Submitted:
07 March 2024
Posted:
08 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Context of Photobiomodulation in Dermatology
1.2. Importance of Light Therapy in Dermatology Practice
1.3. Review Objectives
2. Basic Molecular Mechanisms of Action
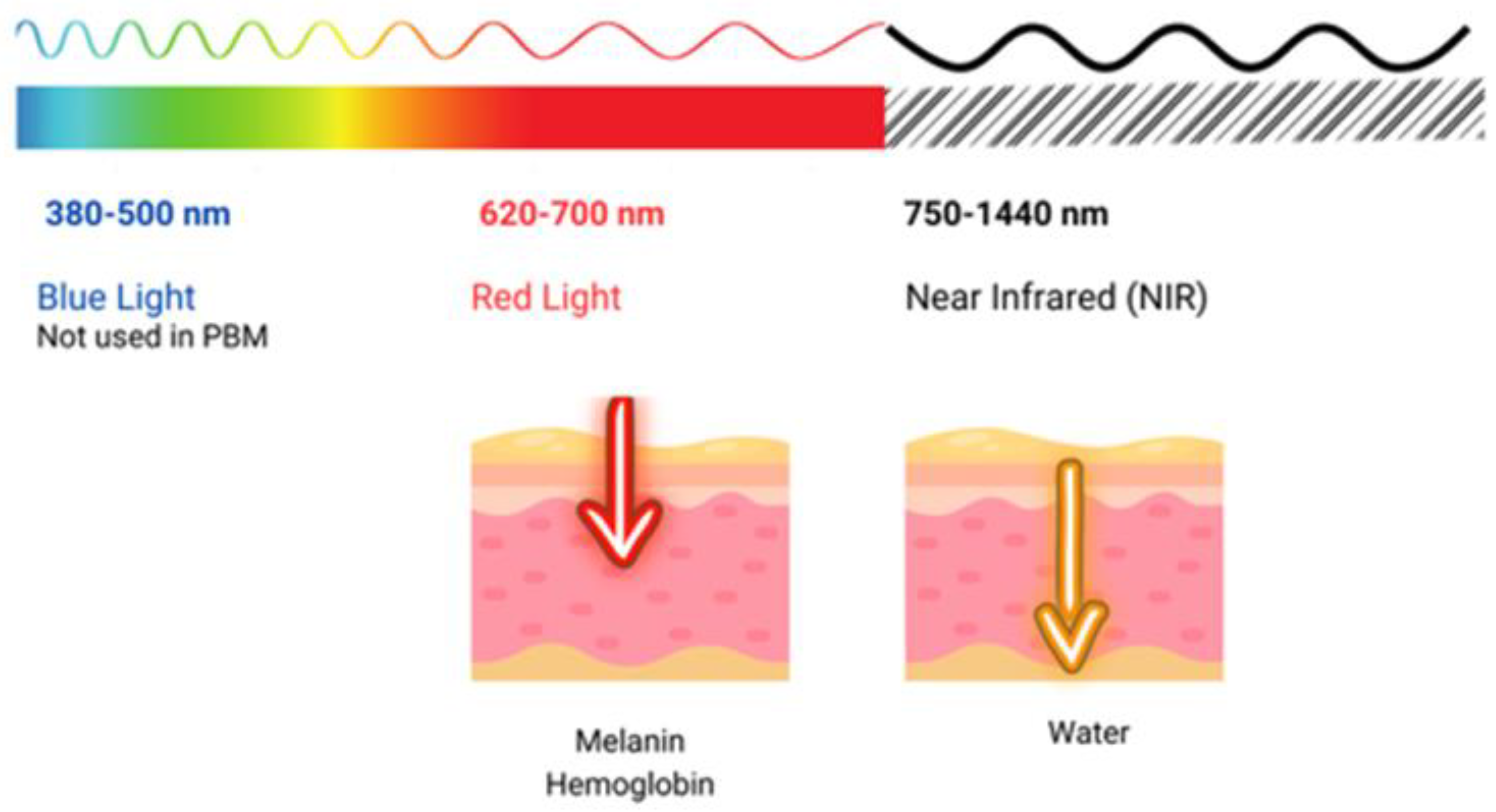
2.1. Blue LED Therapy
2.2. Photodynamic Therapy
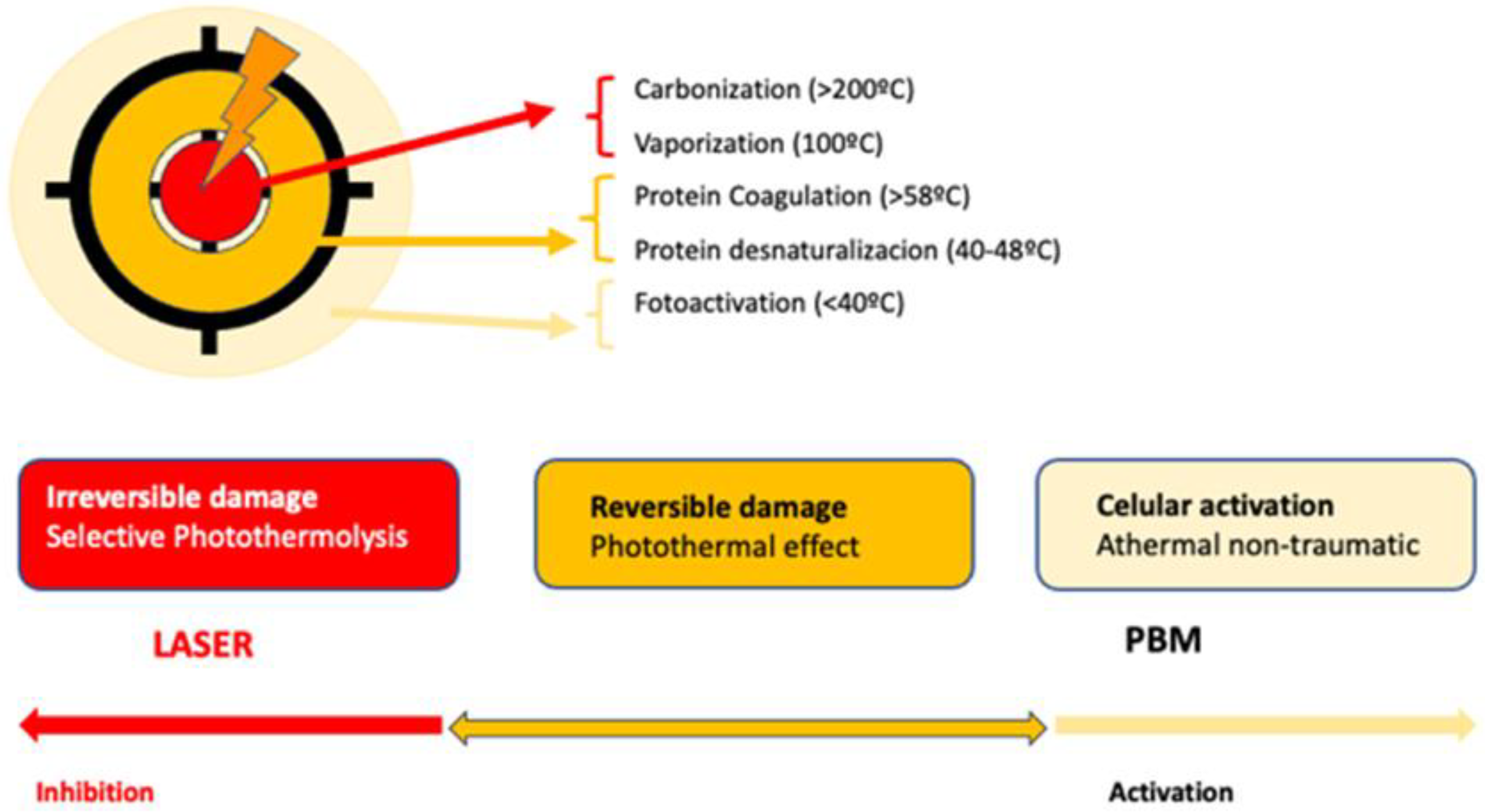
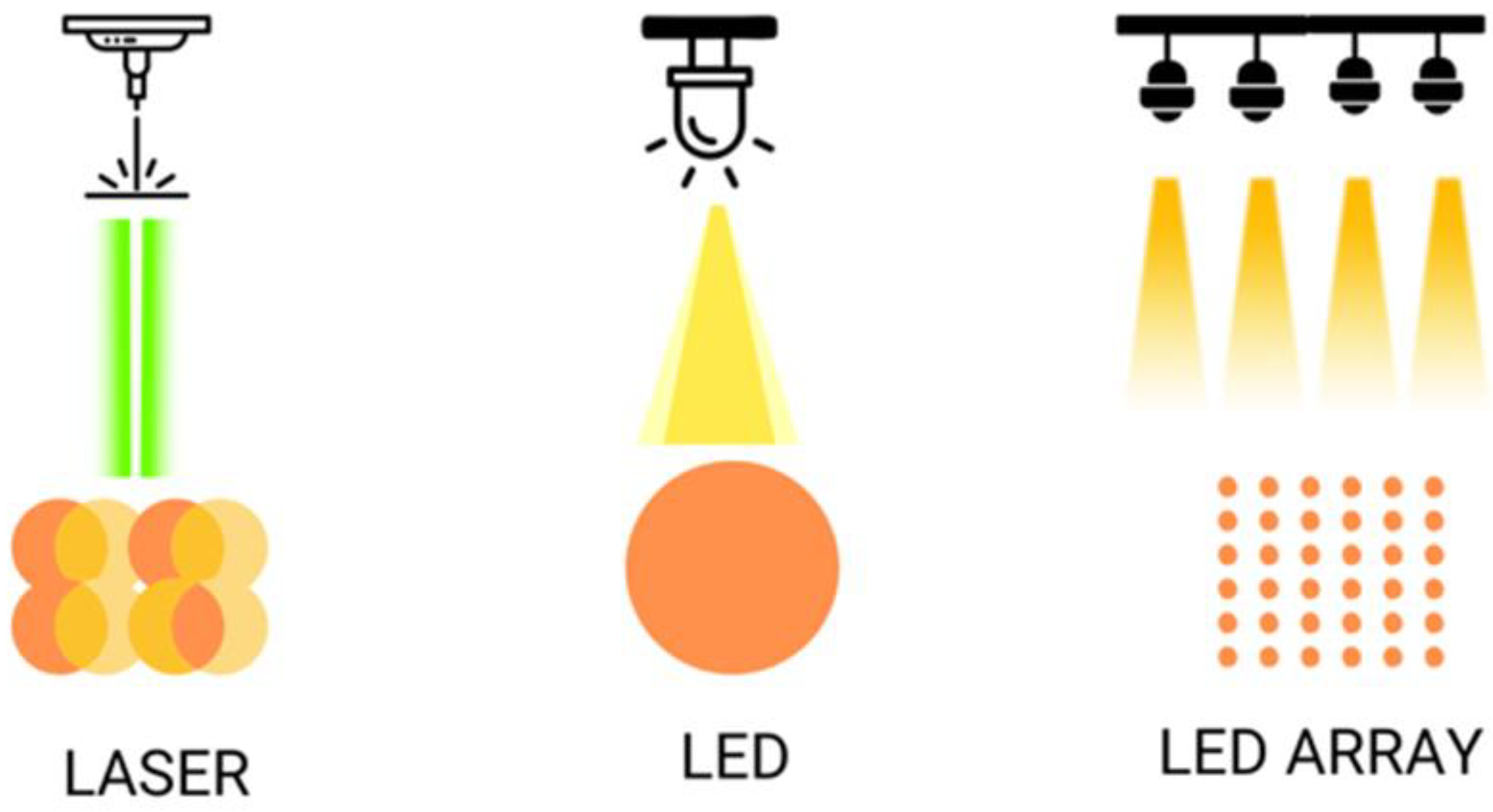
2.3. LED vs. Low-Level Laser Light Therapy Comparison
3. Current Applications in Dermatology
3.1. Acne Treatment
3.1.1. Effectiveness of Photobiomodulation in Acne
3.1.2. Underlying Mechanisms in Acne Treatment
3.2. Photorejuvenation
3.2.1. Reduction of Fine Lines and Wrinkles
3.2.2. Stimulation of Collagen Production
3.3. Wound Healing
3.3.1. Chronic Skin Lesions
3.3.2. Reduction of Hypertrophic Scars and Keloids
3.4. Psoriasis
3.5. Radiation Dermatitis
4. Ethical and Safety Considerations
5. Conclusions
6. Future Directions
6.1. Technological Advances in Photobiomodulation
6.1.1. Development with New Wavelengths
6.1.2. Improvements in Device Portability
6.2. Personalized Therapy in Dermatology
Use of Genomics and Skin Profiling for Targeted Treatments
6.3. Potential in Treating Severe Skin Conditions
6.3.1. Plaque Psoriasis
6.3.2. Severe Atopic Dermatitis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maghfour, J.; Ozog, D.M.; Mineroff, J.; Jagdeo, J.; Kohli, I.; Lim, H.W. Photobiomodulation CME Part I: Overview and Mechanism of Action. Journal of the American Academy of Dermatology 2024, S0190962224001865. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.E. Photobiomodulation: The Clinical Applications of Low-Level Light Therapy. Aesthetic Surgery Journal 2021, 41, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.; Geisler, A.N.; Nguyen, J.; Kohli, I.; Hamzavi, I.; Lim, H.W.; Jagdeo, J. Visible Light. Part I: Properties and Cutaneous Effects of Visible Light. Journal of the American Academy of Dermatology 2021, 84, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Mineroff, J.; Maghfour, J.; Ozog, D.D.; Lim, H.W.; Kohli, I.; Jagdeo, J. Photobiomodulation CME Part II: Clinical Applications in Dermatology. Journal of the American Academy of Dermatology 2024, S0190962224001877. [Google Scholar] [CrossRef] [PubMed]
- Tunér, J. Is Photobiomodulation Therapy Cost Effective? Photobiomodulation, Photomedicine, and Laser Surgery 2020, 38, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.E. Photobiomodulation: A Systematic Review of the Oncologic Safety of Low-Level Light Therapy for Aesthetic Skin Rejuvenation. Aesthetic Surgery Journal 2023, 43, NP357–NP371. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Mostafavinia, A.; Abdollahifar, M.-A.; Amini, A.; Ghoreishi, S.K.; Chien, S.; Hamblin, M.R.; Bayat, S.; Bayat, M. Combined Effects of Metformin and Photobiomodulation Improve the Proliferation Phase of Wound Healing in Type 2 Diabetic Rats. Biomedicine & Pharmacotherapy 2020, 123, 109776. [Google Scholar] [CrossRef] [PubMed]
- Salameh, F.; Shumaker, P.R.; Goodman, G.J.; Spring, L.K.; Seago, M.; Alam, M.; Al-Niaimi, F.; Cassuto, D.; Chan, H.H.; Dierickx, C.; et al. Energy-based Devices for the Treatment of Acne Scars: 2022 International Consensus Recommendations. Lasers Surg Med 2022, 54, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Lim, H.W. Ultraviolet B Phototherapy for Psoriasis: Review of Practical Guidelines. Am J Clin Dermatol 2016, 17, 125–133. [Google Scholar] [CrossRef]
