Submitted:
22 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction.
1.1. The Importance of autism spectrum disorder (ASD) as a mental health comorbidity in adolescent girls and young women.
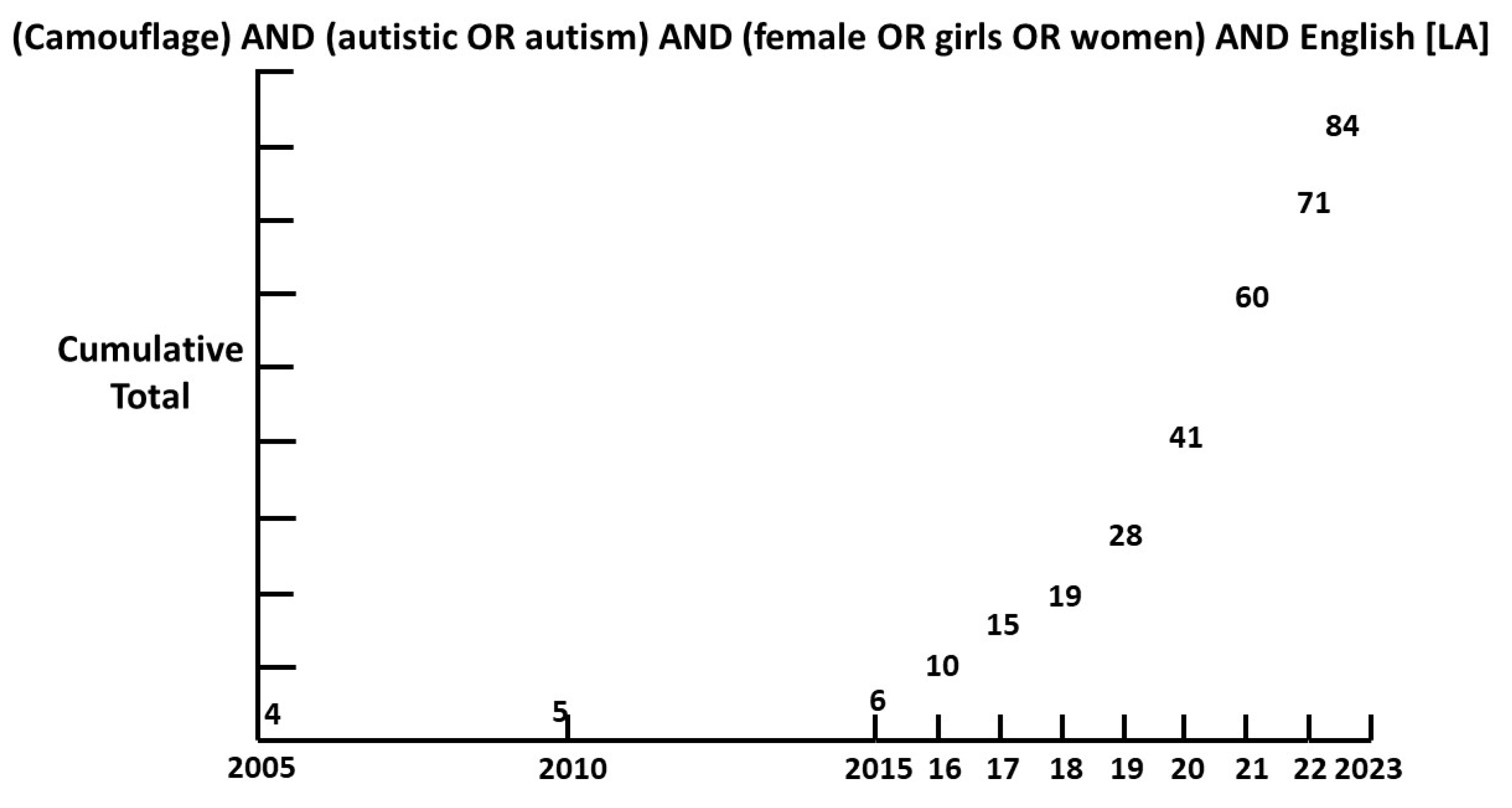
1.2. Camouflage.
1.3. Neurodiversity.
1.4. ASD and Attention Deficit Hyperactivity Disorder (ADHD).
1.5. Guidelines and ASD.
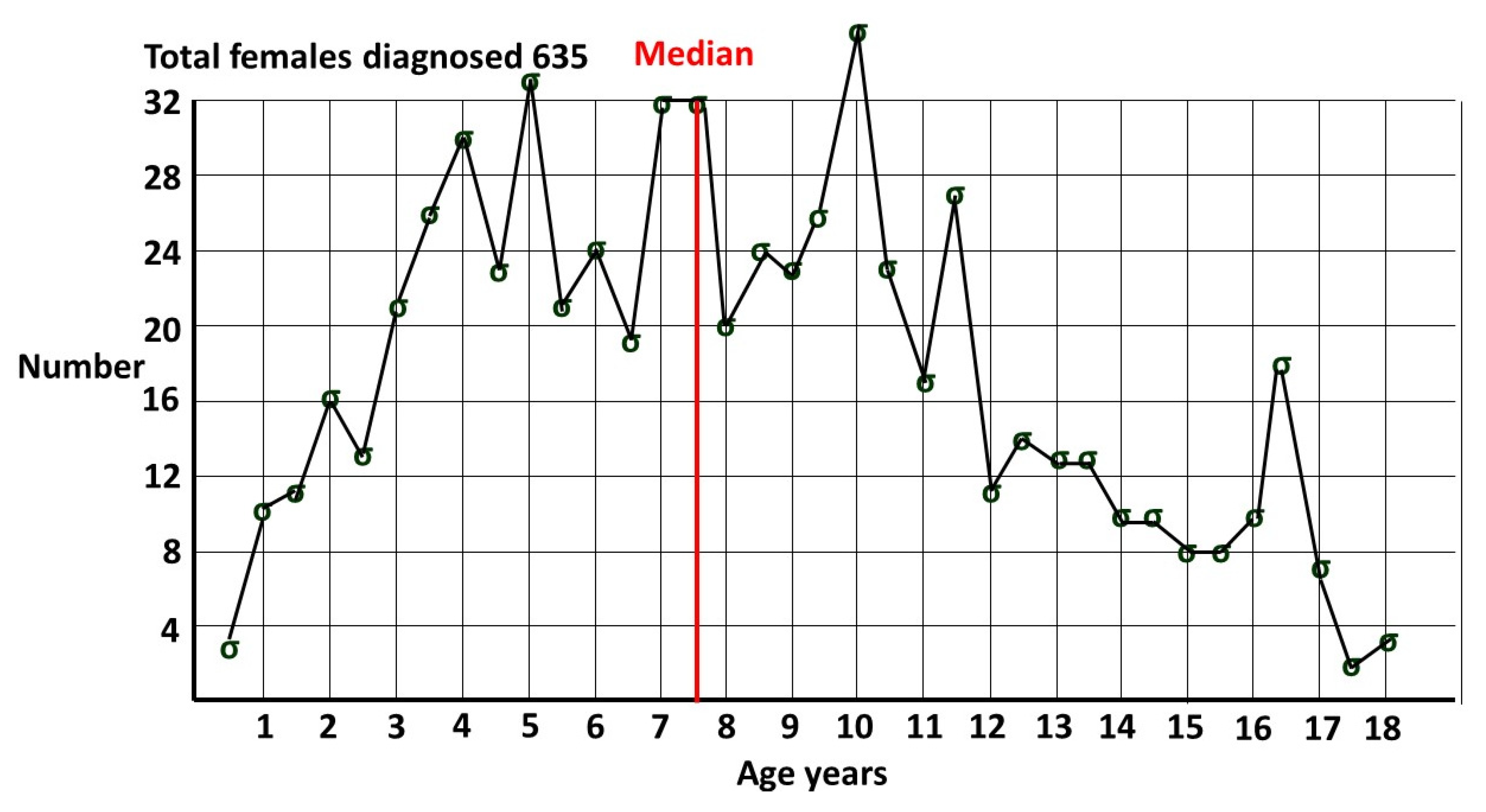
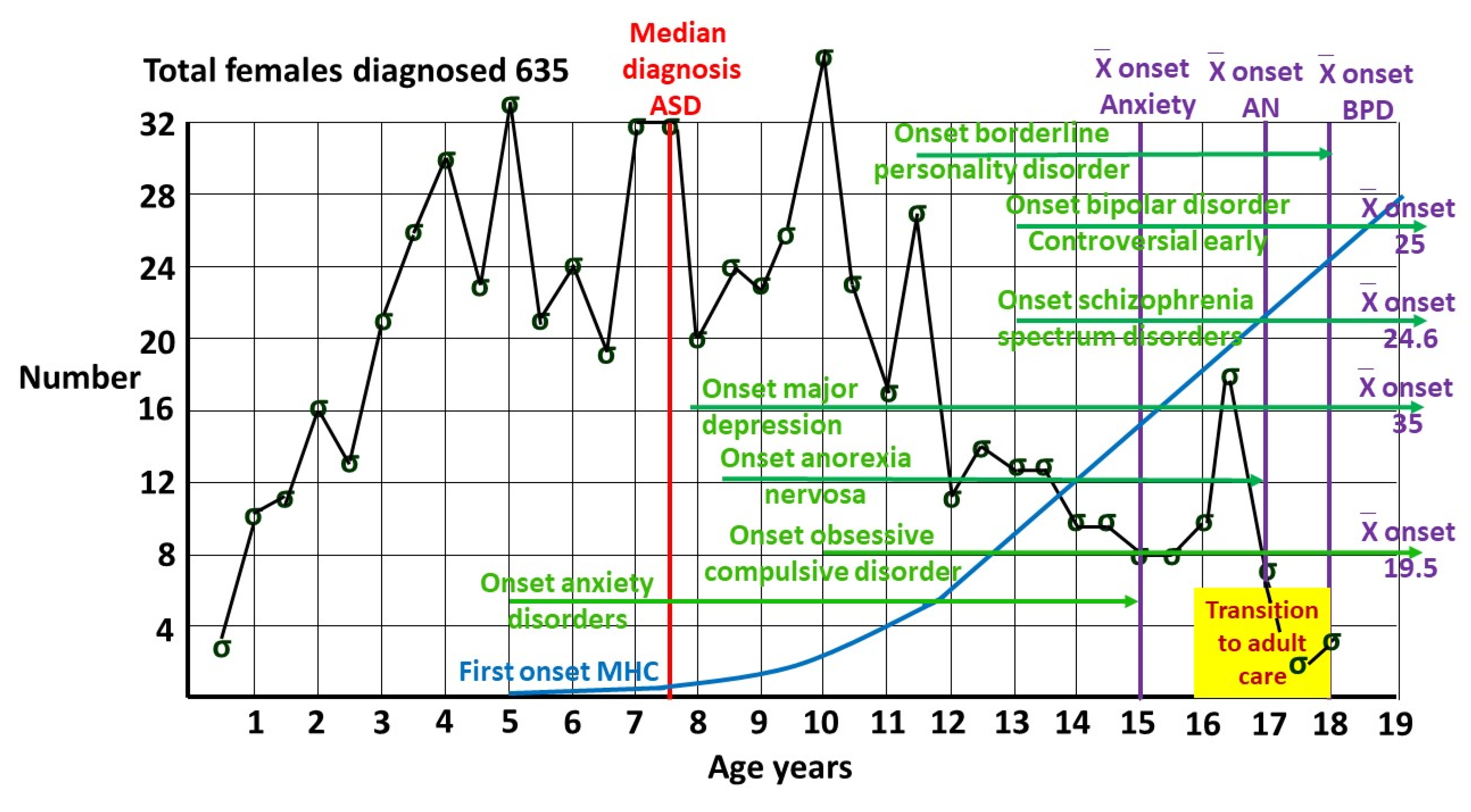
