Submitted:
29 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
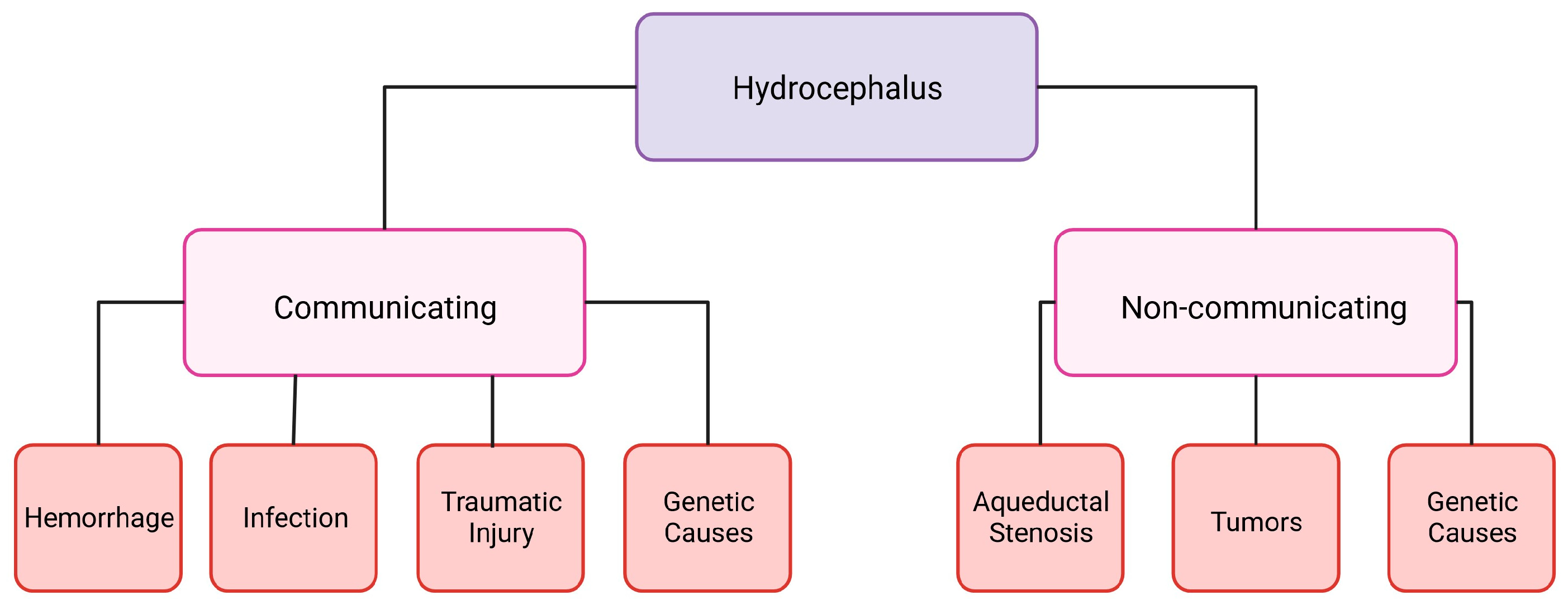
1. Hydrocephalus
2. Hydrocephalus Treatments
3. Animal Models
4. Genetic Animal Models
5. The Choroid Plexus Epithelium
6. Cell Culture Models
7. Electrolyte Transporters and Potential Roles in Fluid/Electrolyte Homeostasis
8. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lifshutz, J.I.; Johnson, W.D. History of hydrocephalus and its treatments. Neurosurgical Focus 2001, 11, 1–5. [Google Scholar] [CrossRef]
- Aschoff, A.; Kremer, P.; Hashemi, B.; Kunze, S. The scientific history of hydrocephalus and its treatment. Neurosurgical Review 1999, 22, 67–93. [Google Scholar] [CrossRef]
- Mokri, B. The Monro–Kellie hypothesis: applications in CSF volume depletion. Neurology 2001, 56, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Hochstetler, A.; Raskin, J.; Blazer-Yost, B.L. Hydrocephalus: historical analysis and considerations for treatment. European Journal of Medical Research 2022, 27, 1–17. [Google Scholar] [CrossRef]
- Koschnitzky, J.E.; Yap, E.; Zhang, Y.; Chau, M.J.; Yerneni, K.; Somera, A.L.; Luciano, M.; Moghekar, A. Inpatient healthcare burden and variables influencing hydrocephalus-related admissions across the lifespan. Journal of Neurosurgery 2022, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karimy, J.K.; Reeves, B.C.; Damisah, E.; Duy, P.Q.; Antwi, P.; David, W.; Wang, K.; Schiff, S.J.; Limbrick Jr, D.D.; Alper, S.L. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nature Reviews Neurology 2020, 16, 285–296. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Mekary, R.; Glancz, L.J.; Yunusa, I.; Baticulon, R.E.; Fieggen, G.; Wellons, J.C.; Park, K.B.; Warf, B.C. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. Journal of Neurosurgery 2018, 130, 1065–1079. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, Z.; Tan, Q.; Guo, J.; Tang, J.; Tan, L.; Feng, H.; Chen, Z. Post-hemorrhagic hydrocephalus: recent advances and new therapeutic insights. Journal of the Neurological Sciences 2017, 375, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Cioca, A.; Gheban, D.; Perju-Dumbrava, D.; Chiroban, O.; Mera, M. Sudden death from ruptured choroid plexus arteriovenous malformation. The American Journal of Forensic Medicine and Pathology 2014, 35, 100–102. [Google Scholar] [CrossRef]
- Karimy, J.K.; Duran, D.; Hu, J.K.; Gavankar, C.; Gaillard, J.R.; Bayri, Y.; Rice, H.; DiLuna, M.L.; Gerzanich, V.; Simard, J.M. Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurgical Focus 2016, 41, E10. [Google Scholar] [CrossRef]
- Bale, J.F. Fetal infections and brain development. Clinics in Perinatology 2009, 36, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, M.C.; Whitney, C.G.; Messonnier, N.E.; Zell, E.R.; Lynfield, R.; Hadler, J.L.; Harrison, L.H.; Farley, M.M.; Reingold, A.; Bennett, N.M. Bacterial meningitis in the United States, 1998–2007. New England Journal of Medicine 2011, 364, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Varagur, K.; Sanka, S.A.; Strahle, J.M. Syndromic hydrocephalus. Neurosurgery Clinics 2022, 33, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Stumpel, C.; Vos, Y.J. L1 Syndrome; University of Washington: Seattle, Seattle (WA), 1993. [Google Scholar]
- Marguet, F.; Vezain, M.; Marcorelles, P.; Audebert-Bellanger, S.; Cassinari, K.; Drouot, N.; Chambon, P.; Gonzalez, B.J.; Horowitz, A.; Laquerriere, A. Neuropathological hallmarks of fetal hydrocephalus linked to CCDC88C pathogenic variants. Acta Neuropathologica Communications 2021, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hale, A.T.; Bastarache, L.; Morales, D.M.; Wellons, J.C.; Limbrick, D.D.; Gamazon, E.R. Multi-omic analysis elucidates the genetic basis of hydrocephalus. Cell Reports 2021, 35, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.M.; Riva-Cambrin, J.; Yavin, D.; Hockley, A.; Pringsheim, T.M.; Jette, N.; Lethebe, B.C.; Lowerison, M.; Dronyk, J.; Hamilton, M.G. Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PloS One 2018, 13, e0204926. [Google Scholar] [CrossRef]
- Tully, H.M.; Dobyns, W.B. Infantile hydrocephalus: a review of epidemiology, classification and causes. European Journal of Medical Genetics 2014, 57, 359–368. [Google Scholar] [CrossRef]
- Wright, Z.; Larrew, T.W.; Eskandari, R. Pediatric hydrocephalus: current state of diagnosis and treatment. Pediatrics in Review 2016, 37, 478–490. [Google Scholar] [CrossRef]
- Ardissino, M.; Moussa, O.; Tang, A.; Muttoni, E.; Ziprin, P.; Purkayastha, S. Idiopathic intracranial hypertension in the British population with obesity. Acta Neurochirurgica 2019, 161, 239–246. [Google Scholar] [CrossRef]
- Andersson, J.; Rosell, M.; Kockum, K.; Lilja-Lund, O.; Söderström, L.; Laurell, K. Prevalence of idiopathic normal pressure hydrocephalus: a prospective, population-based study. PloS One 2019, 14, e0217705. [Google Scholar] [CrossRef]
- Fowler, J.B.; De Jesus, O.; Mesfin, F.B. Ventriculoperitoneal Shunt; StatPearls Publishing: StatPearls [Internet]. Treasure Island (FL), 2023. [Google Scholar]
- Akyol, M.E.; Cetin, E. Effects of shunt types used in idiopathic normal pressure hydrocephalus on patients’ clinical outcomes. Annals of Medical Research 2023, 30, 146–281. [Google Scholar] [CrossRef]
