Submitted:
12 March 2024
Posted:
12 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
2.2. Plasmids and Transient Transfection
2.3. Live-Cell Fluorescence Imaging
2.4. Phase Transition Experiments
2.5. Immunofluorescence Imaging
2.6. Quantitation of Relative Amounts of GFP-MxA in Condensates vs Dispersed State in a Cell
2.7. VSV Stock and Virus Infection
2.8. Antibody Reagents and Chemicals
2.9. Statistical Testing
3. Results
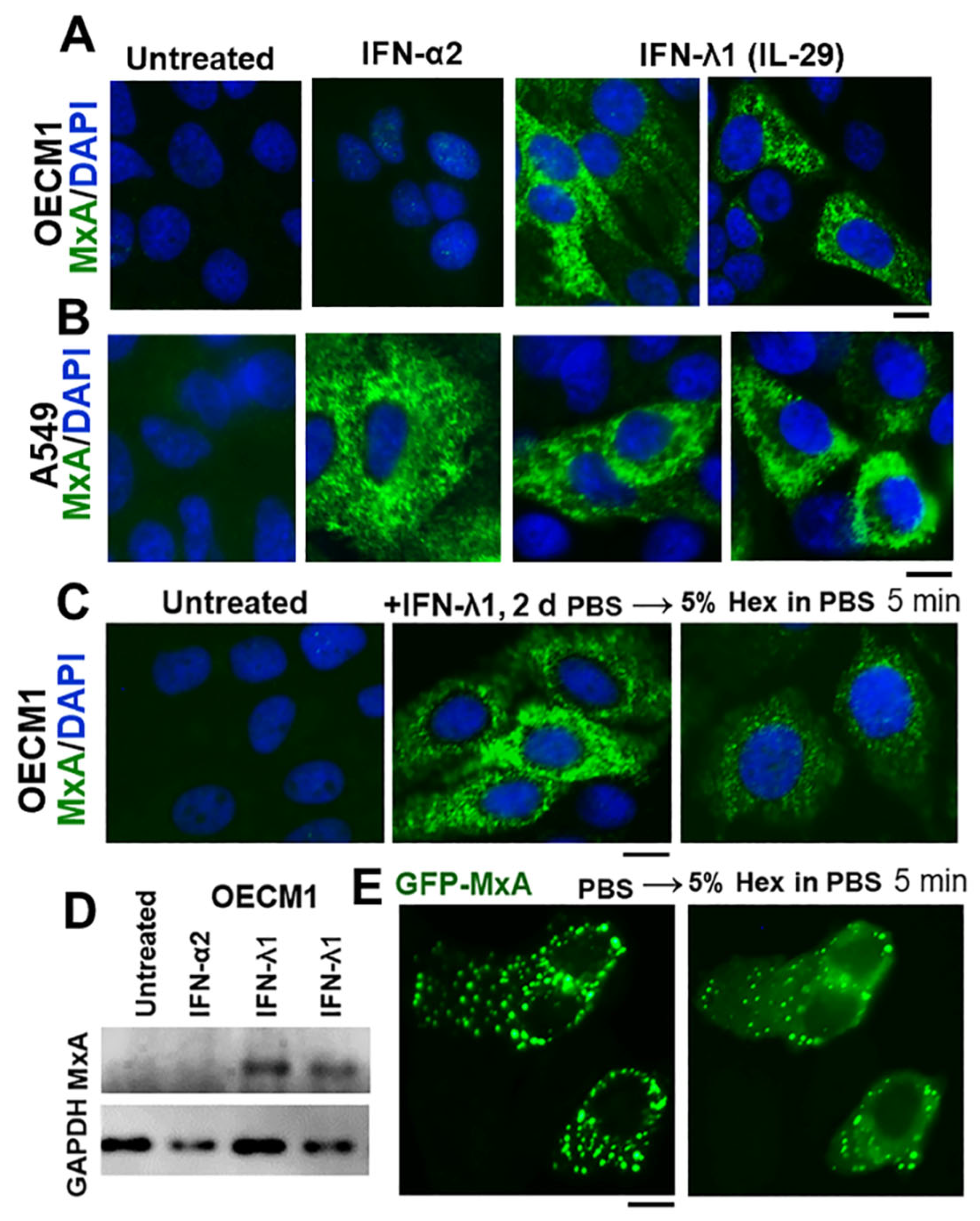
3.1. IFN-λ1 (IL-29), but not IFN-α2, Induces Expression of MxA to Form Endogenous Cytoplasmic Condensates in Oral Epithelial Cells (OECM1 Cell Line)

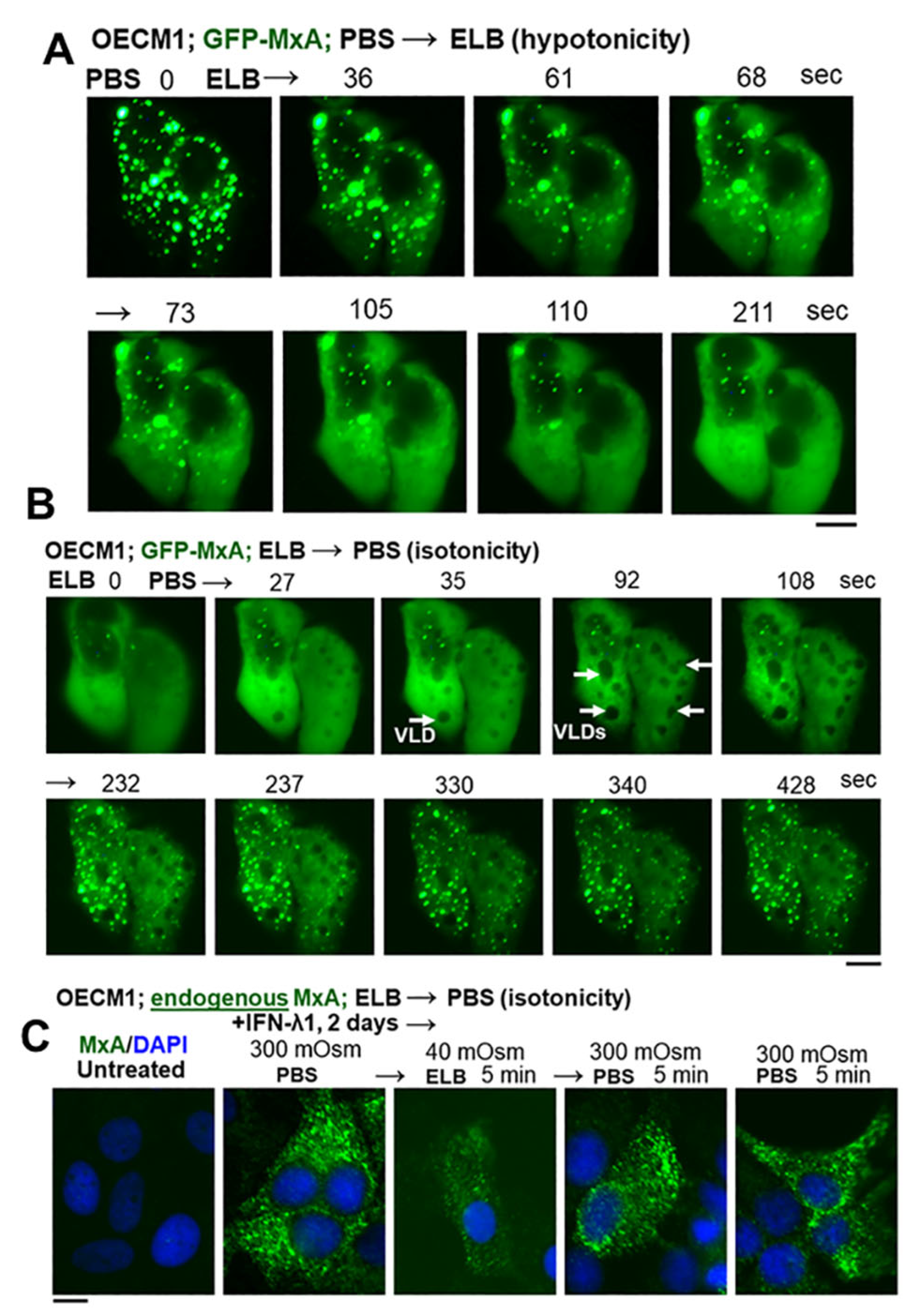
3.2. Hypotonicity Sensing by GFP-MxA and Endogenous MxA Condensates
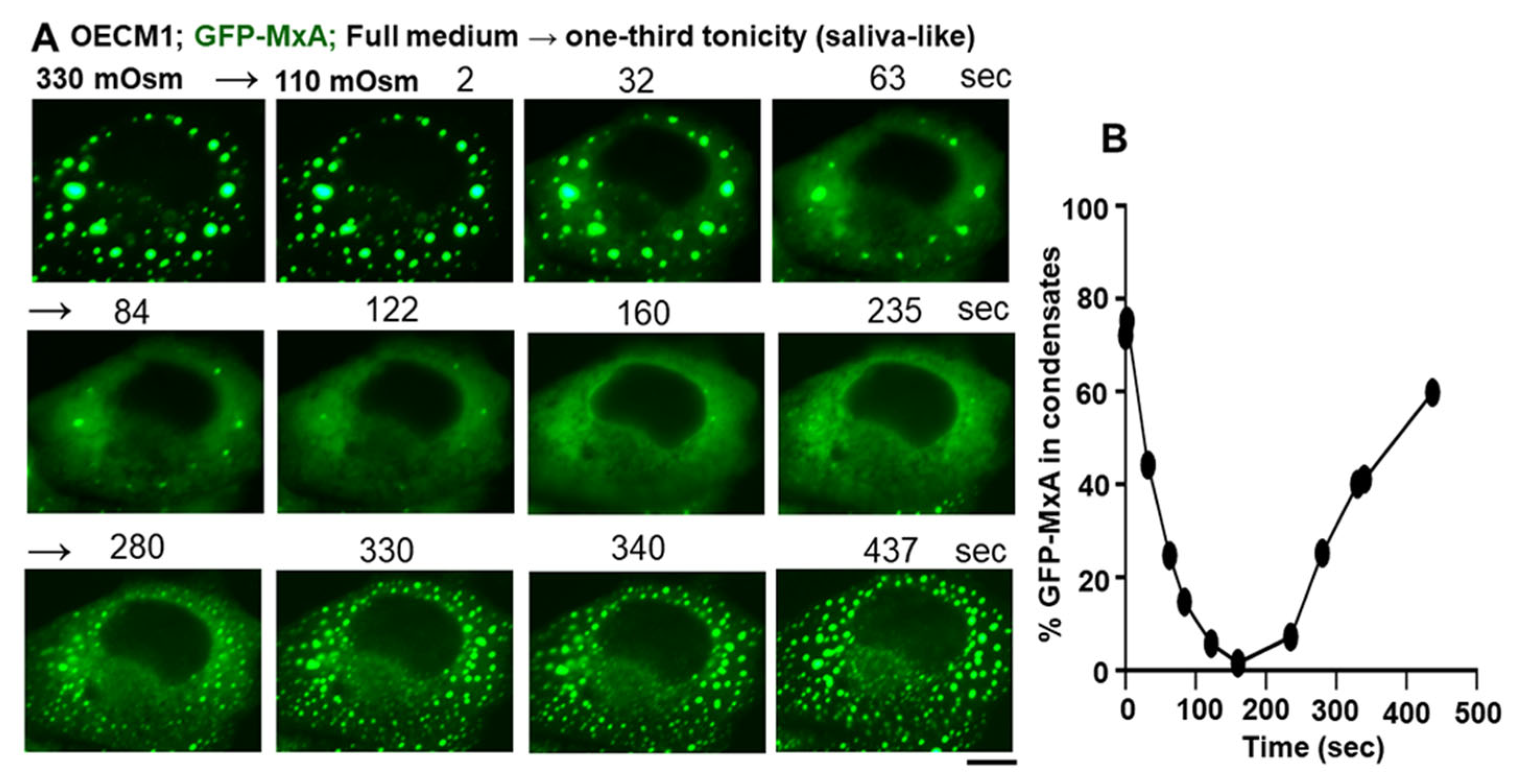
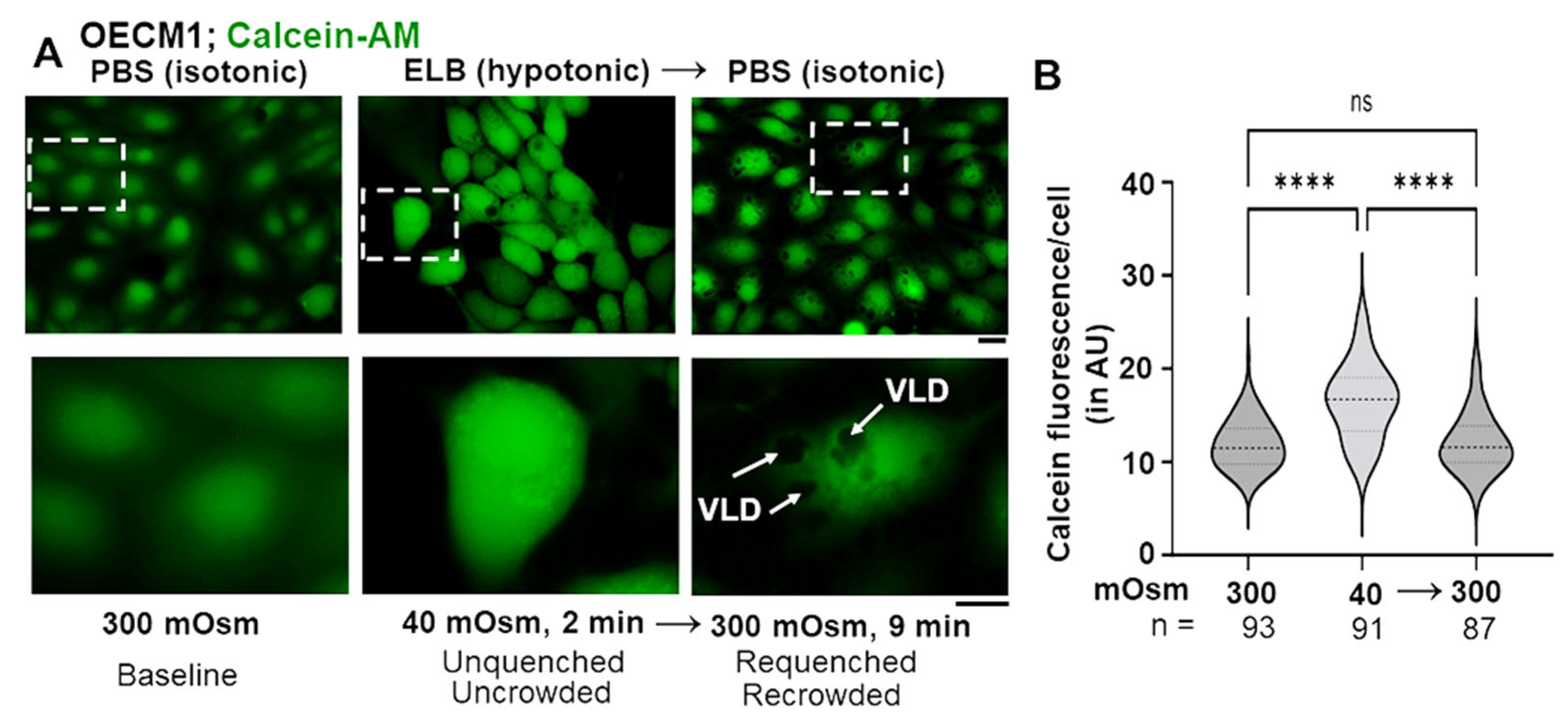
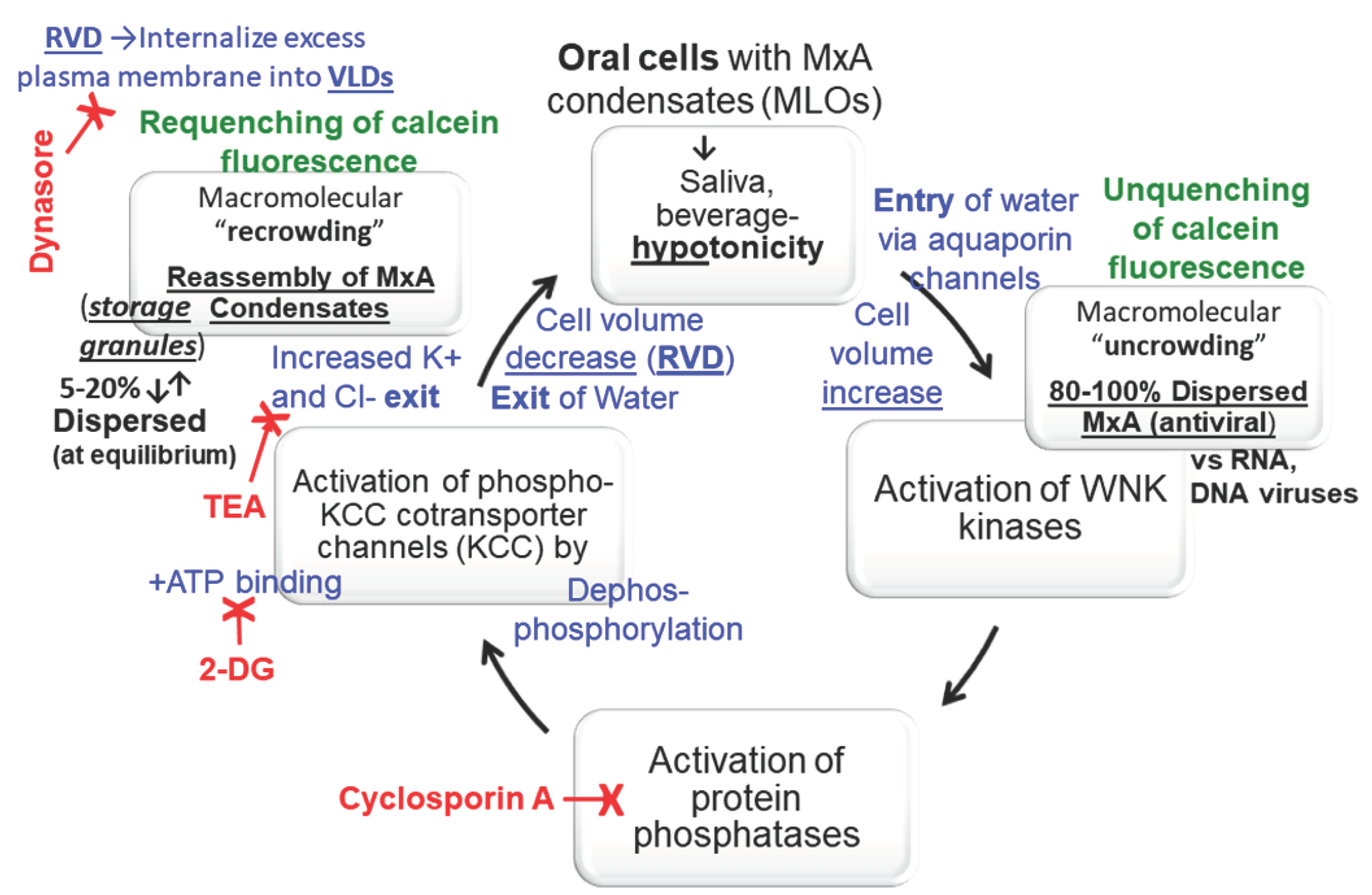
3.3. Biophysical Mechanism: Macromolecular Uncrowding and Recrowding during Regulated Volume Changes
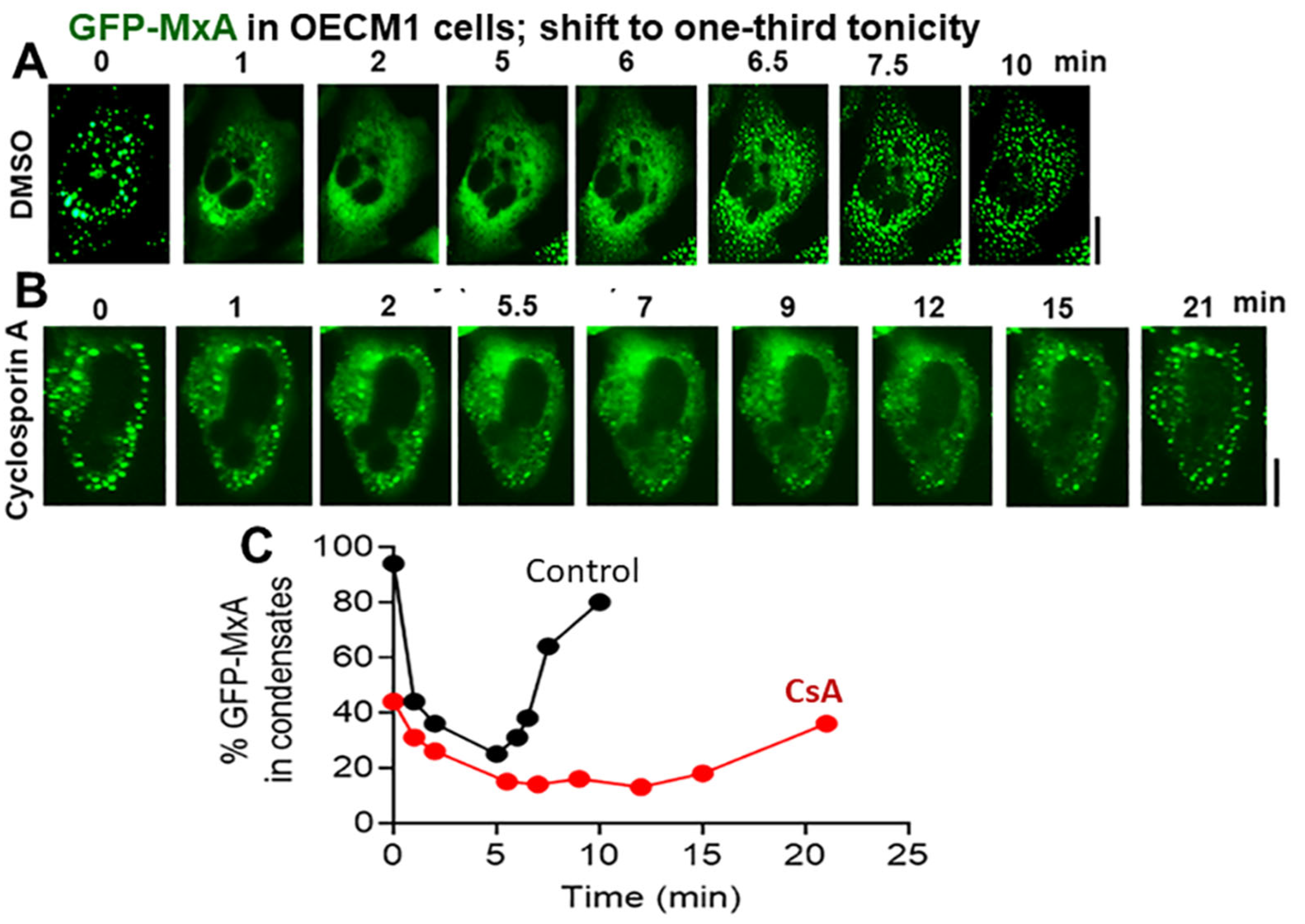
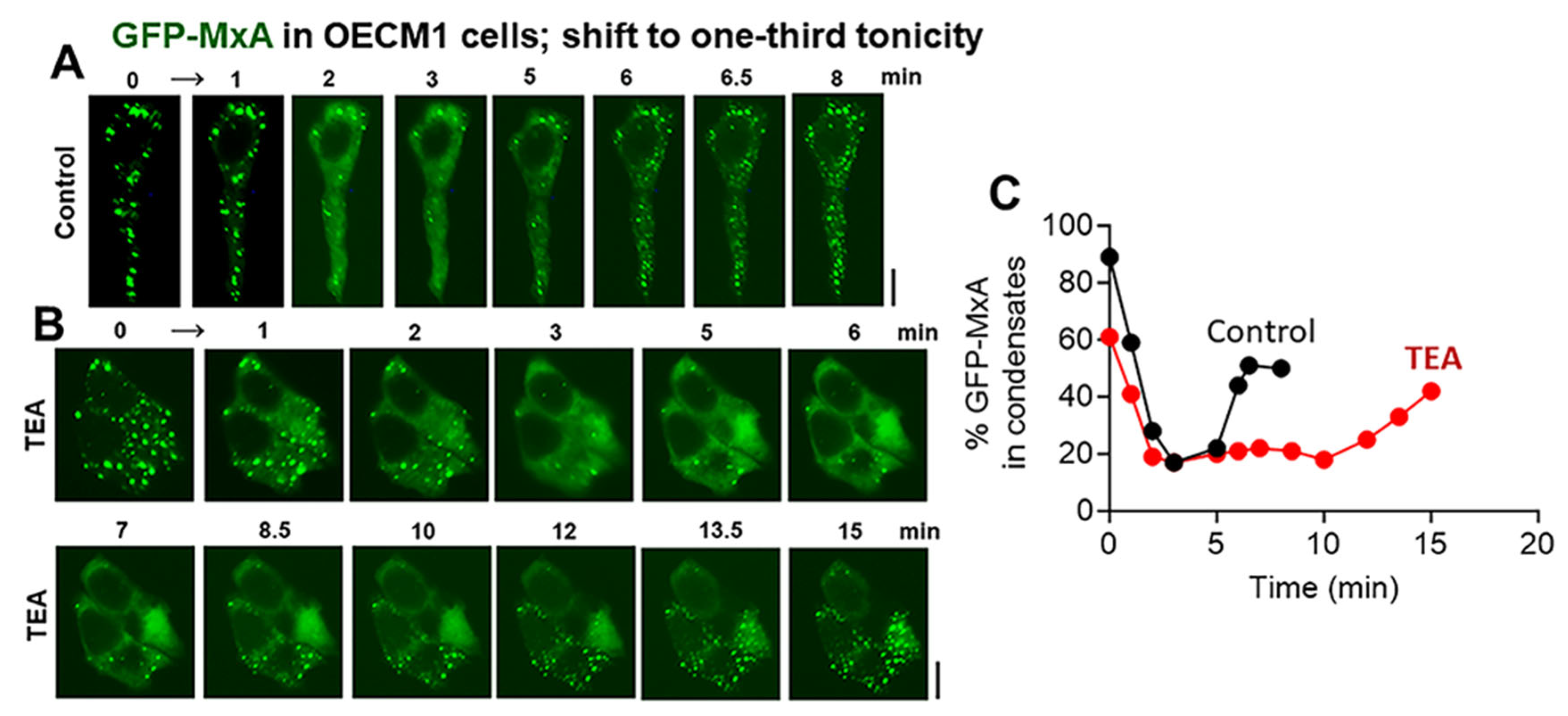
3.4. Biochemical Mechanisms: Volume Sensitive WNK Kinase-Protein Phosphatase-K-Cl-Cotransporter Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rodriquez-Hernandez, C.J.; Sokolski, K.J.; Stocke, K.S. Microbiome-mediated incapacitation of interferon lambda production in the oral mucosa. Proc Natl Acad Sci USA 2021, 118, e2105170118. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Thornton-Evans, G.O.; Genco, R.J. CDC Periodontal Disease Work Group Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012, 91, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Abiko, Y.; Shimizu, N.; Yamaguchi, M.; Suzuki, H.; Takiguchi, H. Effect of aging on functional changes of periodontal tissue cells. Acta Periodontol 1998, 3, 350–369. [Google Scholar] [CrossRef]
