1. Introduction
Neurological disorders are generally linked to the abnormal conformational changes of various proteins. Tau protein is one of these molecules. It contains 441 amino acids and 12 of them are histidine which is the most common metal binding site in various proteins. As a consequence, tau protein is a potentially effective metal binding molecule, but its coordination behaviour is much less studied as compared to other biomolecules related to neurodegeneration. In the case of amyloid-β and prion protein huge number of studies are available for the metal binding ability of both the whole protein and various peptide fragments. The most important results obtained in these fields are summarized in various reviews and strongly support the high metal ion affinity of these substances [
1,
2,
3,
4,
5]. In a more recent review we compared the copper(II) and zinc(II) binding ability of various peptide fragments of amyloid-β, prion and tau proteins [
6]. It turned out from this comparison that imidazole-N donors are the primary metal binding sites for all peptides and for both metal ions. The stoichiometry and coordination modes of the different peptides, however, can be significantly different. The number and location of histidyl residues in the sequence and the presence of other coordinating side chains being the governing factors during complex formation. In case of prion protein the well-separated histidyl residues and the absence of other strongly coordinating side chains results in relatively simple complex formation processes and all histidyl moieties can be independent metal binding site at high copper abundancy, while the formation of macrochelates is favoured under low copper occupancy. Amyloid-β is richer in possible coordination sites involving terminal amino and carboxylate groups of aspartyl and glutamyl residues and the histidine can be either in vicinal or distant positions. Thus, coordination chemistry of amyloid-β is more complexed than that of prion protein fragments and can effectively bind both copper(II) and zinc(II) ions in contrast with prion peptides where the preference for copper(II) binding is much more preferred.[
7,
8,
9]. The amino acid sequence of tau protein shows also a big variety including both distant and vicinal histidyl residues and rich in polar side chains of aspartyl, glutamyl, seryl and threonyl residues. It is also important to note that tau protein contains also cysteinyl residues which can be potential binding sites both for copper(II) and zinc(II) ions and can contribute to tau aggregation [
10,
11,
12]. Former literature studies on the metal ion-tau interactions are briefly summarized in our previous review [
6]. Most of these previous studies were focused on the R3, R4 region of the protein which are considered as the microtubule binding domains of the protein [
13,
14,
15], but the possible biological role of the N-terminal region of the protein was also investigated [
16,
17].
According to literature review and clinical observations, the oxidative stress is suggested as the dominant factor for the pathogenesis of Alzheimer’s Disease [
18,
19]. E.g. metal ions bound to the amyloid-β peptide are involved in the production of reactive oxygen species. This may contribute to the oxidative damage on both the amyloid-β peptide itself and the surrounding molecules [
20]. Similar process may occur with tau protein. However, the metal ion catalysed oxidation of tau protein is especially poorly characterized. Nevertheless, the comparison of this process with other neuronal proteins such as prion protein or amyloid-β peptides has been summarized in our recent review [
6]. But systematic studies are not available in the literature at this field.
In the last few years, we started systematic studies on the metal complexes of various regions of tau protein for the better understanding and comparison of the metal binding ability of the various peptide fragments. Tau(9-16) and tau(26-33) containing His14 and His32 residues, respectively, and their mutants were involved in the first study and the results revealed a slight preference for copper(II) binding at His14 over His32 in slightly acidic samples. The stability order, however, changed by the physiological pH range when the amide bonded species are formed [
21]. Interestingly this preference remained intact even in the presence of the fragment tau(326-333) although the latter peptide contains two vicinal histidyl residues (His329 and His330) [
22]. On the contrary, His14 and His32 has only low zinc(II) binding affinity and the Zn(II)-N
im coordinated species cannot compete with the hydrolytic reactions of the metal ions. The amide coordinated zinc(II) complexes of tau(326-333), however, predominate in the slightly alkaline samples [
23]. Now in this paper we report the results of combined potentiometric and spectroscopic studies for the copper (II), zinc(II) and nickel(II) complexes of three other peptide fragments of tau protein: tau(91-97) (Ac-AQPHTEI-NH
2), tau(385-390) (Ac-KTDHGA-NH
2) and tau(404-409) (Ac-SPRHLS-NH
2). All these peptides contain only one histidyl residue (H95, H388 and H407, respectively) but they are present in different chemical environments.
2. Experimental
2.1. Materials
The peptides were purchased from SynPeptide Co. Ltd. with purity level above 95%. Concentration of the peptide stock solutions was determined by pH-potentiometric titrations.
As concerns Cu(II)-, Zn(II)- and Ni(II)-ions, stock solution (CuCl2, NiCl2, ZnCl2) was prepared from analytical grade reagents (Reanal Zrt.) and its concentration was verified gravimetrically via the precipitation of oxinate or using pH-potentiometric titrations with EDTA. KOH and KCl, used during pH-potentiometric titrations, were purchased from Merck.
2.2. Potentiometric Measurements
pH-potentiometric titrations were completed on 3.00 mL samples at 1-2 mM total ligand concentration with the aid of carbonate free stock solution of potassium hydroxide of known concentration (approximately 0.2 M). The metal to ligand ratios were selected between 1:3 and 1:1. During titration, argon was being bubbled through the sample to ensure the absence of oxygen and carbon dioxide. The samples were being stirred by a VELP scientific magnetic stirrer.
All pH-potentiometric measurements were carried out at 298 K and at constant ionic strength of 0.2 M KCl. pH measurements were made with a MOLSPIN pH-meter equipped with a 6.0234.100 and 6.0234.110 combination glass electrode (Metrohm) and the titrant dispensed by means of a computer controlled MOL-ACS burette. The recorded pH readings were converted into hydrogen ion concentration as described by Irving et al. [
24]. Protonation constants of the ligands and overall stability (logβ
pqr) constants of the metal complexes were calculated by means of general computational programs (SUPERQUAD [
25] and PSEQUAD [
26]) based on the Eqs. (1) and (2).
2.3. Spectroscopic Measurements (UV-Vis- and CD-Spectroscopy)
The same concentration range used for the pH-potentiometry was selected to register the UV-Vis spectra of the copper(II) complexes. The measurements were carried out using a Perkin Elmer Lambda 25 scanning spectrophotometer at a wavelength range of 200-900 nm.
A JASCO J-810 spectropolarimeter was used to perform the circular dichroism (CD) measurements. CD spectra of the copper(II) complexes were acquired from 220 to 800 nm using 1 cm and 1 mm cells and at the same concentration as applied for pH-potentiometric measurements.
2.4. Oxidation of Tau Fragment
The reaction mixtures containing 1.0 mM of peptide at metal to ligand molar ratio 1:1 were incubated at 25 °C for different time periods in the presence of hydrogen peroxide at ligand to H2O2 molar ratio 1:4 and in simultaneous presence of hydrogen peroxide and ascorbic acid at ligand to H2O2 and ascorbic acid molar ratio 1:4:20. The pH was adjusted to 7.4. The reaction was started by the addition of 1% hydrogen peroxide solution, which was freshly prepared. After incubation the reaction was stopped by addition of Na2EDTA at ligand to Na2EDTA ratio 1:5. In the case of reaction mixtures containing ascorbic acid the ligand to ascorbic acid molar ratio was 1:20. The reaction process was monitored by RP-HPLC at different time periods.
2.5. Isolation of Oxidized Products
The samples were analyzed by analytical RP-HPLC using a Jasco instrument, equipped with a Jasco MD-2010 plus multiwavelength detector. The oxidized products were conducted on a Teknokroma Europa Protein 300 C8 (250 × 4.6 mm, 300 Å, 5 μm) at a flow rate of 1 mL·min−1, monitoring the absorbance at 222 nm. Mobile phases were water (A) and acetonitrile (B) containing 0.1% TFA. Gradient: 0.0-1.0-5.0-8.0-23.0-25.0-29.5 min, 100-100-80-80-100-100% water containing 0.1% TFA for the peptide.
2.6. HPLC-MS Measurements
HPLC-MS measurements were performed by a MicroTOF-Q type Qq-TOF MS instrument (Bruker Daltonik, Bremen, Germany) operated in positive ion mode and a Waters 2695 Separations Module with a thermostable autosampler (5 °C), a column module (35 °C) and a Waters 2996 Photodiode-array detector (PDA). The MS instrument was equipped with an electrospray ion source where the spray voltage was 4 kV. N2 was utilized as drying gas. The drying temperature was 200 °C and the flow rate was 9.0 L/min using the same HPLC method described above. The mass spectra were calibrated externally using the exact masses of clusters [(NaTFA)n+Na]+ generated from the electrosprayed solution of sodium trifluoroacetate (NaTFA). The spectra were evaluated with the DataAnalysis 3.4 software from Bruker. The analytes were detected with PDA detector at l = 222 nm while the flow rate and the injection volume were 1.0 mL/min and 10 mL, respectively.
3. Results and Discussion
3.1. Acid-Base Properties of the Peptides
Protonation constants of the peptides have been determined by potentiometric titrations by the procedure described in the experimental section. All peptides were prepared in N- and C-terminally protected form and in addition to the histidyl sites only the side chain carboxylate groups of aspartyl or glutamyl and amino group of lysyl residues can take part in protonation equilibria of these molecules. Corresponding p
K values are listed in
Table 1 and the data published earlier for tau(9-16) and tau(26-33) are also involved in this Table for comparison.
It is clear from
Table 1 that the number of protonation sites is very different for the peptide fragments but with a comparison with previous literature data these values can be easily assigned to the preferred protonation sites. p
K values of imidazole-N donors in the absence of other side chains are generally around 6-6.3 and the value measured for tau(404-409) is in a good agreement with this expectation. In the case of tau(91-97) and tau(385-390) the imidazole p
K values are slightly higher because of the presence of the acidic aspartyl or glutamyl residues. The carboxylic groups of these residues can form a weak hydrogen bond with the imidazole-N atom and slightly hinder its deprotonation. The high p
K value for the lysyl ammonium group is also characteristic for peptides.