- Shannon, C.N.; Carr, K.R.; Tomycz, L.; Wellons, J.C.; Tulipan, N. Time to first shunt failure in pediatric patients over 1 year old: a 10-year retrospective study. Pediatric Neurosurgery 2015, 49, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.C.; Guo, W. Have we made progress in preventing shunt failure? A critical analysis. Journal of Neurosurgery: Pediatrics 2008, 1, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Vinzani, M.; Alshareef, M.; Eskandari, R. Use of a Prophylactic Retrograde-Flushing Device in High-Risk Pediatric Patients with Ventriculoperitoneal Shunts: A Technical Note. Pediatric Neurosurgery 2023, 58, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.J.; Chiang, W.C.; Huang, H.Y.; Lin, S.Z.; Tsai, S.T. Effectiveness and safety of ventriculoperitoneal shunt versus lumboperitoneal shunt for communicating hydrocephalus: A systematic review and meta-analysis with trial sequential analysis. CNS Neuroscience & Therapeutics 2023, 29, 804–815. [Google Scholar]
- Yang, T.-H.; Chang, C.-S.; Sung, W.-W.; Liu, J.-T. Lumboperitoneal shunt: a new modified surgical technique and a comparison of the complications with ventriculoperitoneal shunt in a single center. Medicina 2019, 55, 1–10. [Google Scholar] [CrossRef]
- Yadav, Y.R.; Parihar, V.; Pande, S.; Namdev, H.; Agarwal, M. Endoscopic third ventriculostomy. Journal of Neurosciences in Rural Practice 2012, 3, 163–173. [Google Scholar] [CrossRef]
- Lu, L.; Chen, H.; Weng, S.; Xu, Y. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in patients with obstructive hydrocephalus: meta-analysis of randomized controlled trials. World Neurosurgery 2019, 129, 334–340. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Drake, J.M.; Mallucci, C.L.; Sgouros, S.; Roth, J.; Constantini, S.; Group, C.P.N.S. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. The Journal of Pediatrics 2009, 155, 254–259.e251. [Google Scholar] [CrossRef]
- Hernandez, N.; Wang, S.; Ragheb, J. The Role of the Pediatric Neurosurgeon in the Management of Hydrocephalus Internationally. Journal of Global Neurosurgery 2022, 2. [Google Scholar] [CrossRef]
- Warf, B.C.; Weber, D.S.; Day, E.L.; Riordan, C.P.; Staffa, S.J.; Baird, L.C.; Fehnel, K.P.; Stone, S.S. Endoscopic third ventriculostomy with choroid plexus cauterization: predictors of long-term success and comparison with shunt placement for primary treatment of infant hydrocephalus. Journal of Neurosurgery: Pediatrics 2023, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, Y.; Brar, K.; Yang, K.; Lee, Y.; Ajani, O. Comparison of endoscopic third ventriculostomy with or without choroid plexus cauterization in pediatric hydrocephalus: a systematic review and meta-analysis. Journal of Neurosurgery: Pediatrics 2020, 26, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Marriott, W.M. The use of theobromin sodio salicylate (diuretin) in the treatment of hydrocephalus. American Journal of Diseases of Children 1924, 28, 479–483. [Google Scholar] [CrossRef]
- Hayden, P.W.; Foltz, E.L.; Shurtleff, D.B. Effect of an oral osmotic agent on ventricular fluid pressure of hydrocephalic children. Pediatrics 1968, 41, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, D.B.; Hayden, P.W. The treatment of hydrocephalus with isosorbide, an oral hyperosmotic agent. Journal of Clinical Pharmacology & New Drugs 1972, 108–114. [Google Scholar]
- Group, I.P.D.T. International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. The Lancet 1998, 352, 433–440. [Google Scholar]
- Bass, N.H.; Fällström, S.; Lundborg, P. Digoxin-induced arrest of the cerebrospinal fluid circulation in the infant rat: implications for medical treatment of hydrocephalus during early postnatal life. Pediatric Research 1979, 13, 26–30. [Google Scholar] [CrossRef]
- Bergold, P.J.; Furhang, R.; Lawless, S. Treating traumatic brain injury with minocycline. Neurotherapeutics 2023, 20, 1546–1564. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Tan, C.; Wang, Y.; Tang, Z.; Zhang, Z.; Liu, J.; Xiao, G. Novel therapeutics for hydrocephalus: Insights from animal models. CNS Neuroscience & Therapeutics 2021, 27, 1012–1022. [Google Scholar]
- McAllister, J.P.; Miller, J.M. Minocycline inhibits glial proliferation in the H-Tx rat model of congenital hydrocephalus. Cerebrospinal Fluid Research 2010, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Winer, J.L.; Kitase, Y.; Brigman, J.L.; Jantzie, L.L. Neonatal administration of erythropoietin attenuates cognitive deficits in adult rats following placental insufficiency. Journal of Neuroscience Research 2022, 100, 2112–2126. [Google Scholar] [CrossRef]
- Robinson, S.; Conteh, F.S.; Oppong, A.Y.; Yellowhair, T.R.; Newville, J.C.; Demerdash, N.E.; Shrock, C.L.; Maxwell, J.R.; Jett, S.; Northington, F.J. Extended combined neonatal treatment with erythropoietin plus melatonin prevents posthemorrhagic hydrocephalus of prematurity in rats. Frontiers in Cellular Neuroscience 2018, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Jantzie, L.L.; Muthukumar, S.; Kitase, Y.; Vasan, V.; Fouda, M.A.; Hamimi, S.; Burkhardt, C.; Burton, V.J.; Gerner, G.; Scafidi, J. Infantile Cocktail of Erythropoietin and Melatonin Restores Gait in Adult Rats with Preterm Brain Injury. Developmental Neuroscience 2022, 44, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Safety of Erythropoietin and Melatonin for Very Preterm Infants With Intraventricular Hemorrhage (SCEMPI). Available online: https://clinicaltrials.gov/study/NCT05617833?term=robinson&intr=erythropoietin&rank=1 (accessed on 1/14/24).
