Submitted:
29 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
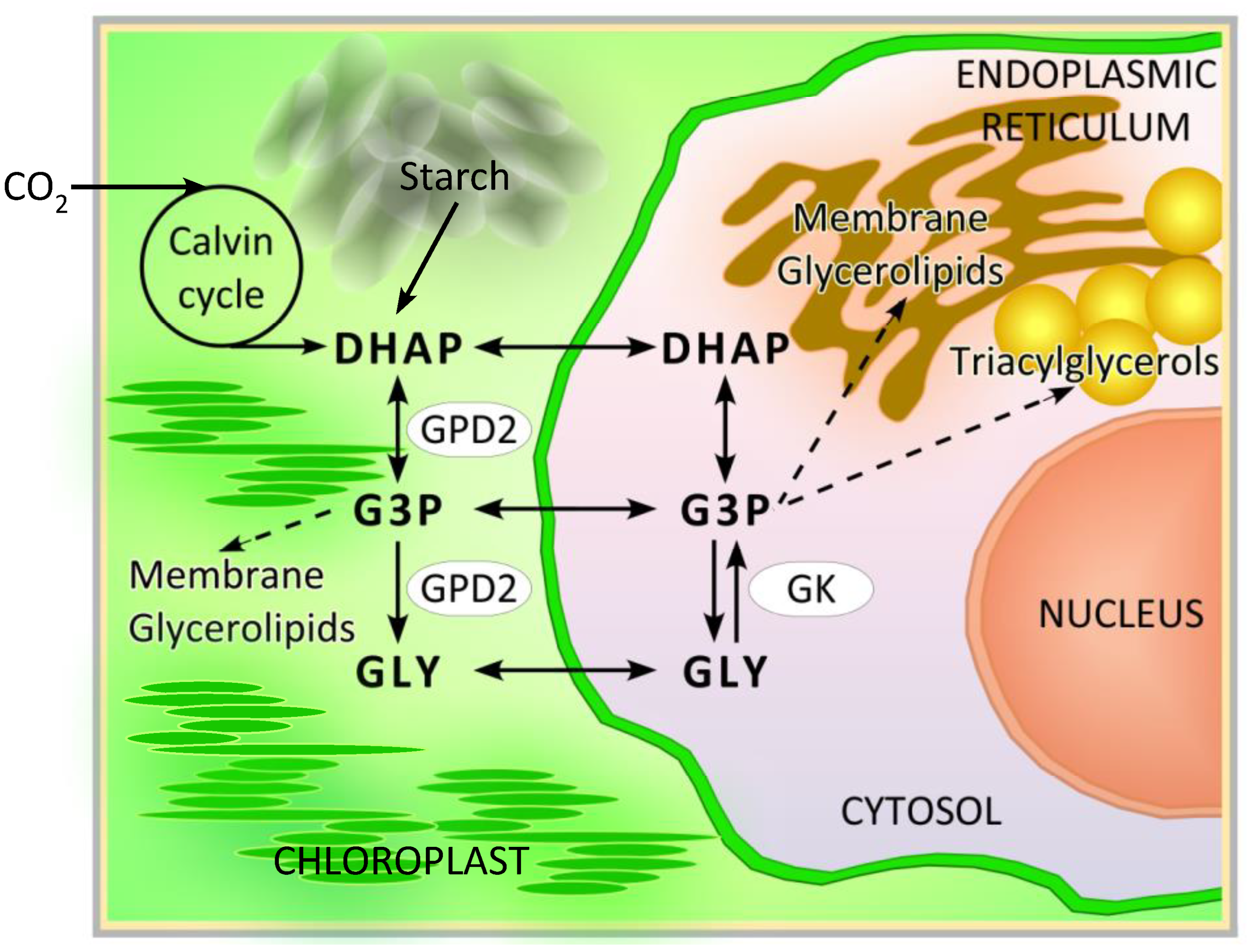
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Construction of the PSAD:GPD2:PSAD Transgene and Generation of Transgenic Strains
2.3. Reverse Transcriptase (RT-) and Quantitative Real Time (qRT-) PCR Assays
2.4. Glycerol and Non-Polar Lipid Analyses
2.5. Immunoblot Analyses
2.6. Treatments with Pharmacological Agents
2.7. Proteomic Analyses
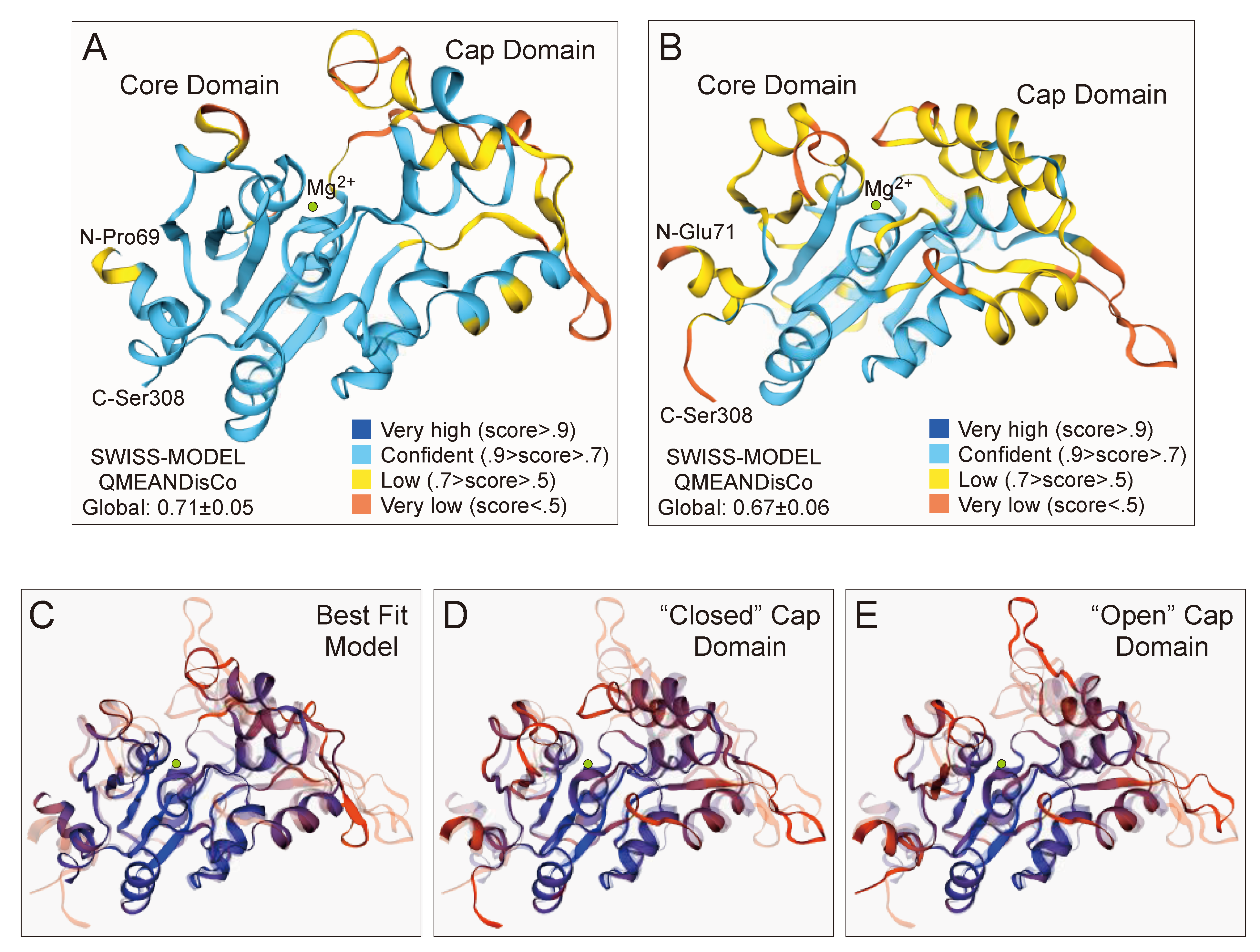
2.8. GPD2 Glycerol-3-Phosphate Phosphatase Domain Structure Predictions
2.9. Statistical Data Analyses
3. Results
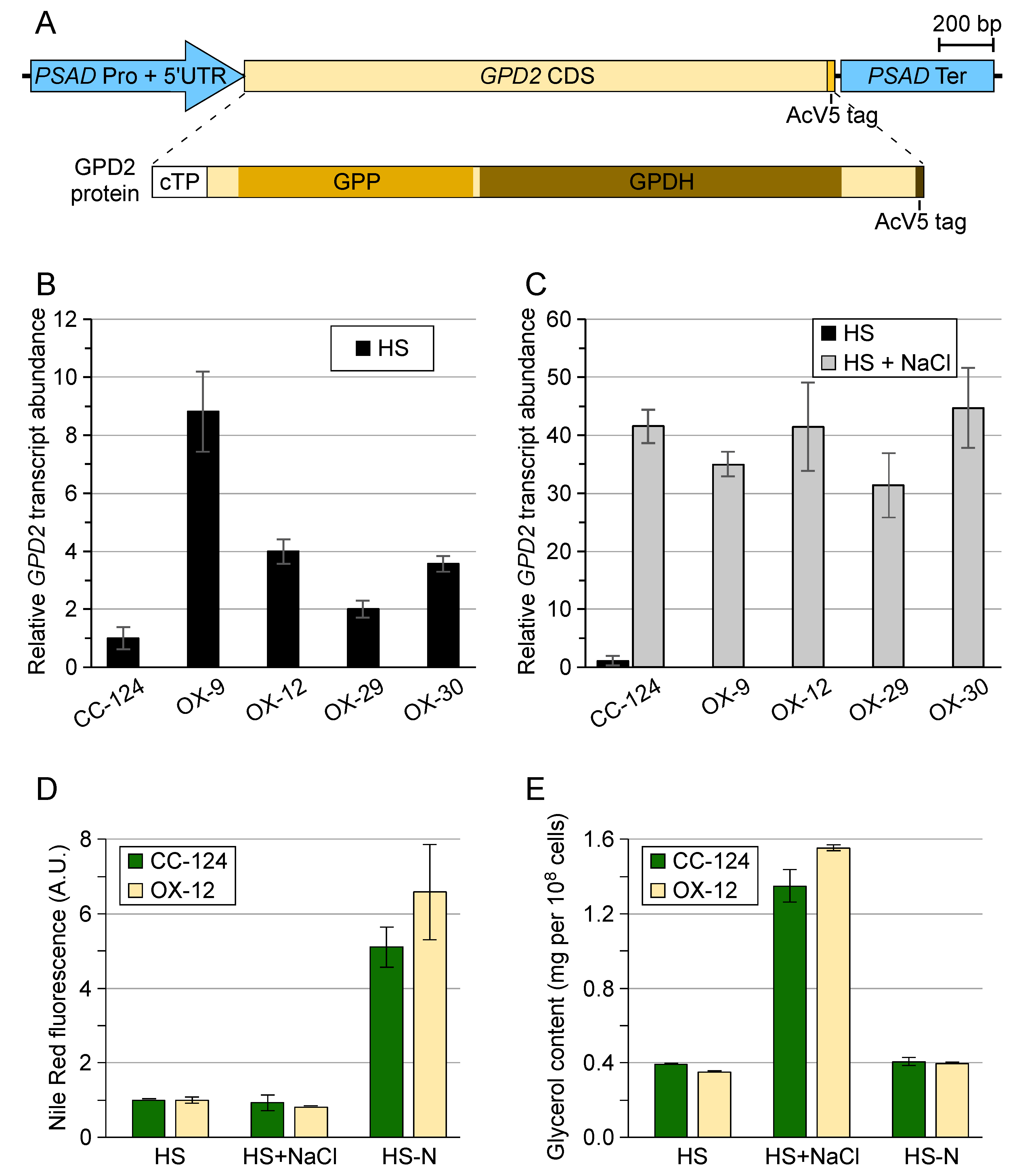
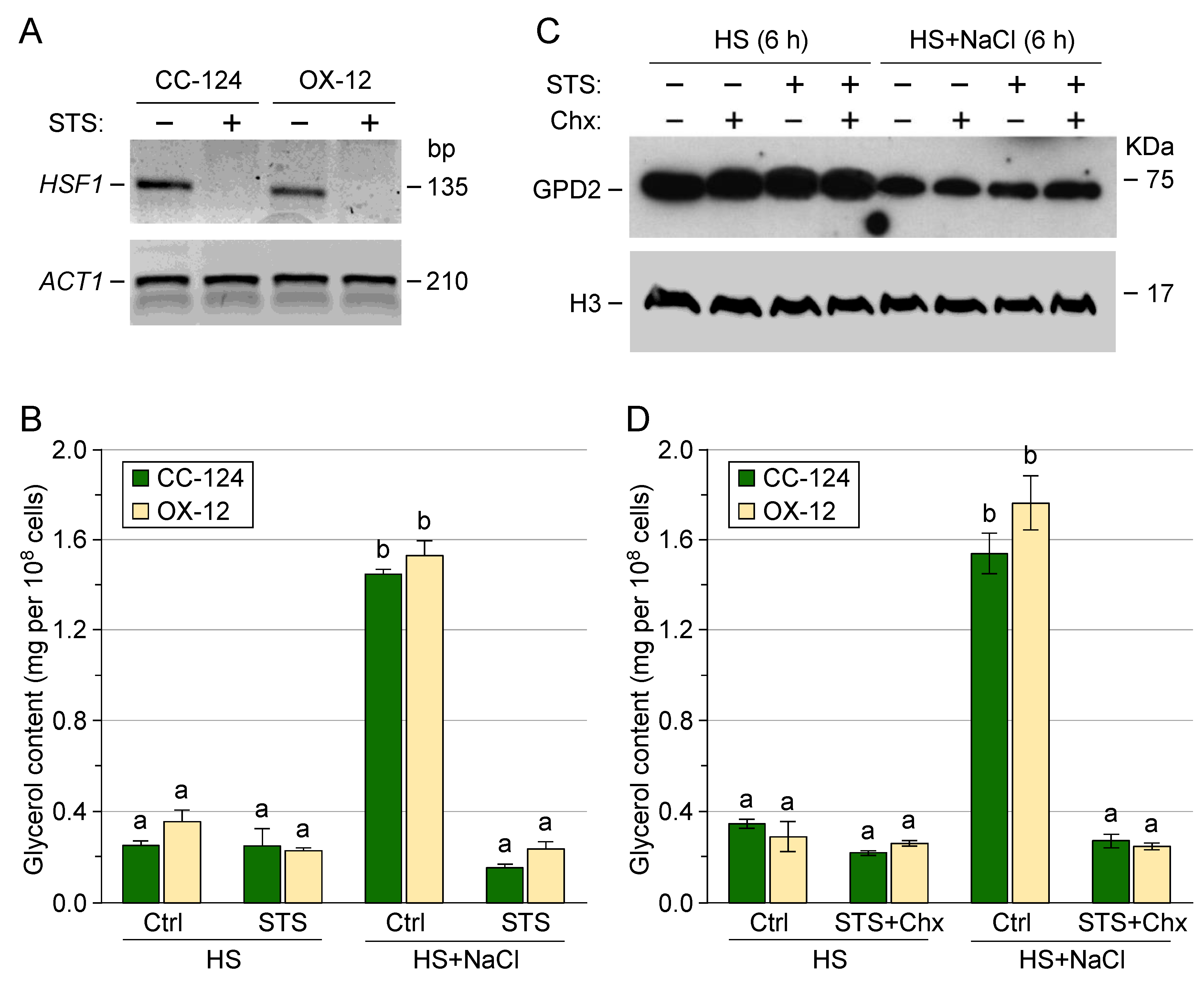
3.1. Chlamydomonas reinhardtii GPD2 Transgenic Strains
3.2. Transgenic GPD2 Protein Abundance and Stability
3.3. Control of Glycerol Accumulation under Salinity Stress
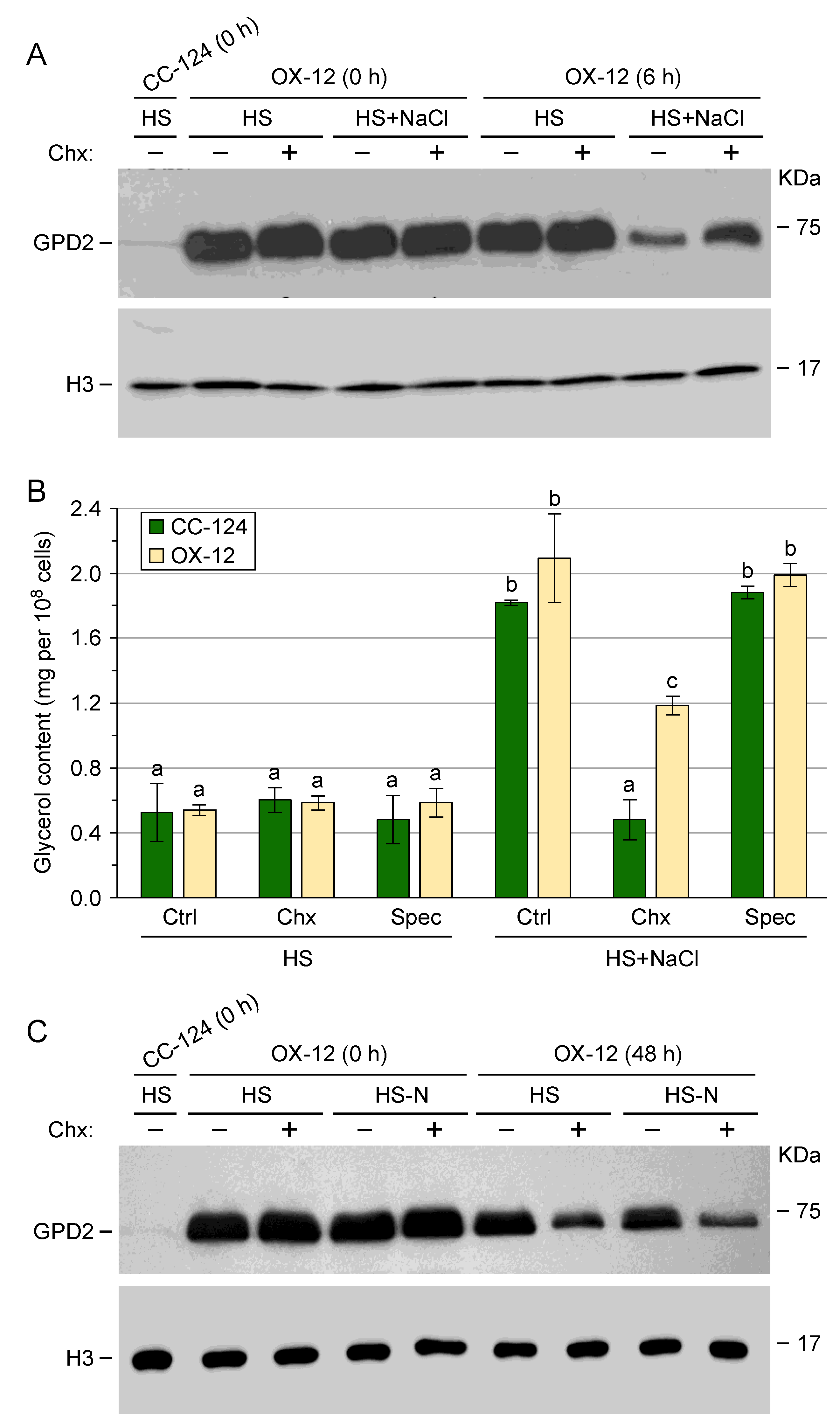
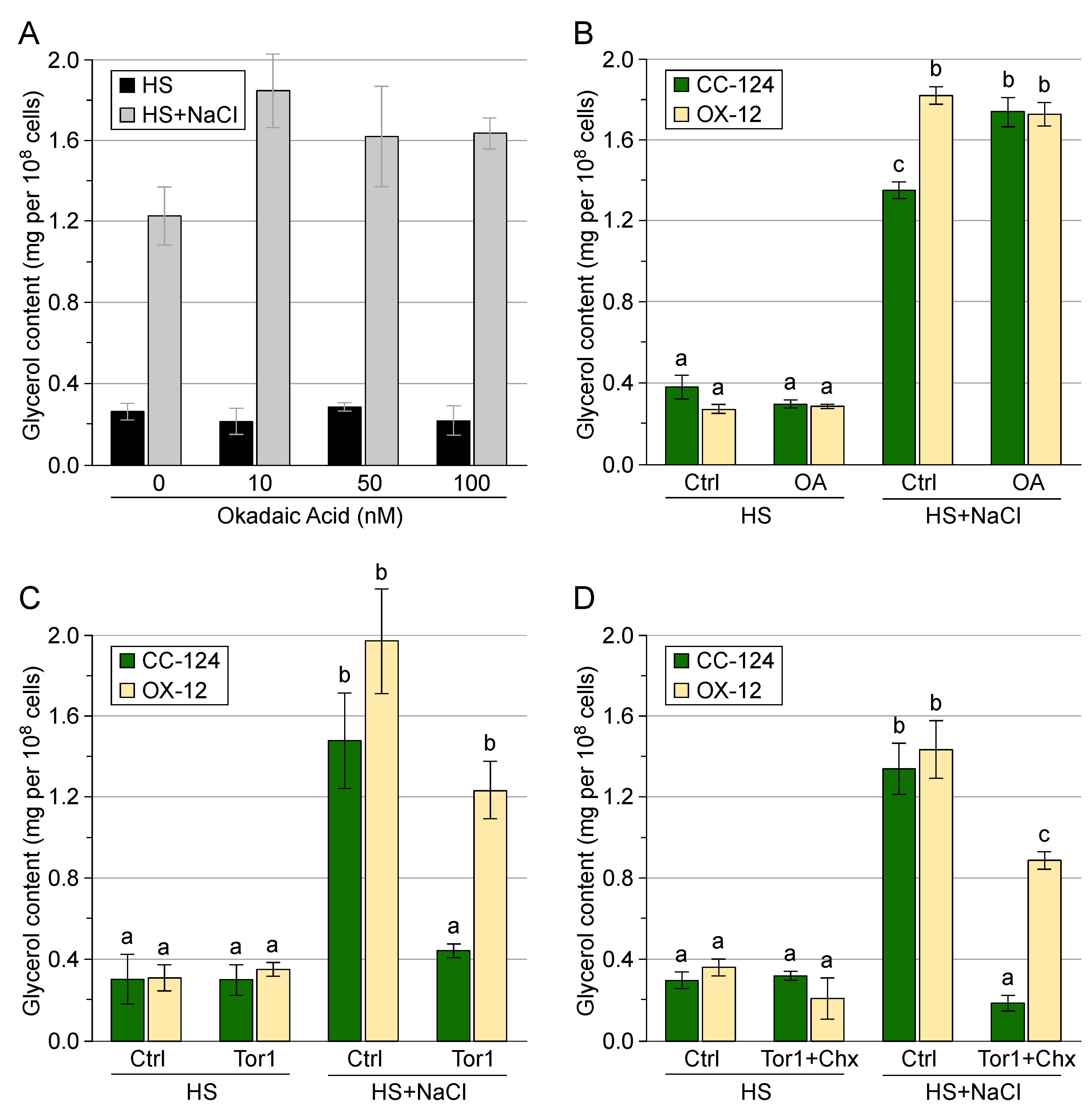
3.4. Post-Translational Regulation of Transgenic GPD2 Protein
3.5. Three-Dimensional Structure Prediction of the GPD2 GPP Domain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, I.; Hellebust, J.A. The Role of Glycerol and Inorganic Ions in Osmoregulatory Responses of the Euryhaline Flagellate Chlamydomonas pulsatilla Wollenweber. Plant Physiol. 1986, 82, 406–410. [Google Scholar] [CrossRef]
- Miyasaka, H.; Ohnishi, Y.; Akano, T.; Fukatsu, K.; Mizoguchi, T.; Yagi, K.; Maeda, I.; Ikuta, Y.; Matsumoto, H.; Shioji, N.; et al. Excretion of glycerol by the marine Chlamydomonas sp. strain W-80 in high CO2 cultures. J. Ferment. Bioeng. 1998, 85, 122–124. [Google Scholar] [CrossRef]
- Sasso, S.; Stibor, H.; Mittag, M.; Grossman, A.R. From molecular manipulation of domesticated Chlamydomonas reinhardtii to survival in nature. Elife. 2018, 7, e39233. [Google Scholar] [CrossRef]
- Salomé, P.A.; Merchant, S.S. A Series of Fortunate Events: Introducing Chlamydomonas as a Reference Organism. Plant Cell. 2019, 31, 1682–1707. [Google Scholar] [CrossRef]
- Raymond, J.A.; Morgan-Kiss, R.; Stahl-Rommel, S. Glycerol Is an Osmoprotectant in Two Antarctic Chlamydomonas Species from an Ice-Covered Saline Lake and Is Synthesized by an Unusual Bidomain Enzyme. Front. Plant. Sci. 2020, 11, 1259. [Google Scholar] [CrossRef]
