Submitted:
07 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Importance of Melatonin in Humans and Animals
2.1. Melatonin Functions
2.2. Factors Affecting Melatonin Synthesis and Release
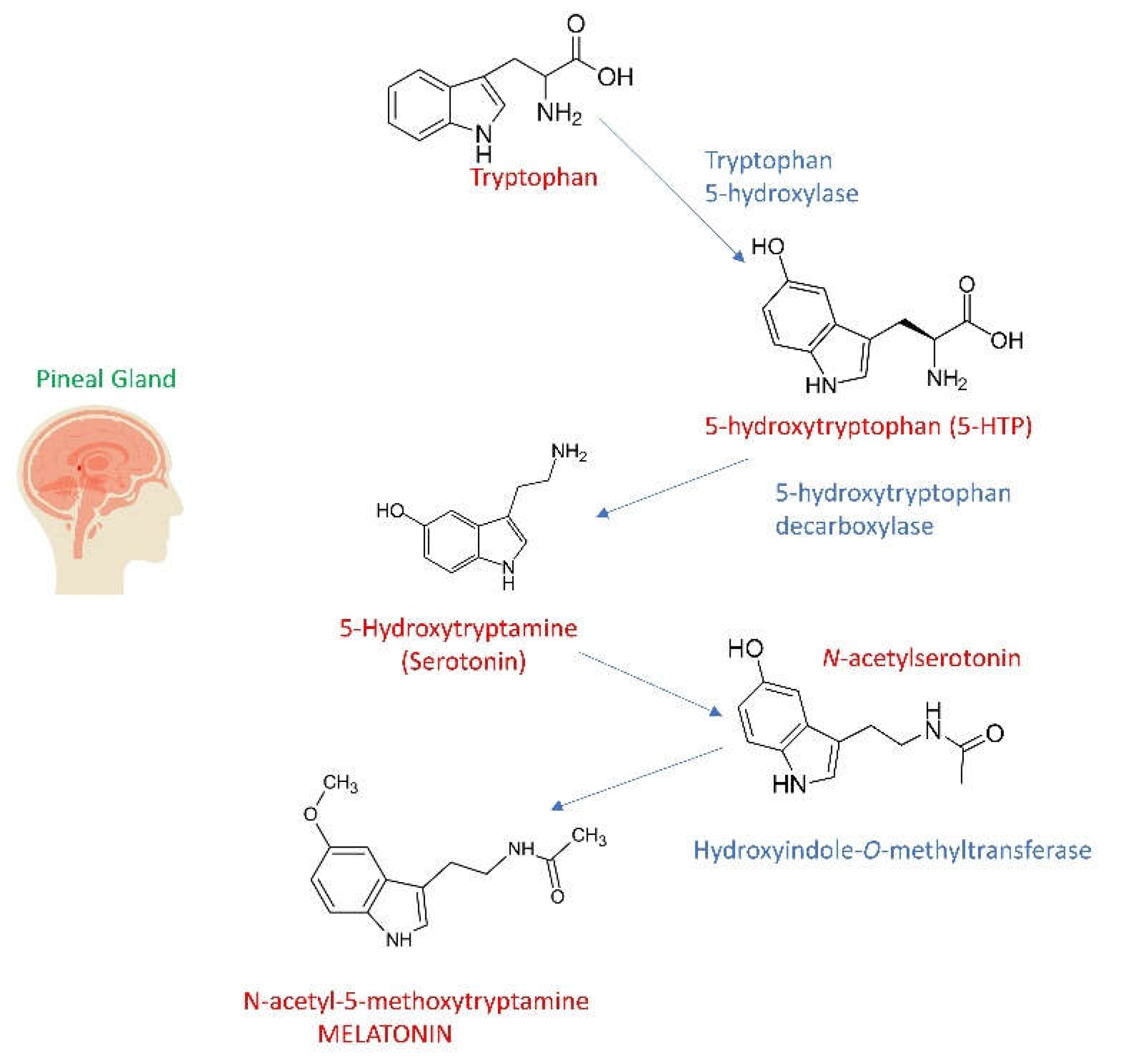
2.3. Melatonin Biosynthesis and Sources
2.4. Melatonin Synthesis Sites
2.5. Effects of Melatonin on Physiological Functions
2.5.1. Effect on Lymphoid Tissues
2.5.2. Presence of Melatonin in the Digestive System
2.5.3. Effect of Melatonin on the Cardiovascular System
2.5.4. Daily and Annual Rhythm Regulation and Photoperiodic Response
2.5.5. Effect of Melatonin on Aging
2.5.6. Effects of Melatonin on the Immune System and Cancer
2.5.7. Free Radical Scavenging and Antioxidant Effect of Melatonin
2.5.8. Effect of Melatonin on Sleep
2.5.9. Melatonin's Relationship with the Reproductive System and Other Hormones
2.5.10. Effect of Melatonin on Aging
2.5.11. Effect of Melatonin on Jetlag
2.5.12. Effect of Melatonin on İnsulin Secretion
3. Presence of Melatonin ın Plants
3.1. Melatonin Formation in Plants
3.2. Edible Vegetable Sources of Melatonin
3.3. Melatonin Uptake in Plants
3.4. Function of Melatonin in the Regulation of Plant Development and Physiology
3.4.1. Function of Melatonin on Seed Germination
3.4.2. Function of Melatonin on Photosynthesis
3.4.3. The Function of Melatonin in the Regulation of Reproduction, Development and Circadian Rhythm of Plants
3.4.4. Function of Melatonin on the Antioxidant Defense System
3.4.5. Effect of Melatonin on Supporting Defense Mechanisms against Various Stress Elements in Plants
3.4.6. Function of Melatonin in Biomass Increase in Plants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiter, R.J. Melatonin: The Chemical Expression of Darkness. Molecular and Cellular Endocrinology 1991, 79, C153–C158. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.B.; Siopes, T.D. Melatonin Enhances Cellular and Humoral Immune Responses in the Japanese Quail (Coturnix Coturnix Japonica) via an Opiatergic Mechanism. General and Comparative Endocrinology 2003, 131, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.-X. Reducing Oxidative/Nitrosative Stress: A Newly-Discovered Genre for Melatonin. Critical Reviews in Biochemistry and Molecular Biology 2009, 44, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Çam, A.; Erdoğan, M.F. Melatonin. Ankara Üniversitesi Tıp Fakültesi Mecmuası 2003, 56. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal Melatonin: Cell Biology of Its Synthesis and of Its Physiological Interactions*. Endocrine Reviews 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; García-Mauriño, S.; Reiter, R.J.; Guerrero, J.M. Evidence of Melatonin Synthesis by Human Lymphocytes and Its Physiological Significance: Possible Role as Intracrine, Autocrine, and/or Paracrine Substance. The FASEB Journal 2004, 18, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. ISOLATION OF MELATONIN, THE PINEAL GLAND FACTOR THAT LIGHTENS MELANOCYTES1. J. Am. Chem. Soc. 1958, 80, 2587–2587. [Google Scholar] [CrossRef]
