Submitted:
12 February 2024
Posted:
12 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Environmental Cadmium and the Increased Prevalence of Chronic Kidney Disease
2.1. Findings from Systematic Reviews and Meta-Analyses
2.2. Exposure Levels of Concern
2.3. Methods of Normalization of Cadmium Excretion Rate
2.5. Demonstrable Dose-Response Relationships
3. Impacts of Cadmium on Tubular Protein Reabsorption
3.1. Cadmium-Induced Albuminuria
3.2. Fractional Reductions in the Reabsorption of Albumin and β2M
3.3. Overall Effects of Cadmium Burden on Tubular Function
3.4. Implication of Albumin Reabsorption for Delivery of Cadmium to Proximal Tubules
3.5. Summary on the Impact of Cadmium on Protien Reabsorptuve Function
4. CKD and the Health Risk Assessment of Environmental Cadmium
4.4. Cadmium Excretion and Glomerular Filtration Rate
4.3. An Acceptable Kidney Burden of Cadmium?
4.4. Past and Present Health Threat of Environemnatl Cadmium
4.4.1. The WHO Exposure Guidelines and the Nephrotoxicity Threshold Level
4.4.2. β2-Microglobulinuria as an Indicator of Toxicity?
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. Estimation of health risks associated with dietary cadmium exposure. Arch. Toxicol. 2023, 97, 329–358. [Google Scholar] [CrossRef]
- Fechner, C.; Hackethal, C.; Höpfner, T.; Dietrich, J.; Bloch, D.; Lindtner, O.; Sarvan, I. Results of the BfR MEAL Study: In Germany, mercury is mostly contained in fish and seafood while cadmium, lead, and nickel are present in a broad spectrum of foods. Food Chem. X 2022, 14, 100326. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Y.; Dong, K.F.; Lu, J. Regional characteristics of cadmium intake in adult residents from the 4th and 5th Chinese Total Diet Study. Environ. Sci. Pollut. Res. 2020, 27, 3850–3857. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Woo, H.D.; Kim, D.W.; Choi, I.J.; Kim, Y.-I.; Kim, J. Association between dietary cadmium intake and early gastric cancer risk in a Korean population: A case–control study. Eur. J. Nutr. 2019, 58, 3255–3266. [Google Scholar] [CrossRef]
- Watanabe, T.; Kataoka, Y.; Hayashi, K.; Matsuda, R.; Uneyama, C. Dietary Exposure of the Japanese General Population to Elements: Total Diet Study 2013–2018. Food Saf. 2022, 10, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Herbrechter, R.; Schlabs, C.; Pethe, A.; Lee, W.K.; Wolff, N.A.; Roussa, E. Role of the SLC22A17/lipocalin-2 receptor in renal endocytosis of proteins/metalloproteins: A focus on iron- and cadmium-binding proteins. Am. J. Physiol. Renal. Physiol. 2023, 325, F564–F577. [Google Scholar] [CrossRef]
- Rentschler, G.; Kippler, M.; Axmon, A.; Raqib, R.; Skerfving, S.; Vahter, M.; Broberg, K. Cadmium concentrations in human blood and urine are associated with polymorphisms in zinc transporter genes. Metallomics 2014, 6, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, G.; Kippler, M.; Axmon, A.; Raqib, R.; Ekström, E.-C.; Skerfving, S.; Vahter, M.; Broberg, K. Polymorphisms in Iron Homeostasis Genes and Urinary Cadmium Concentrations among Nonsmoking Women in Argentina and Bangladesh. Environ. Health Perspect. 2013, 121, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.; Lind, P.M.; Lindgren, C.; Ingelsson, E.; Mahajan, A.; Morris, A.; Lind, L. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum. Mol. Genet. 2015, 24, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, C.; Zhao, D.; Huang, L. Associations of micronutrients exposure with cadmium body burden among population: A systematic review. Ecotoxicol. Environ. Saf. 2023, 256, 114878. [Google Scholar] [CrossRef]
- Fujita, Y.; el Belbasi, H.I.; Min, K.S.; Onosaka, S.; Okada, Y.; Matsumoto, Y.; Mutoh, N.; Tanaka, K. Fate of cadmium bound to phytochelatin in rats. Res. Commun. Chem. Pathol. Pharmacol. 1993, 82, 357–365. [Google Scholar]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Thévenod, F. Expression and function of the lipocalin-2 (24p3/NGAL) receptor in rodent and human intestinal epithelia. PLoS ONE 2013, 8, e71586. [Google Scholar] [CrossRef] [PubMed]
- Langelueddecke, C.; Lee, W.K.; Thévenod, F. Differential transcytosis and toxicity of the hNGAL receptor ligands cadmi-um-metallothionein and cadmium-phytochelatin in colon-like Caco-2 cells: Implications for in vivo cadmium toxicity. Toxicol. Lett. 2014, 226, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Wolff, N.A.; Lee, W.-K.; Thevenod, F. Lipocalin-2 (24p3/Neutrophil Gelatinase-associated Lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J. Biol. Chem. 2012, 287, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Guevara, I.P.; Ortega-Romero, M.S.; Narváez-Morales, J.; Jacobo-Estrada, T.L.; Lee, W.-K.; Arreola-Mendoza, L.; Thévenod, F.; Barbier, O.C. Increased Endocytosis of Cadmium-Metallothionein through the 24p3 Receptor in an In Vivo Model with Reduced Proximal Tubular Activity. Int. J. Mol. Sci. 2021, 22, 7262. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Fresquez, M.R.; Watson, C.H. Cigarette Smoke Cadmium Breakthrough From Traditional Filters: Implications for Exposure. J. Anal. Toxicol. 2015, 39, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Repić, A.; Bulat, P.; Antonijević, B.; Antunović, M.; Džudović, J.; Buha, A.; Bulat, Z. The influence of smoking habits on cadmium and lead blood levels in the Serbian adult people. Environ. Sci. Pollut. Res. 2020, 27, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, R.; Trojanowska, M. Chemical fractionation in environmental studies of potentially toxic particulate-bound elements in urban air: A critical review. Toxics 2022, 10, 124. [Google Scholar] [CrossRef]
- Kim, J.; Song, H.; Lee, J.; Kim, Y.J.; Chung, H.S.; Yu, J.M.; Jang, G.; Park, R.; Chung, W.; Oh, C.-M.; et al. Smoking and passive smoking increases mortality through mediation effect of cadmium exposure in the United States. Sci. Rep. 2023, 13, 3878. [Google Scholar] [CrossRef]
- Nakajima, M.; Kobayashi, E.; Suwazono, Y.; Uetani, M.; Oishi, M.; Inaba, T.; Kido, T.; Zahir A Shaikh, Z.A.; Nogawa, K. Excretion of urinary cadmium, copper, and zinc in cadmium-exposed and nonexposed subjects, with special reference to urinary excretion of beta2-microglobulin and metallothionein. Biol. Trace Elem. Res, 2005; 18, 17–31. [Google Scholar] [CrossRef]
- Aoyagi, T.; Hayakawa, K.; Miyaji, K.; Ishikawa, H.; Hata, M. Cadmium nephrotoxicity and evacuation from the body in a rat modeled subchronic intoxication. Int. J. Urol. 2003, 10, 332–338. [Google Scholar] [CrossRef]
- Elinder, C.G.; Lind, B.; Kjellstorm, T.; Linnman, L.; Friberg, L. Cadmium in kidney cortex, liver and pancreas from Swedish autopsies: Estimation of biological half time in kidney cortex, considering calorie intake and smoking habits. Arch. Environ. Health 1976, 31, 292–301. [Google Scholar] [CrossRef]
- Elinder, C.-G.; Kjellstöm, T.; Lind, B.; Molander, M.-L.; Silander, T. Cadmium concentrations in human liver, blood, and bile: Comparison with a metabolic model. Environ. Res. 1978, 17, 236–241. [Google Scholar] [CrossRef]
- Jarup, L.; Rogenfelt, A.; Elinder, C.; Nogawa, K.; Kjellstrom, T. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand. J. Work. Environ. Health 1983, 9, 327–331. [Google Scholar] [CrossRef]
- Suwazono, Y.; Kido, T.; Nakagawa, H.; Nishijo, M.; Honda, R.; Kobayashi, E.; Dochi, M.; Nogawa, K. Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers 2009, 14, 77–81. [Google Scholar] [CrossRef]
- Ishizaki, M.; Suwazono, Y.; Kido, T.; Nishijo, M.; Honda, R.; Kobayashi, E.; Nogawa, K.; Nakagawa, H. Estimation of biological half-life of urinary cadmium in inhabitants after cessation of environmental cadmium pollution using a mixed linear model. Food Addit. Contam. Part A 2015, 32, 1273–1276. [Google Scholar] [CrossRef]
- Satarug, S.; Nishijo, M.; Ujjin, P.; Vanavanitkun, Y.; Baker, J.R.; Moore, M.R. Evidence for Concurrent Effects of Exposure to Environmental Cadmium and Lead on Hepatic CYP2A6 Phenotype and Renal Function Biomarkers in Nonsmokers. Environ. Health Perspect. 2004, 112, 1512–1518. [Google Scholar] [CrossRef]
