Submitted:
21 February 2024
Posted:
23 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
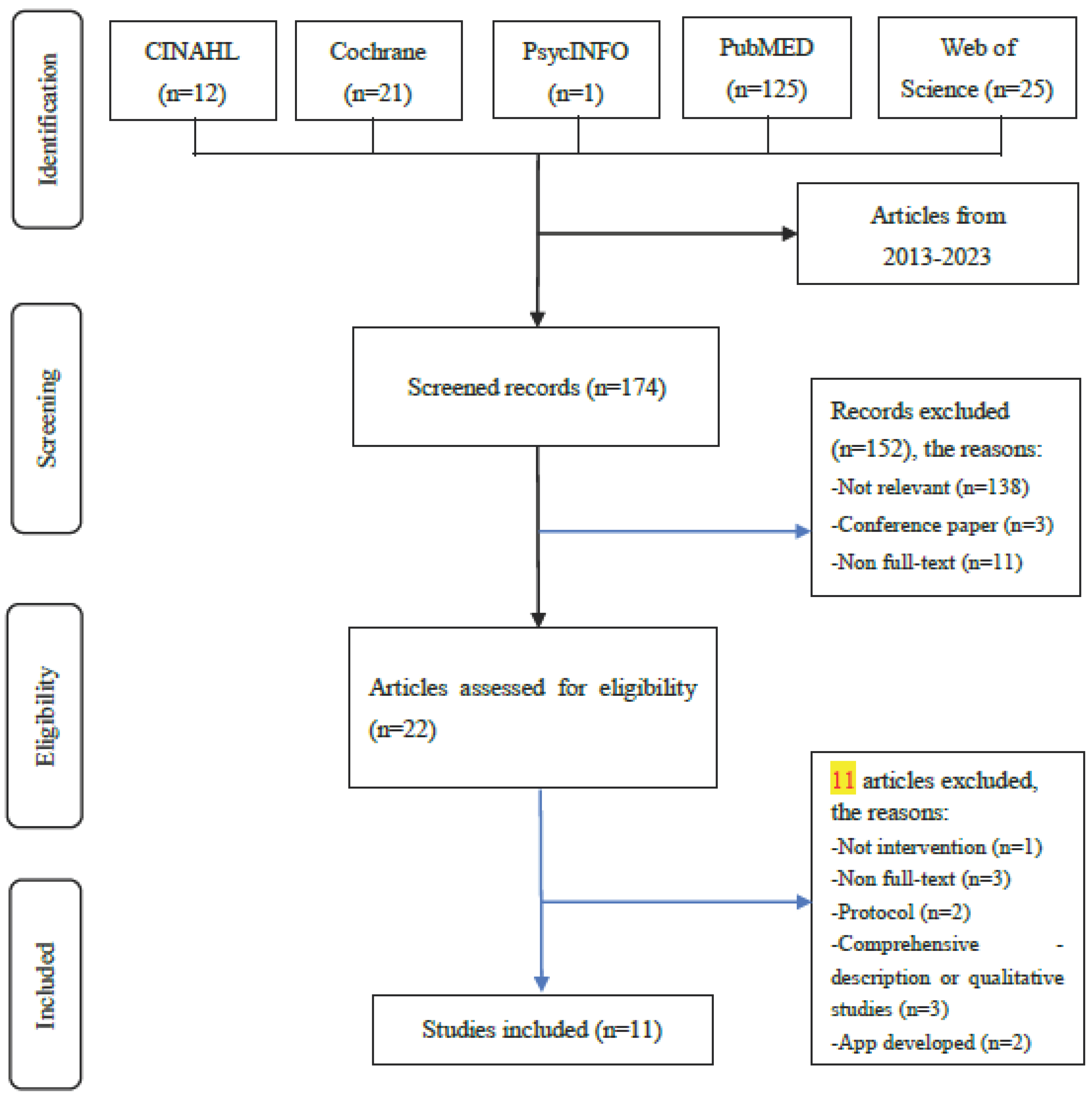
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Screening and Data Extraction
2.4. Selection Process
2.5. Functionality Assessment
2.6. Usability Assessment
3. Results
3.1. Characteristics of the Included Studies
3.2. Interventions Description
3.3. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgart, A.; E Manera, K.; Johnson, D.W.; Craig, J.C.; I Shen, J.; Ruiz, L.; Wang, A.Y.-M.; Yip, T.; Fung, S.K.S.; Tong, M.; et al. Meaning of empowerment in peritoneal dialysis: Focus groups with patients and caregivers. Nephrol. Dial. Transplant. 2020, 35, 1949–1958. [Google Scholar] [CrossRef]
- Tang C. H., et al., Out-of-pocket costs and productivity losses in hemodialysis and peritoneal dialysis from a patient interview survey in Taiwan. BMJ open 2019, 9, e023062. [CrossRef]
- Talbot, B.; Farnbach, S.; Tong, A.; Chadban, S.; Sen, S.; Garvey, V.; Gallagher, M.; Knight, J. Patient and Clinician Perspectives on the use of Remote Patient Monitoring in Peritoneal Dialysis. Can. J. Kidney Heal. Dis. 2022, 9, 20543581221084499. [Google Scholar] [CrossRef] [PubMed]
- Biebuyck, G.K.M.; Neradova, A.; de Fijter, C.W.H.; Jakulj, L. Impact of telehealth interventions added to peritoneal dialysis-care: A systematic review. BMC Nephrol. 2022, 23, 292. [Google Scholar] [CrossRef]
- He, T.; Liu, X.; Li, Y.; Wu, Q.; Liu, M.; Yuan, H. Remote home management for chronic kidney disease: A systematic review. J. Telemed. Telecare 2017, 23, 3–13. [Google Scholar] [CrossRef]
- Markossian, T.W.; Boyda, J.; Taylor, J.; Etingen, B.; Modave, F.; Price, R.; Kramer, H.J. A Mobile App to Support Self-management of Chronic Kidney Disease: Development Study. JMIR Hum. Factors 2021, 8, e29197. [Google Scholar] [CrossRef]
