Submitted:
13 February 2024
Posted:
13 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
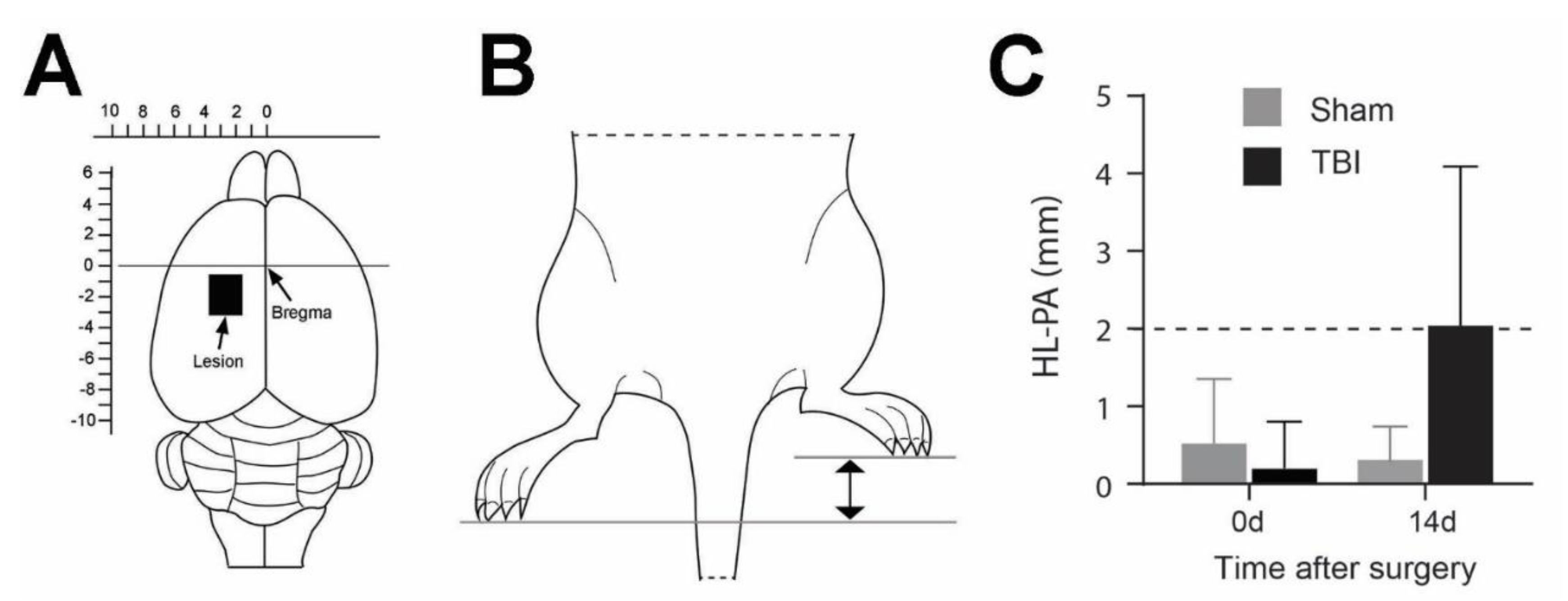
2.1. Traumatic Brain Injury and Sham Animal Model
2.2. Analysis of hindlimb postural asymmetry (HL-PA)
2.3. Tissue collection and muscle tissue preparation
| Muscle | Function | Acting joints |
|---|---|---|
| Biceps femoris (BF) | Flexion Extension |
Knee Hip |
| Extensor digitorum longus (EDL) | Dorsal flexion | Ankle |
| Lateral head of gastrocnemius (LG) | Flexion Plantar flexion |
Knee Ankle |
| Medial head of gastrocnemius (MG) | Flexion Plantar flexion |
Knee Ankle |
| Peroneus longus (PL) | Plantar flexion & eversion | Ankle |
| Semitendinosus (ST) | Flexion Extension |
Knee Hip |
| Soleus (SOL) | Plantar flexion | Ankle |
| Vastus lateralis (VL) | Extension | Knee |
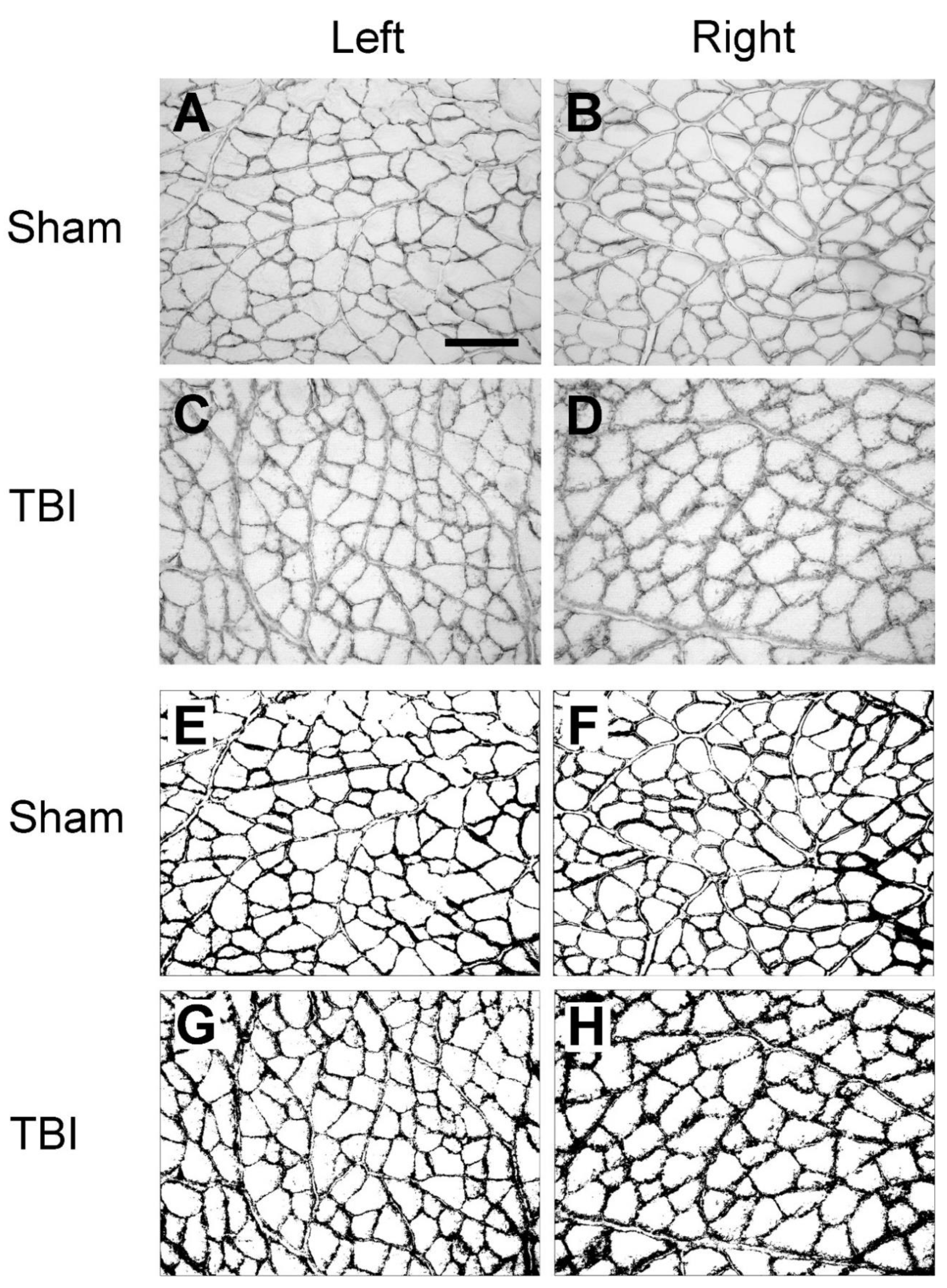
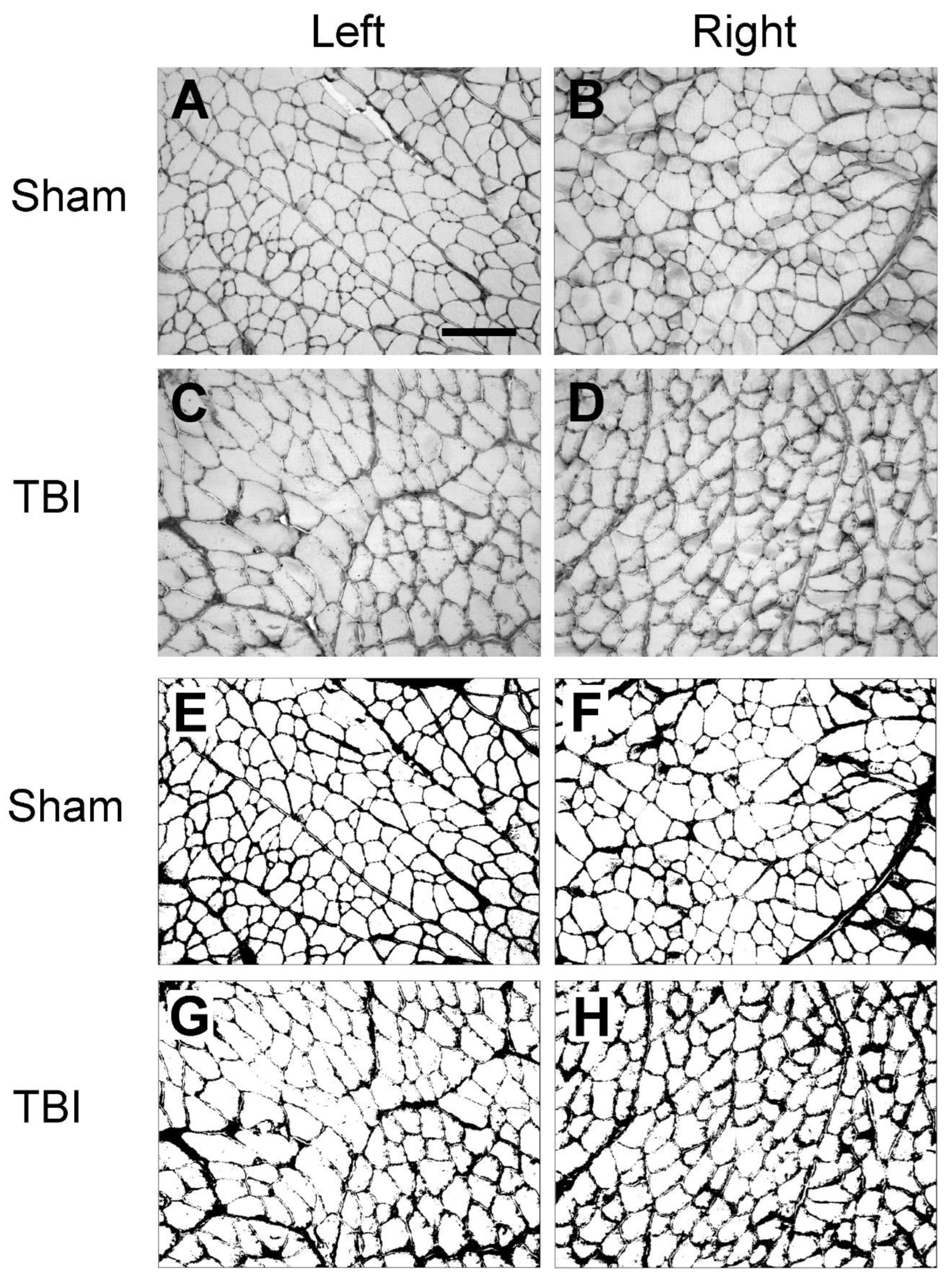
2.4. Immunohistochemistry for detection of collagen IV and laminin
2.5. Data acquisition and analysis
3. Results
3.1. Development of hindlimb postural asymmetry (HL-PA) following traumatic brain injury
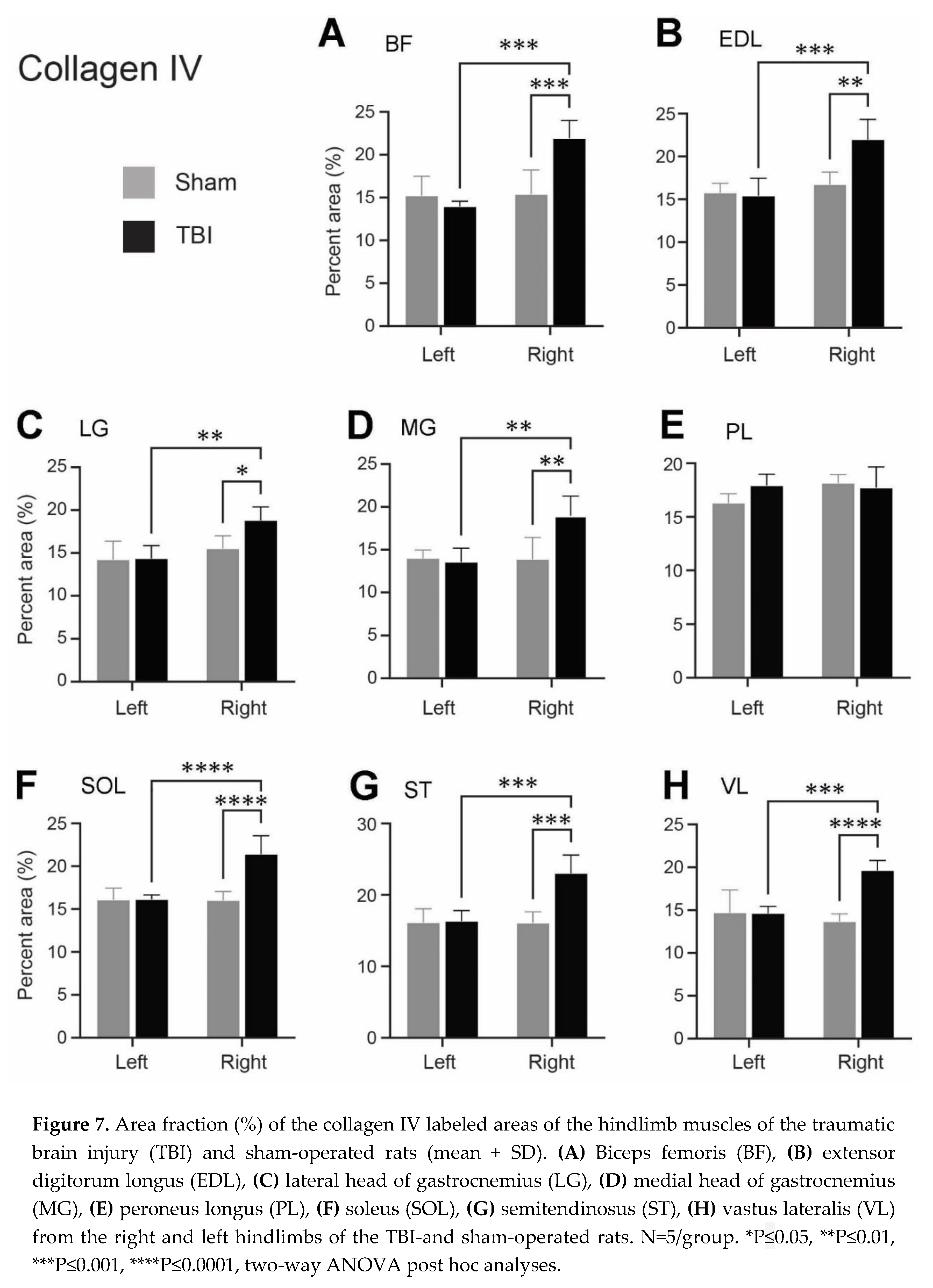
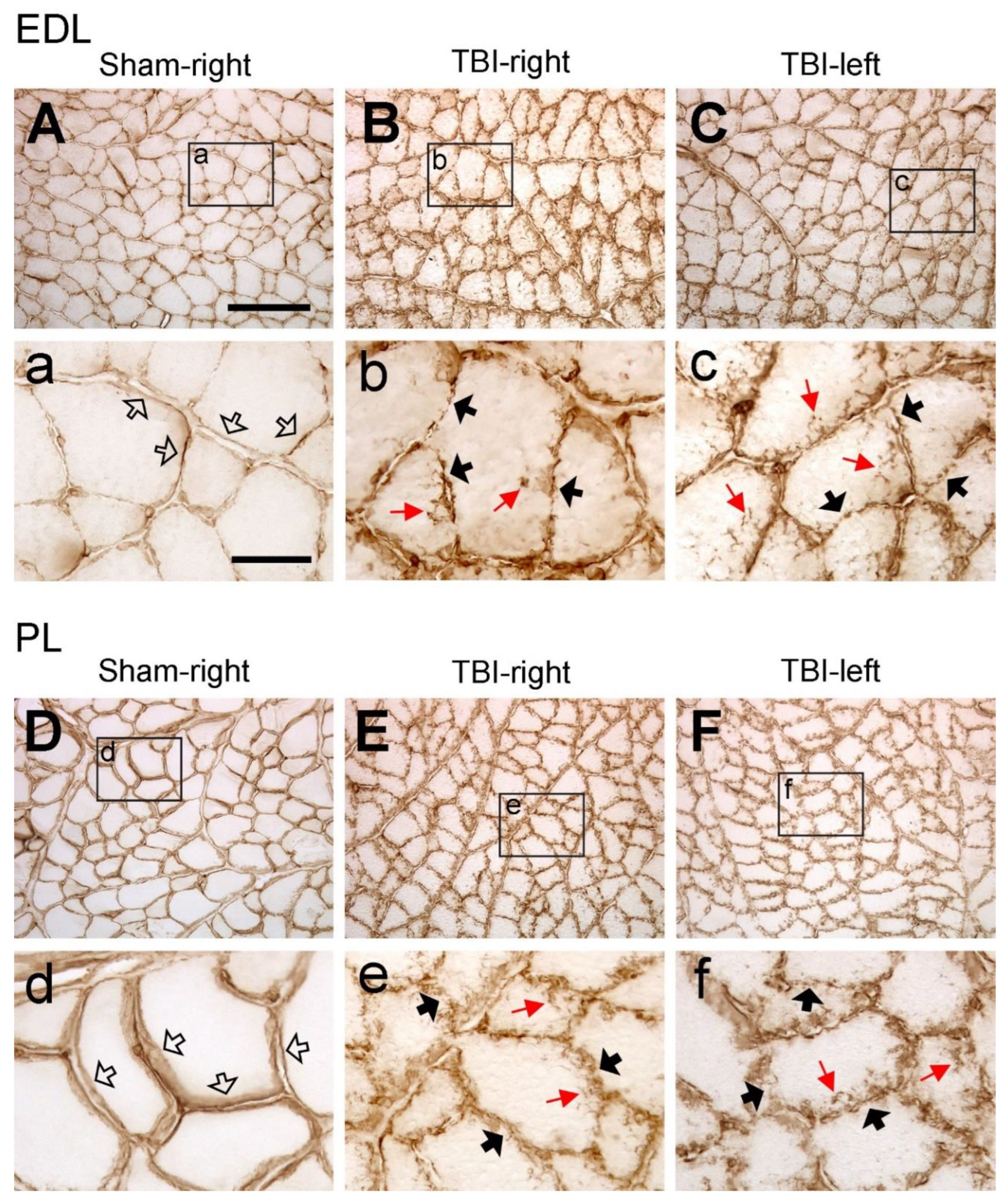
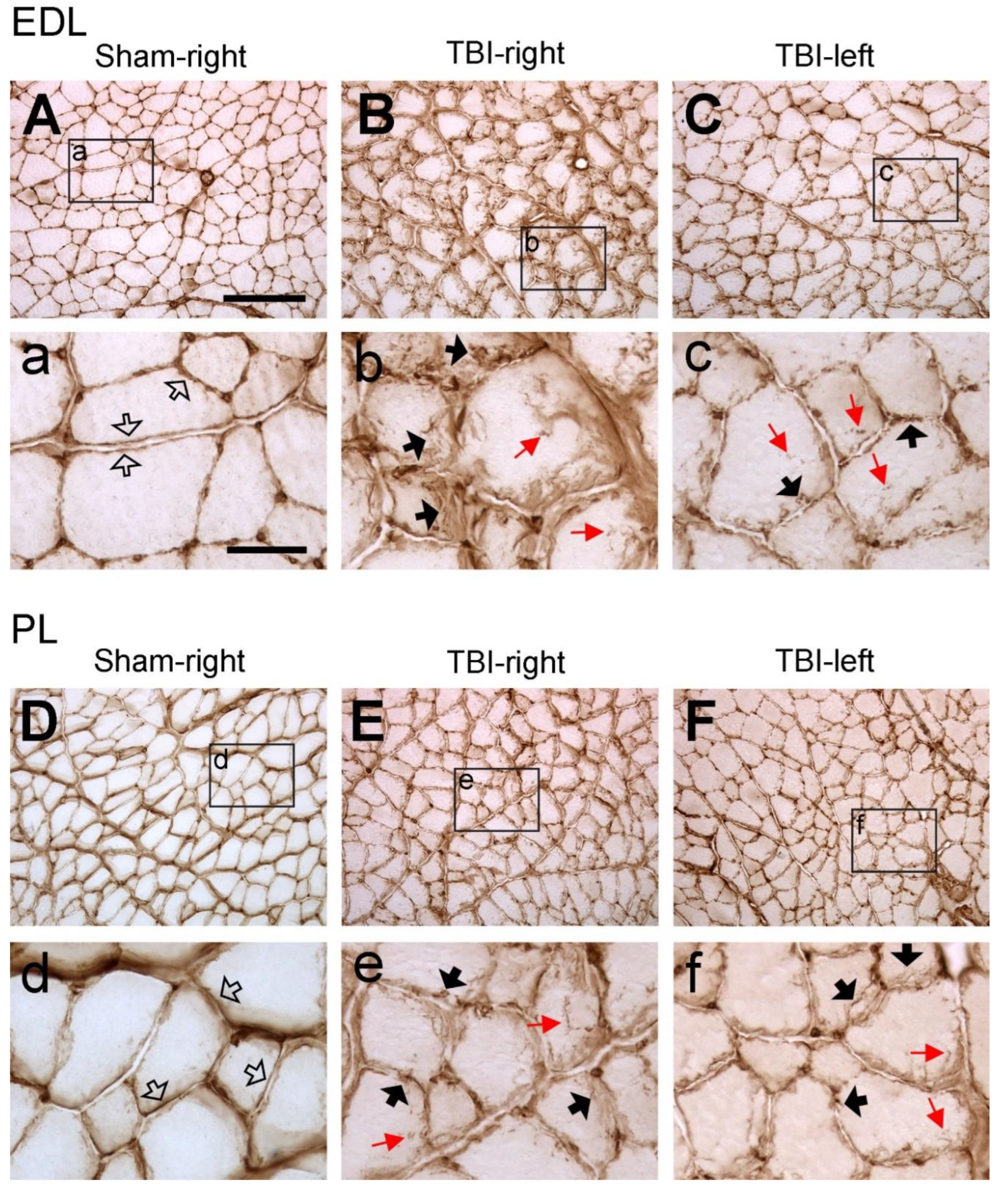
3.2. Traumatic brain injury interrupts the integrity of laminin and collagen IV expression in the basement membrane of hindlimb muscle fibers
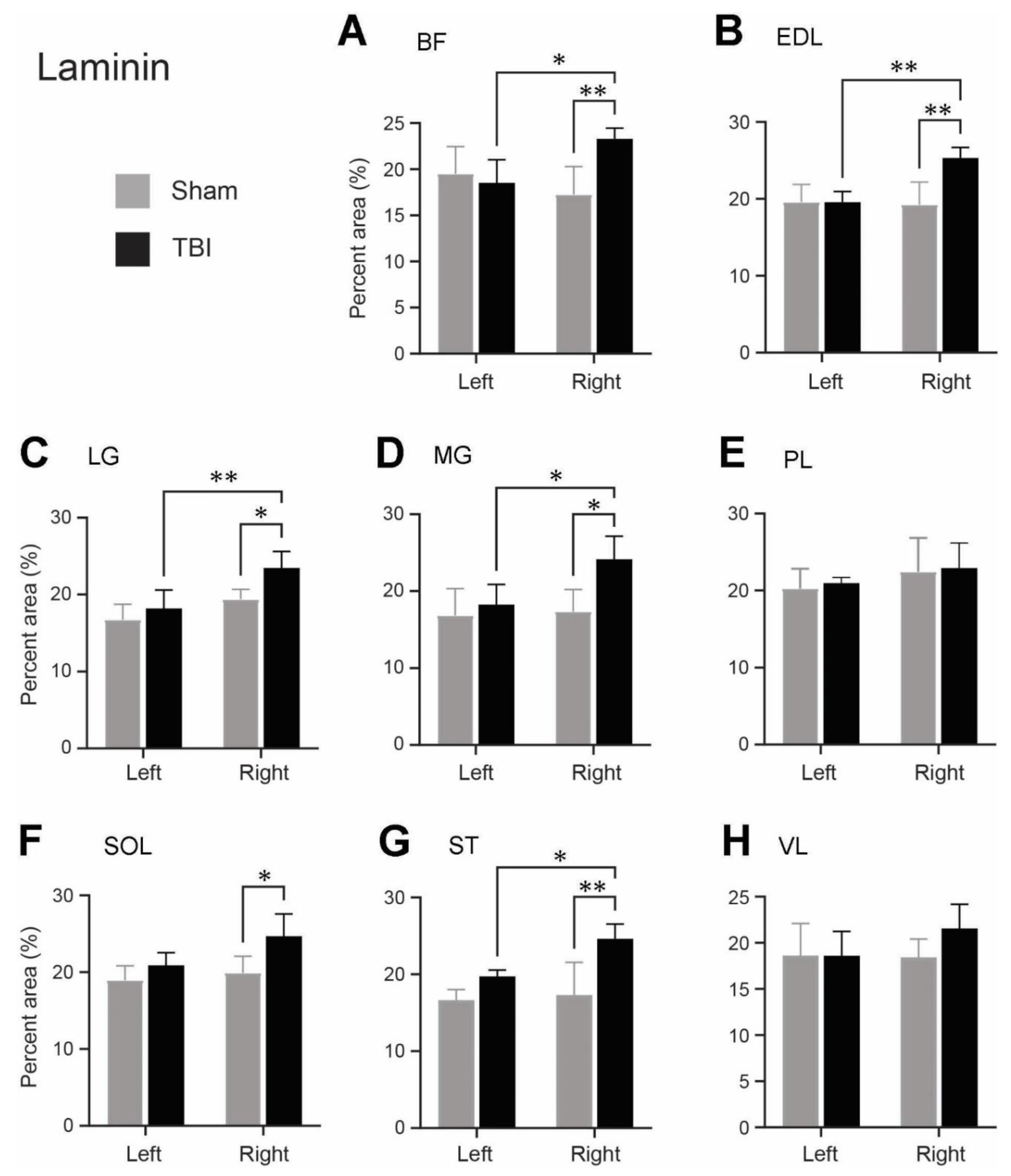
3.3. Significant increase of laminin and collagen IV expression in the ECM of multiple contralateral hindlimb muscles following TBI
| Muscle | Sham | TBI | TBI/Sham | |
|---|---|---|---|---|
