Submitted:
07 March 2024
Posted:
08 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
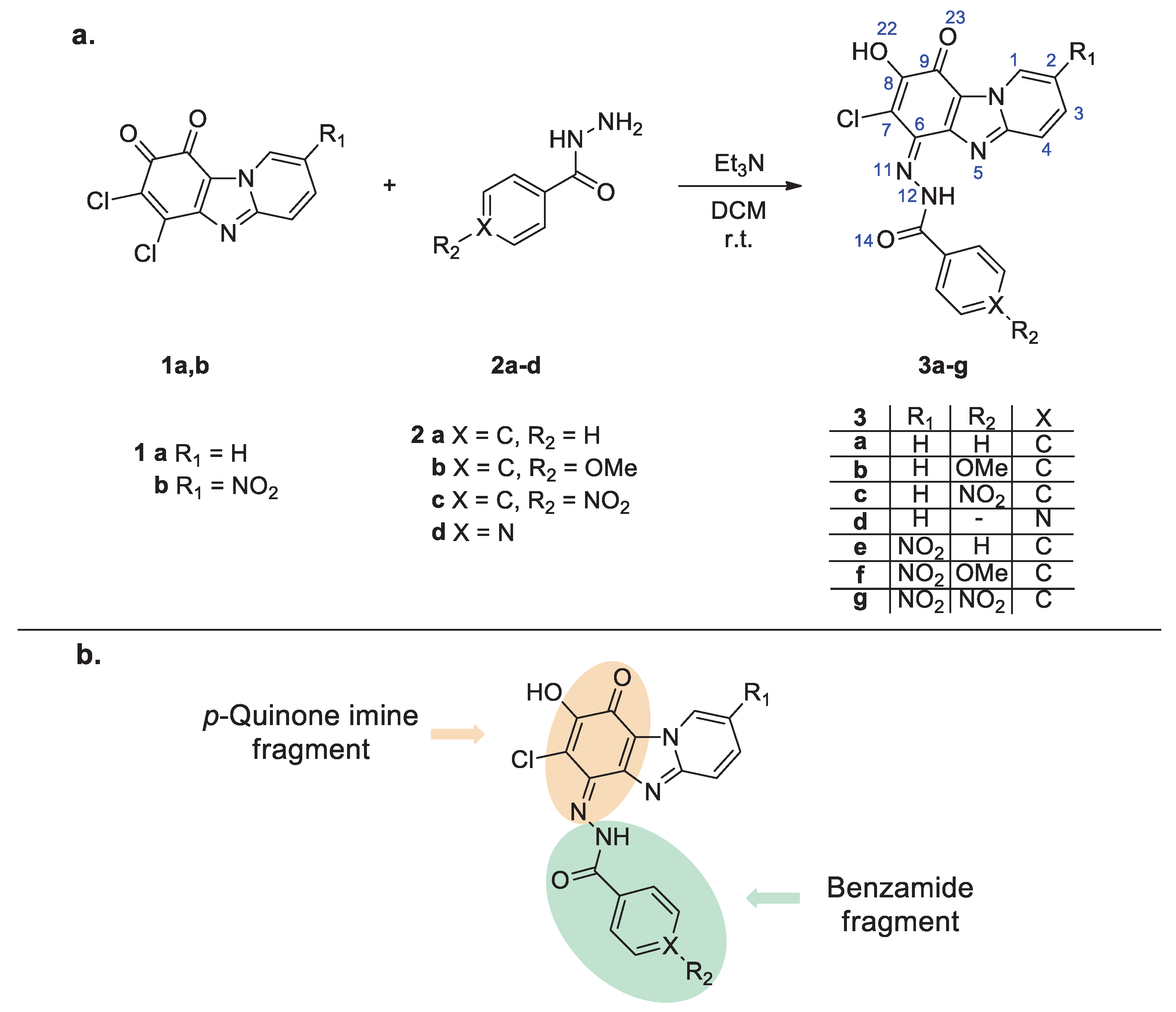
2.1. Synthesis and Structural Studies of Quinone Derivatives 3a-g
2.1.1. Synthesis of Quinone Derivatives 3a-g
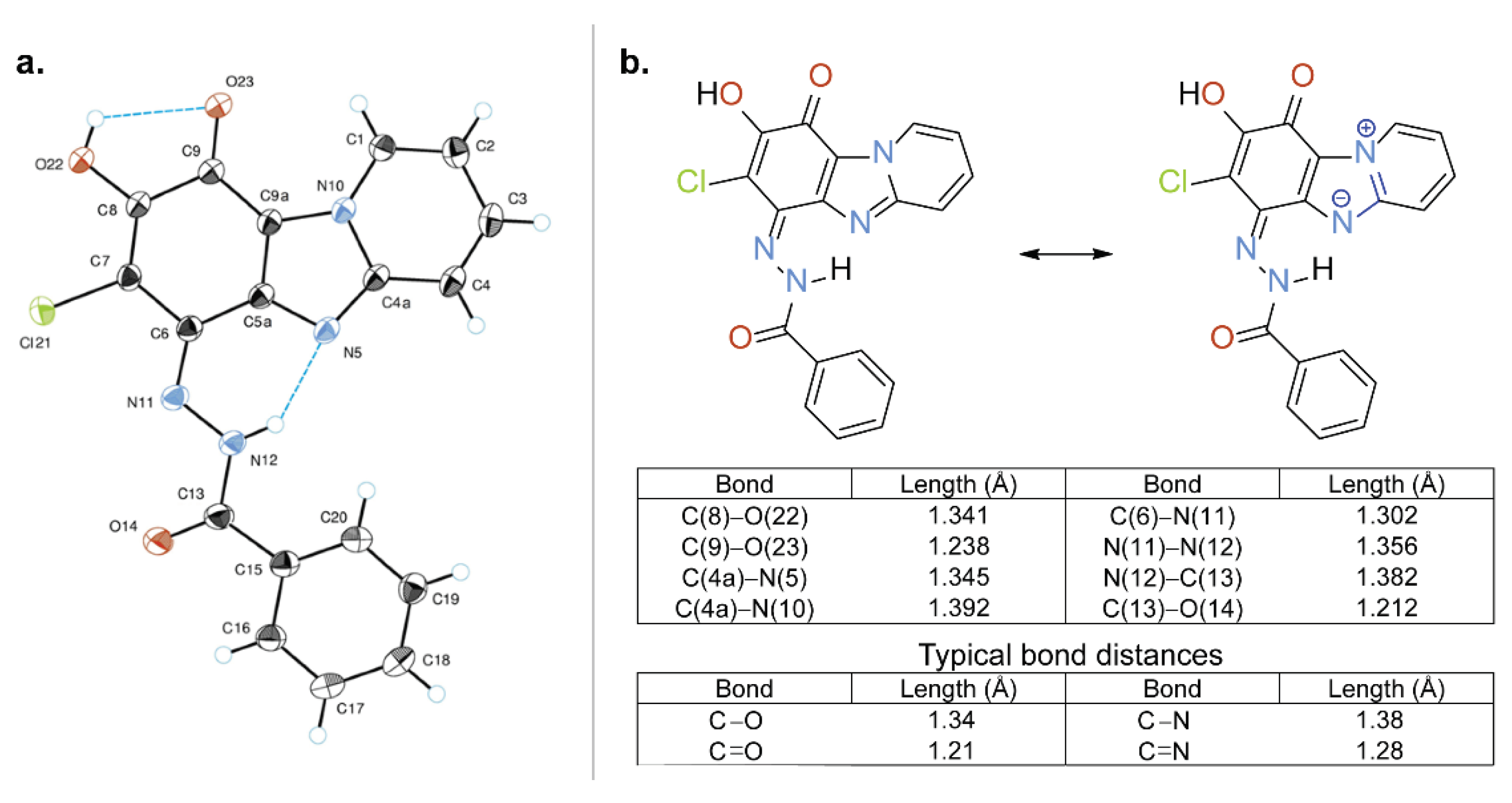
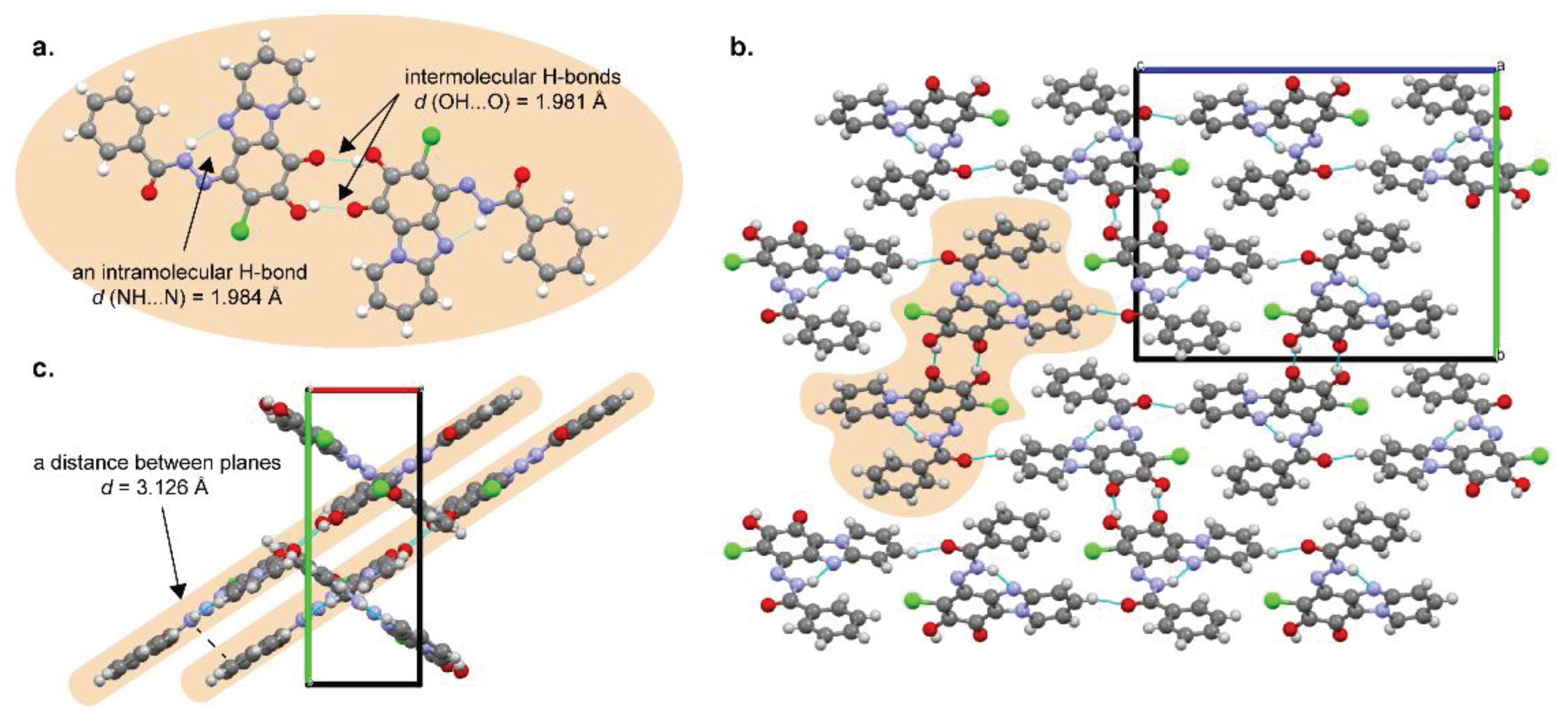
2.1.2. Single Crystal X-ray Analysis of Quinone Derivative 3a
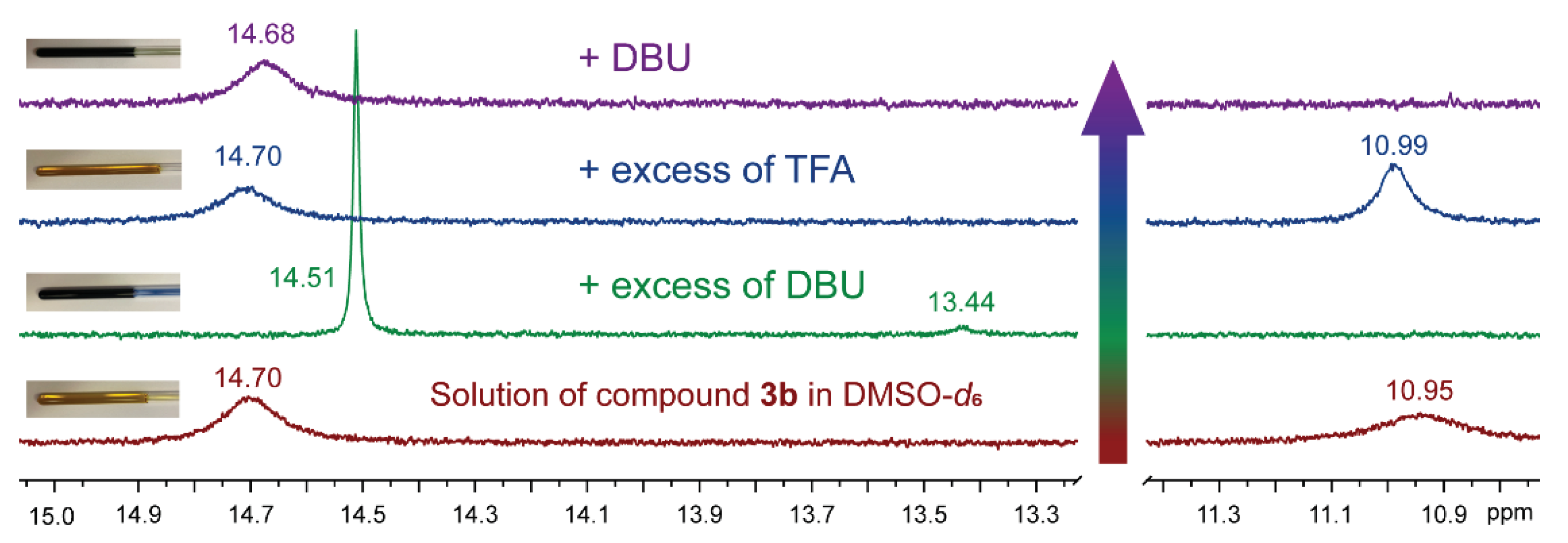
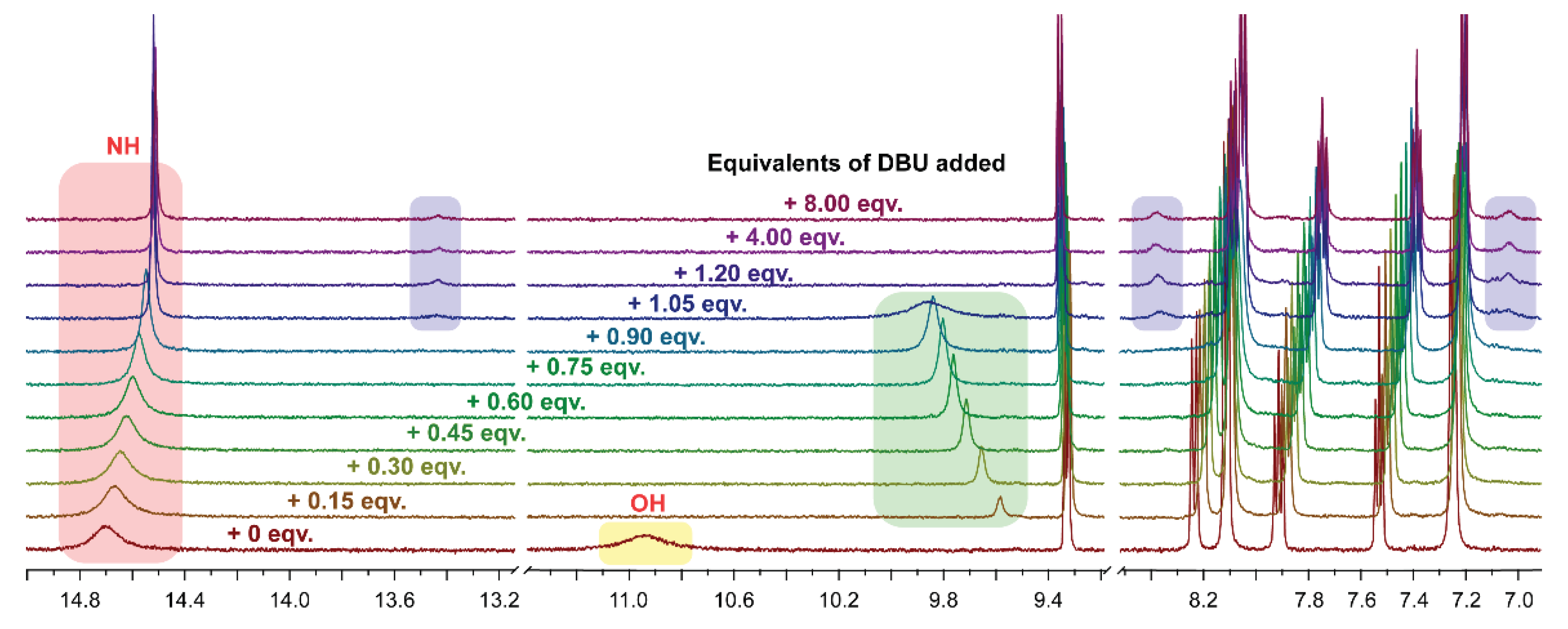
2.1.3.1. H NMR Spectroscopy Analysis of Quinone Derivatives 3a-g
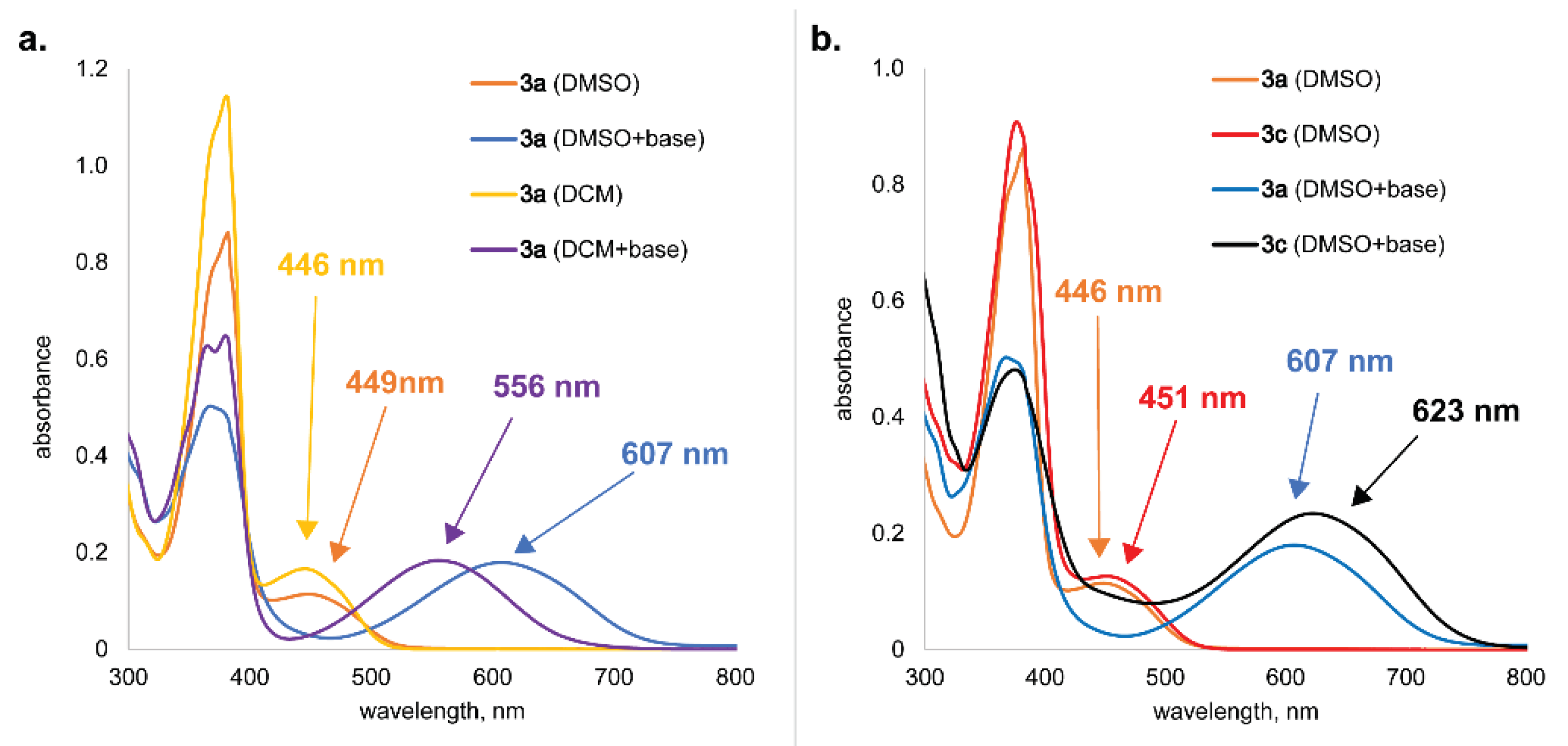
2.1.4. Electronic Absorption
2.2. Electrochemistry/Redox Chemistry Studies of Quinone Derivatives 1a and 3a
2.2.1. Open Circuit Potential Measurements
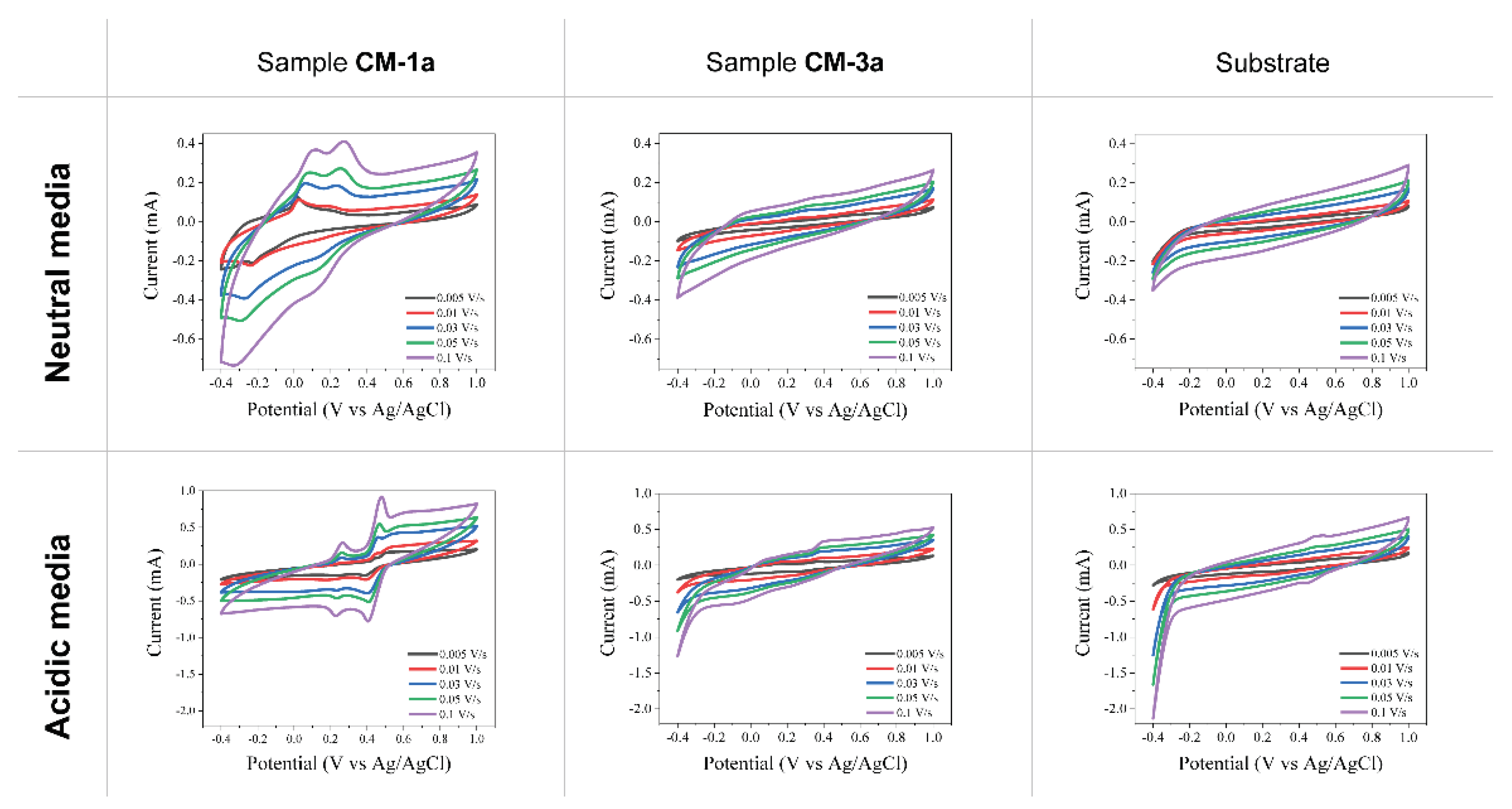
2.2.2. Cyclic Voltammetry Measurements
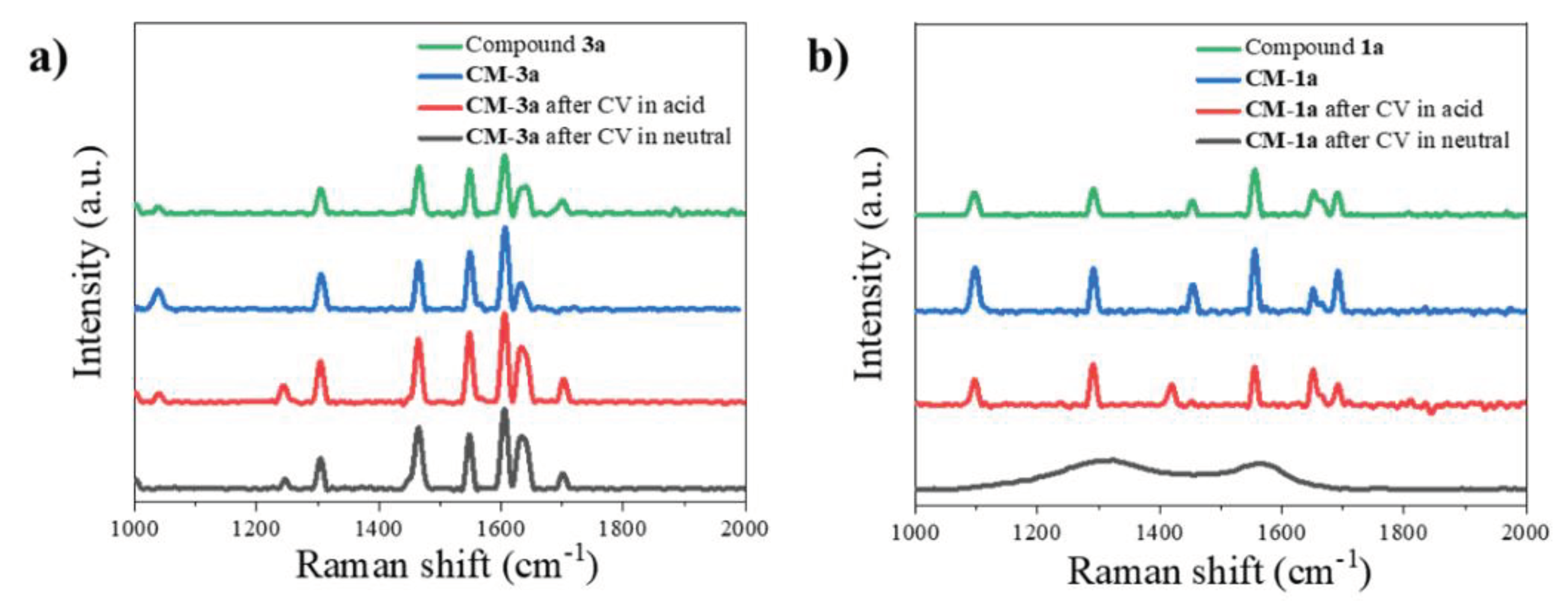
2.2.2. Raman Measurements
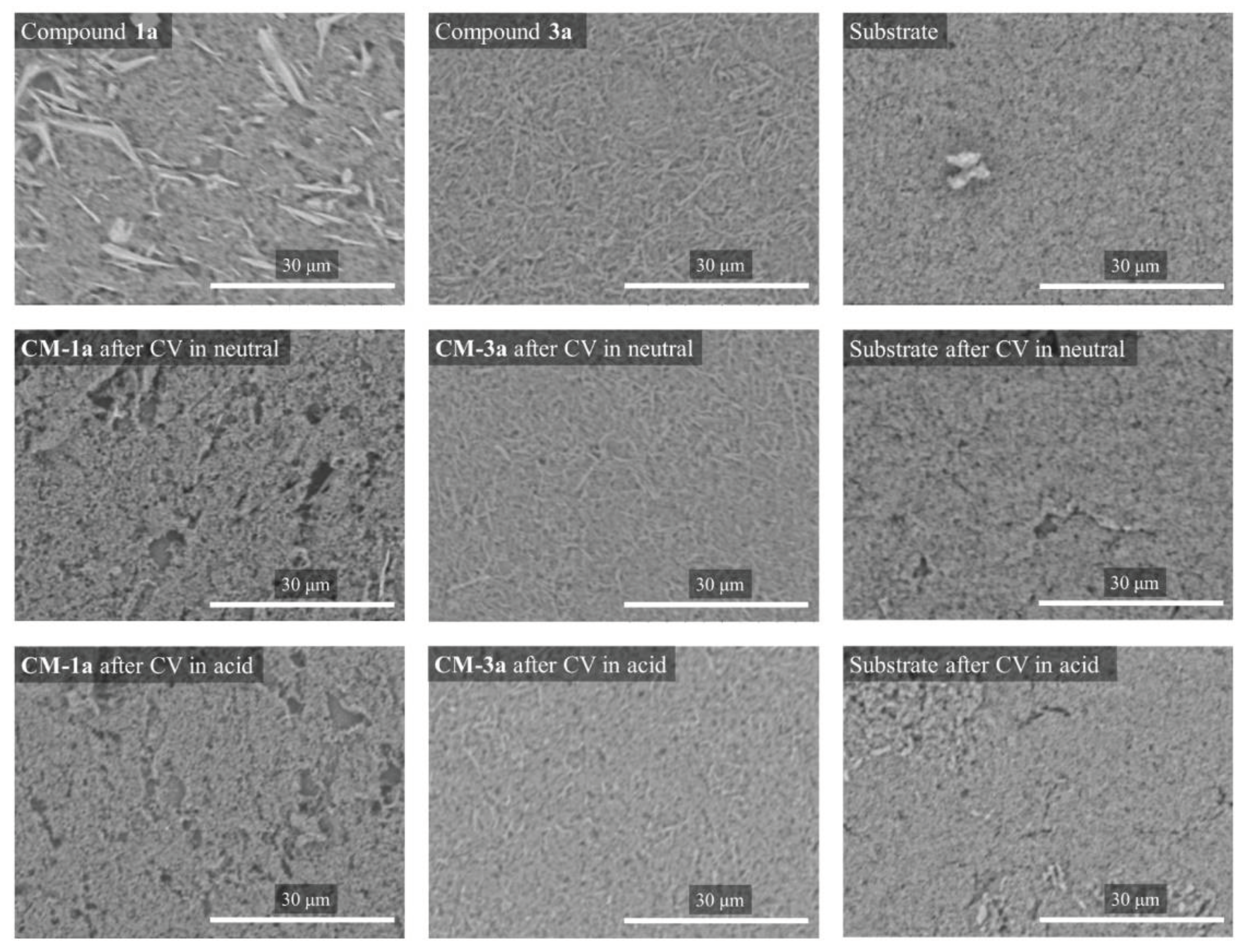
2.2.3. Scanning Electron Microscopy Measurements