- Indrawijaya, Y.Y.A.; Sumarno, S.; Suryaningtyas, W.; Husna, N.A. Drug utilization study of diuretics in children with hydrocephalus. In Proceedings of the Proceedings of International Pharmacy Ulul Albab Conference and Seminar (PLANAR), 2021; pp. 77–87.
- Del Bigio, M.R.; Di Curzio, D.L. Nonsurgical therapy for hydrocephalus: a comprehensive and critical review. Fluids and Barriers of the CNS 2015, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Diringer, M.N.; Edwards, D.F.; Zazulia, A.R. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke 1998, 29, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Z.; Xi, G.; Keep, R.; Hua, Y. Deferoxamine attenuates acute hydrocephalus after traumatic brain injury in rats. Transl Stroke Res 2014, 5, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Zhao, G. Adult posthaemorrhagic hydrocephalus animal models. Journal of the Neurological Sciences 2017, 379, 39–43. [Google Scholar] [CrossRef] [PubMed]
- NS, V. Experimental hydrocephalus in young rats. Arkhiv Patologii 1957, 19, 44–52. [Google Scholar]
- Pudenz, R.H. Experimental and clinical observations on the shunting of cerebrospinal fluid into the circulatory system. Neurosurgery 1958, 5, 98–115. [Google Scholar] [CrossRef]
- McAllister, J.P.; Talcott, M.R.; Isaacs, A.M.; Zwick, S.H.; Garcia-Bonilla, M.; Castaneyra-Ruiz, L.; Hartman, A.L.; Dilger, R.N.; Fleming, S.A.; Golden, R.K. A novel model of acquired hydrocephalus for evaluation of neurosurgical treatments. Fluids and Barriers of the CNS 2021, 18, 1–17. [Google Scholar] [CrossRef]
- da Silva Lopes, L.; Slobodian, I.; Del Bigio, M.R. Characterization of juvenile and young adult mice following induction of hydrocephalus with kaolin. Experimental Neurology 2009, 219, 187–196. [Google Scholar] [CrossRef]
- Gonzalez-Darder, J.; Barbera, J.; Cerda-Nicolas, M.; Segura, D.; Broseta, J.; Barcia-Salorio, J.L. Sequential morphological and functional changes in kaolin-induced hydrocephalus. Journal of Neurosurgery 1984, 61, 918–924. [Google Scholar] [CrossRef]
- Nakayama, D.K.; Harrison, M.R.; Berger, M.S.; Chinn, D.H.; Halks-Miller, M.; Edwards, M.S. Correction of congenital hydrocephalus in utero I. The model: intracisternal kaolin produces hydrocephalus in fetal lambs and rhesus monkeys. Journal of Pediatric Surgery 1983, 18, 331–338. [Google Scholar] [CrossRef]
- Marlin, A.; Wald, A.; Hochwald, G.; Malhan, C. Kaolin-induced hydrocephalus impairs CSF secretion by the choroid plexus. Neurology 1978, 28, 945–945. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Cui, W.; Cao, Y.; Zhao, L.; Wang, H.; Liu, X.; Fan, S.; Huang, K.; Tong, A. Inhibition of neuronal necroptosis mediated by RIP1/RIP3/MLKL provides neuroprotective effects on kaolin-induced hydrocephalus in mice. Cell proliferation 2021, 54, e13108. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Guo, J.; Yu, C.; Yang, J. Molecular mechanisms and risk factors for the pathogenesis of hydrocephalus. Frontiers in Genetics 2022, 12, 777926. [Google Scholar] [CrossRef]
- Yamada, H.; Oi, S.; Tamaki, N.; Matsumoto, S.; Taomoto, K. Embryopathoetiology of Congenital Hydrocephalus in Experimental Models: A Comparative Morphological Study in Two Different Models. In Proceedings of the Hydrocephalus; 1991; pp. 27–35. [Google Scholar]
- Stambolliu, E.; Ioakeim-Ioannidou, M.; Kontokostas, K.; Dakoutrou, M.; Kousoulis, A.A. The most common comorbidities in Dandy-Walker syndrome patients: a systematic review of case reports. Journal of Child Neurology 2017, 32, 886–902. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, J. Early neurovascular abnormalities underlying 6-aminonicotinamide (6-AN)-induced congenital hydrocephalus in rats. Teratology 1970, 3, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Gattone, V.H.; Tourkow, B.A.; Trambaugh, C.M.; Yu, A.C.; Whelan, S.; Phillips, C.L.; Harris, P.C.; Peterson, R.G. Development of multiorgan pathology in the wpk rat model of polycystic kidney disease. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology: An Official Publication of the American Association of Anatomists 2004, 277, 384–395. [Google Scholar] [CrossRef]
- Shim, J.W.; Territo, P.R.; Simpson, S.; Watson, J.C.; Jiang, L.; Riley, A.A.; McCarthy, B.; Persohn, S.; Fulkerson, D.; Blazer-Yost, B.L. Hydrocephalus in a rat model of Meckel Gruber syndrome with a TMEM67 mutation. Scientific Reports 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
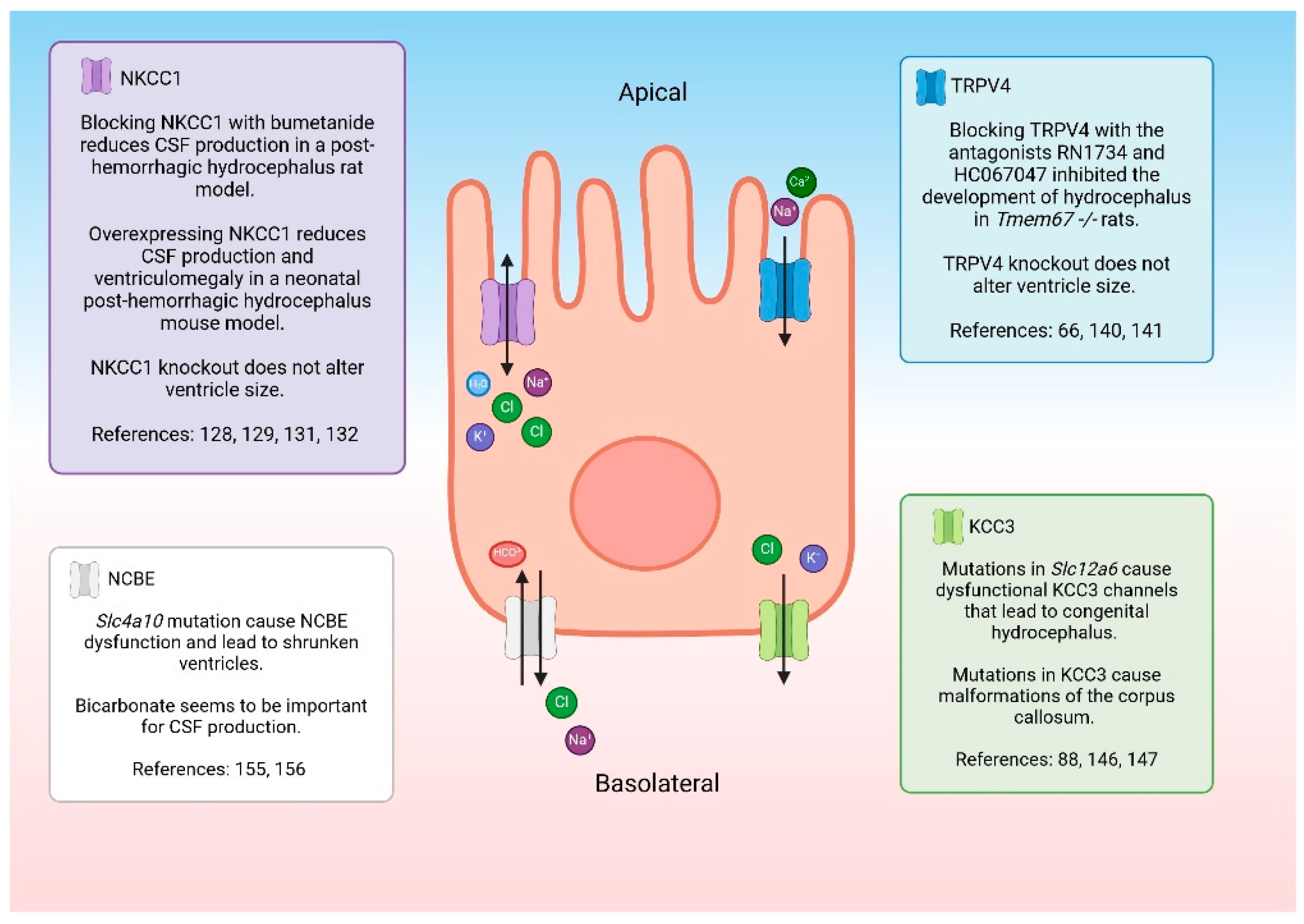
- Hochstetler, A.E.; Smith, H.M.; Preston, D.C.; Reed, M.M.; Territo, P.R.; Shim, J.W.; Fulkerson, D.; Blazer-Yost, B.L. TRPV4 antagonists ameliorate ventriculomegaly in a rat model of hydrocephalus. Jci Insight 2020, 5, 1–14. [Google Scholar] [CrossRef]