| Right/left | Right/left | Left/left | Right/right | |
| Biceps femoris (BF) | 0.88 | 1.26 | 0.95 | 1.35 |
| Extensor digitorum longus (EDL) | 0.98 | 1.29 | 1 | 1.31 |
| Lateral head of gastrocnemius (LG) | 1.15 | 1.29 | 1.08 | 1.21 |
| Lateral head of gastrocnemius (MG) | 1.03 | 1.32 | 1.08 | 1.39 |
| Peroneus longus (PL) | 1.12 | 1.09 | 1.04 | 0.93 |
| Soleus (SOL) | 1.05 | 1.18 | 1.1 | 1.24 |
| Semitendinosus (ST) | 1.04 | 1.25 | 1.18 | 1.42 |
| Vastus lateralis (VL) | 1 | 1.16 | 1 | 1.01 |
| Muscle | Sham | TBI | TBI/Sham | |
|---|---|---|---|---|
| Right/left | Right/left | Left/left | Right/right | |
| Biceps femoris (BF) | 1.08 | 1.57 | 1.02 | 1.42 |
| Extensor digitorum longus (EDL) | 1.06 | 1.43 | 0.98 | 1.31 |
| Lateral head of gastrocnemius (LG) | 1.09 | 1.31 | 1.01 | 1.21 |
| Lateral head of gastrocnemius (MG) | 0.99 | 1.3 | 0.97 | 1.37 |
| Peroneus longus (PL) | 1.11 | 0.99 | 1.1 | 0.98 |
| Soleus (SOL) | 1 | 1.33 | 1 | 1.34 |
| Semitendinosus (ST) | 1 | 1.41 | 1.01 | 1.43 |
| Vastus lateralis (VL) | 0.93 | 1.34 | 0.99 | 1.44 |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Current Understanding of Neuroinflammation after Traumatic Brain Injury and Cell-Based Therapeutic Opportunities. Chin. J. Traumatol. Zhonghua Chuang Shang Za Zhi 2018, 21, 137–151. [Google Scholar] [CrossRef]
- Andersen, M.S.; Güler, D.B.; Larsen, J.; Rich, K.K.; Svenningsen, Å.F.; Zhang, M. The Development of Hindlimb Postural Asymmetry Induced by Focal Traumatic Brain Injury Is Not Related to Serotonin 2A/C Receptor Expression in the Spinal Cord. Int. J. Mol. Sci. 2022, 23, 5358. [Google Scholar] [CrossRef]
