Submitted:
11 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Statins: General Presentation
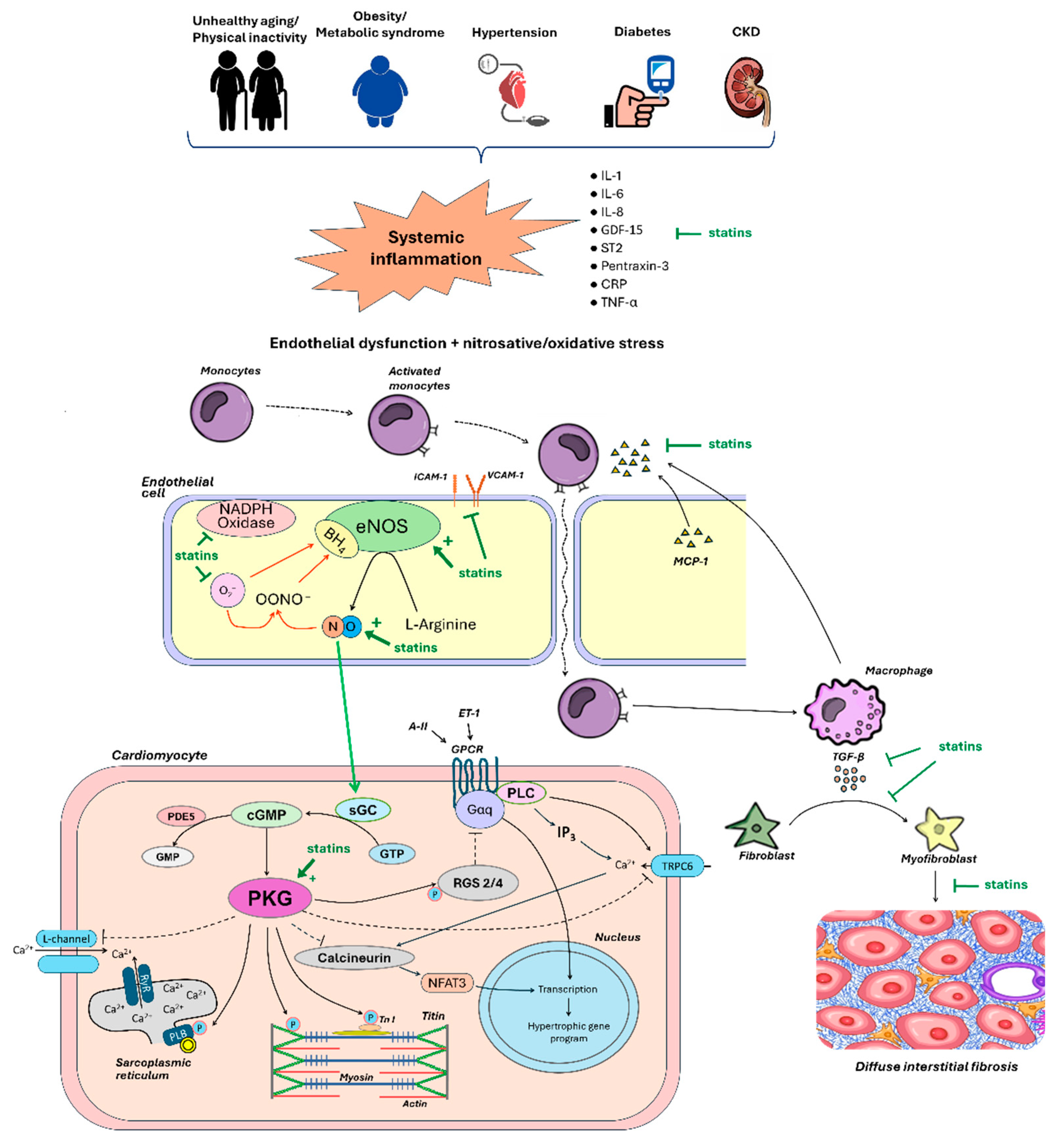
3. Microcirculatory Endothelial Dysfunction in HFpEF and Endothelial Effects of Statins
4. Microcirculatory Inflammation in HFpEF and Anti-Inflammatory Effects of Statins
5. Low NO-cGMP-PKG Pathway Activity Contributes to Myocardial Dysfunction. Role of Statins
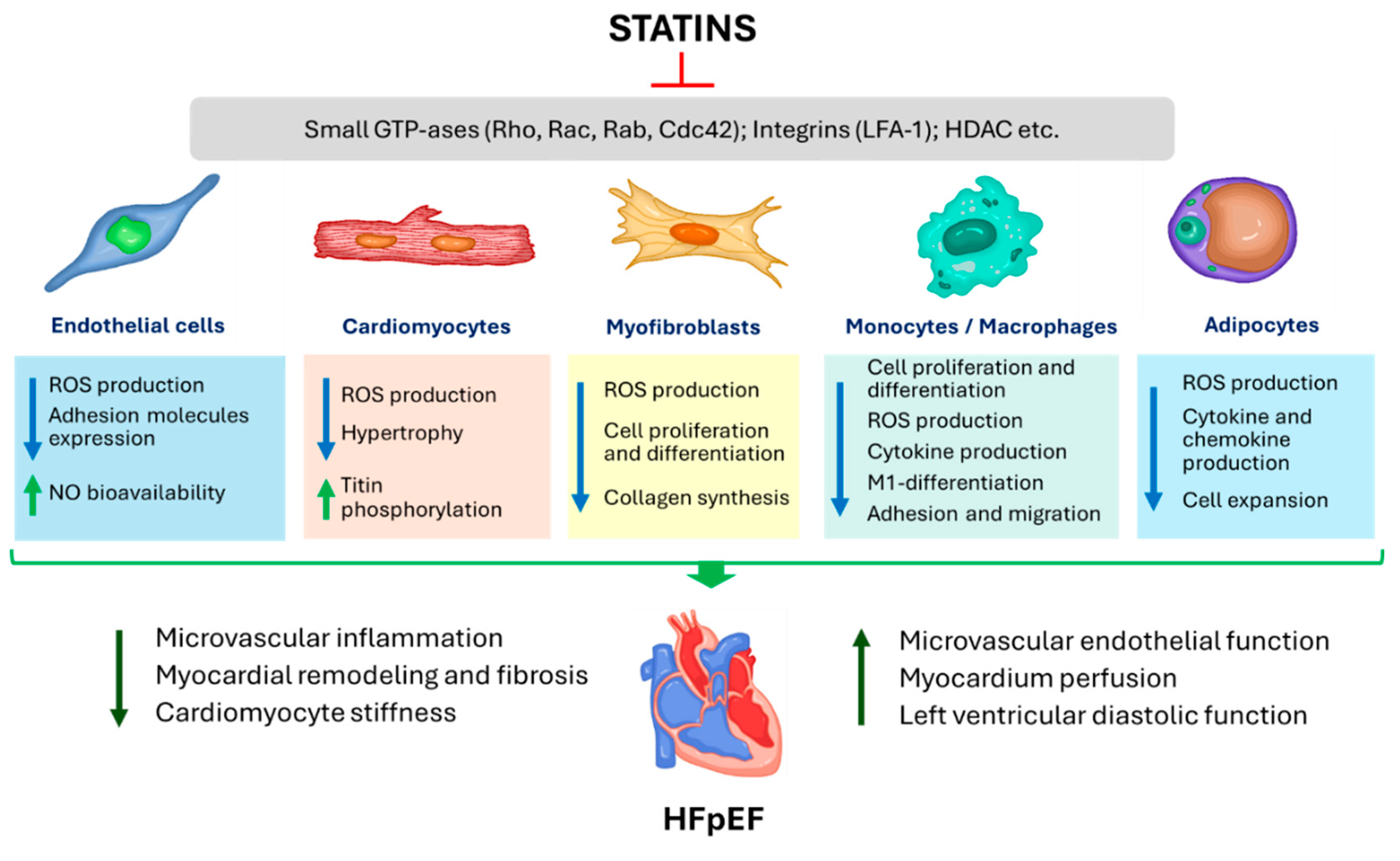
6. Effect of Statins on Myocardial Hypertrophy and Fibrosis
7. Effect of Statins on Diastolic Dysfunction and Epicardial Adipose Tissue
8. Statins in Heart Failure
9. Which Statins are Better for HFpEF?
10. Potential Adverse Effects of Statins in HFpEF
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasan, R.S.; Xanthakis, V.; Lyass, A.; Andersson, C.; Tsao, C.; Cheng, S.; Aragam, J.; Benjamin, E.J.; Larson, M.G. Epidemiology of Left Ventricular Systolic Dysfunction and Heart Failure in the Framingham Study: An Echocardiographic Study Over 3 Decades. JACC Cardiovasc. Imaging. 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Panahi, M.; Papanikolaou, A.; Torabi, A.; Zhang, J.G.; Khan, H.; Vazir, A.; Hasham, M.G.; Cleland, J.G.F.; Rosenthal, N.A.; Harding, S.E.; Sattler, S. Immunomodulatory interventions in myocardial infarction and heart failure: a systematic review of clinical trials and meta-analysis of IL-1 inhibition. Cardiovasc. Res. 2018, 114, 1445–1461. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Pellicori, P.; Filidei, F.; De Biase, N.; Maffia, P.; Guzik, T.J.; Masi, S.; Taddei, S.; Cleland, J.G.F. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: implications for future interventions. Cardiovasc. Res. 2023, 118, 3536–3555. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; Paulus, W.J.; Hamdani, N. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; Poller, W.; Schultheiss, H.P.; Tschöpe, C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef] [PubMed]
- van Heerebeek, L.; Hamdani, N.; Falcão-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.J.; Laarman, G.J.; Somsen, A.; Verheugt, F.W.; Niessen, H.W.; Paulus, W.J. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012, 126, 830–839. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; Davis, B.R.; Braunwald, E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef]
- Lee, M.S.; Duan, L.; Clare, R.; Hekimian, A.; Spencer, H.; Chen, W. Comparison of Effects of Statin Use on Mortality in Patients With Heart Failure and Preserved Versus Reduced Left Ventricular Ejection Fraction. Am. J. Cardiol. 2018, 122, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Koushki, K.; Shahbaz, S.K.; Mashayekhi, K.; Sadeghi, M.; Zayeri, Z.D.; Taba, M.Y.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Anti-inflammatory Action of Statins in Cardiovascular Disease: the Role of Inflammasome and Toll-Like Receptor Pathways. Clin. Rev. Allergy Immunol. 2021, 60, 175–199. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Bakogiannis, C.; Leeson, P.; Guzik, T.J.; Zhang, M.H.; Tousoulis, D.; Antonopoulos, A.S.; Demosthenous, M.; Marinou, K.; Hale, A.; Paschalis, A.; Psarros, C.; Triantafyllou, C.; Bendall, J.; Casadei, B.; Stefanadis, C.; Channon, K.M. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation. 2011, 124, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sola, S.; Mir, M.Q.; Lerakis, S.; Tandon, N.; Khan, B.V. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J. Am. Coll. Cardiol. 2006, 47, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Jiang, H.; Sun, A.; Wang, Y.; Zou, Y.; Ge, J.; Chen, H. Effects of statin therapy on inflammatory markers in chronic heart failure: a meta-analysis of randomized controlled trials. Arch. Med. Res. 2010, 41, 464–471. [Google Scholar] [CrossRef]
- Akahori, H.; Tsujino, T.; Naito, Y.; Matsumoto, M.; Sasaki, N.; Iwasaku, T.; Eguchi, A.; Sawada, H.; Hirotani, S.; Masuyama, T. Atorvastatin ameliorates cardiac fibrosis and improves left ventricular diastolic function in hypertensive diastolic heart failure model rats. J. Hypertens. 2014, 32, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Garre, D.; Gonzalez-Rubio, M.L.; Munoz-Pacheco, P.; Caro-Vadillo, A.; Aragoncillo, P.; Fernandez-Cruz, A. Rosuvastatin added to standard heart failure therapy improves cardiac remodelling in heart failure rats with preserved ejection fraction. Eur. J. Heart Fail. 2010, 12, 903–912. [Google Scholar] [CrossRef]
- Arefieva, T.I.; Filatova, A.Y.; Potekhina, A.V.; Shchinova, A.M. Immunotropic Effects and Proposed Mechanism of Action for 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase Inhibitors (Statins). Biochemistry (Mosc). 2018, 83, 874–889. [Google Scholar] [CrossRef]
- Xu, N.; Shen, N.; Wang, X.; Jiang, S.; Xue, B.; Li, C. Protein prenylation and human diseases: a balance of protein farnesylation and geranylgeranylation. Sci. China Life Sci. 2015, 58, 328–335. [Google Scholar] [CrossRef]
- Soh, J.E.C.; Shimizu, A.; Sato, A.; Ogita, H. Novel cardiovascular protective effects of RhoA signaling and its therapeutic implications. Biochem. Pharmacol. 2023, 218, 115899. [Google Scholar] [CrossRef]
- Seccia, T.M.; Rigato, M.; Ravarotto, V.; Calò, L.A. ROCK (RhoA/Rho Kinase) in Cardiovascular-Renal Pathophysiology: A Review of New Advancements. J. Clin. Med. 2020, 9, 1328. [Google Scholar] [CrossRef]
- Liu, P.Y.; Chen, J.H.; Lin, L.J.; Liao, J.K. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J. Am. Coll. Cardiol. 2007, 49, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Soga, J.; Hidaka, T.; Idei, N.; Fujii, Y.; Fujimura, N.; Mikami, S.; Maruhashi, T.; Kihara, Y.; Chayama, K.; Kato, H.; Noma, K.; Liao, J.K.; Higashi, Y.; ROCK Study Group. Calcium channel blocker and Rho-associated kinase activity in patients with hypertension. J. Hypertens. 2011, 29, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Grunert, M.E.; Rikitake, Y.; Noma, K.; Prsic, A.; Ganz, P.; Liao, J.K.; Creager, M.A. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ. Res. 2006, 99, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, J.H.; Chou, C.W.; Chang, Y.F.; Yeh, S.H.; Chen, C.C. Statins increase p21 through inhibition of histone deacetylase activity and release of promoter-associated HDAC1/2. Cancer Res. 2008, 68, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; Hill, T.M.; Mammen, P.P.A.; Huang, J.; Lee, D.I.; Hahn, V.S.; Sharma, K.; Kass, D.A; Lavandero, S.; Gillette, T.G.; Hill, J.A. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Breitkreuz, M.; Hamdani, N. A change of heart: oxidative stress in governing muscle function? Biophys Rev. 2015, 7, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Banach, M.; Penson, P.E.; Jamialahmadi, T.; Sahebkar, A. Statin-Induced Nitric Oxide Signaling: Mechanisms and Therapeutic Implications. J. Clin. Med. 2019, 8, 2051. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, X.B.; Bi, S.J.; Lu, Q.H.; Zhang, J. Inhibition of Rho-kinase is involved in the therapeutic effects of atorvastatin in heart ischemia/reperfusion. Exp. Ther. Med. 2020, 20, 3147–3153. [Google Scholar] [CrossRef]
