Submitted:
22 March 2024
Posted:
25 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
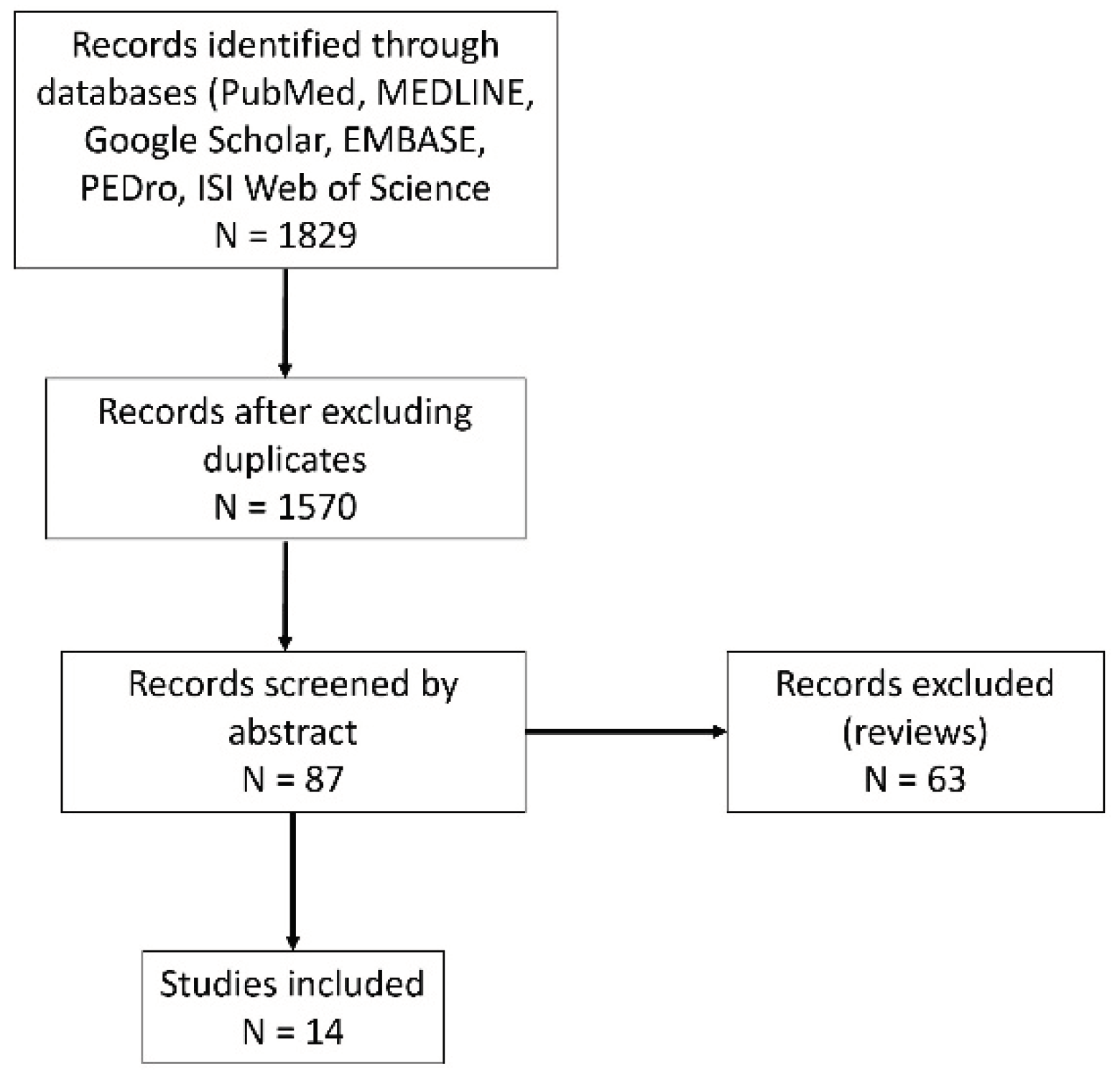
2. Materials and Methods
3. Results
Intratendinous Injection
Intramuscular Administration
Comparative Drug-Controlled Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalichman, L.; Bannuru, R.R.; Severin, M.; Harvey, W. Injection of botulinum toxin for treatment of chronic lateral epicondylitis: systematic review and meta-analysis. Semin Arthritis Rheum. 2011, 40, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Day, D.; Jayaram, P. Efficacy of Botulinum Toxin in Treating Lateral Epicondylitis-Does Injection Location Matter?: A Systematic Review. Am J Phys Med Rehabil. 2020, 99, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, E.; Gustafsson, B.E.; Pettersson, K.; Aulin, K.P. Decreased intramuscular blood flow in patients with lateral epicondylitis. Scand J Med Sci Sports. 2007, 17, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carnero, J.; Fernández-de-Las-Peñas, C.; de la Llave-Rincón, A.I.; Ge, H.Y.; Arendt-Nielsen, L. Widespread mechanical pain hypersensitivity as sign of central sensitization in unilateral epicondylalgia: a blinded, controlled study. Clin J Pain. 2009, 25, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.H.; Gong, H.S.; Baek, G.H. The Prognostic Value of Pain Sensitization in Patients With Lateral Epicondylitis. J Hand Surg Am. 2019, 44, e1–e250. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chung, M.E. Botulinum Toxin for Central Neuropathic Pain. Toxins 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Matak, I.; Rossetto, O.; Lacković, Z. Botulinum toxin type A selectivity for certain types of pain is associated with capsaicin-sensitive neurons. Pain. 2014, 155, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, B.; Machado, D. Treatment of refractory pain with botulinum toxins--an evidence-based review. Pain Med. 2011, 12, 1594–1606. [Google Scholar] [CrossRef] [PubMed]
- Morré, H.H.; Keizer, S.B.; van Os, J.J. Treatment of chronic tennis elbow with botulinum toxin. Lancet. 1997, 349, 1746. [Google Scholar] [CrossRef] [PubMed]
- Keizer, S.B.; Rutten, H.P.; Pilot, P.; Morre, H.H.; v Os, J.J. Verburg AD. Botulinum toxin injection versus surgical treatment for tennis elbow: a randomized pilot study. Clin Orthop. 2002, 401, 125–31. [Google Scholar] [CrossRef]
- Placzek, R.; Lindner, M.S.; Deuretzbacher, G.; Meiss, A.L. Therapie der chronischen Epicondylopathia humeri radialis mit Botulinumtoxin A -- eine Therapiestudie mit 2 Jahren Nachuntersuchungszeitraum [Therapy for chronic radial epicondylitis with botulinum toxin A]. Z Orthop Ihre Grenzgeb. 2004, 142, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.M.; Hui, A.C.; Tong, P.Y.; Poon, D.W.; Yu, E.; Wong, L.K. Treatment of lateral epicondylitis with botulinum toxin: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2005, 143, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Hayton, M.J.; Santini, A.J.; Hughes, P.J.; Frostick, S.P.; Trail, I.A.; Stanley, J.K. Botulinum toxin injection in the treatment of tennis elbow. A double-blind, randomized, controlled, pilot study. J Bone Joint Surg Am. 2005, 87, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Placzek, R.; Drescher, W.; Deuretzbacher, G.; Hempfing, A.; Meiss, A.L. Treatment of chronic radial epicondylitis with botulinum toxin A. A double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg Am. 2007, 89, 255–260. [Google Scholar] [CrossRef]
