1. Introduction
For some material goods production, waste cooking oils represent a more environmentally friendly alternative to mineral oils. First, vegetable oils are raw materials for a sustainable economy, unlike mineral oils obtained from crude oil, which is increasingly difficult to find, at higher and higher prices. Secondly, vegetable oils and the products obtained from them are more easily biodegradable than mineral oils and have minimal toxicity [1-2], while keeping the good characteristics of mineral lubricant oils [
3], so in case of accidental pollution, they are less dangerous than petroleum products. It is even more important to use raw materials from the second generation, such as waste vegetable oils, thus avoiding the allocation of agricultural areas namely for the cultivation of plants not ascribed for food.
The need to introduce vegetable oils to replace mineral lubricants (oils and lubricant greases) emerged during the crises caused by the two world wars and by the 1973 oil crisis [
4]. After a period of unconcern, in the first decade of this century, an increasing interest for replacing mineral oils with bio-lubricants was manifested, especially for those obtained from vegetable oils [5-8], the research focusing on their rheological properties and on increasing some of their performances: poor oxidation stability, poor performance at low temperature or high production costs [
6,
7]. These disadvantages can be mitigated by chemical modifications, thermal treatment or by additives [9-10].
Like mineral oils, the function of a grease is to provide lubrication to machine components by reducing friction and wear. Lubricating greases are used when it is not possible to use lubricating oil in a mechanism or when these mechanisms work intermittently and are inactive for a long period of time [
11].
Classical lubricating greases are produced from mineral oil, thickening soap and possibly, solid additives. Usually, the thickening soap is prepared in situ, in the base oil, from a saturated or an unsaturated fatty acid reacting with a lithium, calcium or natrium base. The preparation from vegetable oils is made generally in the same manner, just replacing the mineral oil with the vegetable one.
The manufacturing process is performed either in batches, using open or closed reactors, or in a slow and continuous process, its productivity being limited by the water evaporating rate, which must be removed from the system.
Besides the raw materials, the preparation mode influences decisively the quality of the obtained grease.
A traditional mode for the preparation of the grease with natrium soap is described by Salahuddin and Majed, in 2016 [
12]: the vegetable oil is mixed from the beginning with all the other materials, in a proportion corresponding to the production of grease with a soap content between 5% and 25% wt.; the mixture is stirred for 125 minutes at 125
oC; this time span is necessary in order to perfect the saponification reaction and to remove the water formed in reaction; then the product is cooled down slowly (during 48 hours). In many cases, the traditional methods use water free, or bound compounds (e.g. monohydrated lithium hydroxide), when starting the saponification reaction, having in view that water contributes to a better dispersion of the soap in the oil. However, from a given moment, the reaction can be inhibited by the formation of a soap layer at the surface of larger hydroxide particles.
The use of an aqueous hydroxide solution to obtain first generation greases had major shortcomings [
13]: a large amount of dispersion to be processed, high mixing energy required, the need to remove large amounts of water at the end of the reaction, a slow and expensive process.
In order to obtain a polar oil more compatible with the thickener, Grignou et al. [
14] considered the epoxidation of a part of the vegetable base oil with hydrogen peroxide in a concentration of 30-70% in an acid medium of formic acid, then obtaining a mixture of 8% epoxidized oil + 90% base oil + 2% lithium hydroxide, followed by saponification with 12-hydroxystearic acid in stoichiometric proportion to the lithium base. Replacing stearic acid with 12-hydroxystearic acid is beneficial because a soap with hydroxyl groups in the molecule is prepared, thus forming hydrogen bonds between the soap fibers with an effect on the grease consistency. Also, after the saponification of the epoxidized oil with 12-hydroxystearic acid, the resulting glycerol does not allow the formation of large soap crystals, and this improves the dropping point from 190
oC to 210
oC, compared to the unmodified base oil.
Also, lubricating greases can be produced by the alcoholysis of the vegetable oil in alkaline catalysis followed by saponification [
15]. By using the correct proportions of oil, alcohol and base, the desired grease composition can be obtained.
Instead of soaps, other thickeners can be synthesized in situ, such as: polyesters resulting from the condensation of dicarboxylic acids (succinic, sebacic, etc.) with diols (1-2 propanediol, 1-4 butanediol) [
16]. Oleo-gels with polyurea are popular thickeners [
17] with better colloidal stability, structural resistance and anti-wear performance; the studies on gel formation from a polyester-based thickener poly(hexane dodecanoate) and three different base oils (a synthetic polyolefin, a mineral oil and castor oil) show a high stability degree of the gel formed following the interaction of polyester with castor oil [
18]. More recently, ionic liquids have been added to the greases produced with lithium and calcium [
19,
20]. LDH (layered double hydroxide) oleo-gels intercalated with organic acids, being inspired by traditional greases but preparing the so-called „prepacked salts”, can replace grease soaps [
21].
Additives are often used in the formulation of greases for improving their performances, such as antiwear agents or friction modifiers among the most important.
Xu et al [
22] added tributyl borate to the classical lithium grease prepared with 12-hydroxystearate and studied the Lewis acid-base interaction between the boron atom and the R-CO
2- group in the soap, observing that this strengthened the fiber network and triggered a very good behavior of the product at high temperatures.