- Andreescu, C.F.; Mihai, L.L.; Raescu, M.; Tuculina, M.J.; Chumpata, C.N.; Ghergic, D.L. Age influence on periodontal tissues: a histological study. Rom J Morphol Embryol 2013, 54 (Suppl. 3), 811–815. [Google Scholar] [PubMed]
- Chen, S.; Zhou, D.; Liu, O.; Chen, H.; Wang, Y.; Zhou, Y. Cellular senescence and periodontitis: mechanisms and therapeutics. Biology 2022, 11, 1419. [Google Scholar] [CrossRef]
- Atukorallya D., S.; Ratyanake, R.K. Oral mucosa, saliva, and COVID-19 infection in oral health care. Front Med 2021, 8, 656926. [Google Scholar] [CrossRef]
- Okui, T.; Matsuda, Y.; Karino, M.; Hideshima, K.; Kanno, T. Oral mucosa could be an infectious target of SARS-CoV-2. Healthcare 2021, 9, 1068. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.T.B.R.; Ferreira, M.C.D.; Guare, R.O. Gingivitis and salivary osmolality in children with cerebral palsy. Int J Paediatr Dent 2016, 26, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.A.; Diniz, M. B, Loyola-Rodriguez, J.P.; et al A controlled study comparing salivary osmolality, caries experience and caries risk in patients with cerebral palsy. Med Oral Patol Oral Cir Bucal 2018, 23, e211-5. [Google Scholar]
- Feldman, M, Barnett, C. Relationships between the acidity and osmolality of popular beverages and reported postprandial heartburn. Gasteroenterology 1995, 108, 125–131. [Google Scholar] [CrossRef]
- Sadowska, A.; Swiderski, F, Laskowski, W. Osmolality of components and their application in the design of functional recovery drinks. Appl Sci 2020, 10, 7663. [Google Scholar] [CrossRef]
- Gresz, V.; Kwon, T.H.; Hurley, P.T.; Varga, G.; Zelles, T.; Nielsen, S.; Case, R.M.; Steward, M.C. Identification and localization of aquaporin water channels in human salivary glands. Am J Physiol Gastroint Liver Physiol 2001, 281, G247–G254. [Google Scholar] [CrossRef] [PubMed]
