Submitted:
18 March 2024
Posted:
18 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Electrode Preparation
2.3. D-XRF and XANES Setup
2.4. PXRD Setup
3. Results and Discussion
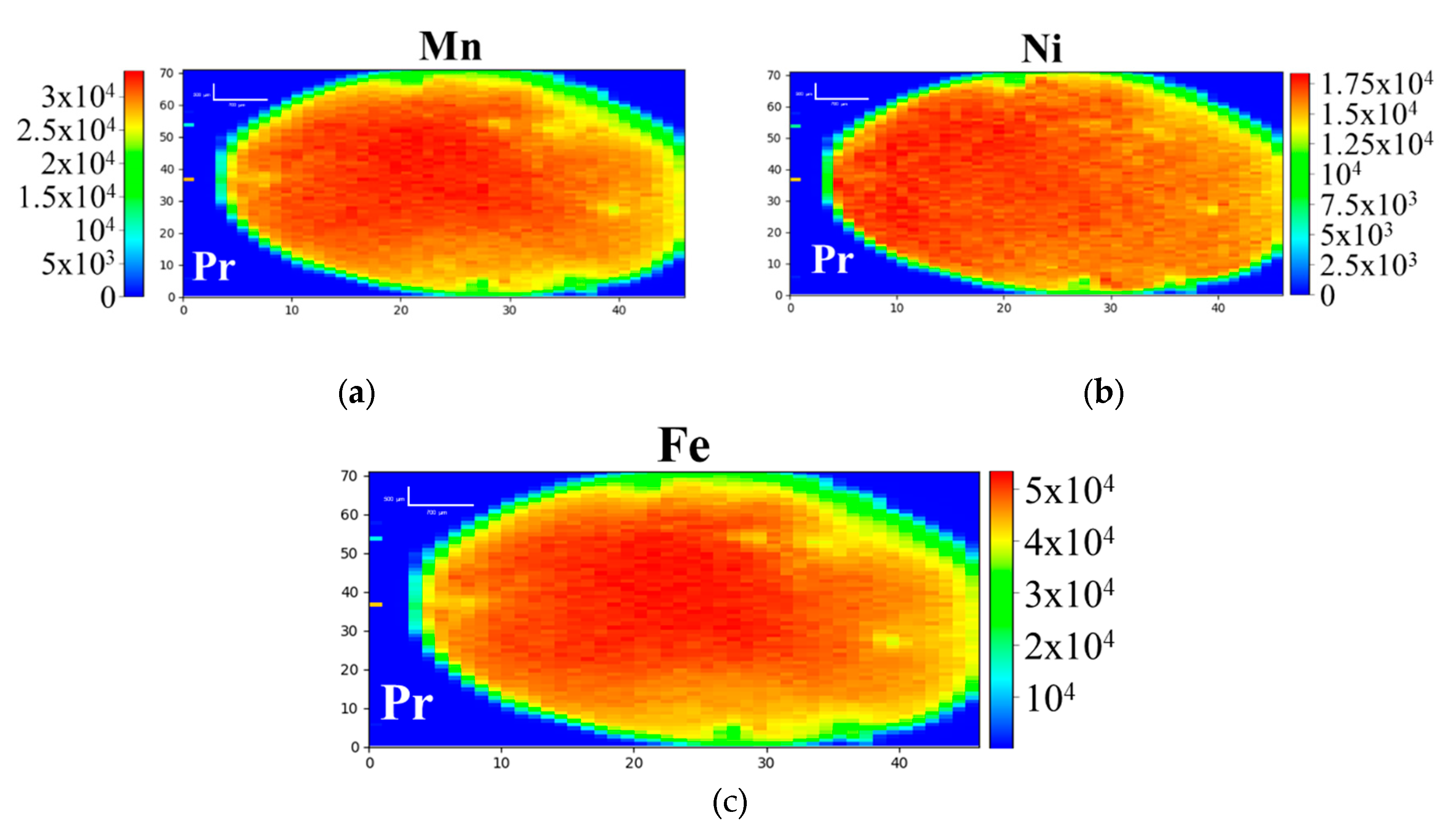