- Zhang, M.; Watanabe, H.; Sarkisyan, D.; Andersen, M.S.; Nosova, O.; Galatenko, V.; Carvalho, L.; Lukoyanov, N.; Thelin, J.; Schouenborg, J.; et al. Hindlimb Motor Responses to Unilateral Brain Injury: Spinal Cord Encoding and Left-Right Asymmetry. Brain Commun. 2020, 2, fcaa055. [Google Scholar] [CrossRef]
- Hollung, S.J.; Hägglund, G.; Gaston, M.S.; Seid, A.K.; Lydersen, S.; Alriksson-Schmidt, A.I.; Andersen, G.L. Point Prevalence and Motor Function of Children and Adolescents with Cerebral Palsy in Scandinavia and Scotland: A CP-North Study. Dev. Med. Child Neurol. 2021, 63, 721–728. [Google Scholar] [CrossRef]
- Singer, B.J.; Jegasothy, G.M.; Singer, K.P.; Allison, G.T.; Dunne, J.W. Incidence of Ankle Contracture after Moderate to Severe Acquired Brain Injury. Arch. Phys. Med. Rehabil. 2004, 85, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, J.; Guo, Y.; Tan, S. Prevalence and Risk Factors for Spasticity After Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 616097. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, N.J.; Ada, L.; Neilson, P.D. Spasticity and Muscle Contracture Following Stroke. Brain J. Neurol. 1996, 119 (Pt 5), 1737–1749. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A Report: The Definition and Classification of Cerebral Palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- Crenna, P. Spasticity and “spastic” Gait in Children with Cerebral Palsy. Neurosci. Biobehav. Rev. 1998, 22, 571–578. [Google Scholar] [CrossRef]
