Submitted:
22 February 2024
Posted:
23 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
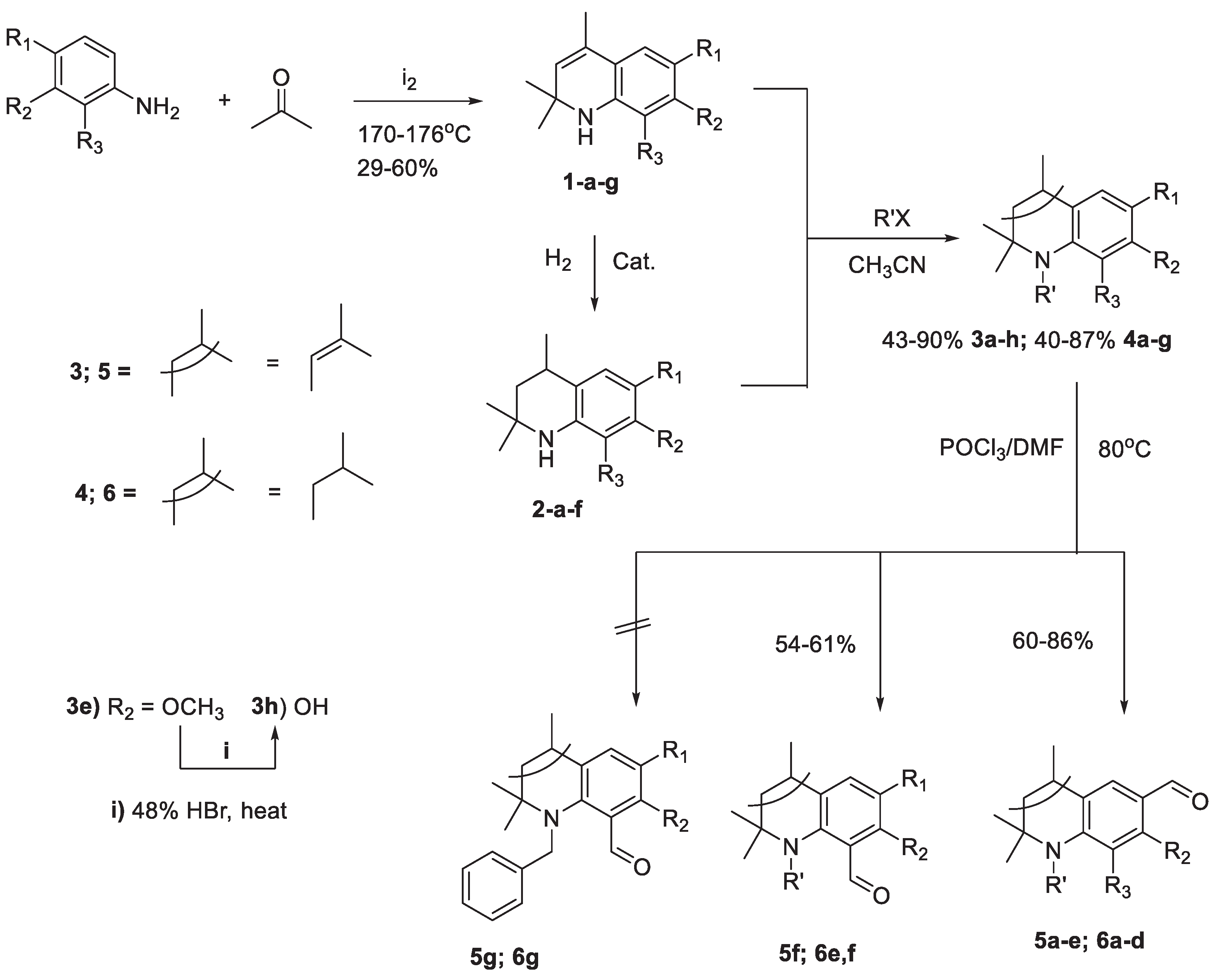
2.1. Chemistry
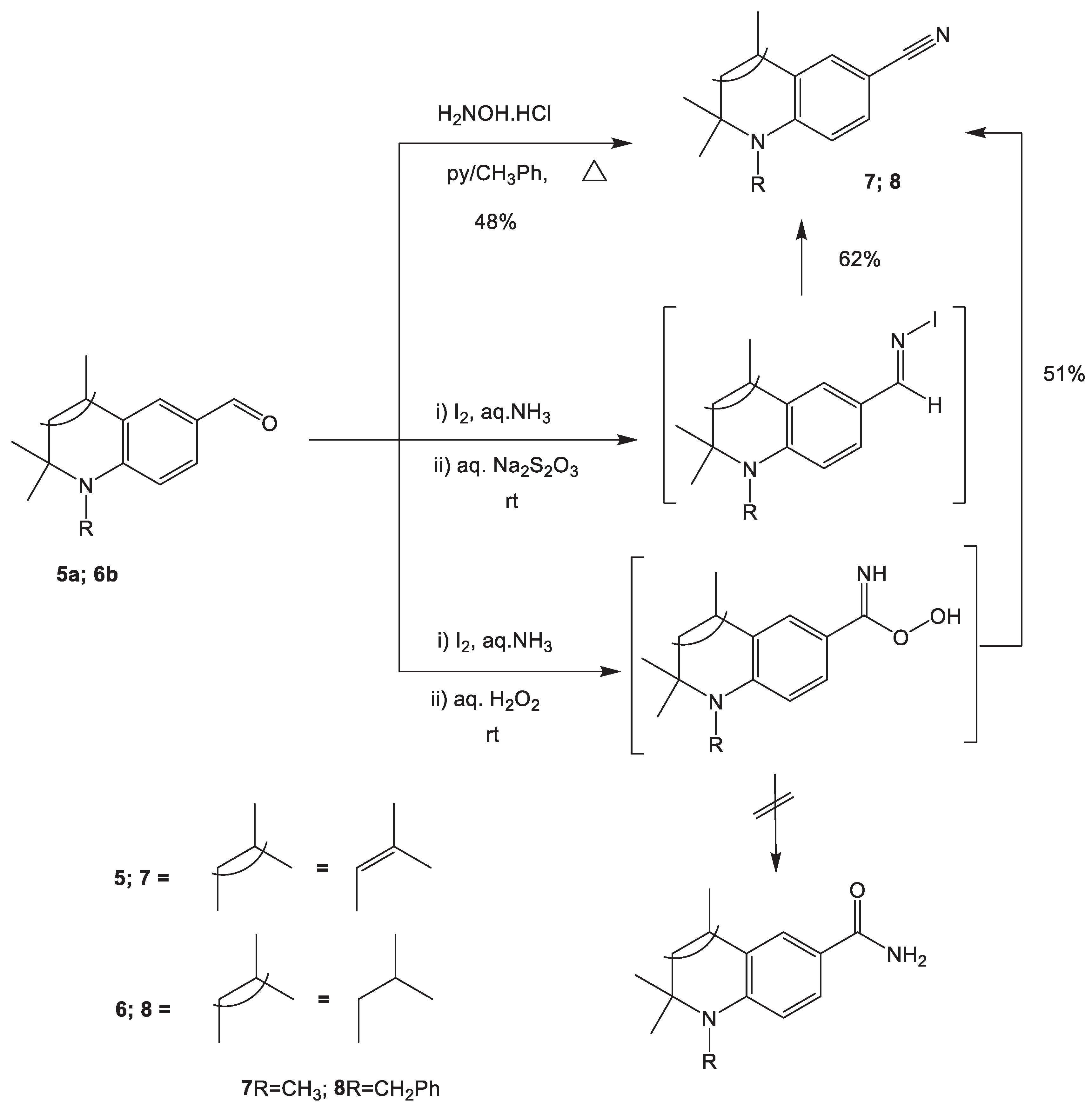
2.1.1. Synthesis of N-alkyl-2,2,4-trimethylhydroquinolin-6-yl-carbonitriles 7, 8
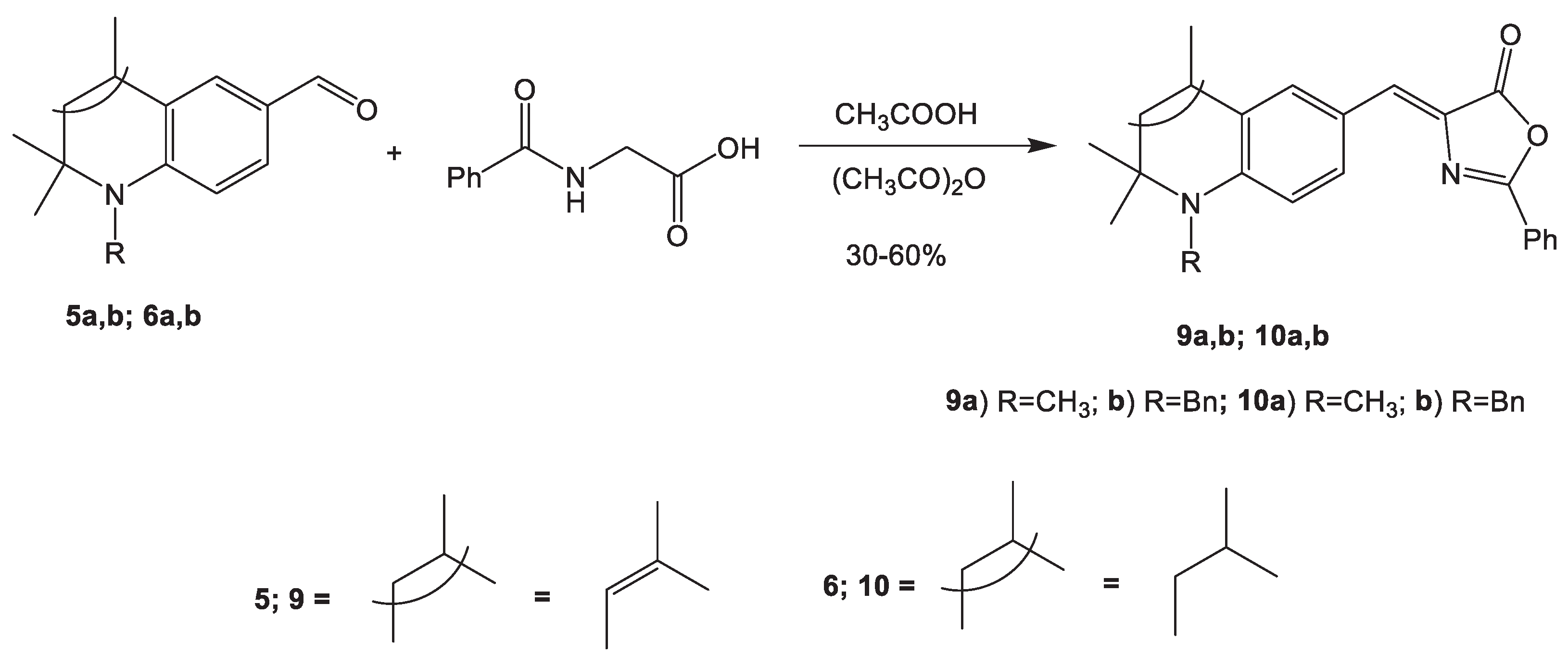
2.1.2. Synthesis of 2-Phenyl-1,3-oxazol-5(4Н)-ones 9, 10
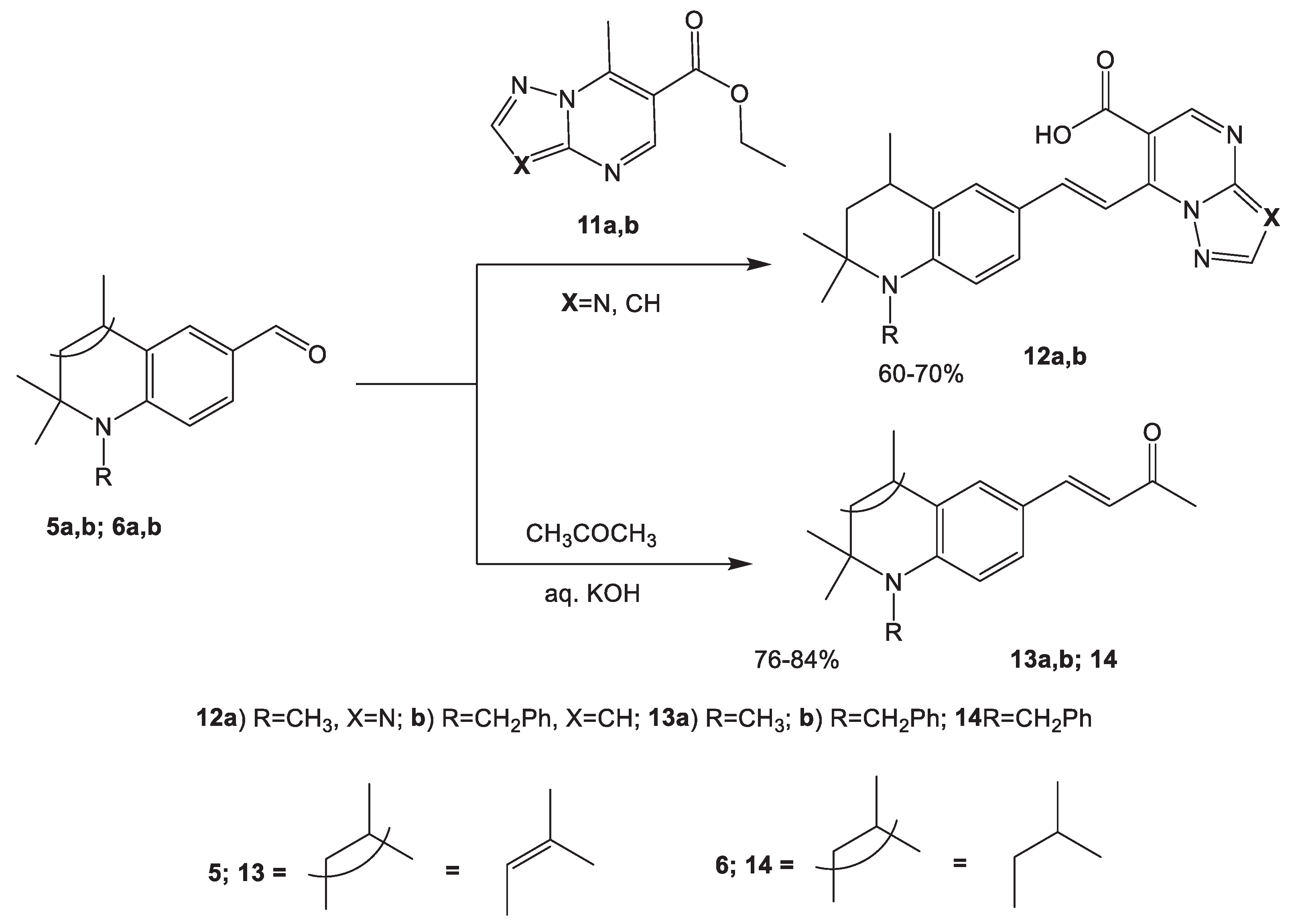
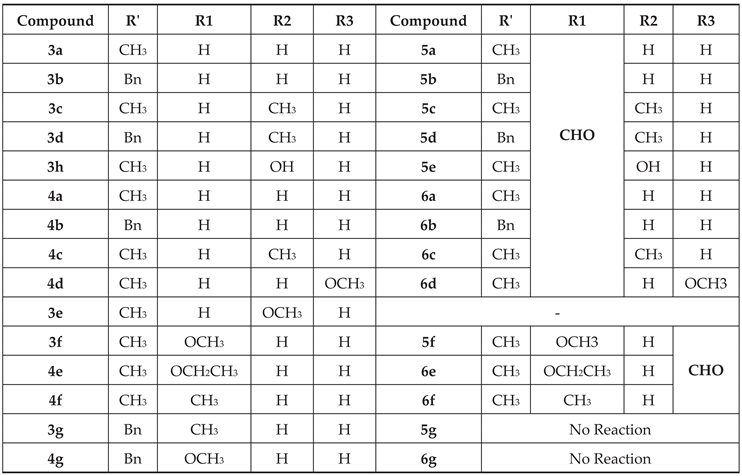
2.1.3. Condensation of Hydroquinolinecarbaldehydes with Reagents Containing an Activated Methyl Group 12, 13 14
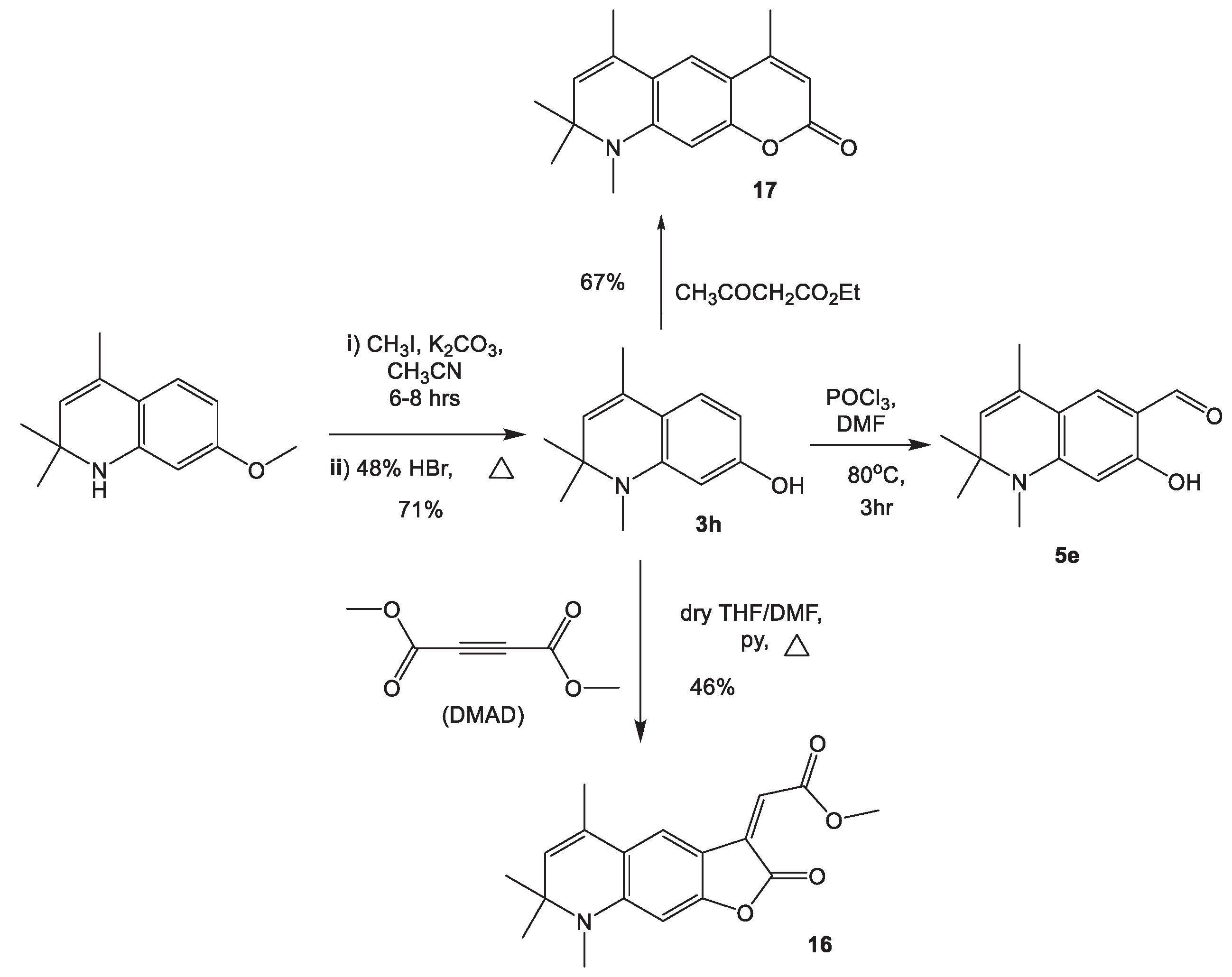
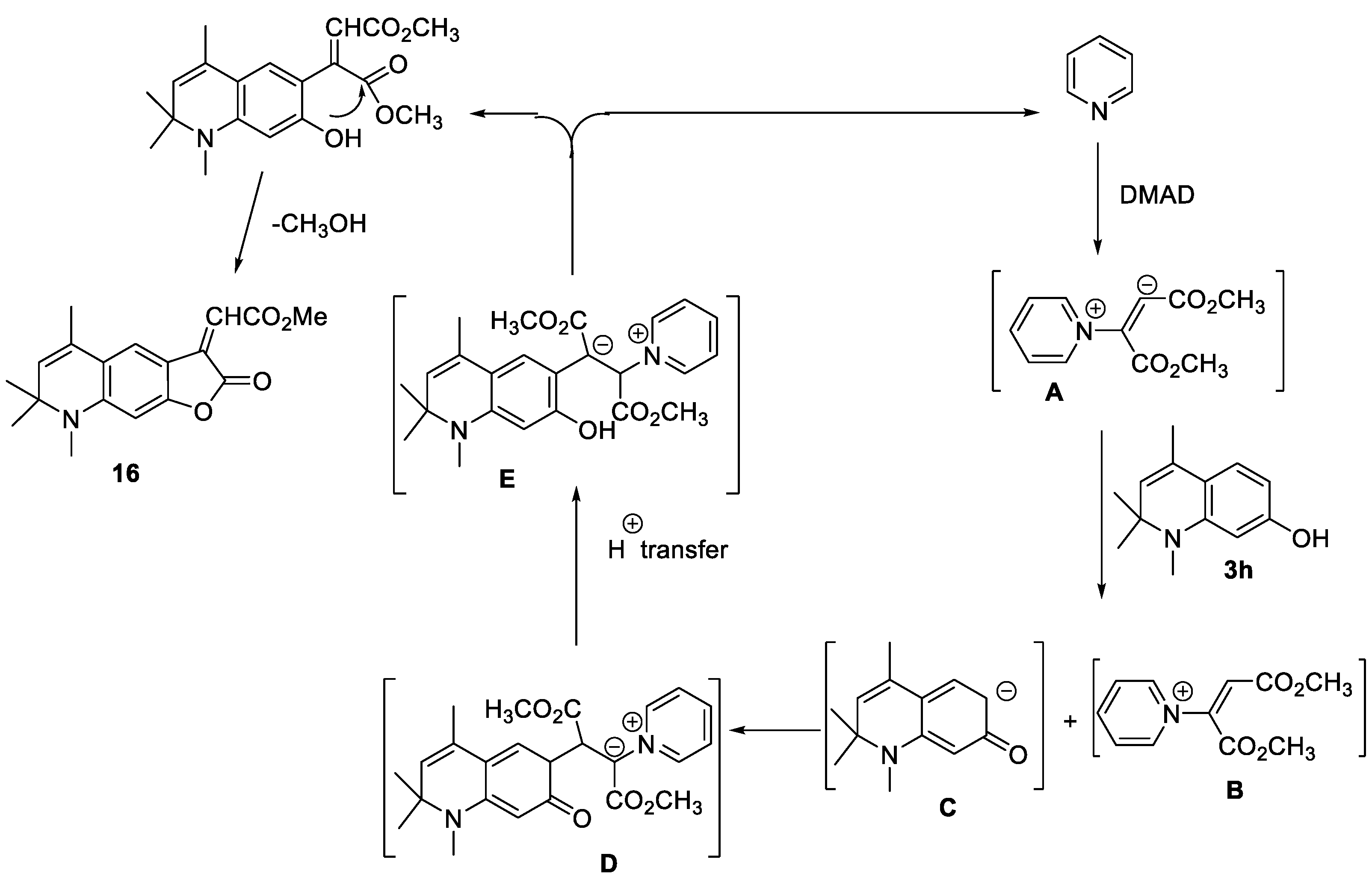
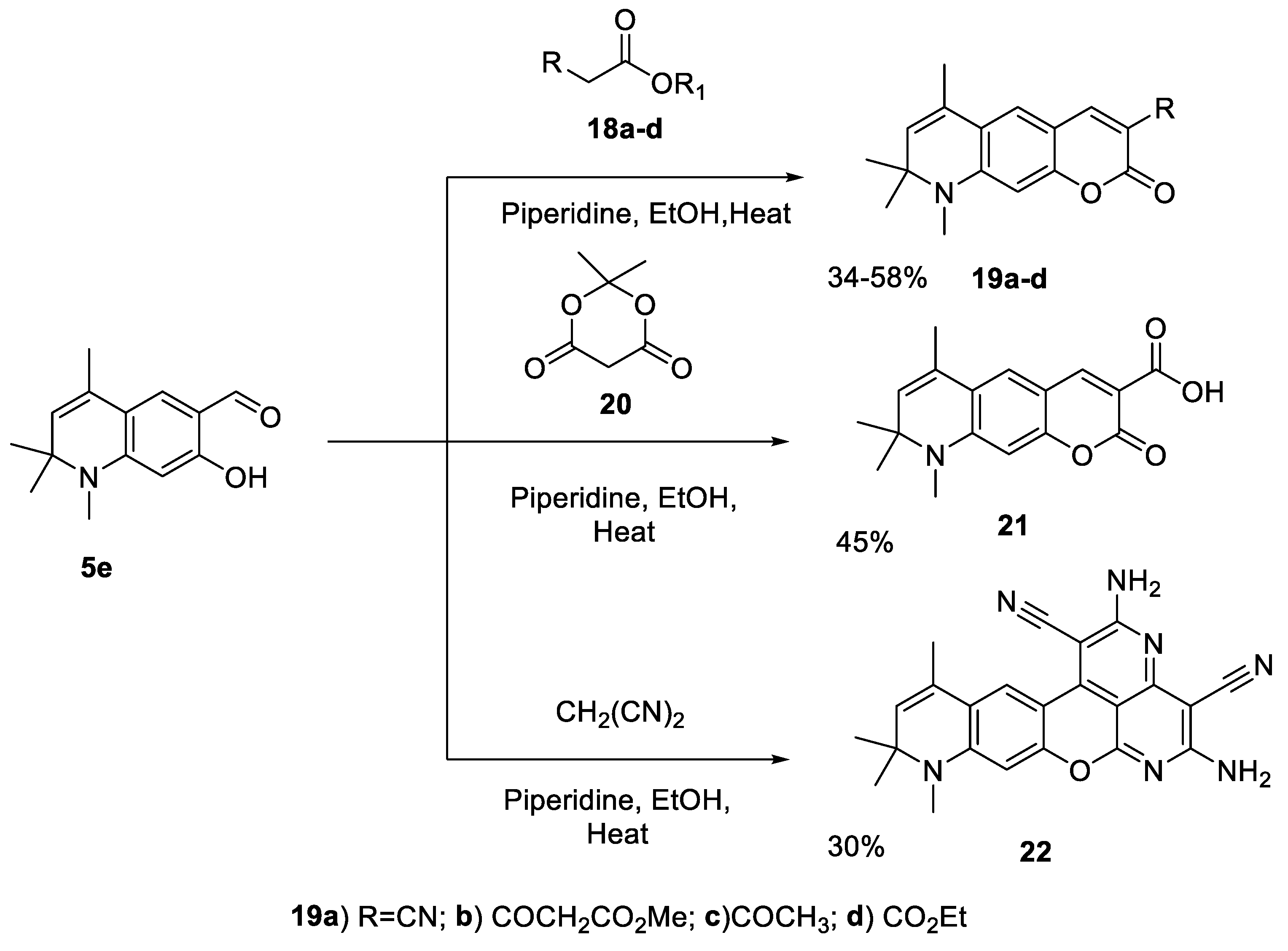
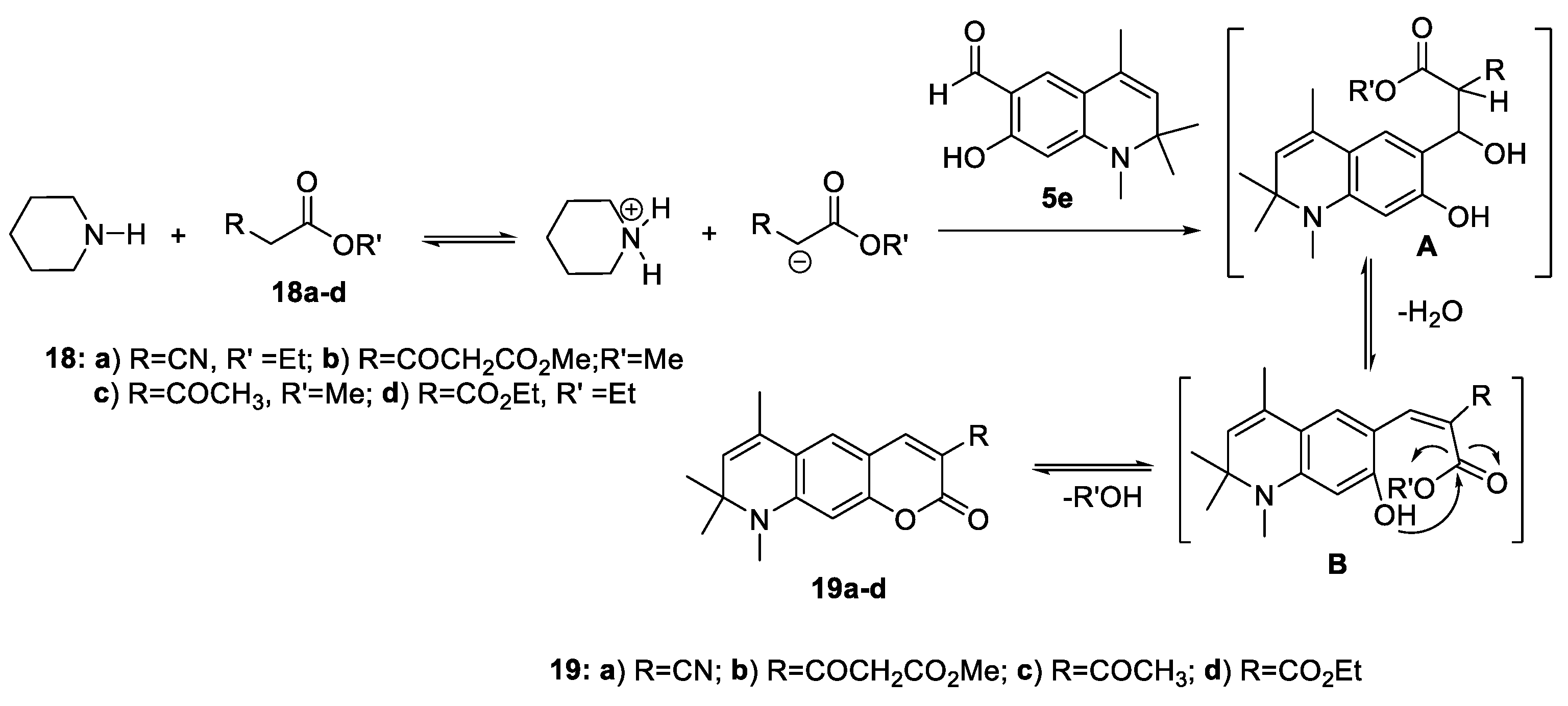
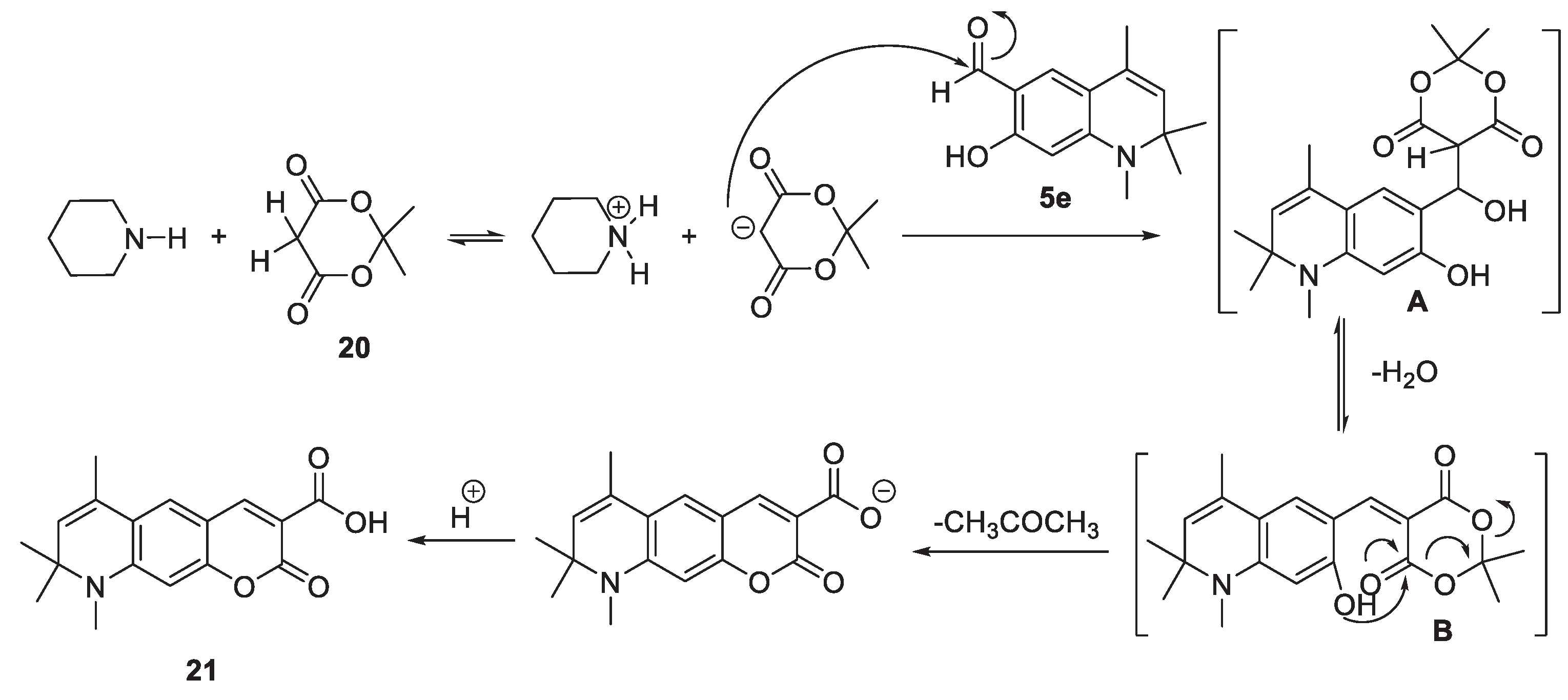
2.1.4. Cyclizations of 7-hydroxy-1,2,2,4-tetramethyl-1,2-dihydroquinoline 3h and 7-hydroxy-1,2,2,4-tetramethyl-1,2-dihydroquinoline-6-carbaldehyde, 5e with Methylene Active Compounds 16, 17, 19, 21, 22
2.2. In-silico studies
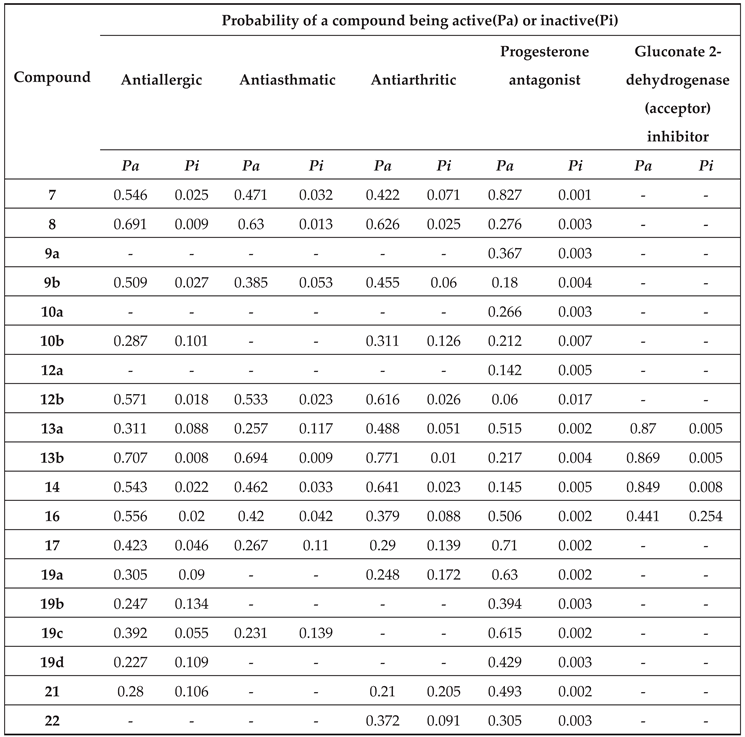
2.2.1. PASS the predicted activity spectrum of test synthesized compounds
3. Experimental Section
3.1. General
3.2. Synthesis of 1,2,2,4-tetramethyl-1,2-dihydroquinoline-6-carbonitrile 7.
3.3. Synthesis of 1-Benzyl-2,2,4-trimethyl-1,2,3,4-tetrahydroquinoline-6-carbonitrile, 8
3.4. Synthesis of 2-phenyl-1,3-oxazol-5(4Н)-ones 9, 10 (General procedure)
3.4.1. (Z)-2-Phenyl-4-((1,2,2,4-tetramethyl-1,2-dihydroquinolin-6-yl)methylene)oxazol-5(4H)-one (9a)
3.4.2. (Z)-4-((1-Benzyl-2,2,4-trimethyl-1,2-dihydroquinolin-6-yl)methylene)-2-phenyloxazol-5(4H)-one (9b)
