Submitted:
21 March 2024
Posted:
22 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
| Tested samples | Tested Parasites | |
|---|---|---|
| L. major | L. tropica | |
| Crude extract | IC50 (µg/mL)SD | IC50 (µg/mL) |
| Hexane fraction | 33.30.7 | 36.60.5 |
| Dichloromethane fraction | NT | NT |
| Ethyl acetate fraction | >100 | >100 |
| N-Butanol fraction | >100 | >100 |
| Water fraction | >100 | >100 |
| Essential oil | 38.30.8 | 55.160.9 |
| Compounds | IC50 (µM/mL) | IC50 (µM/mL) |
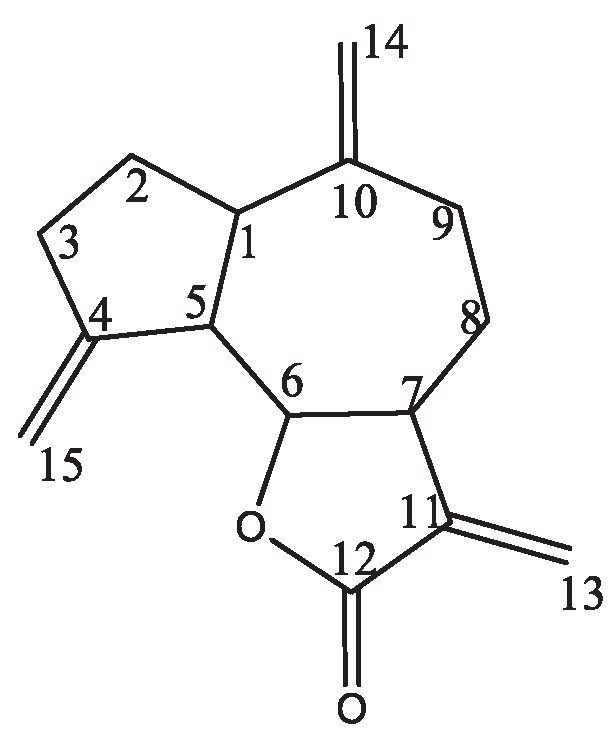
| E.k-cpd1 | 15.30.03 | 14.20.2 |
| Amphotericin B | 3.390.03 | 3.410.02 |
| Pentamidine | 4.560.01 | 4.560.01 |
| Miltefosine | 31.80.2 | 27.20.6 |
| No. | RT (minute) | MW (g/mole) | MF | Name of Compounds | Relative Concentration (%) |
|---|---|---|---|---|---|
| 1 | 18.1 | 152 | C10H16O | (S)-cis-Verbenol | 0.21 |
| 2 | 19.1 | 154 | C10H18O | Borneol | 0.18 |
| 3 | 23.5 | 154 | C10H18O | p-Menth-2-en-7-ol, trans- | 0.28 |
| 4 | 24.4 | 196 | C12H20O2 | Borneol, acetate | 2.08 |
| 5 | 26.7 | 204 | C15H24 | (±)-Cadinene | 3.94 |
| 6 | 27.1 | 204 | C15H24 | δ-Neoclovene | 3.68 |