- Smith, L.R.; Lee, K.S.; Ward, S.R.; Chambers, H.G.; Lieber, R.L. Hamstring Contractures in Children with Spastic Cerebral Palsy Result from a Stiffer Extracellular Matrix and Increased in Vivo Sarcomere Length. J. Physiol. 2011, 589, 2625–2639. [Google Scholar] [CrossRef]
- Pingel, J.; Bartels, E.M.; Nielsen, J.B. New Perspectives on the Development of Muscle Contractures Following Central Motor Lesions. J. Physiol. 2017, 595, 1027–1038. [Google Scholar] [CrossRef]
- Pingel, J.; Kampmann, M.-L.; Andersen, J.D.; Wong, C.; Døssing, S.; Børsting, C.; Nielsen, J.B. Gene Expressions in Cerebral Palsy Subjects Reveal Structural and Functional Changes in the Gastrocnemius Muscle That Are Closely Associated with Passive Muscle Stiffness. Cell Tissue Res. 2021, 384, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Cortina-Borja, M.J.; Theologis, T.N. Collagen Accumulation in Muscles of Children with Cerebral Palsy and Correlation with Severity of Spasticity. Dev. Med. Child Neurol. 2001, 43, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Hamill, K.J.; Kligys, K.; Hopkinson, S.B.; Jones, J.C.R. Laminin Deposition in the Extracellular Matrix: A Complex Picture Emerges. J. Cell Sci. 2009, 122, 4409–4417. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and Function of the Skeletal Muscle Extracellular Matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular Matrix: An Important Regulator of Cell Functions and Skeletal Muscle Development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef]
- Sanes, J.R. The Basement Membrane/Basal Lamina of Skeletal Muscle. J. Biol. Chem. 2003, 278, 12601–12604. [Google Scholar] [CrossRef]