- Mahanonda, R.; Sa-Ard-Iam, N.; Rerkyen, P.; et al. MxA expression induced by α-defensin in healthy human periodontal tissue. Eur J Immunol 2012, 42, 946–956. [Google Scholar] [CrossRef]
- Imangulli, M.M.; Swaim, W.D.; League, S.C.; Gress, R.E.; Pavletic, S.Z. Hakim, F.T. (2009 Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood 113, 3620-3630. [CrossRef]
- Holzinger, D.; Jorna, C.; Stertz, S.; Boisson-Dupuis, S.; Thimme, R. Induction of MxA gene expression by influenza A virus requires Type I or Type III interferon signaling. J Virol 2007, 81, 7776–7785. [Google Scholar] [CrossRef] [PubMed]
- Pervolaraki, K.; Talemi, S.R.; Albrecht, D. Differential induction of interferon stimulated genes between type I and type III interferons is independent of interferon receptor abundance. PLoS Pathogen 2018, 14, e1007420. [Google Scholar] [CrossRef]
- Haller, O. and Kochs, G. (2002). Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic 3, 710-717. [CrossRef]
- Haller, O.; Staeheli, P.; Kochs, G. Interferon-induced Mx proteins in antiviral host defense. Biochimie 2007, 89, 812–818. [Google Scholar] [CrossRef]
- Haller, O.; Staeheli, P.; Schwemmle, M. and Kochs, G. Mx GTPases: dynamin- like antiviral machines of innate immunity. Trends Microbiol 2015, 23, 154–163. [Google Scholar] [CrossRef]
- Verhelst, J.; Hulpiau, P. and Saelens, X. Mx proteins: antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Biol. Rev. 2013, 77, 551–566. [Google Scholar] [CrossRef]
- Staeheli, P.; Pavlovic, J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol 1991, 65, 4498–4501. [Google Scholar] [CrossRef]
- Schwemmle, M.; Weining, K.C.; Richter, M.F.; Shumacher, B.; Staeheli, P. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology 1996, 206, 545–554. [Google Scholar] [CrossRef]
