Submitted:
28 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
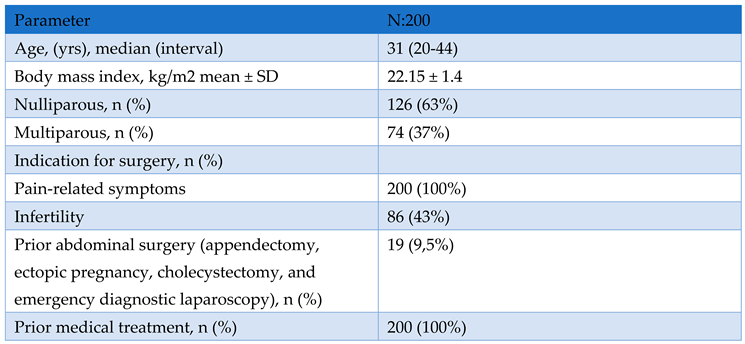
Study Design and Population
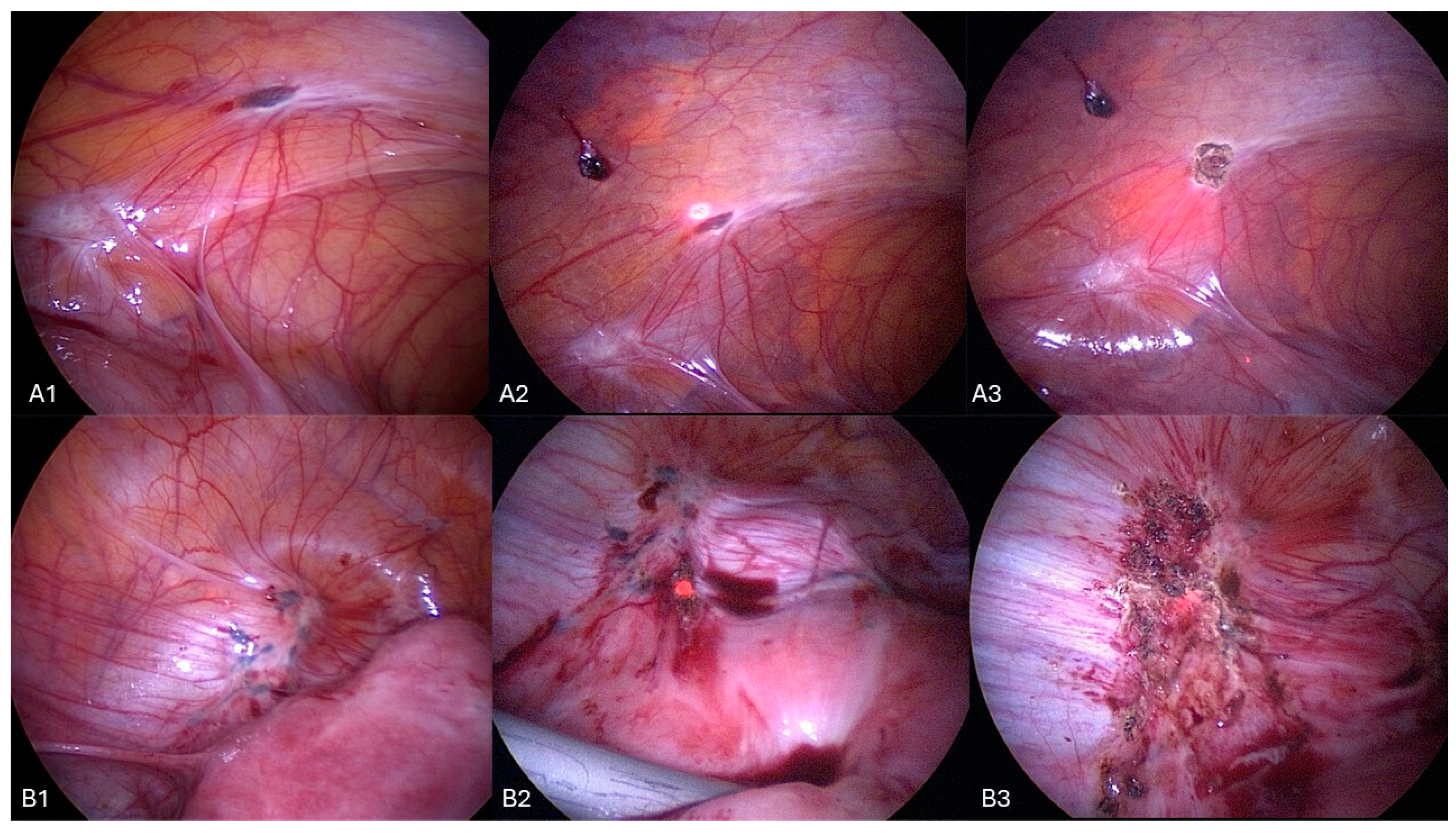
Surgical Procedure
Data Collection