3.4.3. (Z)-2-Phenyl-4-((1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinolin-6-yl)methylene)oxazol-5(4H)-one (10a)
3.4.4. (Z)-4-((1-Benzyl-2,2,4-trimethyl-1,2,3,4-tetrahydroquinolin-6-yl)methylene)-2-phenyloxazol-5(4H)-one (10b)
3.5. Synthesis of (E)-7-[2,2,4-trimethylhydroquinolin-6-ylidenemethyl]triazolo(pyrazolo)[1,5-a]pyrimidin-6-ylcarboxylic acid 12 (General procedure)
3.5.1. (E)-7-(2-(1,2,2,4-Tetramethyl-1,2,3,4-tetrahydroquinolin-6-yl)vinyl)-[1,2,4]triazolo[1,5-a]pyrimidine-6-carboxylic acid (12a)
3.5.2. (E)-7-(2-(1-Benzyl-2,2,4-trimethyl-1,2,3,4-tetrahydroquinolin-6-yl)vinyl)pyrazolo[1,5-a]pyrimidine-6-carboxylic acid (12b)
3.6. Synthesis of (E)-4-(2,2,4-trimethylhydroquinolin-6-yl)-3-buten-2-ones 13, 14.
3.6.1. (E)-4-(1,2,2,4-Tetramethyl-1,2-dihydroquinolin-6-yl)but-3-en-2-one (13a)
3.6.2. (E)-4-(1-Benzyl-2,2,4-trimethyl-1,2-dihydroquinolin-6-yl)but-3-en-2-one (13b)
3.6.3. (E)-4-(1-Benzyl-2,2,4-trimethyl-1,2,3,4-tetrahydroquinolin-6-yl)but-3-en-2-one (14)
3.8. Synthesis of methyl (Z)-2-(5,7,7,8-tetramethyl-2-oxo-7,8-dihydrofuro[3,2-g]quinoline-3(2H)-ylidene)acetate 16
3.9. Synthesis of 4,6,8,8,9-pentamethyl-8,9-dihydro-2H-pyrano[3,2-g]quinolin-2-one 17
3.10. General procedure for the synthesis of 6,8,8,9-tetramethyl-8,9-dihydropyrano[3,2-g]quinolin-2-ones 19, 21 and, 22
3.10.1. 6,8,8,9-Tetramethyl-2-oxo-8,9-dihydro-2H-pyrano[3,2-g]quinoline-3-carbonitrile (19a)
3.10.2. Methyl 3-oxo-3-(6,8,8,9-tetramethyl-2-oxo-8,9-dihydro-2H-pyrano[3,2-g]quinolin-3-yl)propanoate (19b)
3.10.3. 3-Acetyl-6,8,8,9-tetramethyl-8,9-dihydro-2H-pyrano[3,2-g]quinolin-2-one (19c)
