Submitted:
24 January 2024
Posted:
25 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Synthesis of Modified Carbons
2.3. Adsorbent Characterization
2.4. Adsorption of Gallic Acid
2.5. Reuse Experiments
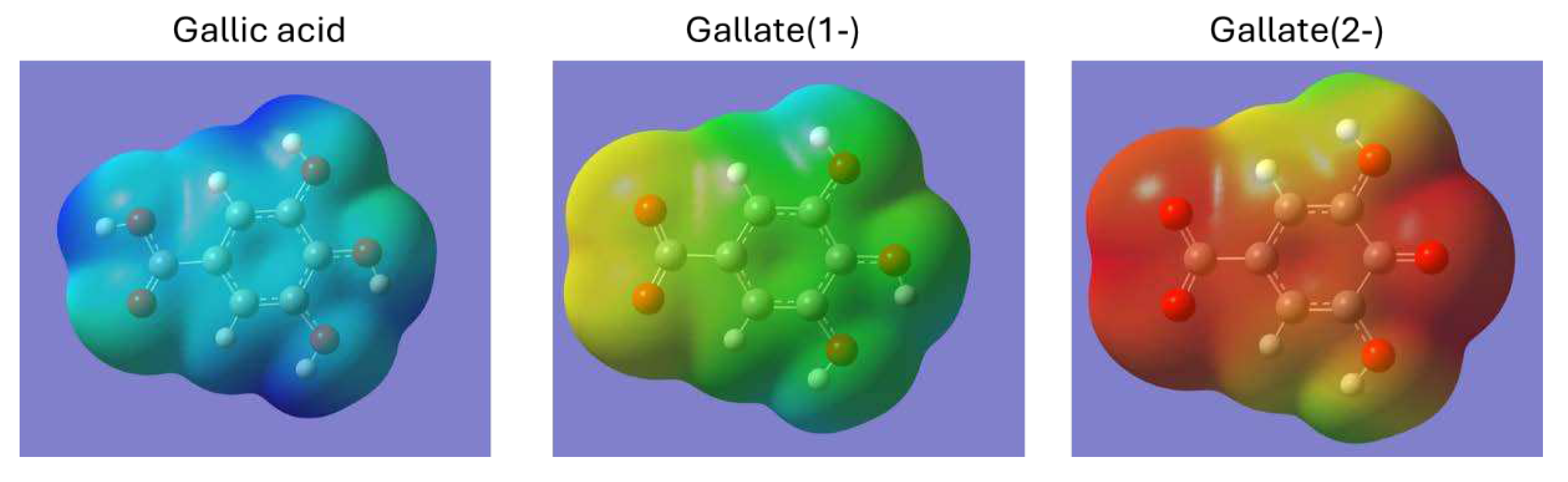
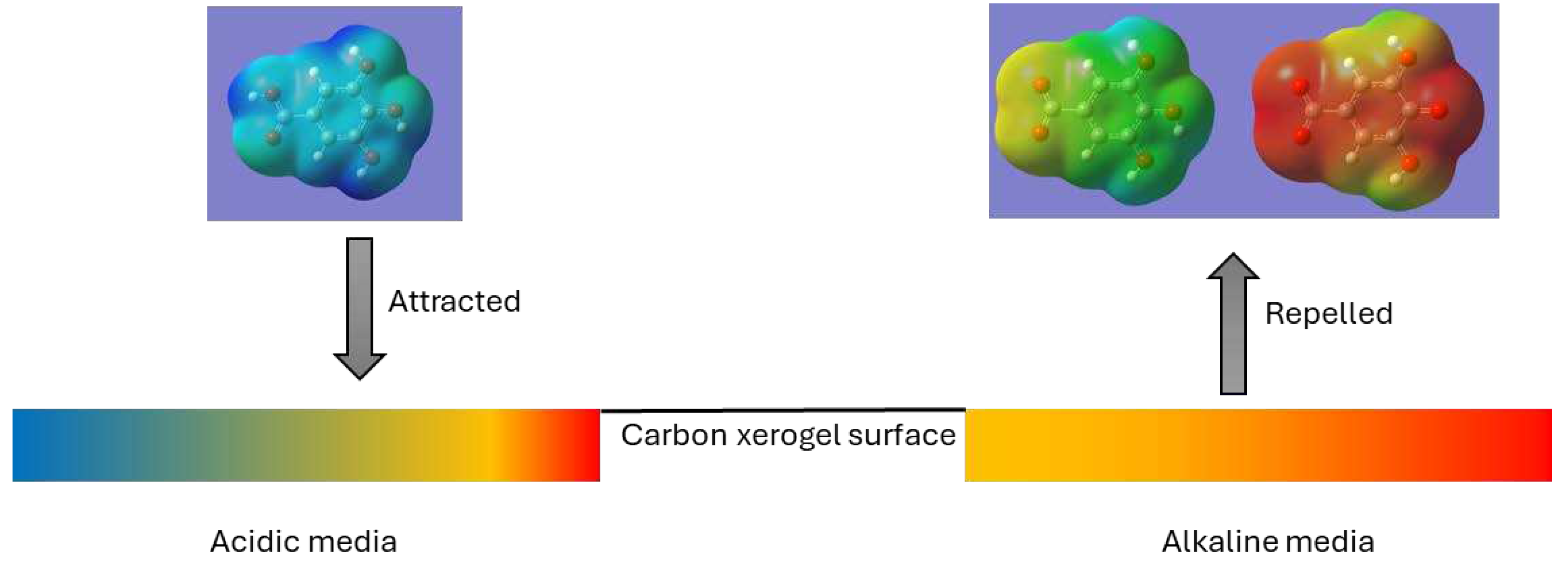
2.6. Computational Study
3. Results and Discussion
3.1. Chemical Characterization of Adsorbents
3.1.1. Composition
3.1.2. Acidic and basic properties
3.2. Structural Characterization
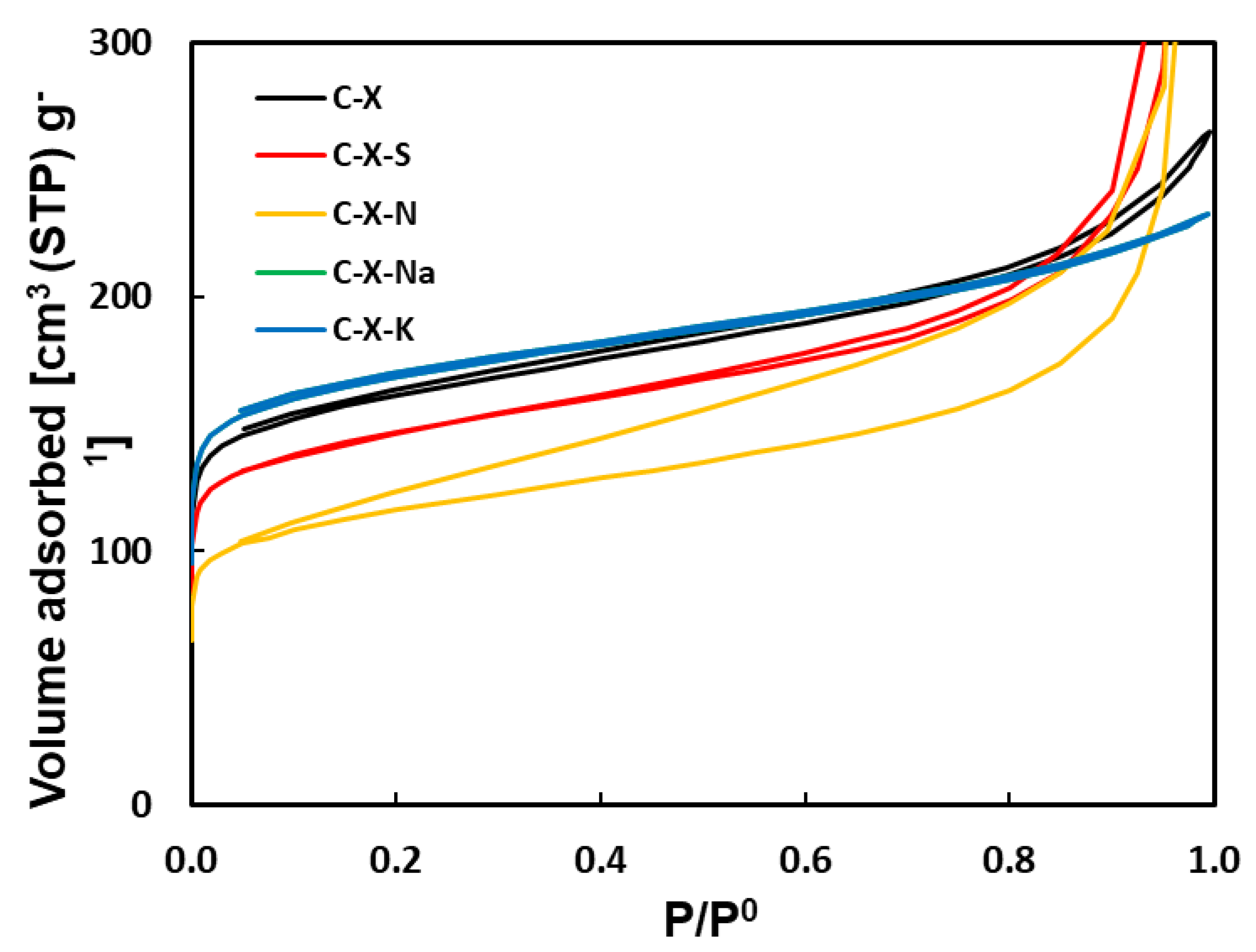
3.2.1. N2 Adsorption
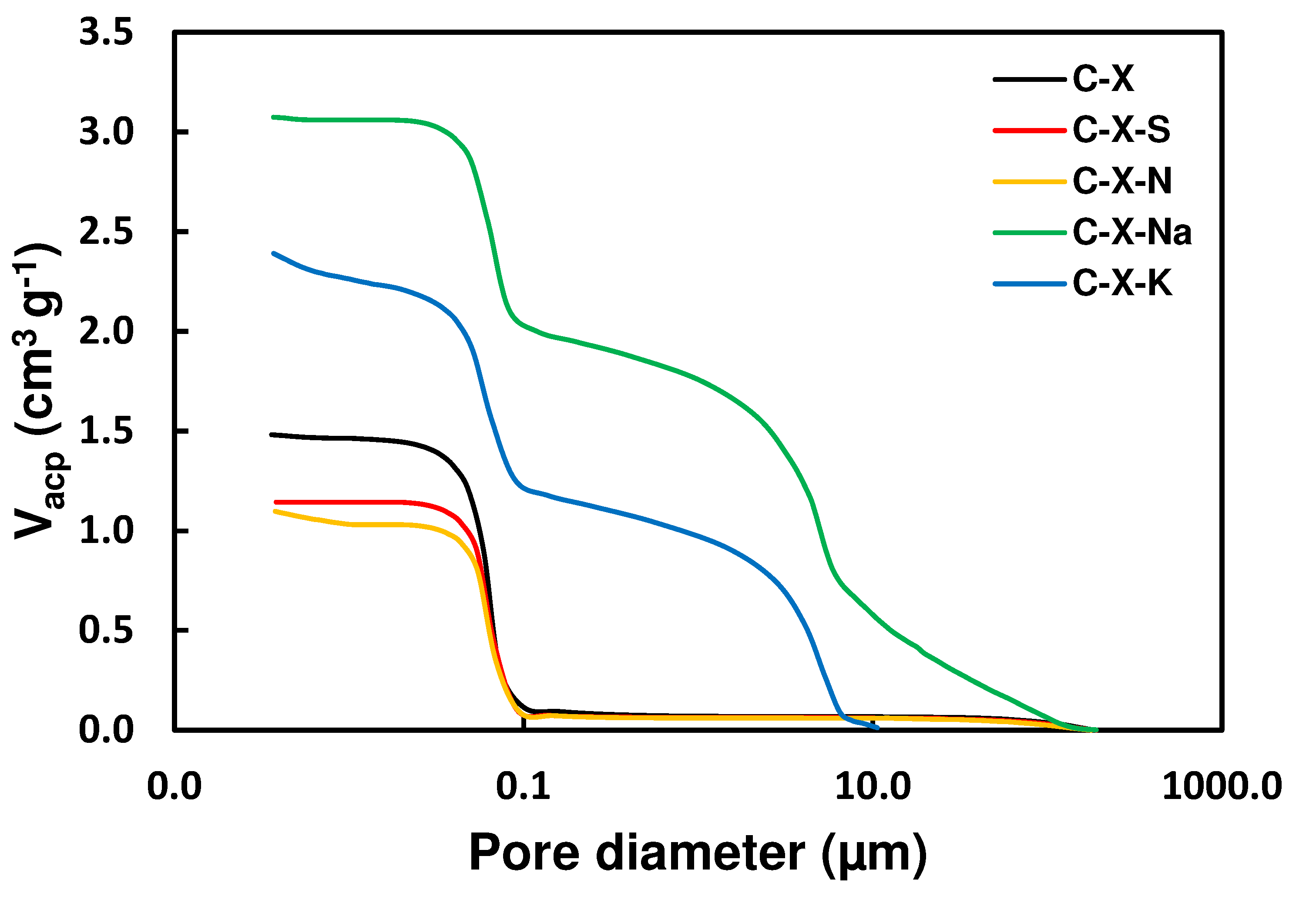