- Bazzani, E.; Lauritano, C.; Mangoni, O.; Bolinesi, F.; Saggiomo, M. Chlamydomonas Responses to Salinity Stress and Possible Biotechnological Exploitation. J. Mar. Sci. Eng. 2021, 9, 1242. [Google Scholar] [CrossRef]
- Pröschold, T.; Marin, B.; Schlösser, U.G.; Melkonian, M. Molecular Phylogeny and Taxonomic Revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and Description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist 2001, 152, 265–300. [Google Scholar] [CrossRef]
- Craig, R.J.; Hasan, A.R.; Ness, R.W.; Keightley, P.D. Comparative genomics of Chlamydomonas. Plant Cell. 2021, 33, 1016–1041. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas Genome Reveals the Evolution of Key Animal and Plant Functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Glycerol metabolism in hypersaline environments. Environ Microbiol. 2017, 19, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, A. Yeast osmoregulation - glycerol still in pole position. FEMS Yeast Res. 2022, 22, foac035. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Avron, M. The Role of Glycerol in the Osmotic Regulation of the Halophitic Alga Dunaliella parva. Plant Physiol. 1973, 51, 875–878. [Google Scholar] [CrossRef] [PubMed]
- León, R.; Galván, F. Halotolerance Studies on Chlamydomonas reinhardtii: Glycerol Excretion by Free and Immobilized Cells. J. Appl. Phycol. 1994, 6, 13–20. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic responses of Dunaliella to the changes of salinity. J. Cell Physiol. 2009, 219, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, D.; Kim, Y.; Terng, E.L.; Peterson, L.; Cerutti, H. A Multidomain Enzyme, with Glycerol-3-Phosphate Dehydrogenase and Phosphatase Activities, Is Involved in a Chloroplastic Pathway for Glycerol Synthesis in Chlamydomonas reinhardtii. Plant. J. 2017, 90, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Colina, F.J.; Carbó, M.; Cañal, M.J.; Valledor, L. A complex metabolic rearrangement towards the accumulation of glycerol and sugars consequence of a proteome remodeling is required for the survival of Chlamydomonas reinhardtii growing under osmotic stress. Environ. Exp. Bot. 2020, 180, 104261. [Google Scholar] [CrossRef]