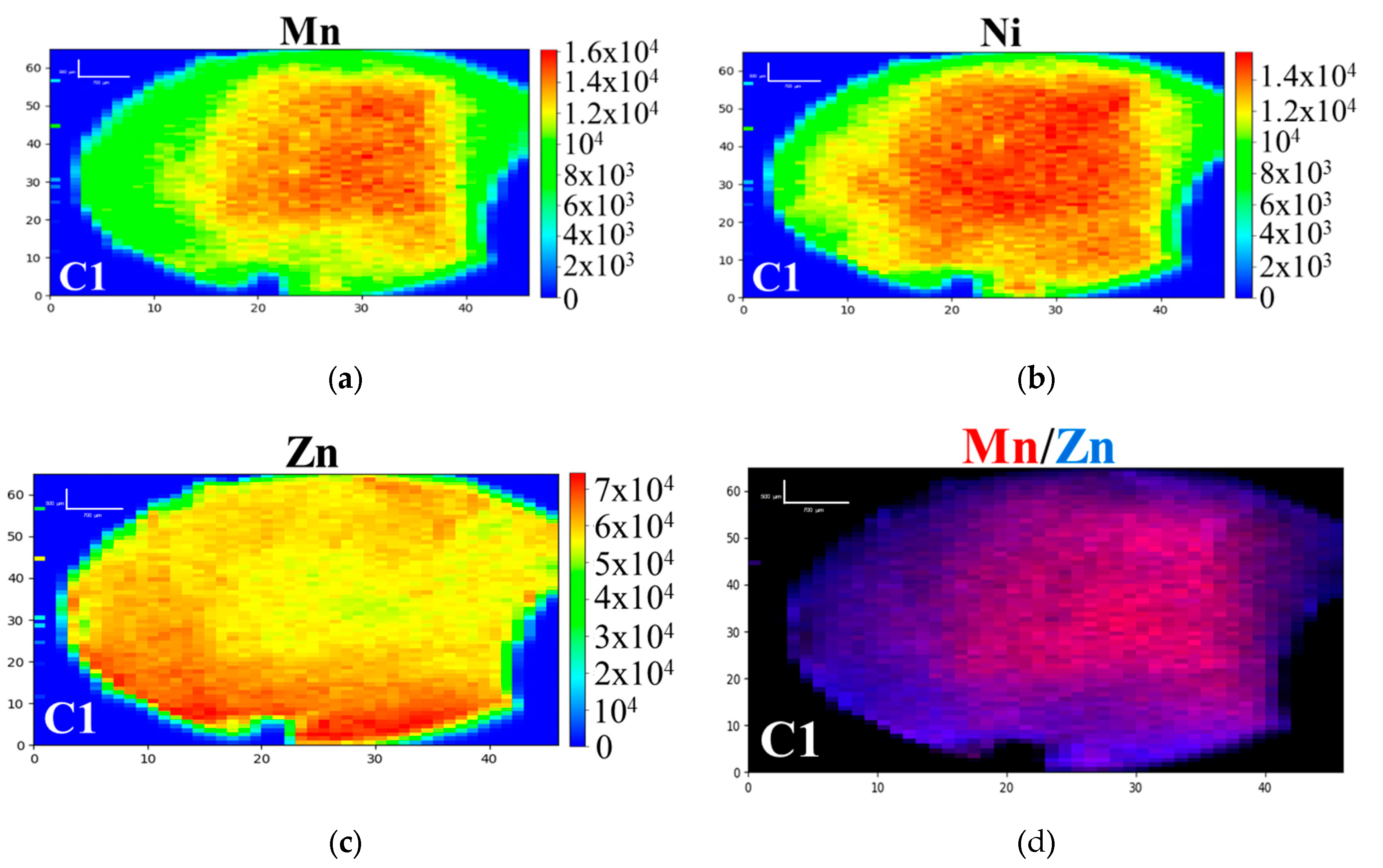
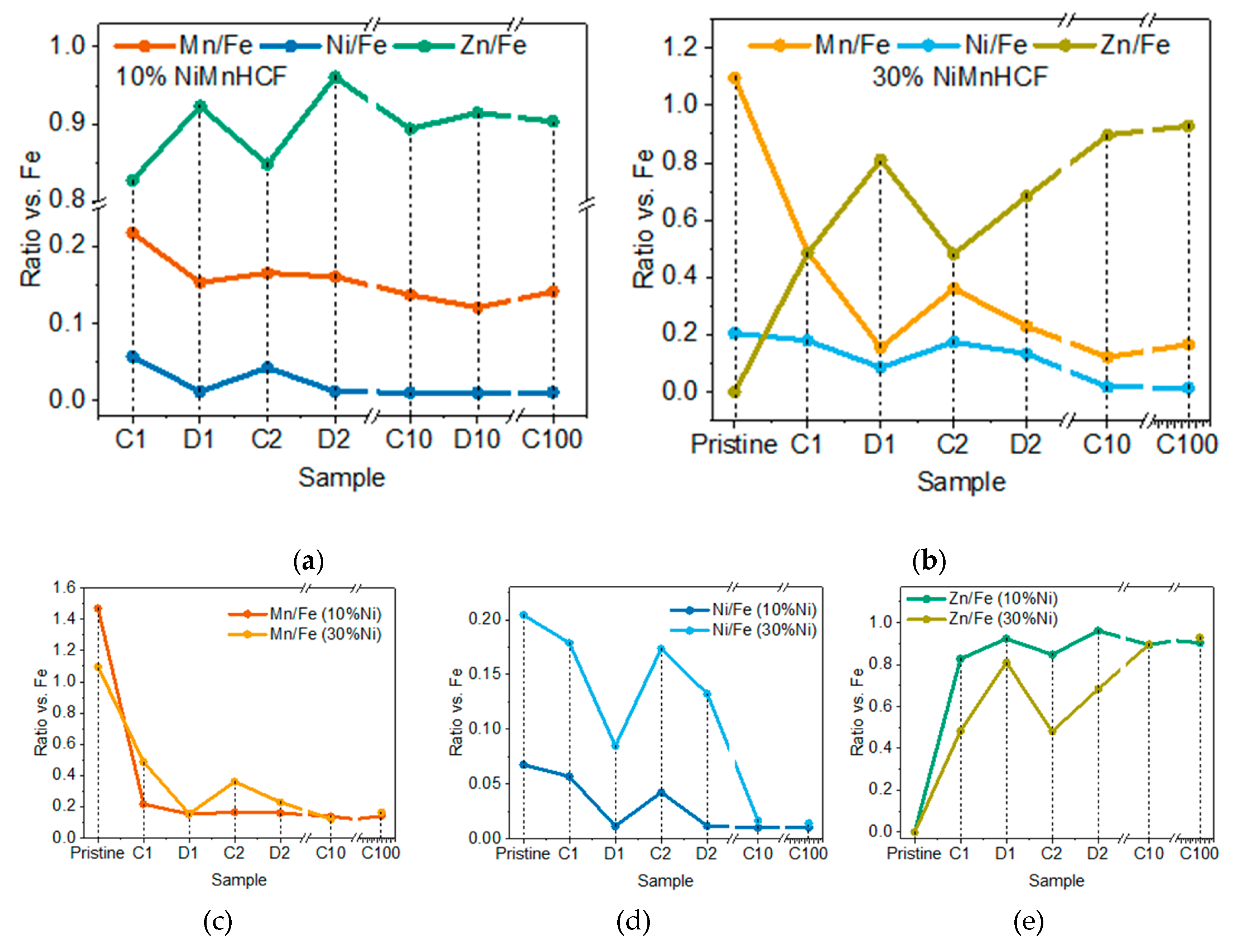
3.1. D-XRF and micro-XANES Analysis
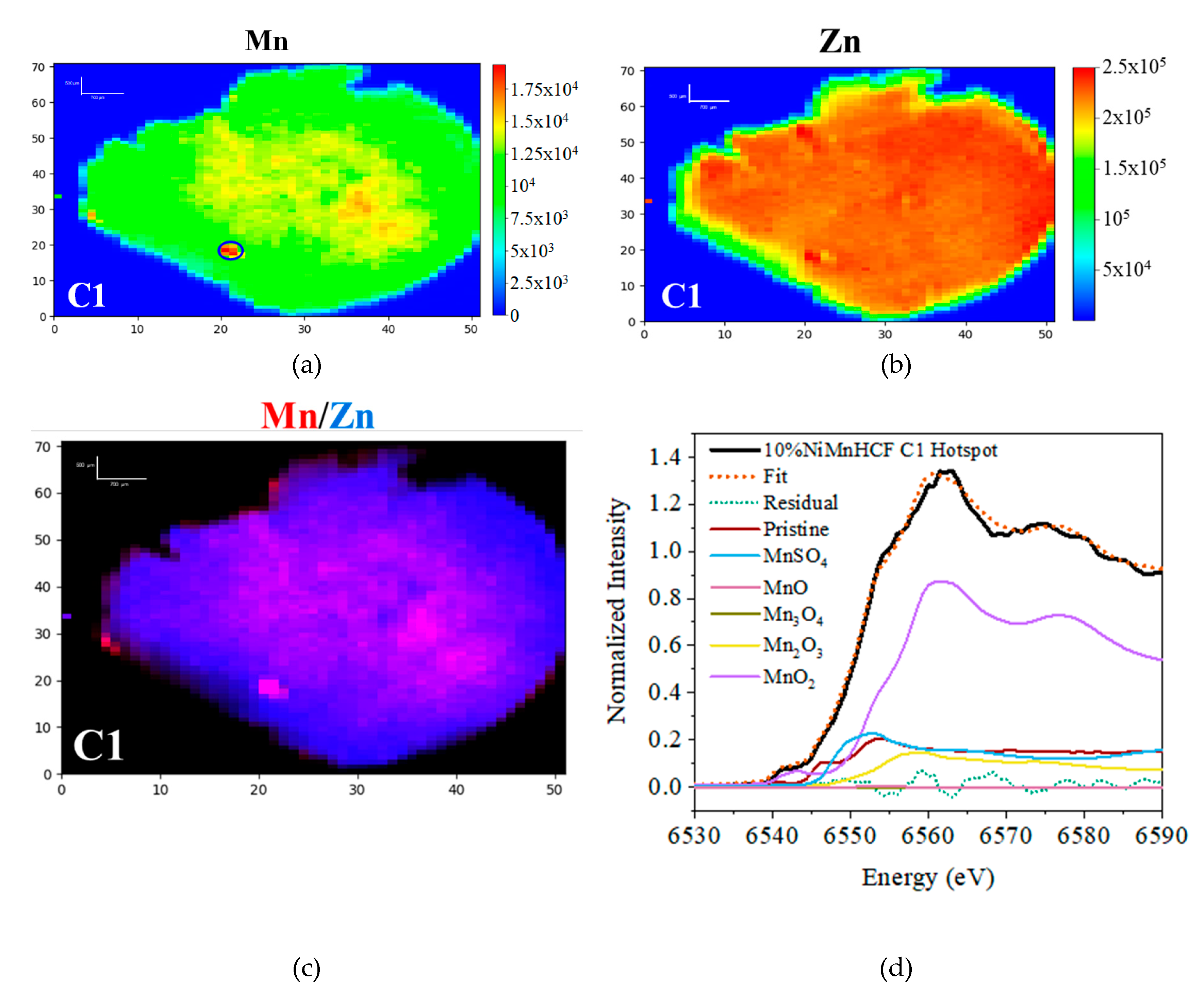
3.2. IR Analysis
3.3. GCPL Analysis
3.4. PXRD Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- EUR-Lex. Available online: https://eur-lex.europa.eu/summary/chapter/20.html#:~:text=The%20European%20climate%20law%20writes,2030%2C%20compared%20to%201990%20levels (accessed 23 02 2024).