- Steiner, F.; Pavlovic, J. Subcellular localization of MxB determines its antiviral potential against influenza virus. J Virol 2020, 94, e00125–e220. [Google Scholar] [CrossRef]
- Davis, D.; Yuan, H.; Liang, F.X.; Yang, Y.M.; Westley, J.; Petzold, C.; Dancel-Manning, K.; Deng, Y.; Sall, J. and Sehgal, P.B. Human antiviral protein MxA forms novel metastable membraneless cytoplasmic condensates exhibiting rapid reversible tonicity- driven phase transitions. J Virol 2019, 93, e01014–19. [Google Scholar] [CrossRef]
- Sehgal, P.B.; Westley, J.; Lerea, K.M. DiSenso-Browne, S. and Etlinger, J.D. Biomolecular condensates in cell biology and virology: phase-separated membraneless organelles (MLOs). Analytical Biochem 2020, 597, 113691. [Google Scholar] [CrossRef]
- Sehgal, P.B. and Lerea, K.L. (2020) Biomolecular condensates and membraneless organelles (MLOs). In Jez. Joseph (Eds) Encyclopedia of Biological Chemistry, 3rd Edn., vol. 1, pp 530-541. Oxford: Elsevier. [CrossRef]
- Sehgal, P.B.; Yuan, H.; Scott, M.F.; Deng, Y.; Liang, F-X. and Mackiewicz, A. Murine GFP-Mx1 forms phase-separated nuclear condensates and associates with cytoplasmic intermediate filaments: novel antiviral activity against vesicular stomatitis virus. J Biol Chem 2020, 294, 15218–15234. [Google Scholar]
- Sehgal, P.B. Metastable biomolecular condensates of interferon-inducible antiviral Mx-family GTPases: a paradigm shift in the last three years. J Biosci 2021, 46, 72. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, P.B.; Yuan, H.; Jin, Y. Rapid reversible osmoregulation of cytoplasmic biomolecular condensates of human interferon-α-induced antiviral MxA GTPase. Intl J Mol Sci 2022, 23, 12739. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.R.; Chou, C.Y.; Ellory, J.C. Volume-sensitive KCl cotransport associated with human cervical carcinogenesis. Pflugers Archiv 2000, 440, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bize, I.; Guvenc, B.; Robb, A.; Buchbinder, G.; Brugnara, C. Serine/threonine protein phosphatases a2d regulation of K-Cl cotransport in human erythrocytes. Am J Physiol 1999, 277, C926–C936. [Google Scholar] [CrossRef] [PubMed]
- Akella, R.; Humphreys J., M.; Sekulski, K.; He, H.; Durbacz, M. Osmosensing by WNK kinases. Mol Biol Cell 2021, 32, 1614–1623. [Google Scholar] [CrossRef]
- Boyd-Shiwarski, C.R.; Shiwarski, D.J.; Griffths S., E.; Beacham, R.T.; Norrell, L. WNK kinases sense molecular crowding and rescue cell volume via phase separation. Cell 2022, 185, 4488–4506. [Google Scholar] [CrossRef] [PubMed]
- Carey, B. L, Ahmed, M.; Puckett, S.; Lyles, D.S. Early steps of the virus replication cycle are inhibited in prostate cancer cells resistant to oncolytic vesicular stomatitis virus. J Virol 2008, 82, 12104–12115. [Google Scholar] [CrossRef]
- Reuzeau, C.; Mills, L.R.; Harris, J.A. Morris, C.E. Discrete and reversible vacuole-like dilations induced by osmomechanical perturbations of neurons. J Membr Biol 1995, 145, 33–47. [Google Scholar] [CrossRef]
- Mola, M.G.; Sparaneo, A.; Gargano, C.D.; Spray, D.C. The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: a different point of view on the role of aquaporins. Glia 2016, 64, 139–154. [Google Scholar] [CrossRef]
- de los Heros, P.; Kahle, K.T.; Rinehart, J.; Bobadilla, N.A.; Vazquez, N.; Cristobal, P.S.; Mount, D.B.; Lifton, R.P.; Hebert, S.C.; Gamba, G. WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci USA 2006, 103, 1976–1981. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Brokl, O.H.; Pannabecker, T.L.; Dantzler, W.H.; Stamer, W.D. Tetraethylammonium block of water flux in aquaporin-1 channels expressed in kidney thin limbs of Henle’s loop and a kidney-deruived cell line. BMC Physiol 2002, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Stertz, S.; Reichelt, M.; Krijnse-Locker, J.; Mackenzie, J.; Simpson, J.C.; Haller, O. and Kochs, G. (2006). Interferon-induced, antiviral human MxA protein localizes to a distinct subcompartment of the smooth endoplasmic reticulum. J. Interferon Cytokine Res. 2006, 26, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Accola, M.A.; Huang, B.; Al Masri, A. and McNiven, M.A. The antiviral dynamin family member, MxA, tubulates lipids and localizes to the smooth endoplasmic reticulum. J. Biol. Chem. 2002, 277, 21829–21835. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A. and Rosen, M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Shin, Y. and Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef]
- Alberti, S. The wisdom of crowds: regulating cell function through condensed states of living matter. J Cell Sci. 2017, 130, 2789–2796. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef]
- Shin, Y.; Berry, J.; Pannucci, N.; Haataja, N.P. Toettcher, J.E. and Brangwynne, C.P. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 2017, 168, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, B.S.; Maliga, Z.; Stein, D.A.; Hyman, A.A. and Whelan, S.P.J. Phase transitions drive the formation of vesicular stomatitis virus replication compartments. MBio 2018, 9, e02290–17. [Google Scholar] [CrossRef]
- Dinh, P.X.; Beurs, L.K.; Das, P.B.; Panda, D.; Das, A. and Pattnaik, A.K. Induction of stress granule-like structures in vesicular stomatitis virus-infected cells. J Virol 2013, 87, 372–383. [Google Scholar] [CrossRef]
