1. Introduction
Alzheimer’s disease (AD) is becoming increasingly common, resulting in a substantial increase in medical expenditures, amounting to
$1.3 trillion, which is expected to increase to
$2.8 trillion by 2030 [
1]. Among the various neurodegenerative diseases, AD is one of the most prevalent among older individuals [
2]. Consequently, health issues related to AD have emerged as a top priority in national public health discussions.
Sleep problems are commonly observed in 45–61.3% of individuals with AD [
3,
4]. Improving sleep duration is crucial for slowing the progression of psychological symptoms and delaying AD-associated deterioration [
5,
6]. AD reduces cholinergic activity and cerebral blood flow and induces a chronic state of vasoconstriction, leading to reduced cerebral perfusion. This can lead to the accumulation of amyloid-beta and tau proteins, resulting in impaired neurological function [
7]. An imbalance in the ANS can lead to sleep disorders. Sufficient sleep duration can serve as an index of parasympathetic nervous system (PSNS) activation and sympathetic nervous system (SNS) deactivation during the night. The PSNS and SNS innervate key points of control for brain perfusion to modulate cerebral blood flow; however, the cerebral vasculature is heavily innervated by PSNS fibers, whose activation can result in cerebral vasodilation. PSNS input increases cerebral perfusion, whereas SNS input reduces cerebral perfusion during sleep [
7]. The PSNS serves as a “brake” for the SNS [
8,
9,
10]. The decline in PSNS activity accelerates with age, particularly in individuals with AD, which gradually results in ANS dysregulation [
9,
11,
12,
13]. Discoordination of SNS and PSNS activities in individuals with AD resulting in ANS dysregulation appears to be associated with unstable and irregular circadian rhythms.
Prolonged sleep or short sleep duration disrupts ANS regulation, leading to bidirectional dysregulation, destabilizing physiological processes, and contributing to various pathologies (such as cognitive impairment, metabolic disorders, oxidative stress, inflammation, immune dysregulation, cardiovascular disease, coronary heart disease, cancer, and mental disorders) commonly associated with < 6 h or > 9 h sleep durations [
2,
4,
14,
15,
16,
17,
18,
19]. In AD, sleep abnormalities manifest as longer sleep latency, longer rapid eye movement (REM) latency, decreased sleep efficiency, decreased deep sleep (stages 3 and 4 sleep), decreased slow-wave sleep (SWS), and increased light sleep (stages 1 and 2 sleep). Other sleep disorders associated with a shortened sleep duration include obstructive sleep apnea (OSA), insomnia, restless legs syndrome (RLS), periodic limb movement disorder (PLMD), and REM sleep behavior disorder (RBD) [
3,
20].
ANS dysregulation is associated with decreased PSNS activity and increased SNS activity during sleep [
11,
13]. At night, increased SNS activity reduces blood flow in the brain, leading to the accumulation of amyloid-beta, deficits in hippocampal synaptic plasticity, cortical thinning, and the inhibition of acetylcholine secretion [
8,
20,
21,
22]. Triggered SNS activity commands load baroreceptors and catecholamine levels to increase and generate a rapid transition to permissive tachycardia, which plays a crucial role in the pathophysiology of diseases associated with cardiovascular alterations, OSA, and hypertension [
23]. Acetylcholine neurotransmitter levels are positively correlated with PSNS activity, suggesting that a reduction in nighttime PSNS activity is a key indicator of a decrease in dementia severity [
10,
24,
25,
26]. Higher PSNS activation at night may indicate good sleep quality [
9,
27,
28]. Reduced sleep duration and SWS activity impair the restoration of the frontal lobe; conversely, treatments for sleep quality that may facilitate amyloid-beta clearance and promote SWS have focused on memory encoding and consolidation [
22].
The ANS comprises the SNS and the PSNS whose activity can be assessed by heart rate variability (HRV), which measures the normalized low-frequency (LF%) and high-frequency (HF) power in HRV data. The LF/HF ratio (low-frequency/high-frequency power ratio) reflects the modulation of sympathovagal balance [
10,
27,
29,
30]. HRV refers to the complex variation in heart rate that is produced by the interaction between the SNS and PSNS (vagal) neural activity at the sinus node [
9,
10,
23,
29,
31]. Previous research has shown that sympathovagal balance is positively correlated with sleep apnea, insomnia, RLS, PLMD, and RBD [
28]. HRV can be measured noninvasively and is used in sleep and cardiovascular research. Sympathovagal balance, reflecting the modulation of the SNS and PSNS, is critical during sleep, especially with respect to ANS dysregulation in patients with AD [
8]. A comprehensive definition of ANS regulation has been presented in previous research: sympathovagal balance during sleep, reflecting the modulation of the SNS and PSNS, is an optimal indicator. This parameter is more effective than using SNS and PSNS activity alone to assess nocturnal ANS regulation, and ANS dysregulation in patients with longer sleep (> 9 h) or shorter sleep duration (< 6 h) disrupts ANS regulation. Discoordination of SNS and PSNS activities in individuals with AD resulting in ANS dysregulation appears to be associated with extreme sleep duration. Therefore, we aimed to examine the correlations among sleep duration, dementia severity, age, and autonomic regulation in patients with probable AD using a noninvasive recording method. We hypothesized that sleep duration and age would affect nocturnal ANS regulation and inadequate sleep duration-related ANS dysregulation. Additionally, we hypothesized that dementia severity would correlate positively with ANS regulation.
2. Results
2.1. Demographic and Clinical Characteristics
Twenty-seven participants successfully completed the study. Each participant underwent 9 h of HRV measurements over one day, resulting in 27 data sets for analysis. Notably, no significant differences in clinicodemographic characteristics, such as sex (χ2 test = 3.0, p = 0.55) or probable AD severity (χ2 test = 6.9, p = 0.86), were observed among the five groups. Overall, 59% of the participants were female, 41% were male, and approximately 8%, 37%, 44%, and 11% had very mild, mild, moderate, or severe probable AD, respectively. The sleep duration was distributed as follows: < 6 h, 29.6% of the participants; 6–7 h, 18.5% of the participants; 7–8 h, 14.8% of the participants; 8–9 h, 11.1% of the participants; and > 9 h, 25.9% of the participants.
To assess the impact of comorbidities, including diabetes, congestive heart failure, and cerebrovascular disease, on sleep duration, the Kruskal‒Wallis test was used to analyze continuous variables such as age, comorbidity index, and medication dosage. No significant differences were found among the groups, underscoring the homogeneity of the participant distribution.