3.2. Copper(II) Complexes of the Peptides
Stability constants of the copper(II) complexes have been determined by potentiometric titrations and the data are included in
Table 2. All peptides have only one effective anchoring site for metal ion coordination and only mononuclear complexes are formed in these systems. Formation of bis(ligand) complexes cannot be ruled out but the bulky side chains of the ligands and the preferred amide coordination suppresses the formation of this type of complexes. Spectroscopic data strongly support that metal ion coordination starts with Cu(II)-N
im binding in acidic media. Stability constant of the Cu(II)-1N
im bonded species is generally around 4 log units [
27] and similar values can be found in
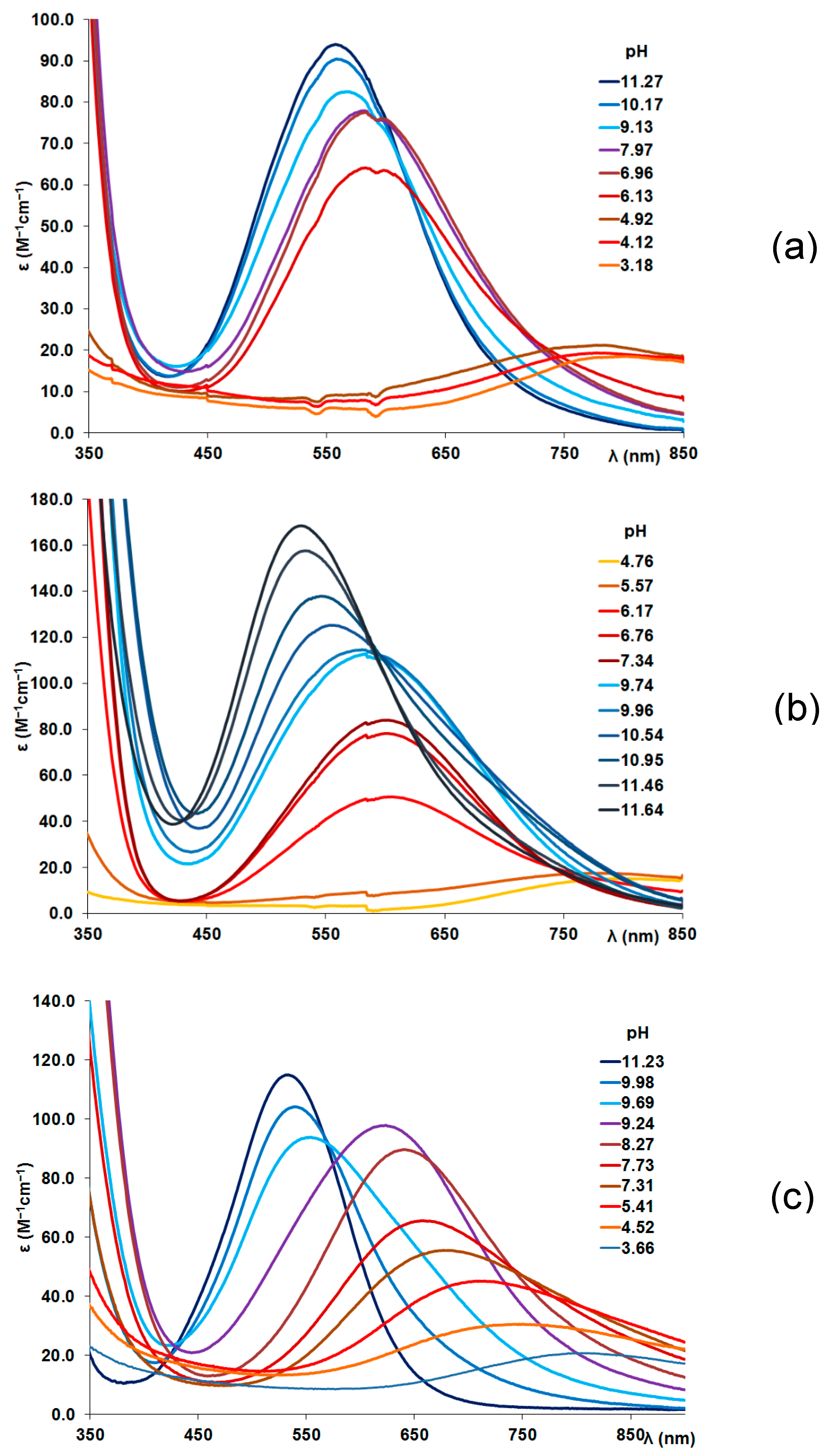
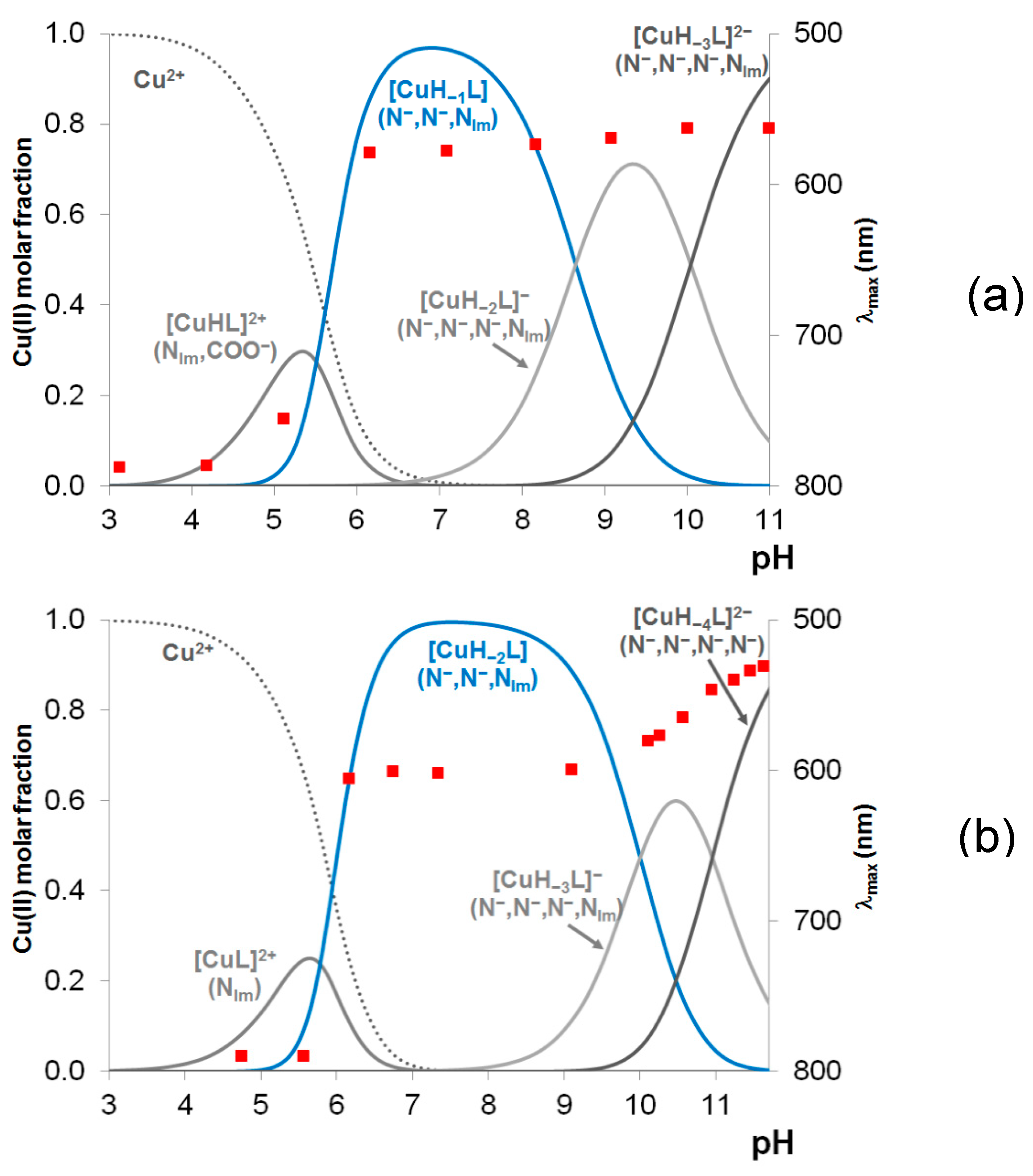
Table 2. The highest value was obtained for the hexapeptide tau(91-97) and it can be easily explained by the presence of a glutamyl carboxylate group in the vicinity of the histidyl residue. The speciation curves for the copper(II)-tau(385-390) and copper(II)-tau(404-409) systems are plotted in Figures 1(a) and 1(b), respectively. It is clear from these Figures that the simple Cu(II)-N
im coordinated complexes ([CuL] or [CuHL]) are present in low concentrations and exist only in acidic media.
The visible absorption maxima of the solutions are also plotted in
Figure 1a,b and it is evident that the formation of the (N
–,N
–,N
im) coordinated species ([CuH
–2L] or [CuH
–2LH]= [CuH
–1L]) from 1N
im coordinated one is accompanied by a significant blue shift of the absorption maxima (from 780 to 580-600 nm; see
Table 3 and
Figure A1 in Appendix).