- He, Q.; Toh, J.D.; Ero, R.; Qiao, Z.; Kumar, V.; Serra, A.; Tan, J.; Sze, S.K.; Gao, Y.G. The unusual di-domain structure of Dunaliella salina glycerol-3-phosphate dehydrogenase enables direct conversion of dihydroxyacetone phosphate to glycerol. Plant J. 2020, 102, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lan, Y.; Cao, X.; Yao, H.; Qiao, D.; Xu, H.; Cao, Y. Characterization and diverse evolution patterns of glycerol-3-phosphate dehydrogenase family genes in Dunaliella salina. Gene. 2019, 710, 161–169. [Google Scholar] [CrossRef]
- Casais-Molina, M.L.; Peraza-Echeverria, S.; Echevarría-Machado, I.; Herrera-Valencia, V.A. Expression of Chlamydomonas reinhardtii CrGPDH2 and CrGPDH3 cDNAs in Yeast Reveals That They Encode Functional Glycerol-3-Phosphate Dehydrogenases Involved in Glycerol Production and Osmotic Stress Tolerance. J. Appl. Phycol. 2016, 28, 219–226. [Google Scholar] [CrossRef]
- Driver, T.; Trivedi, D.K.; McIntosh, O.A.; Dean, A.P.; Goodacre, R.; Pittman, J.K. Two Glycerol-3-Phosphate Dehydrogenases from Chlamydomonas Have Distinct Roles in Lipid Metabolism. Plant. Physiol. 2017, 174, 2083–2097. [Google Scholar] [CrossRef]
- Mastrobuoni, G.; Irgang, S.; Pietzke, M.; Aßmus, H.E.; Wenzel, M.; Schulze, W.X.; Kempa, S. Proteome Dynamics and Early Salt Stress Response of the Photosynthetic Organism Chlamydomonas reinhardtii. BMC Genom. 2012, 13, 215. [Google Scholar] [CrossRef]
- Tietel, Z.; Wikoff, W.R.; Kind, T.; Fiehn, O. Hyperosmotic stress in Chlamydomonas induces metabolomic changes in biosynthesis of complex lipids. Eur. J. Phycol. 2020, 55, 11–29. [Google Scholar] [CrossRef]
- Yokthongwattana, C.; Mahong, B.; Roytrakul, S.; Phaonaklop, N.; Narangajavana, J.; Yokthongwattana, K. Proteomic Analysis of Salinity-Stressed Chlamydomonas reinhardtii Revealed Differential Suppression and Induction of a Large Number of Important Housekeeping Proteins. Planta 2012, 235, 649–659. [Google Scholar] [CrossRef]
- He, Q.; Lin, Y.; Tan, H.; Zhou, Y.; Wen, Y.; Gan, J.; Li, R.; Zhang, Q. Transcriptomic profiles of Dunaliella salina in response to hypersaline stress. BMC Genomics. 2020, 21, 115. [Google Scholar] [CrossRef]
- He, Y.; Meng, X.; Fan, Q.; Sun, X.; Xu, Z.; Song, R. Cloning and characterization of two novel chloroplastic glycerol-3-phosphate dehydrogenases from Dunaliella viridis. Plant Mol Biol. 2009, 71, 193–205. [Google Scholar] [CrossRef]