- Dimmeler, S.; Assmus, B.; Hermann, C.; Haendeler, J.; Zeiher, A.M. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ. Res. 1998, 83, 334–341. [Google Scholar] [CrossRef]
- Liu, P.Y.; Liu, Y.W.; Lin, L.J.; Chen, J.H.; Liao, J.K. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009, 119, 131–138. [Google Scholar] [CrossRef]
- Nohria, A.; Grunert, M.E.; Rikitake, Y.; Noma, K.; Prsic, A.; Ganz, P.; Liao, J.K.; Creager, M.A. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ. Res. 2006, 99, 1426–1432. [Google Scholar] [CrossRef]
- Rawlings, R.; Nohria, A.; Liu, P.Y.; Donnelly, J.; Creager, M.A.; Ganz, P.; Selwyn, A.; Liao, J.K. Comparison of effects of rosuvastatin (10 mg) versus atorvastatin (40 mg) on rho kinase activity in caucasian men with a previous atherosclerotic event. Am. J. Cardiol. 2009, 103, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, M.; Channon, K.M.; Antoniades, C. Statins as regulators of redox state in the vascular endothelium: beyond lipid lowering. Antioxid. Redox Signal. 2014, 20, 1198–215. [Google Scholar] [CrossRef]
- Pelat, M.; Dessy, C.; Massion, P.; Desager, J.P.; Feron, O.; Balligand, J.L. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E-/- mice in vivo. Circulation. 2003, 107, 2480–2486. [Google Scholar] [CrossRef]
- Rossi, J.; Rouleau, L.; Tardif, J.C.; Leask, R.L. Effect of simvastatin on Kruppel-like factor2, endothelial nitric oxide synthase and thrombomodulin expression in endothelial cells under shear stress. Life Sci. 2010, 87, 92–99. [Google Scholar] [CrossRef]
- Bleda, S.; De Haro, J.; Florez, A.; Varela, C.; Esparza, L.; Acin, F. Long-term pleiotropic effect of statins upon nitric oxide and C-reactive protein levels in patients with peripheral arterial disease. Heart Asia. 2011, 3, 130–134. [Google Scholar] [PubMed]
- Vasa, M.; Fichtlscherer, S.; Adler, K.; Aicher, A.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001, 103, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, P.; Taccardi, A.A.; Grilli, A.; De Lutiis, M.A.; Barsotti, A.; Felaco, M.; De Caterina, R. Chronic treatment with rosuvastatin modulates nitric oxide synthase expression and reduces ischemia-reperfusion injury in rat hearts. Cardiovasc. Res. 2005, 66, 462–471. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Anker, S.D.; Bassenge, E. Statins and the role of nitric oxide in chronic heart failure. Heart Fail. Rev. 2003, 8, 99–106. [Google Scholar] [CrossRef]
- Leick, M.; Azcutia, V.; Newton, G.; Luscinskas, F.W. Leukocyte recruitment in inflammation: basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell Tissue Res. 2014, 355, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail. Rev. 2014, 19, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Sager, H.B.; Roh, J.D.; Valero-Muñoz, M.; Houstis, N.E.; Iwamoto, Y.; Sun, Y.; Wilson, R.M.; Wojtkiewicz, G.; Tricot, B.; Osborne, M.T.; Hung, J.; Vinegoni, C.; Naxerova, K.; Sosnovik, D.E.; Zile, M.R.; Bradshaw, A.D.; Liao, R.; Tawakol, A.; Weissleder, R.; Rosenzweig, A.; Swirski, F.K.; Sam, F.; Nahrendorf, M. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 2018, 215, 423–440. [Google Scholar] [CrossRef] [PubMed]
- González, G.E.; Rhaleb, N.E.; D'Ambrosio, M.A.; Nakagawa, P.; Liao, T.D.; Peterson, E.L.; Leung, P.; Dai, X.; Janic, B.; Liu, Y.H.; Yang, X.P.; Carretero, O.A. Cardiac-deleterious role of galectin-3 in chronic angiotensin II-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1287–H1296. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, U.; Libby, P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004, 109, II18–II26. [Google Scholar] [CrossRef] [PubMed]
- Parsamanesh, N.; Karami-Zarandi, M.; Banach, M.; Penson, P.E.; Sahebkar, A. Effects of statins on myocarditis: A review of underlying molecular mechanisms. Prog. Cardiovasc. Dis. 2021, 67, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yuan, Y.; Sun, Z.L. Cholesterol Contributes to Diabetic Nephropathy through SCAP-SREBP-2 Pathway. Int. J. Endocrinol. 2013, 2013, 592576. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.; Resende, R.; Oliveira, C.R.; Pereira, C.M. Cholesterol and statins in Alzheimer's disease: current controversies. Exp. Neurol. 2010, 223, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Chamani, S.; Liberale, L.; Mobasheri, L.; Montecucco, F.; Al-Rasadi, K.; Jamialahmadi, T.; Sahebkar, A. The role of statins in the differentiation and function of bone cells. Eur. J. Clin. Invest. 2021, 51, e13534. [Google Scholar] [CrossRef]
- Lee, Y.G.; Lee, J.; Byeon, S.E.; Yoo, D.S.; Kim, M.H.; Lee, S.Y.; Cho, J.Y. Functional role of Akt in macrophage-mediated innate immunity. Front. Biosci. (Landmark Ed). 2011, 16, 517–530. [Google Scholar] [CrossRef]
- Ovchinnikov, A.G.; Arefieva, T.I.; Potekhina, A.V.; Filatova, A.Y.; Ageev, F.T.; Boytsov, S.A. The Molecular and Cellular Mechanisms Associated with a Microvascular Inflammation in the Pathogenesis of Heart Failure with Preserved Ejection Fraction. Acta Nat. 2020, 12, 40–51. [Google Scholar] [CrossRef]
- Romano, M.; Diomede, L.; Sironi, M.; Massimiliano, L.; Sottocorno, M.; Polentarutti, N.; Guglielmotti, A.; Albani, D.; Bruno, A.; Fruscella, P.; et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab. Investig. 2000, 80, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Weitz-Schmidt, G.; Welzenbach, K.; Brinkmann, V.; Kamata, T.; Kallen, J.; Bruns, C.; Cottens, S.; Takada, Y.; Hommel, U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001, 7, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Veillard, N.R.; Braunersreuther, V.; Arnaud, C.; Burger, F.; Pelli, G.; Steffens, S.; Mach, F. Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis. 2006, 188, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.Z.; Xin, S.L.; Chen, C.; Liu, T. Effect of atorvastatin on expression of TLR4 and NF-κB p65 in atherosclerotic rabbits. Asian Pac. J. Trop. Med. 2013, 6, 493–496. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hoshi, K.; Ichihara, K. Fluvastatin, an inhibitor of 3-hydroxy-3-methylglutaryl-CoA reductase, scavenges free radicals and inhibits lipid peroxidation in rat liver microsomes. Eur. J. Pharmacol. 1998, 361, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Presa, M.A.; Martín-Ventura, J.L.; Ortego, M.; Gómez-Hernández, A.; Tuñón, J.; Hernández-Vargas, P.; Blanco-Colio, L.M.; Mas, S.; Aparicio, C.; Ortega, L.; et al. Atorvastatin reduces the expression of cyclooxygenase-2 in a rabbit model of atherosclerosis and in cultured vascular smooth muscle cells. Atherosclerosis. 2002, 160, 49–58. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Mummidi, S.; Mahimainathan, L.; Patel, D.N.; Bailey, S.R.; Imam, S.Z.; Greene, W.C.; Valente, A.J. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-κB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J. Biol. Chem. 2006, 281, 15099–15109. [Google Scholar] [CrossRef]