- Oskarsson, E.; Piehl Aulin, K.; Gustafsson, B.E.; Pettersson, K. Improved intramuscular blood flow and normalized metabolism in lateral epicondylitis after botulinum toxin treatment. Scand J Med Sci Sports. 2009, 19, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Espandar, R.; Heidari, P.; Rasouli, M.R.; Saadat, S.; Farzan, M.; Rostami, M.; Yazdanian, S.; Mortazavi, S.M. Use of anatomic measurement to guide injection of botulinum toxin for the management of chronic lateral epicondylitis: a randomized controlled trial. CMAJ. 2010, 182, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Tu, Y.K.; Chen, S.S.; Lin, I.L.; Chen, S.C.; Guo, H.R. Comparison between botulinum toxin and corticosteroid injection in the treatment of acute and subacute tennis elbow: a prospective, randomized, double-blind, active drug-controlled pilot study. Am J Phys Med Rehabil. 2010, 89, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Kuan, T.S.; Chen, K.L.; Lien, W.C.; Hsieh, P.C.; Hsieh, I.C.; Chiu, S.H.; Lin, Y.C. Comparison Between Steroid and 2 Different Sites of Botulinum Toxin Injection in the Treatment of Lateral Epicondylalgia: A Randomized, Double-Blind, Active Drug-Controlled Pilot Study. Arch Phys Med Rehabil. 2017, 98, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Creuzé, A.; Petit, H.; de Sèze, M. Short-Term Effect of Low-Dose, Electromyography-Guided Botulinum Toxin A Injection in the Treatment of Chronic Lateral Epicondylar Tendinopathy: A Randomized, Double-Blinded Study. J Bone Joint Surg Am. 2018, 100, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Galván Ruiz, A.; Vergara Díaz, G.; Rendón Fernández, B.; Echevarría Ruiz De Vargas, C. Effects of Ultrasound-Guided Administration of Botulinum Toxin (IncobotulinumtoxinA) in Patients with Lateral Epicondylitis. Toxins (Basel) 2019, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, H.H.; Chang, M.C. The Effect of Botulinum Toxin Injection into the Common Extensor Tendon in Patients with Chronic Lateral Epicondylitis: A Randomized Trial. Pain Med. 2020, 21, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Cogné, M.; Creuzé, A.; Petit, H.; Delleci, C.; Dehail, P.; de Seze, M. Number of botulinum toxin injections needed to stop requests for treatment for chronic lateral epicondylar tendinopathy. A 1-year follow-up study. Ann Phys Rehabil Med. 2019, 62, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Keijsers, R.; Koenraadt, K.; Turkenburg, J.; Beumer, A.; Bertram, T.; Eygendaal, D. Ultrasound Measurements of the ECRB Tendon Shows Remarkable Variations in Patients with Lateral Epicondylitis. Arch Bone Jt Surg. 2020, 8, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Keizer, S.B.; Rutten, H.P.; Pilot, P.; Morre, H.H.; v Os, J.J.; Verburg, A.D. Botulinum toxin injection versus surgical treatment for tennis elbow: a randomized pilot study. Clin Orthop. 