- Keil, L.; Mehlmer, N.; Cavelius, P.; Garbe, D.; Haack, M.; Ritz, M.; Awad, D.; Brück, T. The Time-Resolved Salt Stress Response of Dunaliella tertiolecta-A Comprehensive System Biology Perspective. Int J Mol Sci. 2023, 24, 15374. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, U.; Blaby, I.; Casero, D.; Gallaher, S.D.; Goodson, C.; Johnson, S.; Lee, J.H.; Merchant, S.S.; Pellegrini, M.; Roth, R.; et al. The path to triacylglyceride obesity in the sta6 strain of Chlamydomonas reinhardtii. Eukaryot. Cell. 2014, 13, 591–613. [Google Scholar] [CrossRef]
- Schmollinger, S.; Mühlhaus, T.; Boyle, N.R.; Blaby, I.K.; Casero, D.; Mettler, T.; Moseley, J.L.; Kropat, J.; Sommer, F.; Strenkert, D.; et al. Nitrogen-Sparing Mechanisms in Chlamydomonas Affect the Transcriptome, the Proteome, and Photosynthetic Metabolism. Plant Cell. 2014, 26, 1410–1435. [Google Scholar] [CrossRef]
- Johnson, X.; Alric, J. Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: metabolic constraints for carbon partitioning between oil and starch. Eukaryot. Cell. 2013, 12, 776–793. [Google Scholar] [CrossRef]
- Msanne, J.; Xu, D.; Konda, A.R.; Casas-Mollano, J.A.; Awada, T.; Cahoon, E.B.; Cerutti, H. Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry. 2012, 75, 50–59. [Google Scholar] [CrossRef]
- Sueoka, N. Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 1960, 46, 83–91. [Google Scholar] [CrossRef]
- Harris, E.H. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Lawrence, S.D.; Novak, N.G.; Slack, J.M. Epitope tagging: a monoclonal antibody specific for recombinant fusion proteins in plants. Biotechniques. 2003, 35, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Rochaix, J.D. The flanking regions of PsaD drive efficient gene expression in the nucleus of the green alga Chlamydomonas reinhardtii. Mol. Genet. Genomics. 2001, 265, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.R.; Rochaix, J.D.; Purton, S. The bacterial phleomycin resistance gene ble as a dominant selectable marker in Chlamydomonas. Mol. Gen. Genet. 1996, 251, 23–30. [Google Scholar] [CrossRef]
