Submitted:
28 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
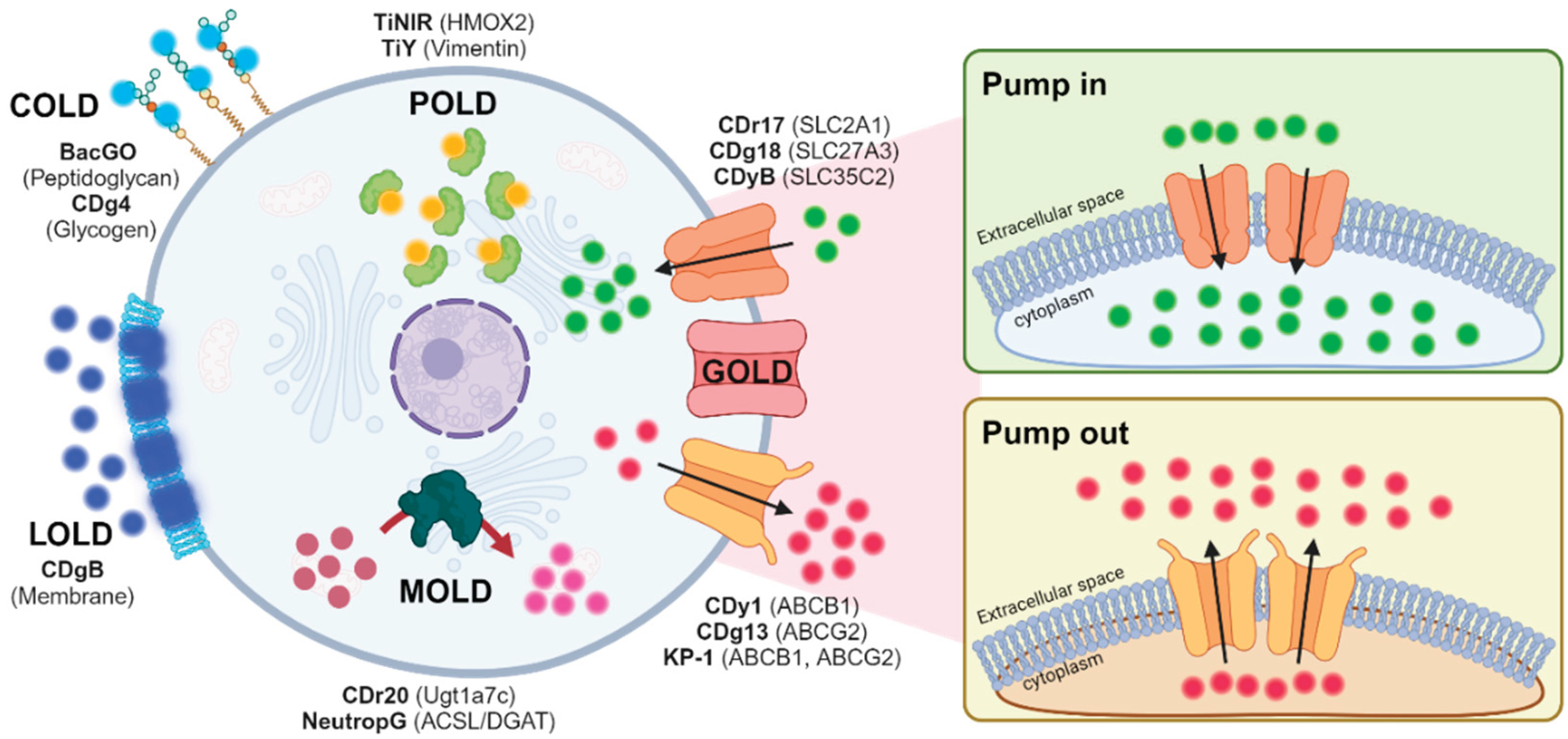
1. Introduction
2. Materials and Methods
Animal experiment
Lymphocyte preparation
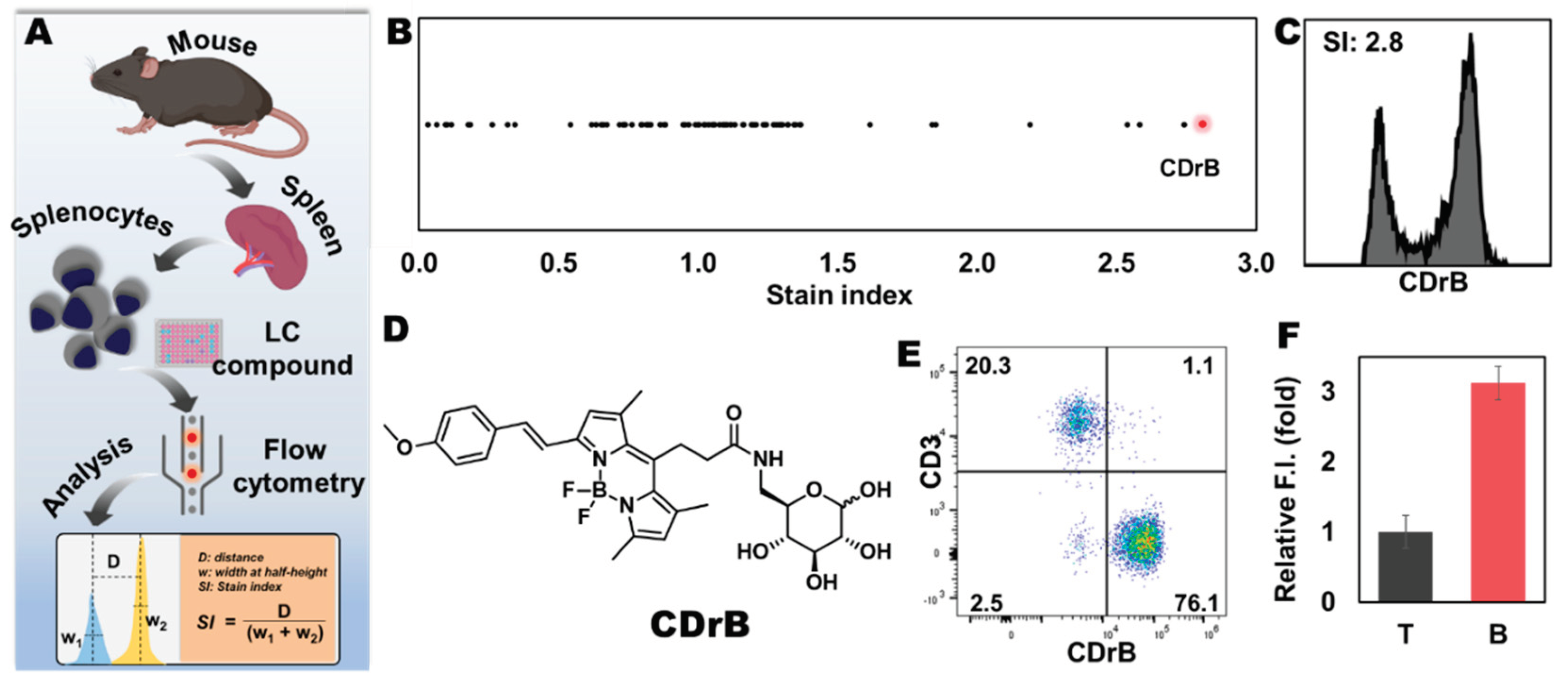
Flow cytometry-based screening
Isolation of B cells and T cells
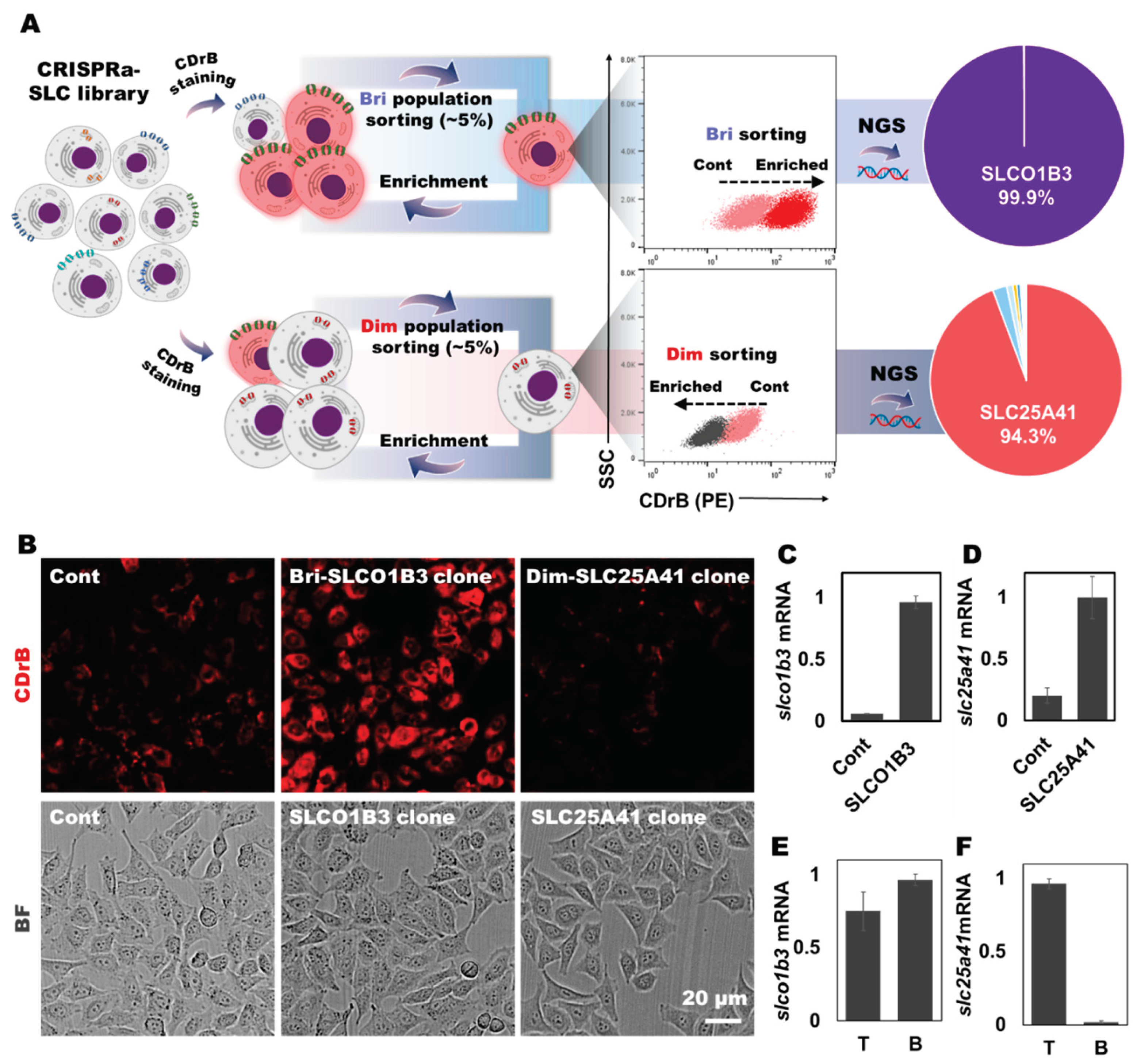
Generation of SLC-CRISPRa pools
FACS
RT-PCR
Chemical materials and general methods for CDrB synthesis
Synthesis of CDrB
3. Results
3.1. Eliciting B cell selective probe
3.2. CDrB selective mechanism identification
3.3. CDrB selective mechanism validation
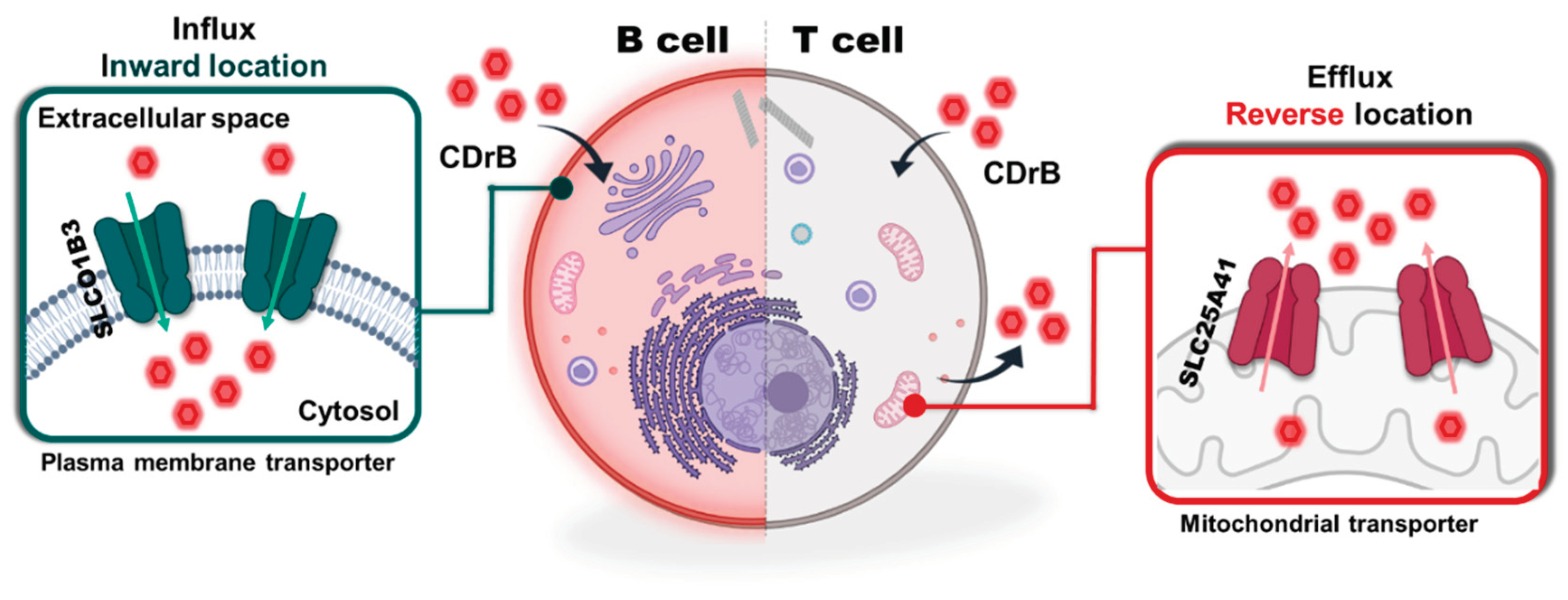
3.4. Proposed mechanism of CDrB
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, S.W.; Kang, N.Y.; Park, S.J.; Ha, H.H.; Kim, Y.K.; Lee, J.S.; Chang, Y.T. Diversity oriented fluorescence library approach (DOFLA) for live cell imaging probe development. Acc Chem Res 2014, 47, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Lee, Y.A.; Su, D.; Lee, J.; Park, S.J.; Kim, B.; Jane