1.3. Prevalence of female ASD.
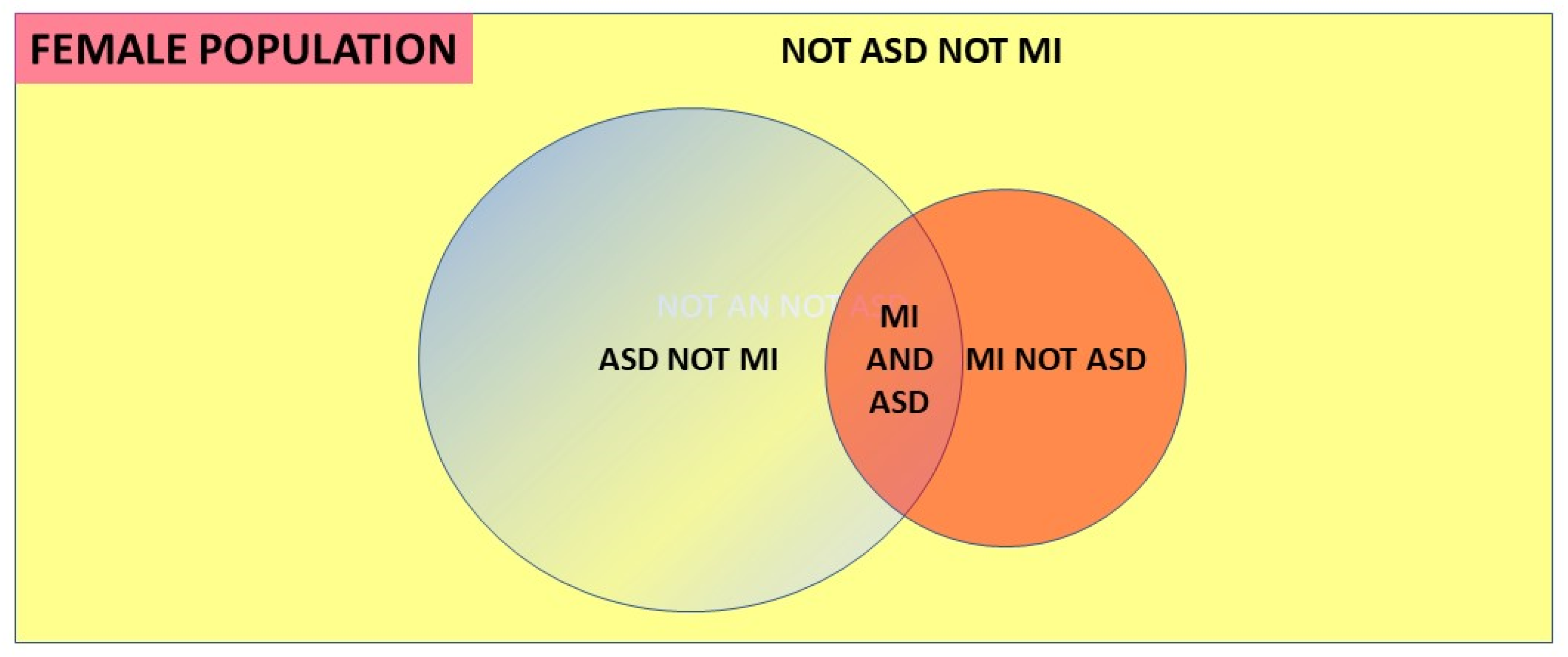
- A discrete comorbid mental illness population (MI) with a known prevalence, with a diagnosed ASD population where the comorbid MI proportion is known, and where ASD in the MI population is carefully looked for. It will be reliably diagnosed because the major bias is initially recognizing that ASD must be sought, rather than in the diagnostic process itself [2].
