Submitted:
02 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Multiple Myeloma
1.2. Wnt Pathway in Multiple Myeloma
1.3. Physical Activity and Wnt Pathway
1.4. Intermittent Fasting
1.5. Nordic Walking
2. Material and Methods
2.1. Study Group Characteristics
2.2. Study Protocol
2.3. Methods
2.4. Statistical Analysis
3. Results
3.1. Body Composition
3.2. Blood Biochemical Parameters Related with MM
3.3. Blood Biochemical Parameters Related with the Wnt Pathway
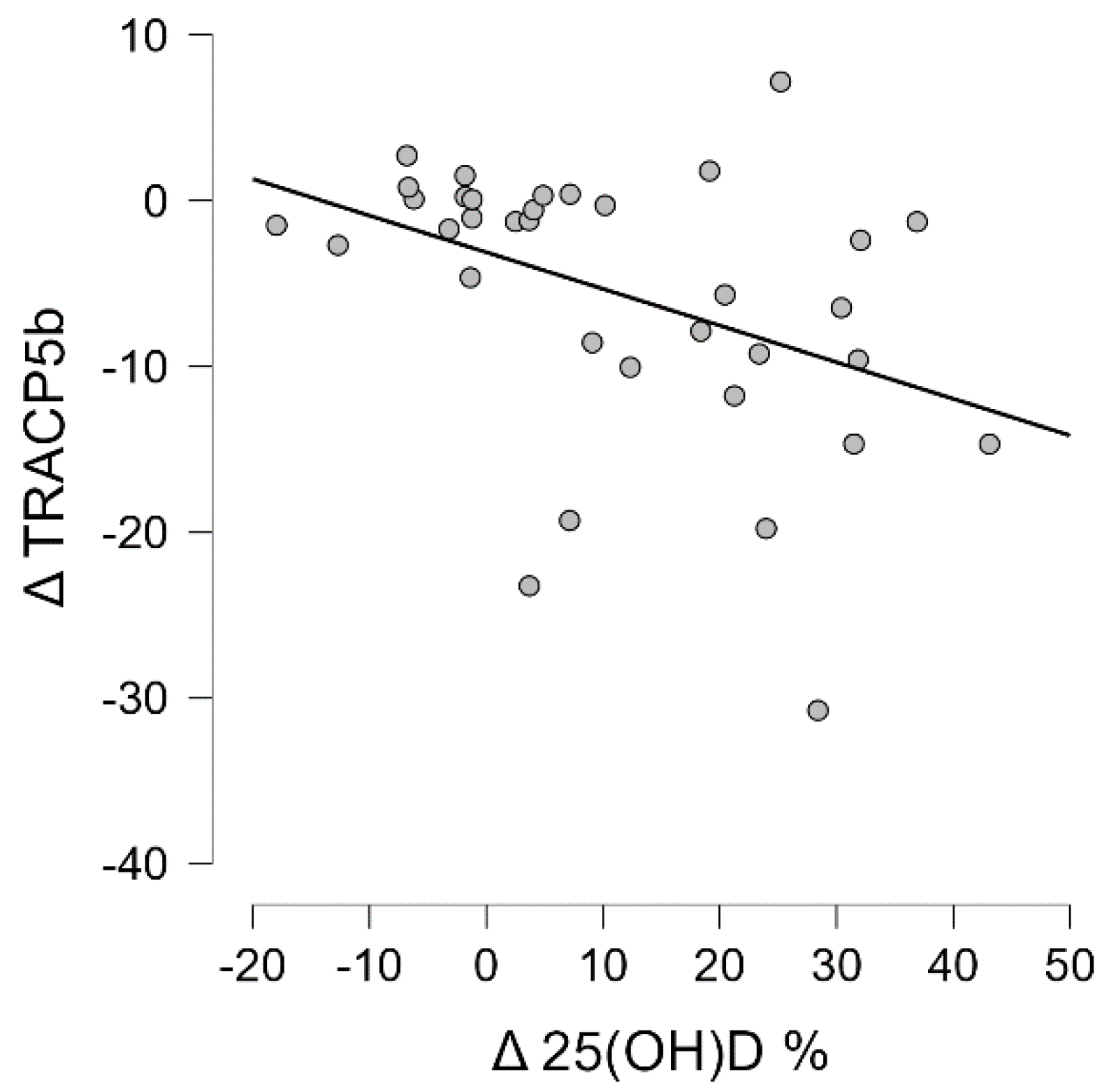
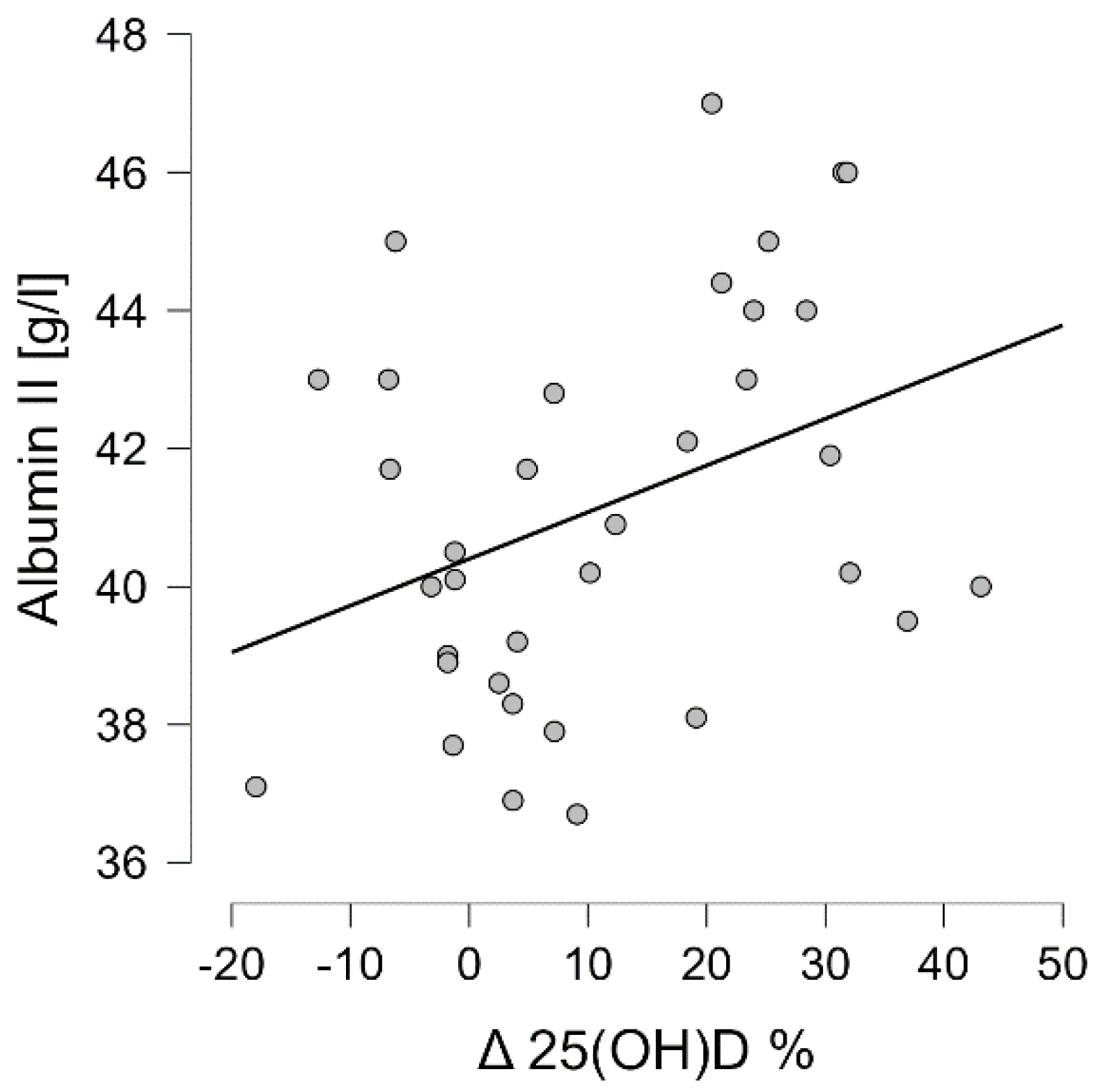
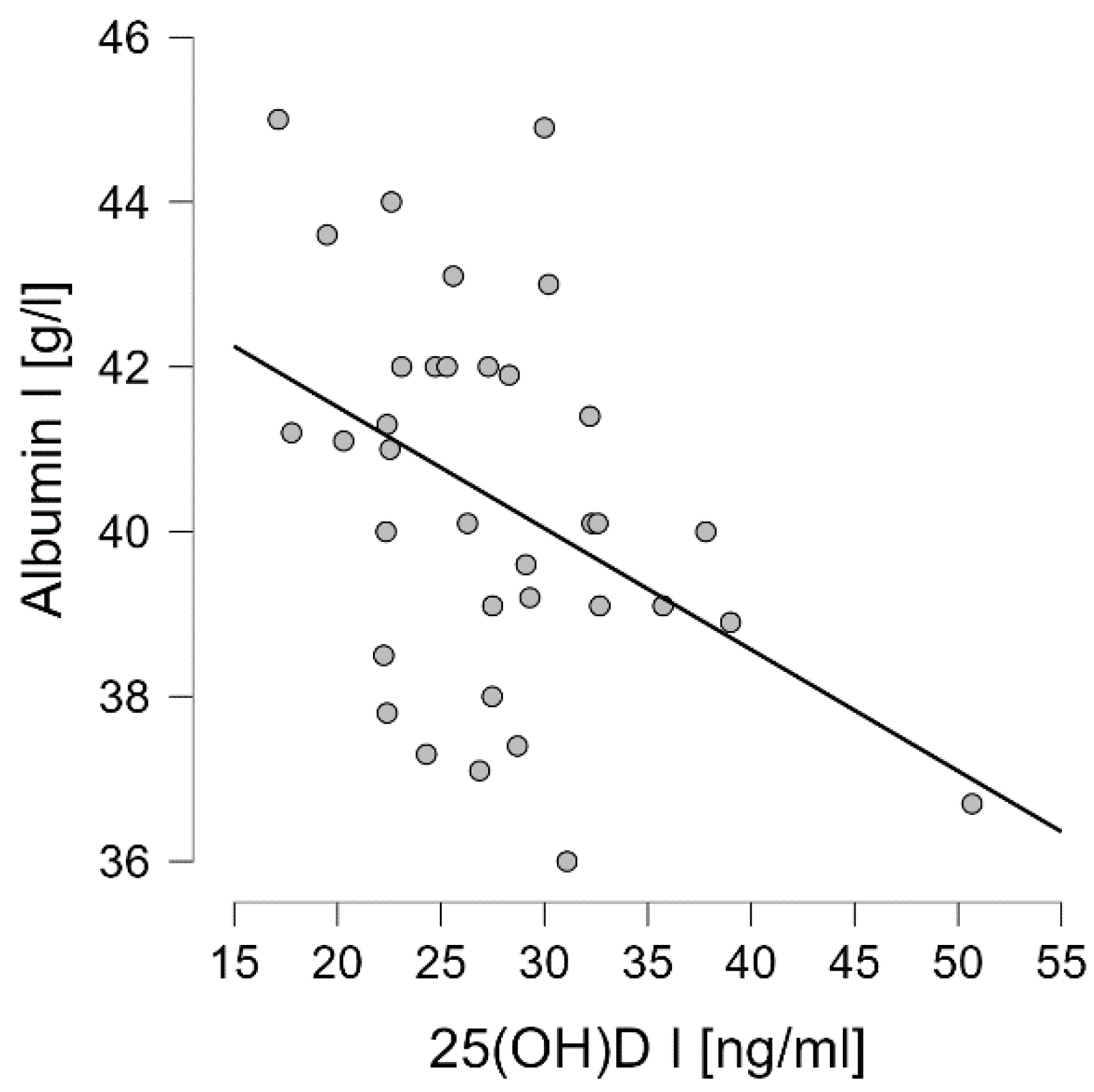
3.4. Correlation Analysis of Wnt Pathway Markers
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojciechowska, U.; Czaderny, K.; Ciuba, A.; Didkowska, J. Nowotwory Złośliwe w Polsce w 2016 Roku. Krajowy rejestr nowotworów.