- Plucinak, T.M.; Horken, K.M.; Jiang, W.; Fostvedt, J.; Nguyen, S.T.; Weeks, D.P. Improved and versatile viral 2A platforms for dependable and inducible high-level expression of dicistronic nuclear genes in Chlamydomonas reinhardtii. Plant J. 2015, 82, 717–729. [Google Scholar] [CrossRef]
- Kim, Y.; Terng, E.L.; Riekhof, W.R.; Cahoon, E.B.; Cerutti, H. Endoplasmic reticulum acyltransferase with prokaryotic substrate preference contributes to triacylglycerol assembly in Chlamydomonas. Proc. Natl. Acad. Sci. USA. 2018, 115, 1652–1657. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular cloning – A laboratory manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Ma, X.; Ibrahim, F.; Kim, E.J.; Shaver, S.; Becker, J.; Razvi, F.; Cerny, R.L.; Cerutti, H. An ortholog of the Vasa intronic gene is required for small RNA-mediated translation repression in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 2020, 117, 761–770. [Google Scholar] [CrossRef]
- Zogli, P.; Alvarez, S.; Naldrett, M.J.; Palmer, N.A.; Koch, K.G.; Pingault, L.; Bradshaw, J.D.; Twigg, P.; Heng-Moss, T.M.; Louis, J.; et al. Greenbug (Schizaphis graminum) herbivory significantly impacts protein and phosphorylation abundance in switchgrass (Panicum virgatum). Sci Rep. 2020, 10, 14842. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Haufroid, M.; Mirgaux, M.; Leherte, L.; Wouters, J. Crystal structures and snapshots along the reaction pathway of human phosphoserine phosphatase. Acta Crystallogr D Struct Biol. 2019, 75, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021, 596, 583–589. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio; PBC: Boston, MA, USA, 2021; Available online: http://www.rtudio.com/.
- Ma, X.; Kim, E.J.; Kook, I.; Ma, F.; Voshall, A.; Moriyama, E.; Cerutti, H. Small interfering RNA-mediated translation repression alters ribosome sensitivity to inhibition by cycloheximide in Chlamydomonas reinhardtii. Plant Cell. 2013, 25, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiol Biochem. 2007, 45, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.W.; Carvalho, B.J.; Jones, H.D.; Singh, S. The role of photo-osmotic adaptation in semi-continuous culture and lipid particle release from Dunaliella viridis. J Appl Phycol. 2015, 27, 109–123. [Google Scholar] [CrossRef]