- Satarug, S.; Tassaneeyakul, W.; Na-Bangchang, K.; Cashman, J.R.; Moore, M.R. Genetic and Environmental Influences on Therapeutic and Toxicity Outcomes: Studies with CYP2A6. Curr. Clin. Pharmacol. 2006, 1, 291–309. [Google Scholar] [CrossRef]
- Apinan, R.; Tassaneeyakul, W.; Mahavorasirikul, W.; Satarug, S.; Kajanawart, S.; Vannaprasaht, S.; Ruenweerayut, R.; Na-Bangchang, K. The influence of CYP2A6 polymorphisms and cadmium on nicotine metabolism in Thai population. Environ. Toxicol. Pharmacol. 2009, 28, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Sanchez, J.J.G.; James, G.; Wittbrodt, E.; Nolan, S.; Sörstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- George, C.; Mogueo, A.; Okpechi, I.; Echouffo-Tcheugui, J.B.; Kengne, A.P. Chronic kidney disease in low-income to middle-income countries: The case for increased screening. BMJ Glob. Health 2017, 2, e000256. [Google Scholar] [CrossRef] [PubMed]
- Doccioli, C.; Sera, F.; Francavilla, A.; Cupisti, A.; Biggeri, A. Association of cadmium environmental exposure with chronic kidney disease: A systematic review and meta-analysis. Sci. Total. Environ. 2024, 906, 167165. [Google Scholar] [CrossRef]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between exposure to heavy metals and the risk of chronic kidney disease: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Byber, K.; Lison, D.; Verougstraete, V.; Dressel, H.; Hotz, P. Cadmium or cadmium compounds and chronic kidney disease in workers and the general population: A systematic review. Crit. Rev. Toxicol. 2016, 46, 191–240. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Yimthiang, S.; Đorđević, A.B. Health Risk in a Geographic Area of Thailand with Endemic Cadmium Contamination: Focus on Albuminuria. Toxics 2023, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease. Toxics 2022, 10, 614. [Google Scholar] [CrossRef]
- Myong, J.-P.; Kim, H.-R.; Baker, D.; Choi, B. Blood cadmium and moderate-to-severe glomerular dysfunction in Korean adults: Analysis of KNHANES 2005–2008 data. Int. Arch. Occup. Environ. Health 2012, 85, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Chung, J.H.; Kim, S.J.; Koh, E.S.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Shin, S.J. Blood lead and cadmium levels and renal function in Korean adults. Clin. Exp. Nephrol. 2014, 18, 726–734. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Hung, C.-H.; Wang, C.-W.; Tu, H.-P.; Li, C.-H.; Tsai, C.-C.; Lin, W.-Y.; Chen, S.-C.; Kuo, C.-H. Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease. Diagnostics 2021, 11, 282. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, R.; Jiang, Q.; Wang, Y.; Yu, C. Analysis of cadmium accumulation in community adults and its correlation with low-grade albuminuria. Sci. Total. Environ. 2022, 834, 155210. [Google Scholar] [CrossRef] [PubMed]
- Grau-Perez, M.; Pichler, G.; Galan-Chilet, I.; Briongos-Figuero, L.S.; Rentero-Garrido, P.; Lopez-Izquierdo, R.; Navas-Acien, A.; Weaver, V.; García-Barrera, T.; Gomez-Ariza, J.L.; et al. Urine cadmium levels and albuminuria in a general population from Spain: A gene-environment interaction analysis. Environ. Int. 2017, 106, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Tellez-Plaza, M.; Guallar, E.; Muntner, P.; Silbergeld, E.; Jaar, B.; Weaver, V. Blood Cadmium and Lead and Chronic Kidney Disease in US Adults: A Joint Analysis. Am. J. Epidemiol. 2009, 170, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Costanzi, S.; Naticchia, A.; Sturniolo, A.; Gambaro, G. Low level exposure to cadmium increases the risk of chronic kidney disease: Analysis of the NHANES 1999-2006. BMC Public Health 2010, 10, 304. [Google Scholar] [CrossRef]
- Madrigal, J.M.; Ricardo, A.C.; Persky, V.; Turyk, M. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ. Res. 2018, 169, 180–188. [Google Scholar] [CrossRef]
- Zhu, X.-J.; Wang, J.-J.; Mao, J.-H.; Shu, Q.; Du, L.-Z. Relationships of Cadmium, Lead, and Mercury Levels With Albuminuria in US Adults: Results From the National Health and Nutrition Examination Survey Database, 2009–2012. Am. J. Epidemiol. 2019, 188, 1281–1287. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Caffrey, J.L.; Lin, J.-W.; Bayliss, D.; Faramawi, M.F.; Bateson, T.F.; Sonawane, B. Increased Risk of Cancer Mortality Associated with Cadmium Exposures in Older Americans with Low Zinc Intake. J. Toxicol. Environ. Health Part A 2012, 76, 1–15. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, C.-W.; Chen, H.-C.; Tu, H.-P.; Chen, S.-C.; Hung, C.-H.; Kuo, C.-H. Gender Difference in the Associations among Heavy Metals with Red Blood Cell Hemogram. Int. J. Environ. Res. Public Health 2021, 19, 189. [Google Scholar] [CrossRef]
- Olsén, L.; Lind, P.M.; Lind, L. Gender differences for associations between circulating levels of metals and coronary risk in the elderly. Int. J. Hyg. Environ. Health 2012, 215, 411–417. [Google Scholar] [CrossRef]
- Sun, H.; Wang, D.; Zhou, Z.; Ding, Z.; Chen, X.; Xu, Y.; Huang, L.; Tang, D. Association of cadmium in urine and blood with age in a general population with low environmental exposure. Chemosphere 2016, 156, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Butler-Dawson, J.; James, K.A.; Krisher, L.; Jaramillo, D.; Dally, M.; Neumann, N.; Pilloni, D.; Cruz, A.; Asensio, C.; Johnson, R.J.; et al. Environmental metal exposures and kidney function of Guatemalan sugarcane workers. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 461–471. [Google Scholar] [CrossRef]
- Win-Thu, M.; Myint-Thein, O.; Win-Shwe, T.-T.; Mar, O. Environmental cadmium exposure induces kidney tubular and glomerular dysfunction in the Myanmar adults. J. Toxicol. Sci. 2021, 46, 319–328. [Google Scholar] [CrossRef]
- Skröder, H.; Hawkesworth, S.; Kippler, M.; El Arifeen, S.; Wagatsuma, Y.; Moore, S.E.; Vahter, M. Kidney function and blood pressure in preschool-aged children exposed to cadmium and arsenic - potential alleviation by selenium. Environ. Res. 2015, 140, 205–213. [Google Scholar] [CrossRef]
- Rodríguez-López, E.; Tamayo-Ortiz, M.; Ariza, A.C.; Ortiz-Panozo, E.; Deierlein, A.L.; Pantic, I.; Tolentino, M.C.; Estrada-Gutiérrez, G.; Parra-Hernández, S.; Espejel-Núñez, A.; et al. Early-Life Dietary Cadmium Exposure and Kidney Function in 9-Year-Old Children from the PROGRESS Cohort. Toxics 2020, 8, 83. [Google Scholar] [CrossRef]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. Chapter 13, General Considerations of Dose-Effect and Dose-Response Relationships. In Handbook on the Toxicology of Metals, 5th ed.; Volume I: General, Considerations; Nordberg, G., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Elsherbiny, H.; Rule, A.D. In-vivo techniques for determining nephron number. Curr. Opin. Nephrol. Hypertens. 2019, 28, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Soveri, I.; Berg, U.B.; Björk, J.; Elinder, C.G.; Grubb, A.; Mejare, I.; Sterner, G.; Bäck, S.E.; SBU GFR Review Group. Measuring GFR: A Systematic Review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef] [PubMed]
- White, C.A.; Allen, C.M.; Akbari, A.; Collier, C.P.; Holland, D.C.; Day, A.G.; Knoll, G.A. Comparison of the new and traditional CKD-EPI GFR estimation equations with urinary inulin clearance: A study of equation performance. Clin. Chim. Acta 2019, 488, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The Source and Pathophysiologic Significance of Excreted Cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Cadmium-Induced Proteinuria: Mechanistic Insights from Dose–Effect Analyses. Int. J. Mol. Sci. 2023, 24, 1893. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. The pathogenesis of albuminuria in cadmium nephropathy. Curr. Res. Toxicol. 2024, 6, 100140. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Navar, L.G.; Maddox, D.A.; Munger, K.A. In: Brenner and Rector’s the kidney, 11th edn. Elsevier, Philadelphia, USA; 2020. Chapter 3, the renal circulations and glomerular filtration, pp 80-114.