By using silica or clay nanoparticles [
23], or ZnO@SiO
2 composite nanoparticles [
24] as additives to lithium or calcium soap greases , their viscosity will increase; in addition, they will have better colloidal stability and, in some cases, friction and wear coefficients will decrease.
Nano and microsize graphite particles are a preferable choice as additives for improving antiwear behavior of a grease [
25], and they are employed to produce greases for hard-loadings.
Biopolymers are attractive as additives due to their abundance in nature and because of various functional groups in their molecular chain adhering to the metallic surface and forming protective layers. However, they are considered challenging due to their hydrophilic moieties which are incompatible with the hydrocarbon chain of the baseoil [
3]. But selecting the appropriate combinations, it is possible to overcome this disadvantage, through synergistic interactions of ingredients. For example, Sexena and co-workers [
3] added Gum Accacia and Guar Gum to a grease prepared with soybean oil and organoclay and the synergistic effect was reflected in increasing of antiwear characteristic by 22% and frictional response improved by 42% [
26].
The cellulose is an abundant natural polymer which meets the requirements of an environmentally friendly additive: available, renewable, nontoxic and biodegradable. But native cellulose tends to agglomerate due to different polarity to the base oil, so it is used in small concentrations, as an additive; also, it can be used as a main thickener after a chemical treatment of the cellulose and/or of the base oil to make them compatible [
27]. The highly crystalline nanocellulose was studied recently as an additive to pristine lithium greases [
28] and found to increase the consistency and greatly improve the mending and protection of metal surface in mechanisms. The nanocellulose is expensive and implies high processing cost to remove water from the grease (the nanocellulose being delivered and used in an aqueous suspension). So, it is worth to find new fabrication methods with inexpensive materials.
A research group from Ovidius University of Constanta (Romania) prepared greases from fresh vegetable oils (olive, corn, palm), thickened with calcium soap [
29] and additivated with microcrystalline cellulose and lignin fibers [30, 31]. Based on a literature review and, consequently, on the encouraging results obtained previously, we decided to continue the research using waste cooking oil, lithium/ calcium stearate prepared in situ, and vegetable additives, for valorizing waste and enhancing the sustainability of lubricating grease production.
The products previously obtained in small batches of 100 g had several characteristics like those of greases obtained from mineral oil [
32]. The addition of lignin or cellulose had a beneficial effect on the rheological properties and consistency, especially when the concentration of additives was higher. This study continues the research work [
32], in larger batches (500 g), having in view the production at larger scale without affecting the quality of the products.
2. Materials and Methods
2.1. Materials
Waste oils resulting from food frying process were procured from restaurants, to synthesize the greases. They were some of the most common in the region: sunflower oil and palm oil waste.
The deterioration of vegetable oils during frying process is caused by heat, moisture and oxygen presence, the oil undergoing thermolytic, hydrolysis and oxidations reactions, thus modifying the composition of the original material.
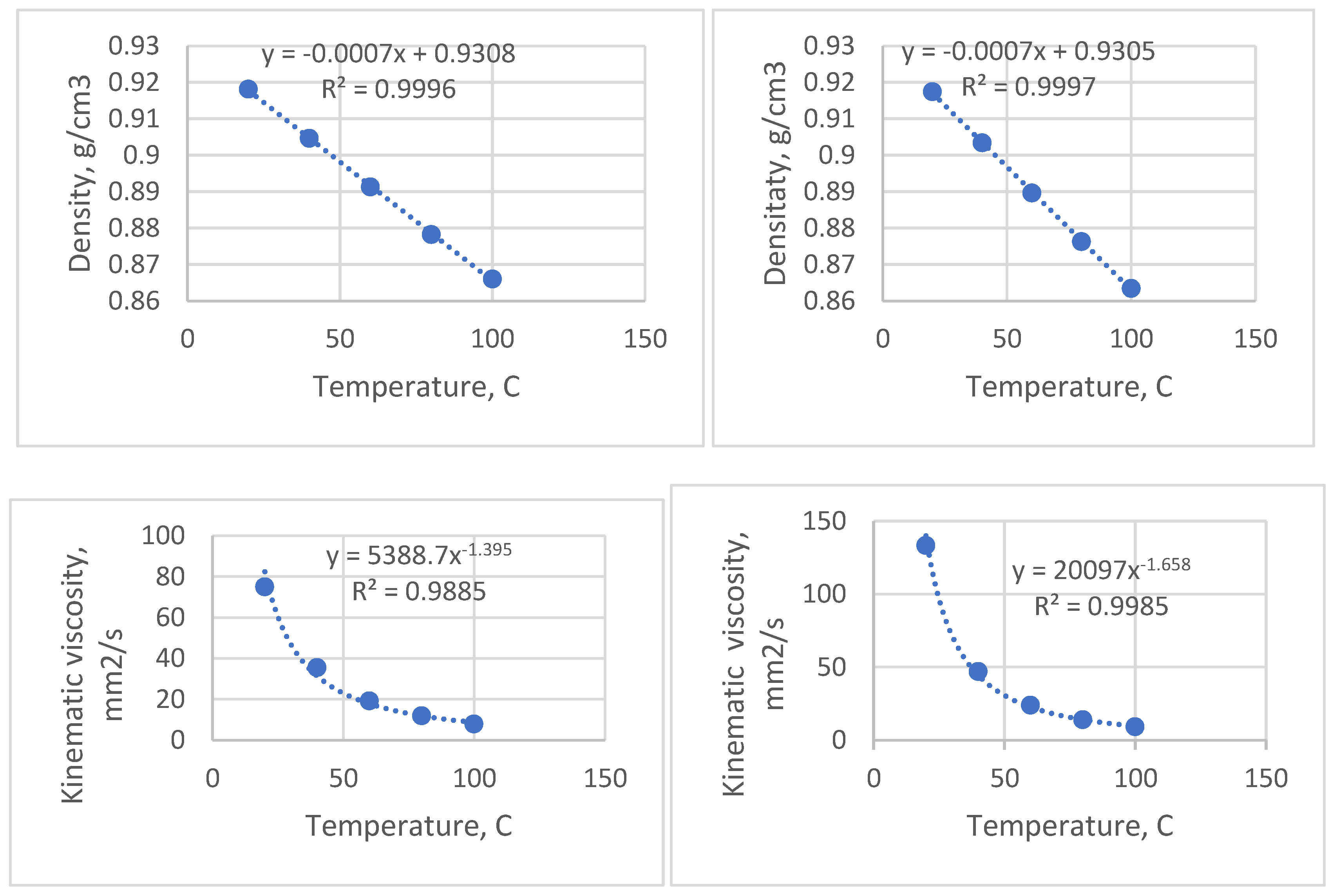
The sunflower oil was taken as a whole, since from the waste palm oil, only the oleic part (the liquid upper layer) of the waste was collected for the manufacture of the greases. The oils were prepared in the laboratory by removing gross particulates through a metallic sieve, then filtering through filtering paper for finer particles separation. Their quality was constant during the experiment, by procuring a large quantity of oils from the beginning of the experiment. The main characteristics of the oils are shown in