- Bubenik, G.A. Localization, Physiological Significance and Possible Clinical Implication of Gastrointestinal Melatonin. Biological Signals and Receptors 2001, 10, 350–366. [Google Scholar] [CrossRef]
- Melatonin Effects on Bone: Experimental Facts and Clinical Perspectives - Cardinali - 2003 - Journal of Pineal Research - Wiley Online Library Available online:. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1034/j.1600-079X.2003.00028.x?sid=nlm%3Apubmed (accessed on 4 March 2024).
- Beyer, C.E.; Steketee, J.D.; Saphier, D. Antioxidant Properties of Melatonin—an Emerging Mystery. Biochemical Pharmacology 1998, 56, 1265–1272. [Google Scholar] [CrossRef]
- Atasoy, N. Melatonin ve Antioksidan Etkileri. Düzce Üniversitesi Sağlık Bilimleri Enstitüsü Dergisi 2019, 9, 196–201. [Google Scholar] [CrossRef]
- Juhnevica-Radenkova, K.; Moreno, D.A.; Ikase, L.; Drudze, I.; Radenkovs, V. Naturally Occurring Melatonin: Sources and Possible Ways of Its Biosynthesis. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 4008–4030. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Hardeland, R. Detection and Quantification of Melatonin in a Dinoflagellate, Gonyaulax Polyedra: Solutions to the Problem of Methoxyindole Destruction in Non-Vertebrate Material. Journal of Pineal Research 1994, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- BAGHURST, R.; COGHILL, R. A STUDY OF MELATONIN IN PLANT TISSUES AND ITS DIETARY AND HEALTH IMPLICATIONS. In Proceedings of the BIOELECTROMAGNETICS Current Concepts; Ayrapetyan, S.N., Markov, M.S., Eds.; Springer Netherlands: Dordrecht, 2006; pp. 405–412. [Google Scholar]
- Hardeland, R. Melatonin and 5-methoxytryptamine in non-metazoans. Reprod. Nutr. Dev. 1999, 39, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Phytomelatonin: Discovery, Content, and Role in Plants. Advances in Botany 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Feng, X.; Wang, M.; Zhao, Y.; Han, P.; Dai, Y. Melatonin from Different Fruit Sources, Functional Roles, and Analytical Methods. Trends in Food Science & Technology 2014, 37, 21–31. [Google Scholar] [CrossRef]
- Claustrat, B.; Geoffriau, M.; Brun, J.; Chazot, G. Melatonin in Humans: A Biochemical Marker of the Circadian Clock and an Endogenous Synchronizer. Neurophysiologie Clinique/Clinical Neurophysiology 1995, 25, 351–359. [Google Scholar] [CrossRef]
- Brzezinski, A. Melatonin in Humans. New England Journal of Medicine 1997, 336, 186–195. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front Endocrinol (Lausanne) 2019, 10, 249. [Google Scholar] [CrossRef]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F.A.J.L. Melatonin Effects on Glucose Metabolism: Time To Unlock the Controversy. Trends in Endocrinology & Metabolism 2020, 31, 192–204. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Czeisler, C.A. Melatonin, Sleep, and Circadian Rhythms. Sleep Medicine Reviews 2005, 9, 5–9. [Google Scholar] [CrossRef]