- Janssens, K. G. X-Ray Fluorescence Analysis. In Handbook of Spectroscopy; Gauglitz G., Vo-Dinh T. Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; Volume 11, pp. 363–419. [Google Scholar]
- Maisuradze M., Carlomagno, I.; Mullaliu, A.; Li, M.; Aquilanti, G.; Giorgetti, M. 2D X-ray fluorescence imaging as a probe for charge state distribution of manganese in aged MnHCF-based electrodes, J. Phys. Chem. C, 2023, 127 (44), 21498. [CrossRef]
- Booth, S. G.; Uehara, A.; Chang, S. Y.; Mosselmans, J. F. W.; Schroeder, S. L. M; Dryfe, R. A. W. Gold Deposition at a Free-Standing Liquid/Liquid Interface: Evidence for the Formation of Au(I) by Microfocus X-ray Spectroscopy (μXRF and μXAFS) and Cyclic Voltammetry, J. Phys. Chem. C, 2015, 119, 16785–16792. [Google Scholar] [CrossRef]
- Yu, X.; Pan, H.; Zhou, Y.; Northrup, P.; Xiao, J.; Bak, S.; Liu, M.; Nam, K- W.; Qu, D.; Liu, J. et al. Direct Observation of the Redistribution of Sulfur and Polysufides in Li–S Batteries During the First Cycle by In Situ X-Ray Fluorescence Microscopy, Adv. Energy Mater. 2015, 5 (16), 1500072. [CrossRef]
- Li, M.; Gaboardi, M.; Mullaliu, A.; Maisuradze, M.; Xue, X.; Aquilanti, G.; Plaisier, J. R.; Passerini, S.; Giorgetti, M. Influence of Vacancies in Manganese Hexacyanoferrate Cathode for Organic Na-ion Batteries: A Structural Perspective. ChemSusChem 2023. [Google Scholar] [CrossRef] [PubMed]
- CATL. Available online: https://www.catl.com/en/news/665.html (accessed 23 02 2024).
- Altris. Available online: https://www.altris.se/technology/ (accessed 23 02 2024).
- Natron Energy. Available online: https://natron.energy/technology/ (accessed 23 02 2024).
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett 2018, 3 (10), 2480–2501. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J. H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett 2018, 3 (10), 2620–2640. [Google Scholar] [CrossRef]
- Grignon, E.; Battaglia, A. M.; Schon, T. B.; Seferos, D. S. Aqueous Zinc Batteries: Design Principles toward Organic Cathodes for Grid Applications. iScience 2022, 25 (5), 104204. [Google Scholar] [CrossRef]
- Shibata, T.; Moritomo, Y. Ultrafast Cation Intercalation in Nanoporous Nickel Hexacyanoferrate. Chem. Commun. 2014, 50 (85), 12941–12943. [Google Scholar] [CrossRef] [PubMed]