- Nikolic, J.; Bars, R.L.; Lama, Z.; Scrima, N.; Lagaudriere-Gesbet, C.; Gaudin, Y. and Blondel, D. Negri bodies are viral factories with properties of liquid organelles. Nat Commun 2017, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, T.; Shabman, R.S.; Groseth, A.; Herwig, A.; Weber, M.; Schudt, G.; Dolnik, O.; Basler, C.F.; Becker, S. and Feldmann, H. Inclusion bodies are a site of ebolavirus replication. J. Virol 2012, 86, 11779–11788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Su, J.M.; Samuel, C.E. and Ma, D. Measles virus forms inclusion bodies with properties of liquid organelles. J Virol 2019, 93, e00948–19. [Google Scholar] [CrossRef] [PubMed]
- Guseva, S.; Milles, G.; Jensen, M.R.; Salvi, N.; Kleman, J.P.; Maurin, D.; Ruigrok, R.W. H. and Blackledge, M. Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Science Advances 2020, 6, eaaz7095. [Google Scholar] [CrossRef]
- Alenquer, M.; Vale-Costa, S.; Sousa, A.L.; Etibor, T.A.; Ferreira, F. and Amorim. M.J. Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nature Comm 2019, 10. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, L.; Qin, Z.; Wang, J.; Zheng X.; Wei, L.; Zhang, X.; Zhang, X. Liu, C.; Li, Z.; Wu, Y.; Li, G.; Yan, Q.; Ma, J. (2020) Phase separation of Epstein-Barr virus EBNA2 and its coactivator EBNALP controls gene expression. J. Virol. [CrossRef]
- Nevers, Q.; Albertini, A.A.; Lagaudriere-Gesbert, C. and Gardin Y. (2020) Negri bodies and other virus membrane-less replication compartments. Biochim. Biophys. Acta (Molecular Cell Research). [CrossRef]
- Cubuk, J.; Alston, J.J.; Incicco, J.J.; Singh, S.; Stuchell-Brereton, M.D.; Ward, M.D.; Zimmerman, M.I.; Vithani, N.; Griffith, D.; Wagoner, J.A.; Bowman, G.R.; Hall, K.B.; Soranno, A. and Holehouse, A.S. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nature Commun 2021, 12, 1936. [Google Scholar] [CrossRef]