- Sun, S.Y. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol. Ther. 2010, 9, 109–110. [Google Scholar] [CrossRef]
- Voigt, J.; Woestemeyer, J. Protease Inhibitors Cause Necrotic Cell Death in Chlamydomonas reinhardtii by Inducing the Generation of Reactive Oxygen Species. J. Eukaryot. Microbiol. 2015, 62, 711–721. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.E.; Lemaire, S.D.; Crespo, J.L. Control of Autophagy in Chlamydomonas Is Mediated through Redox-Dependent Inactivation of the ATG4 Protease. Plant Physiol. 2016, 172, 2219–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ng, D.H.P.; Fang, L.; Chow, Y.Y.S.; Lee, Y.K. MAPK in Dunaliella tertiolecta regulates glycerol production in response to osmotic shock. Eur. J. Phycol. 2016, 51, 119–128. [Google Scholar] [CrossRef]
- Tang, Z.; Cao, X.; Zhang, Y.; Jiang, J.; Qiao, D.; Xu, H.; Cao, Y. Two splice variants of the DsMEK1 mitogen-activated protein kinase kinase (MAPKK) are involved in salt stress regulation in Dunaliella salina in different ways. Biotechnol Biofuels. 2020, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Dounay, A.B.; Forsyth, C.J. Okadaic acid: the archetypal serine/threonine protein phosphatase inhibitor. Curr Med Chem. 2002, 9, 1939–1980. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pásaro, E.; Méndez, J.; Laffon, B. Okadaic acid: more than a diarrheic toxin. Mar Drugs. 2013, 11, 4328–4349. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, X.; Wu, Q.; Pan, J. Ciliary Length Sensing Regulates IFT Entry via Changes in FLA8/KIF3B Phosphorylation to Control Ciliary Assembly. Curr Biol. 2018, 28, 2429–2435. [Google Scholar] [CrossRef]
- Lin, H.; Miller, M.L.; Granas, D.M.; Dutcher, SK. Whole genome sequencing identifies a deletion in protein phosphatase 2A that affects its stability and localization in Chlamydomonas reinhardtii. PLoS Genet. 2013, 9, e1003841. [Google Scholar] [CrossRef]
- Werth, E.G.; McConnell, E.W.; Couso Lianez, I.; Perrine, Z.; Crespo, J.L.; Umen, J.G.; Hicks, L.M. Investigating the effect of target of rapamycin kinase inhibition on the Chlamydomonas reinhardtii phosphoproteome: from known homologs to new targets. New Phytol. 2019, 221, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Henriques, R.; Calderan-Rodrigues, M.J.; Crespo, J.L.; Baena-González, E.; Caldana, C. Growing of the TOR world. J Exp Bot. 2022, 73, 6987–6992. [Google Scholar] [CrossRef]