- A total population with a discrete population of diagnosed ASD where there is an easily recognized comorbidity and the proportion of the comorbid condition in that ASD population is known, together with the prevalence of the comorbid condition in the balance of the population without ASD [13] eg the Danish [44] and Swedish [45] national registers including ASD and anorexia nervosa (AN).
1.6. Conditional probability and Bayes’ theorem.
“… you should say what you mean,” the March Hare went on.“I do,” Alice hastily replied; “at least—at least I mean what I say—that’s the same thing, you know.”“Not the same thing a bit!” said the Hatter. “You might just as well say that ‘I see what I eat’ is the same thing as ‘I eat what I see’!”“You might just as well say,” added the March Hare, “that ‘I like what I get’ is the same thing as ‘I get what I like’!”“You might just as well say,” added the Dormouse, who seemed to be talking in his sleep, “that ‘I breathe when I sleep’ is the same thing as ‘I sleep when I breathe’!”
2. Methods and results.
2.1. Context.
- P(ASD|MI) may have been directly measured. For the reasons described this is uncommon.
- It is relatively common to find values for P(MI|ASD) because in the adolescent literature it has long been known comorbid mental illness is common in this ASD population. In the adult literature researchers with a primary ASD focus are now assessing for comorbidities. Bayes’ theorem can be used if we know P(MI|ASD), P(ASD) and P(MI).
- If a hazard ratio is available:
2.2. P(ASD|MI) for selected mental illnesses in adolescents and young adults.
- 1.
- Anorexia nervosa (AN).
- 2.
- Schizophrenia spectrum disorder (SSD).
= 0.434
- 3.
- Borderline personality disorder (BPD).
- 4.
- Bipolar disorder (BP).
- 5.
- Depression (DP).
- 6.
- Anxiety disorders (ANX)
- 7.
- Obsessive compulsive disorder (OCD)
- 8.
- Social anxiety disorder (SA)
- 9.
- Perinatal depression (PND).
= 0.60 x 0.060/0.115
= 0.313
- 10.
- Post traumatic stress disorder (PTSD)
= 0.60 x 0.06/0.12
= 0.30
- 11.
- Any mental health disorder (MI)