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. X-ray Crystallography Analysis
3.3. Cathode Material Preparation
3.4. Cyclic Voltammetry
3.5. Raman Spectroscopy
3.6. Scanning Electron Microscopy
3.7. Synthesis of Quinone Derivatives 1a,b and 3a-g
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- P. Cao et al., “Structural basis for the assembly and quinone transport mechanisms of the dimeric photosynthetic RC–LH1 supercomplex,” Nat. Commun., vol. 13, no. 1, pp. 1–12, 2022. [CrossRef]
- J. Gutiérrez-Fernández et al., “Key role of quinone in the mechanism of respiratory complex I,” Nat. Commun., vol. 11, no. 1, pp. 1–17, 2020. [CrossRef]
- J. L. Bolton and T. Dunlap, “Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects,” Chem. Res. Toxicol., vol. 30, no. 1, pp. 13–37, Jan. 2017. [CrossRef]
- M. Mansha et al., “Recent Developments on Electroactive Organic Electrolytes for Non-Aqueous Redox Flow Batteries: Current Status, Challenges, and Prospects,” Chem. Rec., vol. 24, no. 1, Jan. 2024. [CrossRef]
- C. Y. Go, J. Shin, M. K. Choi, I. H. Jung, and K. C. Kim, “Switchable Design of Redox-Enhanced Nonaromatic Quinones Enabled by Conjugation Recovery,” Adv. Mater., Dec. 2023. [CrossRef]
- P. Simon and Y. Gogotsi, “Perspectives for electrochemical capacitors and related devices,” Nat. Mater., vol. 19, no. 11, pp. 1151–1163, Nov. 2020. [CrossRef]
- J. J. Shea and C. Luo, “Organic Electrode Materials for Metal Ion Batteries,” ACS Appl. Mater. Interfaces, vol. 12, no. 5, pp. 5361–5380, Feb. 2020. [CrossRef]
- R. B. Jethwa, D. Hey, R. N. Kerber, A. D. Bond, D. S. Wright, and C. P. Grey, “Exploring the Landscape of Heterocyclic Quinones for Redox Flow Batteries,” ACS Appl. Energy Mater., Dec. 2023. [CrossRef]
- L. E. Blanc, D. Kundu, and L. F. Nazar, “Scientific Challenges for the Implementation of Zn-Ion Batteries,” Joule, vol. 4, no. 4, pp. 771–799, Apr. 2020. [CrossRef]
- Y. Tsao et al., “Designing a Quinone-Based Redox Mediator to Facilitate Li2S Oxidation in Li-S Batteries,” Joule, vol. 3, no. 3, pp. 872–884, Mar. 2019. [CrossRef]