- Gauthier, C.; Nguyen-Simonnet, H.; Vincent, C.; Revillard, J.-P.; Pellet, M.V. Renal tubular absorption of β2-microglobulin. Kidney Int. 1984, 26, 170–175. [Google Scholar] [CrossRef]
- Norden, A.G.; Lapsley, M.; Lee, P.J.; Pusey, C.D.; Scheinman, S.J.; Tam, F.W.; Thakker, R.V.; Unwin, R.J.; Wrong, O. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 2001, 60, 1885–1892. [Google Scholar] [CrossRef]
- Nielsen, R.; Christensen, E.I.; Birn, H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int. 2016, 89, 58–67. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef] [PubMed]
- Eshbach, M.L.; Weisz, O.A. Receptor-Mediated Endocytosis in the Proximal Tubule. Annu. Rev. Physiol. 2017, 79, 425–448. [Google Scholar] [CrossRef]
- Bökenkamp, A. Proteinuria-take a closer look! Pediatr. Nephrol. 2020, 35, 533–541. [Google Scholar] [CrossRef]
- Comper, W.D.; Vuchkova, J.; McCarthy, K.J. New insights into proteinuria/albuminuria. Front. Physiol. 2022, 13, 991756. [Google Scholar] [CrossRef]
- Gburek, J.; Konopska, B.; Gołąb, K. Renal Handling of Albumin—From Early Findings to Current Concepts. Int. J. Mol. Sci. 2021, 22, 5809. [Google Scholar] [CrossRef] [PubMed]
- Benzing, T.; Salant, D. Insights into Glomerular Filtration and Albuminuria. New Engl. J. Med. 2021, 384, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Clapp, W.L.; Park, C.H.; Madsen, K.M.; Tisher, C.C. Axial heterogeneity in the handling of albumin by the rabbit proximal tubule. Lab. Invest. 1988, 58, 549–558. [Google Scholar] [PubMed]
- Schuh, C.D.; Polesel, M.; Platonova, E.; Haenni, D.; Gassama, A.; Tokonami, N.; Ghazi, S.; Bugarski, M.; Devuyst, O.; Ziegler, U.; et al. Combined Structural and Functional Imaging of the Kidney Reveals Major Axial Differences in Proximal Tubule Endocytosis. J. Am. Soc. Nephrol. 2018, 29, 2696–2712. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.C.; Sandoval, R.M.; Yadav, S.P.S.; Campos, S.B.; Rhodes, G.J.; Phillips, C.L.; Molitoris, B.A. Lrpap1 (RAP) Inhibits Proximal Tubule Clathrin Mediated and Clathrin Independent Endocytosis, Ameliorating Renal Aminoglycoside Nephrotoxicity. Kidney360 2023, 4, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Phelps, K.R. Chapter 14, Cadmium exposure and toxicity. In Metal Toxicology; Bagchi, M., Bagchi, D., Eds.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Polesel, M.; Kaminska, M.; Haenni, D.; Bugarski, M.; Schuh, C.; Jankovic, N.; Kaech, A.; Mateos, J.M.; Berquez, M.; Hall, A.M. Spatiotemporal organisation of protein processing in the kidney. Nat. Commun. 2022, 13, 5732. [Google Scholar] [CrossRef]
- Sarav, M.; Wang, Y.; Hack, B.K.; Chang, A.; Jensen, M.; Bao, L.; Quigg, R.J. Renal FcRn Reclaims Albumin but Facilitates Elimination of IgG. J. Am. Soc. Nephrol. 2009, 20, 1941–1952. [Google Scholar] [CrossRef]
- Tenten, V.; Menzel, S.; Kunter, U.; Sicking, E.-M.; van Roeyen, C.R.C.; Sanden, S.K.; Kaldenbach, M.; Boor, P.; Fuss, A.; Uhlig, S.; et al. Albumin is recycled from the primary urine by tubular transcytosis. J. Am. Soc. Nephrol. 2013, 24, 1966–1980. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.-H.; Roumelioti, M.-E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef]
- Tojo, A.; Endou, H.; Nolin, A.C.; Mulhern, R.M.; Panchenko, M.V.; Pisarek-Horowitz, A.; Wang, Z.; Shirihai, O.; Borkan, S.C.; Havasi, A.; et al. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am. J. Physiol. Physiol. 1992, 263, F601–F606. [Google Scholar] [CrossRef]
- Carlson, L.A.; Friberg, L. The Distribution of Cadmium in Blood After Repeated Exposure. Scand. J. Clin. Lab. Investig. 1957, 9, 67–70. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Piscator, M.; Nordberg, M. On the Distribution of Cadmium in Blood. Acta Pharmacol. et Toxicol. 1971, 30, 289–295. [Google Scholar] [CrossRef]
- Gibson, M.A.; Sarpong-Kumankomah, S.; Nehzati, S.; George, G.N.; Gailer, J. Remarkable differences in the biochemical fate of Cd2+, Hg2+, CH3Hg+ and thimerosal in red blood cell lysate. Metallomics 2017, 9, 1060–1072. [Google Scholar] [CrossRef]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology—Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef]
- Scott, B.J.; Bradwell, A.R. Identification of the serum binding proteins for iron, zinc, cadmium, nickel, and calcium. Clin. Chem. 1983, 29, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Horn, N.M.; Thomas, A.L. Interactions between the histidine stimulation of cadmium and zinc influx into human erythrocytes. J. Physiol. 1996, 496, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.T.; Keir, J.L.; Boshart, S.J.; Lobanov, V.P.; Ruhland, A.M.; Bahl, N.; Gailer, J. Mobilization of Cd from human serum albumin by small molecular weight thiols. J. Chromatogr. B 2014, 958, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Sagmeister, P.; Gibson, M.A.; McDade, K.H.; Gailer, J. Physiologically relevant plasma d,l-homocysteine concentrations mo-bilize Cd from human serum albumin. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1027, 181–186. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free. Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef]
- Shen, X.-C.; Liou, X.-Y.; Ye, L.-P.; Liang, H.; Wang, Z.-Y. Spectroscopic studies on the interaction between human hemoglobin and CdS quantum dots. J. Colloid Interface Sci. 2007, 311, 400–406. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.; Jiang, L.; Song, L. New insight into molecular interaction of heavy metal pollutant—cadmium(II) with human serum albumin. Environ. Sci. Pollut. Res. 2014, 21, 6994–7005. [Google Scholar] [CrossRef]
- Fels, J.; Scharner, B.; Zarbock, R.; Guevara, I.P.Z.; Lee, W.-K.; Barbier, O.C.; Thévenod, F. Cadmium Complexed with β2-Microglubulin, Albumin and Lipocalin-2 rather than Metallothionein Cause Megalin:Cubilin Dependent Toxicity of the Renal Proximal Tubule. Int. J. Mol. Sci. 2019, 20, 2379. [Google Scholar] [CrossRef]
- Edwards, A.; Long, K.R.; Baty, C.J.; Shipman, K.E.; Weisz, O.A. Modelling normal and nephrotic axial uptake of albumin and other filtered proteins along the proximal tubule. J. Physiol. 2022, 600, 1933–1952. [Google Scholar] [CrossRef]
- Castrop, H.; Schießl, I.M. Novel routes of albumin passage across the glomerular filtration barrier. Acta Physiol. 2016, 219, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Sánchez, M.P.; Pedraza-Chaverri, J.; Molina-Jijón, E.; Arreola-Mendoza, L.; Rodríguez-Muñoz, R.; Barbier, O.C. Impaired endocytosis in proximal tubule from subchronic exposure to cadmium involves angiotensin II type 1 and cubilin receptors. BMC Nephrol. 2013, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Gena, P.; Calamita, G.; Guggino, W.B. Cadmium Impairs Albumin Reabsorption by Down-regulating Megalin and ClC5 Channels in Renal Proximal Tubule Cells. Environ. Health Perspect. 2010, 118, 1551–1556. [Google Scholar] [CrossRef]
- Li, L.; Dong, F.; Xu, D.; Du, L.; Yan, S.; Hu, H.; Lobe, C.G.; Yi, F.; Kapron, C.M.; Liu, J. Short-term, low-dose cadmium exposure induces hyperpermeability in human renal glomerular endothelial cells. J. Appl. Toxicol. 2016, 36, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, L.; Tao, T.; Su, W.; Guo, Y.; Yu, H.; Qin, J. Assessment of cadmium-induced nephrotoxicity using a kidney-on-a-chip device. Toxicol. Res. 2017, 6, 372–380. [Google Scholar] [CrossRef]
- Simmons, R.W.; Noble, A.D.; Pongsakul, P.; Sukreeyapongse, O.; Chinabut, N. Cadmium-hazard mapping using a general linear regression model (Irr-Cad) for rapid risk assessment. Environ. Geochem. Health 2009, 31, 71–79. [Google Scholar] [CrossRef]
- Simmons, R.; Pongsakul, P.; Saiyasitpanich, D.; Klinphoklap, S. Elevated Levels of Cadmium and Zinc in Paddy Soils and Elevated Levels of Cadmium in Rice Grain Downstream of a Zinc Mineralized Area in Thailand: Implications for Public Health. Environ. Geochem. Health 2005, 27, 501–511. [Google Scholar] [CrossRef]
- Suwatvitayakorn, P.; Ko, M.-S.; Kim, K.-W.; Chanpiwat, P. Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ. Geochem. Health 2020, 42, 2331–2344. [Google Scholar] [CrossRef]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling Cadmium Exposures in Low- and High-Exposure Areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Swaddiwudhipong, W.; Nguntra, P.; Kaewnate, Y.; Mahasakpan, P.; Limpatanachote, P.; Aunjai, T.; Jeekeeree, W.; Punta, B.; Funkhiew, T.; Phopueng, I. Human health effects from cadmium exposure: Comparison between persons living in cadmium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J. Trop. Med. Publ. Health 2015, 46, 133–142. [Google Scholar]
- Nishijo, M.; Suwazono, Y.; Ruangyuttikarn, W.; Nambunmee, K.; Swaddiwudhipong, W.; Nogawa, K.; Nakagawa, H. Risk assessment for Thai population: benchmark dose of urinary and blood cadmium levels for renal effects by hybrid approach of inhabitants living in polluted and non-polluted areas in Thailand. BMC Public Health 2014, 14, 702–702. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-C.; Liou, X.-Y.; Ye, L.-P.; Liang, H.; Wang, Z.-Y. Spectroscopic studies on the interaction between human hemoglobin and CdS quantum dots. J. Colloid Interface Sci. 2007, 311, 400–406. [Google Scholar] [CrossRef]