3.10.4. Ethyl 6,8,8,9-tetramethyl-2-oxo-8,9-dihydro-2H-pyrano[3,2-g]quinoline-3-carboxylate (19d)
3.10.5. 6,8,8,9-Tetramethyl-2-oxo-8,9-dihydro-2H-pyrano[3,2-g]quinoline-3-carboxylic acid (21)
3.10.6. 2,5-Diamino-9,10,10,12-tetramethyl-9,10-dihydropyrido[4′,3′,2′:8,1]isochromeno[4,3-g]quinoline-1,4-dicarbonitrile (22)
3.11. In Silico PASS Prediction
4. Conclusion
Supplementary Materials
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022, 4. [Google Scholar] [CrossRef]
- Al-Mulla, A. A Review: Biological Importance of Heterocyclic Compounds. Der Pharma Chem. 2017, 9. [Google Scholar]
- Qadir, T.; Amin, A.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on Medicinally Important Heterocyclic Compounds. Open Med. Chem. J. 2022, 16. [Google Scholar] [CrossRef]
- Mermer, A.; Keles, T.; Sirin, Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review. Bioorganic Chem. 2021, 114, 105076. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Fang, K.C.; Sheu, J.Y.; Hsu, S.L.; Tzeng, C.C. Synthesis and antibacterial evaluation of certain quinolone derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef]
- Yadav, P.; Shah, K. Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorganic Chem. 2021, 109, 104639. [Google Scholar] [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorganic Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef] [PubMed]
- Dib, K.; Ennibi, O.; Alaoui, K.; Cherrah, Y.; Filali-Maltouf, A. Antibacterial activity of plant extracts against periodontal pathogens: A systematic review. J. Herb. Med. 2021, 29, 100493. [Google Scholar] [CrossRef]
