Submitted:
19 March 2024
Posted:
21 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
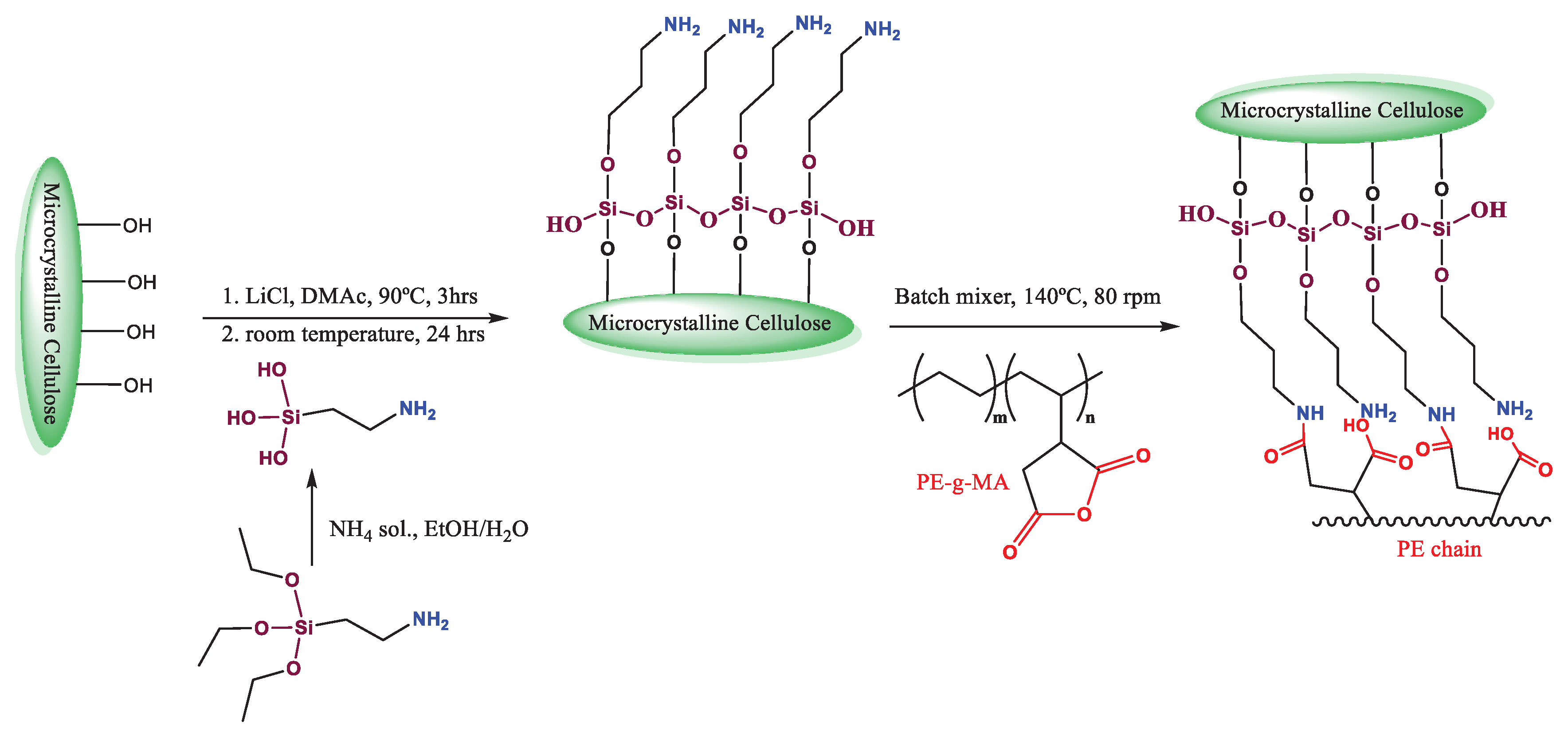
2.2.1. Microcrystalline Cellulose Modification with 3-Aminopropyltriethoxysilane Subsubsection
2.2.2. Microcomposites Preparation
2.3. Characterization
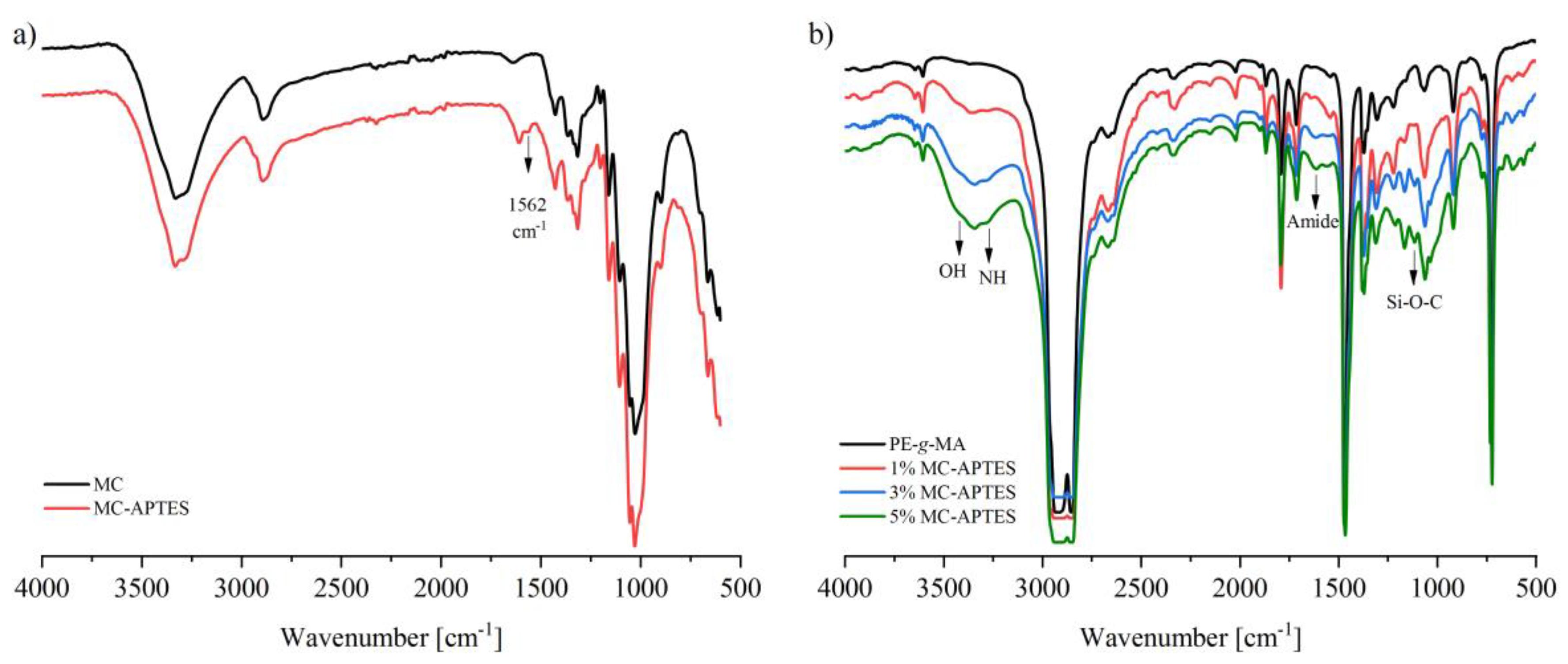
2.3.1. Fourier Transformed Infrared Analysis (FTIR)
2.3.2. Themal Analysis
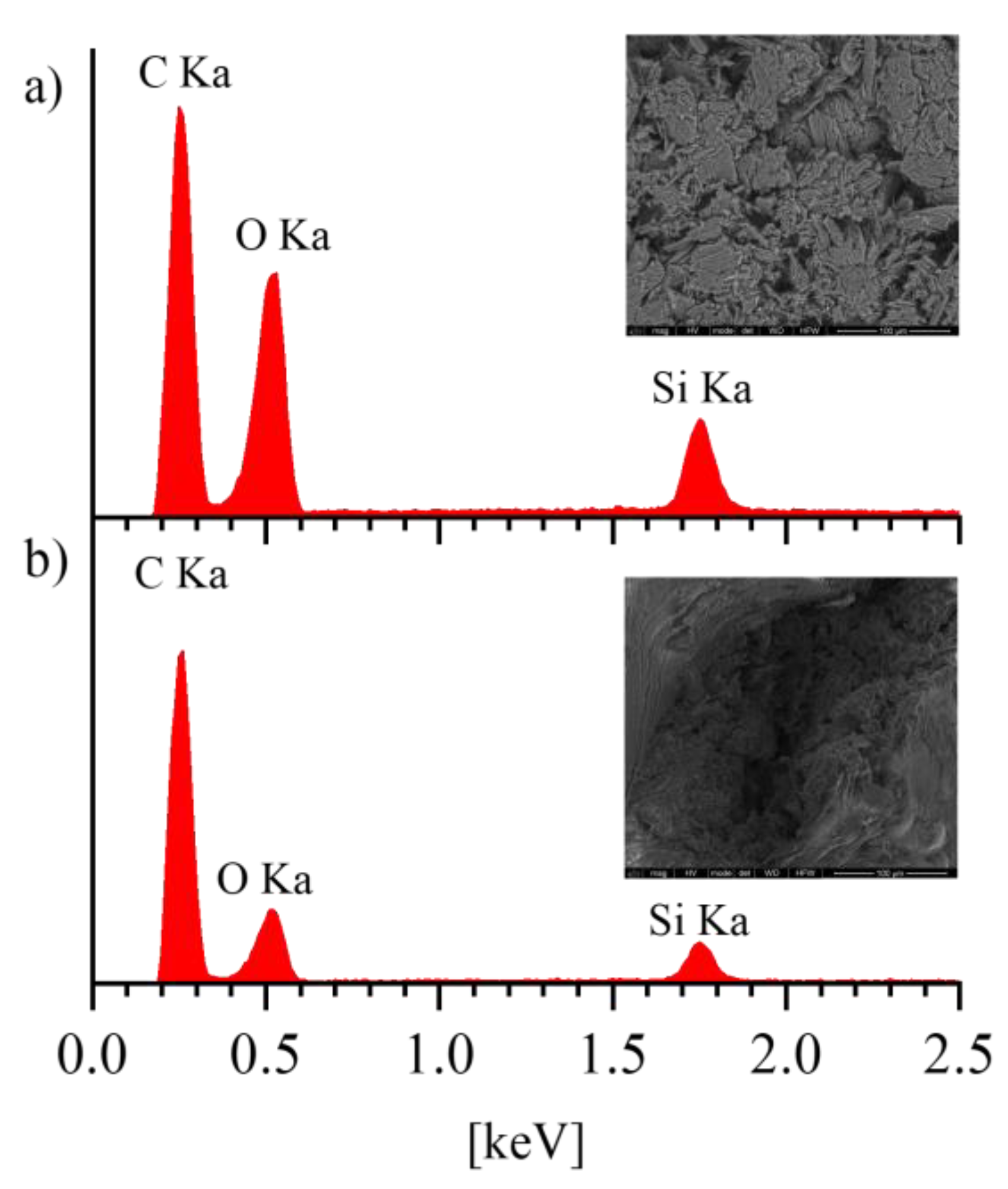
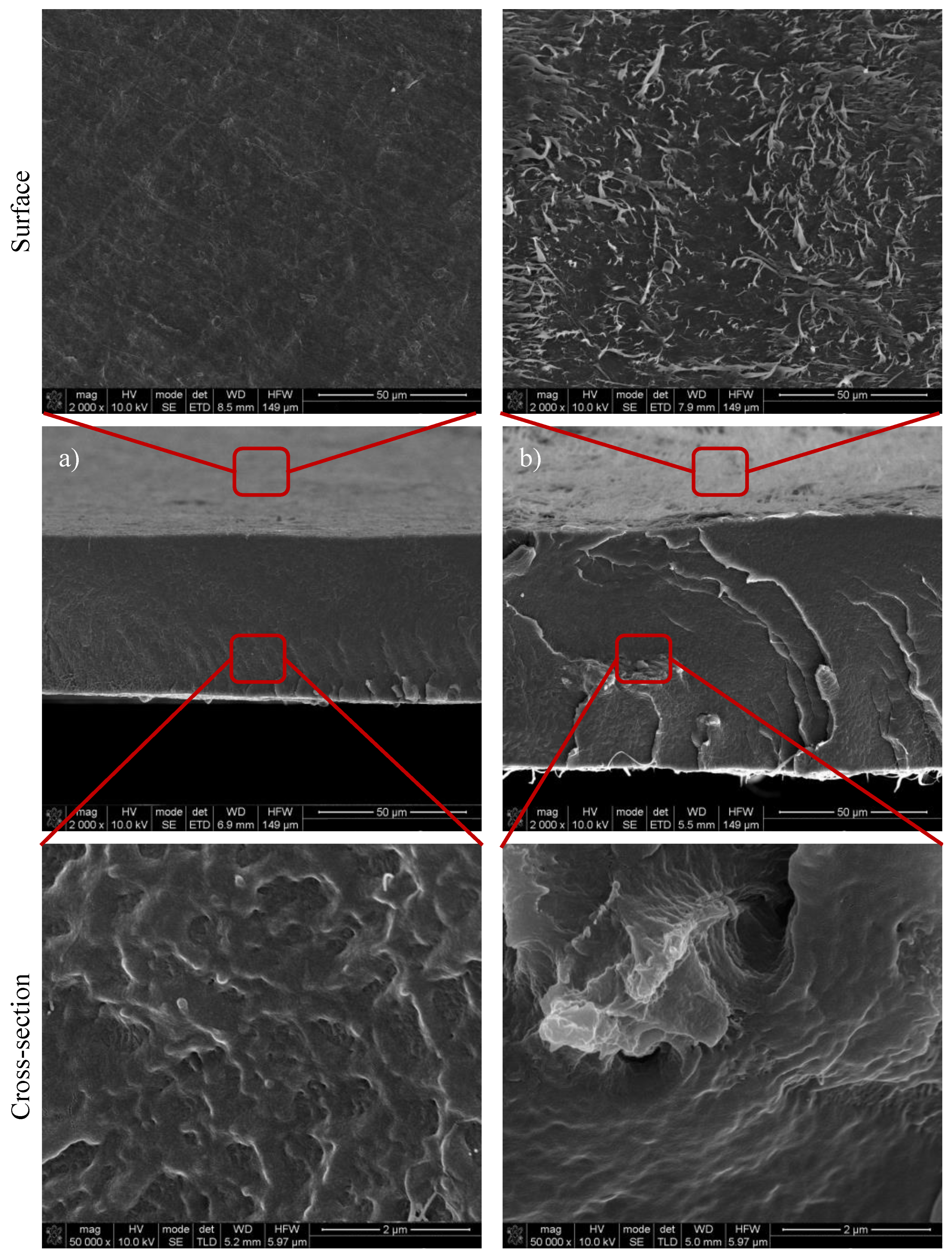
2.3.3. Scanning Electron Spectroscopy Analysis (SEM)
2.3.4. Dynamic Mechanical Analysis (DMA)
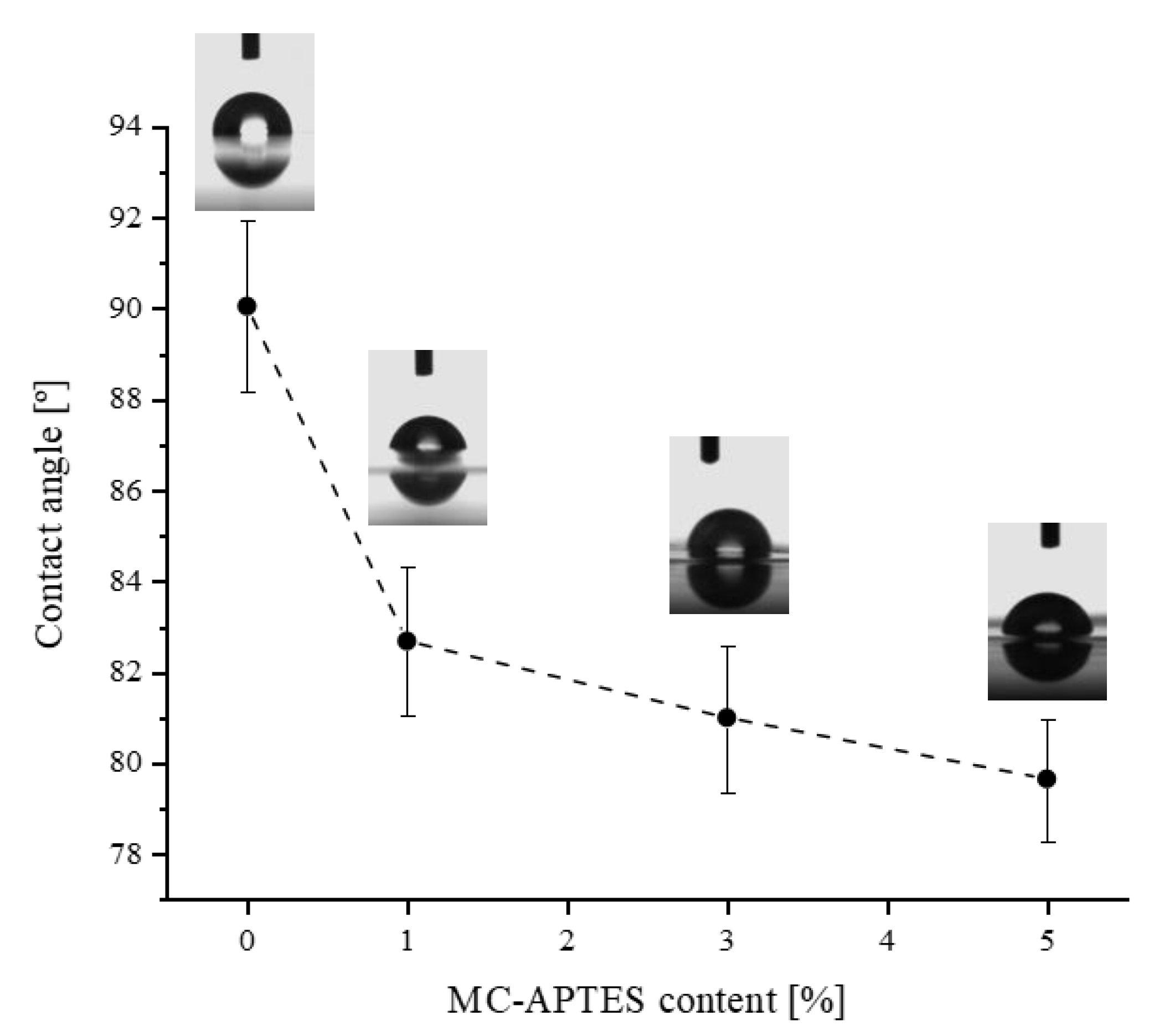
2.3.5. Contact Angle Measurements (CA)
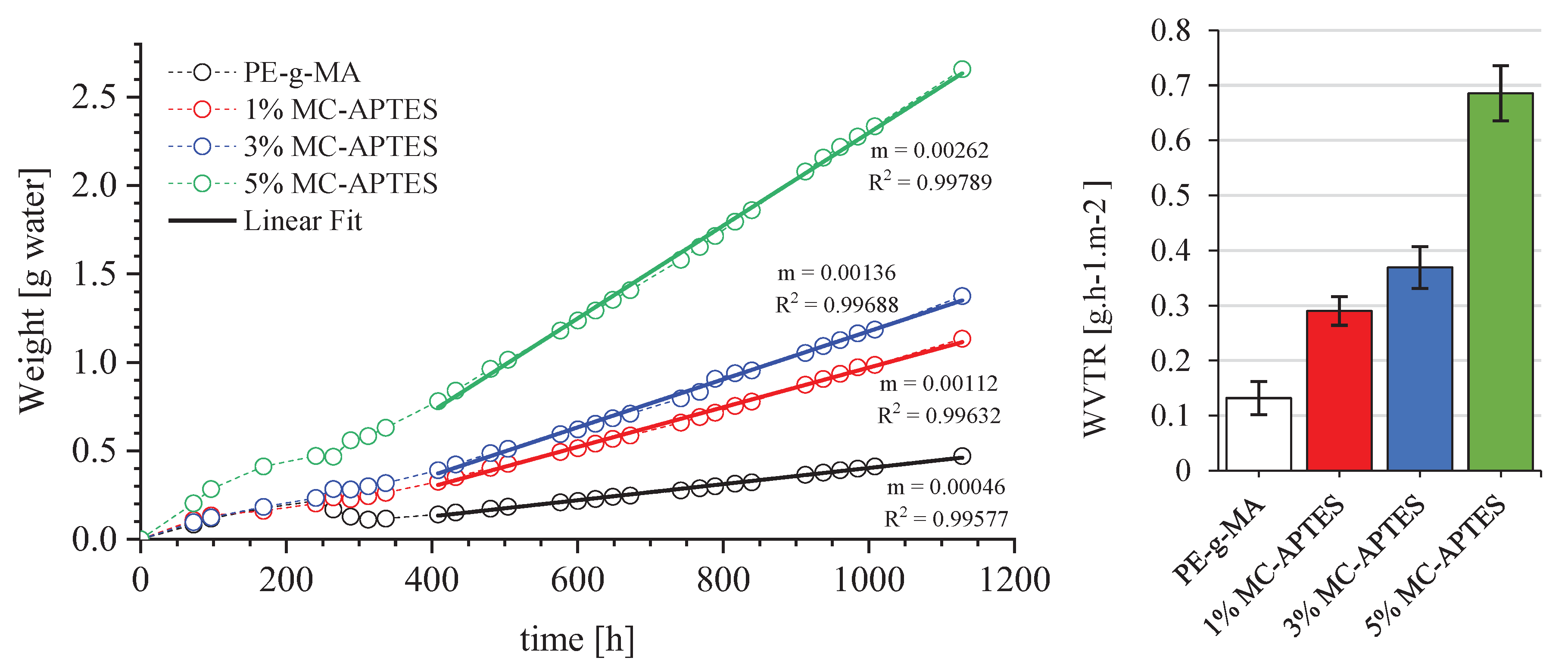