- Ross, E.V. Extended Theory of Selective Photothermolysis: A New Recipe for Hair Cooking? Lasers Surg Med 2001, 29, 413–415. [Google Scholar] [CrossRef]
- Ross, E.V.; Smirnov, M.; Pankratov, M.; Altshuler, G. Intense Pulsed Light and Laser Treatment of Facial Telangiectasias and Dyspigmentation: Some Theoretical and Practical Comparisons. Dermatologic Surgery 2005, 31, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Morita, A. Current Developments in Phototherapy for Psoriasis. The Journal of Dermatology 2018, 45, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Mester, E.; Szende, B.; Gärtner, P. [The effect of laser beams on the growth of hair in mice]. Radiobiol Radiother (Berl) 1968, 9, 621–626. [Google Scholar] [PubMed]
- Tripodi, N.; Corcoran, D.; Antonello, P.; Balic, N.; Caddy, D.; Knight, A.; Meehan, C.; Sidiroglou, F.; Fraser, S.; Kiatos, D.; et al. The Effects of Photobiomodulation on Human Dermal Fibroblasts in Vitro: A Systematic Review. Journal of Photochemistry and Photobiology B: Biology 2021, 214, 112100. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P. Photobiomodulation in Promoting Wound Healing: A Review. Regenerative Medicine 2016, 11, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Shaikh-Kader, A.; Houreld, N.N. Photobiomodulation, Cells of Connective Tissue and Repair Processes: A Look at In Vivo and In Vitro Studies on Bone, Cartilage and Tendon Cells. Photonics 2022, 9, 618. [Google Scholar] [CrossRef]
- Yeh, G.; Wu, C.-H.; Cheng, T.-C. Light-Emitting Diodes--Their Potential in Biomedical Applications. Renewable and Sustainable Energy Reviews 2010, 14, 2161–2166. [Google Scholar] [CrossRef]
- Wang, H.-C.; Chen, Y.-T. Optimal Lighting of RGB LEDs for Oral Cavity Detection. Opt Express 2012, 20, 10186–10199. [Google Scholar] [CrossRef] [PubMed]
- Cicchi, R.; Rossi, F.; Alfieri, D.; Bacci, S.; Tatini, F.; De Siena, G.; Paroli, G.; Pini, R.; Pavone, F.S. Observation of an Improved Healing Process in Superficial Skin Wounds after Irradiation with a blue-LED Haemostatic Device. J. Biophoton 2016, 9, 645–655. [Google Scholar] [CrossRef]
- Passarella, S.; Karu, T. Absorption of Monochromatic and Narrow Band Radiation in the Visible and near IR by Both Mitochondrial and Non-Mitochondrial Photoacceptors Results in Photobiomodulation. J Photochem Photobiol B 2014, 140, 344–358. [Google Scholar] [CrossRef]
- Mason, M.G.; Nicholls, P.; Cooper, C.E. Re-Evaluation of the near Infrared Spectra of Mitochondrial Cytochrome c Oxidase: Implications for Non Invasive in Vivo Monitoring of Tissues. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2014, 1837, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Banchelli, M.; Cherchi, F.; Coppi, E.; Fraccalvieri, M.; Pugliese, A.M.; Pedata, F.; Mangia, A.; Gasperini, S.; Pavone, F.S.; et al. Human Keloid Cultured Fibroblasts Irradiated with Blue LED Light: Evidence from an in Vitro Study. In Proceedings of the Medical Laser Applications and Laser-Tissue Interactions IX (2019), paper 11079_31; Optica Publishing Group, June 23 2019; p. 11079_31.