- Synthetic Approaches and Biological Activities of Quinoline Derivatives: A Review. Int. J. Pharm. Res. 2021, 13. [CrossRef]
- Updates on Synthesis and Biological Activities of Quinoline Derivatives: A Review. Int. J. Pharm. Res. 2021, 13. [CrossRef]
- Kaur, T.; Bhandari, D.D. Annotated Review on Various Biological Activities of Quinoline Molecule. Biointerface Res. Appl. Chem. 2023, 13. [Google Scholar] [CrossRef]
- Patel, A.; Patel, S.; Mehta, M.; Patel, Y.; Patel, R.; Shah, D.; Patel, D.; Shah, U.; Patel, M.; Patel, S.; et al. A review on synthetic investigation for quinoline- recent green approaches. Green Chem. Lett. Rev. 2022, 15, 337–372. [Google Scholar] [CrossRef]
- Marella, A.; Tanwar, O.P.; Saha, R.; Ali, M.R.; Srivastava, S.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Quinoline: A versatile heterocyclic. Saudi Pharm. J. 2013, 21. [Google Scholar] [CrossRef]
- Yang, J.M.; Wu, L.; Fang, D.; Cao, J.; Zhou, Y.J.; Wang, X.S. Iodine-catalyzed Povarov reaction for synthesis of cyclobuta[c]quinoline derivatives. Res. Chem. Intermed. 2014, 40, 1103–1113. [Google Scholar] [CrossRef]
- Ghoshal, A.; Yugandhar, D.; Nanubolu, J.B.; Srivastava, A.K. An Efficient one-pot synthesis of densely functionalized fused-quinolines via sequential Ugi4CC and acid-mediated povarov-type reaction. ACS Comb. Sci. 2017, 19, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, S.M.; Plaksina, M.E.; Shikhaliev, K.S. The synthesis of 6-R-2,2,4-trimethyl-1,2-dihydroquinoline- and 6-R-4-R’-2,2,4-trimethyl-1,2,3,4-tetrahydroquinoline-8-carboxylic acids—The structural analogues of helquinoline. Žurnal organìčnoï ta Farm. hìmìï 2017, 13, 21–25. [Google Scholar] [CrossRef]