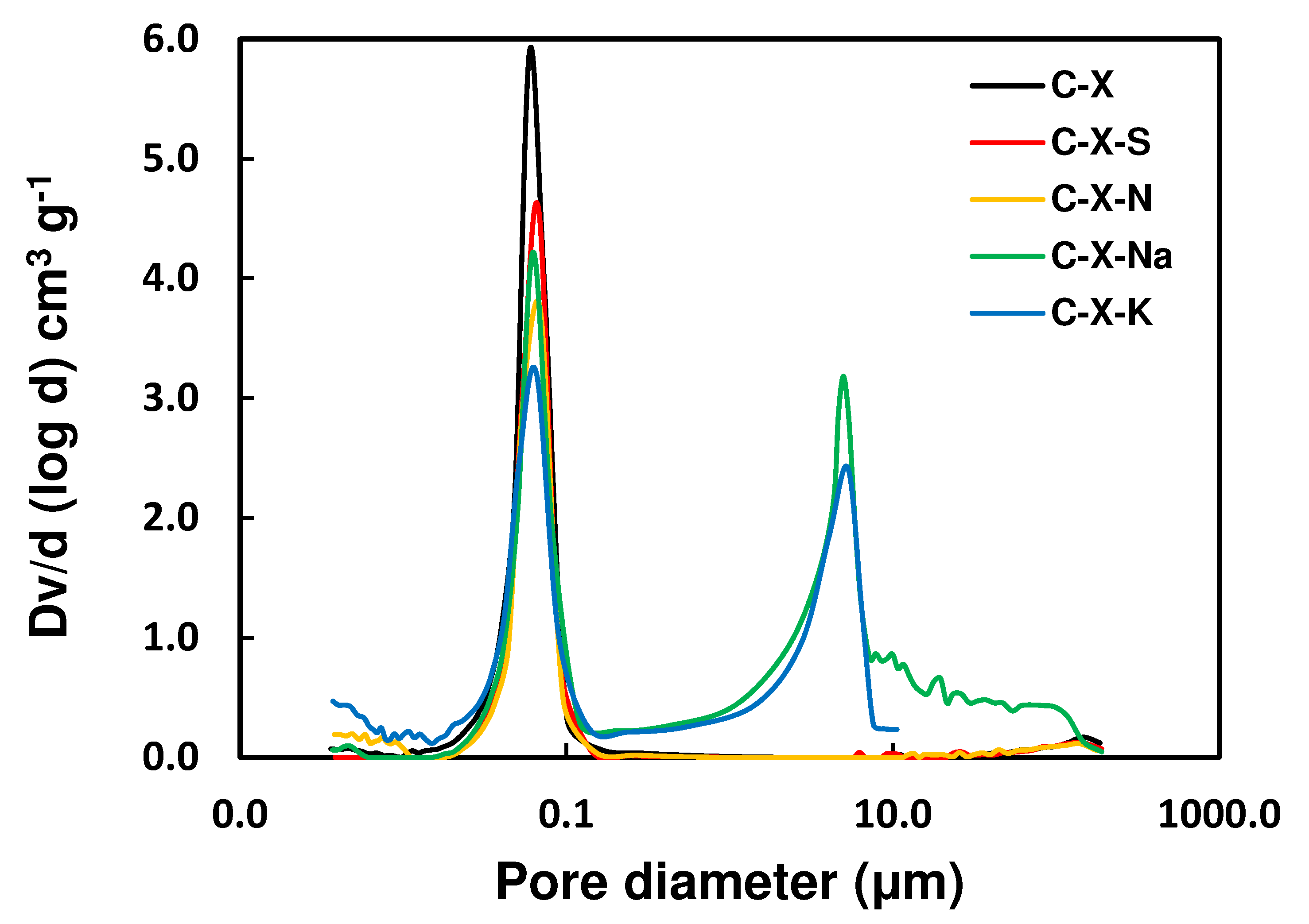
3.2.2. Mercury Porosimetry
3.2.3. Scanning Electron Microscopy
3.3. Gallic Acid Adsorption
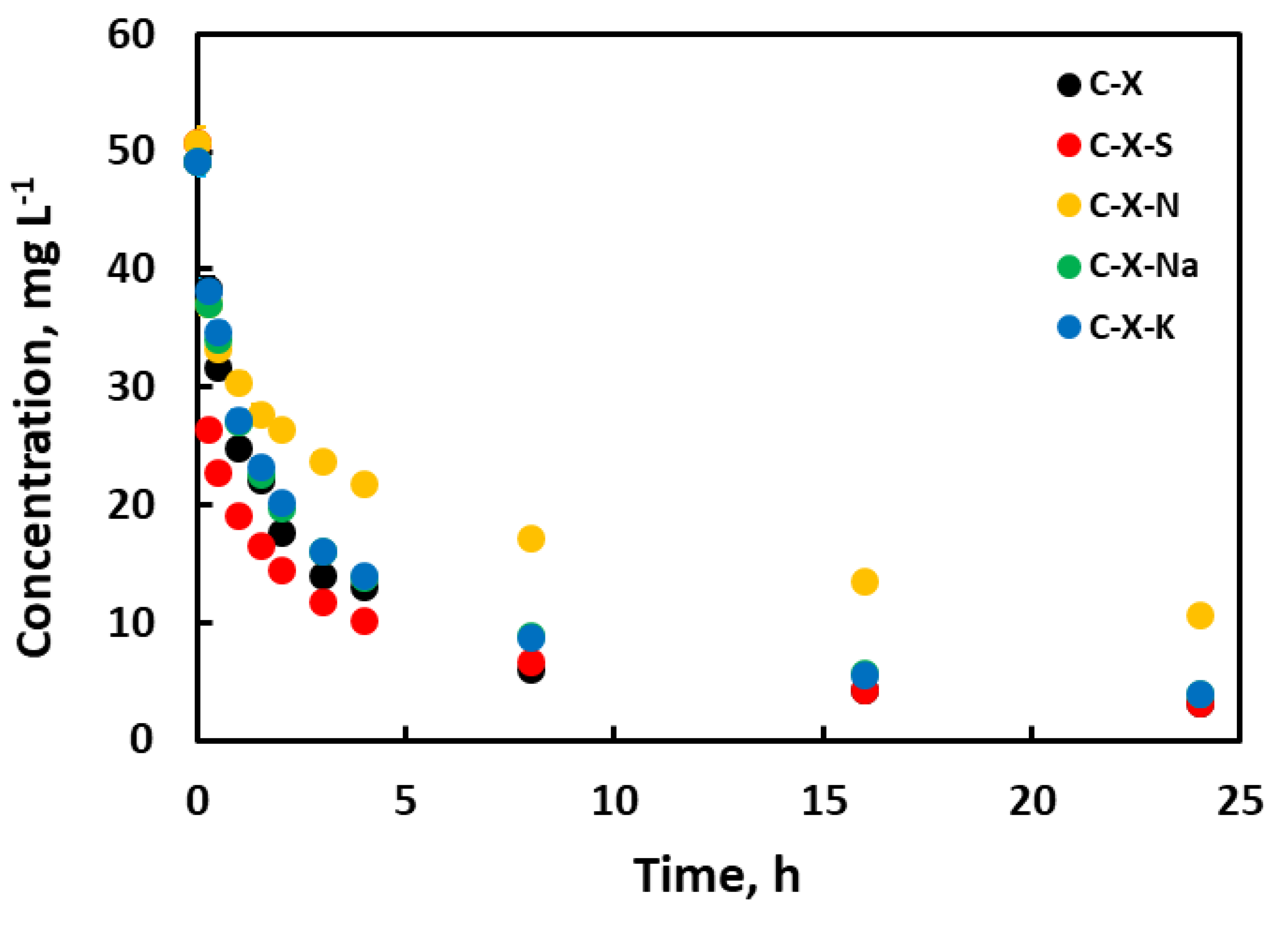
3.3.1. Adsorption Kinetics
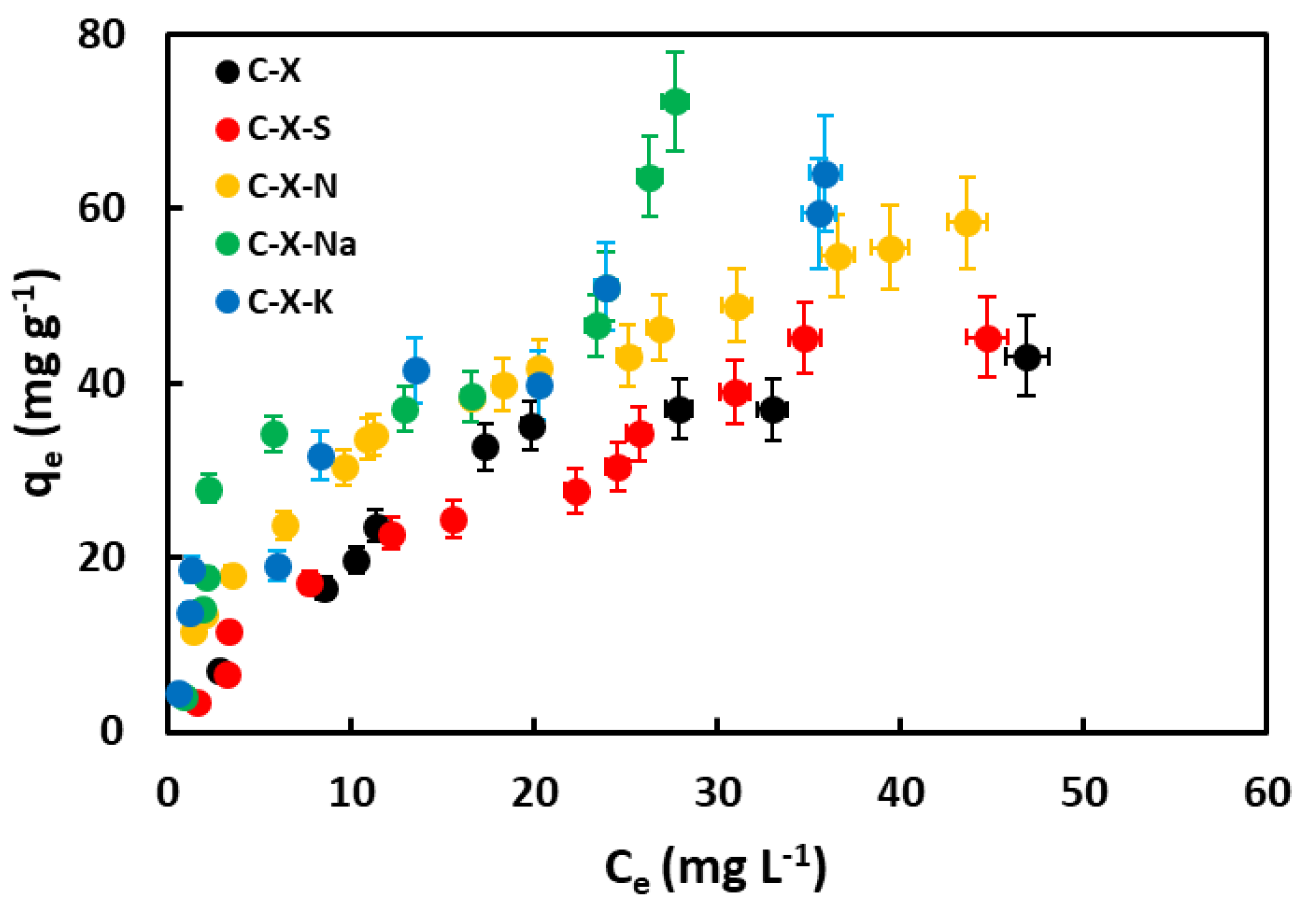
3.3.2. Adsorption Isotherms
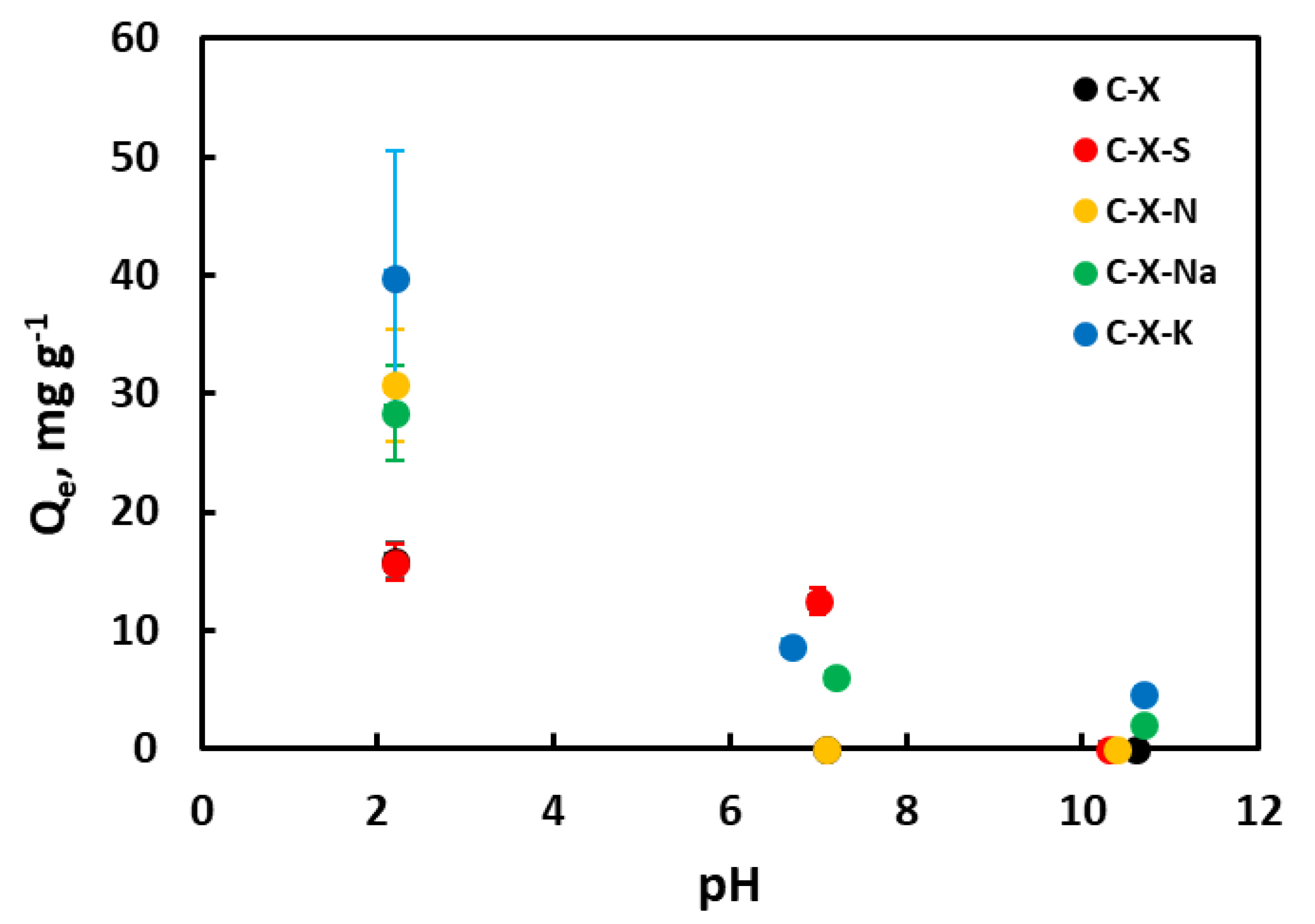
3.3.3. Influence of pH
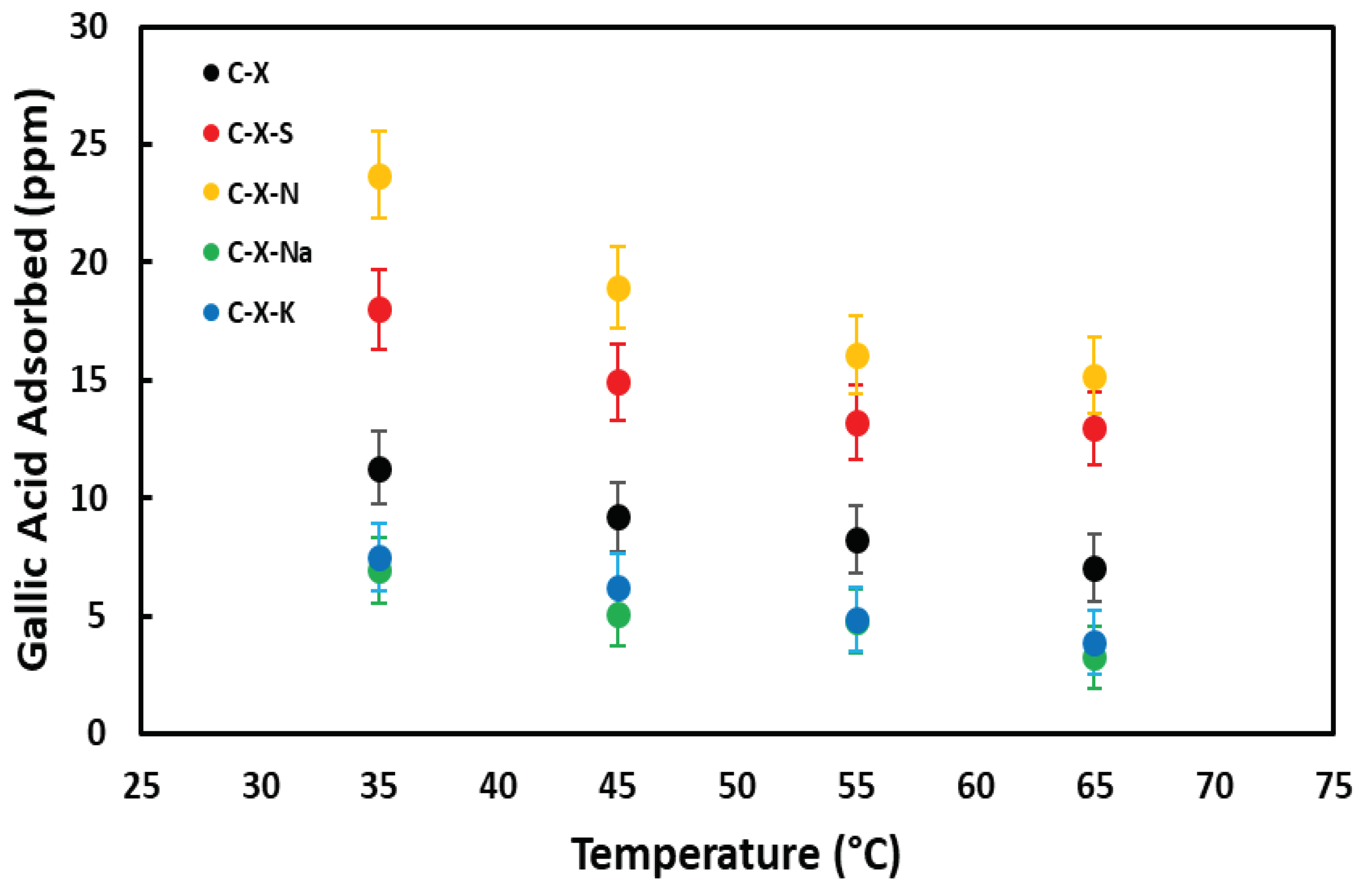
3.3.4. Influence of Temperature
3.3.5. Reuse Experiment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chedri Mammar, A.; Mouni, L.; Bollinger, J.