- Giannopoulos, K.; Jamroziak, K.; Usnarska-Zubkiewicz, L.; Dytfeld, D.; Jurczyszyn, A.; Walewski, J.; Lech-Marańda, E.; Walter-Croneck, A.; Pieńkowska-Grela, B.; Wróbel, T.; et al. Recommendations of Polish Myeloma Group Concerning Diagnosis and Therapy of Multiple Myeloma and Other Plasmacytic Dyscrasias for 2018/2019. Acta Haematologica Polonica 2018, 49, 157–206. [Google Scholar] [CrossRef]
- Jurczyszyn, A.; Gdula-Argasińska, J.; Kosmaczewska, A.; Skotnicki, A.B. Rola Mikrośrodowiska Szpiku Kostnego w Patogenezie Szpiczaka Plazmocytowego. Postepy Higieny i Medycyny Doswiadczalnej 2015, 69, 521–533. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Minter, A.R.; Korde, N.; Tan, E.; Landgren, O. Bone Disease in Multiple Myeloma and Precursor Disease: Novel Diagnostic Approaches and Implications on Clinical Management. Expert Review of Molecular Diagnostics 2011, 11, 593–603. [Google Scholar] [CrossRef]
- Koziński, K.; Dobrzyń, A. Wnt Signaling Pathway – Its Role in Regulation of Cell Metabolism. Postępy Higieny i Medycyny Doświadczalnej 2013, 67, 1098–1108. [Google Scholar] [CrossRef]
- Rossini, M.; Gatti, D.; Adami, S. Involvement of WNT / b -Catenin Signaling in the Treatment of Osteoporosis. Calcified Tissue International 2013, 93, 121–132. [Google Scholar] [CrossRef]
- Glass, D.A.; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt Signaling in Differentiated Osteoblasts Controls Osteoclast Differentiation. Developmental Cell 2005, 8, 751–764. [Google Scholar] [CrossRef]
- Mierzwińska, E.; Hryszko, T.; Szablak-Uliszewska, E.; Naumnik, B. Sclerostin and Chronic Kidney Disease. Postępy Higieny i Medycyny Doświadczalnej 2017, 71, 0–0. [Google Scholar] [CrossRef]
- Gooding, S.; Edwards, C.M. New Approaches to Targeting the Bone Marrow Microenvironment in Multiple Myeloma. Current Opinion in Pharmacology 2016, 28, 43–49. [Google Scholar] [CrossRef]
- Oshima, T.; Abe, M.; Asano, J.; Hara, T.; Kitazoe, K.; Sekimoto, E.; Tanaka, Y.; Shibata, H.; Hashimoto, T.; Ozaki, S.; et al. Myeloma Cells Suppress Bone Formation by Secreting a Soluble Wnt Inhibitor, SFRP-2. Blood 2005, 106, 3160–3165. [Google Scholar] [CrossRef]
- Tian, E.; Zhan, F.; Walker, R.; Rasmussen, E.; Ma, Y.; Barlogie, B.; Shaughnessy, J.D. The Role of the Wnt-Signaling Antagonist DKK1 in the Development of Osteolytic Lesions in Multiple Myeloma. New England Journal of Medicine 2003, 349, 687–696. [Google Scholar] [CrossRef]
- Kaiser, M.; Mieth, M.; Liebisch, P.; Oberländer, R.; Rademacher, J.; Jakob, C.; Kleeberg, L.; Fleissner, C.; Braendle, E.; Peters, M.; et al. Serum Concentrations of DKK-1 Correlate with the Extent of Bone Disease in Patients with Multiple Myeloma. European Journal of Haematology 2008, 80, 490–494. [Google Scholar] [CrossRef]