- Durgadas, S.; Chatare, V.K.; Mukkanti, K.; Pal, S. Ceric Ammonium Nitrate: An Efficient Catalyst for One-Pot Synthesis of 2,2,4-Trimethyl-1,2-dihydroquinolines. Lett. Org. Chem. 2010, 7, 306–310. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, L.J. Design, synthesis, molecular docking, and antibacterial evaluation of some novel flouroquinolone derivatives as potent antibacterial agent. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, J.; Jaroszewska, K.; Nowakowska-Bogdan, E.; Szmatoła, M.; Iłowska, J. Synthesis of 2,2,4-trimethyl-1,2- H -dihydroquinoline (TMQ) over selected organosulfonic acid silica catalysts: Selectivity aspects. Mol. Catal. 2018, 454, 94–103. [Google Scholar] [CrossRef]
- Dadhania, H.; Raval, D.; Dadhania, A. A Highly Efficient and Solvent-Free Approach for the Synthesis of Quinolines and Fused Polycyclic Quinolines Catalyzed by Magnetite Nanoparticle-Supported Acidic Ionic Liquid. Polycycl. Aromat. Compd. 2021, 41, 440–453. [Google Scholar] [CrossRef]
- Dabiri, M.; Azimi, S.C.; Bazgir, A. An efficient and rapid approach to quinolines via Friedländer synthesis catalyzed by silica gel supported sodium hydrogen sulfate under solvent-free conditions. Monatshefte Fuer Chemie/chemical Mon. 2007, 138, 659–661. [Google Scholar] [CrossRef]