Table 1.
As shown in the table above, the vegetable oils are comparable in terms of density at 20
oC, while the viscosity of the palm oil at 20
oC is significantly higher than that of the sunflower oil, observation also valid for fresh oils. The flash points are still high, even if the oils used underwent slight decomposition during the frying process [
34]. The viscosity indices calculated from the viscosity values at 40
oC and 100
oC are very good for un-additivated oils: 202.3 for sunflower oil and 180.8 for palm oil, indicating good lubricating properties of the base oils at high temperatures. This has a great impact because, during the working process, some of the oil “bleeds” and is found in the lower parts of the mechanism, ensuring thus their good lubrication. The iodine number, which measures the susceptibility to oxidation due to the large number of double bonds, is lower in the palm oil, whose molecules have a more saturated nature. During the frying process, the iodine number decreases due to the oxidation of double bonds [
35]. FAO and WHO recommend the following standards for the iodine value for fresh edible oils: 50-55 g I
2/100 g oil in the case of palm oil and 110- 143 g I
2/100 g for the sunflower oil [
36]. From our analyses, it is obvious that the iodine value for the sunflower oil decreased during frying; in the case of palm oil, it must be considered that we used only the oleic part having a more unsaturated character than the whole oil, so the iodine value of 60 g I
2/100 g is explainable.
The density versus temperature and kinematic viscosity versus temperature curves (
Figure 1) were plotted with the same Anton Parr SMV 3000 apparatus. These curves show how the properties are kept at acceptable values when the temperature increases. They are also important for the calculations linked to the running of the mechanisms; for calculations purposes, the equations describing the variation of the properties as a function of temperature can be useful.
For the in-situ preparation of soaps, a cheap available fatty acid was used, i.e., the stearic acid, even if most recipes use the 12-hydroxistearic acid, with the advantage mentioned in Introduction. The use of solid bases with a minimum concentration of water was considered, to perform a rapid process and to minimize the energy consumption. For the lithium soap preparation, anhydrous lithium hydroxide was used since for the calcium soap, a paste of slaked lime with 47.7%wt water content was the least wet material available. The lithium hydroxide was purchased from Merck and the slaked lime was supplied by Carpat Var SRL.
To have an environmentally friendly solution, additives of vegetal origin were chosen, microcrystalline cellulose and lignin, taking into account that cellulose was reported in other studies as effective [37-39], and expecting that lignin, a cross-linked type of polymer, be similar, if not better . Microcrystalline cellulose 102 was purchased from DFE PHARMA GMBH& Co. – Germany, with molecular weight of 160.255 g/mol and the molecular formula (C6H10O5)
n/2, where n=229. Calcium lignosulfonate in powder form with purity > 93% was purchased from Carl Roth & Co. KG – Germany. The sulphate form of the lignin was preferred to the Kraft lignin used in previous studies [
30,
31], due to a better compatibility with the greases.
2.2. The preparation procedure
The un-additivated greases (base greases) were prepared in 500 g batches; then 15% wt. of additive was added, cellulose or lignin; thus, 4 simple greases were obtained: one with 20% wt. lithium soap in sunflower oil, one with 20%wt. calcium soap in sunflower oil, one with 20% wt. lithium soap in palm oil, one with 20%wt. calcium soap in palm oil; other 8 additivated greases were obtained by adding 88.23g cellulose or lignin, in order to obtain 588.23 g grease with 15% wt. additive.