- Özçelik, F.; Erdem, M.; Bolu, A.; Gülsün, M. Melatonin: Genel Özellikleri ve Psikiyatrik Bozukluklardaki Rolü. Psikiyatride Güncel Yaklaşımlar 2013, 5, 179–203. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Neuroactive Compounds in Foods: Occurrence, Mechanism and Potential Health Effects. Food Research International 2020, 128, 108744. [Google Scholar] [CrossRef] [PubMed]
- Kleszczyński, K.; Slominski, A.T.; Steinbrink, K.; Reiter, R.J. Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed. Nutrients 2020, 12, 2561. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Franceschetti, L.; Buffoli, B.; Moghadasian, M.H.; Reiter, R.J.; Rodella, L.F.; Rezzani, R. Melatonin: Protection against Age-Related Cardiac Pathology. Ageing Research Reviews 2017, 35, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L. de; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-Inflammatory Effects of Melatonin: A Mechanistic Review. Critical Reviews in Food Science and Nutrition 2019, 59, S4–S16. [Google Scholar] [CrossRef]
- Karunanithi, D.; Radhakrishna, A.; Sivaraman, K.P.; Biju, V.M.N. Quantitative Determination of Melatonin in Milk by LC-MS/MS. J Food Sci Technol 2014, 51, 805–812. [Google Scholar] [CrossRef] [PubMed]
- ÖZTÜRK, G.; AKBULUT, K.; GÜNEY, Ş. Melatonin, Aging, and COVID-19: Could Melatonin Be Beneficial for COVID-19 Treatment in the Elderly? Turkish Journal of Medical Sciences 2020, 50, 1504–1512. [Google Scholar] [CrossRef]
- Yakupoğlu, G.; Köklü, Ş.; Korkmaz, A. Bitkilerde Melatonin ve Üstlendiği Görevler. Kahramanmaraş Sütçü İmam Üniversitesi Doğa Bilimleri Dergisi 2018. [Google Scholar] [CrossRef]
- Brainard, G.C.; Rollag, M.D.; Hanifin, J.P. Photic Regulation of Melatonin in Humans: Ocular and Neural Signal Transduction. J Biol Rhythms 1997, 12, 537–546. [Google Scholar] [CrossRef]
- Koçak, D.A.; Çolak, D.A. Melatonin ve Santral Sinir Sistemi. Turgut Özal Tıp Merk Derg 1996, 3. [Google Scholar]
- Erzincan Binali Yildirim Universitesi Tip Fakultesi, Fizyoloji Ana Bilim Dali, Erzincan, Turkiye; Ustundag, H.; Senturk, E.; Agri Ibrahim Cecen Universitesi Saglik Bilimleri Fakultesi, Hemsirelik Bolumu, Agri, Turkiye; Gul, M.; Ataturk Universitesi Tip Fakultesi, Fizyoloji Ana Bilim Dali, Ezurum, Turkiye Melatonin and Hyperthyroidism. Arch Basic Clin Res 2020, 2, 59–64. [CrossRef]
- Bernard, M.; Guerlotté, J.; Grève, P.; Gréchez-Cassiau, A.; Iuvone, M.P.; Zatz, M.; Chong, N.W.; Klein, D.C.; Voisin, P. Melatonin synthesis pathway: circadian regulation of the genes encoding the key enzymes in the chicken pineal gland and retina. Reprod. Nutr. Dev. 1999, 39, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Bolliet, V.; Ali, M.A.; Lapointe, F.-J.; Falcón, J. Rhythmic Melatonin Secretion in Different Teleost Species: An in Vitro Study. J Comp Physiol B 1996, 165, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. Melatonin Receptors in Humans: Biological Role and Clinical Relevance. Biomedicine & Pharmacotherapy 2006, 60, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Şahin, D. In vitro koşullarda sirkadien melatonin etkisine maruz bırakılan embriyolarda SOD ve HMGB1 genlerinin ekspresyonları ile melatonin etkisinin takibi. masterThesis, İstanbul Bilim Üniversitesi, Sağlık Bilimleri Enstitüsü., 2014.