- Ozbek, E.; Cekmen, M.; Ilbey, Y.O.; Simsek, A.; Polat, E.C.; Somay, A. Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-κB pathways. Ren. Fail. 2009, 31, 382–392. [Google Scholar] [CrossRef]
- Sheridan, A.; Wheeler-Jones, C.P.D.; Gage, M.C. The Immunomodulatory Effects of Statins on Macrophages. Immuno. 2022, 2, 317–343. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging. Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Lim, K.H.; Staudt, L.M. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a011247. [Google Scholar]
- Wang, S.; Xie, X.; Lei, T.; Zhang, K.; Lai, B.; Zhang, Z.; Guan, Y.; Mao, G.; Xiao, L.; Wang, N. Statins Attenuate Activation of the NLRP3 Inflammasome by Oxidized LDL or TNFα in Vascular Endothelial Cells through a PXR-Dependent Mechanism. Mol. Pharmacol. 2017, 92, 256–264. [Google Scholar] [CrossRef]
- Bahrami, A.; Parsamanesh, N.; Atkin, S.L.; Banach, M.; Sahebkar, A. Effect of statins on toll-like receptors: a new insight to pleiotropic effects. Pharmacol. Res. 2018, 135, 230–238. [Google Scholar] [CrossRef]
- Moutzouri, E.; Tellis, C.C.; Rousouli, K.; Liberopoulos, E.N.; Milionis, H.J.; Elisaf, M.S.; Tselepis, A.D. Effect of simvastatin or its combination with ezetimibe on Toll-like receptor expression and lipopolysaccharide-induced cytokine production in monocytes of hypercholesterolemic patients. Atherosclerosis. 2012, 225, 381–387. [Google Scholar] [CrossRef]
- Dichtl, W.; Dulak, J.; Frick, M.; Alber, H.F.; Schwarzacher, S.P.; Ares, M.P.; Nilsson, J.; Pachinger, O.; Weidinger, F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 58–63. [Google Scholar] [CrossRef]
- Arévalo-Lorido, J.C. Clinical relevance for lowering C-reactive protein with statins. Ann. Med. 2016, 48, 516–524. [Google Scholar] [CrossRef]
- Calabró, P.; Willerson, J.T.; Yeh, E.T. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003, 108, 1930–1932. [Google Scholar] [CrossRef]
- DuBrock, H.M.; AbouEzzeddine, O.F.; Redfield, M.M. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS One. 2018, 13, e0201836. [Google Scholar] [CrossRef]
- Lakhani, I.; Wong, M.V.; Hung, J.K.F.; Gong, M.; Waleed, K.B.; Xia, Y.; Lee, S.; Roever, L.; Liu, T.; Tse, G.; Leung, K.S.K.; Li, K.H.C. Diagnostic and prognostic value of serum C-reactive protein in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart Fail. Rev. 2021, 26, 1141–1150. [Google Scholar] [CrossRef]
- Puri, R.; Nissen, S.E.; Libby, P.; Shao, M.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.; Raichlen, J.S.; Uno, K.; Kataoka, Y.; Nicholls, S.J. C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation. 2013, 128, 2395–2403. [Google Scholar] [CrossRef]
- Komukai, K.; Kubo, T.; Kitabata, H.; Matsuo, Y.; Ozaki, Y.; Takarada, S.; Okumoto, Y.; Shiono, Y.; Orii, M.; Shimamura, K.; et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: The EASY-FIT study. J. Am. Coll. Cardiol. 2014, 64, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Tanaka, A.; Kawasaki, T.; Goto, Y.; Morita, Y.; Asaumi, Y.; Nakao, K.; Fujiwara, R.; Nishimura, K.; Miyamoto, Y.; Ishihara, M.; Ogawa, H.; Koga, N.; Narula, J.; Yasuda, S. Effect of Intensive Statin Therapy on Coronary High-Intensity Plaques Detected by Noncontrast T1-Weighted Imaging: The AQUAMARINE Pilot Study. J. Am. Coll. Cardiol. 2015, 66, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Bonsu, K.O.; Reidpath, D.D.; Kadirvelu, A. Effects of Statin Treatment on Inflammation and Cardiac Function in Heart Failure: An Adjusted Indirect Comparison Meta-Analysis of Randomized Trials. Cardiovasc. Ther. 2015, 33, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Burger, F.; Pelli, G.; Poku, N.K.; Berlier, C.; Steffens, S.; Mach, F. Statins inhibit C-reactive protein-induced chemokine secretion, ICAM-1 upregulation and chemotaxis in adherent human monocytes. Rheumatology (Oxford). 2009, 48, 233–242. [Google Scholar] [CrossRef]
- Park, J.J.; Yoon, M.; Cho, H.W.; Cho, H.J.; Kim, K.H.; Yang, D.H.; Yoo, B.S.; Kang, S.M.; Baek, S.H.; Jeon, E.S.; Kim, J.J.; Cho, M.C.; Chae, S.C.; Oh, B.H.; Choi, D.J. C-reactive protein and statins in heart failure with reduced and preserved ejection fraction. Front. Cardiovasc. Med. 2022, 9, 1064967. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Y. , Fard J.K.; Ghafoor D.; Eid A.H.; Sahebkar A. Paradoxical effects of statins on endothelial and cancer cells: the impact of concentrations. Cancer Cell Int. 2023, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Walkowski, B.; Kleibert, M.; Majka, M.; Wojciechowska, M. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells. 2022, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Qin, H.; Benveniste, E.N. The IFN-γ-Induced Transcriptional Program of the CIITA Gene Is Inhibited by Statins. Eur. J. Immunol. 2008, 38, 2325. [Google Scholar] [CrossRef]
- Leuenberger, T.; Pfueller, C.F.; Luessi, F.; Bendix, I.; Paterka, M.; Prozorovski, T.; Treue, D.; Luenstedt, S.; Herz, J.; Siffrin, V.; Infante-Duarte, C.; Zipp, F.; Waiczies, S. Modulation of dendritic cell immunobiology via inhibition of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase. PLoS One. 2014, 9, e100871. [Google Scholar] [CrossRef]
- Frostegård, J.; Zhang, Y.; Sun, J.; Yan, K.; Liu, A. Oxidized Low-Density Lipoprotein (OxLDL)-Treated Dendritic Cells Promote Activation of T Cells in Human Atherosclerotic Plaque and Blood, Which Is Repressed by Statins: MicroRNA let-7c Is Integral to the Effect. J. Am. Heart Assoc. 2016, 5, e003976. [Google Scholar] [CrossRef] [PubMed]