- Patton, B.L.; Miner, J.H.; Chiu, A.Y.; Sanes, J.R. Distribution and Function of Laminins in the Neuromuscular System of Developing, Adult, and Mutant Mice. J. Cell Biol. 1997, 139, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Leivo, I.; Engvall, E. Merosin, a Protein Specific for Basement Membranes of Schwann Cells, Striated Muscle, and Trophoblast, Is Expressed Late in Nerve and Muscle Development. Proc. Natl. Acad. Sci. U. S. A. 1988, 85, 1544–1548. [Google Scholar] [CrossRef]
- Sanes, J.R. Laminin, Fibronectin, and Collagen in Synaptic and Extrasynaptic Portions of Muscle Fiber Basement Membrane. J. Cell Biol. 1982, 93, 442–451. [Google Scholar] [CrossRef]
- Guicheney, P.; Vignier, N.; Helbling-Leclerc, A.; Nissinen, M.; Zhang, X.; Cruaud, C.; Lambert, J.C.; Richelme, C.; Topaloglu, H.; Merlini, L.; et al. Genetics of Laminin Alpha 2 Chain (or Merosin) Deficient Congenital Muscular Dystrophy: From Identification of Mutations to Prenatal Diagnosis. Neuromuscul. Disord. NMD 1997, 7, 180–186. [Google Scholar] [CrossRef]
- Tomé, F.M.; Evangelista, T.; Leclerc, A.; Sunada, Y.; Manole, E.; Estournet, B.; Barois, A.; Campbell, K.P.; Fardeau, M. Congenital Muscular Dystrophy with Merosin Deficiency. C. R. Acad. Sci. III 1994, 317, 351–357. [Google Scholar]
- Guiraud, S.; Migeon, T.; Ferry, A.; Chen, Z.; Ouchelouche, S.; Verpont, M.-C.; Sado, Y.; Allamand, V.; Ronco, P.; Plaisier, E. HANAC Col4a1 Mutation in Mice Leads to Skeletal Muscle Alterations Due to a Primary Vascular Defect. Am. J. Pathol. 2017, 187, 505–516. [Google Scholar] [CrossRef]