- Deprost, D.; Yao, L.; Sormani, R.; Moreau, M.; Leterreux, G.; Nicolaï, M.; Bedu, M.; Robaglia, C.; Meyer, C. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep. 2007, 8, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, C.M.; Aznar, N.R.; Rodriguez, M.S.; Salerno, G.L.; Martínez-Noël, G.M.A. Target of rapamycin signaling is tightly and differently regulated in the plant response under distinct abiotic stresses. Planta. 2019, 251, 21. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Moin, M.; Madhav, M.S.; Datla, R.; Kirti, P.B. Target of Rapamycin (TOR) negatively regulates chlorophyll degradation and lipid peroxidation and controls responses under abiotic stress in Arabidopsis thaliana. Plant Stress. 2021, 2, 100020. [Google Scholar] [CrossRef]
- Couso, I.; Pérez-Pérez, M.E.; Ford, M.M.; Martínez-Force, E.; Hicks, L.M.; Umen, J.G.; Crespo, J.L. Phosphorus Availability Regulates TORC1 Signaling via LST8 in Chlamydomonas. Plant Cell. 2020, 32, 69–80. [Google Scholar] [CrossRef]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef]
- Schmollinger, S.; Schulz-Raffelt, M.; Strenkert, D.; Veyel, D.; Vallon, O.; Schroda, M. Dissecting the heat stress response in Chlamydomonas by pharmaceutical and RNAi approaches reveals conserved and novel aspects. Mol Plant. 2013, 6, 1795–1813. [Google Scholar] [CrossRef]
- Wang, W.; Cho, H.S.; Kim, R.; Jancarik, J.; Yokota, H.; Nguyen, H.H.; Grigoriev, I.V.; Wemmer, D.E.; Kim, S.H. Structural characterization of the reaction pathway in phosphoserine phosphatase: crystallographic "snapshots" of intermediate states. J Mol Biol. 2002, 319, 421–431. [Google Scholar] [CrossRef]
- Shetty, P.; Gitau, M.M.; Maróti, G. Salinity Stress Responses and Adaptation Mechanisms in Eukaryotic Green Microalgae. Cells. 2019, 8, 1657. [Google Scholar] [CrossRef]
- Gee, R.; Goyal, A.; Byerrum, R.U.; Tolbert, N.E. Two Isoforms of Dihydroxyacetone Phosphate Reductase from the Chloroplasts of Dunaliella tertiolecta. Plant Physiol. 1993, 103, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Sussman, I.; Avron, M. Characterization and partial purification of DL-glycerol-1-phosphatase from Dunaliella salina. Biochim. Biophys. Acta. 1981, 661, 199–204. [Google Scholar] [CrossRef]