Follow-Up (FU)
Statistical Analysis
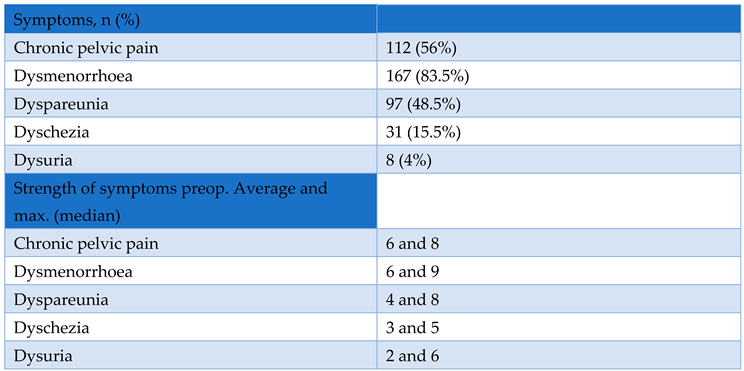
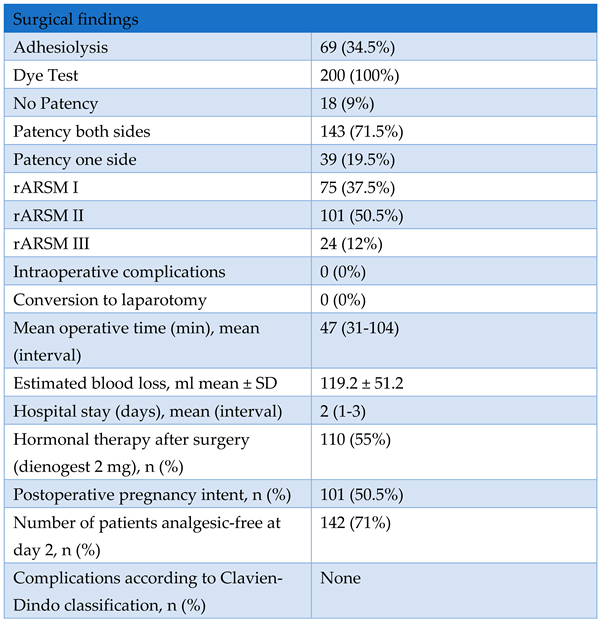
3. Results
4. Discussion
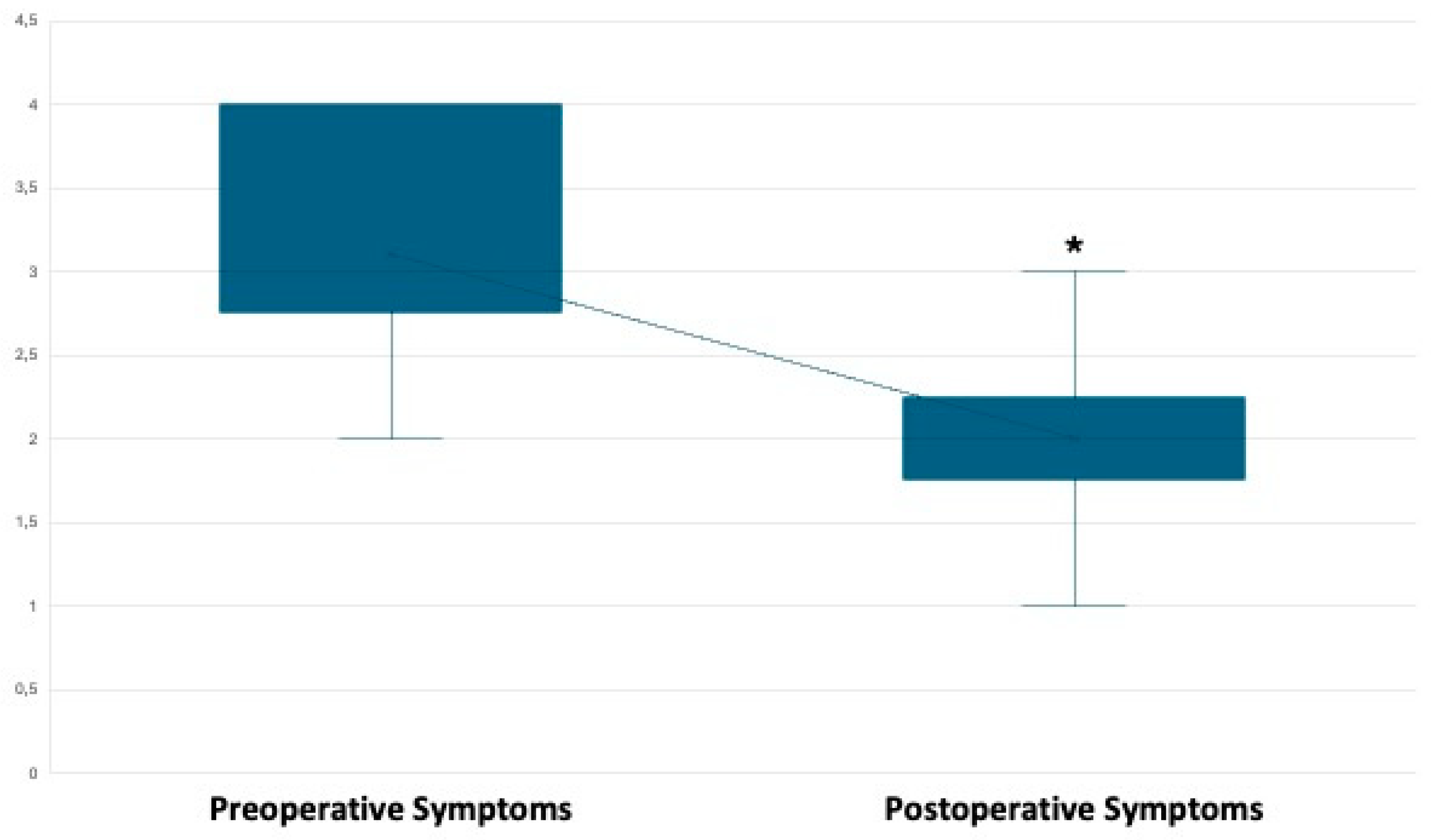
Primary Outcome: Pain Management.