- Lukkanalikitkul, E.; Kongpetch, S.; Chotmongkol, W.; Morley, M.G.; Anutrakulchai, S.; Srichan, C.; Thinkhamrop, B.; Chunghom, T.; Wiangnon, P.; Thinkhamrop, W.; et al. Optimization of the Chronic Kidney Disease–Peritoneal Dialysis App to Improve Care for Patients on Peritoneal Dialysis in Northeast Thailand: User-Centered Design Study. JMIR Form. Res. 2022, 6, e37291. [Google Scholar] [CrossRef]
- Cartwright, E.J.; Goh, Z.Z.; Foo, M.; Chan, C.M.; Htay, H.; Griva, K. eHealth interventions to support patients in delivering and managing peritoneal dialysis at home: A systematic review. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2020, 41, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Schrauben, S.J.; Appel, L.; Rivera, E.; Lora, C.M.; Lash, J.P.; Chen, J.; Hamm, L.L.; Fink, J.C.; Go, A.S.; Townsend, R.R.; et al. Mobile Health (mHealth) Technology: Assessment of Availability, Acceptability, and Use in CKD. Am. J. Kidney Dis. 2021, 77, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Cao, F., et al., Application of instant messaging software in the follow-up of patients using peritoneal dialysis, a randomized controlled trial. Journal of Clinical Nursing 2018, 27, 3001–3007. [CrossRef]
- Dey, V.; Jones, A.; Spalding, E.M. Telehealth: Acceptability, clinical interventions and quality of life in peritoneal dialysis. SAGE Open Med. 2016, 4, 2050312116670188. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.J. Süle, and S. Kohl, eHealth and mHealth. European Journal of Hospital Pharmacy 2019, 26, 57–58. [Google Scholar] [CrossRef]
- Lewis, R.A.; Lunney, M.; Chong, C.; Tonelli, M. Identifying Mobile Applications Aimed at Self-Management in People With Chronic Kidney Disease. Can. J. Kidney Heal. Dis. 2019, 6, 2054358119834283. [Google Scholar] [CrossRef]
- Quach, S.; Benoit, A.; Oliveira, A.; Packham, T.L.; Goldstein, R.; Brooks, D. Features and characteristics of publicly available mHealth apps for self-management in chronic obstructive pulmonary disease. Digit. Heal. 2023, 9, 20552076231167007. [Google Scholar] [CrossRef]
- World Health Organization, mhealth Use of appropriate digital technologies for public health. 2018.