2.3.6. Water Vapor Permeability (WVP)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czerwiński, K.; Rydzkowski, T.; Wróblewska-Krepsztul, J.; Thakur, V.K. Towards Impact of Modified Atmosphere Packaging (MAP) on Shelf-Life of Polymer-Film-Packed Food Products: Challenges and Sustainable Developments. Coatings 2021, 11, 1504. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, F.A.-J.; Opara, U.L. Modified Atmosphere Packaging Technology of Fresh and Fresh-cut Produce and the Microbial Consequences—A Review. Food and Bioprocess Technology 2013, 6, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Hamad, W. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20. [Google Scholar] [CrossRef]
- Yano, H.; Omura, H.; Honma, Y.; Okumura, H.; Sano, H.; Nakatsubo, F. Designing cellulose nanofiber surface for high density polyethylene reinforcement. Cellulose 2018, 25. [Google Scholar] [CrossRef]
- Zimniewska, M.; Wladyka-Przybylak, M.; Mankowski, J. Cellulose Fibers: Bio- and Nano-Polymer Composites. 2011, 97-119.
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-reinforced polymer composites: Manufacturing, properties, and applications. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef]
- Mohit, H.; Selvan, V.A.M. A comprehensive review on surface modification, structure interface and bonding mechanism of plant cellulose fiber reinforced polymer based composites. Composite Interfaces 2018, 25, 629–667. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed]
- Abushammala, H.; Mao, J. A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules 2019, 24, 2782. [Google Scholar] [CrossRef]