- Radyukhina, N.V.; Ruleva, N.Yu.; Filatova, A.Yu.; Arefieva, T.I. Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) suppress human CD4+ T lymphocytes proliferation and motility in vitro. Bull. Exp. Biol. Med. 2021, 172, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ruleva, N.Yu.; Radyukhina, N.V.; Zubkova, E.S.; Filatova, A.Yu.; Arefieva, T.I. Inhibitors of 3-Hydroxy-3-Methylglutaryl Coenzyme a Reductase (Statins) Suppress Differentiation and Reduce LPS IFNγ-Induced Cytokine Production in Human Monocyte Macrophage Culture. Bull. Exp. Biol. Med. 2020, 170, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ghittoni, R.; Napolitani, G.; Benati, D.; Ulivieri, C.; Patrussi, L.; Laghi Pasini, F.; Lanzavecchia, A.; Baldari, C.T. Simvastatin inhibits the MHC class II pathway of antigen presentation by impairing Ras superfamily GTPases. Eur. J. Immunol. 2006, 36, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Weitz-Schmidt, G. Statins as anti-inflammatory agents. Trends Pharmacol. Sci. 2002, 23, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wu, C.; Xu, Y.; Cai, J.; Zhao, M.; Zu, L. The NO-cGMP-PKG Axis in HFpEF: From Pathological Mechanisms to Potential Therapies. Aging Dis. 2023, 14, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Lugnier, C.; Meyer, A.; Charloux, A.; Andrès, E.; Gény, B.; Talha, S. The Endocrine Function of the Heart: Physiology and Involvements of Natriuretic Peptides and Cyclic Nucleotide Phosphodiesterases in Heart Failure. J. Clin. Med. 2019, 8, 1746. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, K.; Liu, M.; Su, J.; Qin, X.; Wang, X.; Zhang, J.; Li, S.; Fan, G. An herbal preparation ameliorates heart failure with preserved ejection fraction by alleviating microvascular endothelial inflammation and activating NO-cGMP-PKG pathway. Phytomedicine. 2021, 91, 153633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Feng, B.; Ma, X.; Sun, K.; Xu, G.; Zhou, Y. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 2019, 18, 107. [Google Scholar] [CrossRef]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.Á.; Falcão-Pires, I.; Reusch, P.H.; Linthout, S.V.; Papp, Z.; van Heerebeek, L.; Vecchione, C.; Maier, L.S.; Ciccarelli, M.; Tschöpe, C.; Mügge, A.; Bagi, Z.; Sossalla, S.; Hamdani, N. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- Takimoto, E.; Koitabashi, N.; Hsu, S.; Ketner, E.A.; Zhang, M.; Nagayama, T.; Bedja, D.; Gabrielson, K.L.; Blanton, R.; Siderovski, D.P.; Mendelsohn, M.E.; Kass, D.A. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J. Clin. Invest. 2009, 119, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Rainer, P.P.; Lee, D.I.; Hao, S.; Bedja, D.; Birnbaumer, L.; Cingolani, O.H.; Kass, D.A. Hyperactive adverse mechanical stress responses in dystrophic heart are coupled to transient receptor potential canonical 6 and blocked by cGMP-protein kinase G modulation. Circ. Res. 2014, 114, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F. A concise discussion of the regulatory role of cGMP kinase I in cardiac physiology and pathology. Basic.Res. Cardiol. 2018, 113, 31. [Google Scholar] [CrossRef] [PubMed]
- Humeres, C.; Venugopal, H.; Frangogiannis, N.G. Smad-dependent pathways in the infarcted and failing heart. Curr. Opin. Pharmacol. 2022, 64, 102207. [Google Scholar] [CrossRef] [PubMed]
- Buxton, I.L.; Duan, D. Cyclic GMP/protein kinase G phosphorylation of Smad3 blocks transforming growth factor-beta-induced nuclear Smad translocation: a key antifibrogenic mechanism of atrial natriuretic peptide. Circ. Res. 2008, 102, 151–153. [Google Scholar] [CrossRef] [PubMed]
- LeWinter, M.M; Granzier, HL. Cardiac titin and heart disease. J. Cardiovasc. Pharm. 2014, 63, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; Bishu, K.G.; von Frieling-Salewsky, M.; Redfield, M.M.; Linke, W.A. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc. Res. 2013, 97, 464–441. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; Redfield, M.M.; Bull, D.A.; Granzier, H.L.; LeWinter, M.M. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015, 131, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P.; Stasch, J.P. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Resp. Med. 2017, 122, S1–S9. [Google Scholar] [CrossRef]
- Wilck, N.; Markó, L.; Balogh, A.; Kräker, K.; Herse, F.; Bartolomaeus, H.; Szijártó, I.A.; Gollasch, M.; Reichhart, N.; Strauss, O.; Heuser, A.; Brockschnieder, D.; Kretschmer, A.; Lesche, R.; Sohler, F.; Stasch, J.P.; Sandner, P.; Luft, F.C.; Müller, D.N.; Dechend, R.; Haase, N. Nitric oxide-sensitive guanylyl cyclase stimulation improves experimental heart failure with preserved ejection fraction. JCI Insight. 2018, 3, e96006. [Google Scholar] [CrossRef]
- Burke, R.M.; Lighthouse, J.K.; Mickelsen, D.M.; Small, E.M. Sacubitril/Valsartan Decreases Cardiac Fibrosis in Left Ventricle Pressure Overload by Restoring PKG Signaling in Cardiac Fibroblasts. Circ. Heart Fail. 2019, 12, e005565. [Google Scholar] [CrossRef] [PubMed]
- Numata, G.; Takimoto, E. Cyclic GMP and PKG Signaling in Heart Failure. Front. Pharmacol. 2022, 13, 792798. [Google Scholar] [CrossRef]
- Ramasubbu, K.; Estep, J.; White, D.L.; Deswal, A.; Mann, D.L. Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 415–426. [Google Scholar] [CrossRef]
- Antoniades, C.; Demosthenous, M.; Reilly, S.; Margaritis, M.; Zhang, M.H.; Antonopoulos, A.; Marinou, K.; Nahar, K.; Jayaram, R.; Tousoulis, D.; Bakogiannis, C.; Sayeed, R.; Triantafyllou, C.; Koumallos, N.; Psarros, C.; Miliou, A.; Stefanadis, C.; Channon, K.M.; Casadei, B. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J. Am. Coll. Cardiol. 2012, 59, 60–70. [Google Scholar] [CrossRef]
- Hahn, V.S.; Yanek, L.R.; Vaishnav, J.; Ying, W.; Vaidya, D.; Lee, Y.Z.J.; Riley, S.J.; Subramanya, V.; Brown, E.E.; Hopkins, C.D.; Ononogbu, S.; Perzel Mandell, K.; Halushka, M.K.; Steenbergen, C. Jr.; Rosenberg, A.Z.; Tedford, R.J.; Judge, D.P.; Shah, S.J.; Russell, S.D.; Kass, D.A.; Sharma, K. Endomyocardial Biopsy Characterization of Heart Failure With Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart Fail. 2020, 8, 712–724. [Google Scholar] [CrossRef]
- Frohlich, E.D.; Apstein, C.; Chobanian, A.V.; Devereux, R.B.; Dustan, H.P.; Dzau, V.; Fauad-Tarazi, F.; Horan, M.J.; Marcus, M.; Massie, B.; et al. The heart in hypertension. N. Engl. J. Med. 1992, 327, 998–1008. [Google Scholar] [CrossRef]
- Hattori, T.; Shimokawa, H.; Higashi, M.; Hiroki, J.; Mukai, Y.; Tsutsui, H.; Kaibuchi, K.; Takeshita, A. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004, 109, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Horinaka, S.; Mita, S.; Nakano, S.; Honda, T.; Yoshida, K.; Kobayashi, T.; Matsuoka, H. Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovasc. Res. 2002, 55, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Chou, T.F.; Tsai, C.H. Association of pravastatin and left ventricular mass in hypercholesterolemic patients: role of 8-iso-prostaglandin f2alpha formation. J. Cardiovasc. Pharmacol. 2002, 40, 868–874. [Google Scholar] [CrossRef]