- Sluijs, A.v.E.v.d.; Vonk, S.; van Jaarsveld, B.C.; Bonenkamp, A.A.; Abrahams, A.C. Good practices for dialysis education, treatment, and eHealth: A scoping review. PLoS ONE 2021, 16, e0255734. [Google Scholar] [CrossRef]
- Shen, H.; van der Kleij, R.M.J.J.; van der Boog, P.J.M.; Chang, X.; Chavannes, N.H. Electronic Health Self-Management Interventions for Patients With Chronic Kidney Disease: Systematic Review of Quantitative and Qualitative Evidence. J. Med Internet Res. 2019, 21, e12384. [Google Scholar] [CrossRef]
- Ryan, S.; Chasaide, N.N.; Hanrahan, S.O.; Corcoran, D.; Caulfield, B.; Argent, R. mHealth Apps for Musculoskeletal Rehabilitation: Systematic Search in App Stores and Content Analysis. JMIR Rehabilitation Assist. Technol. 2022, 9, e34355. [Google Scholar] [CrossRef]
- Wilson, J. , et al., Barriers and facilitators to the use of e-health by older adults: A scoping review. BMC Public Health 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Wang, C.-s. and E. Ku, eHealth in kidney care. Nature Reviews Nephrology 2020, 16, 368–370. [Google Scholar] [CrossRef]
- IMS Institute for Healthcare Informatics. Patient Apps for Improved Healthcare. 2013.
- Farzandipour, M., et al., Patient self-management of asthma using mobile health applications: A systematic re-view of the functionalities and effects. Applied clinical informatics 2017, 8, 1068–1081. [CrossRef]
- Creber, R.M.M.; Maurer, M.S.; Reading, M.; Hiraldo, G.; Hickey, K.T.; Iribarren, S. Review and Analysis of Existing Mobile Phone Apps to Support Heart Failure Symptom Monitoring and Self-Care Management Using the Mobile Application Rating Scale (MARS). JMIR mHealth uHealth 2016, 4, e74. [Google Scholar] [CrossRef]
- Harrison, R.; Flood, D.; Duce, D. Usability of mobile applications: Literature review and rationale for a new usability model. J. Interact. Sci. 2013, 1, 1. [Google Scholar] [CrossRef]
- Weichbroth, P. Usability of Mobile Applications: A Systematic Literature Study. IEEE Access 2020, 8, 55563–55577. [Google Scholar] [CrossRef]
- Moher, David, et al., Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine 2009, 151, 264–269. [CrossRef]
- Moher, David, et al, Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International journal of surgery 2010, 8, 336–341. [CrossRef]
- Joanna Briggs Institute, JBI levels of evidence. 2014.