The base grease was obtained from 400 g waste oil, plus stearic acid and solid base (considered as dry matter) in stoichiometric proportion, to yield 100 g soap. The procedure was the following:
-the waste frying oil is introduced in an agitator open vessel (
Figure 2),
-it is heated up to 65oC (0.4oC/min); then stearic acid is added, and the heating continues until it dissolves in the reaction mass.
-at 80oC, the solution is cleared, and the base is added gradually for 15 minutes while stirring continuously.
-the heating and stirring continue up to 95-100oC for the calcium hydroxide or 85oC for the lithium hydroxide respectively, maintaining this temperature for the finalization of the saponification reaction, moment visualized by the change in texture of the grease (it becomes consistent and homogenous).
-If applicable, the additive is introduced and kept stirred continuously for 30 minutes, then the heating turns off and the grease is allowed to cool down naturally.
2.3. Characterization methods for greases
The rheological tests were performed with the rotative rheometer Anton Parr, MCR 72, , with a plate of 50 mm in diameter, and a gap of 1 mm between plates. The rheometer measures the variation shear of stress ( [Pa] versus the shear rate [s-1], the variation of dynamic viscosity (η) [mPa] versus the shear rate ; it also calculates the hysteresis area of plastic fluids. It serves to detail the rheological characterization and, due to the acquisition of all data in an Excel file, it further allows the mathematical modelling.
The cone penetrometer APA-OA represented in
Figure 3, made by the Cangzou Oubeiruike Instrument and Equipment Co. Ldt., served to determine the consistency of greases in accordance with ASTM D217-02 [
40]. The penetration values were determined at 25
oC, both for un-worked and worked greases. The greases were “worked” in the mixing vessel from
Figure 4, by applying 10000 strokes (200 rotations per minute, for 50 minutes). This analysis is named “prolonged work penetration”.
The characterization method used in order to determine grease consistency at rising temperatures, specific to running mechanisms, is represented by the measurement of the dropping point. This is a static method which measures the temperature at which the first drop of oil separates; thus, its relevance for the grease thermal stability during mechanism operation is limited. However, this is a standardized method for grease stability characterization [
41].
The oil separation capacity was tested via the bleeding test SKF Ma Pro [
42]. A fixed volume of grease is placed on a piece of absorbent paper and kept at 60
oC in the oven, for two hours. Part of the base oil is released, creating a greasy stain whose diameter was measured. Measurements were made both for un-worked and worked greases. Based on these data, the researchers estimated the change in the oil separation capacity from the grease after running in a mechanism, as follows:
where
represent the area of the stain for the un-worked (fresh) grease and for the worked (used) grease respectively;
are their corresponding diameters;
is the relative difference of the wet areas, offering a measure of change in oil separation capacity before and after working the grease.
2.4. The mechanical tests
2.4.1. The High Frequency Reciprocating Rig (HFRR) test
The high frequency reciprocating rig test (HFRR) is a test that involves rubbing a steel ball (AISI-E 52100/535A99, roughness Ra=0.050μm, hardness RC 58-66) against a steel disk (AISI-E 52100/535A99 with 10 mm Diameter, roughness Ra=0.020μm, hardness RC 76-79) under the test conditions of the chosen standard. Before each test, all the test materials are cleaned thoroughly with acetone. The sample is loaded into the sample recipient, coating the steel disk with a uniform layer of grease. Then, the steel ball is loaded into its holder which is connected to the apparatus (
Figure 5) allowing it to oscillate at the required test frequency. The system is then subjected to the required load according to the test conditions, as can be seen in the
Figure 5. The data are then entered into the system with all the test conditions, according to the standard. The analyses were performed according to ASTM D 6079 (Standard Test Method for Evaluating Lubricity of Diesel Fuels by the High-Frequency Reciprocating Rig) using a PCS Instruments D985 apparatus. Tests were run under the following conditions: 60°C, 200 μm stroke, 50 Hz frequency, 400g load, for 60 minutes.
After the system reaches the required test temperature, the test begins and runs for the allocated time. As the test is running, the software provides constant readings for the coefficient of friction and lubricant film percentage and once the test finishes, it provides the final readings for the average coefficient of friction and average lubricant film percentage throughout the whole test. After the test, the steel ball is cleaned thoroughly with acetone and put under an optical microscope in order to visualize and determine the wear scar diameter, by framing the scar between two parallel horizontal lines and two parallel vertical lines; then, the software provides the final value.
2.4.2. Standard Test Method for Wear Preventive Characteristics of Lubricating Grease (Four-Ball Method)- ASTM D2266-1
The test involves rotating a steel ball at a set rotational speed according to the test standard, against three fixed steel balls that have been covered by the lubricant that is being tested. Before each test, all the materials used are cleaned thoroughly with hexane. The three balls are loaded into the sample recipient and fixed using a steel ring after which they are covered with the sample (see
Figure 6). The fourth ball is placed into a holder which is inserted and fixed into the apparatus, allowing it to rotate at the required rotational speed.