- Perdikari, T.M.; Murthy, A.C.; Ryan, V.H.; Watters, S.; Naik, M.T. and Fawzi, N.L. SARSCoV-2 nucleocapsid protein undergoes liquid-liquid phase separation stimulated by RNA and partitions into phases of human ribonucleoproteins. EMBO J 2020, 39, e106478. [Google Scholar] [CrossRef] [PubMed]
- Iserman, C.; Roden, C.; Boerneke, M.; Sealfon, R.; McLaughlin, G.; Jungreis, I.; Park, C.; Boppana, A.; Fritch, E.; Hou, Y. J, Theesfeld, C.; Troyanskaya, O.G.; Baric, R.S.; Sheahan, T.P.; Weeks, K. and Gladfelter, A.S. Genomic RNA elements drive the phase separation of the SARS CoV-2 nucleocapsid. Mol Cell 2020, 80, 1078–1091.e6.
- 58 Carlson, C.R.; J. B. Asfaha, C.M. Ghent, C.J. Howard, N. Hartooni, D.O. Morgan Phosphoregulation of phase separation by the SARS-CoV-2 N protein suggests a biophysical basis for its dual functions. Mol Cell 2020, 80, 1092–1103e4. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Graf, L.; Olal, D.; von der Malsburg, A.; Gao, S.; Kochs, G. and Daumke, O. Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity. J Biol Chem 2015, 290, 12779–12792. [Google Scholar] [CrossRef]
- von der Malsburg, A.; Abutbul-Ionita, I.; Haller, O.; Kochs, G. and Danino, D. Stalk domain of the dynamin-like MxA GTPase protein mediates membrane binding and liposome tubulation via the unstructured L4 loop. J Biol Chem 2011, 286, 37858–37865. [Google Scholar] [CrossRef] [PubMed]
- Moschonas, G.D.; Delhaye, L.; Cooreman, R.; Bhat, A.; Sutter, D.D.; Parthoens, E. ; Desmer, A, Maciejczak, A, Grzek, H.; Lippens, S.; Debyser, Z.; Eyckerman, S.; Saelens, X. (2023) MX2 restricts HIV-1 and herpes simplex virus-1 by forming cytoplasmic biomolecular conednsates that mimic nuclear pore complexes. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