- Emerald, B.S.; Al Jailani, M.A.; Ibrahim, M.F.; Kumar, C.A.; Allouh, M.Z. Cellular and Molecular Variations in Male and Female Murine Skeletal Muscle after Long-Term Feeding with a High-Fat Diet. Int. J. Mol. Sci. 2022, 23, 9547. [Google Scholar] [CrossRef]
- Waisman, A.; Norris, A.M.; Elías Costa, M.; Kopinke, D. Automatic and Unbiased Segmentation and Quantification of Myofibers in Skeletal Muscle. Sci. Rep. 2021, 11, 11793. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Dye, D.E.; Kinnear, B.F.; van Kuppevelt, T.H.; Grounds, M.D.; Coombe, D.R. Interactions between Skeletal Muscle Myoblasts and Their Extracellular Matrix Revealed by a Serum Free Culture System. PloS One 2015, 10, e0127675. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, Y.; Miyachi, R.; Higuchi, T.; Sato, H. Effects of Aging on Collagen in the Skeletal Muscle of Mice. Int. J. Mol. Sci. 2023, 24, 13121. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Duhaime, A.-C.; Bullock, R.; Maas, A.I.R.; Valadka, A.; Manley, G.T. Workshop Scientific Team and Advisory Panel Members Classification of Traumatic Brain Injury for Targeted Therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Mavropalias, G.; Boppart, M.; Usher, K.M.; Grounds, M.D.; Nosaka, K.; Blazevich, A.J. Exercise Builds the Scaffold of Life: Muscle Extracellular Matrix Biomarker Responses to Physical Activity, Inactivity, and Aging. Biol. Rev. Camb. Philos. Soc. 2023, 98, 481–519. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, R.P.; Brashear, S.E.; Smith, L.R. Alignment, Cross Linking, and beyond: A Collagen Architect’s Guide to the Skeletal Muscle Extracellular Matrix. Am. J. Physiol. Cell Physiol. 2023, 325, C1017–C1030. [Google Scholar] [CrossRef]