- García, M.A.M.; Rosales, M.S.F.; Domínguez, E.L.; Velázquez, Y.H.; Isidro, S.D. Telemonitoring system for patients with chronic kidney disease undergoing peritoneal dialysis: Usability assessment based on a case study. PLoS ONE 2018, 13, e0206600. [Google Scholar] [CrossRef]
- Olivares-Gandy, H.J. , et al., A telemonitoring system for nutritional intake in patients with chronic kidney dis-ease receiving peritoneal dialysis therapy. Computers in Biology and Medicine 2019, 109, 1–13. [Google Scholar] [CrossRef]
- Polanco, E. , et al., A COVID-19 pandemic-specific, structured care process for peritoneal dialysis patients facili-tated by telemedicine: Therapy continuity, prevention, and complications management. Therapeutic Apheresis and Dialysis 2021, 25, 970–978. [Google Scholar] [CrossRef]
- Farfan-Ruiz, A.C.; Czikk, D.; Leidecker, J.; Ramsay, T.; McCormick, B.; Wilson, K.; Zimmerman, D. Multidisciplinary Team versus a “Phosphate-Counting” App for Serum Phosphate Control: A Randomized Controlled Trial. Kidney360 2021, 2, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Kiberd, J.; Khan, U.; Stockman, C.; Radhakrishnan, A.; Phillips, M.; Kiberd, B.A.; West, K.A.; Soroka, S.; Chan, C.; Tennankore, K.K. Effectiveness of a Web-Based eHealth Portal for Delivery of Care to Home Dialysis Patients: A Single-Arm Pilot Study. Can. J. Kidney Heal. Dis. 2018, 5, 2054358118794415. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ma, T.; Tian, X.; Li, S.; Pei, H.; Zhao, J.; Xiong, Z.; Liao, Y.; Li, Y.; Lin, Q.; et al. Telemedicine and Clinical Outcomes in Peritoneal Dialysis: A Propensity-Matched Study. Am. J. Nephrol. 2022, 53, 663–674. [Google Scholar] [CrossRef]
- Chae, Y.J.; Kim, H.S. Effects of a mobile application on improving self-management of adult patients receiving peritoneal dialysis: A randomized controlled trial. Jpn. J. Nurs. Sci. 2023, 21, e12555. [Google Scholar] [CrossRef]
- Viglino, G.; Neri, L.; Barbieri, S.; Tortone, C. Videodialysis: A pilot experience of telecare for assisted peritoneal dialysis. J. Nephrol. 2020, 33, 177–182. [Google Scholar] [CrossRef]
- Eberle, C.; Löhnert, M.; Stichling, S. Effectiveness of Disease-Specific mHealth Apps in Patients With Diabetes Mellitus: Scoping Review. JMIR mHealth uHealth 2021, 9, e23477. [Google Scholar] [CrossRef]
| Authors/Year/CountryRef no | App name/ Device /Platform/ Objectives | Design/ Sample Size / Duration | Intervention Content | Outcome variables/ Results |
| Cao et al./2018 / China10 | ·The QQ Application · Smartphone & tablet · Android & ·To investigate the effectiveness of the QQ application. |
·A randomized controlled trial design ·the QQ (experimental) group (n=80) the traditional follow-up group (n=80) ·11.4 months |
Nurses provide health education and disease-related information to patients through the QQ application, engaging in online conversations to address their health concerns. | ·Satisfaction, underwent kidney transplantation and switched to hemodialysis, rehospitalisation, infection rates, serum values ·(1) Patients in the QQ intervention group showed significantly higher levels of serum albumin, hemoglobin, and satisfaction; as well as lower levels of phosphorus and calcium-phosphorus products compared to those in the control group. (2) There was no difference in underwent kidney transplantation and switched to hemodialysis, as well serum calcium levels between the two groups. |
| Chae & Kim/2023 / Korea35 | · PD With You · N/A · Android 9.0 ·Developing a mobile application for enhancing self-management and assessing its effectiveness for patients with PD. |
·A randomized controlled trial ·the experimental group (n =27) the control group (n =26) ·10 weeks |
Patients used the self-management mobile application to record PD dialysis (i.e., replacement time and amount of removed fluid) and observe the physical indicators (i.e., body weight and blood pressure). | ·self-efficacy, serum values, knowledge, health behavior, QOL ·(1) The experimental group showed a significant improvement in PD-related knowledge, PD-related health behavior, serum albumin, hemoglobin level, and the domain symptoms/problems of kidney disease and disease impact on daily activity of HRQOL, compared to the control group. (2) There were no significant differences between the two groups in PD-related self-efficacy, serum K, serum P, the domains of physical, psychological, and burden of kidney disease of HRQoL. |