- Takachi, M.; Fukuzumi, Y.; Moritomo, Y. Na+ Diffusion Kinetics in Nanoporous Metal-Hexacyanoferrates. Dalton Transactions 2016, 45 (2), 458–461. [Google Scholar] [CrossRef]
- You, Y.; Wu, X.-L.; Yin, Y.-X.; Guo, Y.-G. A Zero-Strain Insertion Cathode Material of Nickel Ferricyanide for Sodium-Ion Batteries. J Mater Chem A Mater 2013, 1 (45), 14061. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian Blue Cathode Materials for Sodium-Ion Batteries and Other Ion Batteries. Adv Energy Mater 2018, 8 (17), 1702619. [Google Scholar] [CrossRef]
- Mullaliu, A.; Aquilanti, G.; Conti, P.; Plaisier, J. R.; Fehse, M.; Stievano L.; Giorgetti M. Copper electroactivity in Prussian blue-based cathode disclosed by operando XAS, J Phys Chem C 2018, 122 (28), 15868-15877. [CrossRef]
- Wessells, C. D.; Peddada, S. V.; Huggins, R. A.; Cui, Y. Nickel Hexacyanoferrate Nanoparticle Electrodes For Aqueous Sodium and Potassium Ion Batteries. Nano Lett 2011, 11 (12), 5421–5425. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, N.; Morikawa, T.; Kondo, J.; Takeda, Y.; Yamamoto, O.; Kinugasa, N.; Yamagishi, T. Lithium Intercalation Behavior into Iron Cyanide Complex as Positive Electrode of Lithium Secondary Battery. J Power Sources 1999, 79 (2), 215–219. [Google Scholar] [CrossRef]
- Eftekhari, A. Potassium Secondary Cell Based on Prussian Blue Cathode. J Power Sources 2004, 126 (1–2), 221–228. [Google Scholar] [CrossRef]
- Wessells, C. D.; Huggins, R. A.; Cui, Y. Copper Hexacyanoferrate Battery Electrodes with Long Cycle Life and High Power. Nat Commun 2011, 2 (1), 550. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Y.; Shyam, B.; Stone, K. H.; Weker, J. N.; Pasta, M.; Lee, H.; Toney, M. F.; Cui, Y. Reversible Multivalent (Monovalent, Divalent, Trivalent) Ion Insertion in Open Framework Materials. Adv Energy Mater 2015, 5 (12). [Google Scholar] [CrossRef]
- Mizuno, Y.; Okubo, M.; Hosono, E.; Kudo, T.; Ohishi, K.; Okazawa, A.; Kojima, N.; Kurono, R.; Nishimura, S.; Yamada, A. Electrochemical Mg2+ Intercalation into a Bimetallic CuFe Prussian Blue Analog in Aqueous Electrolytes. J Mater Chem A Mater 2013, 1 (42), 13055. [Google Scholar] [CrossRef]
- Park, H.; Lee, Y.; Ko, W.; Choi, M.; Ku, B.; Ahn, H.; Kim, J.; Kang, J.; Yoo, J.; Kim, J. Review on Cathode Materials for Sodium- and Potassium-Ion Batteries: Structural Design with Electrochemical Properties. Batter Supercaps 2023, 6 (3). [Google Scholar] [CrossRef]