- L. Sieuw et al., “A H-bond stabilized quinone electrode material for Li–organic batteries: the strength of weak bonds,” Chem. Sci., vol. 10, no. 2, pp. 418–426, 2019. [CrossRef]
- M. Sugumaran, “Reactivities of quinone methides versus o-Quinones in catecholamine metabolism and eumelanin biosynthesis,” Int. J. Mol. Sci., vol. 17, no. 9, pp. 1–23, 2016. [CrossRef]
- I. Poddel’sky, N. O. Druzhkov, G. K. Fukin, V. K. Cherkasov, and G. A. Abakumov, “Bifunctional iminopyridino-catechol and its o-quinone: Synthesis and investigation of coordination abilities,” Polyhedron, vol. 124, pp. 41–50, Mar. 2017. [CrossRef]
- T. V. Astaf’eva, M. V. Arsenyev, R. V. Rumyantcev, G. K. Fukin, V. K. Cherkasov, and A. I. Poddel’sky, “Imine-Based Catechols and o -Benzoquinones: Synthesis, Structure, and Features of Redox Behavior,” ACS Omega, vol. 5, no. 35, pp. 22179–22191, Sep. 2020. [CrossRef]
- R. C. B. Ribeiro, P. G. Ferreira, A. De A. Borges, L. Da S. M. Forezi, F. De Carvalho da Silva, and V. F. Ferreira, “1,2-Naphthoquinone-4-sulfonic acid salts in organic synthesis,” Beilstein J. Org. Chem., vol. 18, pp. 53–69, 2022. [CrossRef]
- Z. Wu et al., “Molecular and Morphological Engineering of Organic Electrode Materials for Electrochemical Energy Storage,” Electrochem. Energy Rev., vol. 5, no. s1, pp. 1–67, 2022. [CrossRef]
- N. Batenko, S. Belyakov, G. Kiselovs, and R. Valters, “Synthesis of 6,7-dichloropyrido [1,2-a]benzimidazole-8,9-dione and its analogues and their reactions with nucleophiles,” Tetrahedron Lett., vol. 54, no. 35, pp. 4697–4699, 2013. [CrossRef]
- N. Batenko, S. Belyakov, G. Kiselovs, and R. Valters, “Synthesis of 6,7-dichloropyrido [1,2-a]benzimidazole-8,9-dione and its analogues and their reactions with nucleophiles,” Tetrahedron Lett., vol. 54, no. 35, pp. 4697–4699, Aug. 2013. [CrossRef]
- Gaile, S. Belyakov, B. Turovska, and N. Batenko, “Synthesis of Asymmetric Coupled Polymethines Based on a 7-Chloropyrido [1,2- a ]benzimidazole-8,9-dione Core,” J. Org. Chem., vol. 87, no. 5, pp. 2345–2355, Mar. 2022. [CrossRef]