- Siu, A.W.; Reiter, R.J.; To, C.H. The Efficacy of Vitamin E and Melatonin as Antioxidants against Lipid Peroxidation in Rat Retinal Homogenates. Journal of Pineal Research 1998, 24, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. The Pineal Gland and Melatonin in Relation to Aging: A Summary of the Theories and of the Data. Experimental Gerontology 1995, 30, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.P.N.; Shiu, S.Y.W.; Chow, P.H.; Pang, S.F. Regional and Diurnal Studies of Melatonin and Melatonin Binding Sites in the Duck Gastro-Lntestinal Tract. Neurosignals 1996, 4, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on Melatonin Receptors: IUPHAR Review 20. British Journal of Pharmacology 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Godson, C.; Mahle, C.D.; Weaver, D.R.; Slaugenhaupt, S.A.; Gusella, J.F. Molecular Characterization of a Second Melatonin Receptor Expressed in Human Retina and Brain: The Mel1b Melatonin Receptor. Proceedings of the National Academy of Sciences 1995, 92, 8734–8738. [Google Scholar] [CrossRef]
- Nosjean, O.; Ferro, M.; Cogé, F.; Beauverger, P.; Henlin, J.-M.; Lefoulon, F.; Fauchère, J.-L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the Melatonin-Binding SiteMT 3 as the Quinone Reductase 2 *. Journal of Biological Chemistry 2000, 275, 31311–31317. [Google Scholar] [CrossRef]
- Uz, T.; Arslan, A.D.; Kurtuncu, M.; Imbesi, M.; Akhisaroglu, M.; Dwivedi, Y.; Pandey, G.N.; Manev, H. The Regional and Cellular Expression Profile of the Melatonin Receptor MT1 in the Central Dopaminergic System. Molecular Brain Research 2005, 136, 45–53. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in Aging and Disease —Multiple Consequences of Reduced Secretion, Options and Limits of Treatment. Aging Dis 2011, 3, 194–225. [Google Scholar]
- Dubocovich, M.L. Melatonin Receptors: Are There Multiple Subtypes? Trends in Pharmacological Sciences 1995, 16, 50–56. [Google Scholar] [CrossRef]
- Morgan, P.J.; Barrett, P.; Howell, H.E.; Helliwell, R. Melatonin Receptors: Localization, Molecular Pharmacology and Physiological Significance. Neurochemistry International 1994, 24, 101–146. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Smith, D.G.; Hardeland, R.; Yang, M.Y.; Xu, H.L.; Zhang, L.; Yin, H.D.; Zhu, Q. Melatonin Receptor Genes in Vertebrates. International Journal of Molecular Sciences 2013, 14, 11208–11223. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Golombek, D.A.; Rosenstein, R.E.; Cutrera, R.A.; Esquifino, A.I. Melatonin Site and Mechanism of Action: Single or Multiple? Journal of Pineal Research 1997, 23, 32–39. [Google Scholar] [CrossRef]
- Slaugenhaupt, S.A.; Roca, A.L.; Liebert, C.B.; Altherr, M.R.; Gusella, J.F.; Reppert, S.M. Mapping of the Gene for the Mel1a-Melatonin Receptor to Human Chromosome 4 (MTNR1A) and Mouse Chromosome 8 (Mtnr1a). Genomics 1995, 27, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 Melatonin Receptors in Mammals. Endocr 2005, 27, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Witt-Enderby, P.A.; Bennett, J.; Jarzynka, M.J.; Firestine, S.; Melan, M.A. Melatonin Receptors and Their Regulation: Biochemical and Structural Mechanisms. Life Sciences 2003, 72, 2183–2198. [Google Scholar] [CrossRef]
- Atasoy, Ö.B.; Erbaş, O. Melatonin hormonunun fizyolojik etkileri. İstanbul Bilim Üniversitesi Florence Nightingale Tıp Dergisi 2017, 3, 52–62. [Google Scholar] [CrossRef]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet Promotes Sleep Duration and Quality. Nutrition Research 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Burgess, H.J.; Fogg, L.F. Individual Differences in the Amount and Timing of Salivary Melatonin Secretion. PLOS ONE 2008, 3, e3055. [Google Scholar] [CrossRef] [PubMed]
- WALDHAUSER, F.; WEISZENBACHER, G.; TATZER, E.; GISINGER, B.; WALDHAUSER, M.; SCHEMPER, M.; FRISCH, H. Alterations in Nocturnal Serum Melatonin Levels In Humans With Growth and Aging*. The Journal of Clinical Endocrinology & Metabolism 1988, 66, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Molina-Carballo, A.; Munoz-Hoyos, A.; Martin-Garcia, J. a.; Uberos-Feritindez, J.; Rodriguez-Cabezas, T.; Acuna-Castroviejo, D. 5-Methoxytryptophol and Melatonin in Children: Differences Due to Age and Sex. Journal of Pineal Research 1996, 21, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Vacas, M.I. Pineal Gland, Photoperiodic Responses, and Puberty. J Endocrinol Invest 1984, 7, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous Melatonin Confers Drought Stress by Promoting Plant Growth, Photosynthetic Capacity and Antioxidant Defense System of Maize Seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem Mol Biol Int 1995, 35, 627–634. [Google Scholar] [PubMed]