2.3. Attempted suicide.
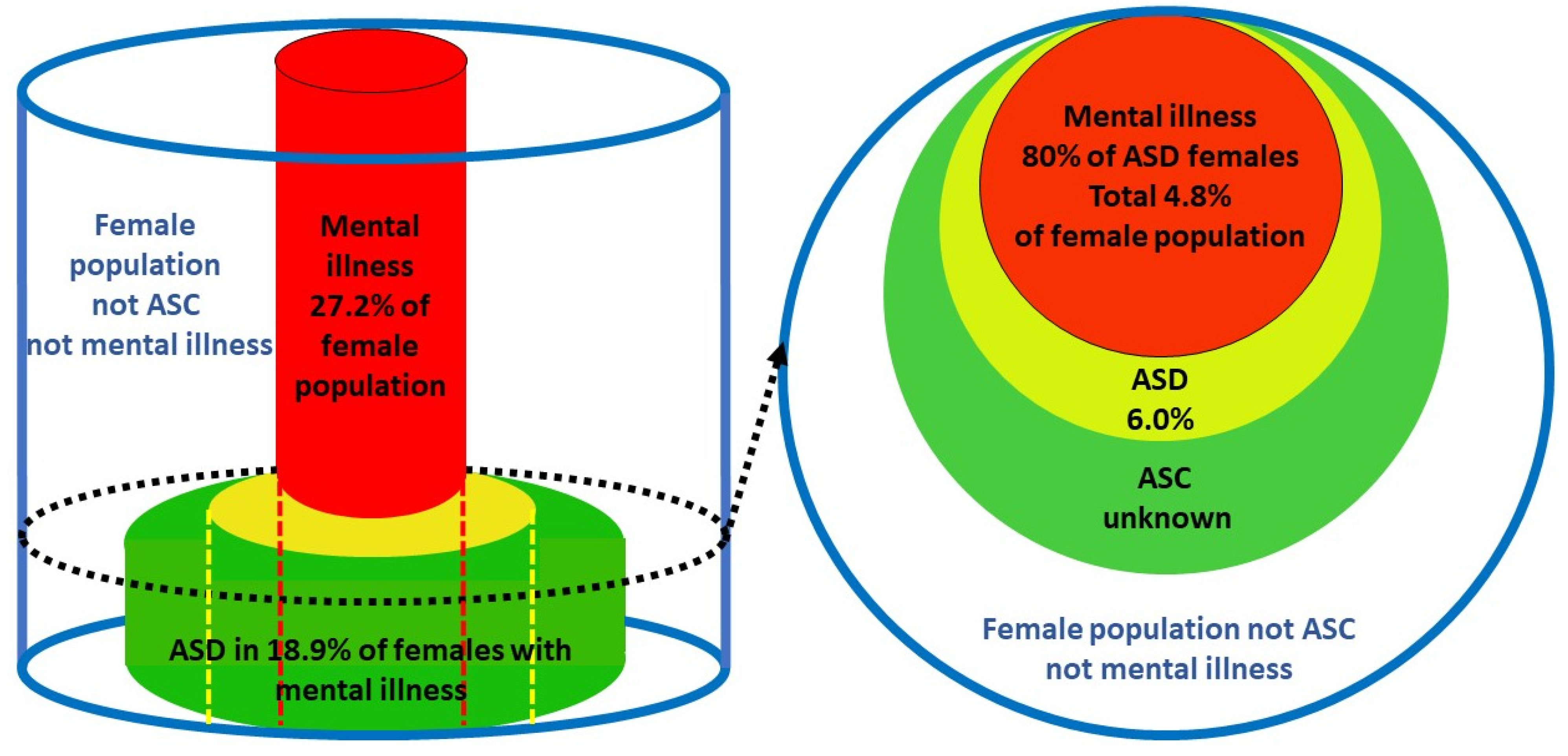
2.4. Validation of the female prevalence value for P(ASD).
= 0.189 x 0.272/0.80
= 0.064
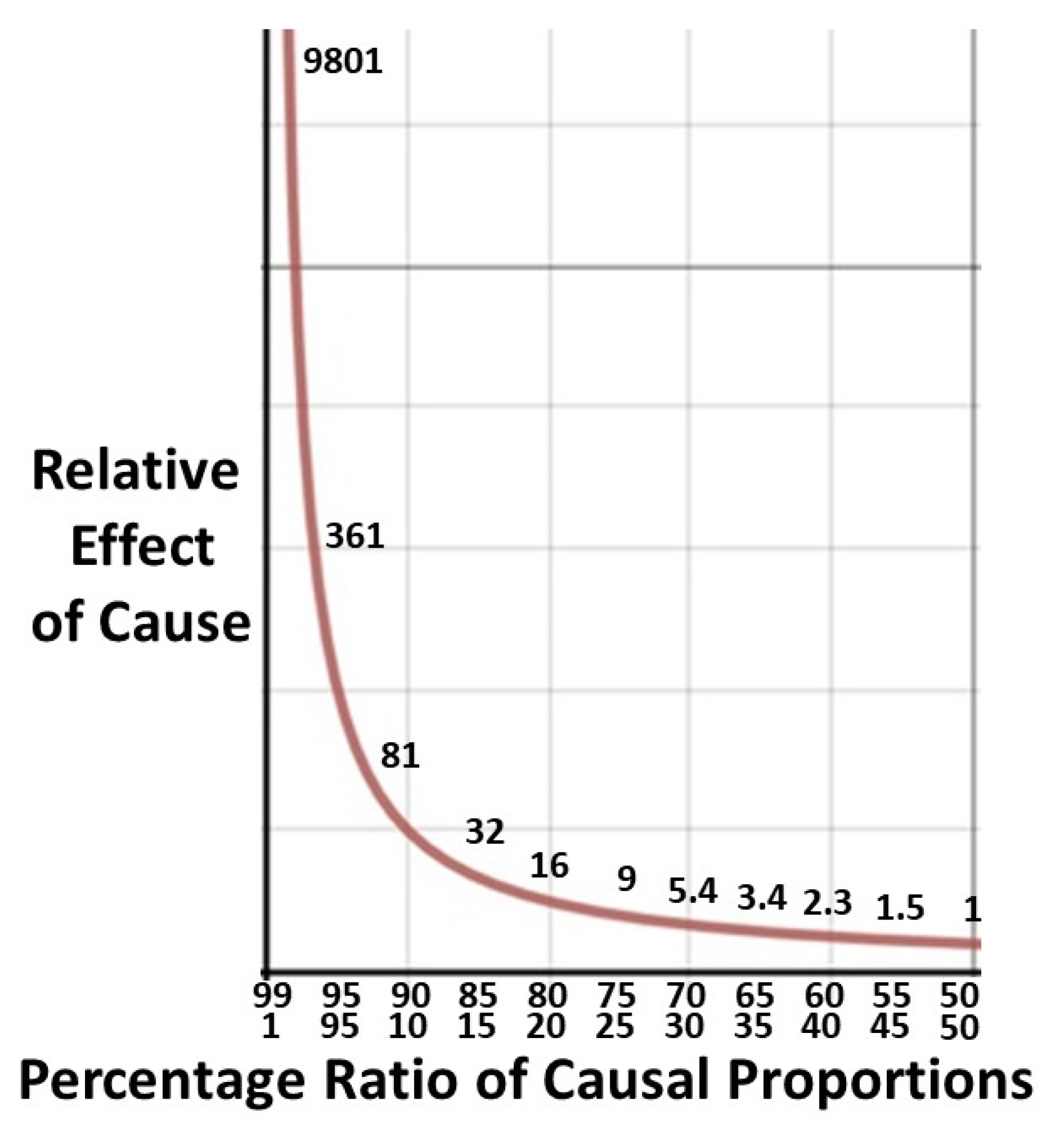
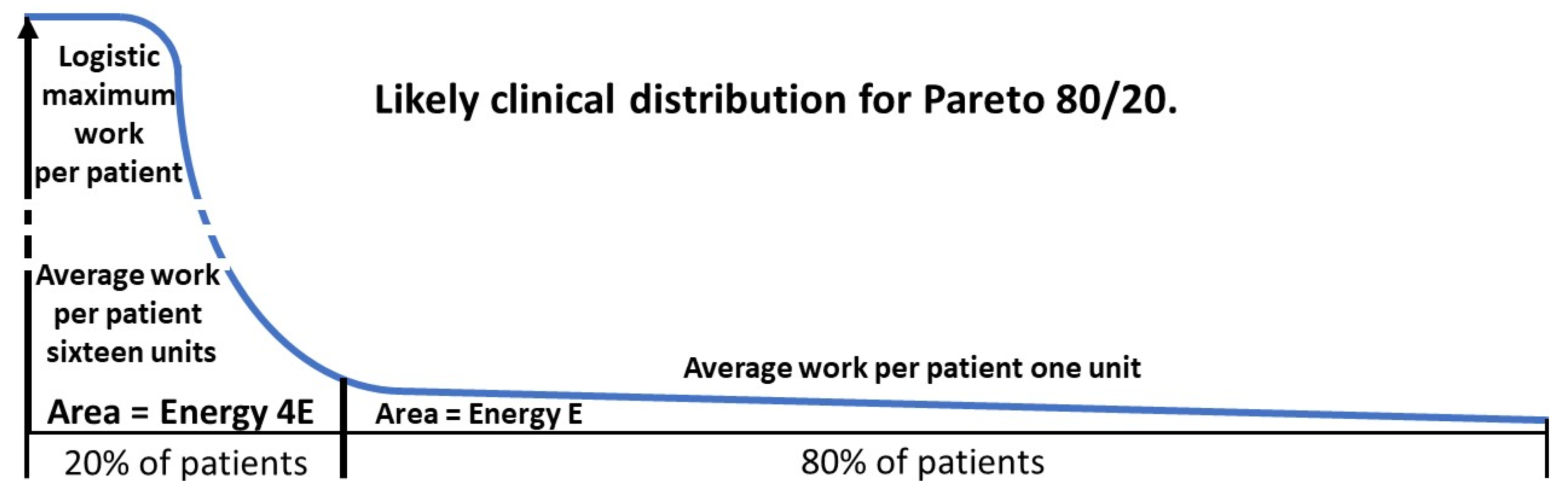
2.5. Degree of benefit: Pareto calculations.
2.5.1. The Pareto principle in health.
2.5.2. Derivation of Pareto formulae.
2.5.3. A worked example.
= 76.4
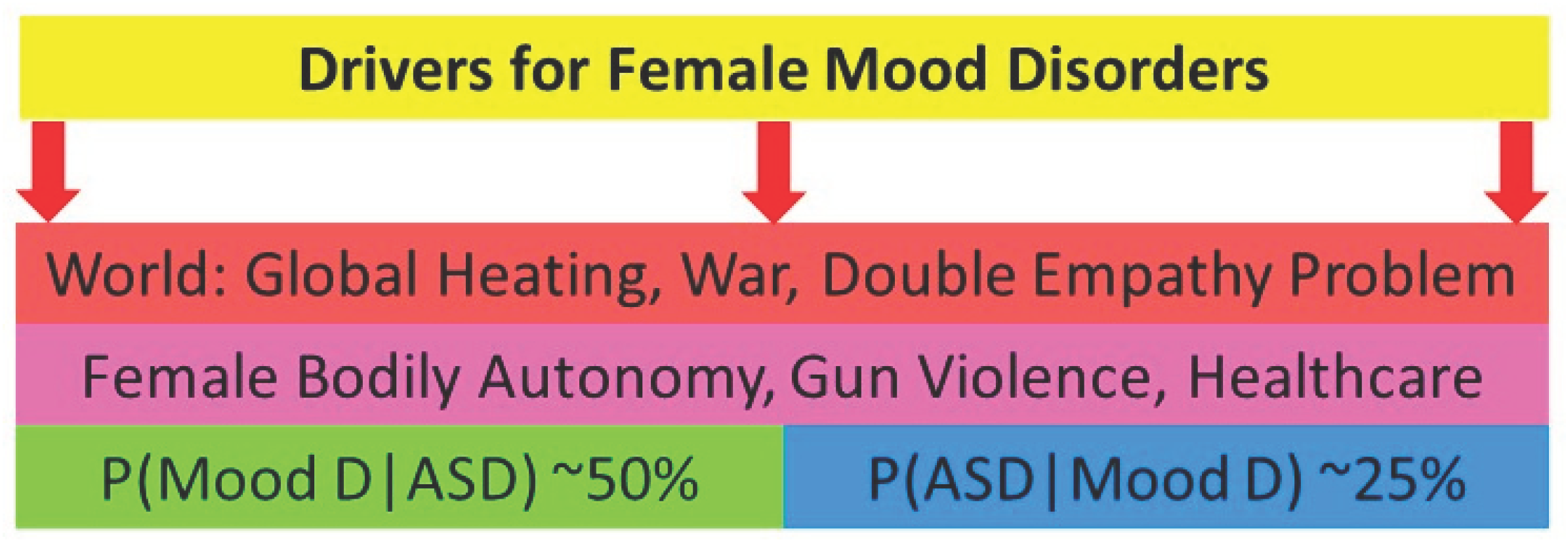
2.5.4. Downstream effects.
3. Discussion.
3.1. Stumbling blocks.
- Services are accessed by only 1/5 of autistic youth.