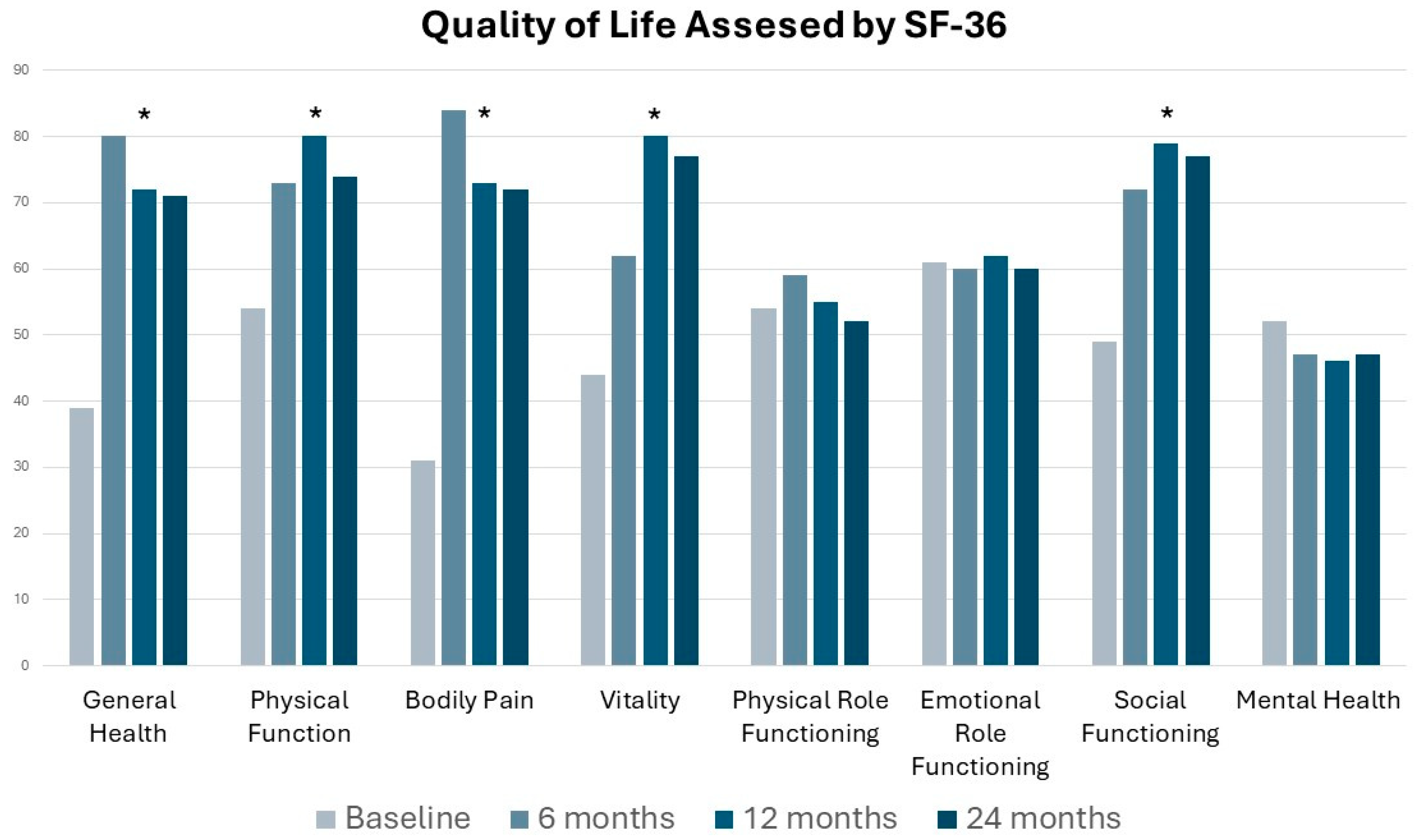
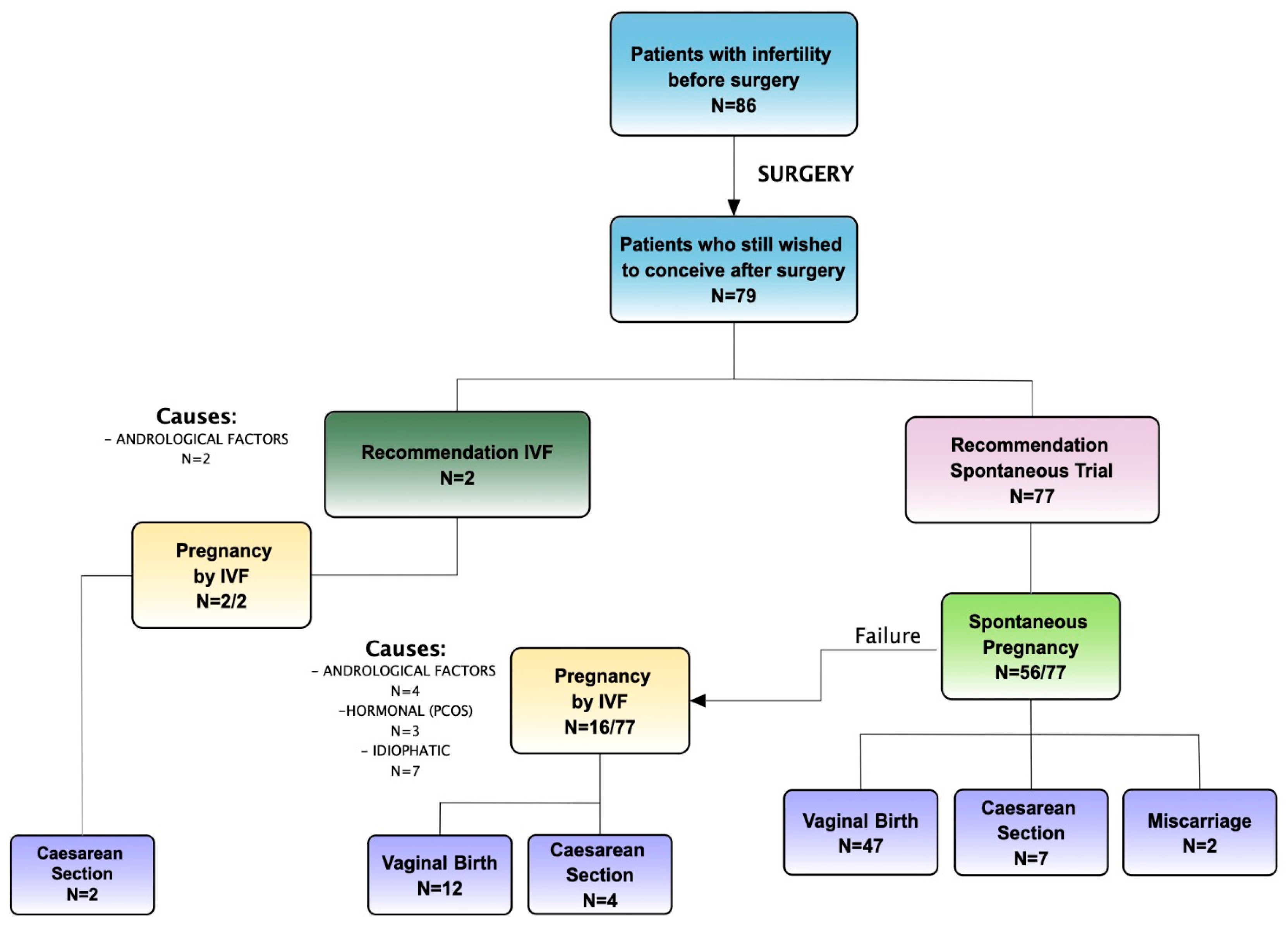
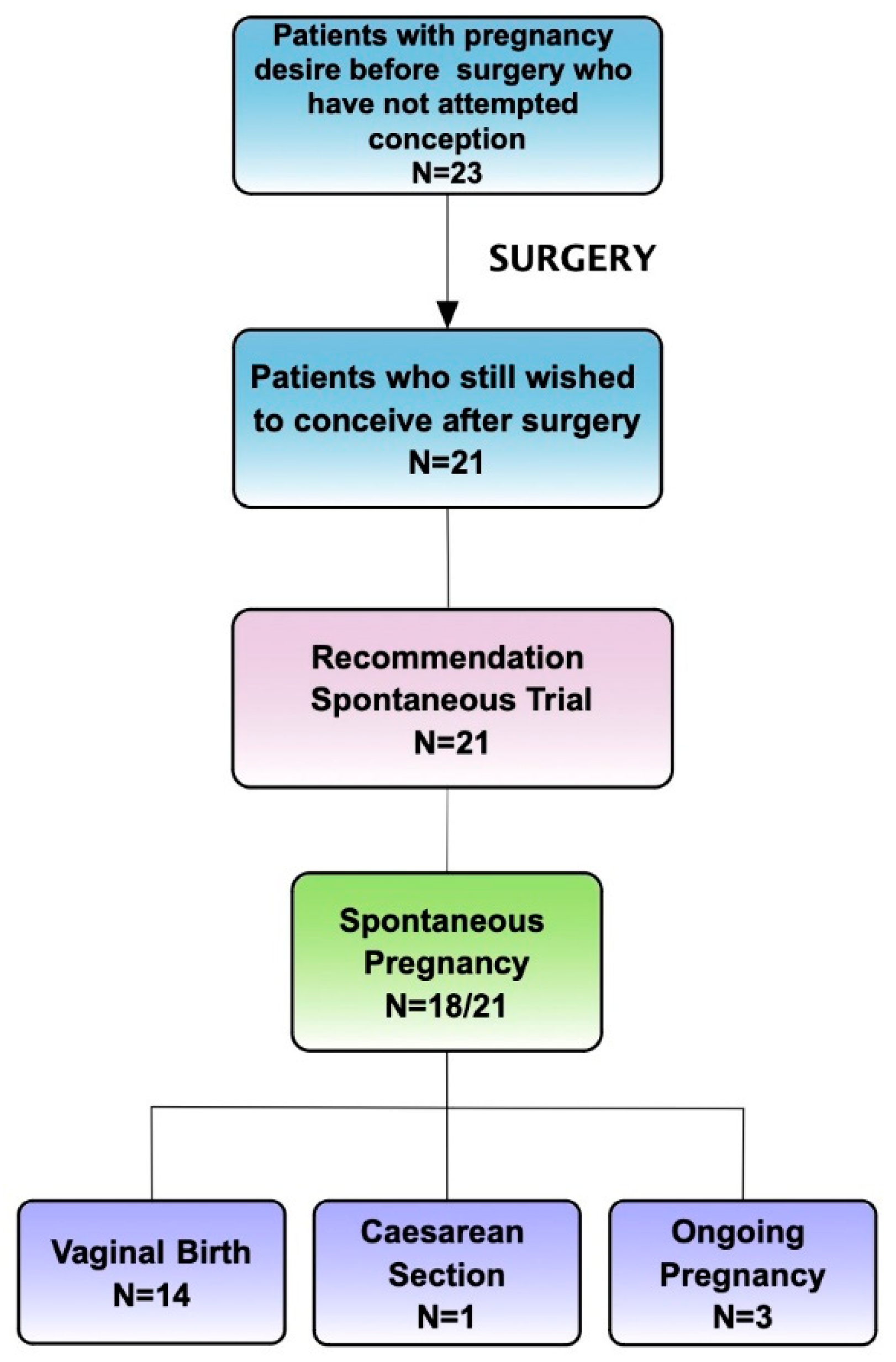
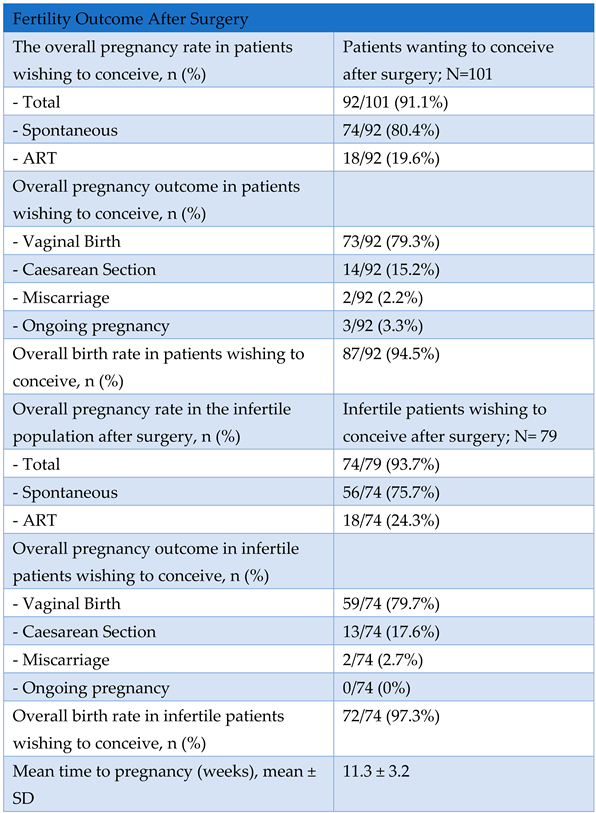
Secondary Outcome: Overall Pregnancy Rate Assessment
5. Conclusions
Author Contributions
Funding
References
- Rahmioglu, N.; Mortlock, S.; Ghiasi, M.; Møller, P.L.; Stefansdottir, L.; Galarneau, G.; Turman, C.; Danning, R.; Law, M.H.; Sapkota, Y.; et al. The Genetic Basis of Endometriosis and Comorbidity with Other Pain and Inflammatory Conditions. Nat. Genet. 2023, 55, 423–436. [Google Scholar] [CrossRef]
- Horne, A.W.; Missmer, S.A. Pathophysiology, Diagnosis, and Management of Endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef]
- Rogers, P.A.W.; D’Hooghe, T.M.; Fazleabas, A.; Gargett, C.E.; Giudice, L.C.; Montgomery, G.W.; Rombauts, L.; Salamonsen, L.A.; Zondervan, K.T. Priorities for Endometriosis Research: Recommendations from an International Consensus Workshop. Reprod. Sci. Thousand Oaks Calif 2009, 16, 335–346. [Google Scholar] [CrossRef]
- Dun, E.C.; Kho, K.A.; Morozov, V.V.; Kearney, S.; Zurawin, J.L.; Nezhat, C.H. Endometriosis in Adolescents. JSLS 2015, 19, e2015.00019. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Metastatic or Embolic Endometriosis, Due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar] [PubMed]
- Tal, A.; Tal, R.; Pluchino, N.; Taylor, H.S. Endometrial Cells Contribute to Preexisting Endometriosis Lesions in a Mouse Model of Retrograde Menstruation†. Biol. Reprod. 2019, 100, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.V.P.; Tokushige, N.; Berbic, M.; Markham, R.; Fraser, I.S. Macrophages and Nerve Fibres in Peritoneal Endometriosis. Hum. Reprod. Oxf. Engl. 2009, 24, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Mechsner, S.; Kaiser, A.; Kopf, A.; Gericke, C.; Ebert, A.; Bartley, J. A Pilot Study to Evaluate the Clinical Relevance of Endometriosis-Associated Nerve Fibers in Peritoneal Endometriotic Lesions. Fertil. Steril. 2009, 92, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The Burden of Endometriosis: Costs and Quality of Life of Women with Endometriosis and Treated in Referral Centres. Hum. Reprod. Oxf. Engl. 2012, 27, 1292–1299. [Google Scholar] [CrossRef]