- Posmyk, M.M.; Janas, K.M. Melatonin in Plants. Acta Physiologiae Plantarum 2009, 31. [Google Scholar] [CrossRef]
- Martin, M.T.; Azpiroz, F.; Malagelada, J.R. Melatonin and the Gastrointestinal Tract. Therapie 1998, 53, 453–458. [Google Scholar]
- Maestroni, G.J.M.; Conti, A.; Pierpaoli, W. The Pineal Gland and the Circadian, Opiatergic, Immunoregulatory Role of Melatonin. Annals of the New York Academy of Sciences 1987, 496, 67–77. [Google Scholar] [CrossRef]
- Sewerynek, E. Melatonin and the Cardiovascular System. Neuro Endocrinol Lett 2002, 23 Suppl 1, 79–83. [Google Scholar]
- Paulis, L.; Simko, F. Blood Pressure Modulation and Cardiovascular Protection by Melatonin: Potential Mechanisms Behind. Physiol Res 2007, 56, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.A.; Dodd, A.N. Circadian Regulation of Chloroplasts. Current Opinion in Plant Biology 2014, 21, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin Suppressed the Heat Stress-Induced Damage in Wheat Seedlings by Modulating the Antioxidant Machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Kolář, J.; Macháčková, I.; Eder, J.; Prinsen, E.; van Dongen, W.; van Onckelen, H.; Illnerová, H. Melatonin: Occurrence and Daily Rhythm in Chenopodium Rubrum. Phytochemistry 1997, 44, 1407–1413. [Google Scholar] [CrossRef]
- Wolf, K.; Kolář, J. a. n.; Witters, E.; van Dongen, W.; van Onckelen, H.; Macháčková, I. Daily Profile of Melatonin Levels in Chenopodium Rubrum L. Depends on Photoperiod. Journal of Plant Physiology 2001, 158, 1491–1493. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Simopoulos, A.P.; Maldonado, M.D.; Flores, L.J.; Terron, M.P. Melatonin in Edible Plants (Phytomelatonin): Identification, Concentrations, Bioavailability and Proposed Functions. World Rev Nutr Diet 2007, 97, 211–230. [Google Scholar] [CrossRef]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT Improves Melatonin Production and Enhances Drought Tolerance in Transgenic Arabidopsis Thaliana Plants. Journal of Pineal Research 2014, 57, 408–417. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Melatonin and Synthetic Melatonergic Agonists: Actions and Metabolism in the Central Nervous System. Central Nervous System Agents in Medicinal Chemistry 12, 189–216.
- Hardeland, R. Melatonin and the Theories of Aging: A Critical Appraisal of Melatonin’s Role in Antiaging Mechanisms. Journal of Pineal Research 2013, 55, 325–356. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.; Osuna, C.; Gitto, E. Actions of Melatonin in the Reduction of Oxidative Stress. J Biomed Sci 2000, 7, 444–458. [Google Scholar] [CrossRef]
- Maestroni, G.J.M. Therapeutic Potential of Melatonin in Immunodeficiency States, Viral Diseases, and Cancer. In Tryptophan, Serotonin, and Melatonin: Basic Aspects and Applications; Huether, G., Kochen, W., Simat, T.J., Steinhart, H., Eds.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, 1999; pp. 217–226. ISBN 978-1-4615-4709-9. [Google Scholar]
- Simonneaux, V.; Ribelayga, C. Generation of the Melatonin Endocrine Message in Mammals: A Review of the Complex Regulation of Melatonin Synthesis by Norepinephrine, Peptides, and Other Pineal Transmitters. Pharmacol Rev 2003, 55, 325–395. [Google Scholar] [CrossRef] [PubMed]
- Gümüşova, S.; Memi̇ş, Y.S. Bazı Viral Enfeksiyonlarda Melatoninin Etkileri. Atatürk Üniversitesi Veteriner Bilimleri Dergisi 2014, 9, 50–54. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annual Review of Pharmacology and Toxicology 2016, 56, 361–383. [Google Scholar] [CrossRef]
- Hotchkiss, A.K.; Nelson, R.J. Melatonin and Immune Function: Hype or Hypothesis? Crit Rev Immunol 2002, 22, 351–371. [Google Scholar]
- Lissoni, P.; Barni, S.; Tancini, G.; Mainini, E.; Piglia, F.; Maestroni, G.J.; Lewinski, A. Immunoendocrine Therapy with Low-Dose Subcutaneous Interleukin-2 plus Melatonin of Locally Advanced or Metastatic Endocrine Tumors. Oncology 1995, 52, 163–166. [Google Scholar] [CrossRef]