The friction couple is then assembled, and the 4 balls are brought into contact. Under the sample holder, a heating plate is inserted that allows the sample to be heated to the required test temperature, the apparatus giving continuous temperature readings throughout the test. The test apparatus is shown in
Figure 7. The sample is given time to reach the desired temperature and the test begins and runs for the specified duration. After the test finishes, the three balls are cleaned with hexane and then they are placed in turn under an optical microscope in order to determine the wear scar diameter. The wear scar diameter is determined for each ball after drawing two perpendicular lines at the extremities of the wear scar, by using the accompanying software. A total of six readings are performed and the average wear scar diameter is calculated.
3. Results and discussion
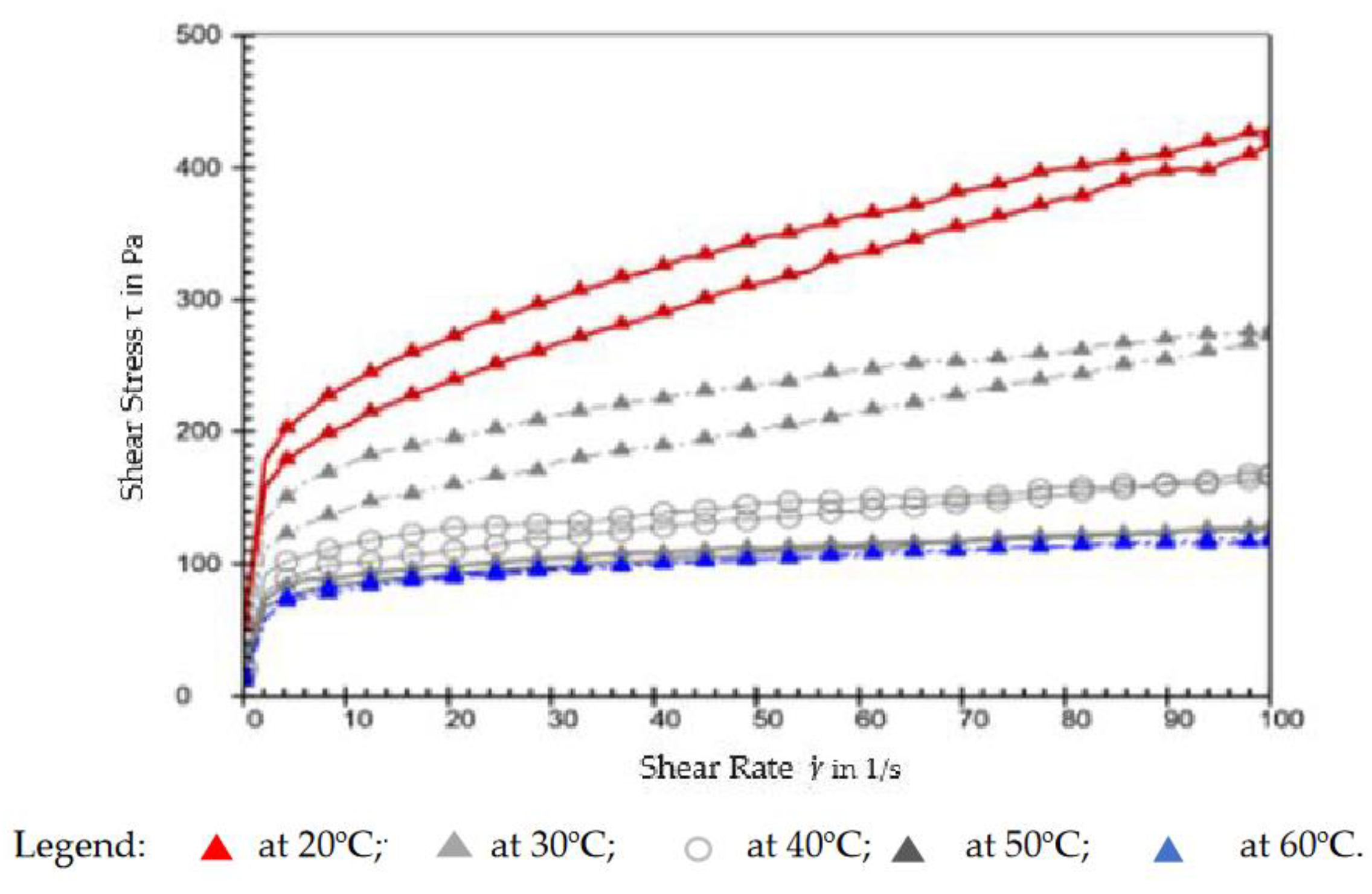
3.1. The rheology of prepared greases
The rheological study consisted in drawing the rheological curves (τ
vs.and η
vs. ) at 20
oC, 30
oC, 40
oC, 50
oC and 60
oC, determining the rheological parameters
and
from the Bingham model, as well as highlighting the values of dynamic viscosity in two reference points, i.e.,
=0,1 s