- Pelletier, S.; Dubourg, L.; Carlier, M.C.; Hadj-Aissa, A.; Fouque, D. The Relation between Renal Function and Serum Sclerostin in Adult Patients with CKD. Clinical Journal of the American Society of Nephrology 2013, 8, 819–823. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical Stimulation of Bone in Vivo Reduces Osteocyte Expression of Sost/Sclerostin. Journal of Biological Chemistry 2008, 283, 5866–5875. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG Pathway. Current Osteoporosis Reports 2007, 5, 98–104. [Google Scholar] [CrossRef]
- Raje, N.S.; Bhatta, S.; Terpos, E. Role of the RANK/RANKL Pathway in Multiple Myeloma. Clinical Cancer Research 2019, 25, 12–20. [Google Scholar] [CrossRef]
- Terpos, E.; Zamagni, E.; Lentzsch, S.; Drake, M.T.; García-Sanz, R.; Abildgaard, N.; Ntanasis-Stathopoulos, I.; Schjesvold, F.; de la Rubia, J.; Kyriakou, C.; et al. Treatment of Multiple Myeloma-Related Bone Disease: Recommendations from the Bone Working Group of the International Myeloma Working Group. The Lancet Oncology 2021, 22, e119–e130. [Google Scholar] [CrossRef]
- Nes, B.M.; Janszky, I.; Wisløff, U.; Støylen, A.; Karlsen, T. Age-Predicted Maximal Heart Rate in Healthy Subjects: The HUNT Fitness Study. Scandinavian Journal of Medicine and Science in Sports 2013, 23, 697–704. [Google Scholar] [CrossRef]
- Emery, A.; Moore, S.; Crowe, J.; Murray, J.; Peacock, O.; Thompson, D.; Betts, F.; Rapps, S.; Ross, L.; Rothschild-Rodriguez, D.; et al. The Effects of Short-Term, Progressive Exercise Training on Disease Activity in Smouldering Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: A Single-Arm Pilot Study. BMC Cancer 2024, 24, 1–18. [Google Scholar] [CrossRef]
- Czerwińska-Ledwig, O.; Vesole, D.H.; Piotrowska, A.; Gradek, J.; Pilch, W.; Jurczyszyn, A. Effect of a 6-Week Cycle of Nordic Walking Training on Vitamin 25(OH)D3, Calcium-Phosphate Metabolism and Muscle Damage in Multiple Myeloma Patients-Randomized Controlled Trial. Journal of clinical medicine 2022, 11, 6534. [Google Scholar] [CrossRef]
- Czerwińska-Ledwig, O.; Jurczyszyn, A.; Piotrowska, A.; Pilch, W.; Antosiewicz, J.; Żychowska, M. The Effect of a Six-Week Nordic Walking Training Cycle on Oxidative Damage of Macromolecules and Iron Metabolism in Older Patients with Multiple Myeloma in Remission—Randomized Clinical Trial. International Journal of Molecular Sciences 2023, 24, 1–15. [Google Scholar] [CrossRef]
- Czerwińska-Ledwig, O.; Gradek, J.; Deląg, J.; Jurczyszyn, A. The Effect of a 6-Week Nordic Walking Training Cycle on Myeloma-Related Blood Parameters, Vitamin 25(OH)D3 Serum Concentration and Peripheral Polyneuropathy Symptoms in Patients with Multiple Myeloma. Clinical Lymphoma Myeloma and Leukemia 2021, 21, S114–S115. [Google Scholar] [CrossRef]