-C.; Belkhiri, L.; Bouzaza, A.; Assadi, A.A.; Belkacemi, H. Modeling and Optimization of Process Parameters in Elucidating the Adsorption Mechanism of Gallic Acid on Activated Carbon Prepared from Date Stones. Sep Sci Technol 2020, 55, 3113–3125. [Google Scholar] [CrossRef]
- Wang, J.; Li, A.; Xu, L.; Zhou, Y. Adsorption of Tannic and Gallic Acids on a New Polymeric Adsorbent and the Effect of Cu(II) on Their Removal. J Hazard Mater 2009, 169, 794–800. [Google Scholar] [CrossRef]
- Lin, C.C.; Liu, Y.T.; Chang, P.H.; Hsieh, Y.C.; Tzou, Y.M. Inhibition of Continuous Cropping Obstacle of Celery by Chemically Modified Biochar: An Efficient Approach to Decrease Bioavailability of Phenolic Allelochemicals. J Environ Manage 2023, 348, 119316. [Google Scholar] [CrossRef] [PubMed]
- Boye, B.; Brillas, E.; Buso, A.; Farnia, G.; Flox, C.; Giomo, M.; Sandonà, G. Electrochemical Removal of Gallic Acid from Aqueous Solutions. Electrochim Acta 2006, 52, 256–262. [Google Scholar] [CrossRef]
- Taoufik, N.; Elmchaouri, A.; Anouar, F.; Korili, S.; Gil, A. Improvement of the Adsorption Properties of an Activated Carbon Coated by Titanium Dioxide for the Removal of Emerging Contaminants. Journal of Water Process Engineering 2019, 31, 100876. [Google Scholar] [CrossRef]