The mean age was 79.5 years (SD = 8.5 years). Antipsychotic medication was administered to 93% of the respondents, with a mean medication-defined daily dose (DDD) of 0.86 (SD = 1.34). In addition, 78% of participants used antidepressants at a mean dose of 0.62 (SD = 0.65), whereas 81% used benzodiazepines at a mean dose of 0.26 (SD = 0.26). The results of the descriptive statistical analysis of all the variables are shown in
Table 1.
2.2. Outcomes
2.2.1. Effect of Sleep Duration on ANS Regulation
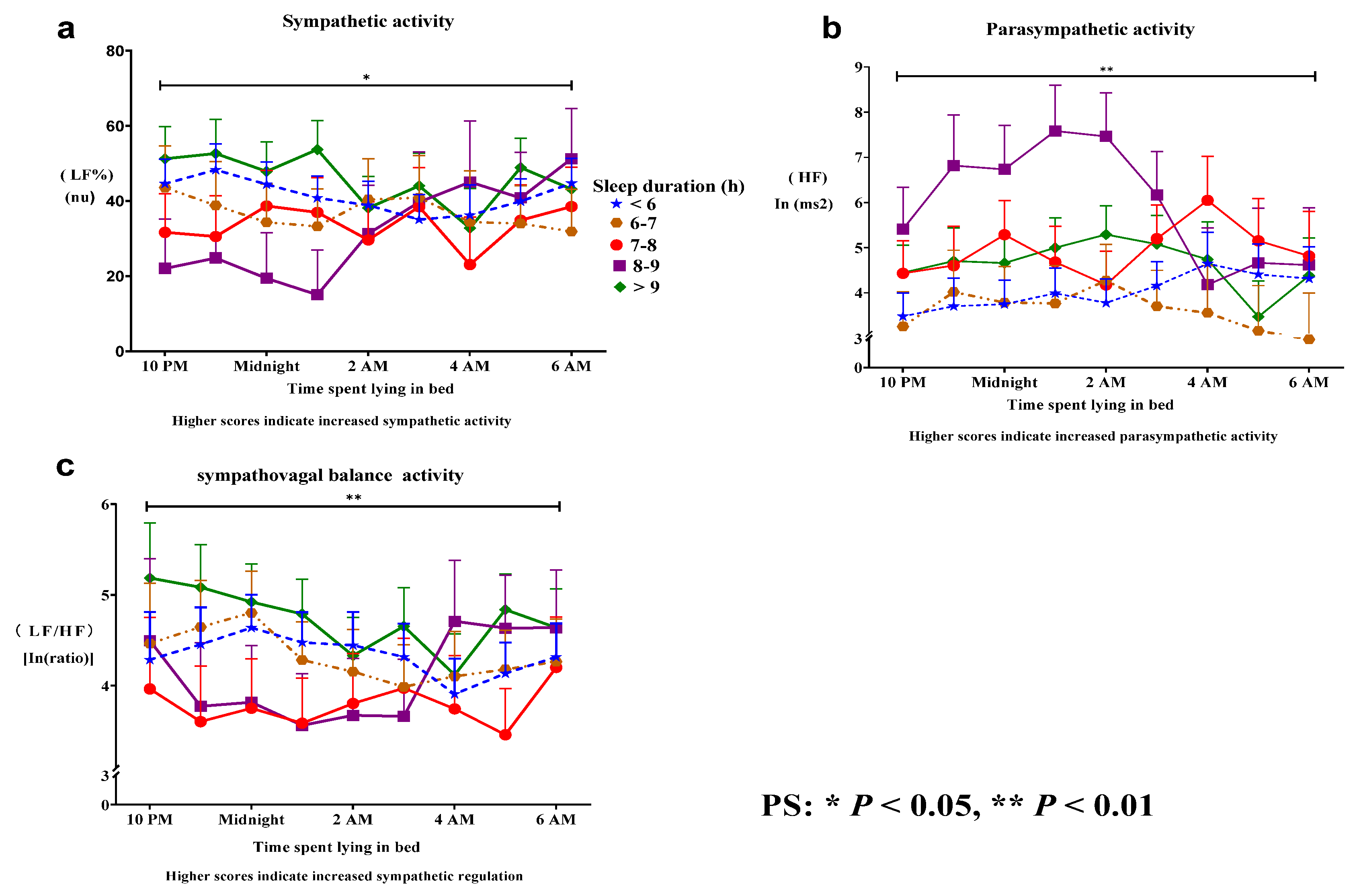
Sleep duration at night was associated with (a) a significant difference in mean SNS activity (Roy's largest root = 3.0, p = 0.03) and (b) a significant difference in PSNS activity (Roy's largest root = 4.7, p = 0. 008), and (c) sympathovagal balance (Roy's largest root = 4.9, p = 0.005) during the bedtime period from 9:00 p.m. to 6:00 a.m. Participants with a sleep duration of 7–8 h per night demonstrated increased ANS regulation compared with those with other sleep durations. Three distinct sleep duration trends were observed: (a) decreased SNS activity, (b) increased PSNS activity, and (c) a significant decrease in sympathovagal balance. A sleep duration of 7–8 h per night was associated with stable and regular circadian rhythms (
Figure 1a–c). Conversely, a sleep duration of 8–9 h, associated with increased PSNS activity, was associated with significant periodic fluctuations and a lack of regular circadian rhythms (
Figure 1). Thus, an inverted U-shaped association between sleep duration and ANS regulation was identified. A longer sleep duration was associated with more severe impairment of ANS regulation than was a shorter sleep duration. A longer sleep duration was characterized by increased SNS regulation (mean of 8.5%), SNS activity (mean of 9.4%), and PSNS activity (13%). The formula used for calculation was: (longer sleep duration ANS – shorter sleep ANS/longer sleep duration ANS) % (
Table 2).