- Fraccalvieri, M.; Amadeo, G.; Bortolotti, P.; Ciliberti, M.; Garrubba, A.; Mosti, G.; Bianco, S.; Mangia, A.; Massa, M.; Hartwig, V.; et al. Effectiveness of Blue Light Photobiomodulation Therapy in the Treatment of Chronic Wounds. Results of the Blue Light for Ulcer Reduction (B.L.U.R.) Study. Ital J Dermatol Venerol 2022, 157, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Tatini, F.; Bacci, S.; Rossi, F. Blue LED Light Affects Mitochondria and Modulates Reactive Oxygen Species: Preliminary in Vitro Results. In Proceedings of the Translational Biophotonics: Diagnostics and Therapeutics III; Lilge, L.D., Huang, Z., Eds.; SPIE: Munich, Germany, August 11, 2023; p. 37. [Google Scholar]
- André-Lévigne, D.; Modarressi, A.; Pepper, M.S.; Pittet-Cuénod, B. Reactive Oxygen Species and NOX Enzymes Are Emerging as Key Players in Cutaneous Wound Repair. Int J Mol Sci 2017, 18, 2149. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int Wound J 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Naik, E.; Dixit, V.M. Mitochondrial Reactive Oxygen Species Drive Proinflammatory Cytokine Production. J Exp Med 2011, 208, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell Mol Life Sci 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Magni, G.; Banchelli, M.; Cherchi, F.; Coppi, E.; Fraccalvieri, M.; Rossi, M.; Tatini, F.; Pugliese, A.M.; Rossi Degl’Innocenti, D.; Alfieri, D.; et al. Experimental Study on Blue Light Interaction with Human Keloid-Derived Fibroblasts. Biomedicines 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Magni, G.; Tatini, F.; Banchelli, M.; Cherchi, F.; Rossi, M.; Coppi, E.; Pugliese, A.M.; Rossi degl’Innocenti, D.; Alfieri, D.; et al. Photobiomodulation of Human Fibroblasts and Keratinocytes with Blue Light: Implications in Wound Healing. Biomedicines 2021, 9, 41. [Google Scholar] [CrossRef]
- Ablon, G. Phototherapy with Light Emitting Diodes: Treating a Broad Range of Medical and Aesthetic Conditions in Dermatology. J Clin Aesthet Dermatol 2018, 11, 21–27. [Google Scholar]
- de Alencar Fernandes Neto, J.; Nonaka, C.F.W.; de Vasconcelos Catão, M.H.C. Effect of Blue LED on the Healing Process of Third-Degree Skin Burns: Clinical and Histological Evaluation. Lasers Med Sci 2019, 34, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, C.; Purpura, V.; Melandri, D. Blue Led Light in Burns: A New Treatment’s Modality. J Clin Investigat Dermatol 2021, 9, 5. [Google Scholar]
- Ankri, R.; Friedman, H.; Savion, N.; Kotev-Emeth, S.; Breitbart, H.; Lubart, R. Visible Light Induces Nitric Oxide (NO) Formation in Sperm and Endothelial Cells. Lasers Surg Med 2010, 42, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem Rev 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Khoo, V.B.; Soon, S.; Yap, C.J.; Chng, S.P.; Tang, T.Y. Use of Blue Light in the Management of Chronic Venous Ulcer in Asian Patients: A Case Series. Cureus 2021, 13, e17703. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.H.; Sensing, W.; Biron, J.A. Clinical Efficacy of Home-Use Blue-Light Therapy for Mild-to Moderate Acne. J Cosmet Laser Ther 2011, 13, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue Light for Infectious Diseases: Propionibacterium Acnes, Helicobacter Pylori, and Beyond? Drug Resist Updat 2012, 15, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Weinstabl, A.; Hoff-Lesch, S.; Merk, H.F.; Von Felbert, V. Prospective Randomized Study on the Efficacy of Blue Light in the Treatment of Psoriasis Vulgaris. Dermatology 2011, 223, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Glitzner, E.; Korosec, A.; Brunner, P.M.; Drobits, B.; Amberg, N.; Schonthaler, H.B.; Kopp, T.; Wagner, E.F.; Stingl, G.; Holcmann, M.; et al. Specific Roles for Dendritic Cell Subsets during Initiation and Progression of Psoriasis. EMBO Mol Med 2014, 6, 1312–1327. [Google Scholar] [CrossRef]
- Pfaff, S.; Liebmann, J.; Born, M.; Merk, H.F.; von Felbert, V. Prospective Randomized Long-Term Study on the Efficacy and Safety of UV-Free Blue Light for Treating Mild Psoriasis Vulgaris. Dermatology 2015, 231, 24–34. [Google Scholar] [CrossRef]
- Félix Garza, Z.C.; Liebmann, J.; Born, M.; Hilbers, P.A.J.; van Riel, N.A.W. A Dynamic Model for Prediction of Psoriasis Management by Blue Light Irradiation. Frontiers in Physiology 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Keemss, K.; Pfaff, S.C.; Born, M.; Liebmann, J.; Merk, H.F.; von Felbert, V. Prospective, Randomized Study on the Efficacy and Safety of Local UV-Free Blue Light Treatment of Eczema. Dermatology 2016, 232, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.-M.; Dräger, J.; Abels, C.; Landthaler, M. Chapter 1 History of Photodynamic Therapy in Dermatology. In Comprehensive Series in Photosciences; Calzavara-Pinton, P., Szeimies, R.-M., Ortel, B., Eds.; Photodynamic Therapy and Fluorescence Diagnosis in Dermatology; Elsevier, 2001; Vol. 2, pp. 3–15.