- Satoh, M.; Ogita, H.; Takeshita, K.; Mukai, Y.; Kwiatkowski, D.J.; Liao, J.K. Requirement of Rac1 in the development of cardiac hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 7432–7437. [Google Scholar] [CrossRef]
- Tanaka, S.; Fukumoto, Y.; Nochioka, K.; Minami, T.; Kudo, S.; Shiba, N.; Takai, Y.; Williams, C.L.; Liao, J.K.; Shimokawa, H. Statins exert the pleiotropic effects through small GTP-binding protein dissociation stimulator upregulation with a resultant Rac1 degradation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1591–600. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Satoh, K.; Nogi, M.; Suzuki, K.; Sunamura, S.; Omura, J.; Kikuchi, N.; Kurosawa, R.; Satoh, T.; Minami, T.; Ikeda, S.; Miyata, S.; Shimokawa, H. SmgGDS as a Crucial Mediator of the Inhibitory Effects of Statins on Cardiac Hypertrophy and Fibrosis: Novel Mechanism of the Pleiotropic Effects of Statins. Hypertension. 2016, 67, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Su, S.F.; Hsiao, C.L.; Chu, C.W.; Lee, B.C.; Lee, T.M. Effects of pravastatin on left ventricular mass in patients with hyperlipidemia and essential hypertension. Am. J. Cardiol. 2000, 86, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Haudek, S.B.; Gupta, D.; Dewald, O.; Schwartz, R.J.; Wei, L.; Trial, J.; Entman, M.L. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc. Res. 2009, 83, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Li, Y.; Noma, K.; Hiroi, Y.; Liu, P.Y.; Taniguchi, M.; Ito, M.; Liao, J.K. FHL2 prevents cardiac hypertrophy in mice with cardiac-specific deletion of ROCK2. FASEB J. 2013, 27, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Surma, M.; Yang, Y.; Wei, L. Disruption of both ROCK1 and ROCK2 genes in cardiomyocytes promotes autophagy and reduces cardiac fibrosis during aging. FASEB J. 2019, 33, 7348–7362. [Google Scholar] [CrossRef]
- Gabrielli, L.; Winter, J.L.; Godoy, I.; McNab, P.; Padilla, I.; Cordova, S.; Rigotti, P.; Novoa, U.; Mora, I.; García, L.; Ocaranza, M.P.; Jalil, J.E. Increased rho-kinase activity in hypertensive patients with left ventricular hypertrophy. Am. J. Hypertens. 2014, 27, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Somers, T.; Siddiqi, S.; Morshuis, W.J.; Russel, F.G.M.; Schirris, T.J.J. Statins and Cardiomyocyte Metabolism, Friend or Foe? J. Cardiovasc. Dev. Dis. 2023, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, Y.; Liang, C.; Wang, H.; Huang, J.; Xue, P.; Luo, M. Inhibition of the mevalonate pathway improves myocardial fibrosis. Exp. Ther. Med. 2021, 21, 224. [Google Scholar] [CrossRef]
- Rizvi, F.; Siddiqui, R.; DeFranco, A.; Homar, P.; Emelyanova, L.; Holmuhamedov, E.; Ross, G.; Tajik, A.J.; Jahangir, A. Simvastatin reduces TGF-β1-induced SMAD2/3-dependent human ventricular fibroblasts differentiation: Role of protein phosphatase activation. Int. J. Cardiol. 2018, 270, 228–236. [Google Scholar] [CrossRef]
- Emelyanova, L.; Sra, A.; Schmuck, E.G.; Raval, A.N.; Downey, F.X.; Jahangir, A.; Rizvi, F.; Ross, G.R. Impact of statins on cellular respiration and de-differentiation of myofibroblasts in human failing hearts. ESC Heart. Fail. 2019, 6, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.F.; Hsieh, C.C.; Wang, S.C.; Chang, C.Y.; Hung, C.H.; Kuo, P.L.; Liu, Y.R.; Li, C.Y.; Liu, P.L. Simvastatin Attenuates Cardiac Fibrosis via Regulation of Cardiomyocyte-Derived Exosome Secretion. J. Clin. Med. 2019, 8, 794. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Denver, R.; Bailey, M.; Krum, H. In vitro inhibitory effects of atorvastatin on cardiac fibroblasts: implications for ventricular remodelling. Clin. Exp. Pharmacol. Physiol. 2005, 32, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Saka, M.; Obata, K.; Ichihara, S.; Cheng, X.W.; Kimata, H.; Nishizawa, T.; Noda, A.; Izawa, H.; Nagata, K.; Murohara, T.; Yokota, M. Pitavastatin improves cardiac function and survival in association with suppression of the myocardial endothelin system in a rat model of hypertensive heart failure. J. Cardiovasc. Pharmacol. 2006, 47, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Habibi, J.; Whaley-Connell, A.; Qazi, M.A.; Hayden, M.R.; Cooper, S.A.; Tramontano, A.; Thyfault, J.; Stump, C.; Ferrario, C.; Muniyappa, R.; Sowers, J.R. Rosuvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, decreases cardiac oxidative stress and remodeling in Ren2 transgenic rats. Endocrinology. 2007, 148, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Marunouchi, T.; Matsumura, K.; Fuji, E.; Iwamoto, A.; Tanonaka, K. Simvastatin Attenuates Cardiac Fibrosis under Pathophysiological Conditions of Heart Failure with Preserved Left Ventricular Ejection Fraction by Inhibiting TGF-β Signaling. Pharmacology. 2024, 109, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Liu, J.; Chen, X.; Duan, Y.; Wang, X.; Shen, Y.; Kuang, Y.; Zhuang, T.; Tomlinson, B.; Chan, P.; Yu, Z.; Cheng, Y.; Zhang, L.; Liu, Z.; Zhang, Y.; Zhao, Z.; Zhang, Q.; Liu, J. Endothelial Klf2-Foxp1-TGFβ signal mediates the inhibitory effects of simvastatin on maladaptive cardiac remodeling. Theranostics. 2021, 11, 1609–1625. [Google Scholar] [CrossRef] [PubMed]
- Pentz, R.; Kaun, C.; Thaler, B.; Stojkovic, S.; Lenz, M.; Krychtiuk, K.A.; Zuckermann, A.; Huber, K.; Wojta, J.; Hohensinner, P.J.; Demyanets, S. Cardioprotective cytokine interleukin-33 is up-regulated by statins in human cardiac tissue. J. Cell Mol. Med. 2018, 22, 6122–6133. [Google Scholar] [CrossRef] [PubMed]
- Mannheim, D.; Herrmann, J.; Bonetti, P.O.; Lavi, R.; Lerman, L.O.; Lerman, A. Simvastatin preserves diastolic function in experimental hypercholesterolemia independently of its lipid lowering effect. Atherosclerosis. 2011, 216, 283–291. [Google Scholar] [CrossRef]
- Lu, J.; Liu, F.; Chen, F.; Jin, Y.; Chen, H.; Liu, D.; Cui, W. Amlodipine and atorvastatin improve ventricular hypertrophy and diastolic function via inhibiting TNF-alpha, IL-1beta and NF-kappaB inflammatory cytokine networks in elderly spontaneously hypertensive rats. Biomed. Pharmacother. 2016, 83, 330–339. [Google Scholar] [CrossRef]
- Yamada, Y.; Takeuchi, S.; Yoneda, M.; Ito, S.; Sano, Y.; Nagasawa, K.; Matsuura, N.; Uchinaka, A.; Murohara, T.; Nagata, K. Atorvastatin reduces cardiac and adipose tissue inflammation in rats with metabolic syndrome. Int. J. Cardiol. 2017, 240, 332–338. [Google Scholar] [CrossRef]
- Okura, H.; Asawa, K.; Kubo, T.; Taguchi, H.; Toda, I.; Yoshiyama, M.; Yoshikawa, J.; Yoshida, K. Impact of statin therapy on systemic inflammation, left ventricular systolic and diastolic function and prognosis in low risk ischemic heart disease patients without history of congestive heart failure. Intern. Med. 2007, 46, 1337–1343. [Google Scholar] [CrossRef]