- About 70% of pediatricians do not support youth during the transition process and >50% of families lack information on how to proceed.
- Little is still known about the effects of comorbid MI. Adult physicians need to monitor ongoing symptoms of ASD-which can intensify and diminish-to guide diagnosis and treatment choices.
- Specific barriers include a shortage of health care services, poor physician knowledge, cost of services, lack of family and individual knowledge, stigma and language barriers.
3.2. A final word on conditional probability.
3.3. Effective therapy.
- Recognize that autistic people are often more deliberative and less intuitive than neurotypical individuals [99].
- Cognitive behavior therapy can be adapted to the core features of ASD [100].
- In designing therapy improvements researchers with lived experience of autism should be involved [101]. We would add autistic clinicians, patients and parents.
-
Specific areas for therapists listed by Gilmore et al. include [102]:
- Be a change agent in the mental health workplace.
- Make thoughtful language choices.
- Individualize treatment.
- Leverage patient strengths.
- Agree on practical goals to navigate life situations.
3.4. The art of the possible.
3.5. It takes a village.
3.6. The long view.
4. Limitations
5. Future Directions.
6. Conclusions.
- The prevalence of ASD in female mental illness is high and ASD must be diligently sought both prior to and after the transition to adult care.
- The solution to facilitating this transition lies within the health system in terms of establishing the extent of the problem and transmitting the information.
- The overall risk in extent and severity of outcome is much higher than generally believed.
- Necessary funding eventually follows convincing evidence.
- Effective therapy is quite feasible. A lot of energy is being wasted on ineffective therapy due to the lack of an ASD diagnosis. That wasted energy can be redirected to effective modes of management with a consequent large positive effect on the relevant system.
- The key to effective diagnosis and therapy is listening, understanding and empathizing with neurodiverse individuals.
- The overall improvement in female mental health and prevention of the downstream effects of mental illness will be substantial.
Funding
Institutional Review Board Statement
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Burrows, C.A.; Grzadzinski, R.L.; Donovan, K.; Stallworthy, I.C.; Rutsohn, J.; St. John, T.; Marrus, N.; Parish-Morris, J.; MacIntyre, L.; Hampton, J.; Botteron, K.N.; Dager, S.R.; Hazlett, H.C.; Pandey, J.S.; Shen, M.D,; Estes, A.M.; Pruett Jr., J.R.; Schultz, R.T.; Zwaigenbaum, L.; Truong, K.N.; Piven, J.; Elison, J.T. A data-driven approach in an unbiased sample reveals equivalent sex ratio of autism spectrum disorder–associated impairment in early childhood. Biol. Psychiatry. 2022; 92:654–662. [CrossRef]
- McCrossin, R. Finding the true number of females with autistic spectrum disorder by estimating the biases in initial recognition and clinical diagnosis. Children. 2022; 9:272. [CrossRef]
- Rodgaard, E-M.; Jensen, K.; Miskowiak, K.W.; Mottron L. Autism comorbidities show elevated female-to-male odds ratios and are associated with the age of first autism diagnosis. Acta Psychiatr. Scand. 2021; 144:475–486. [CrossRef]
- Gesi, C.; Migliarese, G.; Torriero, S.; Capellazzi, M.; Omboni, A.C.; Cerveri, G.; Mencacci, C. Gender differences in misdiagnosis and delayed diagnosis among adults with autism spectrum disorder with no language or intellectual disability. Brain Sci. 2021; 11:912. [CrossRef]
- Downs, J.M.; Lehler, S.; Dean, H.; Sears, N.; Patel, R.; Shetty, H.; Simonoff, E.; Hotopf, M.; Ford, T.J.; Diaz-Caneja, C.M.; Arango, C.; MacCabe, J.H.; Hayes, R.D.; Pina-Camacho, L. The association between comorbid autism spectrum disorders and antipsychotic treatment failure in early-onset psychosis: a historical cohort using electronic health records. J. Clin. Psychiatry. 2017; 78:e1233-e1241. [CrossRef]
- Kinnaird, E.; Norton, C.; Tchanturia, K. Clinicians’ views on working with anorexia nervosa and autism spectrum disorder comorbidity: a qualitative study. BMC Psychiatry. 2017; 17:292. [CrossRef]
- Kinnaird, E.; Oakley, M.; Lawrence, V.; Shergill, S.; Tchanturia, K. A peer interview qualitative study exploring support for carers of people with comorbid autism and eating disorders. J. Eat. Disord. 2021; 9:42. [CrossRef]
- Leppanen, J.; Sedgewick, F.; Halls D.; Tchanturia K. Autism and anorexia nervosa: Longitudinal prediction of eating disorder outcomes. Front. Psychiatry. 2022; 13:985867. [CrossRef]
- Nazar, B.P.; Peynenburg, V.; Rhind, C.; Hibbs, R.; Schmidt, U.; Gowers, S.; Macdonald, P.; Goddard, E.; Todd, G.; Micali, N.; Treasure J. An examination of the clinical outcomes of adolescents and young adults with broad autism spectrum traits and autism spectrum disorder and anorexia nervosa: a multi centre study. Int. J. Eat. Disord. 2018; 51:174–179. [CrossRef]
- El Baou, C.; Bell, G.; Saunders. R.; Buckman, J.E.J.; Mandy, W.; Dagnan, D.; O’Nions, E.; Pender, R.; Clements, H.; Pilling, S.; Richards, M.; John, A.; Stott, J. Effectiveness of primary care services for treating depression and anxiety in autistic adults in England: a retrospective, matched, observational cohort study of national health-care records. Lancet Psychiatry. 2023; 10: 944–54. [CrossRef]
- Secci, I.; Petigas, L.; Cuenod, A.; Klauser, P.; Kapp, C.; Novatti, A.; Armando, M. Case report: treatment-resistant depression, multiple trauma exposure and suicidality in an adolescent female with previously undiagnosed autism spectrum disorder. Front. Psychiatry. 2023; 14:1151293. [CrossRef]
- Cassidy, S.A.; Gould, K.; Townsend, E.; Pelton M.; Robertson, A.E.; Rodgers J. Is camouflaging autistic traits associated with suicidal thoughts and behaviours? Expanding the interpersonal psychological theory of suicide in an undergraduate student sample. J. Autism Dev. Disord. 2020; 50:3638–3648. [CrossRef]
- McCrossin, R. Finding the proportion of females with autistic spectrum disorder who develop anorexia nervosa, the true prevalence of female ASD and its clinical significance. Children. 2023; 1:272. [CrossRef]
- Yanai, I.; Lercher M. A hypothesis is a liability. Genome Biology. 2020; 21:231. [CrossRef]
- Hernan, M.A.; Greenland S. Why stating hypotheses in grant applications is unnecessary. JAMA. 2024; E1-E2. [CrossRef]
- Harmens, M.; Sedgewick, F.; Hobson, H. Autistic women’s diagnostic experiences: interactions with identity and impacts on well-being. Women’s Health. 2022; 18:1–11. [CrossRef]
- Bargiela, S.; Steward, R.; Mandy, W. The experiences of late-diagnosed women with autism spectrum conditions: an investigation of the female autism phenotype. J. Autism Dev. Disord. 2016; 46:3281–3294. [CrossRef]
- Hull, L.; Petrides, K.V.; Allison, C.; Smith, P.; Baron-Cohen, S.; Lai, M.C.; Mandy W. “Putting on my Best normal”: social camouflaging in adults with autism spectrum conditions. J. Autism Dev. Disord. 2017; 47:2519–2534. [CrossRef]
- Zubia, A.G.; y Carrillo Saucedo, I.C. Exploración del enmascaramiento en mujeres autistas y la presencia de sintomatología ansiosa y depresiva. Ehquidad. International Welfare Policies and Social Work Journal. 2023; 20:99-110. [CrossRef]
- Wing, L. Sex ratios in early childhood autism and related conditions. Psychiatry Research. 1981; 5:129-137. [CrossRef]
- Cage, E.; Troxell-Whitman, Z. Understanding the reasons, contexts and costs of camouflaging for autistic adults. J. Autism Dev. Disord. 2019; 49:1899–1911. [CrossRef]
- Jacobs, E.G. Bridging the neuroscience gender divide. Nature. 2023; 623:667. [CrossRef]
- Singer, J. ”why can’t you be normal for once in your life?” From a problem with no name to the emergence of a new category of difference. In: Corker M, French S. (eds). Disability Discourse. Buckingham, UK: Open University Press; 1999; 59-67.