- Pilch, W.; Tyka, A.; Cebula, A.; Śliwicka, E.; Pilaczyńska-Szcześniak, Ł.; Tyka, A. Effects of a 6-Week Nordic Walking Training on Changes in 25(OH)D Blood Concentration in Women Aged over 55. Journal of Sports Medicine and Physical Fitness 2017, 57, 124–129. [Google Scholar] [CrossRef]
- Pilch, W.; Kita, B.; Piotrowska, A.; Tota, Ł.; Maciejczyk, M.; Czerwińska-Ledwig, O.; Sadowska- Krepa, E.; Kita, S.; Pałka, T. The Effect of Vitamin D Supplementation on the Muscle Damage after Eccentric Exercise in Young Men: A Randomized, Control Trial. Journal of the International Society of Sports Nutrition 2020, 17, 53. [Google Scholar] [CrossRef]
- Prusik, K.; Kortas, J.; Prusik, K.; Mieszkowski, J.; Jaworska, J.; Skrobot, W.; Lipinski, M.; Ziemann, E.; Antosiewicz, J. Nordic Walking Training Causes a Decrease in Blood Cholesterol in Elderly Women Supplemented with Vitamin D. Frontiers in Endocrinology 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Alrowaili, M.G.; Hussein, A.M.; Eid, E.A.; Serria, M.S.; Abdellatif, H.; Sakr, H.F. Effect of Intermittent Fasting on Glucose Homeostasis and Bone Remodeling in Glucocorticoid-Induced Osteoporosis Rat Model. Journal of Bone Metabolism 2021, 28, 307–316. [Google Scholar] [CrossRef]
- Janckila, A.J.; Nakasato, Y.R.; Neustadt, D.H.; Yam, L.T. Disease-Specific Expression of Tartrate-Resistant Acid Phosphatase Isoforms. Journal of Bone and Mineral Research 2003, 18, 1916–1919. [Google Scholar] [CrossRef]
- Terpos, E.; De La Fuente, J.; Szydlo, R.; Hatjiharissi, E.; Viniou, N.; Meletis, J.; Yataganas, X.; Goldman, J.M.; Rahemtulla, A. Tartrate-Resistant Acid Phosphatase Isoform 5b: A Novel Serum Marker for Monitoring Bone Disease in Multiple Myeloma. International Journal of Cancer 2003, 106, 455–457. [Google Scholar] [CrossRef]
- Manolagas, S.C. Wnt Signaling and Osteoporosis. Maturitas 2014, 78, 233–237. [Google Scholar] [CrossRef]
- Seefried, L.; Genest, F.; Strömsdörfer, J.; Engelmann, B.; Lapa, C.; Jakob, F.; Baumann, F.T.; Sperlich, B.; Jundt, F. Impact of Whole-Body Vibration Exercise on Physical Performance and Bone Turnover in Patients with Monoclonal Gammopathy of Undetermined Significance. Journal of Bone Oncology 2020, 25, 100323. [Google Scholar] [CrossRef]
- Janik, M.; Stuss, M.; Michalska-Kasiczak, M.; Jegier, A.; Sewerynek, E. Effects of Physical Activity on Sclerostin Concentrations. Endokrynologia Polska 2018, 69, 142–149. [Google Scholar] [CrossRef]