- Muthukrishnan, I.; Sridharan, V.; Menéndez, J.C. Progress in the Chemistry of Tetrahydroquinolines. Chem. Rev. 2019, 119, 5057–5191. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rachwal, S.; Rachwal, B. Recent progress in the synthesis of 1,2,3,4,-tetrahydroquinolines. Tetrahedron 1996, 52, 15031–15070. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Sweidan, K.A.; Mubarak, M.S. Recent advances in the synthesis and biological activity of 8-hydroxyquinolines. Molecules 2020, 25, 4321. [Google Scholar] [CrossRef] [PubMed]
- Kljun, J.; León, I.E.; Peršič, Š.; Cadavid-Vargas, J.F.; Etcheverry, S.B.; He, W.; Bai, Y.; Turel, I. Synthesis and biological characterization of organoruthenium complexes with 8-hydroxyquinolines. J. Inorg. Biochem. 2018, 186, 187–196. [Google Scholar] [CrossRef]
- Heiskanen, J.P.; Omar, W.A.E.; Ylikunnari, M.K.; Haavisto, K.M.; Juan, M.J.; Hormi, O.E.O. Synthesis of 4-alkoxy-8-hydroxyquinolines. J. Org. Chem. 2007, 72, 920–922. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, G.; Xiu, Q.; Guo, L.; Deng, J.; Zhong, C. Synthesis and photovoltaic properties of polymeric metal complexes containing 8-hydroxyquinoline as dye sensitizers for dye-sensitized solar cells. J. Co-ord. Chem. 2012, 65, 1632–1644. [Google Scholar] [CrossRef]
- Chen, R.; Yang, X.; Tian, H.; Wang, X.; Hagfeldt, A.; Sun, L. Effect of tetrahydroquinoline dyes structure on the performance of organic dye-sensitized solar cells. Chem. Mater. 2007, 19, 4007–4015. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Cong, J.; Chen, R.; Teng, C.; Liu, J.; Hao, Y.; Wang, L.; Sun, L. Effect of different electron donating groups on the performance of dye-sensitized solar cells. Dye. Pigment. 2010, 84, 62–68. [Google Scholar] [CrossRef]