- Mitchell, P.; Sheppard, E.; Cassidy S. Autism and the double empathy problem: implications for development and mental health. British Journal of Developmental Psychology. 2021; 39:1–18. [CrossRef]
- Babb, C.; Brede, J.; Jones, C.R.G.; Elliott, M.; Zanker, C.; Tchanturia, K.; Serpell, L.; Mandy, W.; Fox, J.R.E. ‘It’s not that they don’t want to access the support . . . it’s the impact of the autism’: The experience of eating disorder services from the perspective of autistic women, parents and healthcare professionals. Autism. 2021; 25:1409–1421. [CrossRef]
- Malik-Soni, N.; Shaker, A.; Luck, H.; Mullin, A.E.; Wiley, R.E.; Lewis, M.E.S.; Fuentes, J.; Frazier, TW. Tackling healthcare access barriers for individuals with autism from diagnosis to adulthood. Pediatric Research. 2022; 91:1028–1035. [CrossRef]
- Bernard, S.; Grosjean, B.; Caravallah, L. Neurodiversity and early autism. JAMA Pediatr. 2022; 176:1272-1273. [CrossRef]
- Hours, C.; Recasens, C.; Baleyte J-M. ASD and ADHD comorbidity: what are we talking about? Front. Psychiatry. 2022; 13:837424. [CrossRef]
- NICE guidelines 2022. www.nice.org.u,k/guidance/ng222. Accessed 01 November 2023.
- Zuckerbrot, R.A.; Cheung, A.; Jensen, P.S.; Stein, R.E.K.; Laraque D. Guidelines for adolescent depression in primary care (GLAD-PC): Part I. Practice preparation, identification, assessment, and initial management. Pediatrics. 2018; 141: e20174081. [CrossRef]
- Wiles, N.; Taylor, A.; Turner, N.; Barnes, M.; Campbell, J.; Lewis, G.; Morrison, J.; Peters, T.J.; Thomas, L.; Turner, K.; Kessler, D. Management of treatment-resistant depression in primary care: a mixed-methods study. Br. J. Gen. Pract. 2018; e673-e681. [CrossRef]
- White, M.J. Treatment-resistant depression: consider autism. Br. J. Gen. Pract. 2019; 14. [CrossRef]
- Chen, M-H. Postpartum depression and psychosis and subsequent severe mental illness in mothers and neurodevelopmental disorders in children. J. Clin. Psychiatry. 2021; 82:20m13735. [CrossRef]
- Scott, K.; Gregory, E.F.; Burrus, H.H. Addressing Maternal Mortality in the US—A Pediatrics Responsibility. JAMA. 2023; 329:1819-1820. [CrossRef]
- Fox, D. Exploring how health inequalities can be addressed through autism training and understanding in maternity services. MIDIRS Midwifery Digest. 2022; 32:279-282.
- Smith, S.; Woodside DB. Characterizing Treatment-Resistant Anorexia Nervosa. Front. Psychiatry. 2021; 11:542206. [CrossRef]
- Brede, J.; Babb, C.; Jones, C.; Elliott, M.; Zanker, C.; Tchanturia, K.; Serpell, L.; Fox, J.; Mandy, W, “For me, the anorexia is just a symptom, and the cause is the autism”: investigating restrictive eating disorders in autistic women. J. Autism Dev. Disord. 2020; 50:4280–4296. [CrossRef]
- Crone, C.; Fochtmann, L.J.; Attia, E.; Boland, R.; Escobar, J.; Formari, V.; Golden, N.; Guarda, A.; Jackson-Triche, M.; Manzo, L.; Mascolo, M.; Pierce, K.; Riddle, M.; Seritan, A.; Uniacke, B.; Zucker, N.; Yager, J.; Craig, T.; Hong, S-H.; Medicus J. The American Psychiatric Association practice guideline for the treatment of patients with eating disorders. Am. J. Psychiatry. 2023; 180:167-171. [CrossRef]
- Suran, M. Treating bipolar disorder is notoriously difficult, but research underway could lead to new options. JAMA. 2023; E1-E4. [CrossRef]
- Howes, O.D.; Thase, M.E.; Pillinger T. Treatment resistance in psychiatry: state of the art and new directions. Molecular Psychiatry. 2022; 27:58–72. [CrossRef]
- Leichsenring, F. Borderline personality disorder. A review. JAMA. 2023; 329:670-679. [CrossRef]
- D’Mello, A.M.; Frosch, I.R.; Li, C.E.; Cardinaux, A.L.; Gabrieli, J.D.E. Exclusion of females in autism research: empirical evidence for a “leaky” recruitment-to-research pipeline. Autism Research. 2022; 15:1929–1940. [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A,R.; Amoakahene, E.; Bakian, A.V.; Bilder, DA. et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill. Summ. 2023; 72(2):1-14. [CrossRef]
- Koch, S.V.; Larsen, J.T.; Mouridsen, S.E.; Bentz, M.; Petersen, L.V.; Bulik, C.; Mortensen, P.B.; Plessen, K.J. Autism spectrum disorder in individuals with anorexia nervosa and in their first- and second-degree relatives: Danish nationwide register-based cohort-study. Br. J. Psychiatry. 2015; 206:401–407. [CrossRef]
- Martini, M.I.; Kuja-Halkola, R.; Butwicka. A.; Du Rietz, E.; D’Onofri, B.M.; Happé, F.; Kanina, A.; Larsson, H.; Lundström, S.; Martin, J.; Rosenqvist, M.A.; Lichtenstein, P.; Taylor, M.J. Sex differences in mental health problems and psychiatric hospitalization in autistic young adults. JAMA Psychiatry. 2022; 79:1188-1198. [CrossRef]
- Carroll, L. Alice’s Adventures in Wonderland. 1st ed. London: Macmillan and Co; 1866.