-1 and
=100 s
-1; everything was automated and performed by the plate-to-plate Anton Parr rheometer. As an example, the results are shown in
Figure 8 (τ
vs. ) and
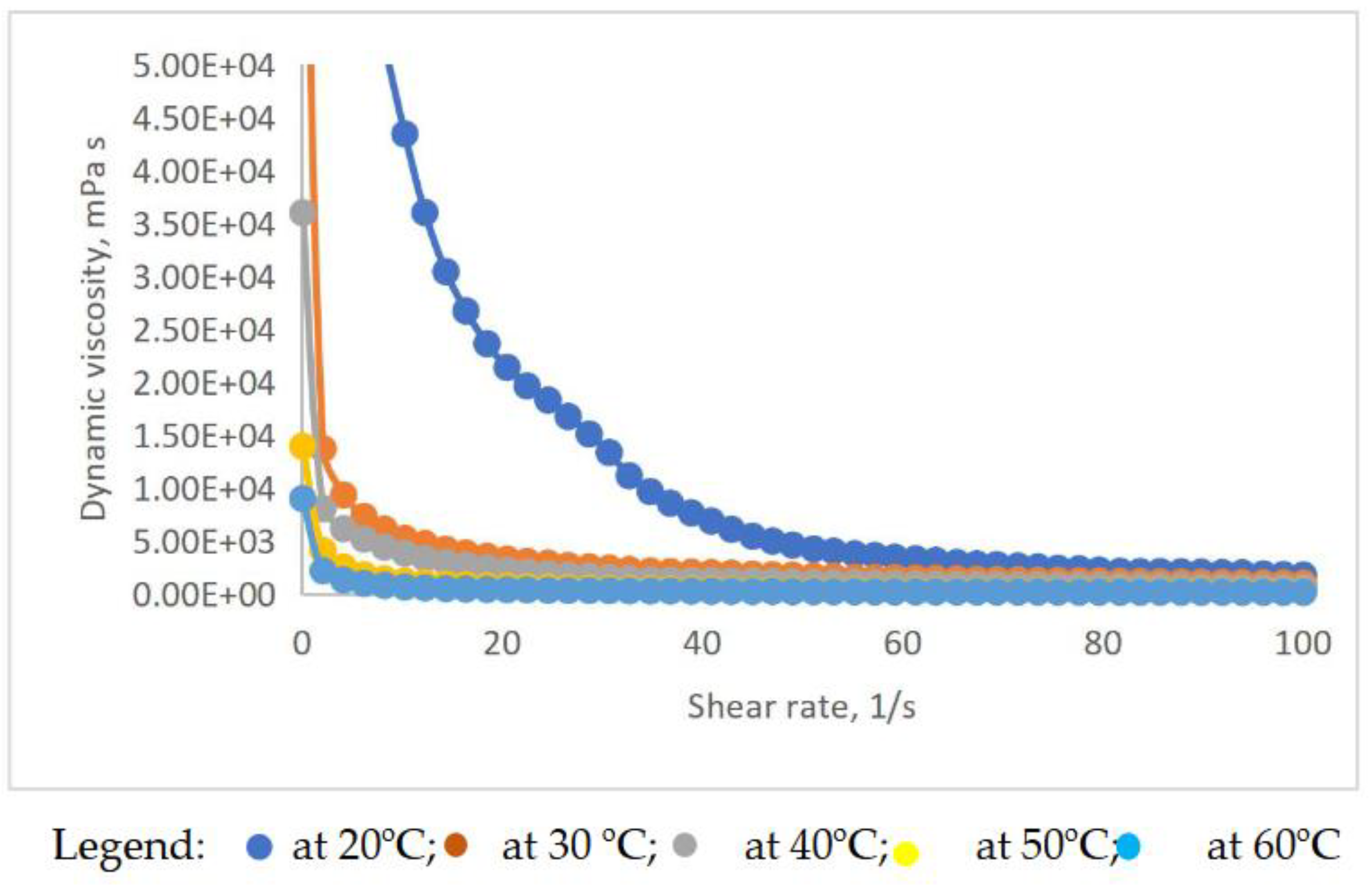
Figure 9 (η
vs.for the grease prepared with 20% wt. Calcium soap and additivated with 15%wt. lignin, but all the curves are shown in
Figure S1 and
Figure S2 (supplementary material). The viscosity at
=0,1 s
-1 and
=100 s
-1, the parameters of the Bingham model plus the hysteresis area are presented in
Table S1 (Supplementary Material).
for the grease prepared with 20% wt. Calcium soap and additivated with 15%wt. lignin (Sample #6)
for the grease prepared with 20% wt. Calcium soap and additivated with 15%wt. lignin (Sample #6)
The curves τ versus have the aspect of non-Newtonian viscoplastic fluids, with an initial shear stress τ0 and hysteresis, its area narrowing down as temperature increases, as observed in Figures 8 and S1. It must be mentioned that the determination at 20oC was repeated, and the second determination was taken into account, this allowing the grease to be churned before the analysis, otherwise the hysteresis area would have been too large at 20oC compared to 30oC, which does not correspond to the reality.