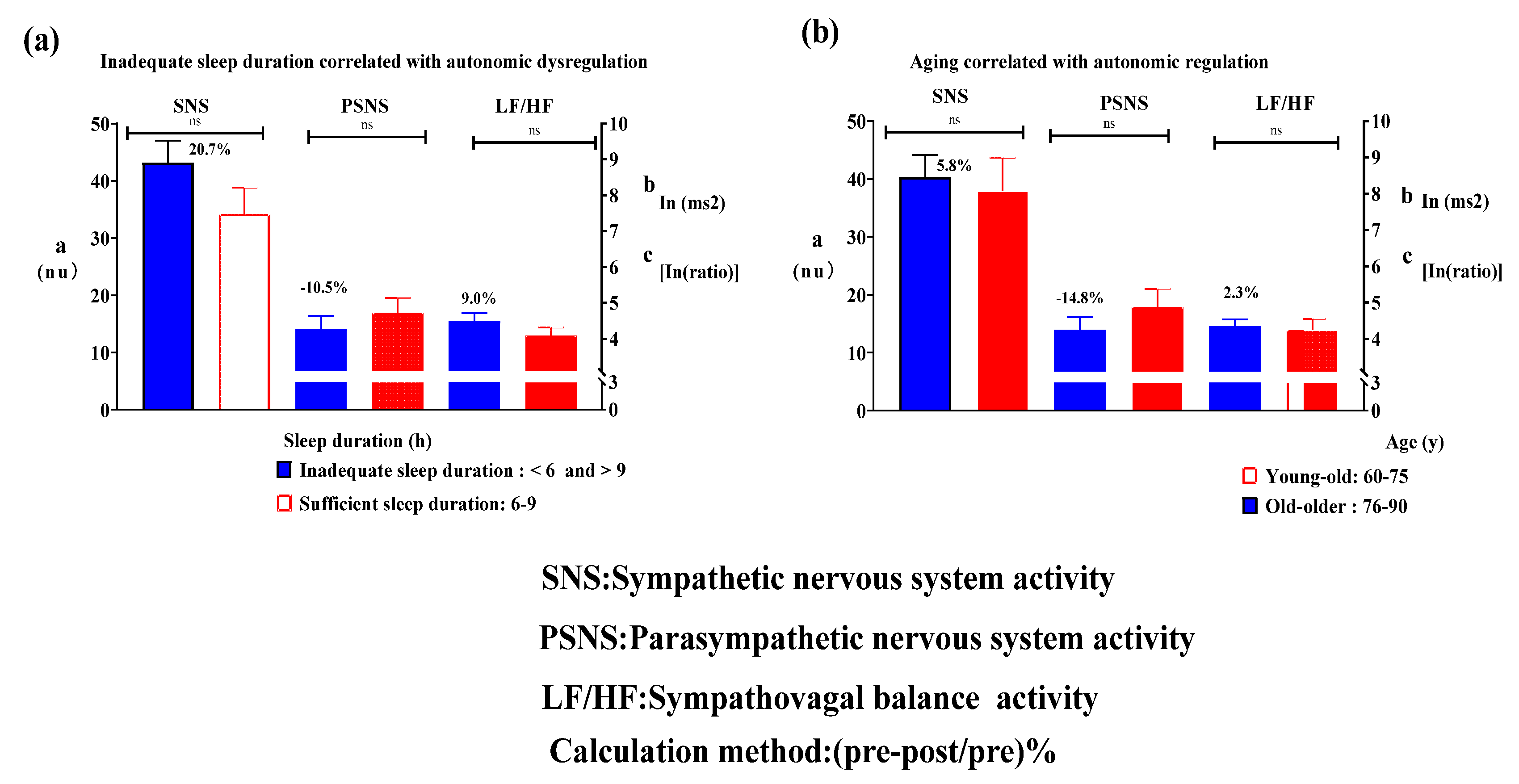
The effect of inadequate sleep duration compared with sufficient sleep duration on ANS dysregulation, as indicated by sympathovagal balance (Roy's largest root = 1.2, p = 0.33), was not statistically significant. These findings are associated with SNS activity (Roy's largest root = 0.9, p = 0.53) and PSNS activity (Roy's largest root = 0.7, p = 0.63). Inadequate sleep duration was characterized by increased SNS regulation (mean of 9.0%), increased SNS activity (mean of 20.7%), and decreased PSNS activity (10.5%). The calculation method was as follows: (pre-post/pre)% (
Figure 2-a).
The difference in ANS regulation between young (60-75 y) and oldest (76-90 y) individuals was nonsignificant, as indicated by sympathovagal balance (Roy's largest root = 1.3, p = 0.30). These findings are associated with SNS activity (Roy's largest root = 0.9, p = 0.48) and PSNS activity (Roy's largest root = 0.2, p = 0.96). The oldest individuals demonstrated a mean increase of 2.3% in SNS regulation, mean increase of 5.8% in SNS activity, and decrease of 14.8% in PSNS activity (
Figure 2- b).