- Hochstetler, A.; Smith, H.; Reed, M.; Hulme, L.; Territo, P.; Bedwell, A.; Persohn, S.; Perrotti, N.; D’Antona, L.; Musumeci, F. Inhibition of serum-and glucocorticoid-induced kinase 1 ameliorates hydrocephalus in preclinical models. Fluids and Barriers of the CNS 2023, 20, 61. [Google Scholar] [CrossRef]
- Mashayekhi, F.; Draper, C.E.; Bannister, C.M.; Pourghasem, M.; Owen-Lynch, P.J.; Miyan, J.A. Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain 2002, 125, 1859–1874. [Google Scholar] [CrossRef]
- Jones, H.; Bucknall, R. INHERITED PRENATAL HYDROCEPHALUS IN THE H–Tx RAT: A MORPHOLOGICAL STUDY. Neuropathology and Applied Neurobiology 1988, 14, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.C.; Carter, B.J.; Morel, L. Characteristics of hydrocephalus expression in the LEW/Jms rat strain with inherited disease. Child's Nervous System 2003, 19, 11–18. [Google Scholar] [CrossRef]
- Itoh, K.; Fushiki, S. The role of L1 cam in murine corticogenesis, and the pathogenesis of hydrocephalus. Pathology International 2015, 65, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Swetloff, A.; Ferretti, P. Changes in E2F5 intracellular localization in mouse and human choroid plexus epithelium with development. International Journal of Developmental Biology 2004, 49, 859–865. [Google Scholar] [CrossRef]
- Banizs, B.; Pike, M.M.; Millican, C.L.; Ferguson, W.B.; Komlosi, P.; Sheetz, J.; Bell, P.D.; Schwiebert, E.M.; Yoder, B.K. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 2005, 132, 5329–5339. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, G.J.; Dagnino, L.; Gaubatz, S.; Xu, Y.; Bronson, R.T.; Warren, H.B.; Livingston, D.M. A specific, nonproliferative role for E2F-5 in choroid plexus function revealed by gene targeting. Genes & Development 1998, 12, 1092–1098. [Google Scholar]
- Lewis, W.R.; Malarkey, E.B.; Tritschler, D.; Bower, R.; Pasek, R.C.; Porath, J.D.; Birket, S.E.; Saunier, S.; Antignac, C.; Knowles, M.R. Mutation of growth arrest specific 8 reveals a role in motile cilia function and human disease. PLoS Genetics 2016, 12, e1006220. [Google Scholar] [CrossRef]
- Hochstetler, A.E.; Whitehouse, L.; Antonellis, P.; Berbari, N.F.; Blazer-Yost, B.L. Characterizing the Expression of TRPV4 in the Choroid Plexus Epithelia as a Prospective Component in the Development of Hydrocephalus in the Gas8GT Juvenile Mouse Model. The FASEB Journal 2018, 32, 750.712–750.712. [Google Scholar] [CrossRef]
- Abdelhamed, Z.; Vuong, S.M.; Hill, L.; Shula, C.; Timms, A.; Beier, D.; Campbell, K.; Mangano, F.T.; Stottmann, R.W.; Goto, J. A mutation in Ccdc39 causes neonatal hydrocephalus with abnormal motile cilia development in mice. Development 2018, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Davy, B.E.; Robinson, M.L. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Human Molecular Genetics 2003, 12, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Bronson, R.T.; Lane, P.W. Hydrocephalus with hop gait (hyh): a new mutation on chromosome 7 in the mouse. Developmental Brain Research 1990, 54, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Di Curzio, D.L. Animal models of hydrocephalus. Open Journal of Modern Neurosurgery 2017, 8, 57–71. [Google Scholar] [CrossRef]
- Kume, T.; Deng, K.-Y.; Winfrey, V.; Gould, D.B.; Walter, M.A.; Hogan, B.L. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell 1998, 93, 985–996. [Google Scholar] [CrossRef]
- Kuwamura, M.; Kinoshita, A.; Okumoto, M.; Yamate, J.; Mori, N. Hemorrhagic hydrocephalus (hhy): a novel mutation on mouse chromosome 12. Developmental Brain Research 2004, 152, 69–72. [Google Scholar] [CrossRef]
- Lin, X.; Liu, B.; Yang, X.; Yue, X.; Diao, L.; Wang, J.; Chang, J. Genetic deletion of Rnd3 results in aqueductal stenosis leading to hydrocephalus through up-regulation of Notch signaling. Proceedings of the National Academy of Sciences 2013, 110, 8236–8241. [Google Scholar] [CrossRef]
- Ramos, C.; Fernández-Llebrez, P.; Bach, A.; Robert, B.; Soriano, E. Msx1 disruption leads to diencephalon defects and hydrocephalus. Developmental Dynamics: An Official Publication of the American Association of Anatomists 2004, 230, 446–460. [Google Scholar] [CrossRef]
- Jones, H.; Dack, S.; Ellis, C. Morphological aspects of the development of hydrocephalus in a mouse mutant (SUMS/NP). Acta Neuropathologica 1987, 72, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Lehtinen, M.K. Experimental approaches for manipulating choroid plexus epithelial cells. Fluids and Barriers of the CNS 2022, 19, 1–15. [Google Scholar] [CrossRef]
- Millar, I.D.; Bruce, J.I.; Brown, P.D. Ion channel diversity, channel expression and function in the choroid plexuses. Cerebrospinal Fluid Research 2007, 4, 1–16. [Google Scholar] [CrossRef]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiological Reviews 2013, 93, 1847–1892. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Z.; Chen, Y.; Liao, J.; Wang, Y.; Liu, J.; Lin, Z.; Xiao, G. Choroid plexus epithelium and its role in neurological diseases. Frontiers in Molecular Neuroscience 2022, 15, 949231. [Google Scholar] [CrossRef]