- Guo, D.; Liu, R. Spectroscopic investigation of the effects of aqueous-phase prepared CdTe quantum dots on protein hemoglobin at the molecular level. J. Biochem. Mol. Toxicol. 2017, 31, 21953. [Google Scholar] [CrossRef] [PubMed]
- Sopjani, M.; Föller, M.; Dreischer, P.; Lang, F. Stimulation of Eryptosis by Cadmium Ions. Cell. Physiol. Biochem. 2008, 22, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Bissinger, R.; Abed, M.; Artunc, F. Eryptosis - the Neglected Cause of Anemia in End Stage Renal Disease. Kidney Blood Press. Res. 2017, 42, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Oguma, E.; Kayama, F. Cadmium Induces Anemia through Interdependent Progress of Hemolysis, Body Iron Accumulation, and Insufficient Erythropoietin Production in Rats. Toxicol. Sci. 2011, 122, 198–210. [Google Scholar] [CrossRef]
- Gray, R.; Stroupe, S. Kinetics and mechanism of bilirubin binding to human serum albumin. J. Biol. Chem. 1978, 253, 4370–4377. [Google Scholar] [CrossRef]
- Petersen, C.E.; Ha, C.-E.; Harohalli, K.; Feix, J.B.; Bhagavan, N.V. A Dynamic Model for Bilirubin Binding to Human Serum Albumin. J. Biol. Chem. 2000, 275, 20985–20995. [Google Scholar] [CrossRef]
- Torra, M.; To-Figueras, J.; Brunet, M.; Rodamilans, M.; Corbella, J. Total and methionein-bound cadmium in the liver and the kidney of a population in Barcelona (Spain). Bull. Environ. Contam. Toxicol. 1994, 53, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Ohta, H.; Yamaguchi, Y.; Seki, Y.; Sagi, M.; Yamazaki, K.; Sumi, Y. Age-dependent changes in metallothionein levels in liver and kidney of the Japanese. Biol. Trace Element Res. 1998, 63, 167–175. [Google Scholar] [CrossRef] [PubMed]
- DelRaso, N.J.; Foy, B.D.; Gearhart, J.M.; Frazier, J.M. Cadmium uptake kinetics in rat hepatocytes: correction for albumin binding. Toxicol. Sci. 2003, 72, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, H.; Liu, Y.; Zhang, Q. Structural changes of human serum albumin induced by calcium acetate. J. Biochem. Mol. Toxicol. 2014, 28, 281–287. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.; Jiang, L.; Song, L. New insight into molecular interaction of heavy metal pollutant—cadmium(II) with human serum albumin. Environ. Sci. Pollut. Res. 2014, 21, 6994–7005. [Google Scholar] [CrossRef]
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, K.H.; More, S.; Mortensen, A.; Naegeli, H.; Noteborn, H.; et al.; EFSA Scientific Committee Update: use of the benchmark dose approach in risk assessment. EFSA J. 2017, 15, e04658. [Google Scholar] [CrossRef] [PubMed]
- Slob, W.; Moerbeek, M.; Rauniomaa, E.; Piersma, A.H. A Statistical Evaluation of Toxicity Study Designs for the Estimation of the Benchmark Dose in Continuous Endpoints. Toxicol. Sci. 2005, 84, 167–185. [Google Scholar] [CrossRef]
- Slob, W.; Setzer, R.W. Shape and steepness of toxicological dose–response relationships of continuous endpoints. Crit. Rev. Toxicol. 2014, 44, 270–297. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, T.; Jelsovsky, J.Z. Bootstrap Estimation of Benchmark Doses and Confidence Limits with Clustered Quantal Data. Risk Anal. 2007, 27, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Baker, J.R.; Reilly, P.E.B.; Moore, M.R.; Williams, D.J. Cadmium Levels in the Lung, Liver, Kidney Cortex, and Urine Samples from Australians without Occupational Exposure to Metals. Arch. Environ. Health Int. J. 2002, 57, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lyon, T.D.B.; Aughey, E.; Scott, R.; Fell, G.S. Cadmium concentrations in human kidney in the UK: 1978-1993. J. Environ. Monit. 1999, 1, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, J.L.; Samuel, O.; Dewailly, E.; Gingras, S.; Lefebvre, M.A. Levels of cadmium in kidney and liver tissues among a Canadian population (province of Quebec). J. Toxicol. Environ. Health, 1999, 56, 145–163. [Google Scholar] [CrossRef]
- Johansen, P.; Mulvad, G.; Pedersen, H.S.; Hansen, J.C.; Riget, F. Accumulation of cadmium in livers and kidneys in Greenlanders. Sci. Total. Environ. 2006, 372, 58–63. [Google Scholar] [CrossRef]
- Barregard, L.; Fabricius-Lagging, E.; Lundh, T.; Mölne, J.; Wallin, M.; Olausson, M.; Modigh, C.; Sallsten, G. Cadmium, mercury, and lead in kidney cortex of living kidney donors: Impact of different exposure sources, Environ. Res. 2010, 110, 47–54. [Google Scholar] [CrossRef]
- Schaefer, H.R.; Flannery, B.M.; Crosby, L.M.; Pouillot, R.; Farakos, S.M.S.; Van Doren, J.M.; Dennis, S.; Fitzpatrick, S.; Middleton, K. Reassessment of the cadmium toxicological reference value for use in human health assessments of foods. Regul. Toxicol. Pharmacol. 2023, 144, 105487. [Google Scholar] [CrossRef]
- Fleming, R.E.; Bacon, B.R. Orchestration of Iron Homeostasis. N. Engl. J. Med. 2005, 352, 1741–1744. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 373, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Kim, N.-S.; Jaar, B.G.; Schwartz, B.S.; Parsons, P.J.; Steuerwald, A.J.; Todd, A.C.; Simon, D.; Lee, B.-K. Associations of low-level urine cadmium with kidney function in lead workers. Occup. Environ. Med. 2011, 68, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhu, X.; Shrubsole, M.J.; Yu, C.; Xia, Z.; Dai, Q. Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003–2012. Environ. Int. 2018, 121, 1355–1362. [Google Scholar] [CrossRef]
- Buser, M.C.; Ingber, S.Z.; Raines, N.; Fowler, D.A.; Scinicariello, F. Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012. Int. J. Hyg. Environ. Health 2016, 219, 261–267. [Google Scholar] [CrossRef]
- Roels, H.; Bernard, A.M.; Cardenas, A.; Buchet, J.P.; Lauwerys, R.R.; Hotter, G.; Ramis, I.; Mutti, A.; Franchini, I.; Bundschuh, I. Markers of early renal changes induced by industrial pollutants. III. Application to workers exposed to cadmium. Occup. Environ. Med. 1993, 50, 37–48. [Google Scholar] [CrossRef]
- Bernard, A.; Thielemans, N.; Roels, H.; Lauwerys, R. Association between NAG-B and cadmium in urine with no evidence of a threshold. Occup. Environ. Med. 1995, 52, 177–180. [Google Scholar] [CrossRef]
- Järup, L.; Hellström, L.; Alfvén, T.; Carlsson, M.D.; Grubb, A.; Persson, B.; Pettersson, C.; Spång, G.; Schütz, A.; Elinder, C.-G. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup. Environ. Med. 2000, 57, 668–672. [Google Scholar] [CrossRef]
- Wallin, M.; Sallsten, G.; Lundh, T.; Barregard, L. Low-level cadmium exposure and effects on kidney function. Occup. Environ. Med. 2014, 71, 848–854. [Google Scholar] [CrossRef]
- Åkesson, A.; Lundh, T.; Vahter, M.; Bjellerup, P.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Strömberg, U.; Skerfving, S. Tubular and Glomerular Kidney Effects in Swedish Women with Low Environmental Cadmium Exposure. Environ. Health Perspect. 2005, 113, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Urinary Cadmium Threshold to Prevent Kidney Disease Development. Toxics 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Boonprasert, K.; Gobe, G.C.; Ruenweerayut, R.; Johnson, D.W.; Na-Bangchang, K.; Vesey, D.A. Chronic exposure to cadmium is associated with a marked reduction in glomerular filtration rate. Clin. Kidney J. 2018, 12, 468–475. [Google Scholar] [CrossRef]
- Saito, H.; Shioji, R.; Hurukawa, Y.; Nagai, K.; Arikawa, T. Cadmium-induced proximal tubular dysfunction in a cadmium-polluted area. Contrib. Nephrol. 1977, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Roels, H.A.; Lauwerys, R.R.; Buchet, J.P.; Bernard, A.M.; Vos, A.; Oversteyns, M. Health significance of cadmium induced renal dysfunction: A five year follow up. Occup. Environ. Med. 1989, 46, 755–764. [Google Scholar] [CrossRef]
- Jarup, L.; Persson, B.; Elinder, C.G. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med. 1995, 52, 818–822. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Limpatanachote, P.; Mahasakpan, P.; Krintratun, S.; Punta, B.; Funkhiew, T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: A five-year follow-up. Environ. Res. 2012, 112, 194–198. [Google Scholar] [CrossRef]
- Schnaper, H.W. The Tubulointerstitial Pathophysiology of Progressive Kidney Disease. Adv. Chronic Kidney Dis. 2017, 24, 107–116. [Google Scholar] [CrossRef]
- Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease. Toxics 2022, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Khamphaya, T.; Pouyfung, P.; Gobe, G.C.; Yimthiang, S. Estimation of the Cadmium Nephrotoxicity Threshold from Loss of Glomerular Filtration Rate and Albuminuria. Toxics 2023, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, K. Epidemiology of renal tubular dysfunction in the inhabitants of a cadmium-polluted area in the Jinzu River basin in Toyama prefecture. Tohoku J. Exp. Med. 1987, 152, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Aoshima, K.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Hosoi, Y.; Katoh, T.; Kayama, F. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int. Arch. Occup. Environ. Health 2010, 83, 953–970. [Google Scholar] [CrossRef] [PubMed]
- JECFA. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010. In Summary and Conclusions; JECFA/73/SC; Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland. 2011. Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 12 February 2024).