- Solmi, M.; Radua, J.; Olivola, M.; Croce, E.; Soardo, L.; de Pablo, G.S.; Shin, J.I.; Kirkbride, J.B.; Jones, P.; Kim, J.H.; Kim, J.Y.; Carvalho, A.F.; Seeman, M.V.; Correll, C.U.; Fusar-Poli, P. Age at onset of mental disorders worldwide: large-scale metaanalysis of 192 epidemiological studies. Molecular Psychiatry. 2022; 27:281–295. [CrossRef]
- Westwood, H.; Tchanturia K. Autism spectrum disorder in anorexia nervosa: an updated literature review. Curr. Psychiatry Rep. 2017; 19:41. [CrossRef]
- Pruccoli, J.; Solari, A.; Terenzi, L.; Malaspina, E.; Angotti, M.; Pignataro, V.; Gualandi, P.; Sacrato, L.; Cordelli, D.M.; Franzoni, E, Parmeggiani, A. Autism spectrum disorder and anorexia nervosa: an Italian prospective study. Ital. J. Pediatr. 2021; 47:59. [CrossRef]
- Margari, L.; Palumbi, R.; Peschechera, A.; Craig, F.; de Giambattista, C.; Ventura, P.; Margari, F. Sex-gender comparisons in comorbidities of children and adolescents with high functioning autism spectrum disorder. Front. Psychiatry. 2019; 10:159. [CrossRef]
- Rapoport, J.; Chavez, A.; Greenstein, D.; Addington A.; Gogtay N. Autism spectrum disorders and childhood onset schizophrenia: clinical and biological contributions to a relationship revisited. J. Am. Acad. Child Adolesc. Psychiatry. 2009: 48:10-16. [CrossRef]
- Kincaid, D.L.; Doris, M.; Shannon, C.; Mulholland, M. What is the prevalence of autism spectrum disorder and ASD traits in psychosis? A systematic review, Psychiatry Research. 2017; 250:99-105. [CrossRef]
- Zheng, Z.; Zheng, P.; Zou X. Association between schizophrenia and autism spectrum disorder: a systematic review and meta-analysis. Autism Res. 2018; 11:1110-1119. [CrossRef]
- Li, X.; Zhou, W.; Yi, Z. A glimpse of gender differences in schizophrenia. General Psychiatry. 2022; 35:e100823. [CrossRef]
- Marin, J.L.; Rodriguez-Franco, M.A.; Chugani, V,M.; Maganto, M.M.; Villoria, E.D.; Bedia RC. Prevalence of schizophrenia disorders in average-IQ adults with autism-spectrum disorders: a meta-analysis. J. Autism Dev. Disord. 2018; 48:239-250. [CrossRef]
- Rees, E.; Creeth, H.D.J.; Hwu, H-G.; Chen, W.J.; Tsuang, M.; Glatt, S.J.; Rey, R.; Kirov, G.; Walters, J.T.R.; Holmans, P.; Owen, M.J.; O’Donovan, M.C. Schizophrenia, autism spectrum disorder sand developmental disorders share specific disruptive coding mutations. Nature Communications. 2021; 1-9. [CrossRef]
- Hofvander, B.; Delorme, R.; Chaste, P.; Nydén, A.; Wentz, E.; Ståhlberg, O.; Herbrecht, E.; Stopin, A.; Anckarsäter, H.; Gillberg, C.; Rastam, M.; Leboyer M. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry 2009; 9:35. [CrossRef]
- Kirsch, A.C.; Huebner, A.R.S.; Mehta, S.Q.; Howie, F.R.; Weaver, A.L.; Myers, S.M.; Voigt, R.G.; Katusic, S.K. Association of comorbid mood and anxiety disorders with autism spectrum disorder. JAMA Pediatr. 2020; 174:63-70. [CrossRef]
- Pezzimenti, F.; Han, G.T.; Vasa, R.A.; Gotham K. Depression in youth with autism spectrum disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2019; 28:397–409. [CrossRef]
- Hudson, C.C.; Hall, L.; Harkness, K.L. Prevalence of depressive disorders in individuals with autism spectrum disorder: a meta-analysis. J. Abnorm. Child Psychol. 2019; 47:165-175. [CrossRef]
- Croen, L.; Zerbo, O.; Qian, Y.; Massolo, M.L.; Rich, S.; Sidney, S.; Kripke C. The health status of adults on the autistic spectrum. Autism. 2015; 19:814-823. [CrossRef]
- Martin, A.F.; Jassi, A.; Cullen, A.E.; Broadbent, M.; Downs, J.; Krebs G. Co-occurring obsessive–compulsive disorder and autism spectrum disorder in young people: prevalence, clinical characteristics and outcomes. European Child Adolescent Psychiatry. 2020; 29:1603–1611. [CrossRef]
- Fawcett, E.J.; Power, H.; Fawcett, J.M. Women are at greater risk of OCD than men: a meta-analytic review of OCD prevalence worldwide. J. Clin. Psychiatry. 2020; 23:19r13085. [CrossRef]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: prevalence, course of Illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011; 45: 1027–1035. [CrossRef]
- Pohl, A.L.; Crockford, S.K.; Blakemore, M.C.; Allison, C.; Baron-Cohen, S. A comparative study of autistic and nonautistic women’s experience of motherhood. Molecular Autism. 2020; 11:3. [CrossRef]
- Luca, D.L.; Margiotta, C.; Staatz, C.; Garlow, E.; Christensen, A.; Zivin, K. Financial toll of untreated perinatal mood and anxiety disorders among 2017 births in the United States. Am. J. Public Health. 2020; 110:888–896. [CrossRef]
- Olff, M. Sex and gender differences in post-traumatic stress disorder: an update. European Journal of Psychotraumatology. 2017; 8:sup4, 1351204. [CrossRef]
- Rumball, F.; Happe, F.; Grey N. Experience of trauma and PTSD symptoms in autistic adults: risk of PTSD development following DSM-5 and non-DSM-5 traumatic life events. Autism Res. 2020; 13:2122-2132. [CrossRef]
- Nyrenius, J.; Eberhard, J.; Ghaziuddin, M.; Gillberg, C.; Billstedt, E. Prevalence of Autism Spectrum Disorders in Adult Outpatient Psychiatry. J. Autism Dev. Disord. 2021; 52:3769-3779. [CrossRef]
- LeCavalier, L.; McCracken, C.E.; Aman, M.G.; McDougle, C.J.; McCracken, J.; Tierney, E.; Smith, T.; Johnson, C.; King, B.; Handen, B.; Swiezy, N.B.; Arnold, L.E.; Bearss, K.; Vitiello, B.; Scahill, L. An exploration of concomitant psychiatric disorders in children with autism spectrum disorder. Comprehensive Psychiatry. 2019; 88:57–64. [CrossRef]
- Lever, A.G.; Geurts, H.M. Psychiatric co-occurring symptoms and disorders in young, middle-aged, and older adults with autism spectrum disorder. J. Autism Dev. Disord. 2016; 46:1916–1930. [CrossRef]
- National Institute of Mental Health: Statistics 2021. https://www.nimh.nih.gov/health/statistics. Accessed 01 November 2023.
- Kalb, L.G.; Contractor, A.; Engel C. Advancing Collaborative Care for Autistic Children and Adolescents. JAMA Pediatrics. 2023; 177:1125-1126. [CrossRef]
- Kolves, K.; Fitzgerald, C.; Nordentoft, M.; Wood, S.J.; Erlangsen, A. Assessment of suicidal behaviors among individuals with autism spectrum disorder in Denmark. JAMA Network Open. 2021; 4:e2033565. [CrossRef]
- Ryan, E.P.; Oquendo, M.A. Suicide risk assessment and prevention: Challenges and opportunities. Focus (Am. Psychiatr. Publ.). 2020; 18:88-99. [CrossRef]
- Krieg, C.; Hudon, C.; Chouinard, M-C.; Dufour, I. Individual predictors of frequent emergency department use: a scoping review. BMC Health Services Research. 2016; 16:594. [CrossRef]
- Naoum,V.; Kyriopoulos, D.; Charonis, A.; Athanasakis, K. Kyriopoulos, J. The Pareto principle (“80−20 rule”) in healthcare services in Greece PHS82. Value In Health. 2016; 19:A618. [CrossRef]
- Hardtstock, F.; Knapp, R.; Maywald, U.; Wilke, T. Health care spending and the Pareto principle -the minority that causes the majority. Value in Health 2020; PM;U8, S604.