- Yagi, S.; Akaike, M.; Aihara, K.-I.; Iwase, T.; Ishikawa, K.; Yoshida, S.; Sumitomo-Ueda, Y.; Kusunose, K.; Niki, T.; Yamaguchi, K.; Koshiba, K.; Taketani, Y.; Tomita, N.; Yamada, H.; Soeki, T.; Wakatsuki, T.; Matsumoto, T.; Sata, M. Effect of low-dose (1 mg/day) pitavastatin on left ventricular diastolic function and albuminuria in patients with hyperlipidemia. Am. J. Cardiol. 2011, 107, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.; Belyavskiy, E.; Potekhina, A.; Ageev, F. Asymptomatic Left Ventricular Hypertrophy Is a Potent Risk Factor for the Development of HFpEF but Not HFrEF: Results of a Retrospective Cohort Study. J. Clin. Med. 2022, 11, 3885. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.K.; Yeh, C.F.; Chiang, J.Y.; Lin, T.T.; Wu, Y.F.; Chiang, C.K.; Kao, T.W.; Hung, K.Y.; Huang, J.W. Effects of atorvastatin treatment on left ventricular diastolic function in peritoneal dialysis patients - The ALEVENT clinical trial. J. Clin. Lipidol. 2017, 11, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Nerlekar, N.; Muthalaly, R.G.; Wong, N.; Thakur, U.; Wong, D.T.L.; Brown, A.J.; Marwick, T.H. Association of Volumetric Epicardial Adipose Tissue Quantification and Cardiac Structure and Function. J. Am. Heart Assoc. 2018, 7, e009975. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, G.; van Veldhuisen, D.J.; Manintveld, O.C.; van Empel, V.P.M.; Willems, T.P.; de Boer, R.A.; Rienstra, M.; Westenbrink, B.D.; Gorter, T.M. Epicardial Adipose Tissue and Outcome in Heart Failure With Mid-Range and Preserved Ejection Fraction. Circ. Heart Fail. 2022, 15, e009238. [Google Scholar] [CrossRef]
- Parisi, V.; Petraglia, L.; D'Esposito, V.; Cabaro, S.; Rengo, G.; Caruso, A.; Grimaldi, M.G.; Baldascino, F.; De Bellis, A.; Vitale, D.; Formisano, R.; Ferro, A.; Paolillo, S.; Davin, L.; Lancellotti, P.; Formisano, P.; Perrone Filardi, P.; Ferrara, N.; Leosco, D. Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue. Int. J. Cardiol. 2019, 274, 326–330. [Google Scholar] [CrossRef]
- Park, J.H.; Park, Y.S.; Kim, Y.J.; Lee, I.S.; Kim, J.H.; Lee, J.H.; Choi, S.W.; Jeong, J.O.; Seong, I.W. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J. Cardiovasc. Ultrasound. 2010, 18, 121–126. [Google Scholar] [CrossRef]
- Soucek, F.; Covassin, N.; Singh, P.; Ruzek, L.; Kara, T.; Suleiman, M.; Lerman, A.; Koestler, C.; Friedman, P.A.; Lopez-Jimenez, F.; Somers, V.K. Effects of Atorvastatin (80 mg) Therapy on Quantity of Epicardial Adipose Tissue in Patients Undergoing Pulmonary Vein Isolation for Atrial Fibrillation. Am. J. Cardiol. 2015, 116, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; Melek, B.H.; Arepalli, C.D.; Hartlage, G.R.; Chen, Z.; Kim, S.; Stillman, A.E.; Raggi, P. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J. Am. Coll. Cardiol. 2013, 61, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Bonsu, K.O.; Kadirvelu, A.; Reidpath, D.D. Statins in heart failure: do we need another trial? Vasc. Health Risk Manag. 2013, 9, 303–319. [Google Scholar] [PubMed]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; Gissi-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008, 372, 1231–1239. [Google Scholar] [PubMed]
- Kjekshus, J.; Apetrei, E.; Barrios, V.; Böhm, M.; Cleland, J.G.; Cornel, J.H.; Dunselman, P.; Fonseca, C.; Goudev, A.; Grande, P.; Gullestad, L.; Hjalmarson, A.; Hradec, J.; Jánosi, A.; Kamenský, G.; Komajda, M.; Korewicki, J.; Kuusi, T.; Mach, F.; Mareev, V.; McMurray, J.J.; Ranjith, N.; Schaufelberger, M.; Vanhaecke, J.; van Veldhuisen, D.J.; Waagstein, F.; Wedel, H.; Wikstrand, J.; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N. Engl. J. Med. 2007, 357, 2248–2261. [Google Scholar] [CrossRef] [PubMed]
- Gastelurrutia, P.; Lupón, J.; de Antonio, M.; Urrutia, A.; Díez, C.; Coll, R.; Altimir, S.; Bayes-Genis, A. Statins in heart failure: the paradox between large randomized clinical trials and real life. Mayo Clin. Proc. 2012, 87, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Maison, P.; Desamericq, G.; Hemery, F.; Elie, N.; Del'volgo, A.; Dubois-Randé, J.L.; Hittinger, L.; Macquin-Mavier, I. Relationship between recommended chronic heart failure treatments and mortality over 8 years in real-world conditions: a pharmacoepidemiological study. Eur. J. Clin. Pharmacol. 2013, 69, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins? Front Cardiovasc Med. 2021, 8, 687585. [Google Scholar] [CrossRef]
- Alehagen, U.; Benson, L.; Edner, M.; Dahlström, U.; Lund, L.H. Association between use of statins and outcomes in heart failure with reduced ejection fraction: prospective propensity score matched cohort study of 21 864 patients in the Swedish Heart Failure Registry. Circ. Heart Fail. 2015, 8, 252–260. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Ibadete Bytyç, I.; Von Haehling, S.; Anker, S.; Jozwiak, J.; Rysz, J.; Hernandez, A.V.; Bajraktari, G.; Mikhalidis, D.M.; Banach, M. Association of statin use and clinical outcomes in heart failure patients: a systematic review and meta-analysis. Lipids Health Dis. 2019, 18, 188. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; Graham, I.M.; Halliday, A.; Landmesser, U.; Mihaylova, B.; Pedersen, T.R.; Riccardi, G.; Richter, D.J.; Sabatine, M.S.; Taskinen, M.R.; Tokgozoglu, L.; Wiklund, O.; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Ohte, N.; Little, W.C. Statins beneficial for heart failure with preserved ejection fraction but not heart failure with reduced ejection fraction? Circ. J. 2015, 79, 508–509. [Google Scholar] [CrossRef]
- Zakeri, R.; Chamberlain, A.M.; Roger, V.L.; Redfield, M.M. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013, 128, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.G.; Dreeva, Z.V.; Potekhina, A.V.; Arefieva, T.I.; Masenko, V.P.; Ageev, F.T. Statins improves functional capacity and restores LV diastolic reserve in patients with heart failure with preserved left ventricular ejection fraction. Eur. J. Heart Fail. 2019, 21 (Suppl 1), 418. [Google Scholar]
- Vanderpool, R.R.; Saul, M.; Nouraie, M.; Gladwin, M.T.; Simon, M.A. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol. 2018, 3, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Holzhauser, L.; Hovnanians, N.; Eshtehardi, P.; Mojadidi, M.K.; Deng, Y.; Goodman-Meza, D.; Msaouel, P.; Ko, Y.A.; Zolty, R. Statin therapy improves survival in patients with severe pulmonary hypertension: a propensity score matching study. Heart Vessels. 2017, 32, 969–976. [Google Scholar] [CrossRef]