| Dey et al./2016/ UK11 | · N/A · Computer tablets · N/A · (1) The application can provide information related to diseases and dietary considerations. (2) Patients can modify the dialysis plan or perform other actions, such as manual exchanges when dialysis resulted in problems. (3) Providing dietary advice through phone consultations by HCP. (4) Recording and accessing personal physiological data and details of dialysis sessions. |
·Observational–Descriptive Studies(A cross-sectional study) ·22 participants ·Over 15 months |
Each patient is provided with a weight scale, blood pressure monitor, and computer tablets equipment. The patients were required to record data, such as weight, blood pressure, dialysis exchanges, and ultrafiltration volume. Additionally, patients need to report on the presence or absence of symptoms, such as swelling, shortness of breath, fever, abdominal pain, tenderness around the catheter site, and other relevant conditions. When the patients’ condition is not well, HCP will provide interventions or treatments. | ·satisfaction, QOL ·(1) No significant improved in QOL. (2) No significant improved in satisfaction with assistive Technology. |
| Kiberd et al./ 2018/ Canda33 | · McKesson · eHealth portal applications · N/A ·To investigate whether eHealth portal applications can effectively enhance the home dialysis care experience for patients. |
·Observational–Analytic Designs, without a control group (A Single-Arm Pilot)Study ·participants (n =27) ·12 months |
Patients and HCP can communicate through a web portal application. HCP can provide suggestions such as medication changes, post-clinic visit explanations, new appointment scheduling, and more. | · satisfaction, acceptance, QOL, consumer quality index · (1) Most participants were satisfied. (2) QOL did not improve, and it was difficult to ascertain the user acceptance due to the small sample size. (3) No significance in the consumer quality index |
| Lukkanalikitkul et al /2022/Thailand7 | ·CKD-PD app ·Smart phone & near-field communication & optical character recognition ·Android & iOS ·To identify Chronic Kidney Disease - Peritoneal Dialysis Application with near-field communication and optical character recognition functions, which can automatically gather the hydration status to enhance care for patients with PD, and improve it. |
·Observational– Analytic Designs (User- Centered Design Study) ·participants (n=10) ·12 months |
Provide an application to collect the peritoneal dialysis fluid and upload the data to HCP. | ·usability ·In the end, participants decreased interest in using this app. Especially, the entering data in NFC and OCR system of the app was difficult for participants compared to the manual data. |
| Martínez García et al/2018 /Mexico29 | ·N/A ·A mobile Web application (for HCP) & Android application(for patients) ·Android ·Evaluating the usability of a remote monitoring system for patients undergoing peritoneal dialysis treatment. |
·Quasi-experimental design (A case study) ·participants (n=24) · 9 months |
Providing applications to patients to monitor the patient’s condition and assess the usability of the app. | ·satisfaction, acceptance ·(1) 94.5% of participants were satisfied with the app among patients with APD and 92.3% among CAPD. (2) 89.5% of participants accepted. |
| Olivares-Gandy et al./2019/ Mexico30 | ·N/A ·Android & iOS · N/A ·To Analyze, design, and develop a mobile health application for the dietary requirements of patients with PD, and then assessing its usability and satisfaction. |
·Observational–Descriptive (A case study) ·one patient & one nutritionist ·N/A |
Patients' experiences with the use of mobile applications. | ·usability ·Patients find the application usable. |
| Polanco et al./2021/ Mexico31 | ·WhatsApp ·Computer, smartphone, tablet · N/A ·The healthcare team utilizes remote healthcare via WhatsApp to reduce hospital visits and the risk of infection during the COVID-19 pandemic. |
·Observational– Analytic Designs (An observational prospective-longitudinal study) ·participants (n=946) ·3 months |
The patients send daily dialysis records and photos of their lower limbs to HCP by using WhatsApp. The medical team members utilize WhatsApp for communication and tracking the patient's condition. | ·switched to hemodialysis, rehospitalisation, peritonitis rate ·The incidence rate of peritonitis, switched to hemodialysis, and hospitalization rates showed no difference. |
| Viglino et al./2020/ Italy36 | ·N/A ·N/A ·N/A ·To explore the reliability, safety, and effectiveness of videodialysis assisted PD patients. To investigate the possibilities of the videodialysis and whether it reduced family care burden and recourse to nurses at home. |