- Li, M.; Bina, A.; Maisuradze, M.; Giorgetti, M. Symmetric Aqueous Batteries of Titanium Hexacyanoferrate in Na+, K+, and Mg2+ Media. Batteries 2021, 8 (1), 1. [Google Scholar] [CrossRef]
- Li, M.; Maisuradze, M.; Sciacca, R.; Hasa, I.; Giorgetti, M. A Structural Perspective on Prussian Blue Analogues for Aqueous Zinc-Ion Batteries. Batter Supercaps 2023. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J. B. Prussian Blue: A New Framework of Electrode Materials for Sodium Batteries. Chemical Communications 2012, 48 (52), 6544. [Google Scholar] [CrossRef]
- Mullaliu, A.; Asenbauer, J.; Aquilanti, G.; Passerini, S.; Giorgetti, M. Highlighting the Reversible Manganese Electroactivity in Na-Rich Manganese Hexacyanoferrate Material for Li- and Na-Ion Storage. Small Methods 2020, 4 (1), 1900529. [Google Scholar] [CrossRef]
- Cao, T.; Zhang, F.; Chen, M.; Shao, T.; Li, Z.; Xu, Q.; Cheng, D.; Liu, H.; Xia, Y. Cubic Manganese Potassium Hexacyanoferrate Regulated by Controlling of the Water and Defects as a High-Capacity and Stable Cathode Material for Rechargeable Aqueous Zinc-Ion Batteries. ACS Appl Mater Interfaces 2021, 13 (23), 26924–26935. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, Z.; Ye, Y.; Zhou, Z.; Li, Y.; Zhang, M.; Yuan, X.; Hu, J.; Zhao, W.; Huang, Z.; Li, C.; Chen, H.; Zheng, J.; Li, R. Zn 2+ Induced Phase Transformation of K 2 MnFe(CN) 6 Boosts Highly Stable Zinc-Ion Storage. Adv Energy Mater 2021, 11 (31). [Google Scholar] [CrossRef]
- Ni, G.; Hao, Z.; Zou, G.; Xu, X.; Hu, B.; Cao, F.; Zhou, C. Potassium Manganese Hexacyanoferrate with Improved Lifespan in Zn(CF 3 SO 3 ) 2 Electrolyte for Aqueous Zinc-Ion Batteries. Sustain Energy Fuels 2022, 6 (5), 1353–1361. [Google Scholar] [CrossRef]
- Fu, H.; Liu, C.; Zhang, C.; Ma, W.; Wang, K.; Li, Z.; Lu, X.; Cao, G. Enhanced Storage of Sodium Ions in Prussian Blue Cathode Material through Nickel Doping. J Mater Chem A Mater 2017, 5 (20), 9604–9610. [Google Scholar] [CrossRef]
- Yang, D.; Xu, J.; Liao, X.-Z.; He, Y.-S.; Liu, H.; Ma, Z.-F. Structure Optimization of Prussian Blue Analogue Cathode Materials for Advanced Sodium Ion Batteries. Chem. Commun. 2014, 50 (87), 13377–13380. [Google Scholar] [CrossRef] [PubMed]
- Maisuradze, M.; Li, M.; Aquilanti, G.; Plaisier, J.; Giorgetti, M. Characterization of Partially Ni Substituted Manganese Hexacyanoferrate Cathode Material. Mater Lett 2023, 330, 133259. [Google Scholar] [CrossRef]