2002, 401, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Hulsopple, C.; Boyce, B. Utilization of Botulinum Toxin for Musculoskeletal Disorders. Current Sports Medicine Reports 2020, 19, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F. Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins (Basel). 2016, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Guo, Y.H.; Hsieh, P.C.; Lin, Y.C. Effectiveness of different doses of botulinum neurotoxin in lateral epicondylalgia: A network meta-analysis. Annals of physical and rehabilitation medicine 2023, 66, 101711. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M.; Jokar, R.; Zamani, M.; Khafri, S.; Esmaeilnejad-Ganji, S.M. Clinical efficacy of local injection therapies for lateral epicondylitis: A systematic review and network meta-analysis. Caspian journal of internal medicine 2022, 13, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Coombes, B.K.; Bisset, L.; Brooks, P.; et al. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia. JAMA 2013, 239, 461. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, W.T.; Hsu, Y.C.; Han, D.S.; Chang, K.V. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018, 32, 131–145. [Google Scholar] [CrossRef] [PubMed]
| Type of study, no. of patients | Inclusion/exclusion criteria | Injection point / targeted structure | Dose | Outcome | Timing | Results | Side effects | |
|---|---|---|---|---|---|---|---|---|
| More, 1997 [9] | Open study/ 14 | Treatment-resistant chronic LE | Electromyographic guidance into extensor fingers III and IV / muscle | 20 – 40 UI BTX-A | Pain | 1 month 3, 6 and 8 months |
Pain improvement | Weakness of fingers III and IV extension (expected) for 3 – 4 months |
| Keizer, 2002 [10] | Pilot, randomized / 40 | Pain older than 6 months, failure of conservative treatment | Anatomic landmark (intramuscular injection ECRB) | 30 – 40 UI BTX-A, 50 UI repeated when necessary / operative (Hohmann procedure) | Pain (VAS*) Grip strength Sick leave ROM* |
Baseline 6 weeks, 3, 6 months, 1 and 2 years |
Pain improvement in all groups and moments Sick leave greater at 3 months in operative group ROM reduced at 3 and 6 months in operative group |
Patients that did not achieve sufficient paresis had a negative postoperative evolution |
| Placzek, 2004 [11] | Pilot, prospective, observational / 16 | Chronic LE, at least 6 months old and failure of 3 therapies | Anatomic landmark: approximately 3 to 4 cm distal to the tender epicondyle, with infiltration of the muscle at two locations; the second location was injected after partial withdrawal of the needle and rotating it in the horizontal plane. | 60 UI aboBTX | Pain (VAS, clinical pain score) Grip strength |
Baseline 2, 6, 10, 14 weeks, 2 years |
Pain reduction from the 2nd week, persisting up to 2 years. Grip strength decreased at 2 and 6 weeks and increased at 10 and 14 weeks. |
At 2 weeks there was significant extension weakness in third finger, it disappeared slowly |
| Wong, 2005 [12] | Randomized, double-blind, placebo-controlled / 60 | Pain longer 3 months No previous injection treatment |
Anatomic landmark: deeply into the subcutaneous tissue and muscle, 1 cm from the lateral epicondyle, and were aimed toward the tender spot / tendon |