| 7 | 27.5 | 204 | C15H24 | β-Elemene | 2.67 |
| 8 | 28.4 | 204 | C15H24 | α-Guaiene | 2.36 |
| 9 | 28.7 | 204 | C15H24 | Caryophyllene | 3.05 |
| 10 | 30.5 | 204 | C15H24 | α-Humulene/α-Caryophyllene | 1.36 |
| 11 | 30.8 | 204 | C15H24 | (-)-Alloaromadendrene | 1.07 |
| 12 | 31.7 | 204 | C15H24 | γ-Muurolene | 0.15 |
| 13 | 32.1 | 204 | C15H24 | β-Eudesmene/β-Selinene | 0.11 |
| 14 | 32.4 | 204 | C15H24 | α-Selinene | 0.43 |
| 15 | 33.5 | 204 | C15H24 | γ-Cadinene | 2.19 |
| 16 | 33.7 | 204 | C15H24 | δ-Cadinene | 0.33 |
| 17 | 34.1 | 204 | C15H24 | β-Guaiene | 11.13 |
| 18 | 35.6 | 222 | C15H26O | ±-trans-Nerolidol | 0.48 |
| 19 | 37.7 | 220 | C15H24O | Caryophyllene oxide | 2.33 |
| 20 | 37.8 | 220 | C15H24O | Aromadendrene oxide-(2) | 0.74 |
| 21 | 38.2 | 220 | C15H24O | 1,4-Methanoazulen-9-one, decahydro-1,5,5,8a-tetramethyl-, [1R-(1α,3aβ,4α,8aβ)]- | 4.98 |
| 22 | 38.7 | 220 | C15H24O | Diepi-α-cedrene epoxide | 0.37 |
| 23 | 38.9 | 222 | C15H26O | Germacrene D-4-ol | 1.84 |
| 24 | 39.1 | 222 | C15H26O | Cubenol | 0.18 |
| 25 | 39.8 | 222 | C15H26O | τ-Cadinol | 0.33 |
| 26 | 39.9 | 222 | C15H26O | τ-Muurolol | 1.32 |