- Karydas, A. G.; Czyzycki, M.; Leani, J. J.; Migliori, A.; Osan, J.; Bogovac, M.; Wrobel, P.; Vakula, N.; Padilla-Alvarez, R.; Menk, R. H.; Gol, M. G.; Antonelli, M.; Tiwari, M. K.; Caliri, C.; Vogel-Mikuš, K.; Darby, I.; Kaiser, R. B. An IAEA Multi-Technique X-Ray Spectrometry Endstation at Elettra Sincrotrone Trieste: Benchmarking Results and Interdisciplinary Applications. J Synchrotron Radiat 2018, 25 (1), 189–203. [Google Scholar] [CrossRef]
- Jark W; Grenci G. Focusing X-Rays in Two Dimensions upon Refraction in an Inclined Prism. In Proc. SPIE – Advances in X-Ray/EUV Optics and Components IX 2014; 9207, p 92070A. [CrossRef]
- Solé, V. A.; Papillon, E.; Cotte, M.; Walter, Ph.; Susini, J. A Multiplatform Code for the Analysis of Energy-Dispersive X-Ray Fluorescence Spectra. Spectrochim Acta Part B At Spectrosc 2007, 62 (1), 63–68. [Google Scholar] [CrossRef]
- Ravel B.; Newville, M. ATHENA , ARTEMIS , HEPHAESTUS : data analysis for X-ray absorption spectroscopy using IFEFFIT, J Synchrotron Radiat 2005, 12 (4), 537–541. [CrossRef]
- Rebuffi, L.; Plaisier, J. R.; Abdellatief, M.; Lausi, A.; Scardi, P. MCX: A Synchrotron Radiation Beamline for X-ray Diffraction Line Profile Analysis. Z Anorg Allg Chem 2014, 640 (15), 3100–3106. [Google Scholar] [CrossRef]
- Plaisier, J. R.; Nodari, L.; Gigli, L.; Rebollo San Miguel, E. P.; Bertoncello, R.; Lausi, A. The X-Ray Diffraction Beamline MCX at Elettra: A Case Study of Non-Destructive Analysis on Stained Glass. ACTA IMEKO 2017, 6 (3), 71. [Google Scholar] [CrossRef]
- Toby, B. H.; Von Dreele, R. B. GSAS-II : The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J Appl Crystallogr 2013, 46 (2), 544–549. [Google Scholar] [CrossRef]
- Li, M.; Sciacca, R.; Maisuradze, M.; Aquilanti, G.; Plaisier, J.; Berrettoni, M.; Giorgetti, M. Electrochemical Performance of Manganese Hexacyanoferrate Cathode Material in Aqueous Zn-Ion Battery. Electrochim Acta 2021, 400, 139414. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Reguera, E.; Lima, E.; Balmaseda, J.; Martínez-García, R.; Yee-Madeira, H. An Atypical Coordination in Hexacyanometallates: Structure and Properties of Hexagonal Zinc Phases. J. Phys. Chem. Solids 2007, 68 (9), 1630–1642. [Google Scholar] [CrossRef]