2.2.2. Correlation Between Dementia Severity and ANS Regulation
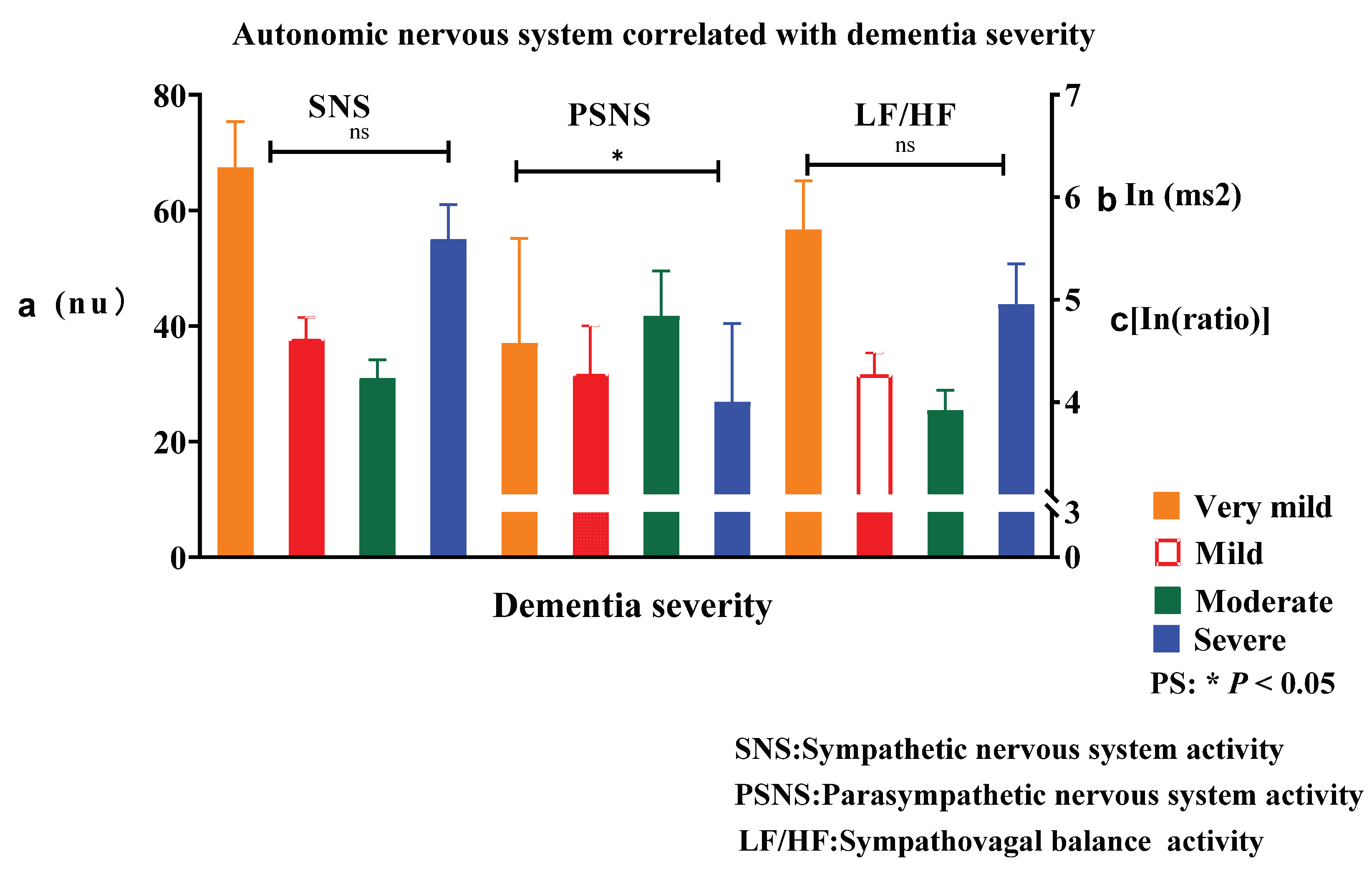
A significant correlation between dementia severity and PSNS activity was observed (Roy’s largest root = 2.8, p = 0.04). However, we found no significant correlation between dementia severity and SNS activity (Roy’s largest root = 1.7, p = 0.16) or sympathovagal balance (Roy’s largest root = 1.5, p = 0.22) (
Figure 3). Among individuals with moderate overall functional impairment, three notable trends were observed compared with individuals in other cognitive groups: (a) decreased SNS activity, (b) increased PSNS activity, and (c) decreased sympathovagal balance. Specifically, individuals with moderate overall functional impairment had significantly greater PSNS activity than those with very mild, mild, or severe impairment. Consequently, the association between dementia severity and PSNS activity was characterized by an inverted U-shaped curve. Individuals with moderate overall functional impairment were more likely to have milder ANS impairment than individuals with other cognitive levels (
Figure 3) functional impairment.
3. Discussion
This pilot study demonstrated that a sleep duration of 7–8 h per night was associated with better ANS regulation than a sleep duration of other durations, as indicated by stable and regular circadian rhythms, followed by sleep durations of 8–9 h, 6–7 h, < 6 h, and > 9 h. A longer sleep duration was associated with more severe impairment of ANS regulation than was a shorter sleep duration. A sleep duration of 7–8 h tended to be associated with increased PSNS activity, decreased SNS activity, and decreased sympathovagal balance compared with other sleep durations.
We found a significant correlation between dementia severity and PSNS activity. Specifically, individuals with moderate overall functional impairment had significantly greater PSNS activity than did those with very mild, mild, or severe impairment. Consequently, the association between dementia severity and PSNS activity was characterized by an inverted U-shaped curve. We found that sleeping 7–8 hours per night was associated with better ANS regulation than was sleeping other durations. These findings are consistent with those of previous studies [
15,
16,
17,
18,
19,
32,
33], which showed that longer or shorter sleep durations can cause ANS dysregulation [
8,
13,
23,
28,
34].
3.1. Inadequate Sleep Duration-Related ANS Dysregulation in Sympathetic-Mediated Diseases
The flip‒flop switch model of sleep–wake regulation suggests that sleep-inducing neurotransmitters, such as gamma-aminobutyric acid and the neuropeptide galanin, are located in the ventrolateral preoptic nucleus in the hypothalamus. The release of these neurotransmitters promotes sleep. Conversely, wakefulness begins with inhibition of the ventrolateral preoptic nucleus involving cholinergic, monoaminergic, and orexinergic neurons. The sleep-wake system is a critical regulator of homeostasis [
10,
23,
34]. An imbalance in the ANS can lead to sleep disturbances.
Inadequate sleep duration-related ANS dysregulation in sympathetic-mediated diseases, i.e., (1) increased levels of proinflammatory cytokines such as interleukin-6, high-sensitivity C-reactive protein, and tumor necrosis factor-alpha; (2) disruption of cyclic adenosine monophosphate and gamma-aminobutyric acid signaling pathways in neurons, affecting synaptic plasticity; (3) potential promotion of tumor growth through altered melatonin secretion; (4) gray matter volume loss and brain atrophy in regions functionally associated with cognition; and (5) increased levels of glucocorticoids and reactive oxygen species; reactive oxygen species can cause DNA damage, which may contribute to telomere shortening and cellular senescence [
11,
13,
16,
19,
23,
31,
35].
Our results suggest that extremely short and long sleep durations may contribute to ANS dysregulation. Such impairments are reflected by a 20.7% increase in ANS activity and an altered sympathovagal balance similar to that observed in patients with probable AD. We speculate that inadequate sleep duration may induce a chronic state of vasoconstriction, leading to reduced cerebral perfusion, a potential marker of impaired autonomic tone, particularly excessive SNS activity [
7] (. This finding is consistent with the findings of a previous study by [
36] Wang et al. (2019), who reported increased mortality and major cardiovascular risks associated with both longer and shorter sleep durations. We found that longer sleep durations were associated with more severe impairment of ANS regulation than shorter sleep durations.
3.2. Medications Disrupt Bidirectional Associations Between Sleep Duration and ANS Regulation
ANS regulation plays an important role during the wake-to-sleep transition through cardiorespiratory and brain regulation [
37], especially for ANS dysregulation in patients with AD [
8].