- Daniilidis, A.; Angioni, S.; Di Michele, S.; Dinas, K.; Gkrozou, F.; D’Alterio, M.N. Deep Endometriosis and Infertility: What Is the Impact of Surgery? J. Clin. Med. 2022, 11, 6727. [Google Scholar] [CrossRef]
- Duffy, J.M.N.; Arambage, K.; Correa, F.J.S.; Olive, D.; Farquhar, C.; Garry, R.; Barlow, D.H.; Jacobson, T.Z. Laparoscopic Surgery for Endometriosis. Cochrane Database Syst. Rev. 2014, CD011031. [Google Scholar] [CrossRef]
- Vercellini, P.; Trespidi, L.; De Giorgi, O.; Cortesi, I.; Parazzini, F.; Crosignani, P.G. Endometriosis and Pelvic Pain: Relation to Disease Stage and Localization. Fertil. Steril. 1996, 65, 299–304. [Google Scholar] [CrossRef]
- Gruppo Italiano per lo Studio dell’Endometriosi Relationship between Stage, Site and Morphological Characteristics of Pelvic Endometriosis and Pain. Hum. Reprod. Oxf. Engl. 2001, 16, 2668–2671. [CrossRef]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, Pathobiology, and Therapeutic Prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef]
- Kuznetsov, L.; Dworzynski, K.; Davies, M.; Overton, C. Guideline Committee Diagnosis and Management of Endometriosis: Summary of NICE Guidance. BMJ 2017, 358, j3935. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE Guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-W. Recurrence of Endometriosis and Its Control. Hum. Reprod. Update 2009, 15, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Bertulessi, C.; Pasini, A.; Rosati, M.; Di Stefano, F.; Shonauer, S.; Vicino, M.; Aguzzoli, L.; Trossarelli, G.F.; Massobrio, M.; et al. Determinants of Short Term Recurrence Rate of Endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 121, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Crowgey, S.R.; Garrison, C.P. Surgical Treatment of Endometriosis via Laser Laparoscopy. Fertil. Steril. 1986, 45, 778–783. [Google Scholar] [CrossRef]