- [Codex Alimentarius, CODEX STAN 193–1995, General Standard for Contaminants and Toxins in Food and Feed. Available online: http://www.fao.org/fileadmin/user_upload/livestockgov/documents/1_CXS_193e.pdf (accessed on 12 February 2024).
- Satarug, S. Is environmental cadmium exposure causally related to diabetes and obesity? Cells 2024, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, A.; Satarug, S. Toxicity Tolerance in the Carcinogenesis of Environmental Cadmium. Int. J. Mol. Sci. 2024, 25, 1851. [Google Scholar] [CrossRef]
- Nogawa, K.; Sakurai, M.; Ishizaki, M.; Kido, T.; Nakagawa, H.; Suwazono, Y. Threshold limit values of the cadmium concentration in rice in the development of itai-itai disease using benchmark dose analysis. J. Appl. Toxicol. 2017, 37, 962–966. [Google Scholar] [CrossRef]
- Nishijo, M.; Nogawa, K.; Suwazono, Y.; Kido, T.; Sakurai, M.; Nakagawa, H. Lifetime Cadmium Exposure and Mortality for Renal Diseases in Residents of the Cadmium-Polluted Kakehashi River Basin in Japan. Toxics 2020, 8, 81. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Su, J.; Bai, Z.; Li, T.; Wu, Y. Maximum cadmium limits establishment strategy based on the dietary exposure estimation: An example from Chinese populations and subgroups. Environ. Sci. Pollut. Res. 2018, 25, 18762–18771. [Google Scholar] [CrossRef]
- Wei, J.; Cen, K. Assessment of human health risk based on characteristics of potential toxic elements (PTEs) contents in foods sold in Beijing, China. Sci. Total. Environ. 2020, 703, 134747. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, J.; Li, S.; Tang, Q.; Shi, W.; Liang, Y.; Shi, G.; Qian, F. Dietary cadmium health risk assessment for the Chinese population. Environ. Sci. Pollut. Res. 2023, 30, 82421–82436. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C. The inverse association of glomerular function and urinary β2-MG excretion and its implications for cadmium health risk assessment. Environ. Res. 2019, 173, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Schardijn, G.H.C.; Statius van Eps, L.W. β2-microglobulin: Its significance in the evaluation of renal function. Kidney Int. 1987, 32, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.W., III; Chung-Park, M.; Vacca, C.V.; London, M.; Crowley, A.Q. The renal handling of beta2-microglobulin in the dog. Kidney Int. 1982, 22, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Wibell, L.; Evrin, P.