In our study, a sleep duration of 7–8 hours were associated with a significant decrease in sympathovagal balance during 1 hour of sleep (11 p.m.). In contrast, a sustained peak in PSNS activity was observed at 4 a.m. Healthy individuals exhibit maximal PSNS activity at 2 a.m. [
38]; lower PSNS activity with insomnia by 5.6 versus 4.6 [
39]; and greater sympathovagal balance with older healthy individuals by 1.1 versus 4.2 [
40]. In individuals with AD, PSNS activity peaks and later exhibits a phase delay, decreasing PSNS activity. Therefore, the underlying mechanism through which increasing PSNS activity promotes sleep and results in physical and mental relaxation differs from that through which sleep medications modulate decreased SNS activity and induce sleep duration. The PSNS is predominantly regulated by the vagus nerve and can inhibit muscle activity, reducing the occurrence of PLMD and RBD during phasic REM sleep [
11,
13,
23,
41].
Sleep medications commonly used to promote sleep include antidepressants, antipsychotics, benzodiazepines, and acetylcholinesterase inhibitors [
20,
36,
42]. These drugs affect ANS regulation by exerting neuroleptic effects and blocking SNS receptors. Antipsychotics with strong anticholinergic properties are associated with a reduction in SNS activity [
43,
44]. However, the use of sleep medications is primarily focused on suppressing SNS activity at night. This disruption disrupts ANS regulation and increases cardiovascular risk. In individuals with AD's, PSNS activity peaks later, exhibits a phase delay, and shows an overall decrease. Therefore, the underlying mechanism of increasing PSNS activity to promote sleep and achieve physical and mental relaxation differs from that of sleep medications to modulate SNS activity and induce sleep.
3.3. Aging-related PSNS Activity Decrement
ANS regulation constantly adjusts the pacemaker rate to regulate sleep-wake cycles and circadian rhythms and maintains homeostasis during the integration of neuroendocrine systems [
34]. We found that sleep duration had a greater beneficial effect on nocturnal ANS regulation. Although moderate overall functional impairment with sufficient sleep duration may still be associated with better ANS regulation results, as a recommendation, maintaining sufficient sleep duration may play a critical role in maintaining optimal ANS regulation. Aging leads to an overall decrease in PSNS activity. PSNS activity decreases with age, particularly in individuals with AD. Nighttime PSNS activity is the key predictive factor for sleep-dependent cognitive function [
10].
This study found that aging decreased PSNS activity by 14.8%; furthermore, the association of dementia severity with PSNS activity was significant and exhibited an inverted U shape. Therefore, we presume that sufficient sleep duration may delay the age-related decline in PSNS activity.
3.4. The Feasibility of this Study
The success rate of wearing an HRV device was 45%. The subjects felt that the device felt like a foreign body when worn on the chest, and it was easy to remove it by themselves. It was necessary to gain the subjects' trust through game interaction and wear the game for many days. To increase adaptability, wearable devices for monitoring HRVs should be improved in the future to include wrist and wrist monitoring.
We found that longer sleep durations were associated with more severe impairment of ANS regulation, suggesting that the possible reasons are as follows: (1) excessive use of sleeping drugs, leading to drowsiness; (2) poor physical fitness, requiring more time to rest; and (3) severe dementia.
3.5. Limitations
This study had several limitations. First, we used a cross-sectional convenience sampling design, which may affect the generalizability of the study results. However, it can still be used to explain specific hospitalized young-old and old-older patients. Second, this study involved longitudinal and continuous monitoring of nighttime HRV in a specific group of participants with probable AD. However, the results are limited by the difficulty in obtaining participants and the relatively small sample size, which slightly affects the accuracy and reliability of the results. However, this high homogeneity among samples improves the sensitivity of statistical tests. Third, only participants with probable AD were included, but the applicability of the findings to patients with other types of dementia can also be used as a reference. Fourth, duration data were collected using objective structured sleep diaries rather than recorded actigraphy data. However, using structured sleep diaries as a research tool is helpful for convenience and practicality in specific groups. Fifth, many interfering factors affect ANS regulation, and it is difficult to comprehensively consider them; this study did not include variables, including OSA, hypertension, RLS, PLMD, or RBD, that affect ANS regulation; these variables were not controlled for. In addition, the design of this study controlled for age, sex, dementia severity, and medication (antipsychotics) use as covariate variables. Finally, in this study, dementia severity was assessed by direct scoring of the CDR as a secondary medical record. The CDR is a multifaceted assessment tool that includes 6 aspects and is evaluated by professional psychologists, doctors, and nurses; therefore, it is still highly objective.
4. Materials and Methods
4.1. Ethics Statement
The research protocol (TCHIRB-10705110) was reviewed and approved by the institutional review board. Prior to participation, both the participants and their caregivers provided written informed consent.
4.2. Study Design
This cross-sectional pilot study was conducted between 2018 and 2020 among 34 patients with plausible ADs hospitalized at a psychiatric center in Taipei, Taiwan. Dementia of the AD type requires a history of cognitive decline and impairment in activities of daily living, confirmed by a neurologist and documented in the medical record. Participants were selected using a cross-sectional convenience sampling design. The primary objective was to measure HRV to quantify ANS regulation during the night. Participants' behavioral and psychological symptoms of dementia typically led to caregiver burden and hospitalization, with an average length of stay of 40 days. Behavioral and psychological symptoms were relatively stable after 14 days of hospitalization. Participants showed no aggressive or disruptive behavior, and they were still in bed at night, which prevents them from removing and destroying the equipment at night (according to the participants' caregivers, who insisted on being with them during the experiment).
To acclimate participants to the feeling of the HRV device on their body, it was recommended that they try it on early between 5:00 p.m. and 6:00 p.m. for 1-5 days until they were willing and able to complete 1 day of 9 hours (9:00 p.m. to 6:00 a.m.) of HRV measurements with the wearable device.
Participants' bedtimes were based on hospital routines, with bedtime medications administered between 8:00 p.m. and 8:30 p.m. and lights turned off at 9:00 p.m. with participants lying in bed. Throughout the night, psychiatric nurses observed eye movement, depth of breathing, and frequency of body movements to determine the difference between falling asleep and waking up. Sleep duration refers to the total amount of sleep obtained during the period between falling asleep and waking up, excluding all periods of wakefulness. Participants were divided into five groups based on their reported sleep duration: < 6 h, 6-7 h, 7-8 h, 8-9 h, and > 9 h. Dementia severity was assessed by direct scoring of the CDR as a secondary medical record and was categorized into four levels: very mild, mild, moderate, and severe. Participants were divided into two age groups: young-old (60–75 y) and old-older (76–90 y). Participants were also divided into two groups based on sleep duration: inadequate sleep duration (< 6 h and > 9 h) and sufficient sleep duration (6-9 h).