- Nezhat, C.; Nezhat, F.R. Safe Laser Endoscopic Excision or Vaporization of Peritoneal Endometriosis. Fertil. Steril. 1989, 52, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.D.; Asmar, P. The Use of CO2 Laser Laparoscopy for Treating Endometriosis. Int. J. Fertil. 1987, 32, 237–239. [Google Scholar]
- Adamyan, L.; Kasyan, V.; Pivazyan, L.; Isaeva, S.; Avetisyan, J. Laser Vaporization Compared with Other Surgical Techniques in Women with Ovarian Endometrioma: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2023, 308, 413–425. [Google Scholar] [CrossRef]
- Mackenzie, S.C.; Stephen, J.; Williams, L.; Daniels, J.; Norrie, J.; Becker, C.M.; Byrne, D.; Cheong, Y.; Clark, T.J.; Cooper, K.G.; et al. Effectiveness of Laparoscopic Removal of Isolated Superficial Peritoneal Endometriosis for the Management of Chronic Pelvic Pain in Women (ESPriT2): Protocol for a Multi-Centre Randomised Controlled Trial. Trials 2023, 24, 425. [Google Scholar] [CrossRef]
- Colasanti, R.; Giannoni, L.; Dallari, S.; Liverotti, V.; Aiudi, D.; Di Rienzo, A.; Rossi, F.; Iacoangeli, M. Application of a Scanner-Assisted Carbon Dioxide Laser System for Neurosurgery. World Neurosurg. 2021, 153, e250–e258. [Google Scholar] [CrossRef] [PubMed]
- Dallari, S.; Giannoni, L.; Filosa, A. Scanning Super/Ultrapulsed CO2 Laser Efficacy in Laryngeal Malignant Lesions. Med. Kaunas Lith. 2022, 58, 200. [Google Scholar] [CrossRef] [PubMed]
- Iacopo, G.; Tommaso, C.; Chiara, L.; Filippo, C.; Paolo, D.; Gianni, R.; Cinzia, T.; Giuseppina, T.; Federico, B.; Alessandra, A.; et al. Scanner-Assisted CO2 Laser Fissurectomy: A Pilot Study. Front. Surg. 2021, 8, 799607. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Mirabella, C.; Scatena, A.; Nreu, B.; Zannoni, S.; Aleffi, S.; Giannoni, L.; Mannucci, E. CO2 Laser for the Treatment of Diabetic Foot Ulcers with Exposed Bone. A Consecutive Series of Type 2 Diabetic Patients. J. Endocrinol. Invest. 