In agreement with previous findings obtained for copper(II) complexes of peptides of histidine, these spectral changes can be explained by the deprotonation and metal ion coordination of the amide bonds of peptides preceding the histidyl residue. It is also evident that the deprotonation and metal ions coordination of the first two amide functions takes place in a cooperative manner resulting in the formation of [CuH
–2L] or [CuH
–2LH]. This is also a common feature of all N-terminally protected peptides of histidine [
5]. In the case of tau(385-390) peptide, deprotonation of the third amide nitrogen occurs above pH 8.5 ([CuH
–2L]), followed by deprotonation of lysine side chain ammonium group ([CuH
–3L]). The absorption maximum characteristic of the (N
–,N
–,N
–,N
im)-coordinated complex appears at a wavelength about 30 nm higher than that of other protected 1-His containing peptides (e.g. tau(9-16)). For [CuH
–3L] of tau(26-33) peptide, which also contains a -TXH- sequence, similar λ
max value is characteristic (
Table 3). In both cases, weak axial coordination of the Thr-OH group may result in a slight red shift of the absorption maximum.
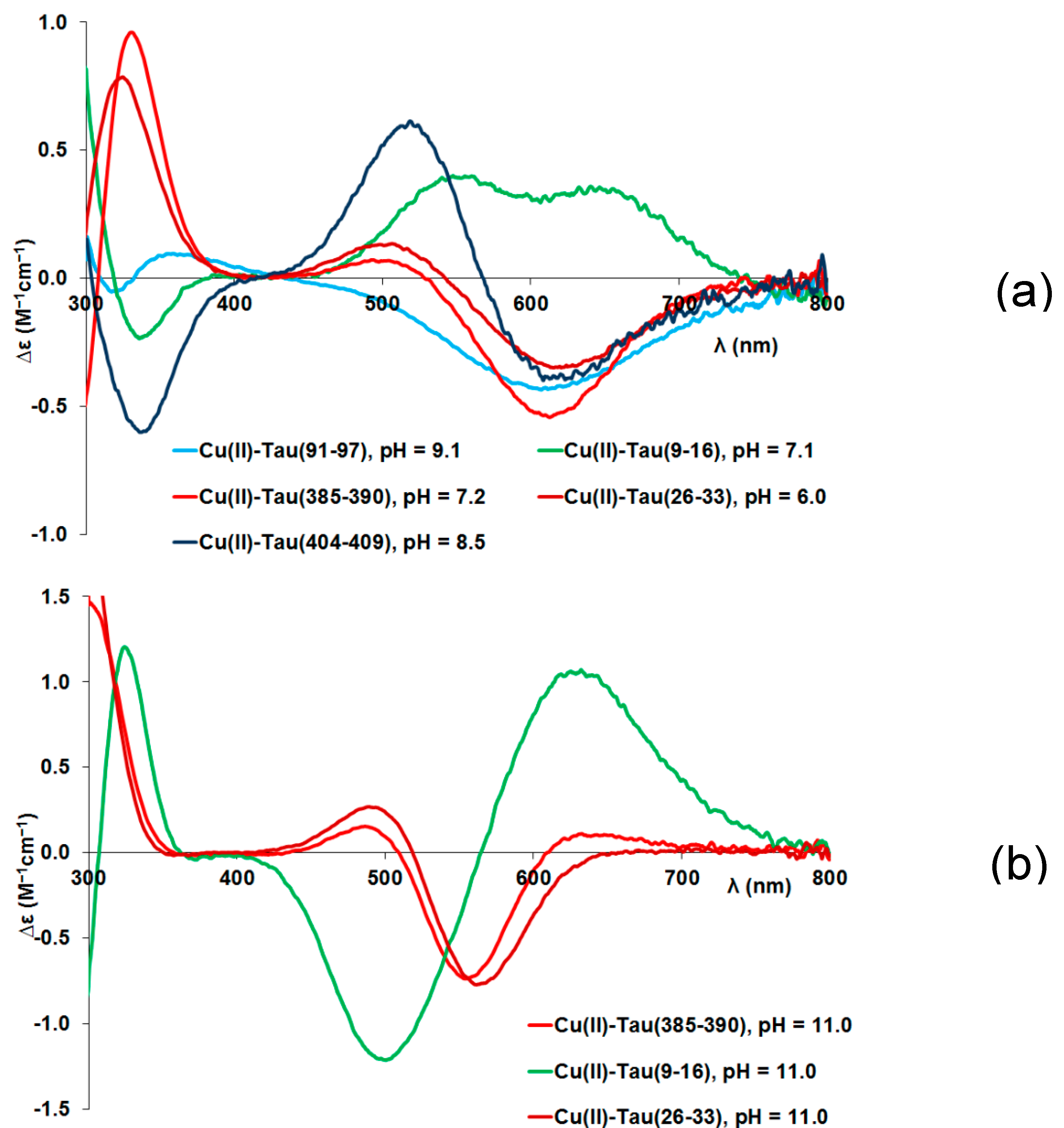
Circular dichroism (CD) spectra provide further support for the above-mentioned coordination modes. Measurable CD spectra can only be detected above pH 5 in parallel with the formation of the amide coordinated complexes (
Figure A2 in Appendix). These spectra are plotted in
Figure 2(a) for the (N
–,N
–,N
im)-coordinated [CuLH
–2] or [CuH
–2LH] (3N complexes) and
Figure 2(b) for the (N
–,N
–,N
–,N
im)-coordinated [CuLH
–3] species (4N complexes). Corresponding spectra of the complexes formed with the N-terminal fragments (tau(9-16) and tau(26-33)) are also shown for comparison.
It is evident that the 3N and 4N coordinated copper(II) complexes have significantly different spectral characteristics supporting that the coordination of the first two amide-N occurs cooperatively in the pH range 5-7, while the last deprotonation process is well separated and shifted to a slightly alkaline media (pH ~ 8-10). It is also obvious from the comparison of CD spectra that the spectra of peptide tau(9-16) differ from those of the others. Similar differences have already been observed for other peptides containing threonyl- or seryl-residues preceding the anchoring histidyl residue by two amino acids in the sequence. This difference was explained by the effect of the presence of alcoholic OH-groups of the threonine and serine amino acids [
28,
29,
30].
In addition to the above-mentioned effect of threonyl residues the complex formation processes of peptides containing proline also differ from the other ones. This difference is reflected in the values of stability constants and in the speciation curves and characteristic spectral parameters, too. Proline is a secondary amine and after its involvement in peptide bond it cannot be deprotonated and cannot take part in metal binding [
31,
32]. As a consequence, the amide coordination can occur only towards the C-terminus in the form of 7-membered chelate. Its thermodynamic stability is much lower than those of the 6- or 5-membered ones and shifts the amide coordination to higher pH values. In the case of tau(404-409), (Ac-SPRHLS-NH
2), where the proline is the second amino acid on the N-terminal side of histidine this affects only the formation of the 4N-coordinated complexes resulting in a much higher p
K3amide value (see first column in
Table 2 and
Figure 2(b)). The effect of proline is even more pronounced if the proline proceeds directly the histidyl residue. This is the case for tau(91-97) (Ac-AQPHTEI-NH
2). The speciation curves for the copper(II)-tau(91-97) system are shown in
Figure A3 in the Appendix. In this case the species [CuL] predominates around pH 7. On one hand it comes from the stabilizing role of the glutamyl carboxylate coordination, on the other hand by the suppression of the amide coordination. It is also a common feature of the two proline containing peptides that at high pH they form the species [CuLH
–4], in which the imidazole-N donors are replaced by the fourth deprotonated amide nitrogen. This change in the coordinated donor groups can also be explained by the low thermodynamic stability of the 7-membered chelates.