- Kang, K.; Bacci, S. Photodynamic Therapy. Biomedicines 2022, 10, 2701. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Applied Sciences 2021, 11, 3626. [Google Scholar] [CrossRef]
- Cappugi, P.; Campolmi, P.; Mavilia, L.; Prignano, F.; Rossi, R. Topical 5-Aminolevulinic Acid and Photodynamic Therapy in Dermatology: A Minireview. J Chemother 2001, 13, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Grandi, V.; Bacci, S.; Corsi, A.; Sessa, M.; Puliti, E.; Murciano, N.; Scavone, F.; Cappugi, P.; Pimpinelli, N. ALA-PDT Exerts Beneficial Effects on Chronic Venous Ulcers by Inducing Changes in Inflammatory Microenvironment, Especially through Increased TGF-Beta Release: A Pilot Clinical and Translational Study. Photodiagnosis Photodyn Ther 2018, 21, 252–256. [Google Scholar] [CrossRef]
- Vallejo, M.C.S.; Moura, N.M.M.; Ferreira Faustino, M.A.; Almeida, A.; Gonçalves, I.; Serra, V.V.; Neves, M.G.P.M.S. An Insight into the Role of Non-Porphyrinoid Photosensitizers for Skin Wound Healing. Int J Mol Sci 2020, 22, 234. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.-M.; Basset-Seguin, N.; Calzavara-Pinton, P.; Gilaberte, Y.; Haedersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum Guidelines on Topical Photodynamic Therapy 2019 Part 1: Treatment Delivery and Established Indications - Actinic Keratoses, Bowen’s Disease and Basal Cell Carcinomas. J Eur Acad Dermatol Venereol 2019, 33, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Szeimies, R.-M.; Basset-Séguin, N.; Calzavara-Pinton, P.G.; Gilaberte, Y.; Haedersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum Guidelines on Topical Photodynamic Therapy 2019 Part 2: Emerging Indications - Field Cancerization, Photorejuvenation and Inflammatory/Infective Dermatoses. J Eur Acad Dermatol Venereol 2020, 34, 17–29. [Google Scholar] [CrossRef]
- Vallejo, M.C.S.; Moura, N.M.M.; Gomes, A.T.P.C.; Joaquinito, A.S.M.; Faustino, M.A.F.; Almeida, A.; Gonçalves, I.; Serra, V.V.; Neves, M.G.P.M.S. The Role of Porphyrinoid Photosensitizers for Skin Wound Healing. Int J Mol Sci 2021, 22, 4121. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J Clin 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Busch, T.M.; Snyder, J.W. Fluence Rate as a Modulator of PDT Mechanisms. Lasers Surg Med 2006, 38, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V.; Chung, T.-Y.; Baxter, G.D. Photodynamic Modulation of Wound Healing: A Review of Human and Animal Studies. Photomed Laser Surg 2012, 30, 118–148. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune Response after Photodynamic Therapy Increases Anti-Cancer and Anti-Bacterial Effects. World J Immunol 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.; Lecci, P.P.; Bacci, S.; Cappugi, P.; Pimpinelli, N. Early Activation of Fibroblasts during PDT Treatment in Leg Ulcers. G Ital Dermatol Venereol 2016, 151, 223–229. [Google Scholar] [PubMed]
- Haensel, D.; Dai, X. Epithelial-to-Mesenchymal Transition in Cutaneous Wound Healing: Where We Are and Where We Are Heading. Dev Dyn 2018, 247, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Nesi-Reis, V.; Lera-Nonose, D.S.S.L.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of Photodynamic Therapy in Wound Healing: A Systematic Review. Photodiagnosis Photodyn Ther 2018, 21, 294–305. [Google Scholar] [CrossRef]
- Oyama, J.; Fernandes Herculano Ramos-Milaré, Á.C.; Lopes Lera-Nonose, D.S.S.; Nesi-Reis, V.; Galhardo Demarchi, I.; Alessi Aristides, S.M.; Juarez Vieira Teixeira, J.; Gomes Verzignassi Silveira, T.; Campana Lonardoni, M.V. Photodynamic Therapy in Wound Healing in Vivo: A Systematic Review. Photodiagnosis Photodyn Ther 2020, 30, 101682. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Grandi, V.; Corsi, A.; Pimpinelli, N.; Bacci, S. Cellular Mechanisms in Acute and Chronic Wounds after PDT Therapy: An Update. Biomedicines 2022, 10, 1624. [Google Scholar] [CrossRef]
- Steinman, L. Elaborate Interactions between the Immune and Nervous Systems. Nat Immunol 2004, 5, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Laverdet, B.; Danigo, A.; Girard, D.; Magy, L.; Demiot, C.; Desmoulière, A. Skin Innervation: Important Roles during Normal and Pathological Cutaneous Repair. Histol Histopathol 2015, 30, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.; Baguneid, M.; Bayat, A. The Role of Neuromediators and Innervation in Cutaneous Wound Healing. Acta Derm Venereol 2016, 96, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front Cell Neurosci 2019, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Grandi, V.; Paroli, G.; Puliti, E.; Bacci, S.; Pimpinelli, N. Single ALA-PDT Irradiation Induces Increase in Mast Cells Degranulation and Neuropeptide Acute Response in Chronic Venous Ulcers: A Pilot Study. Photodiagnosis Photodyn Ther 2021, 34, 102222. [Google Scholar] [CrossRef]
- Lee, M.; Rey, K.; Besler, K.; Wang, C.; Choy, J. Immunobiology of Nitric Oxide and Regulation of Inducible Nitric Oxide Synthase. Results Probl Cell Differ 2017, 62, 181–207. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.P.; Most, D.; Efron, D.T.; Tantry, U.; Fischel, M.H.; Barbul, A. The Role of iNOS in Wound Healing. Surgery 2001, 130, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, A.F. Nitric Oxide: A Newly Discovered Function on Wound Healing. Acta Pharmacol Sin 2005, 26, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Notari, L.; Nardini, P.; Grandi, V.; Corsi, A.; Pimpinelli, N.; Bacci, S. Neuroimmunomodulation in Chronic Wound Healing after Treatment with Photodynamic Therapy: The Role of iNOs. Medical Sciences Forum 2023, 21, 44. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Correction: Photobiomodulation: Lasers vs. Light Emitting Diodes? Photochem Photobiol Sci 2019, 18, 259–259. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation or Low-level Laser Therapy. Journal of Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, Y.; Meng, Z. Successful Treatment of Hyperpigmentation from Fixed Drug Eruption with a Low-dose and Large-spot Q-switched 1064 Nm Nd: YAG Laser. J of Cosmetic Dermatology 2023, 22, 2128–2130. [Google Scholar] [CrossRef]
- Doppegieter, M.; Van Der Beek, N.; Bakker, E.N.T.P.; Neumann, M.H.A.; Van Bavel, E. Effects of Pulsed Dye Laser Treatment in Psoriasis: A Nerve-wrecking Process? Experimental Dermatology 2023, 32, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guarino, M.; Jaén, P. Laser in Psoriasis. G Ital Dermatol Venereol 2009, 144, 573–581. [Google Scholar] [PubMed]
- Fernández-Guarino, M.; Harto, A.; Sánchez-Ronco, M.; García-Morales, I.; Jaén, P. Pulsed Dye Laser vs. Photodynamic Therapy in the Treatment of Refractory Nail Psoriasis: A Comparative Pilot Study. Acad Dermatol Venereol 2009, 23, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Mosca, R.C.; Ong, A.A.; Albasha, O.; Bass, K.; Arany, P. Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach. Adv Skin Wound Care 2019, 32, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Chamayou-Robert, C.; DiGiorgio, C.; Brack, O.; Doucet, O. Blue Light Induces DNA Damage in Normal Human Skin Keratinocytes. Photoderm Photoimm Photomed 2022, 38, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Barolet, D.; Boucher, A. Radiant near Infrared Light Emitting Diode Exposure as Skin Preparation to Enhance Photodynamic Therapy Inflammatory Type Acne Treatment Outcome. Lasers Surg Med 2010, 42, 171–178. [Google Scholar] [CrossRef]
- Ash, C.; Harrison, A.; Drew, S.; Whittall, R. A Randomized Controlled Study for the Treatment of Acne Vulgaris Using High-Intensity 414 Nm Solid State Diode Arrays. Journal of Cosmetic and Laser Therapy 2015, 17, 170–176. [Google Scholar] [CrossRef]
- Gold, M.H.; Biron, J.A.; Sensing, W. Clinical and Usability Study to Determine the Safety and Efficacy of the Silk’n Blue Device for the Treatment of Mild to Moderate Inflammatory Acne Vulgaris. Journal of Cosmetic and Laser Therapy 2014, 16, 108–113. [Google Scholar] [CrossRef]