-E.; Berggård, I. Serum β2-Microglobulin in Renal Disease. Nephron 1973, 10, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Wibell, L.B. Studies on β2-microglobulin in patients and normal subjects. Acta Clin. Belg. 1976, 31, 14–26. [Google Scholar]
- Wibell, L. The serum level and urinary excretion of β2-microglobulin in health and renal disease. Pathol. Biol. (Paris) 1978, 26, 295–301. [Google Scholar] [PubMed]
- Hall, P.W., III; Ricanati, E.S. Renal handling of β2-microglobulin in renal disorders with special reference to hepatorenal syndrome. Nephron 1981, 27, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Portman, R.J.; Kissane, J.M.; Robson, A.M. Use of β2 microglobulin to diagnose tubulo-interstitial renal lesions in children. Kidney Int. 1986, 30, 91–98. [Google Scholar] [CrossRef]
- Bernard, A. Renal dysfunction induced by cadmium: biomarkers of critical effects. BioMetals 2004, 17, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, K.; Kobayashi, E.; Honda, R. A study of the relationship between cadmium concentrations in urine and renal effects of cadmium. Environ. Health Perspect. 1979, 28, 161–168. [Google Scholar] [CrossRef]
- Ikeda, M.; Ezaki, T.; Moriguchi, J.; Fukui, Y.; Ukai, H.; Okamoto, S.; Sakurai, H. The threshold cadmium level that causes a substantial increase in β2-microglobulin in urine of general populations. Tohoku J. Exp. Med. 2005, 205, 247–261. [Google Scholar] [CrossRef]
- Peterson, P.A.; Evrin, P.-E.; Berggard, I. Differentiation of glomerular, tubular, and normal proteinuria: Determinations of urinary excretion of β2-microglobulin, albumin, and total protein. J. Clin. Investig. 1969, 48, 1189–1198. [Google Scholar] [CrossRef]
- Jarup, L.; Persson, B.; Elinder, C.G. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med. 1995, 52, 818–822. [Google Scholar] [CrossRef]
- Elinder, C.G.; Edling, C.; Lindberg, E.; Kagedal, B.; Vesterberg, O. Assessment of renal function in workers previously exposed to cadmium. Occup. Environ. Med. 1985, 42, 754–760. [Google Scholar] [CrossRef]