60 UI aboBTX / saline (placebo) | Pain (VAS) Grip strength |
Baseline 4 weeks 12 weeks |
Pain reduction (significant improvement at 4 weeks, maintained at 12 weeks). No diff in grip strength |
Weakness of finger extension and paresis of digits: 33% at 4 weeks, 7% at 12 weeks, with 3% interfering with job activity |
| Hayton, 2005 [13] | Randomized, double-blinded, placebo-controlled, pilot / 40 | LE older than 6 months, with failure of therapies | Anatomic landmark: 5 cm distal to the area of maximal tenderness at the lateral epicondyle, in line with the middle of the wrist, the needle inserted deep to the forearm fascia / muscle | 50 UI onaBTX-A / saline (placebo) | Pain (VAS) Grip strength General health questionnaire (SF-12) |
Baseline 3 months |
No significant differences between groups | Extensor lag |
| Placzek, 2007 [14] | Prospective, placebo-controlled, double-blinded, multicentric / 132 | Chronic LE; older than 4 months, failure of at least 3 modalities of therapy | Anatomic landmark: approximately 3 to 4 cm distal to the tender epicondyle, with infiltration of the muscle at two locations; the second location was injected after partial withdrawal of the needle and rotating it in the horizontal plane. | 60 UI aboBTX A / placebo (saline) | Pain (VAS, clinical pain score) Grip strength Subjective assessment |
Baseline, 2, 6, 12 and 18 weeks | Pain improved at all moments. Grip strength did not differ at any moment. Subjective assessment improved from week 6 |
Weakness of extension 3rd finger from 2nd week up to 14th week |
| Oskarson, 2009 [15] | Prospective, observational / 10 | Chronic pain with failure of previous therapies and surgical reference | ECRB* guided injection under electromyografic stimulation / muscle | 1 UI/kg onaBTX-A, maximum 100 UI / muscle / contralateral normal elbow | Pain Function (DASH, COPD*). Grip strength. Muscle strength ECRB blood flow and lactate concetration |
Baseline, 3, 12 months | Blood flow increased at 3 and 12 months. Lactate decreased at 12 months Pain decreased at 3 and 12 months. Function improved at 12 months. Grip strength declined at 3 months and increased at 12 months. |
One patient had abnormal blood flow initially and developed bilateral involvement. |
| Espandar, 2010 [16] | Randomized, placebo-controlled / 48 | Failure of previous therapies | Anatomic landmark: distance of one-third the length of the forearm from the tip of the lateral epicondyle on the course of the posterior inter - osseus nerve / muscle | 60 UI BTX-A / saline | Pain at rest Pain at maximum grip and pinch Grip strength Extensor lag for 3rd and 4th finger. |
Baseline 4 weeks 8 weeks 16 weeks |
Pain at rest and at maximum pinch decreased significantly. Grip strength decreased transitory (4 and 8 weeks) but not significantly. |
Extensor lag (weakness of extension of digits 3 and 4) was largely present at 4 weeks, resolved at 8 and 16 weeks. |