- Annab, H.; Fiol, N.; Villaescusa, I.; Essamri, A. A Proposal for the Sustainable Treatment and Valorisation of Olive Mill Wastes. J Environ Chem Eng 2019, 7, 102803. [Google Scholar] [CrossRef]
- Fu, Y.; Gong, H.; Su, S.; Lei, T.; Zhong, S. Fabrication of O-Enriched Macroporous Polymer for the Efficient Adsorption Organic Acid from Aqueous Solution. Journal of Polymer Research 2023, 30, 1–10. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Adame-Pereira, M.; Alexandre-Franco, M.; Fernández-González, C. Adsorption of Bisphenol A by Activated Carbon Developed from PET Waste by KOH Activation. Environmental Science and Pollution Research 2021, 28, 24342–24354. [Google Scholar] [CrossRef]
- Mendoza-Carrasco, R.; Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C.; Gómez-Serrano, V. Preparation of High-Quality Activated Carbon from Polyethyleneterephthalate (PET) Bottle Waste. Its Use in the Removal of Pollutants in Aqueous Solution. J Environ Manage 2016, 181, 522–535. [Google Scholar] [CrossRef]
- Cheng, G.; Sun, M.; Ge, X.; Ou, Y.; Xu, X.; Lin, Q.; Lou, L. Adsorption-Desorption Characteristics of Nonylphenol