- Yilmaz, O.; Senturk, N.; Yuksel, E.P.; Aydin, F.; Ozden, M.G.; Canturk, T.; Turanli, A. Evaluation of 532-Nm KTP Laser Treatment Efficacy on Acne Vulgaris with Once and Twice Weekly Applications. Journal of Cosmetic and Laser Therapy 2011, 13, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Lekwuttikarn, R.; Tempark, T.; Chatproedprai, S.; Wananukul, S. Randomized, Controlled Trial Split-faced Study of 595-nm Pulsed Dye Laser in the Treatment of Acne Vulgaris and Acne Erythema in Adolescents and Early Adulthood. Int J Dermatology 2017, 56, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Ko, E.J.; Seo, S.J.; Hong, C.K. Comparison of Fractional, Nonablative, 1550-Nm Laser and 595-Nm Pulsed Dye Laser for the Treatment of Facial Erythema Resulting from Acne: A Split-Face, Evaluator-Blinded, Randomized Pilot Study. Journal of Cosmetic and Laser Therapy 2014, 16, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Chalermsuwiwattanakan, N.; Rojhirunsakool, S.; Kamanamool, N.; Kanokrungsee, S.; Udompataikul, M. The Comparative Study of Efficacy between 1064-nm Long-pulsed Nd:YAG Laser and 595-nm Pulsed Dye Laser for the Treatment of Acne Vulgaris. J of Cosmetic Dermatology 2021, 20, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Choi, S.C.; Jung, J.Y.; Bae, Y.; Park, G.-H. A Novel Combined Light-Based Treatment of Acne Vulgaris With 1,450-Nm Diode Laser and 450-Nm Blue Light. Dermatol Surg 2019, 45, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Eid, M.M.; Saleh, M.S.; Allam, N.M.; Elsherbini, D.M.; Abdelbasset, W.K.; Eladl, H.M. Narrow Band Ultraviolet B Versus Red Light-Emitting Diodes in the Treatment of Facial Acne Vulgaris: A Randomized Controlled Trial. Photobiomodulation, Photomedicine, and Laser Surgery 2021, 39, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Nitayavardhana, S.; Manuskiatti, W.; Cembrano, K.A.G.; Wanitphadeedecha, R. A Comparative Study Between Once-Weekly and Alternating Twice-Weekly Regimen Using Blue (470 Nm) and Red (640 Nm) Light Combination LED Phototherapy for Moderate-to-Severe Acne Vulgaris. Lasers Surg Med 2021, 53, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, X.; An, Y.; Zhang, J.; Wang, C.; Yang, R. Randomized Trial of Three Phototherapy Methods for the Treatment of Acne Vulgaris in C Hinese Patients. Photoderm Photoimm Photomed 2014, 30, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Lee, J.B.; Yoon, J.Y.; Park, S.Y.; Ryu, H.H.; Park, B.M.; Kim, Y.J.; Suh, D.H. The Clinical and Histological Effect of Home-Use, Combination Blue-Red LED Phototherapy for Mild-to-Moderate Acne Vulgaris in Korean Patients: A Double-Blind, Randomized Controlled Trial: Blue-Red LED Phototherapy in the Treatment of Acne. Br J Dermatol 2013, 168, 1088–1094. [Google Scholar] [CrossRef]
- Li, Y.; Xia, J.; Zhu, Y.; He, S.; Liu, J.; Zeng, W.; Wang, Z. Efficacy and Safety of Low-Level Light Therapy by Delicate Pulsed Light Combined with Low-Dose Oral Isotretinoin for the Treatment of Acne Vulgaris: A Randomized Split-Face Study. Lasers Med Sci 2022, 37, 3221–3229. [Google Scholar] [CrossRef]
- Alba, M.N.; Gerenutti, M.; Yoshida, V.M.H.; Grotto, D. Clinical Comparison of Salicylic Acid Peel and LED-Laser Phototherapy for the Treatment of Acne Vulgaris in Teenagers. Journal of Cosmetic and Laser Therapy 2017, 19, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne Vulgaris: A Review of the Pathophysiology, Treatment, and Recent Nanotechnology Based Advances. Biochemistry and Biophysics Reports 2023, 36, 101578. [Google Scholar] [CrossRef]
- Szymańska, A.; Budzisz, E.; Erkiert-Polguj, A. The Anti-Acne Effect of Near-Infrared Low-Level Laser Therapy. CCID 2021, Volume 14, 1045–1051. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Suh, D.-H. Study of the Photoinactivation Effect on Propionibacterium acnes after Light Irradiation with Variable Wavelengths. Korean Journal of Dermatology 2006, 1332–1338. [Google Scholar]
- Jung, Y.R.; Kim, S.J.; Sohn, K.C.; Lee, Y.; Seo, Y.J.; Lee, Y.H.; Whang, K.U.; Kim, C.D.; Lee, J.H.; Im, M. Regulation of Lipid Production by Light-Emitting Diodes in Human Sebocytes. Arch Dermatol Res 2015, 307, 265–273. [Google Scholar] [CrossRef]
- Bonnans, M.; Fouque, L.; Pelletier, M.; Chabert, R.; Pinacolo, S.; Restellini, L.; Cucumel, K. Blue Light: Friend or Foe ? Journal of Photochemistry and Photobiology B: Biology 2020, 212, 112026. [Google Scholar] [CrossRef] [PubMed]
- de Arruda, L.H.F.; Kodani, V.; Bastos Filho, A.; Mazzaro, C.B. [A prospective, randomized, open and comparative study to evaluate the safety and efficacy of blue light treatment versus a topical benzoyl peroxide 5% formulation in patients with acne grade II and III]. An Bras Dermatol 2009, 84, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Rojanamatin, J.; Choawawanich, P. Treatment of Inflammatory Facial Acne Vulgaris with Intense Pulsed Light and Short Contact of Topical 5-Aminolevulinic Acid: A Pilot Study. Dermatol Surg 2006, 32, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, K.-H.; Choi, J.-W.; Kwon, J.-K.; Lee, D.R.; Shin, M.S.; Lee, J.S.; You, C.E.; Park, M.Y. A Prospective, Randomized, Placebo-Controlled, Double-Blinded, and Split-Face Clinical Study on LED Phototherapy for Skin Rejuvenation: Clinical, Profilometric, Histologic, Ultrastructural, and Biochemical Evaluations and Comparison of Three Different Treatment Settings. Journal of Photochemistry and Photobiology B: Biology 2007, 88, 51–67. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Amin, S.; Russell, B.A.; Phelps, R.; Kellett, N.; Reilly, L.A. Combined 633-Nm and 830-Nm Led Treatment of Photoaging Skin. J Drugs Dermatol 2006, 5, 748–753. [Google Scholar]
- Baez, F.; Reilly, L.R. The Use of Light-emitting Diode Therapy in the Treatment of Photoaged Skin. J of Cosmetic Dermatology 2007, 6, 189–194. [Google Scholar] [CrossRef]
- Wunsch, A.; Matuschka, K. A Controlled Trial to Determine the Efficacy of Red and Near-Infrared Light Treatment in Patient Satisfaction, Reduction of Fine Lines, Wrinkles, Skin Roughness, and Intradermal Collagen Density Increase. Photomedicine and Laser Surgery 2014, 32, 93–100. [Google Scholar] [CrossRef]
- Weiss, R.A.; McDaniel, D.H.; Geronemus, R.G.; Weiss, M.A. Clinical Trial of a Novel Non-thermal LED Array for Reversal of Photoaging: Clinical, Histologic, and Surface Profilometric Results. Lasers Surg Med 2005, 36, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.A.; Kellett, N.; Reilly, L.R. A Study to Determine the Efficacy of Combination LED Light Therapy (633 Nm and 830 Nm) in Facial Skin Rejuvenation. Journal of Cosmetic and Laser Therapy 2005, 7, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.H.; Park, B.C.; Kim, M.H.; Choi, E.H.; Hong, S.P. The Efficacy and Safety of 660 Nm and 411 to 777 Nm Light-Emitting Devices for Treating Wrinkles. Dermatol Surg 2017, 43, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Mota, L.R.; Duarte, I.D.S.; Galache, T.R.; Pretti, K.M.D.S.; Neto, O.C.; Motta, L.J.; Horliana, A.C.R.T.; Silva, D.D.F.T.D.; Pavani, C. Photobiomodulation Reduces Periocular Wrinkle Volume by 30%: A Randomized Controlled Trial. Photobiomodulation, Photomedicine, and Laser Surgery 2023, 41, 48–56. [Google Scholar] [CrossRef]
- Manuskiatti, W. Treatment Response of Keloidal and Hypertrophic Sternotomy Scars: Comparison Among Intralesional Corticosteroid, 5-Fluorouracil, and 585-Nm Flashlamp-Pumped Pulsed-Dye Laser Treatments. Arch Dermatol 2002, 138, 1149. [Google Scholar] [CrossRef]
- Friedman, P.M.; Polder, K.D.; Sodha, P.; Geronemus, R.G. The 1440 Nm and 1927 Nm Nonablative Fractional Diode Laser: Current Trends and Future Directions. J Drugs Dermatol 2020, 19, s3–s11. [Google Scholar]