- Bralten, J.; van Hulzen, K.L.; Martens, M.B, Galesloot, T.L.; Arias Vasquez, A.; Kiemeney, L.A.; Buitelaar, J.K.; Muntjewerff, J.W.; Franke, B.; Poelmans, G. Autism spectrum disorders and autistic traits share genetics and biology. Molecular Psychiatry. 2018; 23:1205–1212. [CrossRef]
- Karazewska, D.; Cleintuar, P.; Oudijn, M.; Lok, A, van Elburg, A.; Denys, D.; Mocking R. Efficacy and safety of deep brain stimulation for treatment refractory anorexia nervosa: a systematic review and metaanalysis. Translational Psychiatry. 2022: 12:333. [CrossRef]
- Babb, C.; Brede, J.; Jones, C.R.G.; Serpell, L.; Mandy, W.; Fox J. A comparison of the eating disorder service experiences of autistic and non-autistic women in the UK. European Eating Disorders Review. 2022; 30:616–627. [CrossRef]
- Tchanturia, K.; Dandil, Y.; Li, Z.; Smith, K.; Leslie, M.; Byford, S. A novel approach for autism spectrum condition patients with eating disorders: analysis of treatment cost savings. Eur. Eat. Disorders Rev. 2020; 1–5. [CrossRef]
- Taylor, H.L.; Menachemi, N.; Gilbert, A.; Chaudhary, J.; Blackburn, J. Economic burden associated with untreated mental illness in Indiana. JAMA Health Forum. 2023; 4:e233535. [CrossRef]
- Moore Simas, T.A.; Whelan, A.; Byatt N. Postpartum depression-new screening recommendations and treatments. JAMA. 2023; E1-E2. [CrossRef]
- O’Nions, E.; Petersen, I.; Buckman, J.E.J.; Charlton, R.; Cooper, C.; Corbett, A.; Happé, F.; Manthorpe, J.; Richards, M.; Saunders, R.; Zanker, C.; Mandy, W.; Stott, J. Autism in England: assessing underdiagnosis in a population based cohort study of prospectively collected primary care data. The Lancet Regional Health – Europe. 2023; 29: 100626. [CrossRef]
- Alkhaldi, R.S.; Sheppard. E.; Burdett, E.; Mitchell, P. Do neurotypical people like or dislike autistic people? Autism In Adulthood. 2021; 3:275-279. [CrossRef]
- Ribolsi, M.; Nastro, F.; Pelle, M.; Medici, C.; Sacchetto, S.; Lisi, G.; Riccioni, A.; Siracusano, M.; Mazzone, L.; Di Lorenzo, G. Recognizing Psychosis in Autism Spectrum Disorder. Front. Psychiatry. 2022; 13:768586. [CrossRef]
- Nyrenius, J.; Eberhard, J.; Ghaziuddin, M.; Gillberg, C.; Billstedt, E. The ‘lost generation” in adult psychiatry: psychiatric, neurodevelopmental and sociodemographic characteristics of psychiatric patients with autism unrecognized in childhood. B. J. Psych. Open. 2023; 9:e89. [CrossRef]
- Maddox, B.B.; Gaus, V.L. Community mental health services for autistic adults: good news and bad news. Autism In Adulthood. 2019; 1. [CrossRef]
- Auger, N.; Potter, B.J.; Ukah, U.V.; Low, N.; Israel, M.; Steiger, H.; Healey-Profitos, J.; Paradis, G. Anorexia and the long-term risk of mortality in women. World Psychiatry. 2021; 20:448-449. [CrossRef]
- Accurso, E.C.; Sim, L.; Muhlheim, L.; Lebow, J. Parents know best: caregiver perspectives on eating disorder recovery. Int. J. Eat. Dis. 2019; 53:1252-1260. [CrossRef]
- Hicks, T.; Buckleton, J.; Castella, V.; Evett, I.; Jackson, G. A logical framework for forensic DNA Interpretation. Genes. 2022; 13:957. [CrossRef]
- Doyle, N. Neurodiversity at work: a biopsychosocial model and the impact on working adults. British Medical Bulletin. 2020; 135:108–125. [CrossRef]
- Lewis DM. WHO definition of health remains fit for purpose. BMJ. 2011; 343:d5357. [CrossRef]
- Pellicano, E.; den Houting, J. Annual Research Review; Shifting from ‘normal science’ to neurodiversity in autism science. J. Child Psychology Psychiatry. 2021; 63:381-396. [CrossRef]
- Field, S.L.; Fox, J.R.E.; Jones, C.R.G.; Williams, M.O. “Work WITH us”: a Delphi study about improving eating disorder treatment for autistic women with anorexia nervosa. J. Eat. Disord. 2023; 11:17. [CrossRef]
- López, B. Commentary on autism and the double-empathy problem: implications for development and mental health. Br. J. Dev. Psychol. 2022; 40:368–370. [CrossRef]
- Hume, R.; Burgess H. ‘‘I’m human after all’’: autism, trauma, and affective empathy. Autism In Adulthood. 2021; 3:221-229. [CrossRef]
- Brosnan, M.; Ashwin, C. Thinking, fast and slow on the autism spectrum. Autism. 2023; 27:1245–1255. [CrossRef]
- Spain, D.; Happé, F. How to optimise cognitive behaviour therapy (CBT) for people with autism spectrum disorders (ASD): a Delphi study. Journal of Rational-Emotive & Cognitive-Behavior Therapy. 2020; 38:184–208. [CrossRef]
- Riches, S.; Hammond, N.; Bianco, M.; Fialho, C.; Acland, J. Adapting cognitive behaviour therapy for adults with autism: a lived experience-led consultation with specialist psychological therapists. The Cognitive Behaviour Therapist. 2023; 16:e13, page 1-12. [CrossRef]
- Gilmore, D.; Longo, A.; Krantz, M.; Radford, D.; Hand, B.N. Five ways providers can improve mental healthcare for autistic adults: a review of mental healthcare use, barriers to care, and evidence-based recommendations. Current Psychiatry Reports. 2022; 24:565–571. [CrossRef]
- Berenson, R.A. If you can’t measure performance, can you improve it? JAMA Forum. 2016; 315:645-646. [CrossRef]
- Marmot, M. Evidence based policy or policy based evidence? BMJ. 2004; 328:906-907. [CrossRef]
- Reupert, A.; Straussner, S.L.; Weimand, B.; Maybery, D. It takes a village to raise a child: understanding and expanding the concept of the “Village”. Front. Public Health. 2022; 10:756066. [CrossRef]
- King, S.L. How do maternity services support autistic women and birthing people now? What improvements could be made to help autistic people who are pregnant and giving birth and the staff who support them? MIDIRS Midwifery Digest. 2022; 32:283-288.
- de Nocker, Y.L.; Toolan, C.K. Using telehealth to provide interventions for children with ASD: a systematic review. Rev. J. Autism Dev. Disord. 2023; 10:82–112. [CrossRef]
- Pellicano, E.; Heyworth M. The foundations of autistic flourishing. Curr. Psychiatry Rep. 2023; 25:419-427. [CrossRef]

| Condition | P(ASD|MI) | References | Given value or Bayes’ calculation |
| Anorexia Nervosa | 0.2-0.3 | 48 | Given |
| 0.23 | 49 | Given | |
| Schizophrenia Spectrum D | 0.43 | 2,52,53,54,55 | Bayes’ probability |
| Borderline Personality D | 0.146 | 57 | Given |
| Bipolar Disorder | 0.272 | 13,58 | Bayes’ odds |
| Depression | 0.127 | 13,58 | Bayes’ odds |
| 0.144 | 13,59 | Bayes’ probability | |
| 0.203 | 13,60 | Bayes’ odds | |
| Anxiety Disorders | 0.157 | 13,58 | Bayes’ odds |
| 0.170 | 13,61 | Bayes’ odds | |
| Obsessive Compulsive D | 0.232 | 62 | Given |
| Social Anxiety D | 0.262 | 2,64 | Bayes’ probability |
| Postnatal Depression | 0.313 | 2,65,66 | Bayes’ probability |
| Post Traumatic Stress D | 0.30 | 2,67,68 | Bayes’ probability |
| Any Mental Illness | 0.189 | 69 | Given |
| 0.176 | 2,70-72 | Bayes’ probability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