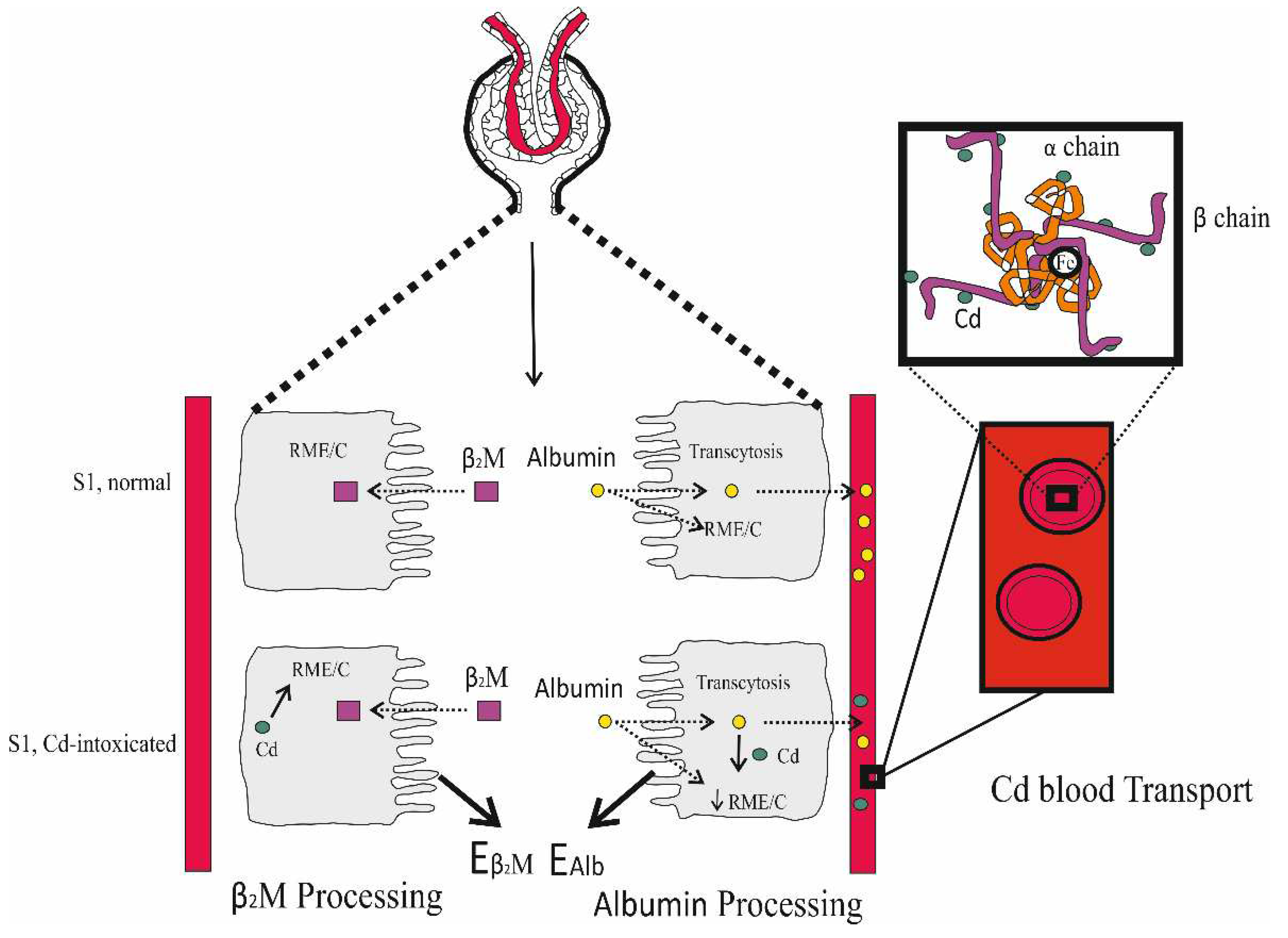
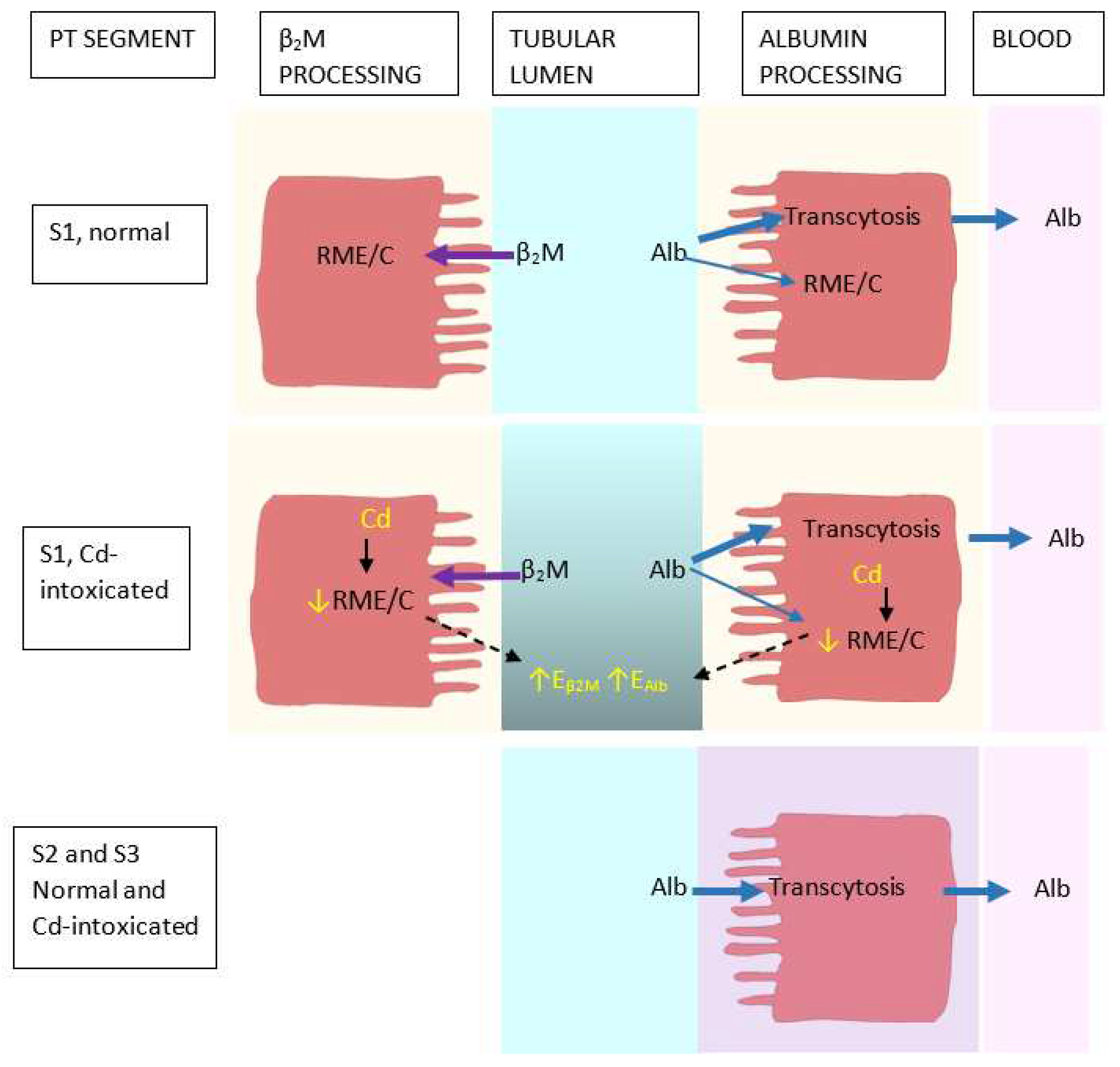
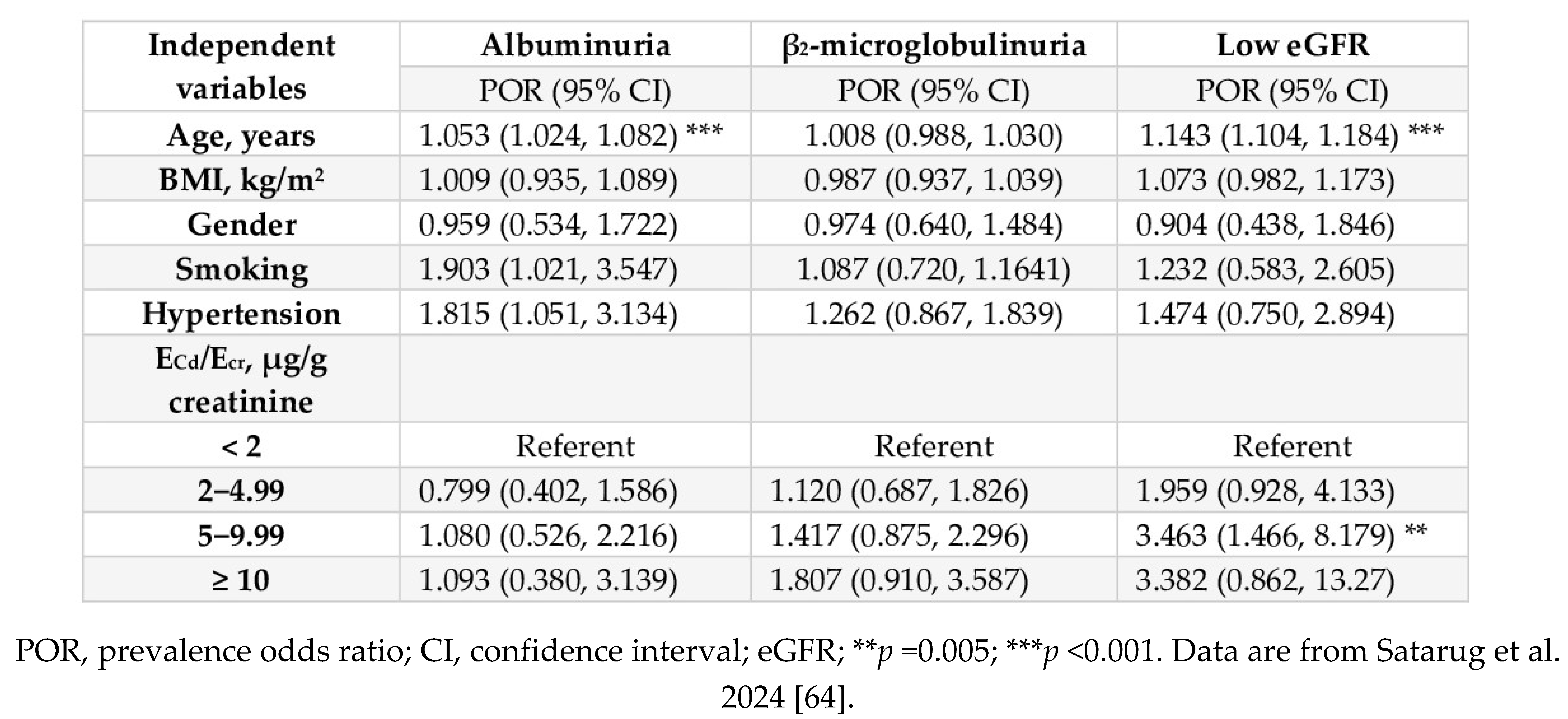
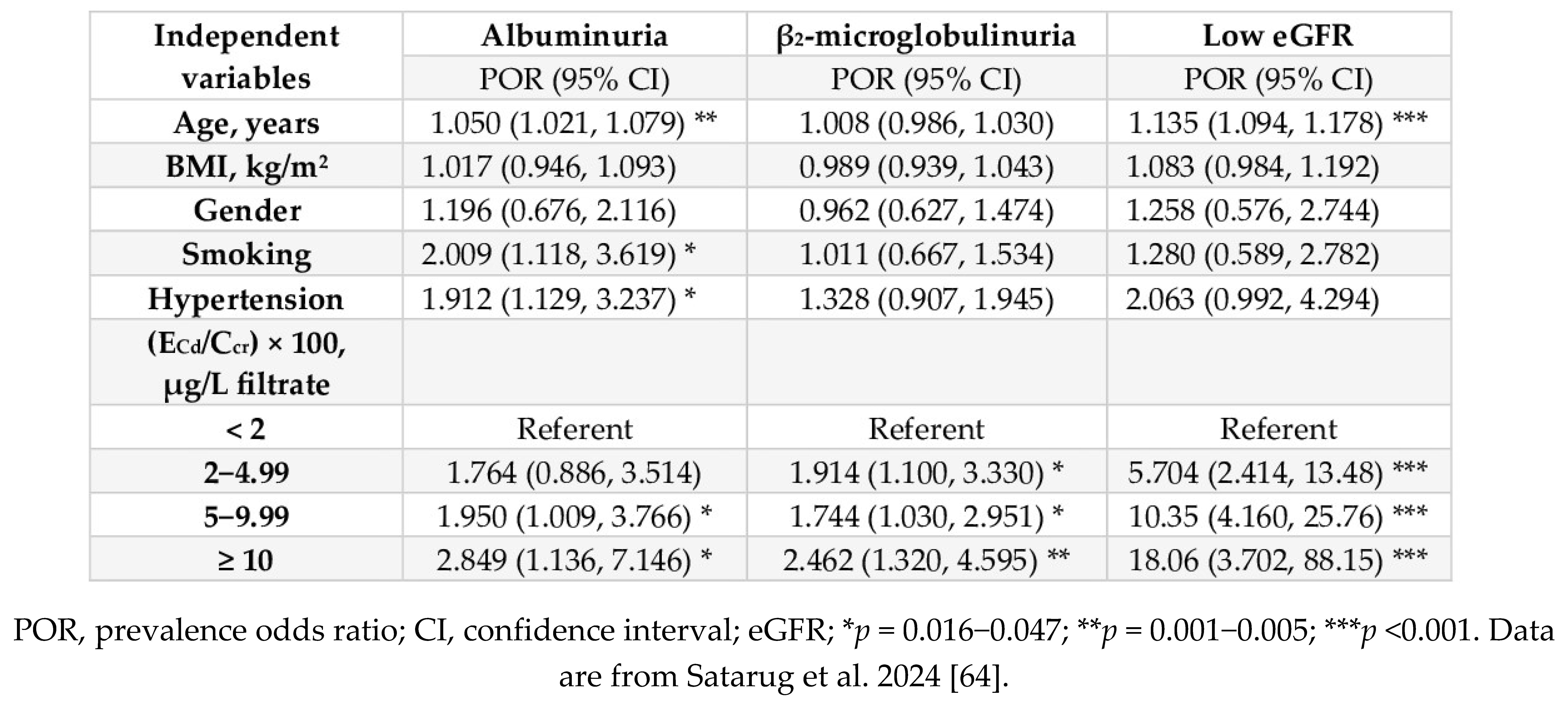
| Study Location | Cadmium Exposure Metrics and Effects Observed | Reference |
|---|---|---|
| Thailand, n 1189 16−87 years mean age 43.2 years |
Risk of low eGFRa increased 6.2-fold and 10.6-fold, comparing urinary Cd levels 0.38–2.49 and ≥ 2.5 µg/g creatinine with ≤ 0.37 µg/g creatinine, respectively. | Satarug et al. 2022 [38]. |
| Korea, n 2992 20–65 years |
Increased risk of low eGFR (OR 1.97) in women was associate with blood Cd levels > 1.74 μg/L. | Myong et al. 2012 [39] |
| Korea, n 2005 ≥ 20 years |
Increased risk of low eGFR (OR 1.93) was associated with blood Cd in the top quartile (mean, 2.08 μg/L). | Chung et al. 2014 [40] |
| Taiwan, n 2447 mean age 55.1 years |
Increased risk of proteinuria was associated with urinary Cd (OR 2.67) and copper (OR 1.94). Mean urinary Cd in subjects with proteinuria (1.1 μg/L) was 27.3% higher than those without proteinuria. | Tsai et al. 2021 [41] |
| China n 683 (64.7% women) mean age 57.4 years |
Risk of elevated albumin excretion increased 2.98-fold, comparing urinary Cd levels ≤ 0.32 with > 1.72 µg/g creatinine. | Feng et al. 2022 [42] |
| Spain, n 1397 age 18–85 years |
Increased risks of albuminuriab by1.58-fold and 4.54-fold were associated with urinary Cd levels > 0.27 and > 0.54 µg/g creatinine, respectively | Grau-Perez et al. 2017 [43] |
| United States NHANES 1999 − 2006 n 14,778, ≥ 20 years |
Blood Cd levels ≥ 0.6 μg/L were associated with low eGFR (OR 1.32), albuminuria (OR 1.92) and low eGFR plus albuminuria (OR 2.91) | Navas-Acien et al. 2009 [44] |
| United States NHANES 1999 − 2006 n 5426, ≥ 20 years |
Blood Cd levels > 1 µg/L plus urinary Cd levels > 1 µg/g creatinine was associated with albuminuria (OR 1.63). Blood Cd levels > 1 µg/L were associated with low eGFR (OR 1.48) and albuminuria (OR 1.41). |
Ferraro et al. 2010 [45] |
| United States NHANES 2007 − 2012 n 12,577, ≥ 20 years |
Blood Cd levels > 0.61 μg/L were associated with low eGFR (OR 1.80) and albuminuria (OR 1.60). eGFR reduction due to Cd was more pronounced in the diabetics, hypertensive, or both |