2017, 40, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, A.; Bianchi, C.; Giannoni, L.; Venzi, R.; Fantappiè, G.; Mecacci, F.; Fambrini, and M. Colposcopic-Magnified Scan-Aided CO2 Laser Vaporization for Genital Warts in Pregnancy: A Propsective Descriptive Evaluation of Safety in a Tertiary Care Obstetrical Hospital. J. Surg. 2023.
- Rosati, M.; Bramante, S.; Conti, F.; Rizzi, M.; Frattari, A.; Spina, T. Laparoscopic Salpingo-Oophorectomy in Conscious Sedation. JSLS 2015, 19, e2015.00031. [Google Scholar] [CrossRef] [PubMed]
- Rosati, M.; Bramante, S.; Conti, F.; Frattari, A.; Rizzi, M.; Roman, R.A. Operative Gynecological Laparoscopy Under Conscious Sedation. JSLS 2020, 24, e2020.00020. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Hoffmann, H.; Clavien, P.-A.; Bucher, H.C.; Dell-Kuster, S. Definition and Classification of Intraoperative Complications (CLASSIC): Delphi Study and Pilot Evaluation. World J. Surg. 2015, 39, 1663–1671. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Coulter, A.; Wright, L. Short Form 36 (SF36) Health Survey Questionnaire: Normative Data for Adults of Working Age. BMJ 1993, 306, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Revised American Fertility Society Classification of Endometriosis: 1985. Fertil. Steril. 1985, 43, 351–352. [CrossRef] [PubMed]
- Centini, G.; Afors, K.; Murtada, R.; Argay, I.M.; Lazzeri, L.; Akladios, C.Y.; Zupi, E.; Petraglia, F.; Wattiez, A. Impact of Laparoscopic Surgical Management of Deep Endometriosis on Pregnancy Rate. J. Minim. Invasive Gynecol. 2016, 23, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ghai, V.; Jan, H.; Shakir, F.; Kent, A. Identifying Preoperative Factors Associated with Nonresponders in Women Undergoing Comprehensive Surgical Treatment for Endometriosis. J. Minim. Invasive Gynecol. 2020, 27, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Xholli, A.; Filip, G.; Previtera, F.; Cagnacci, A. Modification of Endometrioma Size during Hormone Therapy Containing Dienogest. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 36, 545–549. [Google Scholar] [CrossRef]