- Yang, Y.; He, J.; Wang, Y.; Wang, C.; Tan, C.; Liao, J.; Tong, L.; Xiao, G. Targeting choroid plexus epithelium as a novel therapeutic strategy for hydrocephalus. Journal of Neuroinflammation 2022, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. European Annals of Otorhinolaryngology, Head and Neck Diseases 2011, 128, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Oernbo, E.K.; Steffensen, A.B.; Razzaghi Khamesi, P.; Toft-Bertelsen, T.L.; Barbuskaite, D.; Vilhardt, F.; Gerkau, N.J.; Tritsaris, K.; Simonsen, A.H.; Lolansen, S.D. Membrane transporters control cerebrospinal fluid formation independently of conventional osmosis to modulate intracranial pressure. Fluids and Barriers of the CNS 2022, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. Mechanisms of ion transport across the choroid plexus. The Journal of Physiology 1972, 226, 545–571. [Google Scholar] [CrossRef] [PubMed]
- Bouillé, C.; Mesnil, M.; Barriere, H.; Gabrion, J. Gap junctional intercellular communication between cultured ependymal cells, revealed by lucifer yellow CH transfer and freeze-fracture. Glia 1991, 4, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Q.; Graziano, J.H. Primary culture of choroidal epithelial cells: characterization of an in vitro model of blood-CSF barrier. In Vitro Cellular & Developmental Biology-Animal 1998, 34, 40–45. [Google Scholar]
- Mayer, S.E.; Sanders-Bush, E. Sodium-dependent antiporters in choroid plexus epithelial cultures from rabbit. Journal of Neurochemistry 1993, 60, 1308–1316. [Google Scholar] [CrossRef]
- Baehr, C.; Reichel, V.; Fricker, G. Choroid plexus epithelial monolayers–a cell culture model from porcine brain. Cerebrospinal Fluid Research 2006, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Harter, D.; Hsu, K.; Rose, H. Immunofluorescence and cytochemical studies of visna virus in cell culture. Journal of Virology 1967, 1, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Crook, R.B.; Kasagami, H. , Prusiner, S.B. Culture and characterization of epithelial cells from bovine choroid plexus. Journal of Neurochemistry 1981, 25, 215–227. [Google Scholar]
- Monnot, A.D.; Zheng, W. Culture of choroid plexus epithelial cells and in vitro model of blood–CSF barrier. Epithelial Cell Culture Protocols: Second Edition 2013, 13–29. [Google Scholar]
- Fejes, Z.; Pócsi, M.; Takai, J.; Erdei, J.; Tóth, A.; Balogh, E.; Rusznyák, Á.; Fenyvesi, F.; Nagy, A.; Kappelmayer, J. Preterm intraventricular hemorrhage-induced inflammatory response in human choroid plexus epithelial cells. International Journal of Molecular Sciences 2021, 22, 8648. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Zhang, J.S.; Duncan, L.H.; Johnston Jr, R.J. Human neural organoids: Models for developmental neurobiology and disease. Developmental Biology 2021, 478, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Schroten, M.; Hanisch, F.-G.; Quednau, N.; Stump, C.; Riebe, R.; Lenk, M.; Wolburg, H.; Tenenbaum, T.; Schwerk, C. A novel porcine in vitro model of the blood-cerebrospinal fluid barrier with strong barrier function. PLoS One 2012, 7, e39835. [Google Scholar] [CrossRef] [PubMed]
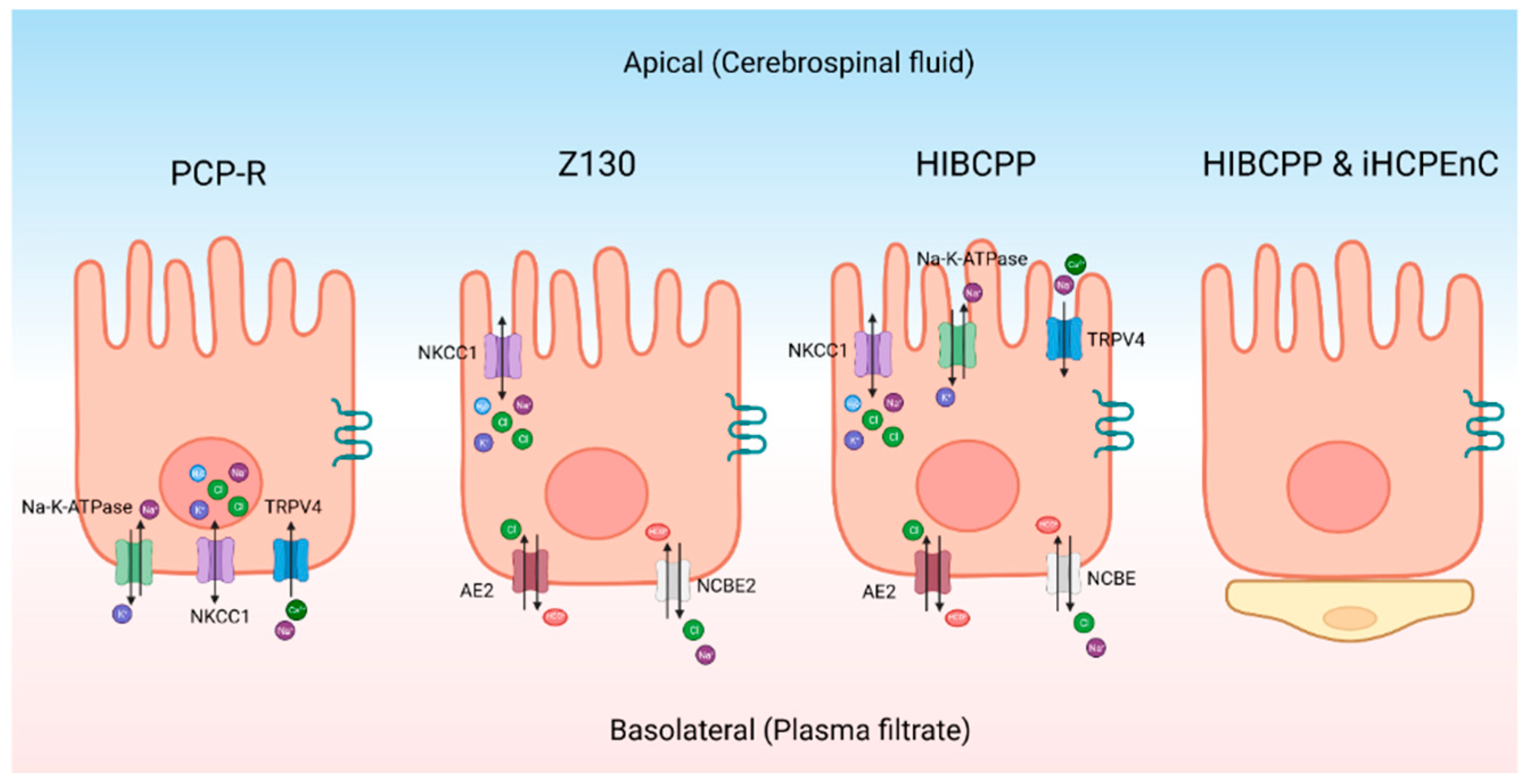
- Hochstetler, A.; Hulme, L.; Delpire, E.; Schwerk, C.; Schroten, H.; Preston, D.; Simpson, S.; Blazer-Yost, B.L. Porcine choroid plexus-riems cell line demonstrates altered polarization of transport proteins compared with the native epithelium. American Journal of Physiology-Cell Physiology 2022, 323, C1–C13. [Google Scholar] [CrossRef]
- Lauer, A.N.; März, M.; Meyer, S.; Meurer, M.; de Buhr, N.; Borkowski, J.; Weiß, C.; Schroten, H.; Schwerk, C. Optimized cultivation of porcine choroid plexus epithelial cells, a blood–cerebrospinal fluid barrier model, for studying granulocyte transmigration. Laboratory Investigation 2019, 99, 1245–1255. [Google Scholar] [CrossRef]
- Hulme, L.; Hochstetler, A.; Schwerk, C.; Schroten, H.; Ishikawa, H.; Tung, C.-Y.; Perrin, B.; Blazer-Yost, B. Characterization of TRPV4-mediated signaling pathways in an optimized human choroid plexus epithelial cell line. American Journal of Physiology-Cell Physiology 2022, 323, C1823–C1842. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Dziegielewska, K.M.; Ek, C.J.; Habgood, M.D.; Bauer, H.; Bauer, H.-C.; Lindsay, H.; Wakefield, M.J.; Strazielle, N.; Kratzer, I. Correction: mechanisms that determine the internal environment of the developing brain: a transcriptomic, functional and ultrastructural approach. PLoS One 2016, 11, e0147680. [Google Scholar] [CrossRef]
- Shi, L.Z.; Zheng, W. Establishment of an in vitro brain barrier epithelial transport system for pharmacological and toxicological study. Brain Research 2005, 1057, 37–48. [Google Scholar] [CrossRef]