| Lin, 2010 [17] | prospective randomized, double-blind, drug-controlled trial / 16 | Acute and subacute LE | Anatomic landmark: ECRB muscle near common origin of wrist and finger extensors. The needle was first inserted into the subcutaneous layer and then pushed further into the ECRB. Localization of needle tip in the ECRB was confirmed by palpation during resisted wrist extension. | 50 UI onaBTX-A / 40 mg triamcinolone (CS*) | Pain (VAS) Grip strength Quality of life (WHOQOL-BREF*) |
Baseline 4, 8, 12 weeks |
Pain improved significantly in both groups at 4 weeks, better in CS group. Grip strength decreased in BTX (4 and 8 weeks) and increased in CS group. At 12 weeks no significant difference. Quality of life improved in both groups |
Grip strength decreased in BTX group (4 and 8 weeks). |
| Guo, 2016 [18] | Randomized, prospective, double-blinded, active drug-controlled pilot study / 26 | LE older than 6 months with failure of physical therapy or oral medication | Anatomic landmark: for the enthesis - 1 cm distal to the lateral epicondyle / for the muscles - the most tender point of the common extensor musclaes (ECRB or EDC) | 20 UI onaBTX-A, 40 mg triamcinolone (CS) |
Pain (VAS) Grip strength PRTEE* |
Baseline 4, 8, 12 and 16 weeks | At 4 weeks: intratendon BTX-A and CS better results than intramuscular BTX-A. At 8, 12 and 16 weeks – no difference. | Intramuscular: extensor lag with full recovery. Grip strength transitory reduced at 4 weeks for intramuscular BTX. |
| Creuze, 2018 [19] | Phase-III, single-center, randomized, double-blinded, placebo-controlled / 60 | Chronic LE 6 months old Failure of previous therapies |
Anatomic landmark: at approximately 5 cm distal to the lateral epicondyle (targeting ECRB), EMG confirmation | 40 UI aboBTX-A / saline | Pain (VAS) Grip strength Quality of life (self-assessment) |
Baseline 30 and 90 days |
Pain improved at both moments. No difference for grip strength. |
No extensor lag. No grip strength alteration. |
| Ruiz, 2019 [20] | Prospective, experimental / 24 | Chronic pain with failure of previous therapies | Ultrasound guided infiltration into specific muscle | Specific doses incoBTX-A per muscle, maximum 80 UI | Pain (VAS) Function (QuickDASH) |
Baseline 1, 3 and 6 months |
Pain and function improved at 1 month and persisted at 6 months | 21% failure 13% required a second dose (positive effect, short duration) |
| Lee, 2019 [21] | Prospective, randomized, comparative / 60 | Pain longer 3 months Failure of previous therapies |
Ultrasound guidance: peppering technique in the tendon in a distal-to-proximal direction | 10 UI aboBTX-A (SD) / 50 UI BTX-A (LD) | Pain (NRS*) Grip strength Motor weakness in extensors |
Baseline 1, 2, 3, 4, 5 and 6 months |
Pain decreased in both groups at all moments, LD group had better results at all moments. Grip strength increase in both groups, all moments, better results in LD |
Extensor weakness 3% in SD and 20% in LD |
| Cogne, 2019 [22] | Open, prospective, observation, continuation of Creuze, 2018 (19) / 50 | Follow-up after first BTX-A injection | Anatomic landmark: at approximately 5 cm distal to the lateral epicondyle (targeting ECRB), EMG confirmation | 40 UI aboBTX-A | Number of required injections | 270 and 365 days | 80% of patients improved after 1 or 2 injections. 2% asked for a third injection |
18% of patients asked for surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