- Laubach, H.-J.; Tannous, Z.; Anderson, R.R.; Manstein, D. Skin Responses to Fractional Photothermolysis. Lasers Surg Med 2006, 38, 142–149. [Google Scholar] [CrossRef]
- Barolet, D.; Roberge, C.J.; Auger, F.A.; Boucher, A.; Germain, L. Regulation of Skin Collagen Metabolism in Vitro Using a Pulsed 660 Nm LED Light Source: Clinical Correlation with a Single-Blinded Study. J Invest Dermatol 2009, 129, 2751–2759. [Google Scholar] [CrossRef]
- Migliario, M.; Rizzi, M.; Rocchetti, V.; Cannas, M.; Renò, F. In Vitro Toxicity of Photodynamic Antimicrobial Chemotherapy on Human Keratinocytes Proliferation. Lasers Med Sci 2013, 28, 565–569. [Google Scholar] [CrossRef]
- Li, W.-H.; Seo, I.; Kim, B.; Fassih, A.; Southall, M.D.; Parsa, R. Low-Level Red plus near Infrared Lights Combination Induces Expressions of Collagen and Elastin in Human Skin in Vitro. Int J Cosmet Sci 2021, 43, 311–320. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J Sel Top Quantum Electron 2016, 22, 7000417. [Google Scholar] [CrossRef]
- Neves, L.M.G.; Parizotto, N.A.; Tim, C.R.; Floriano, E.M.; Lopez, R.F.V.; Venâncio, T.; Fernandes, J.B.; Cominetti, M.R. Polysaccharide-Rich Hydrogel Formulation Combined with Photobiomodulation Repairs UV-Induced Photodamage in Mice Skin. Wound Repair Regen 2020, 28, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and Molecular Processes in Wound Healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef] [PubMed]
- Bacci, S. Fine Regulation during Wound Healing by Mast Cells, a Physiological Role Not Yet Clarified. IJMS 2022, 23, 1820. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int J Inflam 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int J Mol Sci 2016, 17, 2085. [Google Scholar] [CrossRef]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic Wounds. Nat Rev Dis Primers 2022, 8, 50. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The Humanistic and Economic Burden of Chronic Wounds: A Protocol for a Systematic Review. Syst Rev 2017, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical Challenges of Chronic Wounds: Searching for an Optimal Animal Model to Recapitulate Their Complexity. Disease Models & Mechanisms 2014, 7, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guarino, M.; Bacci, S.; Pérez González, L.A.; Bermejo-Martínez, M.; Cecilia-Matilla, A.; Hernández-Bule, M.L. The Role of Physical Therapies in Wound Healing and Assisted Scarring. IJMS 2023, 24, 7487. [Google Scholar] [CrossRef]
- Grandi, V.; Corsi, A.; Pimpinelli, N.; Bacci, S. Cellular Mechanisms in Acute and Chronic Wounds after PDT Therapy: An Update. Biomedicines 2022, 10, 1624. [Google Scholar] [CrossRef]
- Asilian, A.; Darougheh, A.; Shariati, F. New Combination of Triamcinolone, 5-Fluorouracil, and Pulsed-Dye Laser for Treatment of Keloid and Hypertrophic Scars. Dermatol Surg 2006, 32, 907–915. [Google Scholar] [CrossRef]
- Manuskiatti, W.; Wanitphakdeedecha, R.; Fitzpatrick, R.E. Effect of Pulse Width of a 595-Nm Flashlamp-Pumped Pulsed Dye Laser on the Treatment Response of Keloidal and Hypertrophic Sternotomy Scars. Dermatol Surg 2007, 33, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Cassuto, D.A.; Scrimali, L.; Siragò, P. Treatment of Hypertrophic Scars and Keloids with an LBO Laser (532 Nm) and Silicone Gel Sheeting. Journal of Cosmetic and Laser Therapy 2010, 12, 32–37. [Google Scholar] [CrossRef]
- Al-Mohamady, A.E.-S.A.E.-H.; Ibrahim, S.M.A.; Muhammad, M.M. Pulsed Dye Laser versus Long-Pulsed Nd:YAG Laser in the Treatment of Hypertrophic Scars and Keloid: A Comparative Randomized Split-Scar Trial. Journal of Cosmetic and Laser Therapy 2016, 18, 208–212. [Google Scholar] [CrossRef]
- Pongcharoen, P.; Pongcharoen, B.; Disphanurat, W. The Effectiveness of a 595 Nm Pulsed-Dye-Laser in the Treatment of Surgical Scars Following a Knee Arthroplasty. Journal of Cosmetic and Laser Therapy 2019, 21, 352–356. [Google Scholar] [CrossRef]
- Ramadan, H.; Saber, M.; Salah, M.; Samy, N. The Effectiveness of Long Pulsed Nd:YAG Laser Alone for Treatment of Keloids and Hypertrophic Scars versus Its Combination with Bleomycin. J of Cosmetic Dermatology 2021, 20, 3899–3906. [Google Scholar] [CrossRef]
- Ilknur, T.; Akarsu, S.; Aktan, Ş.; Özkan, Ş. Comparison of the Effects of Pulsed Dye Laser, Pulsed Dye Laser+Salicylic Acid, and Clobetasole Propionate+Salicylic Acid on Psoriatic Plaques: PULSED DYE LASER AND PSORIASIS. Dermatologic Surgery 2008, 32, 49–55. [Google Scholar] [CrossRef] [PubMed]
- van Lingen, R.G.; de Jong, E.M.G.J.; van Erp, P.E.J.; van Meeteren, W.S.E.C.; van De Kerkhof, P.C.M.; Seyger, M.M.B. Nd: YAG Laser (1,064 Nm) Fails to Improve Localized Plaque Type Psoriasis: A Clinical and Immunohistochemical Pilot Study. Eur J Dermatol 2008, 18, 671–676. [Google Scholar] [CrossRef]
- De Leeuw, J.; Van Lingen, R.G.; Both, H.; Tank, B.; Nijsten, T.; Martino Neumann, H.A. A Comparative Study on the Efficacy of Treatment with 585 Nm Pulsed Dye Laser and Ultraviolet B-TL01 in Plaque Type Psoriasis. Dermatologic Surgery 2009, 35, 80–91. [Google Scholar] [CrossRef]
- Treewittayapoom, C.; Singvahanont, P.; Chanprapaph, K.; Haneke, E. The Effect of Different Pulse Durations in the Treatment of Nail Psoriasis with 595-Nm Pulsed Dye Laser: A Randomized, Double-Blind, Intrapatient Left-to-Right Study. Journal of the American Academy of Dermatology 2012, 66, 807–812. [Google Scholar] [CrossRef]
- Fife, D.; Rayhan, D.J.; Behnam, S.; Ortiz, A.; Elkeeb, L.; Aquino, L.; Roa, E.D.; Ramsinghani, N.; Kuo, J.; Newcomb, R.; et al. A Randomized, Controlled, Double-Blind Study of Light Emitting Diode Photomodulation for the Prevention of Radiation Dermatitis in Patients with Breast Cancer. Dermatologic Surgery 2010, 36, 1921–1927. [Google Scholar] [CrossRef]
- Strouthos, I.; Chatzikonstantinou, G.; Tselis, N.; Bon, D.; Karagiannis, E.; Zoga, E.; Ferentinos, K.; Maximenko, J.; Nikolettou-Fischer, V.; Zamboglou, N. Photobiomodulation Therapy for the Management of Radiation-Induced Dermatitis: A Single-Institution Experience of Adjuvant Radiotherapy in Breast Cancer Patients after Breast Conserving Surgery. Strahlenther Onkol 2017, 193, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Silva, S.B.; Quinto, A.L.P.; Pasquinelli, P.F.S.; De Queiroz Dos Santos, V.; De Cássia Santos, G.; Veiga, D.F. Phototherapy 660 Nm for the Prevention of Radiodermatitis in Breast Cancer Patients Receiving Radiation Therapy: Study Protocol for a Randomized Controlled Trial. Trials 2014, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Robijns, J.; Censabella, S.; Claes, S.; Pannekoeke, L.; Bussé, L.; Colson, D.; Kaminski, I.; Lodewijckx, J.; Bulens, P.; Maes, A.; et al. Biophysical Skin Measurements to Evaluate the Effectiveness of Photobiomodulation Therapy in the Prevention of Acute Radiation Dermatitis in Breast Cancer Patients. Support Care Cancer 2019, 27, 1245–1254. [Google Scholar] [CrossRef]
- Robijns, J.; Lodewijckx, J.; Claes, S.; Van Bever, L.; Pannekoeke, L.; Censabella, S.; Bussé, L.; Colson, D.; Kaminski, I.; Broux, V.; et al. Photobiomodulation Therapy for the Prevention of Acute Radiation Dermatitis in Head and Neck Cancer Patients (DERMISHEAD Trial). Radiotherapy and Oncology 2021, 158, 268–275. [Google Scholar] [CrossRef]
- Robijns, J.; Lodewijckx, J.; Puts, S.; Vanmechelen, S.; Van Bever, L.; Claes, S.; Pannekoeke, L.; Timmermans, A.; Noé, L.; Govers, M.; et al. Photobiomodulation Therapy for the Prevention of Acute Radiation Dermatitis in Breast Cancer Patients Undergoing Hypofractioned Whole-breast Irradiation (LABRA Trial). Lasers Surg Med 2022, 54, 374–383. [Google Scholar] [CrossRef]
- Are LED Lights Safe for Human Health? - European Commission Available online:. Available online: https://health.ec.europa.eu/scientific-committees/easy-read-summaries-scientific-opinions/are-led-lights-safe-human-health_en (accessed on 3 March 2024).