Lee, J.H.; Liu, X.; Kim, S.S.; Bae, M.A.; et al. A Near-Infrared Probe Tracks and Treats Lung Tumor Initiating Cells by Targeting HMOX2. J Am Chem Soc 2019, 141, 14673–14686. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Kim, J.J.; Lee, J.; Lee, J.H.J.; Sahu, S.; Kwon, H.Y.; Park, S.J.; Jang, S.Y.; Lee, J.S.; Wang, Z.; et al. Identification of Tumor Initiating Cells with a Small-Molecule Fluorescent Probe by Using Vimentin as a Biomarker. Angew Chem Int Ed Engl 2018, 57, 2851–2854. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, B.; Choi, S.; Balasubramaniam, S.; Lee, S.C.; Lee, J.Y.; Kim, H.S.; Kim, J.Y.; Kim, J.J.; Lee, Y.A.; et al. Imaging inflammation using an activated macrophage probe with Slc18b1 as the activation-selective gating target. Nat Commun 2019, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Liu, X.; Choi, E.G.; Lee, J.Y.; Choi, S.Y.; Kim, J.Y.; Wang, L.; Park, S.J.; Kim, B.; Lee, Y.A.; et al. Development of a Universal Fluorescent Probe for Gram-Positive Bacteria. Angew Chem Int Ed Engl 2019, 58, 8426–8431. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kang, N.Y.; Park, S.J.; Yun, S.W.; Chandran, Y.; Chang, Y.T. Development of a fluorescent chalcone library and its application in the discovery of a mouse embryonic stem cell probe. Chem Commun (Camb) 2012, 48, 6681–6683. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Kumar Das, R.; Jung, G.T.; Lee, H.G.; Lee, S.H.; Berry, S.N.; Tan, J.K.S.; Park, S.; Yang, J.S.; Park, S.; et al. Lipid-Oriented Live-Cell Distinction of B and T Lymphocytes. J Am Chem Soc 2021, 143, 5836–5844. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Fukuda, M.; Lee, J.Y.; Su, D.; Sanu, S.; Silvin, A.; Khoo, A.T.T.; Kwon, T.; Liu, X.; Chi, W.; et al. Visualizing Microglia with a Fluorescence Turn-On Ugt1a7c Substrate. Angew Chem Int Ed Engl 2019, 58, 7972–7976. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lee, S.H.; Park, S.H.; Ciaramicoli, L.M.; Kwon, H.Y.; Cho, H.; Jeong, J.; Chang, Y.T. Neutrophil-Selective Fluorescent Probe Development through Metabolism-Oriented Live-Cell Distinction. Angew Chem Int Ed Engl 2021, 60, 23743–23749. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kwon, H.Y.; Sharma, A.; Lee, S.H.; Liu, X.; Miyamoto, N.; Kim, J.J.; Im, S.H.; Kang, N.Y.; Chang, Y.T. Visualizing inflammation with an M1 macrophage selective probe via GLUT1 as the gating target. Nat Commun 2022, 13, 5974. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kwon, H.Y.; Lee, S.H.; Lee, H.G.; Kang, N.Y.; Chang, Y.T. Development of a Fluorescent Probe for M2 Macrophages via Gating-Oriented Live-Cell Distinction. J Am Chem Soc 2023, 145, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lee, S.H.; Das, R.K.; Kwon, H.Y.; Kim, H.S.; Chang, Y.T. A SLC35C2 Transporter-Targeting Fluorescent Probe for the Selective Detection of B Lymphocytes Identified by SLC-CRISPRi and Unbiased Fluorescence Library Screening. Angew Chem Int Ed Engl 2022, 61, e202202095. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Go, Y.H.; Ciaramicoli, L.M.; Kwon, H.Y.; Kim, H.S.; Bi, X.; Yu, Y.H.; Kim, B.; Ha, H.H.; Kang, N.Y.; et al. Target identification of mouse stem cell probe CDy1 as ALDH2 and Abcb1b through live-cell affinity-matrix and ABC CRISPRa library. RSC Chem Biol 2021, 2, 1590–1593. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Feng, S.; Yun, S.W.; Leong, C.; Satapathy, R.; Wan, S.Y.; Chang, Y.T. A Fluorescent Probe for Neural Stem/Progenitor Cells with High Differentiation Capability into Neurons. Chembiochem 2016, 17, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Hirata, N.; Nakagawa, M.; Fujibayashi, Y.; Yamauchi, K.; Murata, A.; Minami, I.; Tomioka, M.; Kondo, T.; Kuo, T.F.; Endo, H.; et al. A chemical probe that labels human pluripotent stem cells. Cell Rep 2014, 6, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