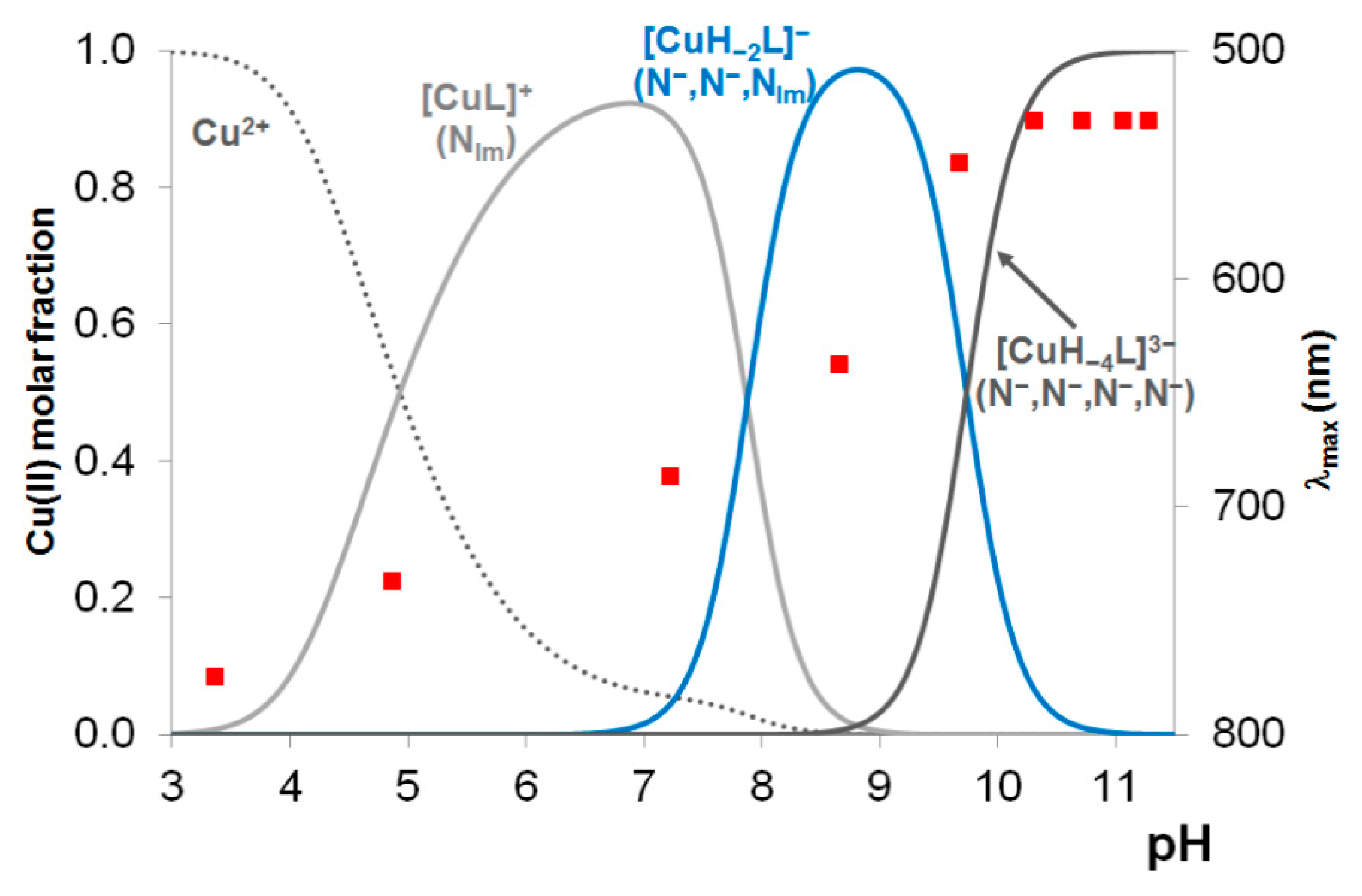
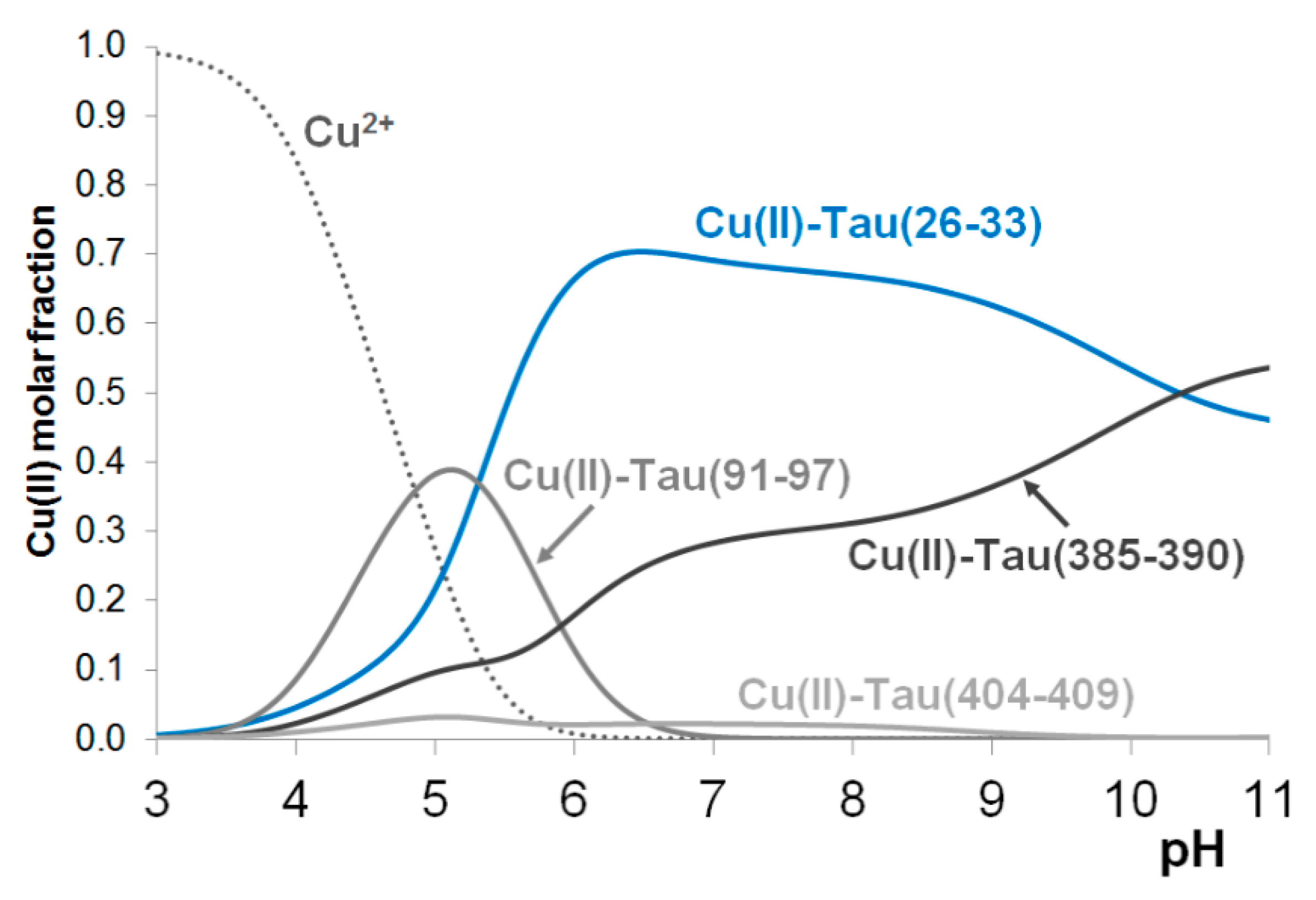
In the Introduction it has already been mentioned that among the previously studied tau fragments the heptapeptide tau(26-33) involving His32 residue was the most effect copper(II) binding ligand.
Figure 3 shows the concentration distribution curves in a hypothetic system where copper(II), tau(26-33), tau(91-97), tau(385-390) and tau(404-409) are present in equimolar concentration (1 mM). It is evident from
Figure 3 that the predominance of His32 binding site remains intact even in the presence of any of the studied 1-histidine containing fragments.
3.3. Copper Catalysed Oxidation of Peptides
The oxidation of the peptides was studied with Cu(II)/H
2O
2 system at pH 7.4 in the absence and presence of ascorbic acid. The coordination modes in the studied systems are quite different at this pH value. In the case of tau(91-97) the Cu-N
im coordinated [CuL] is formed in the highest concentration (80%) besides the (N
–,N
–,N
im)-coordinated [CuLH
–2] species (see
Figure A3), while this latter one is the main species with the other two studied peptides, forming in 94% and 98 % besides the (N
–,N
–,N
–,N
im)-coordinated [CuLH
–3] species with tau(385-390) and tau(404-409), respectively (
Figure 1). However, the oxidation processes of these peptides are similar. In the absence of ascorbic acid, fragmentation of the peptides occurred, there is not any main product, several products are formed. These fragments eluate either with very small retention time or overlapping with the peak of the non-oxidized ligand, therefore the identification of these fragments with small molecular weights and/or very low intensity is not possible. However, in the presence of ascorbic acid a new peek appears in the HPLC chromatogram of each system. The retention time of this peek is always higher than the retention time of the peptide. The
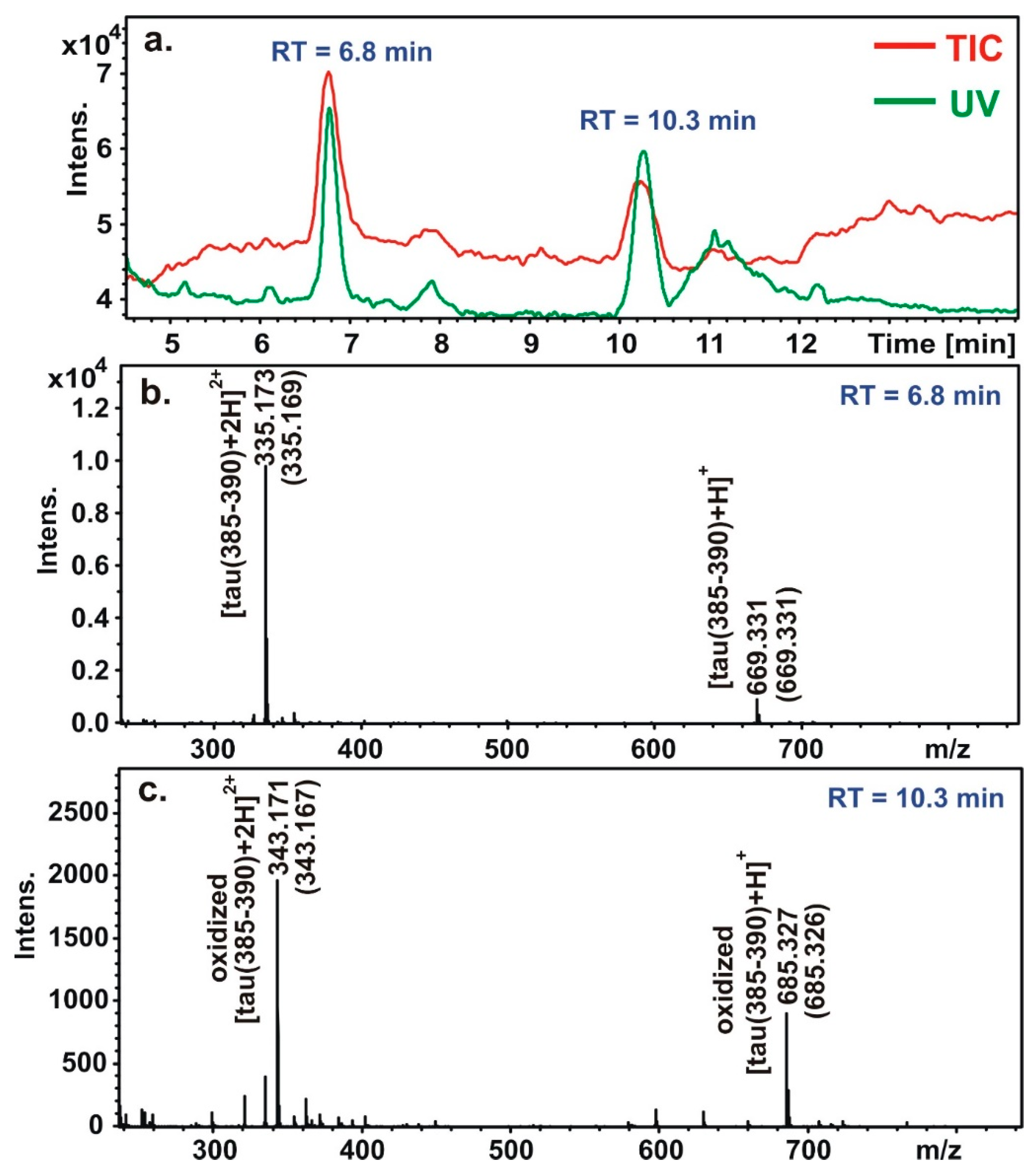
m/z values are higher with 16 and 8 Da in the case of ions with 1 and 2 positive charges, respectively. This main oxidation product was identified as the 2-oxo-histidine derivative of the peptides. A representative LC-MS chromatogram of the tau(385-390) is presented in
Figure 4.