- Topal, T.; Öter, S.; Korkmaz, A. Melatonin ve Kanserle Ilişkisi: Journal of General Medicine / Genel Tıp Dergisi. Journal of General Medicine / Genel Tıp Dergisi 2009, 19, 137–143. [Google Scholar]
- Escames, G.; Guerrero, J.M.; Reiter, R.J.; Garcia, J.J.; Munoz-Hoyos, A.; Ortiz, G.G.; Oh, C.S. Melatonin and Vitamin E Limit Nitric Oxide-Induced Lipid Peroxidation in Rat Brain Homogenates. Neuroscience Letters 1997, 230, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y. Human Aging and Melatonin. Clinical Relevance. Experimental Gerontology 2001, 36, 1083–1100. [Google Scholar] [CrossRef] [PubMed]
- Salt, A.; Çenesiz, M.; Çenesiz, S. Melatonin, Etkileri ve Kullanım Alanları. Etlik Vet. Mik. Derg. 2017, 28, 7–12. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of Melatonin in the Reduction of Oxidative Stress. A Review. J Biomed Sci 2000, 7, 444–458. [Google Scholar] [CrossRef]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and Its Relation to the Immune System and Inflammation. Annals of the New York Academy of Sciences 2000, 917, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Interactions of the Pineal Hormone Melatonin with Oxygen-Centered Free Radicals: A Brief Review. Braz J Med Biol Res 1993, 26, 1141–1155. [Google Scholar] [PubMed]
- Garfinkel, D.; Laudon, M.; Nof, D.; Zisapel, N. Improvement of Sleep Quality in Elderly People by Controlled-Release Melatonin. Lancet 1995, 346, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Macchi, M.M.; Bruce, J.N. Human Pineal Physiology and Functional Significance of Melatonin. Frontiers in Neuroendocrinology 2004, 25, 177–195. [Google Scholar] [CrossRef]
- Arendt, J. Melatonin, Circadian Rhythms, and Sleep. New England Journal of Medicine 2000, 343, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Oztekin, E.; Mogulkoc, R.; Baltaci, A.K.; Tiftik, A.M. The Influence of Estradiol and Progesterone and Melatonin Supplementation on TNF-Alpha Levels in Ovariectomized and Pinealectomized Rats. Acta Biol Hung 2006, 57, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Oztürk, A.; Baltaci, A.K.; Bediz, C.S.; Mogulkoc, R.; Güngör, S. Effects of Zinc and Melatonin Deficiency on Testicular Tissue of Rats. Biol Trace Elem Res 2003, 96, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K. Melatonin, immün sistem ve çinko. Selcuk Med J 2001, 17, 267–272. [Google Scholar]
- Wurtman, R.J.; Axelrod, J.; Chu, E.W. Melatonin, a Pineal Substance: Effect on the Rat Ovary. Science 1963, 141, 277–278. [Google Scholar] [CrossRef]
- Nagy, P.; Guillaume, D.; Daels, P. Seasonality in Mares. Animal Reproduction Science 2000, 60–61, 245–262. [Google Scholar] [CrossRef]
- Uyar, A.; Alan, M. Koyunlarda Erken Anöstrüs Döneminde Melatonin Uygulamalarının Ovulasyon Ve Gebelik Üzerine Etkisi. YYU Vet Fak Derg 2008, 19, 47–54. [Google Scholar]
- Ölmez, E.; Şahna, E.; Ağkadi̇r, M.; Acet, A. MELATONİN: EMEKLİLİK YAŞI 80 OLUR MU? Turgut Özal Tıp Merk Derg 2000, 7. [Google Scholar]
- Ninomiya, T.; Iwatani, N.; Tomoda, A.; Miike, T. Effects of Exogenous Melatonin on Pituitary Hormones in Humans. Clinical Physiology 2001, 21, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Turgut, M.; Uysal, A.; Yurtseven, M. Epifiz Bezinin Morfolojik Özellikleri, Embriyolojik Gelişimi ve Deneysel Greftleme İşlemleri. aktd 2003, 12. [Google Scholar]
- Mollaoğlu, H.; Özgüner, M. fehmi Yaşlanma Sürecinde Melatoninin Rolü. SDÜ Tıp Fak Derg 2009, 12, 52–56. [Google Scholar] [CrossRef]
- Kerman, M.; Cirak, B.; Ozguner, M.F.; Dagtekin, A.; Sutcu, R.; Altuntas, I.; Delibas, N. Does Melatonin Protect or Treat Brain Damage from Traumatic Oxidative Stress? Exp Brain Res 2005, 163, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Jet Lag: Therapeutic Use of Melatonin and Possible Application of Melatonin Analogs. Travel Medicine and Infectious Disease 2008, 6, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Diabetic Goto Kakizaki Rats as Well as Type 2 Diabetic Patients Show a Decreased Diurnal Serum Melatonin Level and an Increased Pancreatic Melatonin-receptor Status - Peschke - 2006 - Journal of Pineal Research - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-079X.2005.00287.x (accessed on 29 March 2024).