- Tavakolian, M.; Jafari, S.; van de Ven, T. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nano-Micro Letters 2020, 12. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Composites Part A: Applied Science and Manufacturing 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Jayasuriya, C.K. Interfacial Bonding in Polymer–Ceramic Nanocomposites☆. in Reference Module in Materials Science and Materials Engineering: Elsevier, 2017.
- Khanjanzadeh, H.; et al. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. International Journal of Biological Macromolecules 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Jia, N.; Li, S.-M.; Ma, M.-G.; Zhu, J.; Sun, R.-C. Synthesis and characterization of cellulose-silica composite fiber in ethanol/water mixed solvents. Bioresources 2011, 6. [Google Scholar] [CrossRef]
- Tarani, E.; Arvanitidis, I.; Christofilos, D.; Bikiaris, D.N.; Chrissafis, K.; Vourlias, G. Calculation of the degree of crystallinity of HDPE/GNPs nanocomposites by using various experimental techniques: a comparative study. Journal of Materials Science 2023, 58, 1621–1639. [Google Scholar] [CrossRef]
- Mirabella, F.; Bafna, A. Determination of the crystallinity of polyethylene/?-olefin copolymers by thermal analysis: Relationship of the heat of fusion of 100% polyethylene crystal and the density. Journal of Polymer Science Part B: Polymer Physics 2002, 40, 1637–1643. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16-Standard Test Methods for Water Vapor Transmission of Materials, 2015.