- Zheng, W. Toxicology of choroid plexus: Special reference to metal-induced neurotoxicities. Microscopy Research and Technique 2001, 52, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, I.; Ishiwat, C.; Ishiwata, E.; Sato, Y.; Kiguchi, K.; Tachibana, T.; Hashimoto, H.; Ishikawa, H. Establishment and characterization of a human malignant choroids plexus papilloma cell line (HIBCPP). Human Cell 2005, 18, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Muranyi, W.; Schwerk, C.; Herold, R.; Stump-Guthier, C.; Lampe, M.; Fallier-Becker, P.; Weiß, C.; Sticht, C.; Ishikawa, H.; Schroten, H. Immortalized human choroid plexus endothelial cells enable an advanced endothelial-epithelial two-cell type in vitro model of the choroid plexus. Iscience 2022, 25, 1–23. [Google Scholar] [CrossRef]
- Giovannucci, T.A.; Leckey, C.A.; Moncur, E.; Tariq, K.; Thorne, L.; Watkins, L.; Toma, A.; Fox, N.C.; Bateman, R.J.; Mills, K. Choroid plexus protein turnover in human choroid plexus organoids recapitulates turnover in humans measured using stable isotope labeling kinetics (SILK). Alzheimer's & Dementia 2023, 19, e074240. [Google Scholar]
- Praetorius, J.; Nielsen, S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. American Journal of Physiology-Cell Physiology 2006, 291, C59–C67. [Google Scholar] [CrossRef]
- MacAulay, N.; Toft-Bertelsen, T.L. Dual function of the choroid plexus: cerebrospinal fluid production and control of brain ion homeostasis. Cell Calcium 2023, 102797. [Google Scholar] [CrossRef] [PubMed]
- Roepke, T.K.; Kanda, V.A.; Purtell, K.; King, E.C.; Lerner, D.J.; Abbott, G.W. KCNE2 forms potassium channels with KCNA3 and KCNQ1 in the choroid plexus epithelium. The FASEB Journal 2011, 25, 4264. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.; Lu, J.; Mount, D.; Delpire, E. Localization of the K+–Cl− cotransporter, KCC3, in the central and peripheral nervous systems: expression in the choroid plexus, large neurons and white matter tracts. Neuroscience 2001, 103, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Leefmans, F.J. CrossTalk proposal: Apical NKCC1 of choroid plexus epithelial cells works in the net inward flux mode under basal conditions, maintaining intracellular Cl− and cell volume. The Journal of Physiology 2020, 598, 4733–4736. [Google Scholar] [CrossRef] [PubMed]
- MacAulay, N.; Rose, C. CrossTalk opposing view: NKCC1 in the luminal membrane of choroid plexus is outwardly directed under basal conditions and contributes directly to cerebrospinal fluid secretion. The Journal of Physiology 2020, 598, 4737–4739. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Leefmans, F.J. Rebuttal from Francisco J. Alvarez-Leefmans. The Journal of Physiology 2020, 598, 4741–4742. [Google Scholar] [CrossRef]
- MacAulay, N.; Rose, C.R. Rebuttal from Nanna MacAulay and Christine R. Rose. The Journal of Physiology 2020, 598, 4743–4743. [Google Scholar] [CrossRef]
- Delpire, E.; Gagnon, K.B. Elusive role of the Na-K-2Cl cotransporter in the choroid plexus. American Journal of Physiology-Cell Physiology 2019, 316, C522–C524. [Google Scholar] [CrossRef]
- Fame, R.M.; Xu, H.; Pragana, A.; Lehtinen, M. Age-appropriate potassium clearance from perinatal cerebrospinal fluid depends on choroid plexus NKCC1. Fluids and Barriers of the CNS 2023, 20, 1–10. [Google Scholar] [CrossRef]
- Zeuthen, T.; MacAulay, N. Cotransport of water by Na+–K+–2Cl− cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. The Journal of Physiology 2012, 590, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, J.; Zhang, Y.; Liu, T.; Friedel, P.; Zhuo, W.; Somasekharan, S.; Roy, K.; Zhang, L.; Liu, Y. The structural basis of function and regulation of neuronal cotransporters NKCC1 and KCC2. Communications Biology 2021, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Macvicar, B.A.; Feighan, D.; Brown, A.; Ransom, B. Intrinsic optical signals in the rat optic nerve: role for K+ uptake via NKCC1 and swelling of astrocytes. Glia 2002, 37, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-L.; Yu, M.-J.; Kassai, E.M.; Morris, R.G.; Hoffert, J.D.; Wall, S.M.; Knepper, M.A. Roles of basolateral solute uptake via NKCC1 and of myosin II in vasopressin-induced cell swelling in inner medullary collecting duct. American Journal of Physiology-Renal Physiology 2008, 295, F192–F201. [Google Scholar] [CrossRef] [PubMed]
- Blazer-Yost, B.L. Consideration of Kinase Inhibitors for the Treatment of Hydrocephalus. International Journal of Molecular Sciences 2023, 24, 1–13. [Google Scholar] [CrossRef]
- Koumangoye, R.; Bastarache, L.; Delpire, E. NKCC1: newly found as a human disease-causing ion transporter. Function 2021, 2, 1–16. [Google Scholar] [CrossRef]
