive on CO2 hydrogenation reactivity of zeolite supported Rh catalysts. Appl. Catal. A: Gen. 1998, 175, 67–81. [CrossRef]
- Kusama, H.; Okabe, K.; Sayama, K.; Arakawa, H., Ethanol synthesis by catalytic hydrogenation of CO2 over Rh-Fe/SiO2 catalysts. Energy 1997, 22, (2-3), 343-348.
- Wang, G.; Luo, R.; Yang, C.; Song, J.; Xiong, C.; Tian, H.; Zhao, Z.-J.; Mu, R.; Gong, J. Active sites in CO2 hydrogenation over confined VOx-Rh catalysts. Sci. China Chem. 2019, 62, 1710–1719. [CrossRef]
- Yang, C.; Mu, R.; Wang, G.; Song, J.; Tian, H.; Zhao, Z.-J.; Gong, J. Hydroxyl-mediated ethanol selectivity of CO2 hydrogenation. Chem. Sci. 2019, 10, 3161–3167. [CrossRef]
- Zheng, K.; Li, Y.; Liu, B.; Jiang, F.; Xu, Y.; Liu, X., Ti-doped CeO2 Stabilized Single-Atom Rhodium Catalyst for Selective and Stable CO2 Hydrogenation to Ethanol. Angew. Chem.-Int. Edit. 2022, 61, (44).
- Chen, J.; Zha, Y.; Liu, B.; Li, Y.; Xu, Y.; Liu, X. Rationally Designed Water Enriched Nano Reactor for Stable CO2 Hydrogenation with Near 100% Ethanol Selectivity over Diatomic Palladium Active Sites. ACS Catal. 2023, 13, 7110–7121. [CrossRef]
- Chen, Y.; Choi, S.; Thompson, L.T. Low temperature CO2 hydrogenation to alcohols and hydrocarbons over Mo2C supported metal catalysts. J. Catal. 2016, 343, 147–156. [CrossRef]
- Okabe, K.; Yamada, H.; Hanaoka, T.; Matsuzaki, T.; Arakawa, H.; Abe, Y., CO2 hydrogenation to alcohols over highly dispersed Co/SiO2 catalysts derived from acetate. Chem Lett 2001, (9), 904-905.
- Gnanamani, M.K.; Jacobs, G.; Keogh, R.A.; Shafer, W.D.; Sparks, D.E.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Fischer-Tropsch synthesis: Effect of pretreatment conditions of cobalt on activity and selectivity for hydrogenation of carbon dioxide. Appl. Catal. A: Gen. 2015, 499, 39–46. [CrossRef]
- Liu, B.; Ouyang, B.; Zhang, Y.; Lv, K.; Li, Q.; Ding, Y.; Li, J. Effects of mesoporous structure and Pt promoter on the activity of Co-based catalysts in low-temperature CO2 hydrogenation for higher alcohol synthesis. J. Catal. 2018, 366, 91–97. [CrossRef]
- Luk, H.T.; Mondelli, C.; Ferré, D.C.; Stewart, J.A.; Pérez-Ramírez, J. Status and prospects in higher alcohols synthesis from syngas. Chem. Soc. Rev. 2017, 46, 1358–1426. [CrossRef]
- Ding, L.; Shi, T.; Gu, J.; Cui, Y.; Zhang, Z.; Yang, C.; Chen, T.; Lin, M.; Wang, P.; Xue, N.; et al. CO2 Hydrogenation to Ethanol over Cu@Na-Beta. Chem 2020, 6, 2673–2689. [CrossRef]
- Iltsiou, D.; Mielby, J.; Kegnaes, S., Direct Conversion of CO2 into Alcohols Using Cu-Based Zeolite Catalysts. ChemPlusChem 2023, 89, (1).
- Aitbekova, A.; Goodman, E. D.; Wu, L.; Boubnov, A.; Hoffman, A. S.; Genc, A.; Cheng, H.; Casalena, L.; Bare, S. R.; Cargnello, M., Engineering of Ruthenium-Iron Oxide Colloidal Heterostructures: Improved Yields in CO2 Hydrogenation to Hydrocarbons. Angew. Chem.-Int. Edit. 2019, 58, (48), 17451-17457.