- Peschke, E.; Stumpf, I.; Bazwinsky, I.; Litvak, L.; Dralle, H.; Mühlbauer, E. Melatonin and Type 2 Diabetes - a Possible Link? J Pineal Res 2007, 42, 350–358. [Google Scholar] [CrossRef]
- Wolden-Hanson, T.; Mitton, D.R.; McCants, R.L.; Yellon, S.M.; Wilkinson, C.W.; Matsumoto, A.M.; Rasmussen, D.D. Daily Melatonin Administration to Middle-Aged Male Rats Suppresses Body Weight, Intraabdominal Adiposity, and Plasma Leptin and Insulin Independent of Food Intake and Total Body Fat. Endocrinology 2000, 141, 487–497. [Google Scholar] [CrossRef]
- Cagnacci, A.; Arangino, S.; Renzi, A.; Paoletti, A.M.; Melis, G.B.; Cagnacci, P.; Volpe, A. Influence of Melatonin Administration on Glucose Tolerance and Insulin Sensitivity of Postmenopausal Women. Clin Endocrinol (Oxf) 2001, 54, 339–346. [Google Scholar] [CrossRef]
- Champney, T.H.; Steger, R.W.; Christie, D.S.; Reiter, R.J. Alterations in Components of the Pineal Melatonin Synthetic Pathway by Acute Insulin Stress in the Rat and Syrian Hamster. Brain Res 1985, 338, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulator during Stress? Trends in Plant Science 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A Multifunctional Factor in Plants. Int J Mol Sci 2018, 19, 1528. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. Novel Perspectives on the Molecular Crosstalk Mechanisms of Serotonin and Melatonin in Plants. Plant Physiology and Biochemistry 2018, 132, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, Y.; Shi, Y.; Li, T.; Chen, Y.; Zhao, D.; Zhao, Z. The Role of Phyto-Melatonin and Related Metabolites in Response to Stress. Molecules 2018, 23, 1887. [Google Scholar] [CrossRef]
- Acharya, D.; Satapathy, S.; Somu, P.; Parida, U.K.; Mishra, G. Apoptotic Effect and Anticancer Activity of Biosynthesized Silver Nanoparticles from Marine Algae Chaetomorpha Linum Extract Against Human Colon Cancer Cell HCT-116. Biol Trace Elem Res 2021, 199, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P.; et al. Melatonin and Its Effects on Plant Systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Van Tassel, D.L.; O’Neill, S.D. Putative Regulatory Molecules in Plants: Evaluating Melatonin. Journal of Pineal Research 2001, 31, 1–7. [Google Scholar] [CrossRef]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and Quantification of the Antioxidant Melatonin in Montmorency and Balaton Tart Cherries (Prunus Cerasus). J Agric Food Chem 2001, 49, 4898–4902. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, G.; Chang, Y.; Lin, D.; Reiter, R.J.; He, C.; Shi, H. Melatonin Biosynthesis Enzymes Recruit WRKY Transcription Factors to Regulate Melatonin Accumulation and Transcriptional Activity on W-Box in Cassava. J Pineal Res 2018, 65, e12487. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and Chloroplasts as the Original Sites of Melatonin Synthesis: A Hypothesis Related to Melatonin’s Primary Function and Evolution in Eukaryotes. Journal of Pineal Research 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Protective Effect of Melatonin against Chlorophyll Degradation during the Senescence of Barley Leaves. Journal of Pineal Research 2009, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Campbell, S.S.B.; Saxena, P.K. The Role of Serotonin and Melatonin in Plant Morphogenesis: Regulation of Auxin-Induced Root Organogenesis in in Vitro-Cultured Explants of St. John’s Wort (Hypericum Perforatum L.). In Vitro Cell.Dev.Biol.