·Observational–Analytic Designs ·the intervention group (n=15) the control group (n=62) ·Follow-up 285 months |
The experimental group was provided with videodialysis, a device consisting of two parts. One part included the equipment that patients should have at home, including a camera, monitor, microphone, and a technical connection box. The other part comprised the equipment required on the healthcare personnel's end, including a high-resolution display, a network camera, a computer with speakers, and software capable of managing six audio streams simultaneously. | ·satisfaction, switched to hemodialysis, Peritonitis rate ·(1) All the patients expressed a satisfaction with the app for enhance confidence. (2) There is no difference in the incidence of peritonitis. (3) Three out of the 15 participants transitioned to hemodialysis. |
| Xu et al./2022/ China34 | ·Manburs ·N/A ·N/A ·To explore the long-term impact of telemedicine (Manburs app) on patients in terms of mortality and technical performance failure. |
·Observational–Analytic Designs (A Propensity-Matched Study) ·Participants (n=7539) ·From June 2016 to December 2020 (4 years) |
Telemedicine, through the application (Manburs), involves self-monitoring records, online educational materials, and real-time doctor-patient communication. | ·switched to hemodialysis, infection rates, mortality, fluid overload, inadequate solute clearance, · The intervention group were observed to have significantly lower risks of all-cause mortality, CVD mortality, all-cause transfer to hemodialysis, transfer to hemodialysis from PD-related infection, severe fluid overload, inadequate solute clearance, and catheter-related noninfectious complications compared with that in the control group. |
| Authors(Year) | Functionality | ||||||
| Inform | Instruct | Record | Display | Guide | Remind/Alert | Communicate | |
| Cao et al.(2018) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Chae & Kim(2023) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Dey et al.(2016) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Farfan-Ruiz et al.(2021) | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Kiberd et al.(2018) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Lukkanalikitkul et al.(2022) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Martínez García et al. (2018) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Olivares-Gandy et al.(2019) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Polanco et al.(2021) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Viglino et al.(2020) | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Xu et al.,(2022) | ✓ | ✓ | ✓ | ✓ | |||
| Outcomes | Statistical significance | |||
| Positive | Negative | No difference | ||
| Efficiency | PD-related self-efficacy | Chae & Kim (2023) | ||
| Usability | Olivares-Gandy et al.(2019) | Lukkanalikitkul et al. (2022) | ||
| Satisfaction | Patients’ satisfaction | Cao et al.(2018) Dey, et al.(2016) Kiberd et al.(2018) Martínez García et al. (2018) Viglino et al.(2020) |
||
| Acceptance | Martínez García et al. (2018) | Kiberd et al.(2018) | ||
|
Effectiveness |
Underwent kidney transplantation and switched to hemodialysis | Xu et al.(2022) | Cao et al.(2018) Polanco et al.(2021) Viglino et al.(2020)b |
|
| Rehospitalisation | Cao et al.(2018) Polanco et al.(2021) |
|||
| Peritonitis rate | Polanco et al.(2021) Viglino et al.(2020) |
|||
| The infection rates at the exit site | Xu et al.(2022) | Cao et al.(2018) |
||
| Mortality | Xu et al.(2022) | Cao et al.(2018) | ||
| Fluid overload | Xu et al.(2022) | |||
| Inadequate solute clearance | Xu et al.(2022) | |||
| Serum albumin | Cao et al.(2018) Chae & Kim/2023 |
|||
| Serum Hemoglobin | Cao et al.(2018) Chae & Kim(2023) |
|||
| Serum calcium | Cao et al.(2018) Farfan-Ruiz et al.(2021) |
|||
| Serum phosphorus | Cao et al.(2018) Farfan-Ruiz et al.(2021)a |
Chae & Kim(2023) | ||
| Serum potassium | Chae & Kim(2023) | |||
| Calcium-phosphorus product |
Cao et al./2018 | |||
| Taking calcium carbonate | Farfan-Ruiz et al.(2021) | |||
| PD-related knowledge | Chae & Kim(2023) | |||
| PD-related health behavior | Chae & Kim(2023) | |||
| Quality of life | Chae & Kim(2023)a | Dey et al.(2016) Kiberd et al.(2018) |
||
| Consumer quality index (CQI) | Kiberd et al.(2018) | |||
| Technology readiness | Farfan-Ruiz et al.(2021) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