| 27 | 40.1 | 222 | C15H26O | δ-Cadinol, (-)- | 0.08 |
| 28 | 40.3 | 222 | C15H26O | α-Cadinol | 1.10 |
| 29 | 40.5 | 220 | C15H24O | γ-Gurjunenepoxide-(2) | 0.90 |
| 30 | 40.8 | 204 | C15H24 | Globulol | 0.33 |
| 31 | 41.0 | 220 | C15H24O | Aromadendrene oxide-(1) | 3.29 |
| 32 | 42.0 | 232 | C16H24O | 9-Methoxycalamenene | 1.43 |
| 33 | 42.6 | 220 | C15H24O | Cedren-13-ol, 8- | 0.09 |
| 34 | 42.8 | 220 | C15H24O | Ledene oxide-(II) | 0.43 |
| 35 | 44.0 | 220 | C15H24O | Lanceol, cis | 5.92 |
| 36 | 44.6 | 220 | C15H24O | Santalol, cis, α- | 0.80 |
| 37 | 45.5 | 220 | C15H24O | 2-(4a,8-Dimethyl-1,2,3,4,4a,5,6,7-octahydro-naphthalen-2-yl)-prop-2-en-1-ol | 1.95 |
| 38 | 46.2 | 236 | C15H24O2 | Bicyclo[4.4.0]dec-5-ene, 1,5-dimethyl-3-hydroxy-8-(1-methylene-2-hydroxyethyl-1)- | 0.39 |
| 39 | 46.6 | 220 | C15H24O | α-Copaen-11-ol | 0.08 |
| 40 | 47.5 | 236 | C15H24 O2 | Bicyclo[4.4.0]dec-2-ene-4-ol, 2-methyl-9-(prop-1-en-3-ol-2-yl)- | 0.18 |
| 41 | 49.0 | 230 | C16H22O | Cycloisolongifolene, 8,9-dehydro-9-formyl- | 0.20 |
| 42 | 50.5 | 202 | C15H22 | Aromadendrene, dehydro- | 0.19 |