- Mineroff, J.; Austin, E.; Jagdeo, J. Cutaneous Effects of Photobiomodulation with 1072 Nm Light. Arch Dermatol Res 2022, 315, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Patra, V.; Bordag, N.; Clement, Y.; Köfeler, H.; Nicolas, J.-F.; Vocanson, M.; Ayciriex, S.; Wolf, P. Ultraviolet Exposure Regulates Skin Metabolome Based on the Microbiome. Sci Rep 2023, 13, 7207. [Google Scholar] [CrossRef]
- Gupta, A.; Avci, P.; Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Ultraviolet Radiation in Wound Care: Sterilization and Stimulation. Advances in Wound Care 2013, 2, 422–437. [Google Scholar] [CrossRef]
- Hart, P.H.; Norval, M. More Than Effects in Skin: Ultraviolet Radiation-Induced Changes in Immune Cells in Human Blood. Front. Immunol. 2021, 12, 694086. [Google Scholar] [CrossRef]
- Schwarz, T.; Schwarz, A. Molecular Mechanisms of Ultraviolet Radiation-Induced Immunosuppression. Eur J Cell Biol 2011, 90, 560–564. [Google Scholar] [CrossRef]
- Purbhoo-Makan, M.; Houreld, N.N.; Enwemeka, C.S. The Effects of Blue Light on Human Fibroblasts and Diabetic Wound Healing. Life 2022, 12, 1431. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, P.; Li, J.; Guo, C.; Zhai, J.; Zhang, Y. Efficacy of Laser Therapy Combined with Topical Antifungal Agents for Onychomycosis: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Lasers Med Sci 2022, 37, 2557–2569. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Can Light-based Approaches Overcome Antimicrobial Resistance? Drug Development Research 2019, 80, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Bumah, V.V.; Morrow, B.N.; Cortez, P.M.; Bowman, C.R.; Rojas, P.; Masson-Meyers, D.S.; Suprapto, J.; Tong, W.G.; Enwemeka, C.S. The Importance of Porphyrins in Blue Light Suppression of Streptococcus Agalactiae. Journal of Photochemistry and Photobiology B: Biology 2020, 212, 111996. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, N.T.A.; Santos, M.F.; Gomes, R.C.; Brandino, H.E.; Martinez, R.; De Jesus Guirro, R.R. Blue Laser Inhibits Bacterial Growth of Staphylococcus Aureus, Escherichia Coli, and Pseudomonas Aeruginosa. Photomedicine and Laser Surgery 2015, 33, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Leong, A.; McMullin, G. Blue Light Therapy in the Management of Chronic Wounds: A Narrative Review of Its Physiological Basis and Clinical Evidence. Wounds 2023, 35, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Naranjo García, P.; López Andrino, R.; Gómez González, C.; Pinto, H. Three Wavelengths Integrated: Efficacy and Safety of a Novel Combination for Hair Removal. J of Cosmetic Dermatology 2022, 21, 259–267. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Keshri, G.K.; Gupta, A. Dual-NIR Wavelength (Pulsed 810 Nm and Superpulsed 904 Nm Lasers) Photobiomodulation Therapy Synergistically Augments Full-Thickness Burn Wound Healing: A Non-Invasive Approach. Journal of Photochemistry and Photobiology B: Biology 2023, 246, 112761. [Google Scholar] [CrossRef] [PubMed]
- Noirrit-Esclassan, E.; Valera, M.C.; Vignes, E.; Munzer, C.; Bonal, S.; Daries, M.; Vaysse, F.; Puiseux, C.; Castex, M.P.; Boulanger, C.; et al. Photobiomodulation with a Combination of Two Wavelengths in the Treatment of Oral Mucositis in Children: The PEDIALASE Feasibility Study. Archives de Pédiatrie 2019, 26, 268–274. [Google Scholar] [CrossRef]
- Nizamutdinov, D.; Ezeudu, C.; Wu, E.; Huang, J.H.; Yi, S.S. Transcranial Near-Infrared Light in Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2022, 13, 965788. [Google Scholar] [CrossRef]
- Hong, N. Photobiomodulation as a Treatment for Neurodegenerative Disorders: Current and Future Trends. Biomed. Eng. Lett. 2019, 9, 359–366. [Google Scholar] [CrossRef]
- Gazor, R.; Asgari, M.; Abdollajhifar, M.-A.; Kiani, P.; Zare, F.; Fadaei Fathabady, F.; Norouzian, M.; Amini, A.; Khosravipour, A.; Atashgah, R.B.; et al. Simultaneous Treatment of Photobiomodulation and Demineralized Bone Matrix With Adipose-Derived Stem Cells Improve Bone Healing in an Osteoporotic Bone Defect. J Lasers Med Sci 2021, 12, e41–e41. [Google Scholar] [CrossRef]
- Vassão, P.G.; Silva, B.A.; De Souza, M.C.; Parisi, J.R.; De Camargo, M.R.; Renno, A.C.M. Level of Pain, Muscle Strength and Posture: Effects of PBM on an Exercise Program in Women with Knee Osteoarthritis – a Randomized Controlled Trial. Lasers Med Sci 2020, 35, 1967–1974. [Google Scholar] [CrossRef]
- Keshmiri, S.; Velayati, M.; Momenzadeh, S. Clinical Effectiveness of Ultrasound-Guided Biolaser Versus Ozone Therapy in Reducing Chronic Pain in Knee Osteoarthritis: A Three-Month Follow-Up Study. Iran J Radiol 2023, 20. [Google Scholar] [CrossRef]
- Rossetti, M.; Napierala, J.; Matuschek, N.; Achatz, U.; Duelk, M.; Vélez, C.; Castiglia, A.; Grandjean, N.; Dorsaz, J.; Feltin, E. Superluminescent Light Emitting Diodes: The Best out of Two Worlds.; Schenk, H., Piyawattanametha, W., Noell, W., Eds.; San Francisco, California, USA, 2012; p. 825208. 9 February.
- Kim, H.-J.; Sritandi, W.; Xiong, Z.; Ho, J.S. Bioelectronic Devices for Light-Based Diagnostics and Therapies. Biophysics Reviews 2023, 4, 011304. [Google Scholar] [CrossRef]
- Cohen, M.; Austin, E.; Masub, N.; Kurtti, A.; George, C.; Jagdeo, J. Home-Based Devices in Dermatology: A Systematic Review of Safety and Efficacy. Arch Dermatol Res 2022, 314, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P. Combinations of Photodynamic Therapy with Other Minimally Invasive Therapeutic Technologies against Cancer and Microbial Infections. IJMS 2023, 24, 10875. [Google Scholar] [CrossRef]
- Lee, G.-H.; Moon, H.; Kim, H.; Lee, G.H.; Kwon, W.; Yoo, S.; Myung, D.; Yun, S.H.; Bao, Z.; Hahn, S.K. Multifunctional Materials for Implantable and Wearable Photonic Healthcare Devices. Nat Rev Mater 2020, 5, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Paradigm Shift in Future Biophotonics for Imaging and Therapy: Miniature Living Lasers to Cellular Scale Optoelectronics. Theranostics 2022, 12, 7335–7350. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Gholipour-Khalili, S.; Farajdokht, F.; Kamari, F.; Walski, T.; Hamblin, M.R.; DiDuro, J.O.; Cassano, P. Therapeutic Potential of Intranasal Photobiomodulation Therapy for Neurological and Neuropsychiatric Disorders: A Narrative Review. Reviews in the Neurosciences 2020, 31, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Jo, I.-Y.; Byeon, H.-K.; Ban, M.-J.; Park, J.-H.; Lee, S.-C.; Won, Y.K.; Eun, Y.-S.; Kim, J.-Y.; Yang, N.-G.; Lee, S.-H.; et al. Effect of a Novel Handheld Photobiomodulation Therapy Device in the Management of Chemoradiation Therapy-Induced Oral Mucositis in Head and Neck Cancer Patients: A Case Series Study. Photonics 2023, 10, 241. [Google Scholar] [CrossRef]
- Langella, L.G.; Casalechi, H.L.; Tomazoni, S.S.; Johnson, D.S.; Albertini, R.; Pallotta, R.C.; Marcos, R.L.; De Carvalho, P.D.T.C.; Leal-Junior, E.C.P. Photobiomodulation Therapy (PBMT) on Acute Pain and Inflammation in Patients Who Underwent Total Hip Arthroplasty—a Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Lasers Med Sci 2018, 33, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu Avsar, N.; Balkaya, U.; Yarali Cevik, Z.B. Design of Portable Multicolor LED-Based Optical System for the Photobiomodulation Therapy on Wound Healing Process. J Intell Syst Appl 2021, 61–67. [Google Scholar] [CrossRef]
- Sutterby, E.; Chheang, C.; Thurgood, P.; Khoshmanesh, K.; Baratchi, S.; Pirogova, E. Investigating the Effects of Low Intensity Visible Light on Human Keratinocytes Using a Customized LED Exposure System. Sci Rep 2022, 12, 18907. [Google Scholar] [CrossRef]
- Phan, D.T.; Nguyen, C.H.; Nguyen, T.D.P.; Tran, L.H.; Park, S.; Choi, J.; Lee, B.; Oh, J. A Flexible, Wearable, and Wireless Biosensor Patch with Internet of Medical Things Applications. Biosensors 2022, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Trajano, L.A.D.S.N.; Sergio, L.P.D.S.; Stumbo, A.C.; Mencalha, A.L.; Fonseca, A.D.S.D. Low Power Lasers on Genomic Stability. Journal of Photochemistry and Photobiology B: Biology 2018, 180, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R.; Kurzrock, R. Dermatologic Disease-Directed Targeted Therapy (D3T2): The Application of Biomarker-Based Precision Medicine for the Personalized Treatment of Skin Conditions—Precision Dermatology. Dermatol Ther (Heidelb) 2022. [Google Scholar] [CrossRef]
- Yu, L.; Li, L. Potential Biomarkers of Atopic Dermatitis. Front. Med. 2022, 9, 1028694. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, N.; Sidiroglou, F.; Apostolopoulos, V.; Feehan, J. Transcriptome Analysis of the Effects of Polarized Photobiomodulation on Human Dermal Fibroblasts. Journal of Photochemistry and Photobiology B: Biology 2023, 242, 112696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, M.X. A Clinical Review of Phototherapy for Psoriasis. Lasers Med Sci 2018, 33, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Lim, H.W. Ultraviolet B Phototherapy for Psoriasis: Review of Practical Guidelines. Am J Clin Dermatol 2016, 17, 125–133. [Google Scholar] [CrossRef]
- Elmets, C.A.; Lim, H.W.; Stoff, B.; Connor, C.; Cordoro, K.M.; Lebwohl, M.; Armstrong, A.W.; Davis, D.M.R.; Elewski, B.E.; Gelfand, J.M.; et al. Joint American Academy of Dermatology-National Psoriasis Foundation Guidelines of Care for the Management and Treatment of Psoriasis with Phototherapy. J Am Acad Dermatol 2019, 81, 775–804. [Google Scholar] [CrossRef] [PubMed]
- Archier, E.; Devaux, S.; Castela, E.; Gallini, A.; Aubin, F.; Le Maître, M.; Aractingi, S.; Bachelez, H.; Cribier, B.; Joly, P.; et al. Efficacy of Psoralen UV-A Therapy vs. Narrowband UV-B Therapy in Chronic Plaque Psoriasis: A Systematic Literature Review. J Eur Acad Dermatol Venereol, 3. [CrossRef]
- R Hamblin, M. ; 1 Wellman Center for Photomedicine, Massachusetts General Hospital, BAR414, 40 Blossom Street, Boston, MA 02114, USA; 2 Department of Dermatology, Harvard Medical School, Boston, MA 02115, USA; 3 Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA 02139, USA Mechanisms and Applications of the Anti-Inflammatory Effects of Photobiomodulation. AIMS Biophysics 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Ablon, G. Combination 830-Nm and 633-Nm Light-Emitting Diode Phototherapy Shows Promise in the Treatment of Recalcitrant Psoriasis: Preliminary Findings. Photomedicine and Laser Surgery 2010, 28, 141–146. [Google Scholar] [CrossRef]
- Niu, T.; Tian, Y.; Cai, Q.; Ren, Q.; Wei, L. Red Light Combined with Blue Light Irradiation Regulates Proliferation and Apoptosis in Skin Keratinocytes in Combination with Low Concentrations of Curcumin. PLoS ONE 2015, 10, e0138754. [Google Scholar] [CrossRef] [PubMed]
- Krings, L.; Liebmann, J.; Born, M.; Leverkus, M. A Randomized Study Comparing the Efficacy and Safety of Blue Light and Topical Vitamin D Treatments for Mild Psoriasis Vulgaris.