- Albrecht, M.; Rodemerck, U.; Schneider, M.; Bröring, M.; Baabe, D.; Kondratenko, E.V. Unexpectedly efficient CO2 hydrogenation to higher hydrocarbons over non-doped Fe2O3. Appl. Catal. B: Environ. 2017, 204, 119–126. [CrossRef]
- Jiang, J.; Wen, C.; Tian, Z.; Wang, Y.; Zhai, Y.; Chen, L.; Li, Y.; Liu, Q.; Wang, C.; Ma, L., Manganese-Promoted Fe3O4 Microsphere for Efficient Conversion of CO2 to Light Olefins. Ind Eng Chem Res 2020, 59, (5), 2155-2162.
- Kasipandi, S.; Bae, J.W. Recent Advances in Direct Synthesis of Value-Added Aromatic Chemicals from Syngas by Cascade Reactions over Bifunctional Catalysts. Adv. Mater. 2019, 31, e1803390. [CrossRef]
- Yao, R.; Wei, J.; Ge, Q.; Xu, J.; Han, Y.; Ma, Q.; Xu, H.; Sun, J. Monometallic iron catalysts with synergistic Na and S for higher alcohols synthesis via CO2 hydrogenation. Appl. Catal. B: Environ. 2021, 298, 120556. [CrossRef]
- Wang, Y.; Wang, W.; He, R.; Li, M.; Zhang, J.; Cao, F.; Liu, J.; Lin, S.; Gao, X.; Yang, G.; Wang, M.; Xing, T.; Liu, T.; Liu, Q.; Hu, H.; Tsubaki, N.; Wu, M., Carbon-Based Electron Buffer Layer on ZnOx-Fe5C2-Fe3O4 Boosts Ethanol Synthesis from CO2 Hydrogenation. Angew. Chem.-Int. Edit. 2023, 62, (46).
- Wang, J.; Zhang, G.; Zhu, J.; Zhang, X.; Ding, F.; Zhang, A.; Guo, X.; Song, C., CO2 Hydrogenation to Methanol over In2O3-Based Catalysts: From Mechanism to Catalyst Development. Acs Catal 2021, 11, (3), 1406-1423.
- Umegaki, T.; Kuratani, K.; Yamada, Y.; Ueda, A.; Kuriyama, N.; Kobayashi, T.; Xu, Q. Hydrogen production via steam reforming of ethyl alcohol over nano-structured indium oxide catalysts. J. Power Sources 2008, 179, 566–570. [CrossRef]
- Lorenz, H.; Jochum, W.; Klötzer, B.; Stöger-Pollach, M.; Schwarz, S.; Pfaller, K.; Penner, S. Novel methanol steam reforming activity and selectivity of pure In2O3. Appl. Catal. A: Gen. 2008, 347, 34–42. [CrossRef]
- Witoon, T.; Numpilai, T.; Nijpanich, S.; Chanlek, N.; Kidkhunthod, P.; Cheng, C.K.; Ng, K.H.; Vo, D.-V.N.; Ittisanronnachai, S.; Wattanakit, C.; et al. Enhanced CO2 hydrogenation to higher alcohols over K-Co promoted In2O3 catalysts. Chem. Eng. J. 2022, 431. [CrossRef]
- Goud, D.; Churipard, S.R.; Bagchi, D.; Singh, A.K.; Riyaz, M.; Vinod, C.P.; Peter, S.C. Strain-Enhanced Phase Transformation of Iron Oxide for Higher Alcohol Production from CO2. ACS Catal. 2022, 12, 11118–11128. [CrossRef]
- Ye, X.; Yang, C.; Pan, X.; Ma, J.; Zhang, Y.; Ren, Y.; Liu, X.; Li, L.; Huang, Y., Highly Selective Hydrogenation of CO2 to Ethanol via Designed Bifunctional Ir1-In2O3 Single-Atom Catalyst. J Am Chem Soc 2020, 142, (45), 19001-19005.
- An, K.; Zhang, S.; Wang, J.; Liu, Q.; Zhang, Z.; Liu, Y. A highly selective catalyst of Co/La4Ga2O9 for CO2 hydrogenation to ethanol. J. Energy Chem. 2020, 56, 486–495. [CrossRef]
- Zhang, S.; Wu, Z.; Liu, X.; Shao, Z.; Xia, L.; Zhong, L.; Wang, H.; Sun, Y. Tuning the interaction between Na and Co2C to promote selective CO2 hydrogenation to ethanol. Appl. Catal. B: Environ. 2021, 293, 120207. [CrossRef]
- Liu, S.; Zhou, H.; Zhang, L.; Ma, Z.; Wang, Y., Activated Carbon-Supported Mo-Co-K Sulfide Catalysts for Synthesizing Higher Alcohols from CO2. Chem Eng Technol 2019, 42, (5), 962-970.