- Kotov, N.; Raus, V.; Dybal, J. Intermolecular Interactions in N,N-Dimethylacetamide without and with LiCl Studied by Infrared Spectroscopy and Quantum Chemical Model Calculations. The Journal of Physical Chemistry B 2018, 122, 8921–8930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; et al. Characterization of Silane Treated and Untreated Natural Cellulosic Fibre from Corn Stalk Waste as Potential Reinforcement in Polymer Composites. Carbohydrate Polymers 2019, 218, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.; Oksman, K. The use of silane technology in crosslinking polyethylene/wood flour composites. Composites Part A: Applied Science and Manufacturing 2006, 37, 752–765. [Google Scholar] [CrossRef]
- Lomakin, S.M.; Rogovina, S.Z.; Grachev, A.V.; Prut, E.V.; Alexanyan, C.V. Thermal degradation of biodegradable blends of polyethylene with cellulose and ethylcellulose. Thermochimica Acta 2011, 521, 66–73. [Google Scholar] [CrossRef]
- Hubbe, M.A.; et al. Nanocellulose in Thin Films, Coatings, and Plies for Packaging Applications: A Review. 2017, Barrier properties; Water vapor transmission; Food shelf life; Oxygen transmission; Packages; Cellulose nanomaterials vol. 12, no. 1, p. 91, 2017-02-01 2017. [Online]. Available: https://ojs.cnr.ncsu.edu/index.php/BioRes/article/view/BioRes_12_1_2143_Hubbe_Review_Nanocellulose_Thin_Films_Coatings_Plies/5093.
- Dang, X.; Cao, X.; Ke, L.; Ma, Y.; An, J.; Wang, F. Combination of cellulose nanofibers and chain-end-functionalized polyethylene and their applications in nanocomposites. Journal of Applied Polymer Science 2017, 134, 45387. [Google Scholar] [CrossRef]
- Junior, O.G.D.S.; de Melo, R.P.; Sales, R.D.B.C.; Ayres, E.; Patricio, P.S.D.O. Processing and characterization of polyethylene/starch/curauá composites: Potential for application as thermal insulated coating. Journal of Building Engineering 2017, 11, 178–186. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. International Journal of Polymer Science 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Fotie, G.; Limbo, S.; Piergiovanni, L. Manufacturing of Food Packaging Based on Nanocellulose: Current Advances and Challenges. Nanomaterials 2020, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
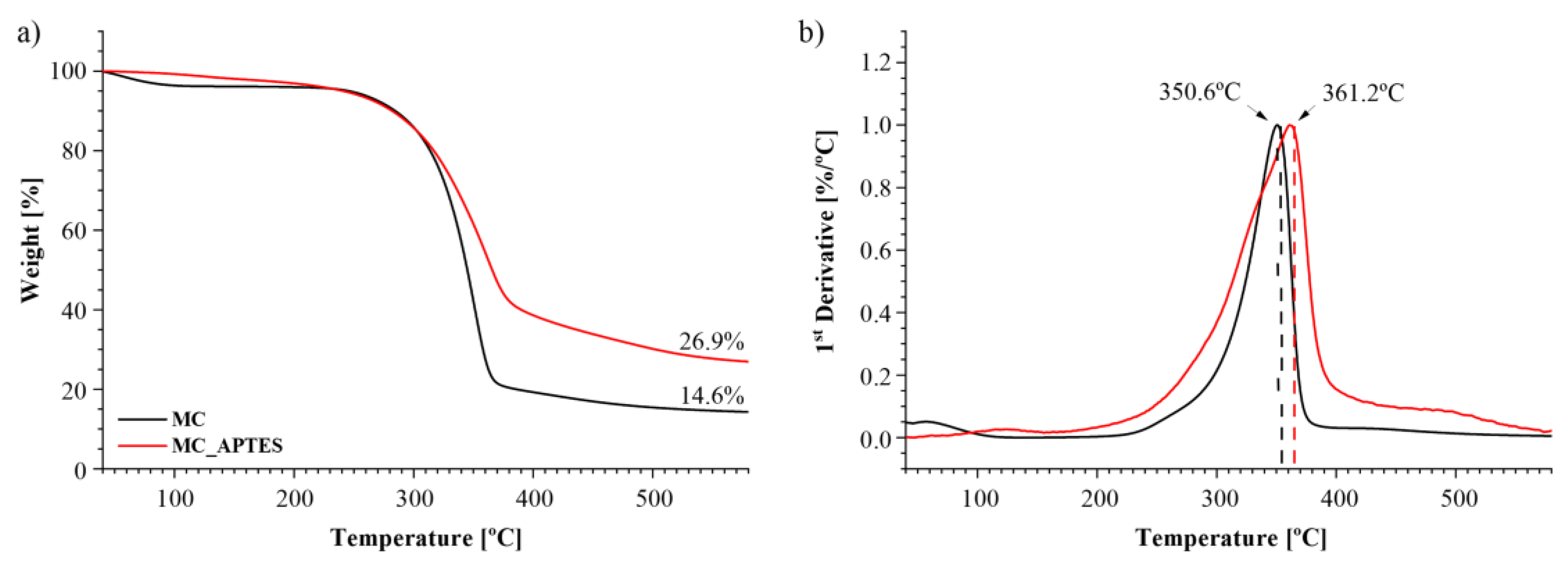
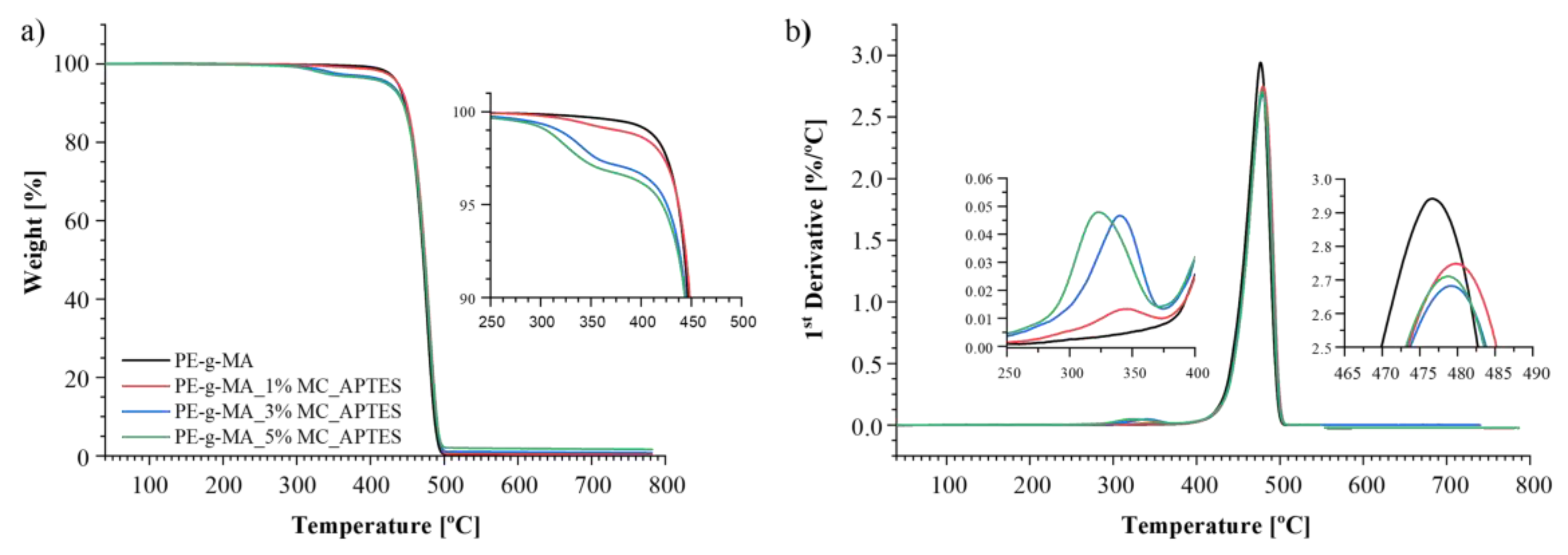
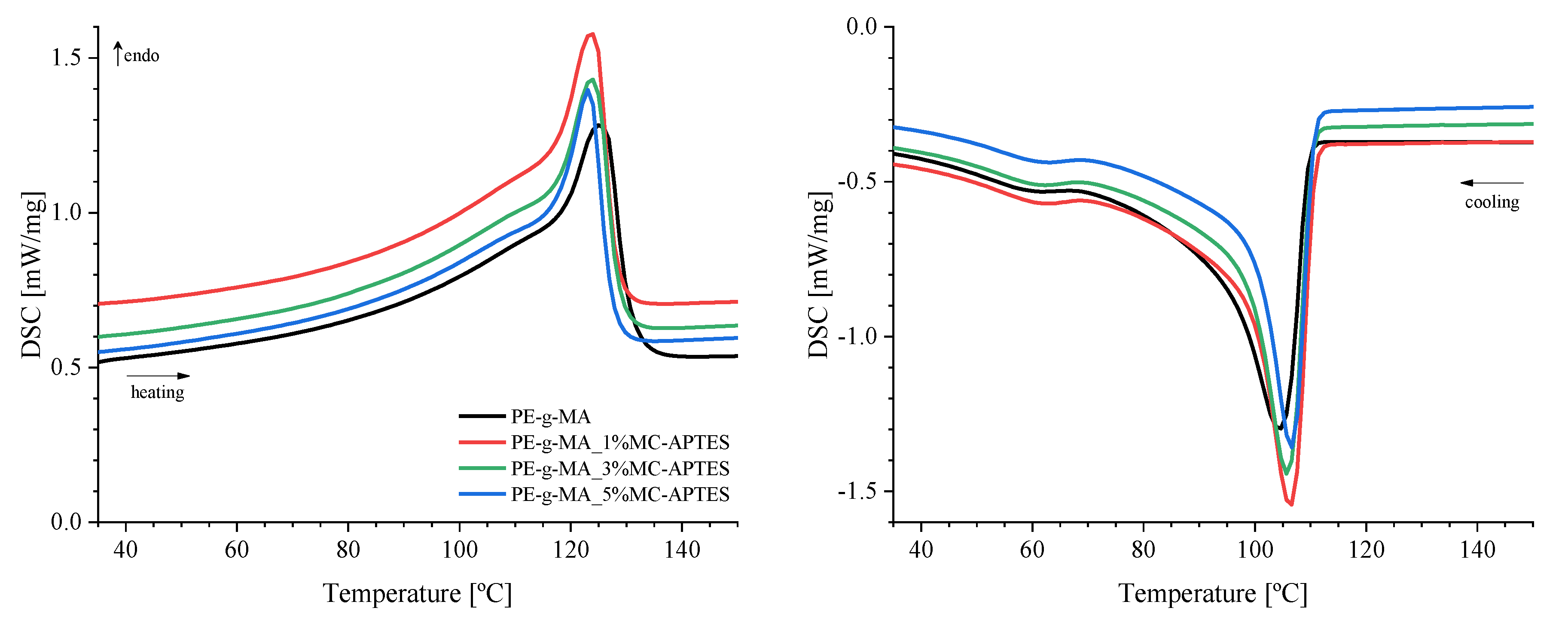
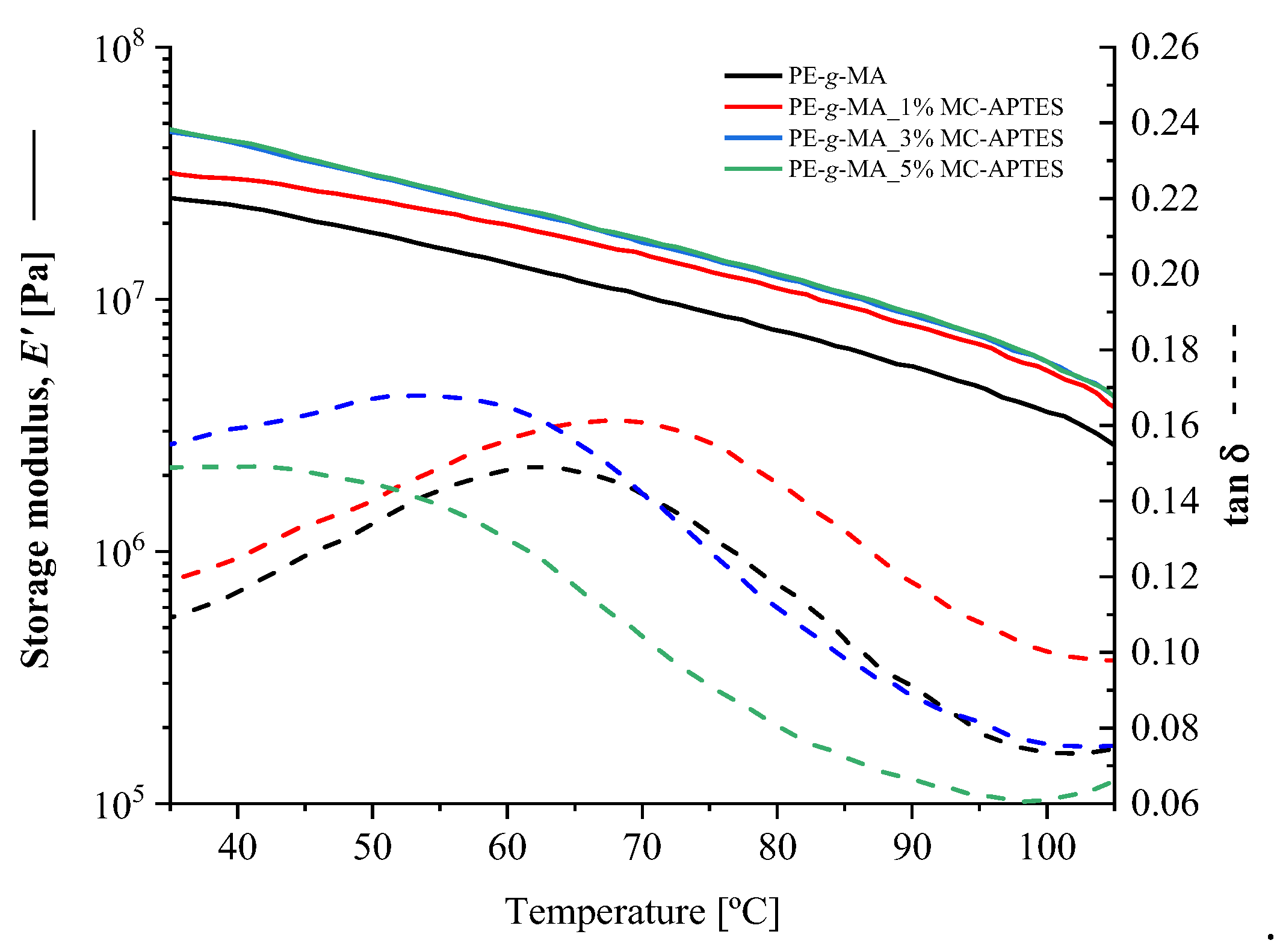
| Composition code | Weight (%) | Processing conditions | ||||
|---|---|---|---|---|---|---|
| PE-g-MA | MA-APTES | Tm (ºC) | Rotors speed (rpm) | tmixing (min) | ||
| PE-g-MA_1% MC-APTES | 99 | 1 | 140 | 80 | 2.5 mixing + 7 reaction | |
| PE-g-MA_3% MC-APTES | 97 | 3 | ||||
| PE-g-MA_5% MC-APTES | 95 | 5 | ||||
| Sample | ΔHm [J/g] | Χc [%] | Tm [ºC] | Tc [ºC] |
|---|---|---|---|---|
| PE-g-MA | 123.9 | 42.3 | 125 | 105 |
| 1% MC-APTES | 126.8 | 43.7 | 124 | 107 |
| 3% MC-APTES | 120.3 | 42.3 | 124 | 106 |
| 5% MC-APTES | 111.8 | 40.2 | 123 | 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