The retention time of the peptide is 6.8 min. It appears both with only 1 or 2 positive charges whose m/z values are 669.331 (calculated value is 669.331) and 335.173 (calculated value is 335.169). The retention time of the oxidized product is 10.3 min. It appears with 685.327 and 343.171 m/z values (the calculated values are 685.326 and 343.167). It refers to the mono oxidized product of the peptide, namely the 2-oxo-derivative of the peptide. The higher retention time of the 2-oxo-derivative can be explained by the reduced basicity of the imidazole ring; the less basic compound eluate later with the acidic, TFA containing eluent.
To sum up, we can conclude that the coordination mode of the formed complexes, and the number of the coordinating amide nitrogen atoms - if coordination sphere of the Cu(II) ion is unsaturated - have no effect on the oxidation of the tau peptides. In the absence of ascorbic acid the fragmentation of the tau peptides occurs; 40% of the peptides remain unchanged during 90 minutes oxidation.
In the presence of ascorbic acid the extent of oxidation is slightly higher (about 30% of the peptides remain unchanged during 90 min oxidation), but the fragmentation of the peptide is less preferred, the formation of 2-oxo-histidine occurs. Ascorbic acid has no protective role during the oxidation of tau peptides in contrast to the methionine containing prion protein fragments [
33].
3.4. Zinc(II) Complexes of Peptides
Zinc is one of the most important trace elements for almost all forms of life. It is the major constituent of huge number of metalloenzymes and takes part in a series of other biochemical processes. Many previous publications prove its involvement in neurodegeneration but the exact role of zinc(II) ions in these disorders is not well understood. On the other hand zinc(II) is an effective complex forming ion with most of the various biomolecules containing either nitrogen, oxygen or sulphur donor atoms. Zinc(II) complexes of peptides are also often studied but the thermodynamic stability of these complexes is generally much lower than those of the corresponding copper(II) species. The major difference in the copper(II) and zinc(II) peptide complexes is related to their affinity for amide binding. Copper(II) can easily promote deprotonation and coordination of amide groups forming stable 5- or 6-membered chelate rings but in case of zinc(II) this binding mode is rather rare and occurs only with specific sequences [
32]. For example, peptide fragments of prion protein have relatively low zinc(II) binding affinity because the histidyl sites are well separated and the number of other polar side chains is low [
9]. On the contrary, amyloid-β can form stable complexes both with copper(II) and zinc(II) ions and this can be explained by the presence of vicinal histidyl residues and a series of polar aspartic acid and glutamic acid side chains [
8]. Recently we reported the results obtained for the zinc(II) complexes of a series of tau peptide fragments including both the N- terminal and the R3 region of the protein [
23]. This study revealed a relatively low zinc(II) binding affinity of the His14 and His32 residues close to the N-terminus. On the contrary, the peptide and its mutants from the R3 region, (tau(326-333)) containing the adjacent His329 and His330 residues formed rather stable zinc(II) complexes with the involvement of imidazole and deprotonated amide nitrogen donors in metal binding.
Potentiometric titrations of the zinc(II) containing systems revealed the very low zinc(II) binding affinity of all three peptides (tau(91-97), tau(385-390) and tau(404-409) involved in this study. In the case of tau(91-97) (Ac-AQPHTEI-NH
2) the formation of zinc(II)-hydroxide precipitate hindered the determination of any equilibrium data for any species, even in the presence of excess of ligand. For tau(385-390) (Ac-KTDHGA-NH
2) log
β(ZnL) = 5.05(5) can obtained but taking into account the lysyl residue which must be protonated in the acidic media the real stoichiometry of this species is [ZnH
–1LH] containing an imidazole coordinated and protonated ligand with a coordinated hydroxide ion. Substracting the p
K value of he lysyl ammonium group log
β = –5.29 can be calculated for the mixed hydroxide complex. Tau(404-409) has only one protonation site and in this case the stability constants both for the simple imidazole-N coordinated and mixed hydroxido complexes can be directly obtained: log
β([ZnL]) = 2.14 and log
β([ZnH
–1L]) = –4.68. These values are rather close to those obtained for other 1-histidine peptides but much lower than those of tau(9-16) (log
β([ZnH
–1L]) = –3.49 [
23]. The latter peptide, however, contains 2 glutamic- and 1 aspartic-acid and their carboxylate functions have a significant contribution to zinc(II) binding in addition to the imidazole-N donor atom. It is also important to note that deprotonation and zinc(II) coordination of peptide amide groups were not observed for any ligands containing a single histidyl residue. The occurrence of this process was, however, reported for the tau(326-333) fragment [
23] containing 2 vicinal histidyl sites. As a consequence, it can be unambiguously stated that the R3 region of tau protein is the most preferred zinc(II) binding domain.
3.5. Nickel(II) Complexes of Peptides
Nickel(II) ions have a versatile coordination chemistry because a great number of octahedral, square planar and tetrahedral complexes have already been characterized. On the other hand, there are significant similarities in the complex formation processes of peptides with copper(II) and nickel(II) ions. Similar to copper(II), nickel(II) can promote the deprotonation and metal ion coordination of amide groups and form four-coordinated square planar complexes with tri- or longer peptides. The thermodynamic stability of the nickel(II)-peptide complexes is, however, smaller than those of the corresponding copper(II) complexes and these species are generally formed slightly above the physiological pH range. Our previous study on the nickel(II) complexes of peptides from the N-terminal and R3 domain of tau-protein revealed that complex formation processes of these peptides are quite similar to those of copper(II) and His32 was identified as the most preferred nickel(II) binding site [
23]. These similarities support that nickel(II) can be effectively used as a probe to understand the complex formation processes of peptides with copper(II), although nickel(II) is not an essential element for human life.
Stability constants of the nickel(II) complexes of tau(91-97), tau(385-390) and tau(404-409) are listed in
Table 4 in comparison with tau(9-16) and a mutant of tau(26-33). (The Gln/Lys mutation increased the aqueous solubility of the peptide without changing the coordination geometry [
21,
23]).
The evaluation of data in
Table 3 leads to some important conclusions:
- (i)
The presence of proline significantly alter the complex formation processes of nickel(II) ions. This is especially true for tau(91-97) where the amide coordination is not possible towards the N-terminus. In principle it can occur on the C-terminal side of histidine but the thermodynamic stability of the 7-membered chelate is much smaller and the corresponding species could be formed only under strongly alkaline conditions without any biological relevance. Thus only the species [NiL] can be detected in the nickel(II)-tau(91-97) system. Its low stability constants, however, cannot prevent the hydrolysis of nickel(II) ions slightly above the physiological pH.
- (ii)
In the nickel(II)-tau(404-409) system the deprotonation and coordination of one amide group is possible, (Nim,N–)-coordination mode, but the nickel(II) ion has 2 free coordination site in this complex resulting in hydrolytic reactions by increasing pH.