- Zhang, H.; Han, H.; Xiao, L.; Wu, W., Highly Selective Synthesis of Ethanol via CO2 Hydrogenation over CoMoCx Catalysts. Chemcatchem 2021, 13, (14), 3333-3339.
- Zhang, G.; Fan, G.; Zheng, L.; Li, F., Ga-Promoted CuCo-Based Catalysts for Efficient CO2 Hydrogenation to Ethanol: The Key Synergistic Role of Cu-CoGaOx Interfacial Sites. Acs Appl Mater Interfaces 2022, 14, (31), 35569-35580.
- Irshad, M.; Chun, H.-J.; Khan, M.K.; Jo, H.; Kim, S.K.; Kim, J. Synthesis of n-butanol-rich C3+ alcohols by direct CO2 hydrogenation over a stable Cu–Co tandem catalyst. Appl. Catal. B: Environ. 2024, 340. [CrossRef]
- Zhang, Q.; Wang, S.; Geng, R.; Wang, P.; Dong, M.; Wang, J.; Fan, W. Hydrogenation of CO2 to higher alcohols on an efficient Cr-modified CuFe catalyst. Appl. Catal. B: Environ. 2023, 337. [CrossRef]
- Si, Z.; Wang, L.; Han, Y.; Yu, J.; Ge, Q.; Zeng, C.; Sun, J. Synthesis of Alkene and Ethanol in CO2 Hydrogenation on a Highly Active Sputtering CuNaFe Catalyst. ACS Sustain. Chem. Eng. 2022, 10, 14972–14979. [CrossRef]
- Yamamoto, T.; Inui, T., Highly effective synthesis of ethanol from CO2 on Fe, Cu-based novel catalysts. In Advances in Chemical Conversions for Mitigating Carbon Dioxide, Inui, T.; Anpo, M.; Izui, K.; Yanagida, S.; Yamaguchi, T., Eds 1998, 114, 513-516.
- Guo, H.; Li, S.; Peng, F.; Zhang, H.; Xiong, L.; Huang, C.; Wang, C.; Chen, X. Roles Investigation of Promoters in K/Cu–Zn Catalyst and Higher Alcohols Synthesis from CO2 Hydrogenation over a Novel Two-Stage Bed Catalyst Combination System. Catal. Lett. 2014, 145, 620–630. [CrossRef]
- Wang, Y.; Wang, K.; Zhang, B.; Peng, X.; Gao, X.; Yang, G.; Hu, H.; Wu, M.; Tsubaki, N. Direct Conversion of CO2 to Ethanol Boosted by Intimacy-Sensitive Multifunctional Catalysts. ACS Catal. 2021, 11, 11742–11753. [CrossRef]
- Xu, D.; Yang, H.; Hong, X.; Liu, G.; Tsang, S.C.E. Tandem Catalysis of Direct CO2 Hydrogenation to Higher Alcohols. ACS Catal. 2021, 11, 8978–8984. [CrossRef]
- Huang, J.; Zhang, G.; Wang, M.; Zhu, J.; Ding, F.; Song, C.; Guo, X. The synthesis of higher alcohols from CO2 hydrogenation over Mn-Cu-K modified Fe5C2 and CuZnAlZr tandem catalysts. Front. Energy Res. 2023, 10. [CrossRef]
- Wang, Y.; Xu, D.; Zhang, X.; Hong, X.; Liu, G., Selective C2+ alcohol synthesis by CO2 hydrogenation via a reaction-coupling strategy. Catalysis Science & Technology 2022, 12, (5), 1539-1550.
- Zhang, S.; Huang, C.; Shao, Z.; Zhou, H.; Chen, J.; Li, L.; Lu, J.; Liu, X.; Luo, H.; Xia, L.; Wang, H.; Sun, Y., Revealing and Regulating the Complex Reaction Mechanism of CO2 Hydrogenation to Higher Alcohols on Multifunctional Tandem Catalysts. Acs Catal 2023, 13, (5), 3055-3065.
- Liu, T.; Xu, D.; Song, M.; Hong, X.; Liu, G., K-ZrO2 Interfaces Boost CO2 Hydrogenation to Higher Alcohols. Acs Cata 2023, 13, 4667-4674.