- Gregoriades, J.M.; Madaris, A.; Alvarez, F.J.; Alvarez-Leefmans, F.J. Genetic and pharmacological inactivation of apical Na+-K+-2Cl− cotransporter 1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. American Journal of Physiology-Cell Physiology 2019, 316, C525–C544. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, J.; Minichiello, L. NKCC1 deficiency in forming hippocampal circuits triggers neurodevelopmental disorder: role of BDNF-TrkB signalling. Brain Sciences 2022, 12, 502. [Google Scholar] [CrossRef]
- Karimy, J.K.; Zhang, J.; Kurland, D.B.; Theriault, B.C.; Duran, D.; Stokum, J.A.; Furey, C.G.; Zhou, X.; Mansuri, M.S.; Montejo, J. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nature Medicine 2017, 23, 997–1003. [Google Scholar] [CrossRef]
- Sadegh, C.; Xu, H.; Sutin, J.; Fatou, B.; Gupta, S.; Pragana, A.; Taylor, M.; Kalugin, P.N.; Zawadzki, M.E.; Alturkistani, O. Choroid plexus-targeted NKCC1 overexpression to treat post-hemorrhagic hydrocephalus. Neuron 2023, 111, 1591–1608.e1594. [Google Scholar] [CrossRef]
- Bothwell, S.W.; Omileke, D.; Patabendige, A.; Spratt, N.J. CSF secretion is not altered by NKCC1 nor TRPV4 antagonism in healthy rats. Brain Sciences 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Savardi, A.; Borgogno, M.; De Vivo, M.; Cancedda, L. Pharmacological tools to target NKCC1 in brain disorders. Trends in Pharmacological Sciences 2021, 42, 1009–1034. [Google Scholar] [CrossRef]
- Shibasaki, K. TRPV4 ion channel as important cell sensors. Journal of Anesthesia 2016, 30, 1014–1019. [Google Scholar] [CrossRef]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Hudspeth, A.; Friedman, J.M.; Heller, S. Vanilloid receptor–related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T. TRPV4 calcium entry channel: a paradigm for gating diversity. American Journal of Physiology-Cell Physiology 2004, 286, C195–C205. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nature Cell Biology 2000, 2, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Darby, W.; Grace, M.; Baratchi, S.; McIntyre, P. Modulation of TRPV4 by diverse mechanisms. The International Journal of Biochemistry & Cell Biology 2016, 78, 217–228. [Google Scholar]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4-/-mice. Proceedings of the National Academy of Sciences 2003, 100, 13698–13703. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, K.; Suzuki, M.; Mizuno, A.; Hara, A. Hearing impairment in TRPV4 knockout mice. Neuroscience Letters 2005, 382, 304–308. [Google Scholar] [CrossRef]
- Goyal, N.; Skrdla, P.; Schroyer, R.; Kumar, S.; Fernando, D.; Oughton, A.; Norton, N.; Sprecher, D.L.; Cheriyan, J. Clinical pharmacokinetics, safety, and tolerability of a novel, first-in-class TRPV4 ion channel inhibitor, GSK2798745, in healthy and heart failure subjects. American Journal of Cardiovascular Drugs 2019, 19, 335–342. [Google Scholar] [CrossRef]
- Lawhorn, B.G.; Brnardic, E.J.; Behm, D.J. TRPV4 antagonists: A patent review (2015–2020). Expert Opinion on Therapeutic Patents 2021, 31, 773–784. [Google Scholar] [CrossRef]
- Park, S.; Ku, S.K.; Ji, H.W.; Choi, J.-H.; Shin, D.M. Ca(2+) is a Regulator of the WNK/OSR1/NKCC Pathway in a Human Salivary Gland Cell Line. The Korean Journal of Physiology and Pharmacology 2015, 19, 249–255. [Google Scholar] [CrossRef]
- Toft-Bertelsen, T.L.; Barbuskaite, D.; Heerfordt, E.K.; Lolansen, S.D.; Andreassen, S.N.; Rostgaard, N.; Olsen, M.H.; Norager, N.H.; Capion, T.; Rath, M.F. Lysophosphatidic acid as a CSF lipid in posthemorrhagic hydrocephalus that drives CSF accumulation via TRPV4-induced hyperactivation of NKCC1. Fluids and Barriers of the CNS 2022, 19, 1–17. [Google Scholar] [CrossRef]
- Jin, S.C.; Furey, C.G.; Zeng, X.; Allocco, A.; Nelson-Williams, C.; Dong, W.; Karimy, J.K.; Wang, K.; Ma, S.; Delpire, E. SLC12A ion transporter mutations in sporadic and familial human congenital hydrocephalus. Molecular Genetics & Genomic Medicine 2019, 7, e892. [Google Scholar]
- Kahle, K.T.; Flores, B.; Bharucha-Goebel, D.; Zhang, J.; Donkervoort, S.; Hegde, M.; Begum, G.; Duran, D.; Liang, B.; Sun, D. Peripheral motor neuropathy is associated with defective kinase regulation of the KCC3 cotransporter. Science Signaling 2016, 9, ra77–ra77. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Alessi, D.R. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signaling pathway. Journal of Cell Science 2008, 121, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Lauf, P.K.; Bauer, J. Direct evidence for chloride-dependent volume reduction in macrocytic sheep reticulocytes. Biochemical and Biophysical Research Communications 1987, 144, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Mercado, A.; Broumand, V.; Zandi-Nejad, K.; Enck, A.H.; Mount, D.B. A C-terminal domain in KCC2 confers constitutive K+-Cl-cotransport. Journal of Biological Chemistry 2006, 281, 1016–1026. [Google Scholar] [CrossRef]
- Quinton, P.M. Chloride impermeability in cystic fibrosis. Nature 1983, 301, 421–422. [Google Scholar] [CrossRef]