Corroborating the aspect of rheological curves from
Figure S1 (supplementary material) with the data in
Table S1 (supplementary material), one can observe that the hysteresis area at 20
oC, 30
oC, and 40
oC is larger at additivated greases, compared to the grease without an additive; then, at 50
oC and 60
oC, the simple grease has a hysteresis area that is larger than the additivated ones, thus indicating a more pronounced viscoplastic nature of the simple grease at higher temperature; moreover, at 60
oC, some greases have negative values for the hysteresis area, which is typical of rheopectic fluids, similar to dilatant fluids, whose characteristics are dependent on time. This is an exception observed at the greases additivated with lignin, indicating that lignin is less compatible with the base grease than cellulose.
As a rule, viscosity values decrease when increasing temperature and shear rate, as seen in
Figure 9 and
Figure S2 (Supplementary Material), and the curves at 50
oC and 60
oC almost overlap. The aspect of the rheological curves η
vs. makes one think of a law power governed process, which was demonstrated by the statistical processing of the experimental data in Excel; the correlation between the dynamic viscosity and the shear rate, at different temperatures resulted and the equations are presented in
Table S2 (supplementary material).
The correlation coefficients between 0.9534 and 1.0000 prove that the power law applies in each case, with accuracy. In general, the constant in front of the variable x decreases when the temperature increases, showing that viscosity decreases with temperature. The exponent also decreases with temperature, in general, showing that at higher temperatures, the variation of viscosity with shear rate is smaller.
By comparing the viscosity values at 20
oC of the greases prepared in 100 g batches [
38] with those prepared in the present study, in batches of 500 g, close values were observed both for the un-additivated greases (ranging from 1.1 to 3.0 Pa
.s for the small batches and 1.2-3.03 Pa
.s for the larger ones, respectively) and for additivated greases (ranging from 2.2 to 7.0 Pa
.s for the small batches and 2.24- 5.46 Pa
.s for the larger ones, respectively). This indicates a controlled preparation process both at a small scale and at a larger one.
3.2. Th consistency of greases
The consistency of greases can be assessed on the one hand, based on the rheological data: the value of the initial shear stress, τ
0, and by comparing the viscosity values in the same temperature and shear rate conditions; on the other hand, the penetration values (in 10
-1 mm or dmm), determined with the cone penetrometer, represents a standardized method for evaluating the consistency. There are two grease conditions that undergo this assessment: the fresh grease (the un-worked grease) and the grease that underwent a “working” process, , namely after the grease was subjected to efforts mimicking those in the lubricated mechanism (the worked grease). In
Table 2, the two sets of penetration values determined by ASTM 217-02 [
40] are presented.
The freshly prepared greases (i.e., the unworked ones) had 3-6 NLGI grades, so they can be described as firm (grade 3), very firm (grade 4), hard (grade 5) or very hard (grade 6); thus, they are characterized as very good for storage before use or for being kept inside the lubricated mechanism in long downtimes.
After the submission to 10000 strokes, mimicked by mixing at 200 rotations per minute for 50 minutes, the greases softened to grade 0 (very soft) or 1 (soft); these grades are recommendable for mild conditions, namely for lighter loads. All additivated greases were characterized by grade 1 and the un-additivated ones - by grade 0, excepting the simple sunflower oil with lithium stearate grease, which was characterized by grade 1. Comparatively, classical greases, based on mineral oils and lithium soap were characterized by grades 1-2; thus, some of the greases prepared here were similar, namely those with grade 1. Greases characterized by grades 0-1 are solid one, unlike the lower categories 00 and 000, which are semi-fluid or fluid greases, respectively.
3.3. Grease stability at high temperature
The dropping point test shows the temperature at which the first drop of oil separates and drops when placed in a device with standard dimensions [
41]; this constitutes an indication of the grease stability at higher temperatures, specific to the operating conditions in the lubricated mechanism.
In
Table 3, the values of the dropping point are presented for the greases prepared here.
The dropping points were included in the interval 87.5-104.5
oC, which indicates a rather low service temperature, specific to the mechanisms working under lighter loads. The un-additivated greases had a lower dropping point, while the additivated ones have a better thermal stability. However, this characteristic is not defining for the quality of the grease, since the separation of the oil is, to some extent, beneficial for the lubrication of the mechanism. Grease softening was observed during the operation of the lubricated mechanism, due to the so-called “churning” inside the mechanism; the grease heats up because of friction; it softens, and the oil separation begins after a certain operation period (“bleeding”) it drains into the lower parts of the mechanism (“leakage”), thus ensuring the lubrication of lower parts [
42], the oil itself having good lubricant qualities; occasionally, refilling the mechanism with some grease is necessary in order to ensure the lubrication of the upper parts of the mechanism.
3.4. The variation of the oil separation capacity
The way in which the mechanism operation is affected by the separation of the oil from the grease can be measured by the SFK Ma Pro bleeding test [
42], which provides a supplementary indication on the grease stability, at moderate temperatures. The diameters of the oil stains (i.e., the traces left by the same quantity of grease) were measured on absorbent paper, at 60
oC, , before and after “working” it. The procedure and calculations were presented in
Section 2. Materials and methods. The results are presented in
Table 2.
As expected, the worked greases have a higher separation oil capacity than the fresh ones. This tendency manifests itself more strongly at the additivated greases compared to those un-additivated, in the same category of greases (based on the same vegetable oil, with the same soap). The palm oil-based greases release less oil than the sunflower oil-based ones, also proving a better cohesion with the lignin. In addition, one can observe that the palm oil-based greases have a weaker cohesion with the added cellulose compared to sunflower oil-based greases. This can be explained by the difference in the composition of oils and hence by the difference in their polarity, which triggers different compatibility with the added material.