- El-Ghaffar, M.A.A.; Shaffei, K.A.; Abdelwahab, N. Evaluation of some conducting polymers as novel antioxidants for rubber vulcanizates. Int. J. Polym. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Šmejkal, F.; Číhová, A.; Popl, M.; Novák, J. The analysis of polymeric 2,2,4-trimethyl-1,2-dihydroquinoline. Die Angew. Makromol. Chemie 1980, 88. [Google Scholar] [CrossRef]
- Saldanha, L.; Langel, Ü.; Vale, N. In Silico Studies to Support Vaccine Development. Pharmaceutics 2023, 15, 654. [Google Scholar] [CrossRef]
- Ratra, S.; Pant, B.; Roy, K.; Manohar, S.; Kumar, P.; Singh, S.; Tumba, K.; Kumari, K.; Singh, P. A review on synthesis of antiviral drugs, in silico studies and their toxicity. J. Indian Chem. Soc. 2023, 100. [Google Scholar] [CrossRef]
- Matin, M.M.; Bhattacharjee, S.C.; Chakraborty, P.; Alam, M.S. Synthesis, PASS predication, in vitro antimicrobial evaluation and pharmacokinetic study of novel n-octyl glucopyranoside esters. Carbohydr. Res. 2019, 485, 107812. [Google Scholar] [CrossRef]
- Tian, K.; Hu, D.; Hu, R.; Wang, S.; Li, S.; Li, Y.; Yang, G. Multiple fluorescence ΔCIE and ΔRGB codes for sensing volatile organic compounds with a wide range of responses. Chem. Commun. 2011, 47, 10052–10054. [Google Scholar] [CrossRef] [PubMed]
- Manahelohe, G.M.; Potapov, A.Y.; Shikhaliev, K.S. Synthesis of new hydroquinolinecarbaldehydes. Russ. Chem. Bull. 2016, 65, 1145–1147. [Google Scholar] [CrossRef]
- Манахелoхе, Г.М. СИНТЕЗ НОВЫХ ГЕТЕРОЦИКЛИЧЕСКИХ СИСТЕМ НА ОСНОВЕ ФОРМИЛГИДРОХИНОЛИНОВ. ДИССЕРТАЦИЯ, ВОРОНЕЖСКИЙ ГОСУДАРСТВЕННЫЙУНИВЕРСИТЕТ, 2015.