- de Oliveira, R.D.J.; de Oliveira, R.G.; de Oliveira, L.C.; Santos-Filho, S.D.; Sá-Caputo, D.C.; Bernardo-Filho, M. Effectiveness of Whole-Body Vibration on Bone Mineral Density in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Osteoporosis International 2023, 34, 29–52. [Google Scholar] [CrossRef]
- Cochrane, D.J. Vibration Exercise: The Potential Benefits. International Journal of Sports Medicine 2011, 32, 75–99. [Google Scholar] [CrossRef]
- Miyakoshi, N.; Masutani, N.; Kasukawa, Y.; Kudo, D.; Saito, K.; Matsunaga, T.; Shimada, Y. Comparison of the Effects of Native Vitamin D and Eldecalcitol on Muscular Strength and Dynamic Balance in Patients with Postmenopausal Osteoporosis. Progress in Rehabilitation Medicine 2020, 5, n. [Google Scholar] [CrossRef]
- Mindikoglu, A.L.; Abdulsada, M.M.; Jain, A.; Jalal, P.K.; Devaraj, S.; Wilhelm, Z.R.; Opekun, A.R.; Jung, S.Y. Intermittent Fasting from Dawn to Sunset for Four Consecutive Weeks Induces Anticancer Serum Proteome Response and Improves Metabolic Syndrome. Scientific Reports 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Descamps, O.; Riondel, J.; Ducros, V.; Roussel, A.M. Mitochondrial Production of Reactive Oxygen Species and Incidence of Age-Associated Lymphoma in OF1 Mice: Effect of Alternate-Day Fasting. Mechanisms of Ageing and Development 2005, 126, 1185–1191. [Google Scholar] [CrossRef]
- Lu, Z.; Xie, J.; Wu, G.; Shen, J.; Collins, R.; Chen, W.; Kang, X.; Luo, M.; Zou, Y.; Huang, L.J.-S.; et al. Fasting Selectively Blocks Development of Acute Lymphoblastic Leukemia via Leptin-Receptor Upregulation. Nature Medicine 2017, 23, 79–90. [Google Scholar] [CrossRef]
- de Groot, S.; Vreeswijk, M.P.G.; Welters, M.J.P.; Gravesteijn, G.; Boei, J.J.W.A.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.M.; Nortier, J.W.R.; et al. The Effects of Short-Term Fasting on Tolerance to (Neo) Adjuvant Chemotherapy in HER2-Negative Breast Cancer Patients: A Randomized Pilot Study. BMC Cancer 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Dorff, T.B.; Groshen, S.; Garcia, A.; Shah, M.; Tsao-Wei, D.; Pham, H.; Cheng, C.W.; Brandhorst, S.; Cohen, P.; Wei, M.; et al. Safety and Feasibility of Fasting in Combination with Platinum-Based Chemotherapy. BMC Cancer 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Badar, T.; Ismail, A.; AlShanqeeti, A. Safety and Feasability of Muslim Fasting While Receiving Chemotherapy. IOSR Journal of Pharmacy (IOSRPHR) 2014, 4, 15–20. [Google Scholar] [CrossRef]
- Marinac, C.R.; Nelson, S.H.; Breen, C.I.; Hartman, S.J.; Natarajan, L.; Pierce, J.P.; Flatt, S.W.; Sears, D.D.; Patterson, R.E. Prolonged Nightly Fasting and Breast Cancer Prognosis. JAMA Oncology 2016, 2, 1049. [Google Scholar] [CrossRef]
| Non-training (n=14) | Training (n=21) | |||
|---|---|---|---|---|
| CG (n=7) | IF (n=7) | NW (n=10) | IF NW (n=11) | |