- Fukuta, H.; Sane, D.C.; Brucks, S.; Little, W.C. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation. 2005, 112, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Roik, M.; Starczewska, M.H.; Huczek, Z.; Kochanowski, J.; Opolski, G. Statin therapy and mortality among patients hospitalized with heart failure and preserved left ventricular function--a preliminary report. Acta Cardiol. 2008, 63, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Wang, Y.; Foody, J.M. Effect of statins, angiotensin-converting enzyme inhibitors, and beta blockers on survival in patients >or=65 years of age with heart failure and preserved left ventricular systolic function. Am. J. Cardiol. 2008, 101, 217–222. [Google Scholar] [CrossRef]
- Tehrani, F.; Morrissey, R.; Phan, A.; Chien, C.; Schwarz, E.R. Statin therapy in patients with diastolic heart failure. Clin Cardiol. 2010, 33, E1–5. [Google Scholar] [CrossRef]
- Gomez-Soto, F.M.; Romero, S.P.; Bernal, J.A.; Escobar, M.A.; Puerto, J.L.; Andrey, J.L.; Ruiz, P.; Gomez, F. Mortality and morbidity of newly diagnosed heart failure treated with statins: a propensity-adjusted cohort study. Int. J. Cardiol. 2010, 140, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Quirós López, R.; García Alegría, J.; Martín Escalante, M.D.; Trujillo Santos, J.; Villena Ruiz, M.Á.; Perea Milla, E. Prognostic factors and long-term survival after initial diagnosis of heart failure. Med. Clin. (Barc). 2012, 138, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Suzuki, S.; Yajima, J.; Oikawa, Y.; Sagara, K.; Otsuka, T.; Matsuno, S.; Kano, H.; Uejima, T.; Koike, A.; Nagashima, K.; Kirigaya, H.; Sawada, H.; Aizawa, T.; Yamashita, T. Clinical characteristics and long-term clinical outcomes of Japanese heart failure patients with preserved versus reduced left ventricular ejection fraction: a prospective cohort of Shinken Database 2004-2011. J. Cardiol. 2013, 62, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Benson, L.; Edner, M.; Dahlström, U.; Lund, L.H. Association Between Use of Statins and Mortality in Patients With Heart Failure and Ejection Fraction of ≥50. Circ. Heart. Fail. 2015, 8, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Duan, L.; Clare, R.; Hekimian, A.; Spencer, H.; Chen, W. Comparison of Effects of Statin Use on Mortality in Patients With Heart Failure and Preserved Versus Reduced Left Ventricular Ejection Fraction. Am. J. Cardiol. 2018, 122, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Nochioka, K.; Sakata, Y.; Miyata, S.; Miura, M.; Takada, T.; Tadaki, S.; Ushigome, R.; Yamauchi, T.; Takahashi, J.; Shimokawa, H.; CHART-2 Investigators. Prognostic impact of statin use in patients with heart failure and preserved ejection fraction. Circ. J. 2015, 79, 574–582. [Google Scholar] [CrossRef]
- Yap, J.; Sim, D.; Lim, C.P.; Chia, S.Y.; Go, Y.Y.; Jaufeerally, F.R.; Sim, L.L.; Liew, R.; Ching, C.K. Predictors of two-year mortality in Asian patients with heart failure and preserved ejection fraction. Int J Cardiol. 2015, 183, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Kajio, H. Favorable effects of statins in the treatment of heart failure with preserved ejection fraction in patients without ischemic heart disease. Int. J. Cardiol. 2018, 255, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Marume, K.; Takashio, S.; Nagai, T.; Tsujita, K.; Saito, Y.; Yoshikawa, T.; Anzai, T. Effect of Statins on Mortality in Heart Failure With Preserved Ejection Fraction Without Coronary Artery Disease- Report From the JASPER Study. Circ. J. 2019, 83, 357–367. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, X.-X.; Xu, Y.-L.; Ru, J.; Hui, R.T.; Huang, X.H. Meta-analysis of the effect of statins on mortality in patients with preserved ejection fraction. Am. J. Cardiol. 2014, 113, 1198–1204. [Google Scholar] [CrossRef]
- Fukuta, H.; Goto, T.; Wakami, K.; Ohte, N. The effect of statins on mortality in heart failure with preserved ejection fraction: a meta-analysis of propensity score analyses. Int. J. Cardiol. 2016, 214, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Ergatoudes, C.; Schaufelberger, M.; Andersson, B.; Pivodic, A.; Dahlström, U.; Fu, M. Non-cardiac comorbidities and mortality in patients with heart failure with reduced vs. preserved ejection fraction: a study using the Swedish Heart Failure Registry. Clin. Res. Cardiol. 2019, 108, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Sanders-van Wijk, S.; Tromp, J.; Beussink-Nelson, L.; Hage, C.; Svedlund, S.; Saraste, A.; Swat, S.A.; Sanchez, C.; Njoroge, J.; Tan, R.S.; Fermer, M.L.; Gan, L.M.; Lund, L.H.; Lam, C.S.P.; Shah, S.J. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure With Preserved Ejection Fraction: Results From the PROMIS-HFpEF Study. Circulation. 2020, 142, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Armitage, J.; Parish, S.; Sleigh, P.; Peto, R.; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003, 361, 2005–2016. [Google Scholar] [PubMed]
- Tonelli, M.; Moyé, L.; Sacks, F.M.; Kiberd, B.; Curhan, G.; Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann. Intern. Med. 2003, 138, 98–104. [Google Scholar] [CrossRef]
- Raymakers, A.J.N.; Sadatsafavi, M.; Sin, D.D.; De Vera, M.A.; Lynd, L.D. The Impact of Statin Drug Use on All-Cause Mortality in Patients With COPD: A Population-Based Cohort Study. Chest. 2017, 152, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Nezasa, K.; Higaki, K.; Matsumura, T.; Inazawa, K.; Hasegawa, H.; Nakano, M.; Koike, M. Liver-specific distribution of rosuvastatin in rats: comparison with pravastatin and simvastatin. Drug Metab. Dispos. 2002, 30, 1158–1163. [Google Scholar] [CrossRef]
- Sahebkar, A.; Kotani, K.; Serban, C.; Ursoniu, S.; Mikhailidis, D.P.; Jones, S.R.; Ray, K.K.; Blaha, M.J.; Rysz, J.; Toth, P.P.; Muntner, P.; Lip, G.Y.; Banach, M.; Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Statin therapy reduces plasma endothelin-1 concentrations: A meta-analysis of 15 randomized controlled trials. Atherosclerosis. 2015, 241, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.J.; Cauthen, C.A.; Biondi-Zoccai, G.G.; Abbate, A.; Vrtovec, B.; Khan, B.V.; Vetrovec, G.W. Meta-analysis of randomized controlled trials of statins versus placebo in patients with heart failure. Am. J. Cardiol. 2009, 104, 1708–1716. [Google Scholar] [CrossRef]
- Morimoto, T.; Katanasaka, Y.; Sunagawa, Y.; Hirano, S.; Miyazaki, Y.; Funamoto, M.; Hojo, Y.; Suzuki, H.; Morimoto, E.; Ueno, M.; Shimatsu, A.; Satoh-Asahara, N.; Yamakage, H.; Wada, H.; Hasegawa, K. Effects of Statins on Left Ventricular Diastolic Function in Patients with Dyslipidemia and Diastolic Dysfunction (Stat-LVDF Study). Biol. Pharm. Bull. 2015, 38, 1404–1409. [Google Scholar] [CrossRef]