- Chen, J.; Zha, Y.; Liu, B.; Li, Y.; Xu, Y.; Liu, X. Rationally Designed Water Enriched Nano Reactor for Stable CO2 Hydrogenation with Near 100% Ethanol Selectivity over Diatomic Palladium Active Sites. ACS Catal. 2023, 13, 7110–7121. [CrossRef]
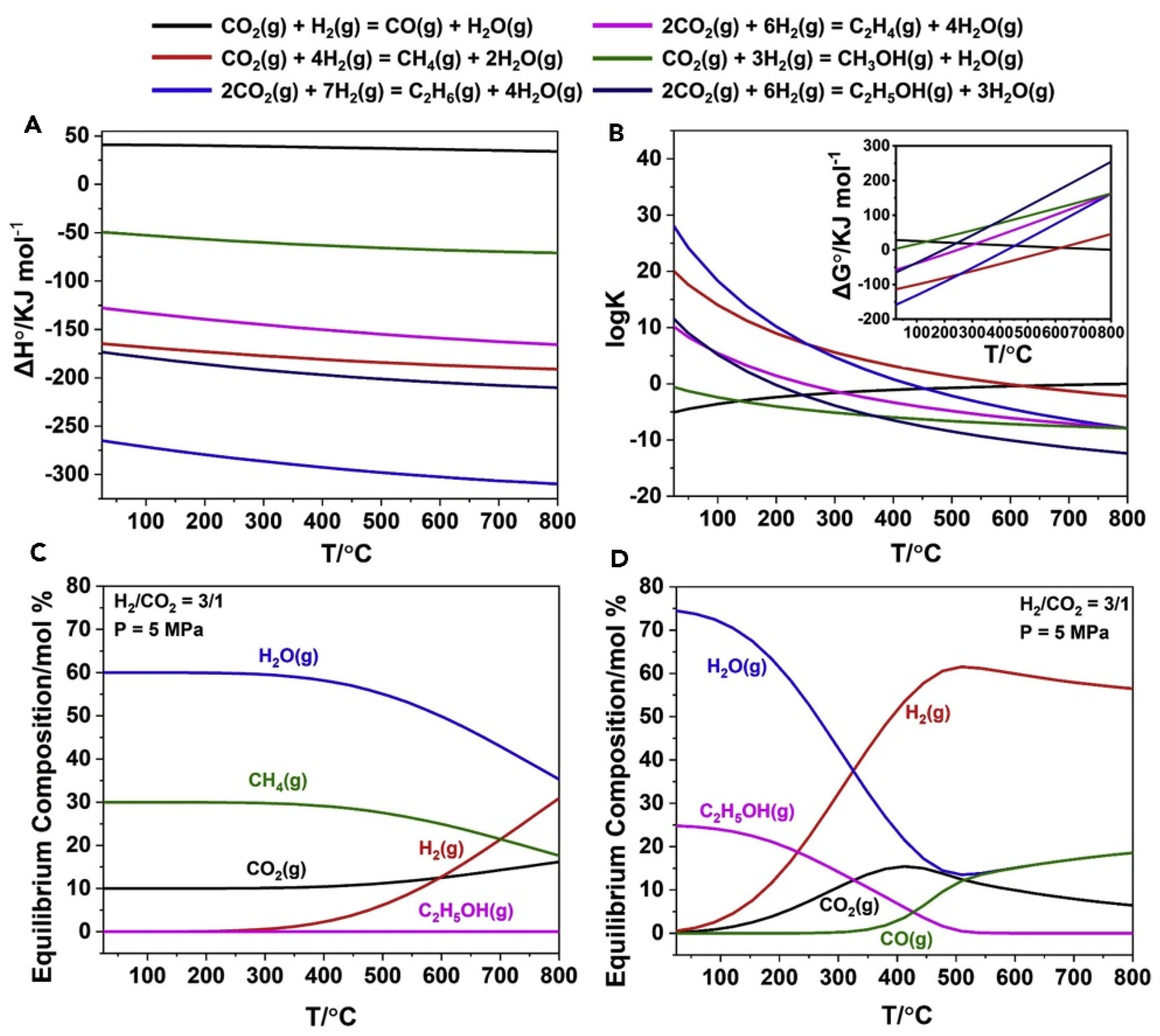
| Reaction Equation | △G298 K (kJ/mol) |
△H298 K (kJ/mol) |
K298K |
|---|---|---|---|
| CO2 + H2 ↔ CO + H2O | 28.6 | 41.1 | 9.67×10-6 |
| CO2 + 4H2 ↔ CH4 + 2H2O | -113.5 | -165.0 | 7.79×1019 |
| n CO2 + (3n+1) H2 ↔ CnH2n+2 + 2n H2O | |||
| n CO2 + 3n H2 ↔ CnH2n + 2n H2O | |||
| CO2 +3H2 ↔ CH3OH + H2O | 3.5 | -49.3 | 2.45×10-1 |
| 2CO2 + 6H2 ↔ C2H5OH +3H2O | -32.4 | -86.7 | 4.70×105 |
| n CO2 + 3n H2 ↔ CnH2n+1OH + (2n-1) H2O |
| Enter | Catalysts | T (℃) |
P (MPa) |
GHSV/ L.g-1h-1 |
H2/ CO2 |
XCO2 (%) |
SCO (%) |
SHC (%) |
SMeOH (%) |
SHA (%) |
STYHA (mmolgcat-1h-1) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | In2O3 | 200 | 5 | / | 4 | 0.1 | / | / | 100 | / | / | [61] |
| 2 | 2.5K5Co-In2O3 | 380 | 4 | / | 3 | 36.6 | 80.8 | 33.9 | 8.2 | 57.9 | 3.73 | [64] |
| 3 | In2Fe/K-Al2O3 | 300 | 2 | 4 | 3 | 36.7 | 7.4 | 37.9 | 2.3 | 42 | / | [65] |
| 4 | Ir1-In2O3 | 200 | 6 | / | 5 | / | / | / | / | 99.7 | 0.99 | [66] |
| 5 | Co/La4Ga2O9 | 280 | 3 | 3 | 3 | 9.6 | 10.8 | 52.2 | 13.7 | 23.3 | / | [67] |
| 6 | Na-Co/SiO2 | 250 | 5 | 6 | 3 | 21.5 | 26.3 | 61.2 | 1.7 | 10.8 | 0.47 | [68] |