4.3. Participants
The required sample size was calculated using G Power 3.1 computer software, which yielded an estimate of 20 participants. The data were analyzed using analysis of variance (ANOVA). Statistical significance was indicated by α = 0.05 and β = 0.2 with an effect size = 0.3 [
45]. F tests and repeated-measures ANOVA were used to examine within-between interactions. The starting sample size was 35 participants across five groups.
Sleep duration, measured from sleep onset to wake onset (09:00 p.m. to 06:00 a.m.), was quantified for each night using sleep diaries. The mean sleep duration over the 5-day observation period was determined for each participant. For the final analysis, one day with at least 9 hours (09:00 p.m. until 6:00 a.m.) of HRV measurements was required to assess ANS regulation at night.
The inclusion criteria for participants were as follows: (1) met the standard clinical criteria for probable AD according to the International Classification of Diseases, 10
th Revision, Clinical Modification [
46]; (2) were acutely hospitalized with probable AD; (3) were aged ≥ 60 years; (4) voluntarily participated in the study (both participants and their caregivers); and (5) were willing to use an HRV instrument at the end of the study to complete 5 days of 9-h HRV measurements. The exclusion criteria were as follows: (1) the presence of a pacemaker or a medical history of arrhythmia [
47], as reported by caregivers and recorded in medical records; and (2) other diseases that affect sleep, such as delirium, RLS, fever, and unstable vital signs.
Among the 34 eligible participants, 7 dropped out of the study because they were unable to complete 1 day of 9-h HRV measurements. Consequently, only 27 participants successfully completed the study.
4.4. Instruments and Outcome Measures
The primary objective was to assess the effect of sleep duration on nocturnal ANS performance and dementia severity.
We determined the primary outcome measures, including the mean values of the trial variables, such as HF power, LF power, and the LF/HF ratio, from the HRV data. PSNS activity was indicated by the HF component, SNS activity was indicated by LF%, and sympathovagal balance was indicated by the LF/HF ratio, reflecting the modulation of SNS activity. Dementia Severity was the secondary outcome, as assessed by the Washington University Clinical Dementia Rating (CDR) score. The CDR rates an individual’s dementia severity in six domains: memory, orientation, judgment and problem solving, community affairs, home, and hobbies. The CDR score indicated very mild (0.5), mild (1), moderate (2), or severe (3) dementia [
48].
4.4.1. Validity and Reliability of the HRV Data
We evaluated one component each in the LF (0.04–0.15 Hz) and HF (0.15–0.40 Hz) ranges. The LF/HF ratio is negatively correlated with delta power (0.5–4.0 Hz), which is measured by electroencephalography during quiet sleep, whereas cardiac sympathovagal balance is negatively correlated with sleep depth [
10,
49,
50].
The HRV device (WG-103A, Taipei, Taiwan) was positioned over the sinus node for continuous recording during overnight bed rest (9:00 p.m. to 6:00 a.m.) for 1 day using the KY laboratory software package (
http://xenon2.ym.edu.tw/hrv/). We adjusted the HRV parameters for confounders, system settings, and statistical settings and excluded extreme values. The real-time signal was transmitted to a smartphone using Xenon Bluetooth Low Energy as a low-cost and high-efficiency measurement tool. HRV data were sent to the respective sensor and mobile device to achieve high validity and reliability.
4.4.2. Validity and Reliability of Sleep Duration Data
The sleep diary is a standard assessment instrument used in psychiatric hospital routines that allows for prospective monitoring of sleep patterns in the hospital. The variables derived from sleep diaries included sleep-onset latency and wake time after sleep onset. Sleep duration refers to the total amount of sleep obtained, the period between sleep-on and sleep-off, excluding all the wake stages [
39].
Each night, two psychiatric nurses took turns visiting the participants every 30 min from 9:00 p.m. to 6:00 a.m. to complete the sleep diaries. The sleep diaries were based on observations of voluntary eye opening, breathing depth, and limb movement frequency. Each psychiatric professional received training prior to performing the sleep diary observation technique. The psychiatric nurses completed the sleep diaries with 80% consistency and accuracy.
4.5. Statistical Analyses
The demographic characteristics are summarized as frequencies and percentages (N, %) for categorical variables and means for continuous variables. The demographic characteristics were compared between the five sleep duration groups using the chi-square test. We used repeated-measures generalized linear models (GLMs) to examine the correlations of sleep duration and ANS regulation with age, sex, dementia severity, and medication (antipsychotics) as covariates, as well as correlations of dementia severity and ANS regulation with age, sex, and medication (antipsychotics) as covariates. Analyses were performed using SPSS 24 software, and a p value < 0.05 indicated statistical significance. We examined the effects of sleep duration on ANS regulation by using the indicators of SNS activity, sympathovagal balance (LF% and LF/HF ratio) and PSNS activity (HF power component from HRV data). We also analyzed the main effects of different sleep durations (6–7, 7–8, 8–9, > 9, and < 6 h) on ANS regulation; the main effects of age (young-old compared with oldest-old) on ANS regulation; and the effects of sleep quality (inadequate sleep duration compared with sufficient sleep duration) on ANS dysregulation.
5. Conclusions
This pilot study demonstrated that a sleep duration of 7–8 h per night was associated with better ANS regulation than other sleep durations. However, there was evidence of inadequate sleep duration, concurrent aging was not significantly associated with ANS dysregulation, and insufficient sleep duration was characterized by increased SNS activity. Specifically, longer sleep duration was associated with more severe impairment of ANS regulation than was shorter sleep duration. An inverted U-shaped relationship was observed between sleep duration and ANS regulation.
This study showed that the association between dementia severity and PSNS activity exhibited an inverted U shape and that aging decreased PSNS activity. Specifically, the combination of moderate overall functional impairment and a sleep duration of 7–8 hours led to better ANS regulation results. As a recommendation, maintaining a sleep duration of 7–8 hours per night may play a critical role in maintaining optimal ANS regulation; however, the present study involved a few participants. Additional research with a larger cohort is needed to confirm these findings. The recommendation to maintain a sleep duration of 7–8 hours per night may guide the adjustment of sleep medications or interventions in older individuals with probable AD.