- (iii)
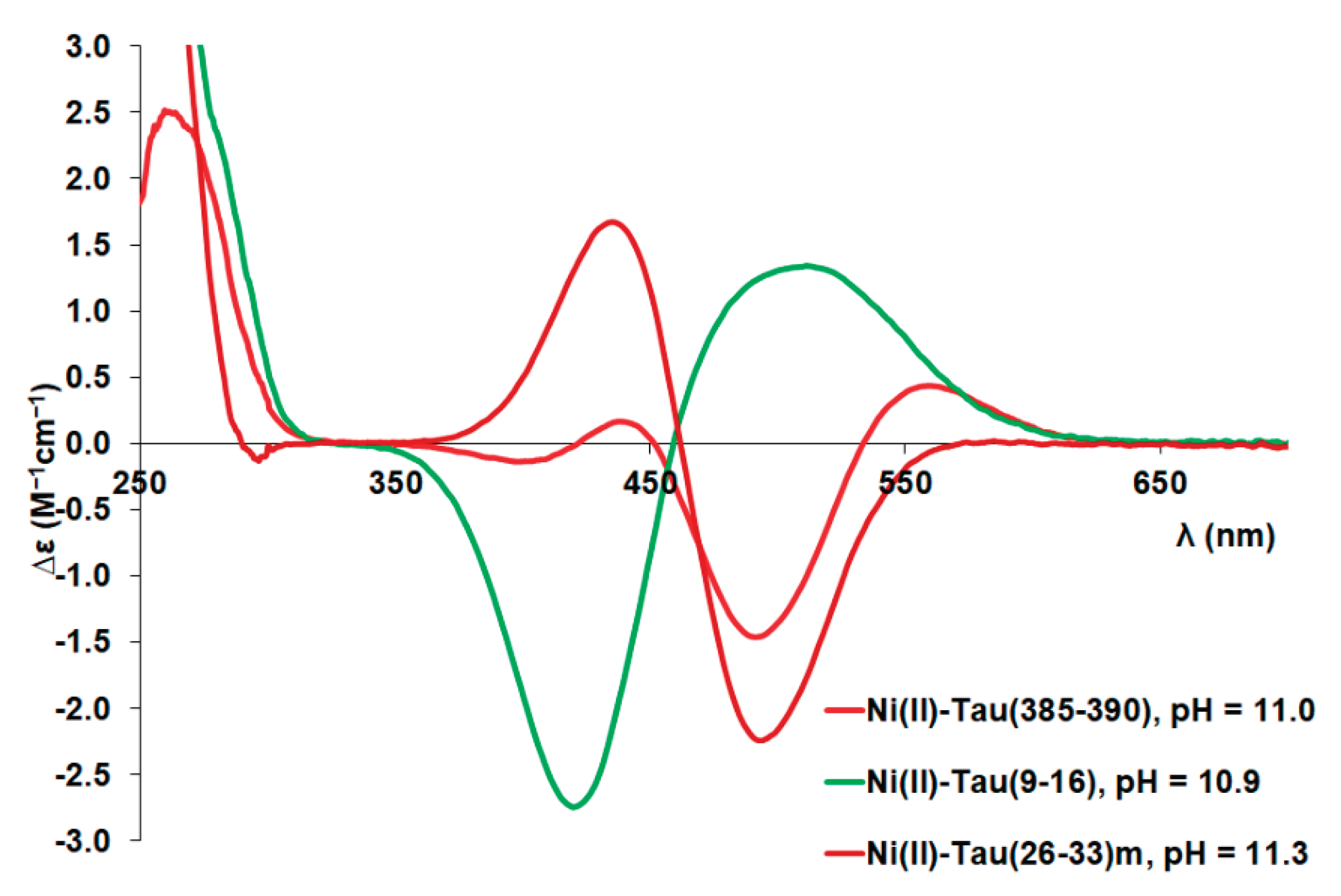
Complex formation processes in the nickel(II)-tau(385-390) and tau(26-33)(mutant) systems are quite similar to each other. Deprotonation of three amide groups is possible resulting the (N
im,3N
–) coordination mode above pH 8. The visible absorption (see
Figure A4) and circular dichroism spectroscopic measurements (see
Figure A5) strongly support the suggested coordination modes. It is also important to note that the pH-dependent changes of CD absorptions are similar for the nickel(II) complexes of tau(385-390) and tau(26-33)mutant, but differ from those of peptides missing the –TXH- sequence (
Figure A6). Detailed investigation of the CD spectra of (N
im,3N
–) coordinated Ni(II) complexes of peptides containing the -TXH- or –SXH- sequence [
34,
35] also shows that, an intense negative extreme appears around 500 nm and a positive peak between 430-451 nm. In some cases an additional positive extrema can be observed around 560 nm. The CD spectral pattern of the [NiH
–3L] complex of tau(385-390) corresponds to the latter case [
34].
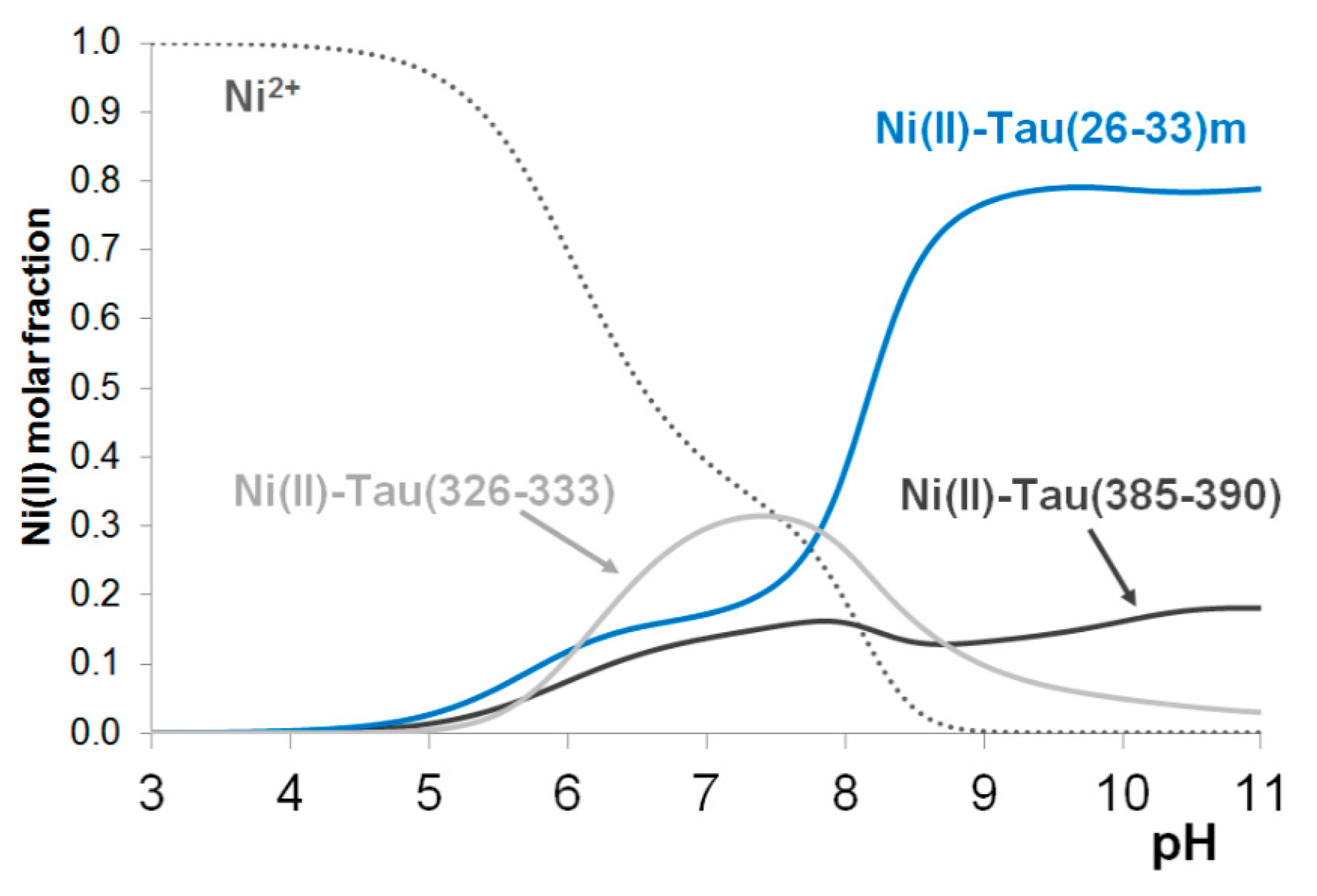
The major conclusions on the nickel(II) complexes, however, can be obtained from the hypothetic concentration distribution curves (
Figure 5) where the distribution of nickel(II) ions between three peptides tau(385-390), tau(26-33)m and tau(326-333) are plotted as a function of pH. It is evident from
Figure 5 that formation of stable nickel(II) complexes occurs only in slightly basic media (above pH ~ 8) when both imidazole and amide nitrogen donors take part in metal binding. It is also obvious that (similar to copper(II) complexes) the fragment tau(26-33), close to the N-terminus, has the highest nickel(II) binding affinity.
4. Conclusions
The studies reported in this manuscript unambiguously support that histidine containing peptide fragments of tau protein outside the N-terminal domain and R1, R3 regions can effectively bind metal ions, especially copper(II). The peptides tau(91-97) (Ac-AQPHTEI-NH2), tau(385-390) (Ac-KTDHGA-NH2) and tau(404-409) (Ac-SPRHLS-NH2) were involved in this study and except tau(385-390) prolyl residue in the N-terminal side of histidine is also present in the sequence. Proline generally works as a break-point for amide coordination and results in lower thermodynamic stability of metal complexes. The reduced stability of complexes is especially true for the nickel(II) and zinc(II) containing systems. Similar to other N-terminally protected peptides of histidine the imidazole-N donors of histidyl residues are the primary metal binding sites but the deprotonated amide nitrogens can also take part in copper(II) and nickel(II) binding. Zinc(II) promoted amide coordination has not been observed for any peptides revealing that these domains of tau protein are not preferred zinc(II) binding sites. Comparison of the metal binding capability of these peptides with those of the N-terminal domain or the R1-R3 regions demonstrates that His32 residue of tau protein has the highest copper(II) and nickel(II) binding affinity. Copper(II) catalyzed oxidation of peptides has also been investigated in the presence of H2O2 and ascorbic acid. Both fragmentation of peptides and the oxidation of histidyl residues to 2-oxohistidine has been detected demonstrating that ascorbic acid has no protective role during the oxidation of tau-peptides in contrast to the methionine containing prion protein fragments.
Author Contributions
Conceptualization, I.S. and K.V.; formal analysis, Z.K., A.B., S.V., C.K. L.N. and K.V.; investigation, Z.K., A.B., S.V. and N.L.; resources, K.V.; writing—original draft preparation, C.K., K.V. and I.S..; writing—review and editing, K.V. and I.S.; visualization, K.V.; supervision, C.K. and K.V.; funding acquisition, C.K., L.N. and K.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
The research was supported by the University of Debrecen Program for Scientific Publication. The authors thank Gréta Dancs and Tamás P. Szák-Kocsis for their help in the UV-Vis and CD spectroscopic measurements and evaluation of data.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Copper(II), Nickel(II) and Zinc(II) Complexes of Peptide Fragments of Tau Protein
Figure A1.
pH dependent UV-Vis spectra of the Cu(II)-tau(385-390) at 1:1 ratio, c(L) = 1.2 mM (a), Cu(II)-tau(404-409) at 1:1 ratio, c(L) = 1.2 mM (b) and Cu(II)-tau(91-97) at 1:2 ratio, c(L) = 0.9 mM (c).