- Wang, Y.; Cong, Y.; Wang, Y.; Guo, Z.; Yue, J.; Xing, Z.; Gao, X.; Chai, X. Identification of Early Salinity Stress-Responsive Proteins in Dunaliella salina by isobaric tags for relative and absolute quantitation (iTRAQ)-Based Quantitative Proteomic Analysis. Int. J. Mol. Sci. 2019, 20, 599. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.W.; Werth, E.G.; Hicks, L.M. The phosphorylated redox proteome of Chlamydomonas reinhardtii: Revealing novel means for regulation of protein structure and function. Redox Biol. 2018, 17, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Sadka, A.; Lers, A.; Zamir, A.; Avron, M. A critical examination of the role of de novo protein synthesis in the osmotic adaptation of the halotolerant alga Dunaliella. FEBS Lett. 1989, 244, 93–98. [Google Scholar] [CrossRef]
- van Schaftingen, E.; van Laere, A.J. Glycerol formation after the breaking of dormancy of Phycomyces blakesleeanus spores. Role of an interconvertible glycerol-3-phosphatase. Eur J Biochem. 1985, 148, 399–404. [Google Scholar] [CrossRef]
- Bayer, R.G.; Stael, S.; Rocha, A.G.; Mair, A.; Vothknecht, U.C.; Teige, M. Chloroplast-localized protein kinases: a step forward towards a complete inventory. J Exp Bot. 2012, 63, 1713–1723. [Google Scholar] [CrossRef]
- White-Gloria, C.; Johnson, J.J.; Marritt, K.; Kataya, A.; Vahab, A.; Moorhead, G.B. Protein Kinases and Phosphatases of the Plastid and Their Potential Role in Starch Metabolism. Front Plant Sci. 2018, 9, 1032. [Google Scholar] [CrossRef]
- Pagliaro, M. Glycerol: a key platform chemical of the forthcoming bioeconomy. In Glycerol: The Renewable Platform Chemical; Pagliaro, M., Ed.; Elsevier, 2017; pp. 109–132. [Google Scholar]
- Demmig-Adams, B.; Burch, T.A.; Stewart, J.J.; Savage, E.L.; Adams III, W.W. Algal glycerol accumulation and release as a sink for photosynthetic electron transport. Algal Res. 2017, 21, 161–168. [Google Scholar] [CrossRef]
- Semkiv, M.V.; Ruchala, J.; Dmytruk, K.V.; Sibirny, A.A. 100 Years Later, What Is New in Glycerol Bioproduction? Trends Biotechnol. 2020, 38, 907–916. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