- Sist, B.; Fouad, K.; Winship, I.R. Plasticity beyond Peri-Infarct Cortex: Spinal up Regulation of Structural Plasticity, Neurotrophins, and Inflammatory Cytokines during Recovery from Cortical Stroke. Exp. Neurol. 2014, 252, 47–56. [Google Scholar] [CrossRef]
- Lukoyanov, N.; Watanabe, H.; Carvalho, L.S.; Kononenko, O.; Sarkisyan, D.; Zhang, M.; Andersen, M.S.; Lukoyanova, E.A.; Galatenko, V.; Tonevitsky, A.; et al. Left-Right Side-Specific Endocrine Signaling Complements Neural Pathways to Mediate Acute Asymmetric Effects of Brain Injury. eLife 2021, 10, e65247. [Google Scholar] [CrossRef] [PubMed]
- Jannapureddy, S.R.; Patel, N.D.; Hwang, W.; Boriek, A.M. Genetic Models in Applied Physiology. Merosin Deficiency Leads to Alterations in Passive and Active Skeletal Muscle Mechanics. J. Appl. Physiol. Bethesda Md 1985 2003, 94, 2524–2533, discussion 2523. [Google Scholar] [CrossRef]
- Rayagiri, S.S.; Ranaldi, D.; Raven, A.; Mohamad Azhar, N.I.F.; Lefebvre, O.; Zammit, P.S.; Borycki, A.-G. Basal Lamina Remodeling at the Skeletal Muscle Stem Cell Niche Mediates Stem Cell Self-Renewal. Nat. Commun. 2018, 9, 1075. [Google Scholar] [CrossRef]
- Silver, J.S.; Günay, K.A.; Cutler, A.A.; Vogler, T.O.; Brown, T.E.; Pawlikowski, B.T.; Bednarski, O.J.; Bannister, K.L.; Rogowski, C.J.; Mckay, A.G.; et al. Injury-Mediated Stiffening Persistently Activates Muscle Stem Cells through YAP and TAZ Mechanotransduction. Sci. Adv. 2021, 7, eabe4501. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.R.; Pichika, R.; Meza, R.C.; Gillies, A.R.; Baliki, M.N.; Chambers, H.G.; Lieber, R.L. Contribution of Extracellular Matrix Components to the Stiffness of Skeletal Muscle Contractures in Patients with Cerebral Palsy. Connect. Tissue Res. 2021, 62, 287–298. [Google Scholar] [CrossRef]