- Murrow, J.R.; Sher, S.; Ali, S.; Uphoff, I.; Patel, R.; Porkert, M.; Le, N.A.; Jones, D.; Quyyumi, A.A. The differential effect of statins on oxidative stress and endothelial function: atorvastatin versus pravastatin. J. Clin. Lipidol. 2012, 6, 42–49. [Google Scholar] [CrossRef]
- Patel, K.K.; Sehgal, V.S.; Kashfi, K. Molecular targets of statins and their potential side effects: Not all the glitter is gold. Eur. J. Pharmacol. 2022, 922, 174906. [Google Scholar] [CrossRef]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-Associated Side Effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef]
- Turner, R.M.; Pirmohamed, M. Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; Macfarlane, P.W.; Packard, C.J.; Stott, D.J.; Westendorp, R.G.; Shepherd, J.; Davis, B.R.; Pressel, S.L.; Marchioli, R.; Marfisi, R.M.; Maggioni, A.P.; Tavazzi, L.; Tognoni, G.; Kjekshus, J.; Pedersen, T.R.; Cook, T.J.; Gotto, A.M.; Clearfield, M.B.; Downs, J.R.; Nakamura, H.; Ohashi, Y.; Mizuno, K.; Ray, K.K.; Ford, I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Mach, F.; Ray, K.K.; Wiklund, O.; Corsini, A.; Catapano, A.L.; Bruckert, E.; De Backer, G.; Hegele, R.A.; Hovingh, G.K.; Jacobson, T.A.; Krauss, R.M.; Laufs, U.; Leiter, L.A.; März, W.; Nordestgaard, B.G.; Raal, F.J.; Roden, M.; Santos, R.D.; Stein, E.A.; Stroes, E.S.; Thompson, P.D.; Tokgözoglu, L.; Vladutiu, G.D.; Gencer, B.; Stock, J.K.; Ginsberg, H.N.; Chapman, M.J.; European Atherosclerosis Society Consensus Panel. Adverse effects of statin therapy: perception vs. the evidence - focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur. Heart J. 2018, 39, 2526–2539. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, S.P. Different effects of statins on induction of diabetes mellitus: an experimental study. Drug Des. Devel. Ther. 2015, 9, 6211–6223. [Google Scholar] [CrossRef]
- Robinson, J.G. Statins and diabetes risk: how real is it and what are the mechanisms? Curr. Opin. Lipidol. 2015, 26, 228–235. [Google Scholar] [CrossRef]
- Drexel, H.; Coats, A.J.S.; Spoletini, I.; Bilato, C.; Mollace, V.; Perrone Filardi, P.; Rosano, G.M.C. An expert opinion paper on statin adherence and implementation of new lipid-lowering medications by the ESC Working Group on Cardiovascular Pharmacotherapy: Barriers to be overcome. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 115–121. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Shahzeb Khan, M.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure, E.; Giannetti, N.; Gomez-Mesa, J.E.; Janssens, S.; Januzzi, J.L.; Gonzalez-Juanatey, J.R.; Merkely, B.; Nicholls, S.J.; Perrone, S.V.; Piña, I.L.; Ponikowski, P.; Senni, M.; Seronde, M.F.; Sim, D.; Spinar, J.; Squire, I.; Taddei, S.; Tsutsui, H.; Verma, S.; Vinereanu, D.; Zhang, J.; Jamal, W.; Schnaidt, S.; Schnee, J.M.; Brueckmann, M.; Pocock, S.J.; Zannad, F.; Packer, M.; EMPEROR-Preserved Trial Committees and Investigators. Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR-Preserved trial. Eur. J. Heart Fail. 2020, 22, 2383–2392. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; Voelkel, N.F.; Dinarello, C.A.; Abbate, A. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Trankle, C.R.; Canada, J.M.; Carbone, S.; Buckley, L.; Kadariya, D.; Del Buono, M.G.; Billingsley, H.; Wohlford, G.; Viscusi, M.; Oddi-Erdle, C.; Abouzaki, N.A.; Dixon, D.; Biondi-Zoccai, G.; Arena, R.; Abbate, A. IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005036. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; Kastelein, J.J.P.; Cornel, J.H.; Pais, P.; Pella, D.; Genest, J.; Cifkova, R.; Lorenzatti, A.; Forster, T.; Kobalava, Z.; Vida-Simiti, L.; Flather, M.; Shimokawa, H.; Ogawa, H.; Dellborg, M.; Rossi, P.R.F.; Troquay, R.P.T.; Libby, P.; Glynn, R.J.; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
| Study, Year [References] | Study Design |
Country | Patients, n |
Proportion on Statins, % | Type of Statin |
Follow-Up, Years | Main Results |
|---|---|---|---|---|---|---|---|
| Fukuta H. et al., 2005 [157] | Prospective | United States | 137 | 50 | Atorvastatin, simvastatin, pravastatin | 2 | ↓All-cause mortality (HR 0.20; 95% CI 0.06 to 0.62; P < 0.01) |
| Roik M. et al., 2008 [158] | Prospective | Poland | 146 | 71 | Simvastatin, atorvastatin, lovastatin | 1 | ↓ All-cause mortality (HR 0.24, 95% CI 0.07 to 0.90; P < 0.05) ↓ CV admission (HR 0.55, 95% CI 0.33 to 0.92; P< 0.05) |
| Shah R. et al., 2008 [159] | Retrospective | United States | 13533 | 17 | N/A | 3 | ↓All-cause mortality (HR 0.73, 95% CI 0.68 to 0.79, P < 0.001) |
| Tehrani F. et al., 2010 [160] | Retrospective | United States | 270 | 30 | N/A | 5 | ↓ All-cause mortality (HR 0.65; 95% CI 0.45 to 0.95; P = 0.029) No significant reduction in CV admission |
| Gomez-Soto F.M. et al., 2010 [161] | Prospective | Spain | 1120 | 50 | Simvastatin, lovastatin, pravastatin | 5 | ↓ All-cause mortality (HR 0.34, 95% CI 0.21 to 0.47, P < 0.001) ↓ HF admission (13.9 vs. 19.7 per 100 persons-year, P < 0.001) |
| Quirós López R. et al., 2012 [162] | Retrospective | Spain | 231 | 21 | N/A | 10 | ↓ All-cause mortality (HR 0.27, 95% CI 0.15 to 0.50, P = 0.01) |
| Kaneko H. et al., 2013 [163] | Prospective | Japan | 1121 | 55 | N/A | 3 | ↓ All-cause mortality (HR 0.26, 95% CI 0.140 to 0.479, P < 0.001) |
| Nochioka K. et al., 2015 [164] | Prospective | Japan | 3124 | 37 | N/A | 3 | ↓All-cause mortality (HR 0.71; 95% CI 0.62 to 0.82; P < 0.001.) No significant reduction in HF admission |
| Alehagen U. et al., 2015 [165] | Prospective | Sweden | 9140 | 38 | N/A | 1 | ↓ All-cause mortality (HR 0.80, 95% CI 0.72 to 0.89, P < 0.001) ↓ All-cause mortality + CV admission (HR 0.89, 95% CI 0.82 to 0.96; P < 0.01) |
| Yap J. et al., 2015 [166] | Prospective | Singapore | 751 | 61 | N/A | 2 | ↓ All-cause mortality (HR 0.59, 95% CI 0.44 to 0.79; P < 0.001) ↓ CV mortality (HR 0.58, 95% CI 0.37-0.89; P = 0.012) |
| Lee M.S.et al., 2018 [167] | Retrospective | United States | 7563 | 72 | N/A | 6.7 | ↓ All-cause mortality (HR 0.73, 95% CI 0.66 to 0.81; P < 0.001) |
| Tsujimoto T. et al., 2018 [168] | Prospective (data of TOPCAT trial) | United States, Canada, Brazil, Argentina, Russia, Georgia | 3378 | 52 | N/A | 3.3 | ↓ All-cause mortality (HR 0.79, 95% CI 0.63 to 0.99; P = 0.04) |
| Marume K. et al., 2019 [169] | Prospective | Japan | 414 | 20 | N/A | 2 | ↓ All-cause mortality (HR 0.21, 95% CI 0.06 to 0.72; P < 0.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