| Age [years] |
65.2±4.8 | 65.4±5.5 | ||
| 65.3±5.0 | 65.1±4.9 | 65.70±6.9 | 65.18±4.2 | |
| BMI [kg/m2] |
28.6±4.2 | 30.2±3.5 | ||
| 29.0±4.4 | 28.2±4.4 | 28.6±3.6 | 31.7±2.8 | |
| Body height [cm] |
166.1±5.2 | 163.5±10.3 | ||
| 164.9±4.4 | 167.3±5.9 | 164.6±10.5 | 162.5±10.6 | |
| MM duration [months] | 56.8±10.7 | 57.2±12.8 | ||
| Disease duration [months] | 54.4±12.2 | 59.1±9.2 | 57.5±11.6 | 57.0±14.3 |
| Type of MM IgG: IgA: Other: |
IgG (n=8) IgA (n=5) light chains (n=1) |
IgG (n=11) IgA (n=8) light chains (n=2) |
||
| Type of MM | κ-chains (n=2) λ-chains (n=1) κ-chains (n=2) λ-chains (n=1) light chains (n=1) |
κ-chains (n=2) λ-chains (n=3) κ-chains (n=1) λ-chains (n=1) - |
κ-chains (n=4)λ-chains (n=2)κ-chains (n=1)λ-chains (n=1)light chains (n=2) | κ-chains (n=2)λ-chains (n=3)κ-chains (n=4)λ-chains (n=2)- |
| Cycles of therapy | median=6 (min=3, max=7) |
median=4 (min=3, max=8) |
||
| Cycles of therapy | median=6 (min=3, max=7) | median=5 (min=4, max=7) | median=4 (min=3, max=6) | median=5 (min=3, max=8) |
| auto-HSCT | 100% 75% - 1 procedure 25% - 2 procedures |
100% 80% - 1 procedure 20% - 2 procedures |
||
| Time | Non-training (n=14) | Training (n=21) | |||
| CG (n=7) | IF CG (n=7) | NW (n=10) | IF NW (n=11) | ||
| Body mass [kg] | I | 79.4±15.5 | 79.1±12.3 | ||
| 79.1±14.0 | 79.7±18.1 | 80.6±14.0 | 77.7±11.0 | ||
| II | 79.8±15.6 | 79.0±12.2 | |||
| 79.6±13.6 | 80.1±18.5 | 80.8±13.9 | 77.4±10.8 | ||
| LBM [kg] |
I | 52.6±9.8 | 51.4±9.6 | ||
| 52.7±10.1 | 52.5±10.4 | 52.5±9.1 | 50.4±10.3 | ||
| II | 55.2±11.5 | 51.8±9.6 | |||
| 56.5±12.4 | 53.9±11.4 | 53.3±9.3 | 50.5±10.2 | ||
| SLM [kg] |
I | 50.0±9.4 | 47.4±9.0 | ||
| 50.1±9.6 | 49.8±9.9 | 48.5±8.7 | 46.5±9.7 | ||
| II | 50.5±9.7 | 47.9±9.2 | |||
| 49.8±9.1 | 51.1±11.0 | 49.4±9.1 | 46.5±9.5 | ||
| TBW [%] |
I | 46.4±4.7 | 46.3±4.6 | ||
| 46.3±5.2 | 46.5±4.4 | 46.4±3.8 | 46.2±5.5 | ||
| II | 46.6±4.6 | 47.0±5.2 | |||
| 46.6±5.5 | 46.6±3.9 | 47.5±5.5 | 46.5±5.2 | ||
| BMI [kg/m2] |
I | 28.6±4.2 | 30.2±3.5 | ||
| 29.0±4.4 | 28.2±4.4 | 28.6±3.6 | 31.7±2.8 | ||
| II | 28.7±4.3 | 30.2±3.4 | |||
| 29.2±4.4 | 28.3±4.5 | 28.7±3.6 | 31.4±2.7 | ||
| PBF [%] |
I | 33.1±7.5 | 31.0±3.9 | ||
| 32.9±8.2 | 33.3±7.3 | 30.9±3.1 | 31.2±4.7 | ||
| II | 32.8±6.2 | 30.9±3.7 | |||
| 33.5±8.0 | 32.2±4.3 | 30.8±2.9 | 31.0±4.4 | ||
| waist circumference | I | 97.6±15.7 | 99.5±10.9 | ||
| 98.3±15.7 | 97.0±16.9 | 101.8±13.0 | 97.4±8.8 | ||
| II | 97.9±16.1 | 97.6±10.7 | |||
| 98.7±15.8 | 97.1±17.7 | 100.2±13.6 | 95.3±7.6 | ||
| hip circumference | I | 103.7± 10.9 | 106.8±8.9 | ||
| 106.2±12.3 | 101.3±9.5 | 106.9±8.0 | 106.7±10.1 | ||
| II | 103.6± 10.8 | 105.2± 7.5 | |||
| 105.0±12.2 | 102.2±9.9 | 104.5±7.1 | 105.8±8.0 | ||