Figure A1.
pH dependent UV-Vis spectra of the Cu(II)-tau(385-390) at 1:1 ratio, c(L) = 1.2 mM (a), Cu(II)-tau(404-409) at 1:1 ratio, c(L) = 1.2 mM (b) and Cu(II)-tau(91-97) at 1:2 ratio, c(L) = 0.9 mM (c).
Figure A2.
pH dependent CD spectra of the Cu(II)-tau(385-390) at 1:1 ratio, c(L) = 1.2 mM (a), Cu(II)-tau(404-409) at 1:1 ratio, c(L) = 1.2 mM (b) and Cu(II)-tau(91-97) at 1:2 ratio, c(L) = 0.9 mM (c).
Figure A2.
pH dependent CD spectra of the Cu(II)-tau(385-390) at 1:1 ratio, c(L) = 1.2 mM (a), Cu(II)-tau(404-409) at 1:1 ratio, c(L) = 1.2 mM (b) and Cu(II)-tau(91-97) at 1:2 ratio, c(L) = 0.9 mM (c).
Figure A3.
Metal ion speciation in the copper(II)-tau(91-97) system (c(L) = c(M) = 0.9 mM) together with the change of absorption maxima (marked with red squares).
Figure A3.
Metal ion speciation in the copper(II)-tau(91-97) system (c(L) = c(M) = 0.9 mM) together with the change of absorption maxima (marked with red squares).
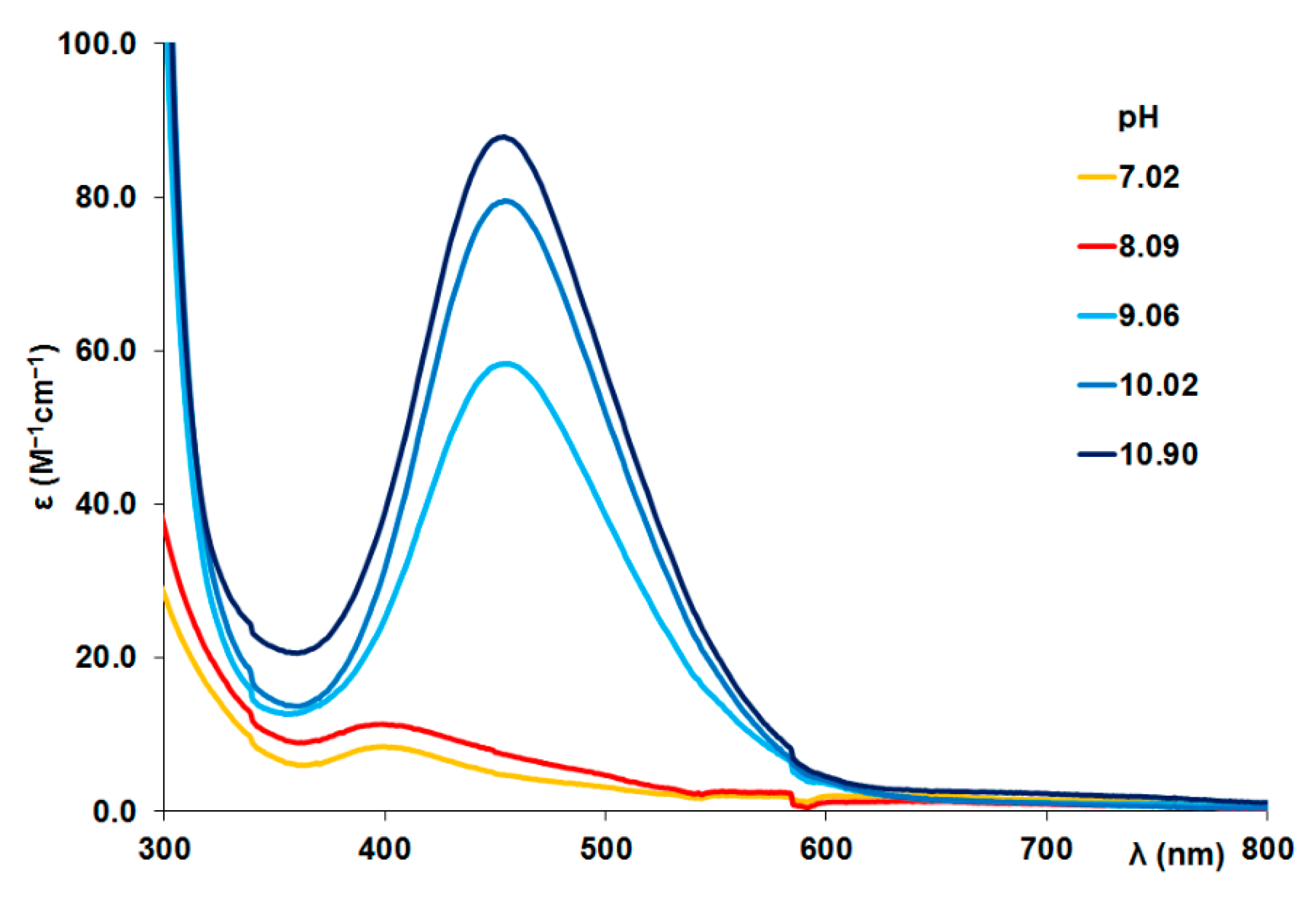
Figure A4.
UV-Vis spectra registered in Ni(II)-tau(385-390) (Ac- KTDHGA-NH2) = 1:1 system (c(L) = 1.2 mM).
Figure A4.
UV-Vis spectra registered in Ni(II)-tau(385-390) (Ac- KTDHGA-NH2) = 1:1 system (c(L) = 1.2 mM).
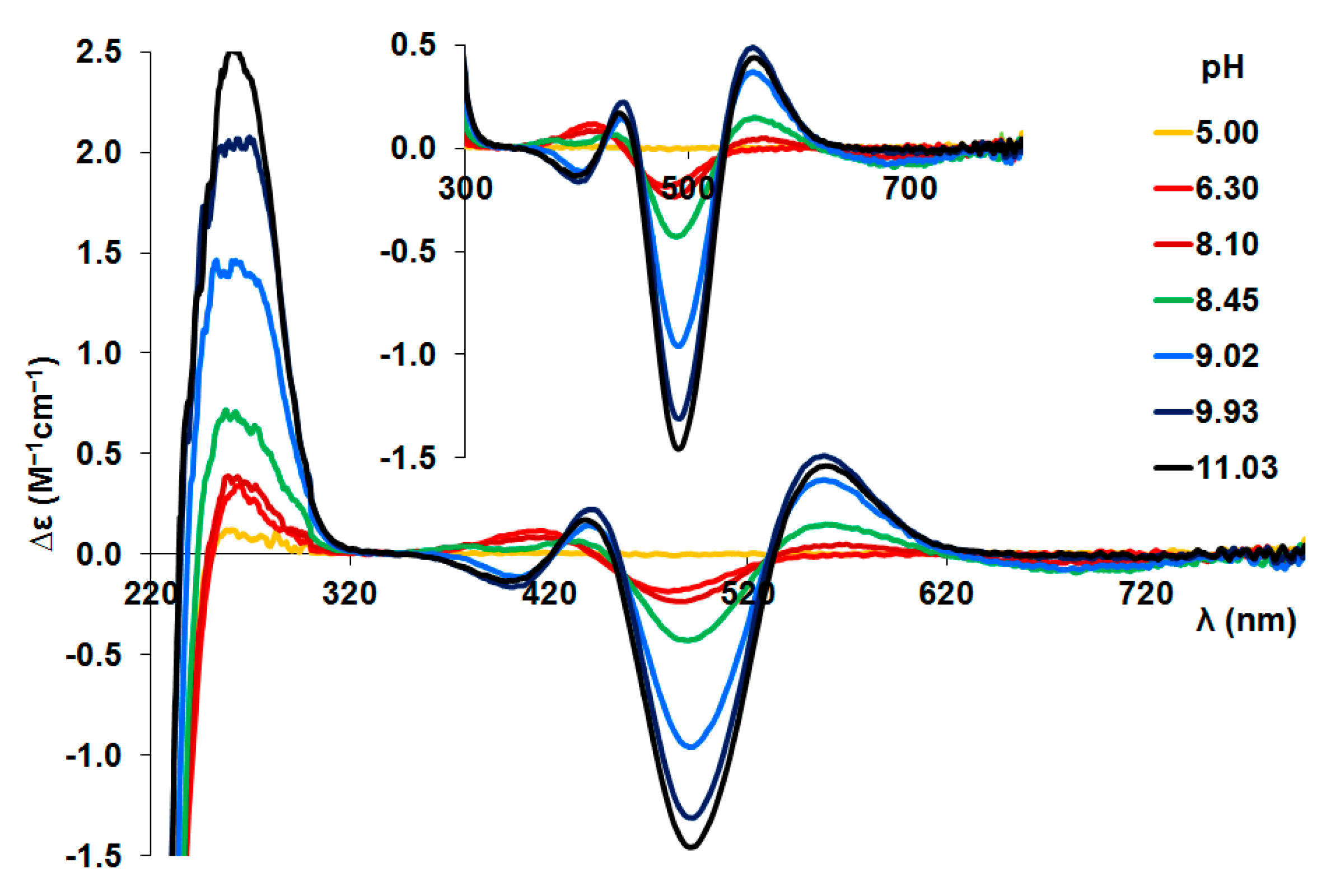
Figure A5.
CD spectra registered in Ni(II)-tau(385-390) (Ac- KTDHGA-NH2) = 1:1 system (c(L) = 1.2 mM).
Figure A5.
CD spectra registered in Ni(II)-tau(385-390) (Ac- KTDHGA-NH2) = 1:1 system (c(L) = 1.2 mM).
Figure A6.
Visible circular dichroism (CD) spectra of the (N–,N–,N–,Nim)-coordinated [NiLH–3] (4N) complexes.