- Sciacca, R.; Zamponi, S.; Berrettoni, M.; Giorgetti, M. Stable Films of Zinc-Hexacyanoferrate: Electrochemistry and Ion Insertion Capabilities. J. Solid State Electrochem. 2022, 26 (1), 63–72. [Google Scholar] [CrossRef]
- Alfaruqi, M. H.; Mathew, V.; Gim, J.; Kim, S.; Song, J.; Baboo, J. P.; Choi, S. H.; Kim, J. Electrochemically Induced Structural Transformation in a γ-MnO 2 Cathode of a High Capacity Zinc-Ion Battery System. Chem. Mater. 2015, 27 (10), 3609–3620. [Google Scholar] [CrossRef]
- Huang, C.; Wu, C.; Zhang, Z.; Xie, Y.; Li, Y.; Yang, C.; Wang, H. Crystalline and Amorphous MnO2 Cathodes with Open Framework Enable High-Performance Aqueous Zinc-Ion Batteries. Front Mater Sci 2021, 15 (2), 202–215. [Google Scholar] [CrossRef]
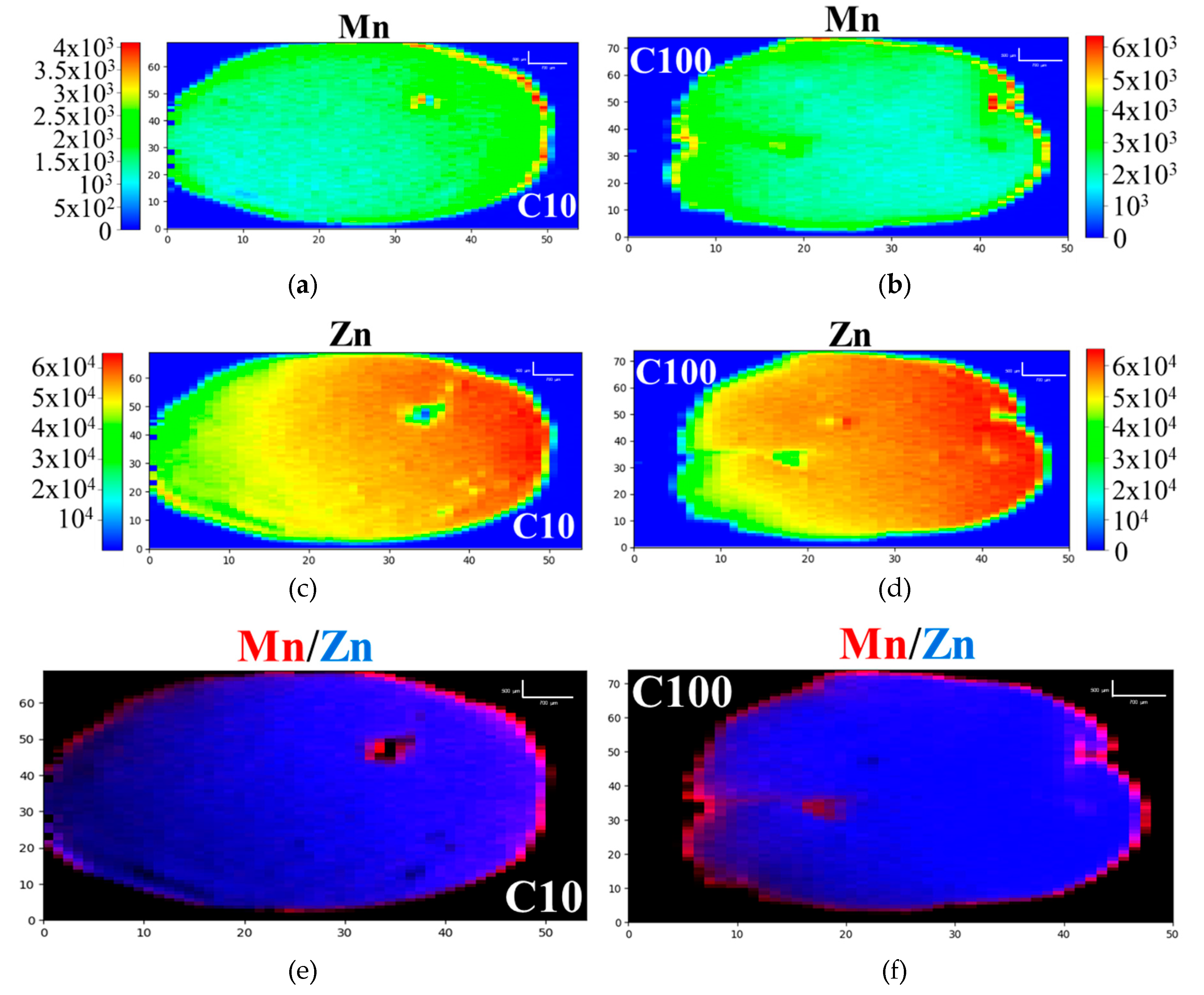
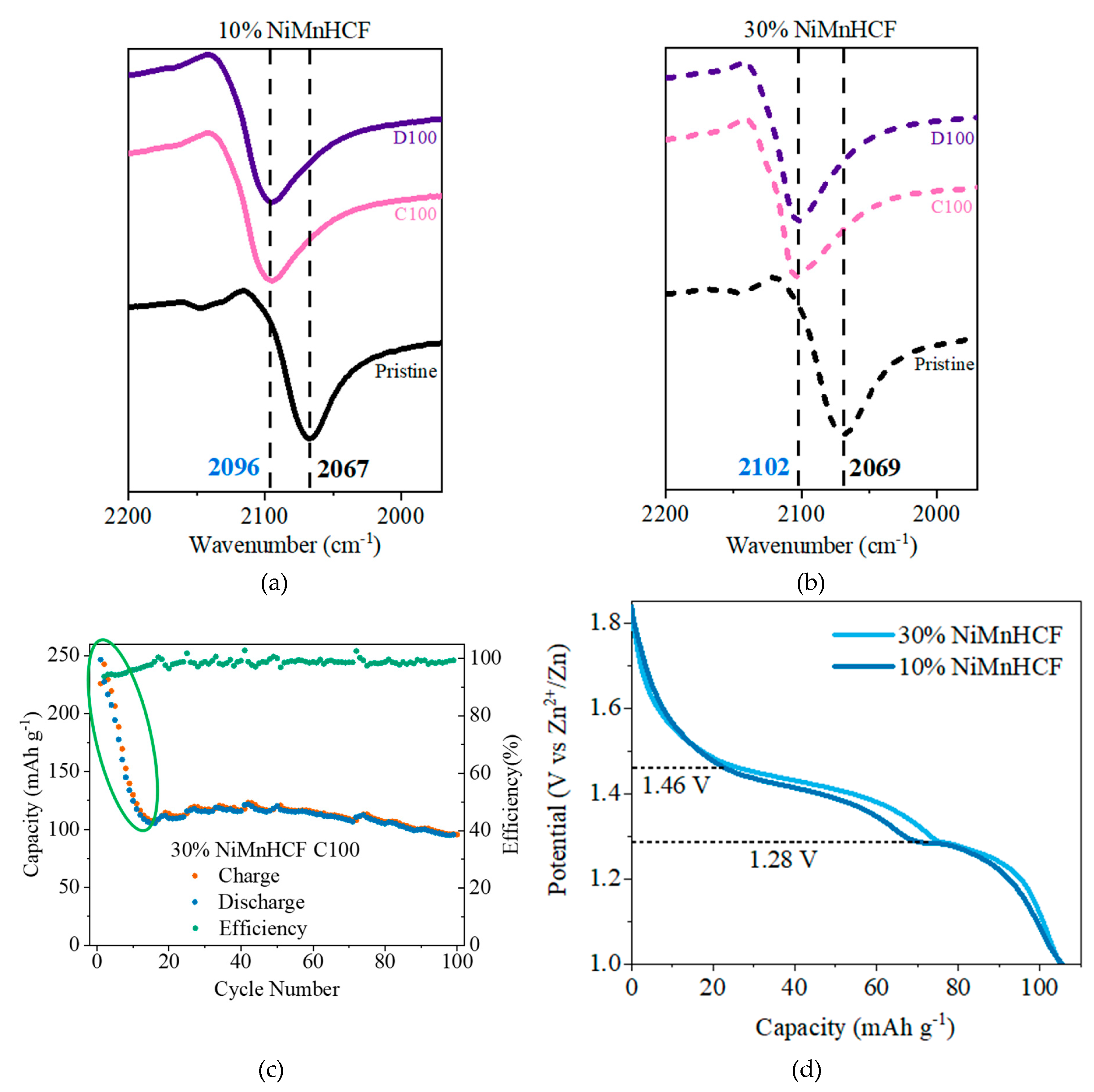

| Samples | Description | 2D-XRF | XANES | ||
|---|---|---|---|---|---|
| 10%NiMnHCF | 30%NiMnHCF | 10%NiMnHCF | 30%NiMnHCF | ||
| Pristine | Fresh electrode | ✓ | ✓ | ✓ | ✓ |
| C1 | Charged after 1st cycle | ✓ | ✓ | ✓ | ✓ |
| D1 | Discharged after 1st cycle | ✓ | ✓ | ✓ | - |
| C2 | Charged after 2nd cycle | ✓ | ✓ | ✓ | - |
| D2 | Discharged after 2nd cycle | ✓ | ✓ | ✓ | - |
| C10 | Charged after 10th cycle | ✓ | ✓ | - | - |
| D10 | Discharged after 10th cycle | ✓ | - | - | - |
| C100 | Charged after 100th cycle | ✓ | ✓ | - | - |
| Pristine | MnSO4 | MnO | Mn3O4 | Mn2O3 | MnO2 | R-factor | Reduced χ2 | |
| Ratio | 0.159 | 0.148 | 0.001 | 0 | 0.089 | 0.604 | 0.00255 | 0.00059 |
| Error | 0.028 | 0.017 | 0.024 | 0.032 | 0.068 | 0.028 |
| 30%NiMnHCF Samples | Cell parameters | ||||||||||
| F m-3m | P m-3m | R -3c | P 21/c | ||||||||
| a (Å) | a (Å) | a (Å) | c (Å) | a (Å) | b (Å) | c (Å) | β (º) | ||||
| Pristine | 10.33 | - | - | - | - | - | - | - | |||
| C1 | 10.29 | 11.82 | - | - | - | - | - | - | |||
| D1 | 10.22 | 11.79 | - | - | - | - | - | - | |||
| C2 | 10.33 | 11.88 | - | - | - | - | - | - | |||
| D2 | 10.16 | 11.76 | - | - | - | - | - | - | |||
| C10 | - | 11.91 | - | - | - | - | - | - | |||
| D10 | - | 11.88 | 12.42 | 32.85 | - | - | - | - | |||
| C50 | - | 11.99 | 12.48 | 32.94 | - | - | - | - | |||
| D50 | - | 12.05 | 12.46 | 32.74 | - | - | - | - | |||
| C100 | - | 11.94 | 12.37 | 33.08 | 6.23 | 13.77 | 9.91 | 125.45 | |||
| D100 | - | 11.99 | 12.48 | 32.94 | 6.16 | 13.69 | 9.77 | 126.84 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