- Gaile, S. Belyakov, V. Rjabovs, I. Mihailovs, B. Turovska, and N. Batenko, “Investigation of Weak Noncovalent Interactions Directed by the Amino Substituent of Pyrido- and Pyrimido-[1,2- a ]benzimidazole-8,9-diones,” ACS Omega, vol. 8, no. 43, pp. 40960–40971, Oct. 2023. [CrossRef]
- T. YAMADA, T. YAMASHITA, M. NAKAMURA, H. SHIMAMURA, A. YAMAGUCHI, and M. TAKAYA, “Synthesis and Hemostatic Activity of 1, 2-Naphthoquinones,” YAKUGAKU ZASSHI, vol. 100, no. 8, pp. 799–806, 1980. [CrossRef]
- F. I. Carroll, H. W. Miller, and R. Meck, “Thiosemicarbazone and amidinohydrazone derivatives of some 1,4-naphthoquinones,” J. Chem. Soc. C Org., vol. 3, no. 15, p. 1993, 1970. [CrossRef]
- K. H. Dudley, H. W. Miller, P. W. Schneider, and R. L. McKee, “Potential Naphthoquinone Antimalarials. 2-Acylhydrazino-1,4-naphthoquinones and Related Compounds,” J. Org. Chem., vol. 34, no. 9, pp. 2750–2755, 1969. [CrossRef]
- M. B. Smith and J. March, March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure: Sixth Edition, vol. 9780471720. 2006.
- M. J. Turner, S. P. Thomas, M. W. Shi, D. Jayatilaka, and M. A. Spackman, “Energy frameworks: Insights into interaction anisotropy and the mechanical properties of molecular crystals,” Chem. Commun., vol. 51, no. 18, pp. 3735–3738, 2015. [CrossRef]
- P. R. Spackman et al., “CrystalExplorer : a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals,” J. Appl. Crystallogr., vol. 54, no. 3, pp. 1006–1011, Jun. 2021. [CrossRef]
- J. Catalán, “Toward a Generalized Treatment of the Solvent Effect Based on Four Empirical Scales: Dipolarity (SdP, a New Scale), Polarizability (SP), Acidity (SA), and Basicity (SB) of the Medium,” J. Phys. Chem. B, vol. 113, no. 17, pp. 5951–5960, Apr. 2009. [CrossRef]
- N. N. Shapet’ko and D. N. Shigorin, “NMR study of intramolecular hydrogen bond protons in quinoid structures,” Zhurnal Strukt. Khimii, vol. 8, no. 3, pp. 538–540, 1967.