Author Contributions
All the authors conceived and contributed to the design of the experiments and the writing of the manuscript and approved it in its final form. All the authors contributed equally to this work and share first authorship. All the authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE ((TCHIRB-10705110) ).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are accessible from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Ya-Hui Shih for her assistance with the data collection as well as all the study participants and their caregivers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Global status report on the public health response to dementia. World Health Organization 2021.
- Zhong, G.; Naismith, S.L.; Rogers, N.L.; Lewis, S.J. Sleep-wake disturbances in common neurodegenerative diseases: A closer look at selected aspects of the neural circuitry. J Neurol. Sci 2011, 307, 9–14. [Google Scholar] [CrossRef]
- Liu, C.R.; Liou, Y.M.; Jou, J.H. Ambient bright lighting in the morning improves sleep disturbances of older adults with dementia. Sleep Med 2022, 89, 1–9. [Google Scholar] [CrossRef]
- Wong, R.; Lovier, M.A. Sleep disturbances and dementia risk in older adults: Findings from 10 years of national U.S. prospective data. Am. J. Prev Med 2023, 64, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.R.; Liou, Y.M.; Jou, J.H. Pilot study of the effects of bright ambient therapy on dementia symptoms and cognitive function. Front Physiol 2021, 12, 782160. [Google Scholar] [CrossRef]
- Van Erum, J.; Van Dam, D.; De Deyn, P.P. Alzheimer's disease: Neurotransmitters of the sleep-wake cycle. Neurosci Biobehav Rev 2019, 105, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Beishon, L.C.; Hosford, P.; Gurung, D.; Brassard, P.; Minhas, J.S.; Robinson, T.G.; et al. The role of the autonomic nervous system in cerebral blood flow regulation in dementia: A review. Auton Neurosci 2022, 240, 102985. [Google Scholar] [CrossRef]
- Chen, P.C.; Simon, K.C.; Sattari, N.; Whitehurst, L.N.; Mednick, S.C. Autonomic central coupling during daytime sleep differs between older and younger people. Neurobiol Learn Mem 2022, 193, 107646. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Wang, Y.; Gao, Z.; Wang, J.; Liu, X.; Pang, G. Effects of Alzheimer's disease of varying severity on cardiac and autonomic function. Braz J Med Biol Res 2022, 55, e11504. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.R.; Kuo, T.B.J.; Jou, J.H.; Lai, C.L.; Chang, Y.K.; Liou, Y.M. Bright morning lighting enhancing parasympathetic activity at night: A pilot study on elderly female patients with dementia without a pacemaker. Healthcare (Basel) 2023, 11, 793. [Google Scholar] [CrossRef]
- Carroll, J.E.; Prather, A.A. Sleep and biological aging: A short review. Curr Opin Endocr Metab Res 2021, 18, 159–164. [Google Scholar] [CrossRef]
- Choi, J.; Cha, W.; Park, M.G. Declining trends of heart rate variability according to aging in healthy Asian adults. Front Aging Neurosci 2020, 12, 610626. [Google Scholar] [CrossRef]
- Choi, S.; Baudot, M.; Vivas, O.; Moreno, C.M. Slowing down as we age: Aging of the cardiac pacemaker's neural control. Geroscience 2022, 44, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yang, N.; Dai, J.; Zhao, Y.; Zhang, X.; Yin, J.; et al. Association of sleep duration with all-cause and cardiovascular mortality: A prospective cohort study. Front Public Health 2022, 10, 880276. [Google Scholar] [CrossRef]
- Leng, Y.; Yaffe, K. Sleep duration and cognitive aging-beyond a U-shaped association. JAMA Netw Open 2020, 3, e2014008. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, D.; Huang, Y.; Chen, Z.; Wang, R.; Dong, Q.; et al. Sleep duration and health outcomes: an umbrella review. Sleep & Breathing 2022, 26, 1479–1501. [Google Scholar]
- Lucey, B.P.; Wisch, J.; Boerwinkle, A.H.; Landsness, E.C.; Toedebusch, C.D.; McLeland, J.S.; et al. Sleep and longitudinal cognitive performance in preclinical and early symptomatic Alzheimer's disease. Brain 2021, 144, 2852–2862. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, L.; Zheng, F.; Shi, L.; Zhong, B.; Xie, W. Association between sleep duration and cognitive decline. JAMA Netw Open 2020, 3, e2013573. [Google Scholar] [CrossRef] [PubMed]
- Pak, V.M.; Paul, S.; Swieboda, D.; Balthazar, M.S.; Wharton, W. Sleep duration and biomarkers of inflammation in African American and white participants with a parental history of Alzheimer's disease. Alzheimer's Dement 2022, 8, e12332. [Google Scholar] [CrossRef]
- Salami, O.; Lyketsos, C.; Rao, V. Treatment of sleep disturbance in Alzheimer's dementia. Int J Geriatr. Psychiatry 2011, 26, 771–782. [Google Scholar] [CrossRef]
- Forte, G.; Favieri, F.; Casagrande, M. Heart rate variability and cognitive function: A systematic review. Front Neurosci 2019, 13, 710. [Google Scholar] [CrossRef]
- Wang, C.; Holtzman, D.M. Bidirectional relationship between sleep and Alzheimer's disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology 2020, 45, 104–120. [Google Scholar] [CrossRef]
- Kim, H.; Jung, H.R.; Kim, J.B.; Kim, D.J. Autonomic dysfunction in sleep disorders: From neurobiological basis to potential therapeutic approaches. J Clin Neurol (Seoul, Korea) 2022, 18, 140–151. [Google Scholar] [CrossRef]
- Chen, W.C.; Wang, X.Y. Longitudinal associations between sleep duration and cognitive impairment in Chinese elderly. Front Aging Neurosci 2022, 2022 14, 1037650. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Q.; Wang, M.; Wang, P.; Shen, N. Prospective association between sleep duration and cognitive impairment: Findings from the China Health and Retirement Longitudinal Study (CHARLS). Front Med (Lausanne) 2022, 9, 971510. [Google Scholar] [CrossRef]
- Tao, R.; Mi, B.; Hu, Y.; Lin, S.; Xiong, Y.; Lu, X.; et al. Hallmarks of peripheral nerve function in bone regeneration. Bone Res 2023, 11, 6. [Google Scholar] [CrossRef]