| 43 | 56.9 | 230 | C15H18O2 | Dehydrocostus lactone | 24.95 |
3. Materials and Methods
3.1. Description of the Experimental Site
3.2. Preparation of Plant Sample
3.3. Essential Oil Extraction
3.4. Chemical Composition Analysis of Essential Oil
3.5. Crude Extraction and Fractionation
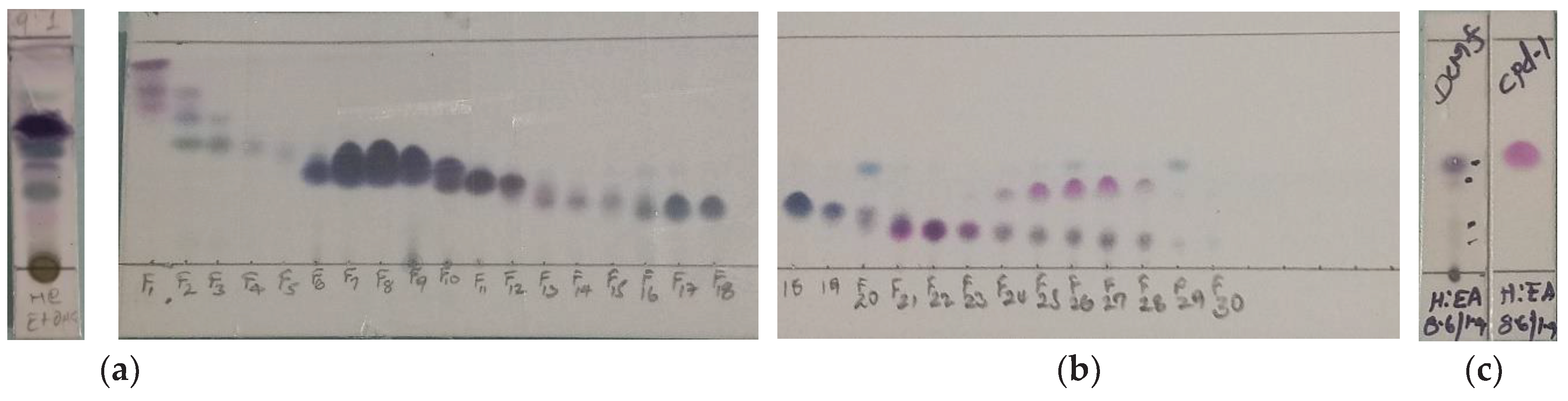
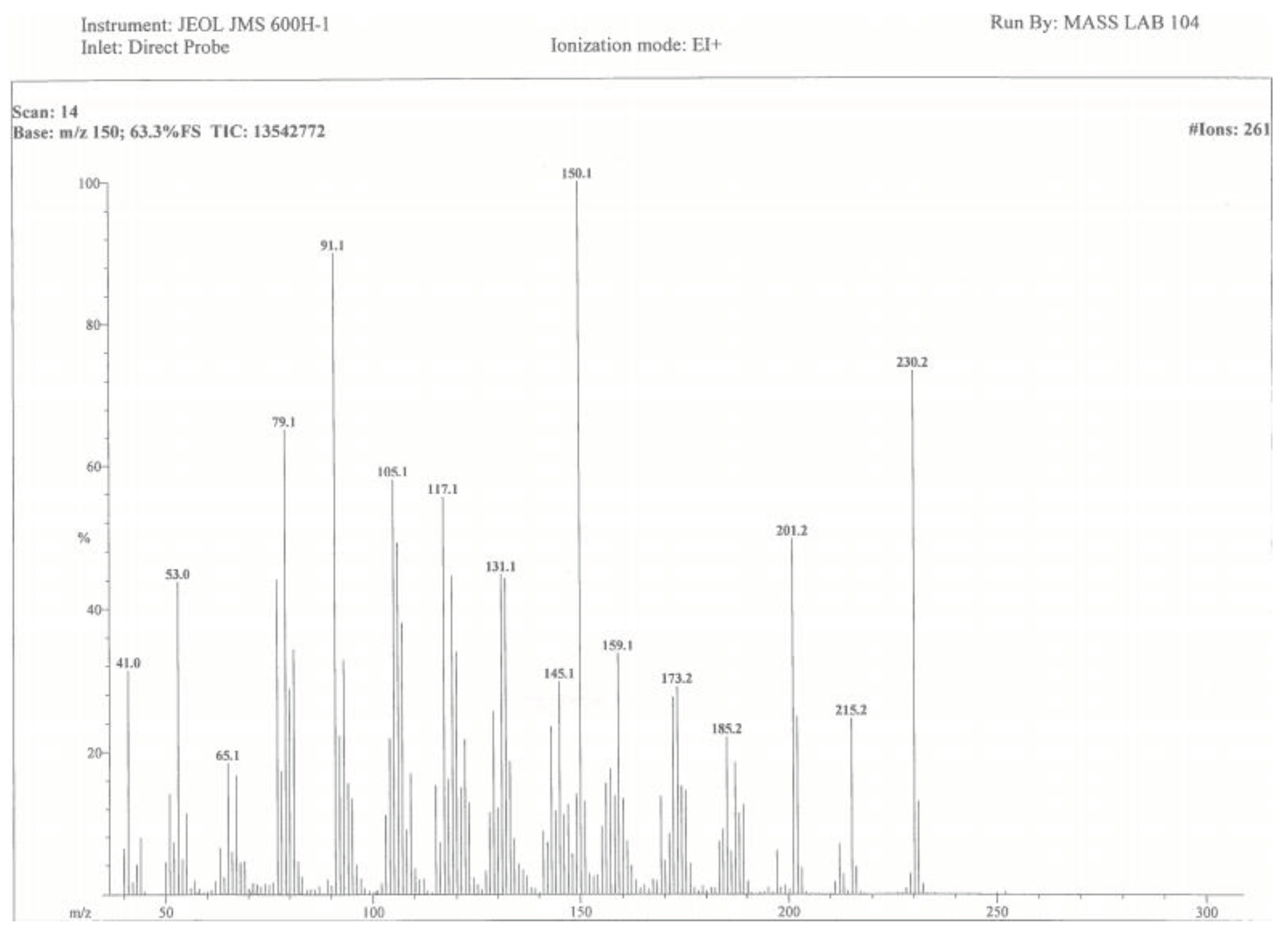
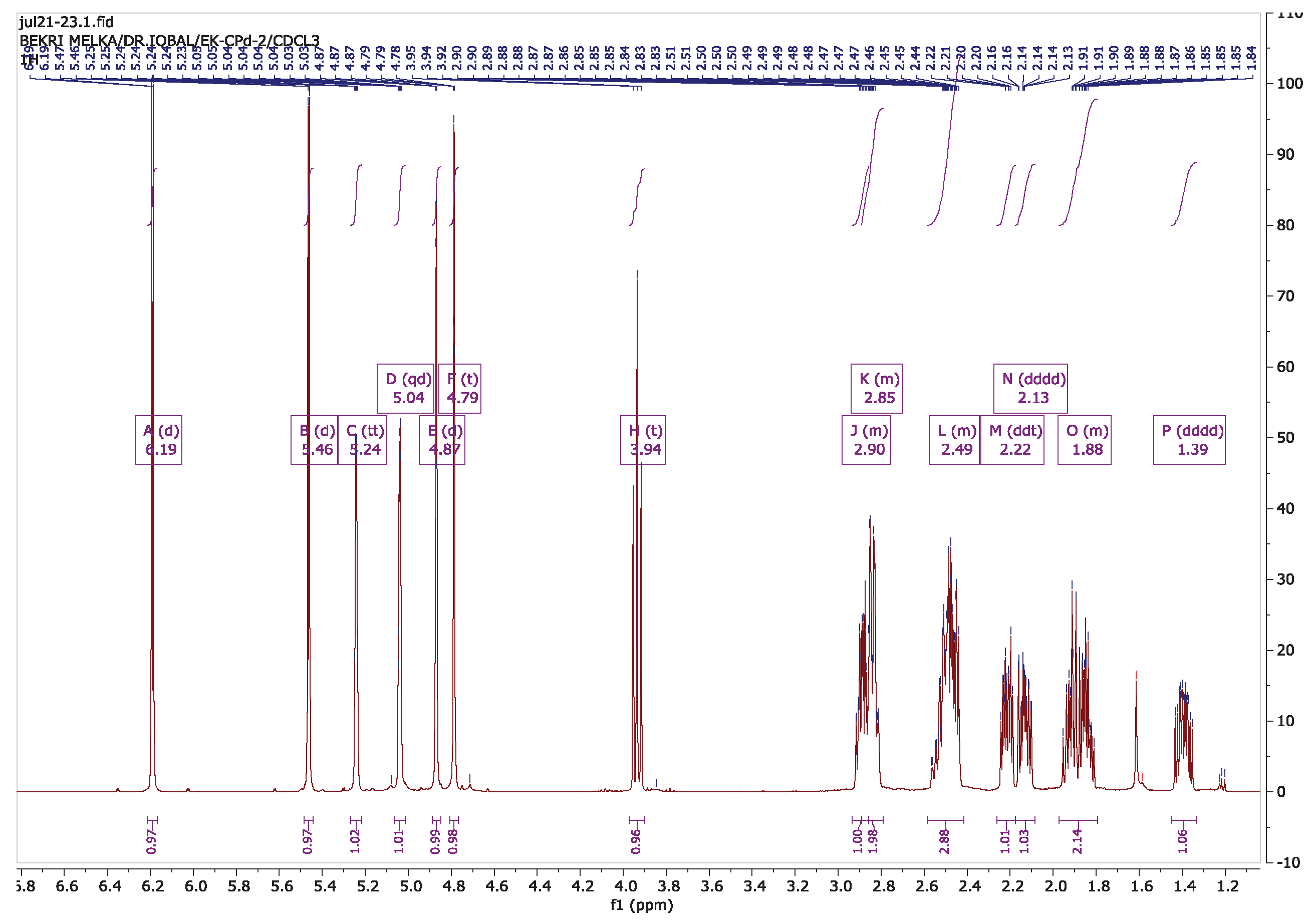
3.6. Isolation of Compounds
3.7. Antileishmanial Activity Test
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Leishmaniasis report. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
- Amare, G. A.; Mekonnen, G. G.; Kassa, M.; Addisu, A.; Kendie, D. A.; Tegegne, B.; Abera, A.; Tadesse, D.; Getahun, S.; Wondmagegn, Y.M.; Merdekios, B.; Asres, M.S.; Van Griensven, J.; Van der Auwera, G.; van Henten, S.; Pareyn, M. First report of cutaneous leishmaniasis caused by Leishmania donovani in Ethiopia. Parasites and Vectors 2023, 16, 1–9. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Regional strategic framework for accelerating and sustaining elimination of kala-azar in the South-East Asia Region, 2022. .