- M. J. Kamlet and R. W. Taft, “Solvent Hydrogen-Bond Acceptor (HBA) Basicities,” J. Am. Chem. Soc., vol. 98, no. 2, pp. 377–383, 1975. [CrossRef]
- X. Su, M. Lõkov, A. Kütt, I. Leito, and I. Aprahamian, “Unusual para-substituent effects on the intramolecular hydrogen-bond in hydrazone-based switches,” Chem. Commun., vol. 48, no. 85, p. 10490, 2012. [CrossRef]
- X. Su and I. Aprahamian, “Hydrazone-based switches, metallo-assemblies and sensors,” Chem. Soc. Rev., vol. 43, no. 6, pp. 1963–1981, 2014. [CrossRef]
- J. E. Johnson, N. M. Morales, A. M. Gorczyca, D. D. Dolliver, and M. A. McAllister, “Mechanisms of Acid-Catalyzed Z / E Isomerization of Imines,” J. Org. Chem., vol. 66, no. 24, pp. 7979–7985, Nov. 2001. [CrossRef]
- X. Su and I. Aprahamian, “Switching Around Two Axles: Controlling the Configuration and Conformation of a Hydrazone-Based Switch,” Org. Lett., vol. 13, no. 1, pp. 30–33, Jan. 2011. [CrossRef]
- Ryabchun, Q. Li, F. Lancia, I. Aprahamian, and N. Katsonis, “Shape-Persistent Actuators from Hydrazone Photoswitches,” J. Am. Chem. Soc., vol. 141, no. 3, pp. 1196–1200, Jan. 2019. [CrossRef]
- J. Kim, J. Ling, Y. Lai, and P. J. Milner, “Redox-Active Organic Materials: From Energy Storage to Redox Catalysis,” ACS Mater. Au, 2023. [CrossRef]
- M. Wang, X. Dong, I. C. Escobar, and Y.-T. Cheng, “Lithium Ion Battery Electrodes Made Using Dimethyl Sulfoxide (DMSO)—A Green Solvent,” ACS Sustain. Chem. Eng., vol. 8, no. 30, pp. 11046–11051, Aug. 2020. [CrossRef]
- Laurence, J. Legros, A. Chantzis, A. Planchat, and D. Jacquemin, “A Database of Dispersion-Induction DI, Electrostatic ES, and Hydrogen Bonding α 1 and β 1 Solvent Parameters and Some Applications to the Multiparameter Correlation Analysis of Solvent Effects,” J. Phys. Chem. B, vol. 119, no. 7, pp. 3174–3184, Feb. 2015. [CrossRef]
- Y. Ooyama and S. Yagi, Progress in the Science of Functional Dyes. 2021. [CrossRef]
- M. S. Miran, H. Kinoshita, T. Yasuda, M. A. B. H. Susan, and M. Watanabe, “Hydrogen bonds in protic ionic liquids and their correlation with physicochemical properties,” Chem. Commun., vol. 47, no. 47, pp. 12676–12678, 2011. [CrossRef]
- R. G. Almeida et al., “Synthesis of quinone imine and sulphur-containing compounds with antitumor and trypanocidal activities: Redox and biological implications,” RSC Med. Chem., vol. 11, no. 10, pp. 1145–1160, 2020. [CrossRef]
- Klopčič and M. S. Dolenc, “Chemicals and Drugs Forming Reactive Quinone and Quinone Imine Metabolites,” Chem. Res. Toxicol., vol. 32, no. 1, pp. 1–34, 2019. [CrossRef]
- N. Batenko, A. Kricka, S. Belyakov, B. Turovska, and R. Valters, “A novel method for the synthesis of benzimidazole-based 1,4-quinone derivatives,” Tetrahedron Lett., vol. 57, no. 3, pp. 292–295, 2016. [CrossRef]
- E. Smith and G. Dent, Modern Raman Spectroscopy – A Practical Approach. Wiley, 2004. [CrossRef]
- N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prud’homme, I. A. Aksay, and R. Car, “Raman spectra of graphite oxide and functionalized graphene sheets,” Nano Lett., vol. 8, no. 1, pp. 36–41, 2008. [CrossRef]
- M. Saravanan, M. Ganesan, and S. Ambalavanan, “An in situ generated carbon as integrated conductive additive for hierarchical negative plate of lead-acid battery,” J. Power Sources, vol. 251, pp. 20–29, 2014. [CrossRef]
- H. E. Gottlieb, V. Kotlyar, and A. Nudelman, “NMR chemical shifts of common laboratory solvents as trace impurities,” J. Org. Chem., vol. 62, no. 21, pp. 7512–7515, 1997. [CrossRef]
- G. M. Sheldrick, “Crystal structure refinement with SHELXL,” Acta Crystallogr. Sect. C Struct. Chem., vol. 71, no. Md, pp. 3–8, 2015. [CrossRef]
- J. Bourhis, O. V. Dolomanov, R. J. Gildea, J. A. K. Howard, and H. Puschmann, “The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment - Olex2 dissected,” Acta Crystallogr. Sect. A Found. Crystallogr., vol. 71, no. 1, pp. 59–75, 2015. [CrossRef]
- F. MacRae et al., “Mercury 4.0: From visualization to analysis, design and prediction,” J. Appl. Crystallogr., vol. 53, pp. 226–235, 2020. [CrossRef]
| 1 |
1H NMR spectrum of DBU and TFA mixture in DMSO-d6 solution was recorded; the NH+ signal appears at 9.69 ppm (Figure S11). |
| Crystal parameter | Compound 3a |
| Empirical formula | C18H11ClN4O3 |
| Calculated density (g/cm3) | 1.556 |
| Formula Weight | 366.766 |
| Color | Red |
| Size/mm3 | 0.18×0.03×0.01 |
| Temperature/K | 150.0(1) |
| Crystal System | monoclinic |
| Space Group | P21/n |
| a/Å | 5.65994(5) |
| b/Å | 14.90948(19) |
| c/Å | 18.5815(2) |
| α/° | 90 |
| β/° | 93.1950(9) |
| γ/° | 90 |
| V/Å3 | 1565.60(3) |
| Wavelength/Å | 1.54184 |
| Radiation type | Cu Kα |
| Absorption coefficient (mm−1) | 2.419 |
| θmin/° | 3.8 |
| 2θmax/° | 155.0 |
| Measured reflections | 17875 |
| Number of independent reflections | 3322 |
| Reflections with I≥2σ(I) | 3089 |
| Rint | 0.0327 |
| Number of refined parameters | 243 |
| Restraints | 0 |
| Largest Peak | 0.3476 |
| Deepest Hole | -0.3143 |
| Goodness of fit | 1.0362 |
| wR2 (all data) | 0.0962 |
| wR2 | 0.0945 |
| R1 (all data) | 0.0367 |
| R1 | 0.0346 |
| CCDC deposition number | 2238663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