Figure A6.
Visible circular dichroism (CD) spectra of the (N–,N–,N–,Nim)-coordinated [NiLH–3] (4N) complexes.
References
- Migliorini, C.; Porciatti, E.; Luczkowski, M.; Valensin, D. Structural characterization of Cu2+, Ni2+ and Zn2+ binding sites of model peptides associated with neurodegenerative diseases. Coord. Chem. Rev. 2012, 256, 352–368. [Google Scholar] [CrossRef]
- Zawisza, I.; Rózga, M.; Bal, W. Affinity of copper and zinc ions to proteins and peptides related to neurodegenerative conditions. Coord. Chem. Rev. 2012, 256, 2297–2307. [Google Scholar] [CrossRef]
- Ayton, S.; Lei, P.; Bush, A.I. Metallostasis in Alzheimer’s disease. Free Radical Biology and Medicine 2013, 62, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Rowinska-Zyrek, M.; Salerno, M.; Kozlowski, H. ; Neurodegenerative diseases – Understanding their molecular bases and progress in the development of potential treatments. Coord. Chem. Rev. 2015, 284, 298–312. [Google Scholar] [CrossRef]
- Sóvágó, I.; Várnagy, K.; Lihi, N.; Grenács, Á. Coordinating properties of peptides containing histidyl residues. Coord. Chem. Rev. 2016, 327–328, 43–54. [Google Scholar] [CrossRef]
- Sóvágó, I.; Várnagy, K.; Kállay, C.; Grenács, Á. Interactions of copper(II) and zinc(II) ions with the peptide fragments of proteins related to neurodegenerative disorders: similarities and differences. Current Med. Chem. 2023, 30, 4050–4071. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hureau, C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide. Dalton Trans. 2009, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.; Pappalardo, G.; Sóvágó, I.; Rizzarelli, E. Copper(II) interaction with amyloid-β: Affinity and speciation. Coord. Chem. Rev. 2012, 256, 3–12. [Google Scholar] [CrossRef]
- Arena, G.; La Mendola, D.; Pappalardo, G.; Sóvágó, I.; Rizzarelli, E. Interactions of Cu2+ with prion family peptide fragments: Considerations on affinity, speciation and coordination. Coord. Chem. Rev. 2012, 256, 2202–2218. [Google Scholar] [CrossRef]
- Mo, Z.-Y.; Zhu, Y.-Z.; Zhu, H.-L.; Fan, J.-B.; Chen, J.; Liang, Y. ; Low micromolar zinc accelerates the fibrillization of human tau via bridging of Cys-291 and Cys-322. J. Biol. Chem. 2009, 284, 34648–34657. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Z.; Cao, Y.; Lang, M.; Lu, B.; Zhou, B. Zinc binding directly regulates tau toxicity independent of tau hyperphosphorylation. Cell Reports 2014, 8, 831–842. [Google Scholar] [CrossRef]
- Moreira, G.G.; Cristóvão, J.S.; Torres, V.M.; Carapeto, A.P.; Rodrigues, M.S.; Landrieu, I.; Cordeiro, C.; Gomes, C.M. Zinc binding to tau influences aggregation kinetics and oligomer distribution. Int. J. Mol. Sci. 2019, 20, 5979–5991. [Google Scholar] [CrossRef]
- Shin, B.; Saxena, S. ; Insight into potential Cu(II)-binding motifs in the four pseudorepeats of tau protein. J. Phys. Chem. B 2011, 115, 15067–15078. [Google Scholar] [CrossRef] [PubMed]
- Bacchella,C. ; Gentili, S; Bellotti,D.; Quartieri, E.; Draghi, S.; Baratto, M.C.; Remelli, M.; Valensin, D.; Monzani, E.; Nicolis, S; Casella, L; Tegoni, M.; Dell’Acqua, S. Binding and reactivity of copper to R1 and R3 fragments of tau protein. Inorg. Chem. 2020, 59, 274–286. [Google Scholar]
- Ahmadi, S.; Wu, B.; Song, R.; Zhu, R.; Simpson, A.; Wilson, D.J.; Kraatz, H.B. ; Exploring the interactions of iron and zinc with the microtubule binding repeats R1 and R4. J. Inorg. Biochem. 2020, 205, 110987. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, G.; Bellia, F.; .Sciacca, M.F.M.; Campagna, T.; Pappalardo, G. Tau-peptide fragments and their copper(II) complexes: Effects on Amyloid-b aggregation. Inorg. Chim. Acta 2018, 472, 82–92. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Di Natale, G.; Tosto, R.; Milardi, D.; Pappalardo, G. Tau/Aβ chimera peptides: Evaluating the dual function of metal coordination and membrane interaction in one sequence. J. Inorg. Biochem. 2020, 205, 110996. [Google Scholar] [CrossRef]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular mechanisms and menetics of oxidative stress in Alzheimer’s Disease. 2019, 72, 981–1017.
- Roy, R.G.; Mandal, P.V.; Maroon, J.C. Oxidative stress occurs prior to amyloid Aβ plaque formation and tau phosphorylation in Alzheimer’s disease: role of glutathione and metal ions. ACS Chem. Neurosci. 2023, 14, 2944–2954. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018, 2018 14, 450–464. [Google Scholar] [CrossRef]
- Lukács, M.; Szunyog, Gy.; Grenács, Á.; Lihi, N.; Kállay, Cs.; Di Natale, G.; Campagna, T.; Lanza, V.; Tabbi, G.; Pappalardo, G.; Sóvágó, I.; Várnagy, K. Copper(II) coordination abilities of the tau protein's N-terminus peptide fragments: a combined potentiometric, spectroscopic and mass spectrometric study. ChemPlusChem. 2018, 84, 1697–1708. [Google Scholar] [CrossRef]
- Balogh, B.D.; Szakács, B.; Di Natale, G.; Tabbi, G.; Pappalardo, G.; Sóvágó, I.; Várnagy, K. Copper(II) binding properties of an octapeptide fragment from the R3 region of tau protein: A combined potentiometric, spectroscopic and mass spectrometric study. J. Inorg. Biochem. 2021, 217, 11358. [Google Scholar] [CrossRef] [PubMed]
- Balogh, B.D.; Szunyog, Gy.; Lukács, M.; Szakács, B.; Sóvágó, I.; Várnagy, K. Thermodynamics and structural characterization of the nickel(II) and zinc(II) complexes of various peptide fragments of tau protein. Dalton Trans. 2021, 50, 14411–14420. [Google Scholar] [CrossRef] [PubMed]
- Irving, H.M.; Miles, M.G. Pettit, L.D.; A study of some problems in determining the stoicheiometric proton dissociation constants of complexes by potentiometric titrations using a glass electrode. Analytica Chimica Acta 1967, 38, 475–488. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. SUPERQUAD: an improved general program for computation of formation constants from potentiometric data. J. Chem. Soc. Dalton Trans. 1985, 6, 1195–1200. [Google Scholar] [CrossRef]
- Zékány L.; Nagypál I. In Computational Methods for the Determination of Formation Constants, Ed.: Leggett D. J.; Plenum Press. 1985, p. 291-353.
- Kállay, C.; Várnagy, K.; Malandrinos, G.; Hadjiliadis, N.; Sanna, D.; Sóvágó, I. Thermodynamic and structural characterization of the macrochelates formed in the reactions of copper(II) and zinc(II) ions with peptides of histidine. Inorg. Chim. Acta 2009, 362, 935–945. [Google Scholar] [CrossRef]
- Gergely, A.; Farkas, E. Studies on transition-metal–peptide complexes. Part 6. Influence of side-chain donor group on the equilibrium and thermodynamics of binary and ternary copper(II)–dipeptide complexes. J. Chem. Soc. Dalton Trans. 1982, 381–386. [Google Scholar] [CrossRef]
- Farkas, E.; Kiss, T. Effects of side-chain donor groups on deprotonation of peptide amide in copper(II) complexes at high pH. Polyhedron 1989, 8, 2463–2467. [Google Scholar] [CrossRef]
- Kállay, C.; Turi, I.; Timári, S.; Nagy, Z.; Sanna, D.; Pappalardo, G.; de Bona, P.; Rizzarelli, E.; Sóvágó, I. The effect of point mutations on copper(II) complexes with peptide fragments encompassing the 106-114 region of human prion protein. Monatsh. Chem. 2011, 142, 411–419. [Google Scholar] [CrossRef]
- Kozlowski, H.; Bal, W.; Dyba, M.; Kowalik-Jankowska, T. Specific structure–stability relations in metallopeptides. Coord. Chem. Rev. 1999, 184, 319–346. [Google Scholar] [CrossRef]
- Sóvágó, I.; Kállay, C.; Várnagy, K. Peptides as complexing agents: Factors influencing the structure and thermodynamic stability of peptide complexes. Coord. Chem. Rev. 2012, 256, 2225–2233. [Google Scholar] [CrossRef]
- Bodnár, N.; Várnagy, K.; Nagy, L.; Csire, G.; Kállay, C. Ambivalent role of ascorbic acid in the metal-catalyzed oxidation of oligopeptides. J. Inorg. Biochem. 2021, 222, 111510. [Google Scholar] [CrossRef] [PubMed]
- Wezynfeld, N.E.; Bossak, K.; Goch, W.; Bonna, A.; Bal, W.; Frączyk, T. ; Human Annexins A1, A2, and A8 as Potential Molecular Targets for Ni(II) Ions. Chem. Res. Toxicol. 2014, 27, 1996–2009. [Google Scholar] [CrossRef] [PubMed]
- Frącyk, T.; Bonna, A.; Stefaniak, E.; Wezynfeld, N.E.; Bal, W. Peptide bond cleavage by Ni(II) ions within the nuclear localization signal sequence. Chem. Biodiversity 2020, 17, e1900652. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).