- Wang, E.-C.; Lin, G.-J. ChemInform Abstract: A New One-Pot Method for the Conversion of Aldehydes into Nitriles Using Hydroxyamine and Phthalic Anhydride. ChemInform 2010, 29. [Google Scholar] [CrossRef]
- Wang, E.-C.; Lin, G.-J. A new one pot method for the conversion of aldehydes into nitriles using hydroxyamine and phthalic anhydride. Tetrahedron Lett. 1998, 39, 4047–4050. [Google Scholar] [CrossRef]
- Sampath Kumar, H.M.; Reddy, B.V.S.; Reddy, P.T.; Yadav, J.S. Efficient one-pot preparation of nitriles from aldehydes using N- methyl-pyrrolidone. Synthesis 1999, 1999, 586–587. [Google Scholar] [CrossRef]
- Sosnovsky, G.; Krogh, J.A.; Umhoefer, S.G. A one-flask conversion of aldehydes to nitriles using hydroxylamine hydrochloride and selenium dioxide. Synthesis (Stuttg) 1979, 1979, 722–724. [Google Scholar] [CrossRef]
- Talukdar, S.; Hsu, J.-L.; Chou, T.-C.; Fang, J.-M. Direct transformation of aldehydes to nitriles using iodine in ammonia water. Tetrahedron Lett. 2001, 42, 1103–1105. [Google Scholar] [CrossRef]
- Shie, J.J.; Fang, J.M. Direct conversion of aldehydes to amides, tetrazoles, and triazines in aqueous media by one-pot tandem reactions. J. Org. Chem. 2003, 68, 1158–1160. [Google Scholar] [CrossRef]
- El-Hady, H.A.; Abubshait, S.A. Synthesis and anticancer evaluation of imidazolinone and benzoxazole derivatives. Arab. J. Chem. 2017, 10, S3725–S3731. [Google Scholar] [CrossRef]
- Rao, Y.S.; Filler, R. Geometric Isomers of 2-Aryl(Aralkyl)-4-arylidene(alkylidene)-5(4H)-oxazolones. Synthesis 1975, 1975, 749–764. [Google Scholar] [CrossRef]
- Yavari, I.; Anary-Abbasinejad, M.; Alizadeh, A. A Simple Synthesis of Stable 1,4-Diionic Pyridinium Betaines. ChemInform 2003, 34. [Google Scholar] [CrossRef]
- Yavari, I.; Hossaini, Z. Synthesis of fused α-methylene-γ-butyrolactone derivatives through pyridine-induced addition of phenols to dimethyl acetylenedicarboxylate. Tetrahedron Lett. 2006, 47, 4465–4468. [Google Scholar] [CrossRef]
- De, S.K.; Gibbs, R.A. An Efficient and Practical Procedure for the Synthesis of 4-Substituted Coumarins. Synthesis (Stuttg) 2005, 2005, 1231–1233. [Google Scholar] [CrossRef]
- Hirbod, K.; Jalili-Baleh, L.; Nadri, H.; Ebrahimi, S.E.S.; Moradi, A.; Pakseresht, B.; Foroumadi, A.; Shafiee, A.; Khoobi, M. Coumarin derivatives bearing benzoheterocycle moiety: synthesis, cholinesterase inhibitory, and docking simulation study. Iran. J. Basic Med. Sci. 2017, 20, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Alheety, K.A.; Jamel, N.M.; Ahmed, B.J. Synthesis of coumarin by Pechman reaction - A Review. J. Pharm. Sci. Res. 2019, 11. [Google Scholar]
- Undale, K.A.; Gaikwad, D.S.; Shaikh, T.S.; Desai, U.V.; Pore, D.M. Potassium phosphate catalyzed efficient synthesis of 3-carboxycoumarins. Indian J. Chem. - Sect. B Org. Med. Chem. 2012, 51. [Google Scholar]
- Prajapati, D.; Sandhu, J.S. Cadmium iodide as a new catalyst for Knoevenagel condensations. J. Chem. Soc. Perkin Trans. 1 1993, 739–740. [Google Scholar] [CrossRef]
- Mhiri, C.; El Gharbi, R.; Le Bigot, Y. Polymer supported reagents: Novel methodology for selective and general synthesis of iminocoumarins. Synth. Commun. 1999, 29, 3385–3399. [Google Scholar] [CrossRef]
- Brahmachari, G.; Das, S. Sodium Formate-Catalyzed One-Pot Synthesis of Benzopyranopyrimidines and 4-Thio-substituted 4H-Chromenes via Multicomponent Reaction at Room Temperature. J. Heterocycl. Chem. 2015, 52, 653–659. [Google Scholar] [CrossRef]
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. Convenient one-step synthesis of a medicinally relevant benzopyranopyridine system. Tetrahedron Lett. 2006, 47, 9309–9312. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. One-Step Synthesis of Heterocyclic Privileged Medicinal Scaffolds by a Multicomponent Reaction of Malononitrile with Aldehydes and Thiols. ChemInform 2007, 38. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