- Bear, C.E.; Li, C.; Kartner, N.; Bridges, R.J.; Jensen, T.J.; Ramjeesingh, M.; Riordan, J.R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 1992, 68, 809–818. [Google Scholar] [CrossRef]
- Genovese, M.; Borrelli, A.; Venturini, A.; Guidone, D.; Caci, E.; Viscido, G.; Gambardella, G.; di Bernardo, D.; Scudieri, P.; Galietta, L.J. TRPV4 and purinergic receptor signalling pathways are separately linked in airway epithelia to CFTR and TMEM16A chloride channels. The Journal of Physiology 2019, 597, 5859–5878. [Google Scholar] [CrossRef]
- Johnsen, L.Ø.; Friis, K.A.; Damkier, H.H. In vitro investigation of the effect of proinflammatory cytokines on mouse choroid plexus membrane transporters Ncbe and NKCC1. Fluids and Barriers of the CNS 2023, 20, 1–14. [Google Scholar] [CrossRef]
- Praetorius, J.; Damkier, H.H. Transport across the choroid plexus epithelium. American Journal of Physiology-Cell Physiology 2017, 312, C673–C686. [Google Scholar] [CrossRef]
- Damkier, H.H.; Praetorius, J. Genetic ablation of Slc4a10 alters the expression pattern of transporters involved in solute movement in the mouse choroid plexus. American Journal of Physiology-Cell Physiology 2012, 302, C1452–C1459. [Google Scholar] [CrossRef]
- Christensen, I.B.; Wu, Q.; Bohlbro, A.S.; Skals, M.G.; Damkier, H.H.; Hübner, C.A.; Fenton, R.A.; Praetorius, J. Genetic disruption of slc4a10 alters the capacity for cellular metabolism and vectorial ion transport in the choroid plexus epithelium. Fluids and Barriers of the CNS 2020, 17, 1–18. [Google Scholar] [CrossRef]
- Yang, D.; Li, Q.; So, I.; Huang, C.-L.; Ando, H.; Mizutani, A.; Seki, G.; Mikoshiba, K.; Thomas, P.J.; Muallem, S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. The Journal of Clinical Investigation 2011, 121, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Ruusuvuori, E.; Sipilä, S.T.; Haapanen, A.; Damkier, H.H.; Kurth, I.; Hentschke, M.; Schweizer, M.; Rudhard, Y.; Laatikainen, L.M. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proceedings of the National Academy of Sciences 2008, 105, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Gurnett, C.A.; Veile, R.; Zempel, J.; Blackburn, L.; Lovett, M.; Bowcock, A. Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental retardation. Archives of Neurology 2008, 65, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Fasham, J.; Huebner, A.K.; Liebmann, L.; Khalaf-Nazzal, R.; Maroofian, R.; Kryeziu, N.; Wortmann, S.B.; Leslie, J.S.; Ubeyratna, N.; Mancini, G.M. SLC4A10 mutation causes a neurological disorder associated with impaired GABAergic transmission. Brain 2023, 146, 4547–4561. [Google Scholar] [CrossRef]
| Treatment Type | Benefits | Complications | References |
|---|---|---|---|
| Ventriculoperitoneal shunts (VPS) | Treats both communicating and non-communicating hydrocephalus Effective for patients suffering from iNPH |
Failure due to mechanical problems, catheter migration, cell overgrowth Fails frequently in pediatric patients; surgery required for shunt revisions Possibility of infection |
[23,24,25] |
| Ventriculoperitoneal shunts and the Reflow® System | Fewer shunt obstructions | Still in clinical trials Can fail from other complications typical of VPS |
[26] |
| Lumboperitoneal shunts (LPS) | Does not require brain surgery Decreases chances of brain hemorrhage |
Cannot be used for non-communicating hydrocephalus Failure due to mechanical problems, catheter migration, cell overgrowth Possibility of infection |
[27,28] |
| Endoscopic third ventriculostomy (ETV) | Does not require a shunt | Pediatric patients do not respond to treatment Requires brain surgery Possibility of infection |
[29,30,31] |
| Endoscopic third ventriculostomy/choroid plexus cauterization (ETV/CPC) | Lower need for re-operation when compared to ETV alone May be more beneficial in developing countries |
Long term effects of CPC have not been studied Possibility of infection |
[32,33,34] |
| Disease Model | Animal Models | Type of Hydrocephalus | References |
|---|---|---|---|
| Hydrocephalus caused by traumatic brain injury | Fluid percussion injury model with injection of FeCl3 | Communicating | [50] |
| Post-hemorrhagic hydrocephalus | Induced models using injections of blood, red blood cells, iron, hemoglobin | Communicating | [8,44,51] |
| Chemically induced hydrocephalus | Kaolin injected models, 6-AN rats | Communicating | [52,53,54,55,56,57,58,59,60,61,62,63] |
| Genetic hydrocephalus models | Rat: Wpk, LEW/Jms Mouse: L1CAM, E2F5, Gas8, CCDC39, Hy-3, Hpy, Hyh, Msx1, SUMS/NP |
Communicating | [64,65,70,71,72,73,74,75,76,77,78,79,80,84,85,] |
| Genetic hydrocephalus models | Rat: H-Tx Mouse: Rnd3, Hhy |
Non-communicating | [60,68,69,82,83] |
| Genetic model of post-hemorrhagic hydrocephalus | Mouse: Mf1 Mouse: Hhy |
Communicating Non-communicating |
[60,81,82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