- Spiegelhalder, K.; Fuchs, L.; Ladwig, J.; Kyle, S.D.; Nissen, C.; Voderholzer, U.; et al. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res 2011, 20, 137–145. [Google Scholar] [CrossRef]
- Yang, H.; Goldstein, M.R.; Vazquez, M.; Williams, J.P.; Mullington, J.M. Effects of sleep and sleep deficiency on autonomic function in humans. Curr Opin Endocr Metab Res 2021, 18, 268–274. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Huang, Y.C.; Huang, W.L. Heart rate variability in patients with dementia or neurocognitive disorders: A systematic review and meta-analysis. Aust N Z J Psychiatry 2022, 56, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Mikail, N.; Bengs, S.; Haider, A.; Treyer, V.; Buechel, R.R.; et al. Heart-brain interactions in cardiac and brain diseases: Why sex matters. Eur Heart J 2022, 43, 3971–3980. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, P.; Mari, D.; Abbate, C.; Inglese, S.; Bertagnoli, L.; Tomasini, E.; et al. Autonomic function in amnestic and non-amnestic mild cognitive impairment: Spectral heart rate variability analysis provides evidence for a brain-heart axis. Sci Rep 2020, 10, 11661. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; He, F.; Calhoun, S.L.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Objective short sleep duration increases the risk of all-cause mortality associated with possible vascular cognitive impairment. Sleep Health 2020, 6, 71–78. [Google Scholar] [CrossRef]
- Li, M.; Wang, N.; Dupre, M.E. Association between the self-reported duration and quality of sleep and cognitive function among middle-aged and older adults in China. J Affect Disord 2022, 304, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, R.; Sawai, S.; Ogata, T.; Iimura, A. Autonomic dysfunction in older individuals: The contributions of multiple brain diseases and diabetes. Neurol Clin Neurosci 2022, 10, 198–209. [Google Scholar] [CrossRef]
- Voumvourakis, K.I.; Sideri, E.; Papadimitropoulos, G.N.; Tsantzali, I.; Hewlett, P.; Kitsos, D.; et al. The dynamic relationship between the glymphatic system, aging, memory, and sleep. Biomedicines 2023, 11, 2092. [Google Scholar] [CrossRef]
- Wang, C.; Bangdiwala, S.I.; Rangarajan, S.; Lear, S.A.; AlHabib, K.F.; Mohan, V.; et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116 632 people from 21 countries. Eur Heart J 2019, 40, 1620–1629. [Google Scholar] [CrossRef]
- Olivares, *!!! REPLACE !!!*; et al. Sleep dysregulation in sympathetic-mediated diseases: implications for disease progression. Sleep 2022, 45. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, P.; Yeh, W.H.; Dumont, G.A.; Boivin, D.B. Circadian variation of heart rate variability across sleep stages. Sleep 2013, 36, 1919–1928. [Google Scholar] [CrossRef]
- Jarrin, D.C.; Ivers, H.; Lamy, M.; Chen, I.Y.; Harvey, A.G.; Morin, C.M. Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J Sleep Res 2018, 27, e12663. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chang, M.C.; Litrownik, D.; Wayne, P.M.; Yeh, G.Y. Day-night patterns in heart rate variability and complexity: differences with age and cardiopulmonary disease. J Clin Sleep Med 2023, 19, 873–882. [Google Scholar] [CrossRef]
- Kong, S.D.X.; Hoyos, C.M.; Phillips, C.L.; McKinnon, A.C.; Lin, P.; Duffy, S.L.; et al. Altered heart rate variability during sleep in mild cognitive impairment. Sleep 2021, 44, zsaa232. [Google Scholar] [CrossRef] [PubMed]
- Camargos, E.F.; Pandolfi, M.B.; Freitas, M.P.; Quintas, J.L.; Jde, O.L.; Miranda, L.C.; et al. Trazodone for the treatment of sleep disorders in dementia: An open-label, observational and review study. Arquivos de Neuro-Psiquiatria 2011, 69, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Dailey, J.H.; Chowdhuri, S. Pharmacology of sleep. In Essentials of sleep medicine; Badr, M.S., Martin, J.L., Eds.; Springer, 2020; pp. 21–46. [Google Scholar] [CrossRef]
- Alvares, G.A.; Quintana, D.S.; Hickie, I.B.; Guastella, A.J. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. J Psychiatry Neurosci 2016, 41, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Kerkering, E.M.; Greenlund, I.M.; Bigalke, J.A.; Migliaccio, G.C.L.; Smoot, C.A.; Carter, J.R. Reliability of heart rate variability during stable and disrupted polysomnographic sleep. Am J Physiol Heart Circ Physiol 2022, 323, H16–h23. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S. ICD-10-CM diagnosis coding for neuropsychological assessment. Arch Clin Neuropsychol 2019, 34, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.D.; Hoyos, C.M.; Phillips, C.L.; McKinnon, A.C.; Palmer, J.R.; Duffy, S.L.; et al. Left amygdala volume moderates the relationship between nocturnal high-frequency heart rate variability and verbal memory retention in older adults with amnestic mild cognitive impairment: Biomarkers (non-neuroimaging)/novel biomarkers. Alzheimer's & Dementia 2020, 16, e044608. [Google Scholar]
- Huang, H.C.; Tseng, Y.M.; Chen, Y.C.; Chen, P.Y.; Chiu, H.Y. Diagnostic accuracy of the Clinical Dementia Rating Scale for detecting mild cognitive impairment and dementia: A bivariate meta-analysis. Int J Geriatr Psychiatry 2021, 36, 239–251. [Google Scholar] [CrossRef]
- Dalise, A.M.; Prestano, R.; Fasano, R.; Gambardella, A.; Barbieri, M.; Rizzo, M.R. Autonomic nervous system and cognitive impairment in older patients: Evidence from long-term heart rate variability in real-life setting. Front Aging Neurosci 2020, 12, 40. [Google Scholar] [CrossRef]
- Silva, L.E.V.; Moreira, H.T.; de Oliveira, M.M.; Cintra, L.S.S.; Salgado, H.C.; Fazan, R. Jr.; et al. Heart rate variability as a biomarker in patients with chronic chagas cardiomyopathy with or without concomitant digestive involvement and its relationship with the Rassi score. BioMed Eng Online 2022, 21, 44. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).