- Hassan, A. A.; Khalid, H. E.; Abdalla, A. H.; Mukhtar, M. M.; Osman, W. J.; Efferth, T. Antileishmanial Activities of Medicinal Herbs and Phytochemicals In Vitro and In Vivo: An Update for the Years 2015 to 2021. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Tabrez, S.; Rahman, F.; Ali, R.; Alouffi, A. S.; Alshehri, B. M.; Alshammari, F. A.; Alaidarous, M. A.; Banawas, S.; Bin Dukhyil, A. A.; Rub, A. Assessment of the Antileishmanial Potential of Cassia fistula Leaf Extract. ACS Omega 2021, 6, 2318–2327. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Kaiser, C. A.; Krieger, M.; Bretscher, A.; Ploegh, H.; Amon, A. Cellular and Molecular Biology 2013, 579–586.
- Abera, B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. Journal of Ethnobiology and Ethnomedicine 2014, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- d’Avigdor, E.; Wohlmuth, H.; Asfaw, Z.; Awas, T. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. Journal of Ethnobiology and Ethnomedicine 2014, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Mesfn, F.; Seta, T.; Assefa, A. An Ethnobotanical Study of Medicinal Plants in Amaro Woreda, Ethiopia. Ethnobotany Research & Applications 2014, 12, 341–354. [Google Scholar]
- Teklehaymanot, T.; Giday, M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J Ethnobiol Ethnomed 2007, 3. [Google Scholar] [CrossRef] [PubMed]
- Teklehaymanot, T.; Giday, M.; Medhin, G.; Mekonnen, Y. Knowledge and use of medicinal plants by people around Debre Libanos monastery in Ethiopia. J Ethno pharmacol. 2007, 111, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Abebe, D.; Debella, A.; Urga, K. Echinops kebericho. In Medicinal Plants and Other Useful Plants of Ethiopia; Camerapix publishers international: Addis Ababa, 2003; Volume 34. [Google Scholar]
- Fullas, F. Ethiopian Traditional Medicine: Common Medicinal Plants in Perspective. 1st edition, 2001.
- Karunamoorthi, K.; Hailu, T. Insect repellent plants traditional usage practices in the Ethiopian malaria epidemic-prone setting: an ethnobotanical survey. Journal of Ethnobiology and Ethnomedicine 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Asfaw, N.; Demissew, S. Echinops kebericho. In Aromatic plants of Ethiopia; Shama Books: Addis ababa, 2009; Volume 77. [Google Scholar]
- Deyno, S.; Tola, M. A.; Bazira, J.; Makonnen, E.; Alele, P. E. Acute and repeated-dose toxicity of Echinops kebericho Mesfin essential oil. Toxicology Report 2021, 8, 131–138. [Google Scholar] [CrossRef]
- Tariku, Y.; Hymete, A.; Hailu, A.; Rohloff, J. In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chemistry and Biodiversit 2011, 8, 614–623. [Google Scholar] [CrossRef]
- Hymete, A.; Rohloff, J.; Iversen, Tor-H.; Kjøsen, H. Volatile constituents of the roots of Echinops kebericho Mesfin. Flavour Fragr. J. 2006, 22, 35–38. [Google Scholar] [CrossRef]
- Tadesse, M.; Abegaz, B.M. AETFAT proceedings, symposium VI, Mittl. inst.Allg.Bot.,Hamberg 1990, 23, 605. [Google Scholar]
- Tariku Yinebeb. In vitro efficacy study of some selected medicinal plants against Leishmania spp. MSc thesis, Addis Ababa University, Ethiopia, 2008; pp. 1–108. [Google Scholar]
- Chan, M. M. Y.; Bulinski, J. C.; Chang, K. P.; Fong, D. A microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitology Research 2003, 89(4), 266–271. [Google Scholar] [CrossRef]