| 7 | Mo-Co-K | 320 | 5 | 3 | 3 | 23.5 | / | 21 | 70 | 8.9 | / | [29] |
| 8 | CoMoCx-800 | 180 | 2 | / | 3 | / | / | / | / | / | 0.53 | [69] |
| 9 10 11 |
Cu-CoGa-0.4 Na-CuCo-9 Cs-C0.8F1.0Z1.0 |
220 330 330 |
3 4 5 |
6 5 4 |
3 1 3 |
17.8 20.1 36.6 |
2.3 26.5 / |
43.5 53.8 / |
27.5 0.5 1.2 |
23.8 26.3 18.6 |
1.35 1.09a 1.47 |
[70] [71] [72] |
| 12 | Cr-CuFe | 320 | 4 | 6 | 3 | 38.4 | 14.8 | / | / | 29.2 | 104.1b | [73] |
| 13 | sp-CuNaFe | 310 | 3 | 28.8 | / | 32.2 | / | / | / | 10 | 153b | [74] |
| Enter | Catalysts | T (℃) |
P (MPa) |
GHSV/ L.g-1h-1 |
H2/ CO2 |
XCO2 (%) |
SCO (%) |
SHC (%) |
SMeOH (%) |
SHA (%) |
STYHA (mmolgcat-1h-1) |
Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CuZnAlK/FeCuAlK | 330 | 8 | 20 | 3 | 54.5 | 9.7 | 64.5 | 5.2 | 17.0 | / | [75] | |
| 2 | CZK||CFCK | 350 | 6 | 5 | 3 | 32.4 | 45.3 | 42.9 | 5.3 | 6.5 | 1.37 | [76] | |
| 3 | Na-Fe@C/KCuZnAl | 320 | 5 | / | 2.8 | 39.2 | 9.4 | 54.5 | 4.6 | 35 | / | [77] | |
| 4 | MnCuK-FeC/CuZnAlZr | 300 | 3 | 6 | 3 | 42.1 | 22.7 | 60.6 | 1.2 | 15.5 | / | [79] | |
| 5 | CuZnAl/K-CuMgZnFe | 320 | 5 | 6 | 3 | 42.3 | 13.8 | 67.6 | 1.3 | 17.4 | 2.24 | [78] | |
| 6 | 4.7KCuFeZn/CuZnAlZr | 300 | 5 | 3 | 3 | 29.4 | 26.1 | 66.7 | 2.1 | 31.2 | / | [80] | |
| 7 | Co2C||CuZnAl | 250 | 5 | 12 | 3 | 21.2 | / | / | / | 18.3 | 2.20 | [81] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