-Plant 2001, 37, 786–793. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The Physiological Function of Melatonin in Plants. Plant Signal Behav 2006, 1, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Promotes Adventitious- and Lateral Root Regeneration in Etiolated Hypocotyls of Lupinus Albus L. J Pineal Res 2007, 42, 147–152. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of Melatonin on Seedling Growth, Mineral Nutrition, and Nitrogen Metabolism in Cucumber under Nitrate Stress. J Pineal Res 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, J.-P.; Scott, E.R.; Liu, J.-W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.-Y. Exogenous Melatonin Alleviates Cold Stress by Promoting Antioxidant Defense and Redox Homeostasis in Camellia Sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef]
- Aguilera, Y.; Herrera, T.; Liébana, R.; Rebollo-Hernanz, M.; Sanchez-Puelles, C.; Martín-Cabrejas, M.A. Impact of Melatonin Enrichment during Germination of Legumes on Bioactive Compounds and Antioxidant Activity. J Agric Food Chem 2015, 63, 7967–7974. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of Melatonin in Plants: A Review. J Pineal Res 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Synthesis from Tryptophan and Its Role in Higher Plant. Amino acids in higher plants 2015, 390–435. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin Enhances Root Regeneration, Photosynthetic Pigments, Biomass, Total Carbohydrates and Proline Content in the Cherry Rootstock PHL-C (Prunus Avium × Prunus Cerasus). Plant Physiology and Biochemistry 2012, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Assessment of Different Sample Processing Procedures Applied to the Determination of Melatonin in Plants. Phytochem Anal 2009, 20, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and Reactive Oxygen and Nitrogen Species: A Model for the Plant Redox Network. Melatonin Research 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Manchester, L.C.; Tan, D.-X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High Levels of Melatonin in the Seeds of Edible Plants: Possible Function in Germ Tissue Protection. Life Sciences 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Protective Effect of Melatonin against Chlorophyll Degradation during the Senescence of Barley Leaves. J Pineal Res 2009, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing Seed Treatment with Melatonin Protects Red Cabbage Seedlings against Toxic Copper Ion Concentrations. J Pineal Res 2008, 45, 24–31. [Google Scholar] [CrossRef]
- Kolář, J.; Johnson, C.H.; Macháčková, I. Exogenously Applied Melatonin (N-Acetyl-5-Methoxytryptamine) Affects Flowering of the Short-Day Plant Chenopodium Rubrum. Physiologia Plantarum 2003, 118, 605–612. [Google Scholar] [CrossRef]
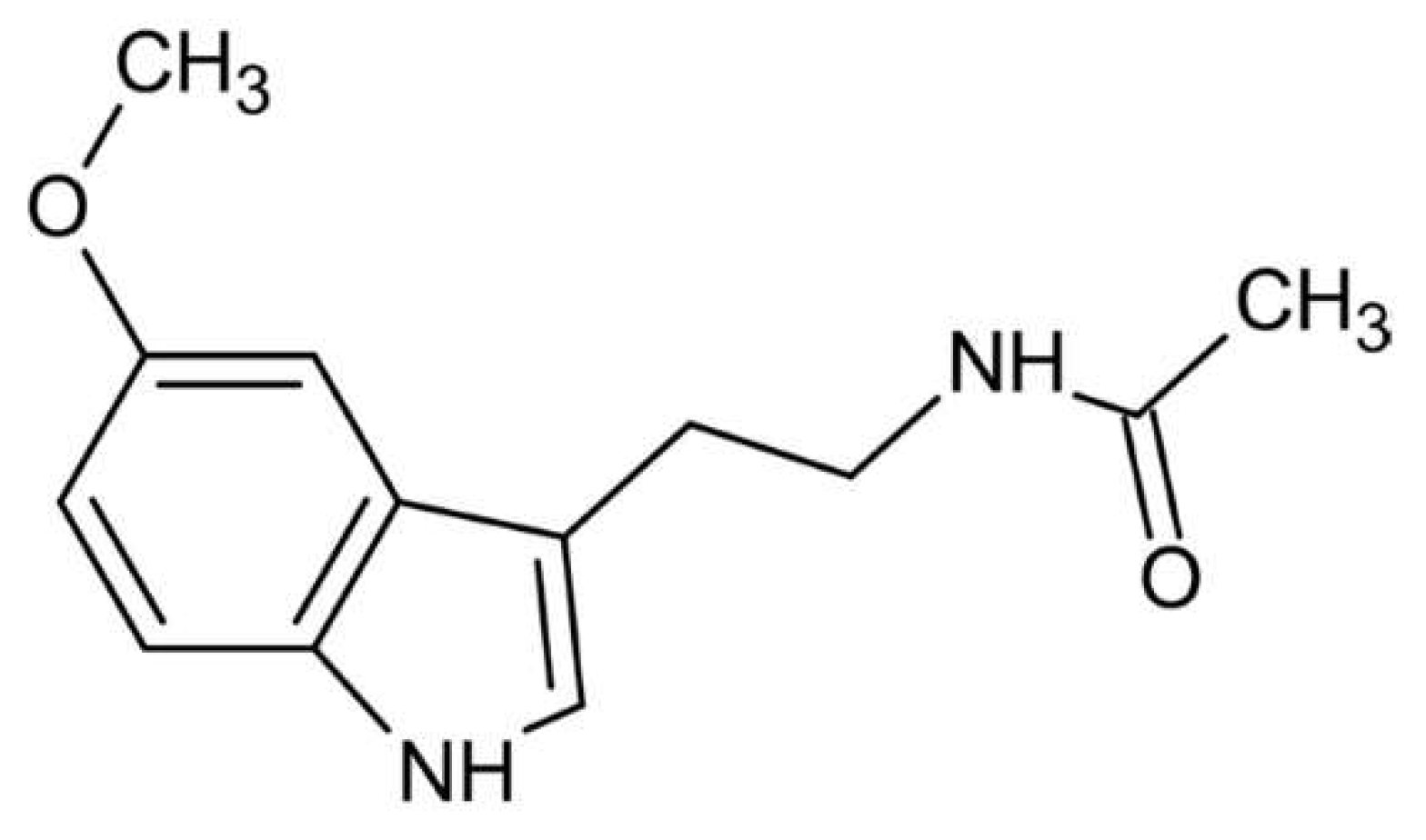
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