- Strowitzki, T.; Marr, J.; Gerlinger, C.; Faustmann, T.; Seitz, C. Detailed Analysis of a Randomized, Multicenter, Comparative Trial of Dienogest versus Leuprolide Acetate in Endometriosis. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2012, 117, 228–233. [Google Scholar] [CrossRef]
- Garry, R. The Effectiveness of Laparoscopic Excision of Endometriosis. Curr. Opin. Obstet. Gynecol. 2004, 16, 299–303. [Google Scholar] [CrossRef]
- He, W.; Liu, X.; Zhang, Y.; Guo, S.-W. Generalized Hyperalgesia in Women with Endometriosis and Its Resolution Following a Successful Surgery. Reprod. Sci. Thousand Oaks Calif 2010, 17, 1099–1111. [Google Scholar] [CrossRef]
- Buchweitz, O.; Wülfing, P.; Malik, E. Interobserver Variability in the Diagnosis of Minimal and Mild Endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 122, 213–217. [Google Scholar] [CrossRef]
- Reis, F.M.; Santulli, P.; Marcellin, L.; Borghese, B.; Lafay-Pillet, M.-C.; Chapron, C. Superficial Peritoneal Endometriosis: Clinical Characteristics of 203 Confirmed Cases and 1292 Endometriosis-Free Controls. Reprod. Sci. Thousand Oaks Calif 2020, 27, 309–315. [Google Scholar] [CrossRef]
- Teodoro, M.C.; Genovese, F.; Rubbino, G.; Palumbo, M.; Zarbo, G. [Chronic pelvic pain in patients with endometriosis: results of laparoscopic treatment]. Minerva Ginecol. 2012, 64, 9–14. [Google Scholar] [PubMed]
- Dückelmann, A.M.; Taube, E.; Abesadze, E.; Chiantera, V.; Sehouli, J.; Mechsner, S. When and How Should Peritoneal Endometriosis Be Operated on in Order to Improve Fertility Rates and Symptoms? The Experience and Outcomes of Nearly 100 Cases. Arch. Gynecol. Obstet. 2021, 304, 143–155. [Google Scholar] [CrossRef]
- Medical Therapy for Preventing Recurrent Endometriosis after Conservative Surgery: A Cost-Effectiveness Analysis - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28613432/ (accessed on 20 February 2024).