- Bhushan, A.; Rani, D.; Tabassum, M.; Kumar, S.; Gupta, P. N.; Gairola, S.; Gupta, A. P.; Gupta, P. in Crude Extract and Fractions of Aucklandia costus Falc . and Cytotoxicity Studies against Cancer Cells. 2023. [Google Scholar] [CrossRef]
- Li, A.; Sun, A.; Liu, R. Preparative isolation and purification of costunolide and dehydrocostuslactone from Aucklandia lappa Decne by high-speed counter-current chromatography. Journal of Chromatography A 2005, 1076, 193–197. [Google Scholar] [CrossRef]
- Deyno, S.; Abebe, A.; Tola, M. A.; Hymete, A.; Bazira, J.; Makonnen, E.; Alele, P. E. Acute and sub-acute toxicity of Echinops kebericho decoction in rats. BMC Complementary Medicine and Therapies 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hao, J.; Wu, Q.; Guo, K.; Wang, C.; Zhang, W. K.; Liu, W.; Wang, Q.; Yang, X. Dehydrocostus lactone inhibits cell proliferation and induces apoptosis by PI3K/Akt/Bad and ERS signalling pathway in human laryngeal carcinoma. Journal of Cellular and Molecular Medicine 2020, 24, 6028–6042. [Google Scholar] [CrossRef] [PubMed]
- Tessema, F. B.; Gonfa, Y. H.; Asfaw, T. B.; Tadesse, M. G.; Bachheti, A. J.; Singab, A. N.; Bachheti, R. K. Dehydrocostus lactone from the root of Ajuga integrifolia (Buch.-Ham. Ex D. Don): Quantitative determination and in- silico study for anti-breast cancer activity. Plant Science Today 2023, 11, 34–44. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xie, Y.; Hu, H. Antitumor activity and mechanism of costunolide and dehydrocostus lactone: Two natural sesquiterpene lactones from the Asteraceae family. Biomedicine and Pharmacotherapy 2020, 125, 109955. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Z.; Wang, C.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Ma, J.; Zhang, B.; Yang, Q.; Ma, X.; Xu, Y. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-κB/COX-2 signaling pathway by targeting IKKβ. American Journal of Cancer Research 2017, 7, 1270–1284. [Google Scholar]
- Hsu, Y. L.; Wu, L. Y.; Kuo, P. L. Dehydrocostuslactone, a medicinal plant-derived sesquiterpene lactone, induces apoptosis coupled to endoplasmic reticulum stress in liver cancer cells. Journal of Pharmacology and Experimental Therapeutics 2009, 329, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Paço, A.; Brás, T.; Santos, J. O.; Sampaio, P.; Gomes, A. C.; Duarte, M. F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, W. X.; He, Z. Q.; Wu, B. S.; Shen, Z. F.; Shang, H. T.; Chen, T.; Wang, Q.; Chen, Y. G.; Han, S. T. The Possible Anti-Inflammatory Effect of Dehydrocostus Lactone on DSS-Induced Colitis in Mice. Evidence-Based Complementary and Alternative Medicine 2020. [Google Scholar] [CrossRef] [PubMed]
- Seo, C. S.; Lim, H. S.; Jeong, S. J.; Shin, H. K. Anti-allergic effects of sesquiterpene lactones from the root of Aucklandia lappa Decne. Molecular Medicine Reports 2015, 12, 7789–7795. [Google Scholar] [CrossRef]
- Abebe, T.; Hymete, A.; Giday, M.; Bisrat, D. Antidepressant-Like Activity and Molecular Docking Analysis of a Sesquiterpene Lactone Isolated from the Root Bark of Ximenia americana (L.). Evidence-Based Complementary and Alternative Medicine 2024, 1–10. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Dakka, N.; Fellah, H.; Abrini, J.; Bakri, Y. The antileishmanial potential of medicinal plant extracts from the North-West of Morocco. Beni-Suef University Journal of Basic and Applied Sciences 2018, 7, 50–54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