| WHR | I | 0.9± 0.1 | 0.9± 0.1 | ||
| 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | ||
| II | 0.9± 0.1 | 0.9± 0.1 | |||
| 0.9±0.1 | 0.9±0.1 | 0.9± 0.1 | 0.9±0.1 | ||
| Time | Non-training (n=14) | Training (n=21) | |||
| CG (n=7) | IF CG (n=7) | NW (n=10) | IF NW (n=11) | ||
| Ca [mmol/l] |
I | 2.37±0.13 | 2.39±0.15 | ||
| 2.36±0.15 | 2.37±0.13 | 2.39±0.10 | 2.38±0.18 | ||
| II | 2.37±0.10 | 2.38±0.19 | |||
| 2.35±0.11 | 2.41±0.07 | 2.36±0.12 | 2.39±0.25 | ||
| P [mmol/l] |
I | 0.97±0.20 | 1.04±0.24 | ||
| 0.95±0.28 | 0.98±0.12 | 1.01±0.25 | 1.06±0.22 | ||
| II | 0.98±0.19 | 1.04±0.21 | |||
| 0.95±0.24 | 1.03±0.12 | 1.02±0.20 | 1.07±0.21 | ||
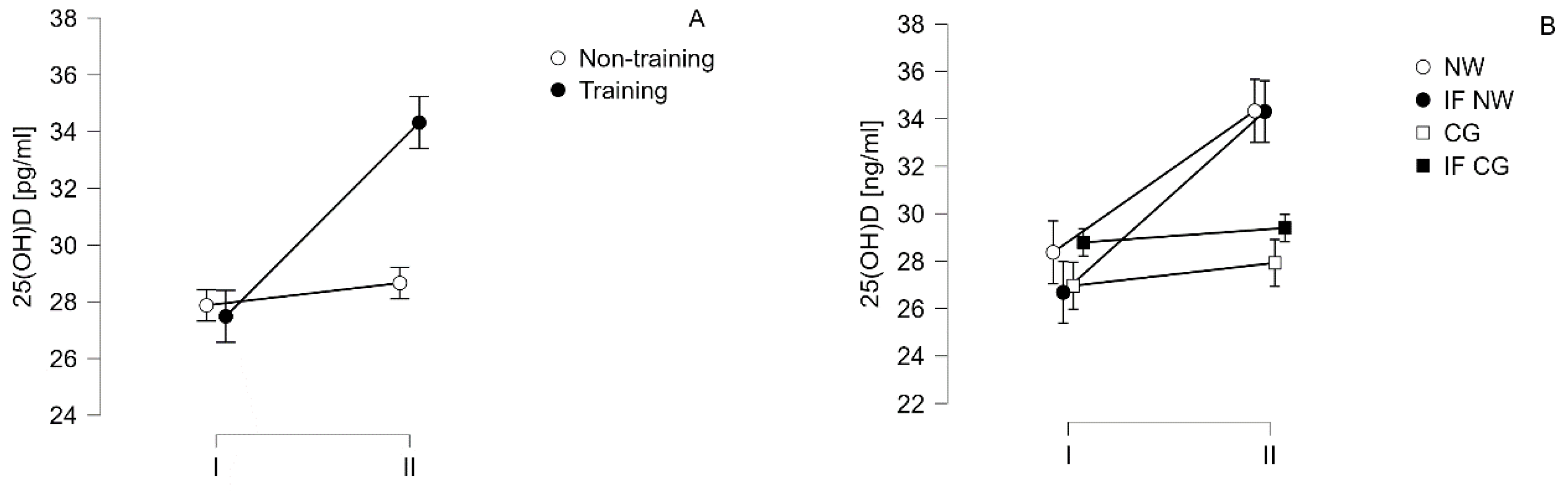
| 25(OH)D [ng/ml] |
I | 27.87±6.11 | 27.49±7.10# | ||
| 26.96±5.06 | 28.79±7.31 | 28.37±8.69* | 26.68±5.61* | ||
| II | 28.66±7.31 | 34.32±10.07# | |||
| 27.92±6.04 | 29.39±8.83 | 34.33±11.90* | 34.31±8.67* | ||
| B2M [mg/l] |
I | 2.15±0.47 | 2.06±0.36 | ||
| 2.20±0.60 | 2.10±0.35 | 2.12±0.43 | 2.01±0.30 | ||
| II | 2.10±0.44 | 2.02±0.41 | |||
| 2.09±0.54 | 2.12±0.35 | 2.08±0.45 | 1.97±0.37 | ||
| Albumin [g/l] |
I | 40.53±2.06 | 40.30±2.52 | ||
| 40.79±1.81 | 40.27±2.41 | 40.17±2.67 | 40.41±2.51 | ||
| II | 40.75±2.28 | 41.42±3.20 | |||
| 40.99±2.10 | 40.51±2.59 | 41.58±3.06 | 41.28±3.46 | ||
| Time | Non-training (n=14) | Training (n=21) | |||
| CG (n=7) | IF CG (n=7) | NW (n=10) | IF NW (n=11) | ||
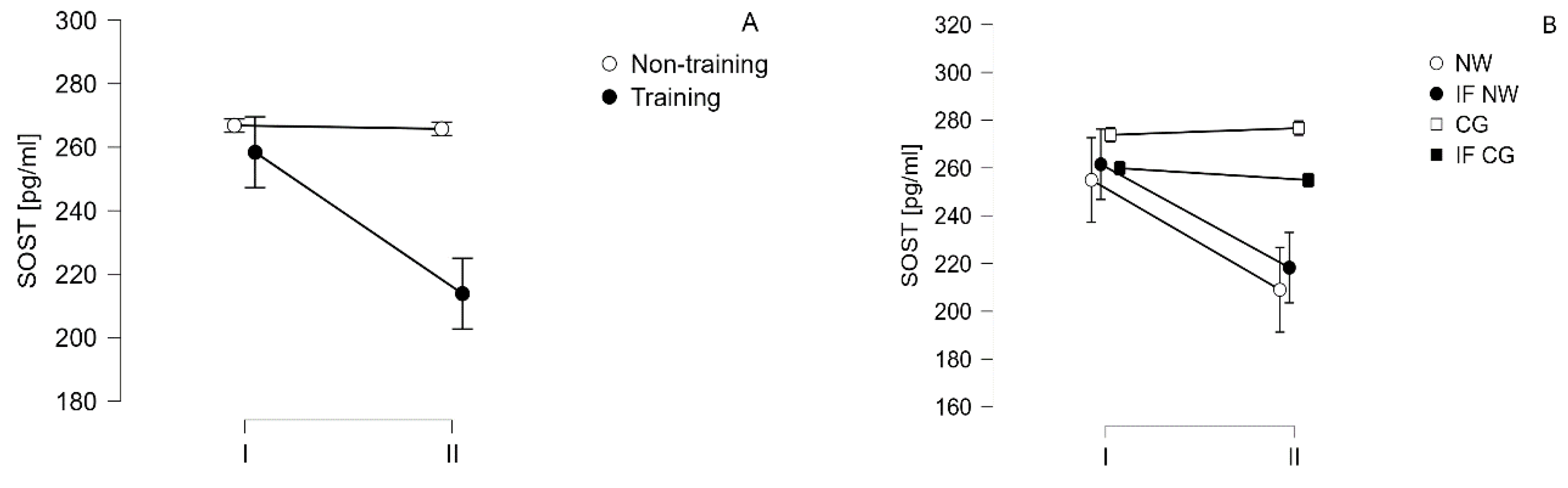
| SOST [pg/ml] |
I | 266.8±100.2 | 258.4±103.3 | ||
| 273.8±93.0 | 259.8±113.9 | 255.0±106.2 | 261.5±105.8 | ||
| II | 265.5±102.8 | 213.9±86.0* | |||
| 276.±99.5 | 254.9±112.8 | 209.0±69.37 | 218.3±102.1 | ||
| DKK-1 [pg/ml] |
I | 4964.9±906.0 | 4866.4±1264.7 | ||
| 5252.2±501.3 | 4677.5±1155.2 | 4704.6±1472.9 | 5013.5±1093.9 | ||
| II | 5099.4±786.6 | 4845.5 ±1362.2 | |||
| 5238.2±591.9 | 4960.5±972.3 | 4750.2±1553.7 | 4932.0±1233.4 | ||
| OPG [pg/ml] |
I | 1155.9±309.7 | 1096.6±316.2 | ||
| 1039.4±269.3 | 1272.5±321.7 | 1109.1±323.3 | 1085.3±325.0 | ||
| II | 1152.1 ±312.7 | 1083.7±262.4 | |||
| 1037.4±266.2 | 1266.8±332.1 | 1089.8±248.3 | 1078.2±286.6 | ||
| OPN [pg/ml] |
I | 21492.6±9121.2 | 21906.5±7420.7 | ||
| 25072.9±5371.4 | 17912.4±9022.6 | 24160.1±6993.5 | 19857.8±7511.3 | ||
| II | 21328.3±8940.6 | 19849.1±8418.0 | |||
| 25313.1±5038.6 | 17343.4±9523.8 | 21344.2±5906.7 | 184890±9298.4 | ||
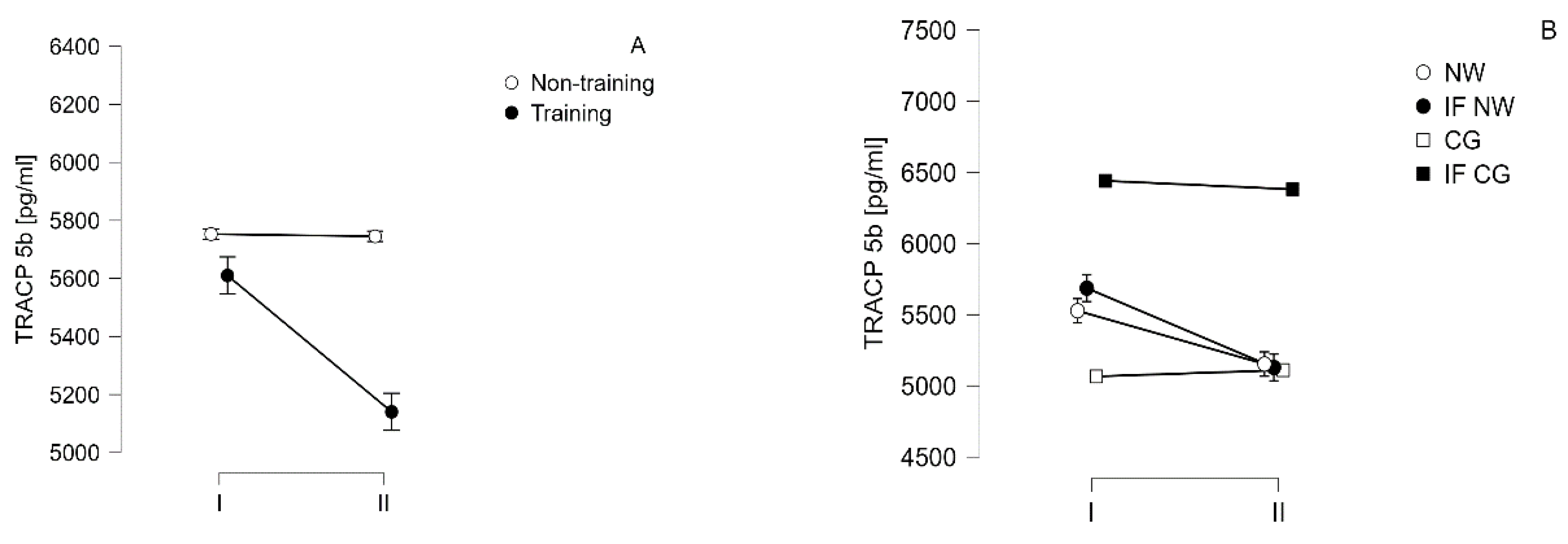
| TRACP-5b [pg/ml] |
I | 5752.8±1375.1 | 5610.2±1281.4 | ||
| 5065.9±1398.6 | 6439.8±1019.7 | 5527.2±1333.6 | 5685.7±1292.3 | ||
| II | 5745.2±1302.5 | 5140.3±1169.5* | |||
| 5110.1±1338.1 | 6380.4±971.4 | 5153.2±1182.5* | 5128.6±1215.3* | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