- Tobiume, T.; Kotani, Y.; Takaya, H.; Nakai, H.; Tsuji, I.; Suzuki, A.; Mandai, M. Determinant Factors of Postoperative Recurrence of Endometriosis: Difference between Endometrioma and Pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 205, 54–59. [Google Scholar] [CrossRef]
- Zakhari, A.; Delpero, E.; McKeown, S.; Tomlinson, G.; Bougie, O.; Murji, A. Endometriosis Recurrence Following Post-Operative Hormonal Suppression: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2021, 27, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Williams, C. Surgical Treatment of Endometriosis: Location and Patterns of Disease at Reoperation. Fertil. Steril. 2010, 93, 57–61. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, Y.; Liu, Z.; Wang, Y. Inflammation and Endometriosis. Front. Biosci. Landmark Ed. 2016, 21, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Coxon, L.; Wiech, K.; Vincent, K. Is There a Neuropathic-Like Component to Endometriosis-Associated Pain? Results From a Large Cohort Questionnaire Study. Front. Pain Res. 2021, 2, 743812. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Yang, H.-L.; Shao, J.; Mei, J.; Chang, K.-K.; Zhu, R.; Li, M.-Q. Anti-Inflammatory Cytokines in Endometriosis. Cell. Mol. Life Sci. CMLS 2019, 76, 2111–2132. [Google Scholar] [CrossRef]
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of Endometriosis and Its Comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef]
- Abbott, J.; Hawe, J.; Hunter, D.; Holmes, M.; Finn, P.; Garry, R. Laparoscopic Excision of Endometriosis: A Randomized, Placebo-Controlled Trial. Fertil. Steril. 2004, 82, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, J.; Mohindra, R.; Ross, S.; Taenzer, P.; Brant, R. Laparoscopy and Reported Pain among Patients with Endometriosis. J. Obstet. Gynaecol. Can. JOGC J. Obstet. Gynecol. Can. JOGC 2005, 27, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Bafort, C.; Beebeejaun, Y.; Tomassetti, C.; Bosteels, J.; Duffy, J.M. Laparoscopic Surgery for Endometriosis. Cochrane Database Syst. Rev. 2020, 10, CD011031. [Google Scholar] [CrossRef]
- Arcoverde, F.V.L.; Andres, M. de P.; Borrelli, G.M.; Barbosa, P. de A.; Abrão, M.S.; Kho, R.M. Surgery for Endometriosis Improves Major Domains of Quality of Life: A Systematic Review and Meta-Analysis. J. Minim. Invasive Gynecol. 2019, 26, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Aimi, G.; Busacca, M.; Apolone, G.; Uglietti, A.; Crosignani, P.G. Laparoscopic Uterosacral Ligament Resection for Dysmenorrhea Associated with Endometriosis: Results of a Randomized, Controlled Trial. Fertil. Steril. 2003, 80, 310–319. [Google Scholar] [CrossRef]
- Angioni, S.; Nappi, L.; Sorrentino, F.; Peiretti, M.; Daniilidis, A.; Pontis, A.; Tinelli, R.; D’Alterio, M.N. Laparoscopic Treatment of Deep Endometriosis with a Diode Laser: Our Experience. Arch. Gynecol. Obstet. 2021, 304, 1221–1231. [Google Scholar] [CrossRef]
- Tahmasbi Rad, M.; Akpinar-Isci, D.; Nobs, T.; Gasimli, K.; Becker, S. Pregnancy after Laparoscopic Surgery for Endometriosis: How Long Should We Wait? A Retrospective Study Involving a Long-Term Follow up at a University Endometriosis Center. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2023, 163, 108–114. [Google Scholar] [CrossRef]
- Nezhat, C.; Khoyloo, F.; Tsuei, A.; Armani, E.; Page, B.; Rduch, T.; Nezhat, C. The Prevalence of Endometriosis in Patients with Unexplained Infertility. J. Clin. Med. 2024, 13, 444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