3.5. The mechanical tests
The twelve prepared samples were first subjected to High Frequency Reciprocating Rig test (HFRR analysis), as a preliminary rheological study. The average values of the contact resistance (grease film, %) resulted. Additionally, the coefficient of friction (COF) was calculated by the software. Then, the wear scar diameters (WSD) were determined by the Four (4) Ball test.
The coefficients of friction (Avg COF) vary from 0.030 to 0.062, depending on the lubricating properties of the prepared greases, all the values being satisfactory by comparing with similar data (between 0.04 and 0.06) for commercial greases with mineral base oil and Li and Ca soaps as thickeners [
43].
The contact resistance of the film (Avg Film) differs a lot from one sample to another and indicates the soft consistency of some prepared materials. In general, sunflower oil-based greases form more resistant films than those based on palm oil, excepting the grease additivated with cellulose (sample 11), which is as resistant as the previous ones (samples 1-6).
The wear scar diameter (WSD) falls within the range 168.5-250 µm, except for the un-additivated sunflower oil-based greases with 20% Calcium soap, which had a smaller wear scar: i.e., 63µm. All the WSD values are low, indicating good lubrification at 60oC. In general, the sunflower oil-based greases have smaller WSD values, but sample 11 fits to the sunflower oil-based grease behavior. Corroborated with good film resistance, sample 11 (palm oil with cu 20% calcium stearate additivated with 15% cellulose) demonstrates a good quality lubricant.
Tribological performances were compared with other results from literature even though the tests were performed in different conditions. Closer conditions were used by Wang et.al [
44]: 5N load and test temperature 25
oC, for paraffin oil based commercial greases thickened with lithium 12- hydroxystearate; their consistency was measured as penetration for the unworked grease, and reveals soft greases: 230, 250, 270 and 300 dmm, respectively. Our greases were more consistent, with penetration between 171 and 240 dmm. The wear spots of our greases were smaller (160.5-250 µm, at 60
oC
) than those of Wu et al. (445-720 µm). Polyurea commercial grease with comparable consistency: 230, 250 and 300 dmm, also tested in work [
44], got even bigger wear spots (867-874 µm).
For the best performing samples with mechanical tests (samples 4 and 5), the 4-ball friction test was performed, which is a harsher test, according to ASTM D 2266-01 Standard Test Method for Anti-Wear Properties of Lubricating Grease (Four Ball Method), at 75°C on a Seta Stanhope 19800-7T. The test was performed in triplicate, and three out of the 4 test balls were cleaned and placed under a microscope in order to determine the average WSD value; the obtained measurements are shown in
Table 6.
Sample 5 was found to be more fluid at the test temperature, but retained a liquid film that provided a smaller wear footprint. Sample 4 is more consistent, and it is possible that metal particles appeared as a result of friction, which further contributed to the increase in wear scar diameters. In this case, the addition of cellulose seems to be beneficial for the lubrication in harsher conditions.
4. Conclusions
Efforts are being made by scientists for manufacturing environmentally friendly greases and for their sustainability. In this study, lubricating greases were prepared and characterized using waste oils from food frying processes and natural polymers (cellulose and lignin sulphate). Following other studies on the preparation of greases from vegetable oils, the present work has some novel aspects: batches of 500g were prepared in view of scaling up production and waste vegetable oils for which no similar studies have been carried out so far.
The preparation process is simple and takes place in mild conditions, at 85-100oC and at atmospheric pressure, provided that heating steps and the order of addition of ingredients be respected and carefully controlled in order to obtain quality products.
Solid bases with water content as low as possible are used, thus reducing the energy required for the removal of water by evaporation.
Viscoplastic fluids, like the lubricating greases described in literature, were obtained. The addition of cellulose or lignin increased grease viscosity. The viscosity variation with the shear rate follows the power law at different temperatures in the range 30-60oC. The Bingham model is applicable to the rheological curves and the model parameters were determined.
The consistency of fresh greases, as measured by the penetration test, was equal to or better in this study compared to greases prepared in smaller batches [
32]. It decreases after working the greases, which is normal, becoming soft greases, prone for the use in lower loads mechanisms and comparable to commercial ones.
Greases stability, measured as dropping points around 100oC, and the variation of the oil separation capacity, measured by the SFK Ma Pro bleeding test, confirm that the greases are “soft”.
The mechanical tests were carried out on the twelve grease samples obtained in the laboratory and revealed that our greases are comparable with the commercial soft greases. The best results were obtained for the samples based on sunflower oil and Ca 20% by weight soap, non-additivated or additivated with 15% by weight cellulose.
In conclusion, the greases prepared in the present study can be used in industrial applications, according to their quality, in mechanisms with lower loads.
The research will continue with efforts to improve the quality of waste oils, by reducing the number of double bonds in molecules and increasing their polarity, possibly through epoxidation, and by performing some changes in the preparation procedure, so that the products can be compared with the best lubricating greases obtained from mineral oils.