Madrigal et al. 2019 [46] |
| United States NHANES 2009 − 2012 n 2926, ≥ 20 years |
Urinary Cd levels > 0.220 μg/L were associated with elevated albumin excretion, compared with urinary Cd levels < 0.126 μg/L. Blood Cd levels > 0.349 μg/L were associated with elevated albumin excretion, compared with blood Cd levels < 0.243 μg/L |
Zhu et al. 2019 [47] |
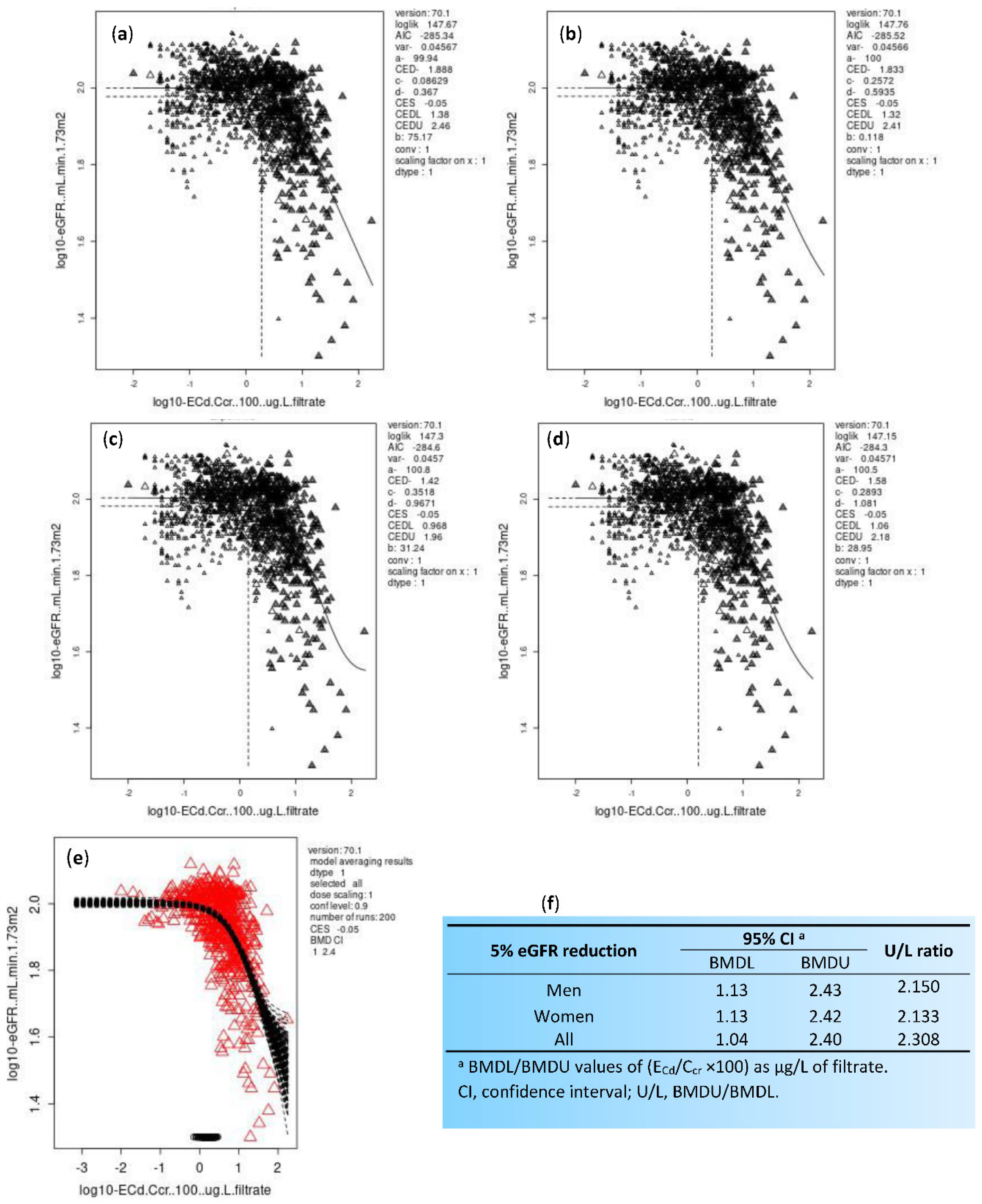
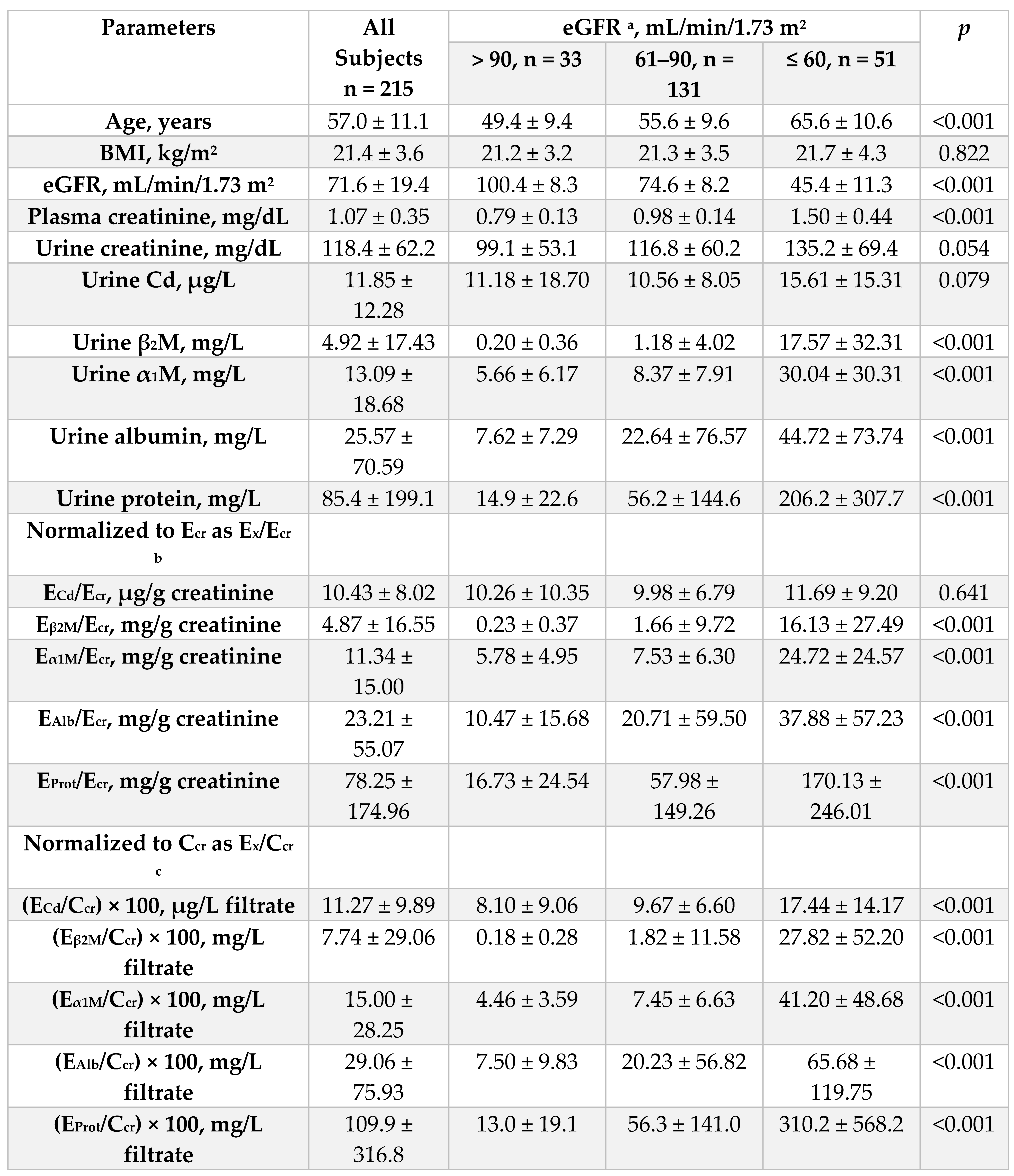 |
 |
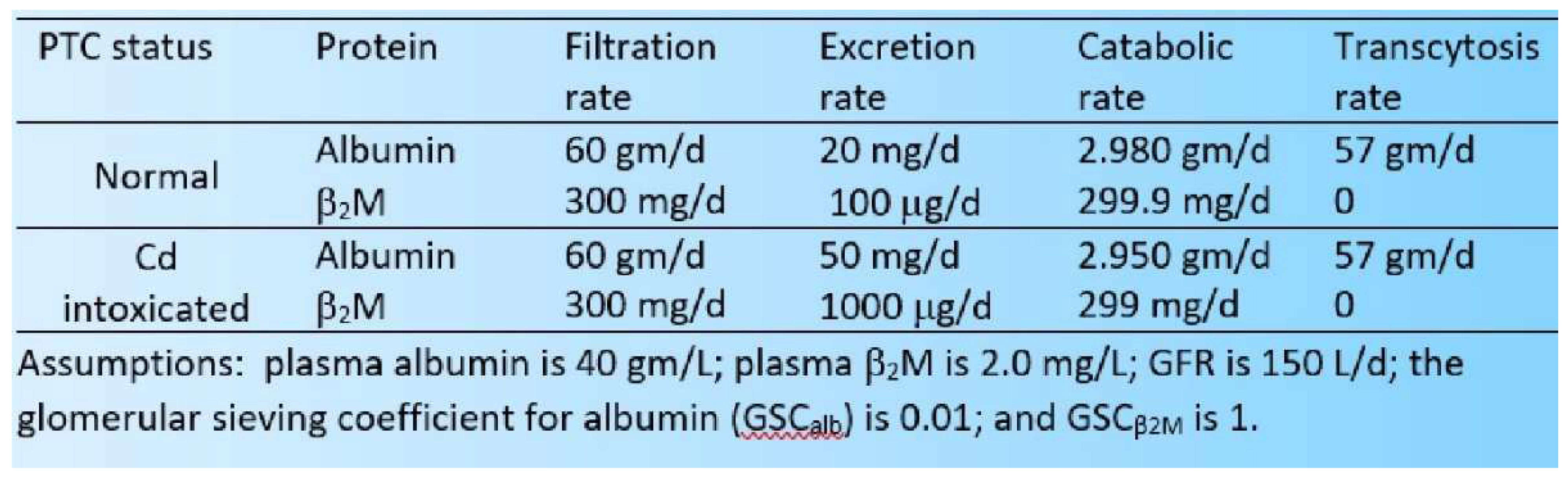
 |
 |
| Country of Origin | Cadmium content, µg/g wet tissue weight | Reference |
|---|---|---|
| Australia, Autopsy, n 61, 2–89 years |
The percentage of kidney Cd content ≥ 50 µg/g was 3.3%.a Mean lung, liver and kidney Cd were 0.13, 0.95, and 15.45 µg/g, respectively. Mean kidney Cd was 16 times higher than liver. Peak hepatic and renal Cd levels were 1.5 and 25.9 µg/g. |
Satarug et al. [126] |
| United Kingdom, Autopsy, n 2700, nationwide (1978−1993) |
The percentage of kidney Cd content ≥ 50 µg/g was 3.9%. Mean kidney Cd content was 19 µg/g. Peak renal Cd level was 23 µg/g. |
Lyon et al. [127] |
| Canada (Quebec) Autopsy, n 314 |
Respective mean liver (kidney) Cd in smokers, ex-smokers and non-smokers were 2.5 (34.5), 1.4 (20.3) and 0.7(7.0) µg/g. Mean liver Cd in female smokers was higher than male smokers (3.6 vs. 2.2 µg/g). Peak hepatic and renal Cd levels were 2.2 and 44.2 µg/g. |
Benedetti et al. [128] |
| Greenland [101] Autopsy, n 95, 19−89 years |
Mean (range) liver Cd content was 5.3 (0.3−24.3) μg/g. Mean (range) kidney Cd content was 43.8 (6.7−126) μg/g. Peak hepatic and renal Cd levels were 1.97 and 22.3 μg/g. |
Johansen et al. [129] |
| Sweden Kidney transplant donors, n 109, 24−70 years, median age 51. |
Median kidney Cd was 12.9 μg/g. In non-smokers, renal Cd accumulation rate was 3.9 μg/g in every 10-year increase in age. An additional 3.7 μg/g accumulation rate in every 10-year smoking. In women who had serum ferritin levels ≤ 20 µg/L (depleted iron stores), renal Cd accumulation rate was 4.5 μg/g in every 10-year increase in age. |
Barregard et al. [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