- Jekal, S.-J.; Park, M.-S.; Kim, D.-J. The Combined Effects of Curcumin Administration and 630 Nm LED Phototherapy against DNCB-Induced Atopic Dermatitis-like Skin Lesions in BALB/c Mice. Korean J Clin Lab Sci 2017, 49, 150–160. [Google Scholar] [CrossRef]
- Kim, Y.L.; Lim, H.S.; Lee, S.M. Effect of Low-Level Laser Intervention on Dermatitis Symptoms and Cytokine Changes in DNCB-Induced Atopy Mouse Model: A Randomized Controlled Trial. Exp Ther Med 2021, 22, 1196. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Alomar, A.; Bieber, T.; Deleuran, M.; Fink-Wagner, A.; Gelmetti, C.; Gieler, U.; Lipozencic, J.; Luger, T.; Oranje, A.P.; et al. Guidelines for Treatment of Atopic Eczema (Atopic Dermatitis) Part II. J Eur Acad Dermatol Venereol 2012, 26, 1176–1193. [Google Scholar] [CrossRef] [PubMed]
- Patrizi, A.; Raone, B.; Ravaioli, G.M. Management of Atopic Dermatitis: Safety and Efficacy of Phototherapy. Clin Cosmet Investig Dermatol 2015, 8, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Holme, S.A.; Anstey, A.V. Phototherapy and PUVA Photochemotherapy in Children. Photodermatol Photoimmunol Photomed 2004, 20, 69–75. [Google Scholar] [CrossRef]
- Kamata, Y.; Tominaga, M.; Takamori, K. Itch in Atopic Dermatitis Management. Curr Probl Dermatol 2016, 50, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.H.Y.; Murrell, D.F. Itch Management: Physical Approaches (UV Phototherapy, Acupuncture). Curr Probl Dermatol 2016, 50, 54–63. [Google Scholar] [CrossRef]
- El Samahy, M.H.; Attia, E.A.S.; Saad, A.A.; Mahmoud, E.Y. Circulating CD4(+) CD25(High) FoxP3(+) T-Regulatory Cells in Patients with Atopic Dermatitis after Narrowband-Ultraviolet B Phototherapy. Int J Dermatol 2015, 54, e424–e429. [Google Scholar] [CrossRef]
- Dogra, S.; Mahajan, R. ; Indian Association of Dermatologists, Venereologists and Leprologists Phototherapy for Atopic Dermatitis. Indian J Dermatol Venereol Leprol 2015, 81, 10–15. [Google Scholar] [CrossRef]
- Garritsen, F.M.; Brouwer, M.W.D.; Limpens, J.; Spuls, P.I. Photo(Chemo)Therapy in the Management of Atopic Dermatitis: An Updated Systematic Review with Implications for Practice and Research. Br J Dermatol 2014, 170, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Alomar, A.; Bieber, T.; Deleuran, M.; Fink-Wagner, A.; Gelmetti, C.; Gieler, U.; Lipozencic, J.; Luger, T.; Oranje, A.P.; et al. Guidelines for Treatment of Atopic Eczema (Atopic Dermatitis) Part I. J Eur Acad Dermatol Venereol 2012, 26, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, M.; Narbutt, J.; Lesiak, A. Blue Light in Dermatology. Life 2021, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, G.H.; Oberg, K.; Tucker, B.; Gaston, M. The Effectiveness and Safety of Topical PhotoActif Phosphatidylcholine-Based Anti-Cellulite Gel and LED (Red and near-Infrared) Light on Grade II-III Thigh Cellulite: A Randomized, Double-Blinded Study. J Cosmet Laser Ther 2007, 9, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Trelles, M.A.; Allones, I.; Mayo, E. Combined Visible Light and Infrared Light-Emitting Diode (LED) Therapy Enhances Wound Healing after Laser Ablative Resurfacing of Photodamaged Facial Skin. Medical Laser Application 2006, 21, 165–175. [Google Scholar] [CrossRef]
- Joshi, A.A.; Vocanson, M.; Nicolas, J.-F.; Wolf, P.; Patra, V. Microbial Derived Antimicrobial Peptides as Potential Therapeutics in Atopic Dermatitis. Front. Immunol. 2023, 14, 1125635. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Williams, D.; Enwemeka, S.K.; Hollosi, S.; Yens, D. Blue 470-Nm Light Kills Methicillin-Resistant Staphylococcus Aureus (MRSA) in Vitro. Photomed Laser Surg 2009, 27, 221–226. [Google Scholar] [CrossRef]
| AUTHOR/YEAR | TYPE OF LED | PATIENTS | DESIGN OF THE STUDY | PROTOCOL OF TREATMENT | RESULTS |
|---|---|---|---|---|---|
| Weis 2005 |
RL 590 nm | N=90 | 8 treatments in 4 weeks 6 months follow-up |
0,1J/cm2 pulsing | 90% improve photoaging. Histological response: -90% improve Collagen I -4% decrease MMPI |
| Rusell 2005 |
RL 630 nm+ NIR 830 nm |
N=31 | 9 light treatments Flow up week 9 and 12 |
RL 126 J/cm2 NIR 66 J/cm2 |
52% improvement in photoaging 81% patient report improvement in periocular wrinkles |
| Golberg 2007 |
RL 630 nm+ NIR 830 nm |
N=36 | 9 treatments in 12 weeks | RL 126 J/cm2 NIR 66 J/cm2 |
Significant improvement of softness, smoothness, and firmness |
| Yoon-Lee 2007 | RL 630 nm+ NIR 830 nm |
N=112 | 4 Groups: NIR, RL, NIR+RL and placebo 8 sesions, 4 weeks and 3 months follow-up |
RL 126 J/cm2 NIR 66 J/cm2 |
Both RL and NIR effective and significant wrinkle reduction Skin elasticity better NIR and NIR+RL Melanin decrease RL |
| Baez 2007 |
RL 630 nm+ NIR 830 nm |
N=30 | 9 sessions, 12 weeks | RL 126 J/cm2 NIR 66 J/cm2 |
91% color improvement 82% smoothness improvement 25-50% investigator assessment improvement |
| Wunsch 2014 |
RLT 611-650 nm ELT 570-850 nm |
N=136 | 2 sessions per week 30 treatments 3 Groups: RLT, ELT and placebo |
No different between wavelengths Both treatments significant differences in wrinkles |
|
| Hee-Naan 2017 | RL 660 nm LED 411-777 nm |
N=52 | 1 sessions/day 12 weeks 2 Groups: RL, LED |
5,17 J/cm2 | Both treatments improve significantly wrinkles |
| Rocha-Mota 2018 | RL 660 nm AL 590 nm |
N=137 Split-face |
10 sessions periocular 4 weeks |
3,8J/cm2 | Significant periocular wrinkles, with RL 31,6% and 29,9% with AL. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