- Gonzalez, D.; Contreras, O.; Rebolledo, D.L.; Espinoza, J.P.; van Zundert, B.; Brandan, E. ALS Skeletal Muscle Shows Enhanced TGF-β Signaling, Fibrosis and Induction of Fibro/Adipogenic Progenitor Markers. PloS One 2017, 12, e0177649. [Google Scholar] [CrossRef]
- Shahidi, B.; Shah, S.B.; Esparza, M.; Head, B.P.; Ward, S.R. Skeletal Muscle Atrophy and Degeneration in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2018, 35, 398–401. [Google Scholar] [CrossRef]
- Ariano, M.A.; Armstrong, R.B.; Edgerton, V.R. Hindlimb Muscle Fiber Populations of Five Mammals. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1973, 21, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Hibino, I.; Okita, M.; Inoue, T.; Banno, Y.; Hoso, M. Effect of Immobilization on Insoluble Collagen Concentration and Type I and Type III Collagen Isoforms of Rat Soleus Muscle. J. Jpn. Phys. Ther. Assoc. Rigaku Ryoho 2008, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rubiano, A.M.; Lee, K. Traumatic Brain Injury Research: Homogenising Heterogeneity. Lancet Neurol. 2023, 22, 973–975. [Google Scholar] [CrossRef]
- Covington, N.V.; Duff, M.C. Heterogeneity Is a Hallmark of Traumatic Brain Injury, Not a Limitation: A New Perspective on Study Design in Rehabilitation Research. Am. J. Speech Lang. Pathol. 2021, 30, 974–985. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