on Two Different Origins of Black Carbon. Water Air Soil Pollut 2017, 228. [Google Scholar] [CrossRef]
- Domínguez-Vargas, J.R.; Navarro-Rodríguez, J.A.; de Heredia, J.B.; Cuerda-Correa, E.M. Removal of Chlorophenols in Aqueous Solution by Carbon Black Low-Cost Adsorbents. Equilibrium Study and Influence of Operation Conditions. J Hazard Mater 2009, 169, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.L. Carbon Xerogels for Catalytic Applications. Boletín del Grupo Español del Carbón 2012, 26, 12–17. [Google Scholar]
- Job, N.; Heinrichs, B.; Lambert, S.; Pirard, J.; Colomer, J.; Vertruyen, B.; Marien, J. Carbon Xerogels as Catalyst Supports: Study of Mass Transfer. AIChE journal 2006, 52, 2663–2676. [Google Scholar] [CrossRef]
- Men’shchikov, I.E.; Shkolin, A. V.; Khozina, E. V.; Grinchenko, A.E.; Fomkin, A.A. Mesoporous Carbon Xerogel as a Promising Adsorbent for Capture and Storage of Liquified Natural Gas Vapors. Adsorption 2023, 29, 255–273. [Google Scholar] [CrossRef]
- Calvo, E.G.; Ania, C.O.; Zubizarreta, L.; Menéndez, J.A.; Arenillas, A. Exploring New Routes in the Synthesis of Carbon Xerogels for Their Application in Electric Double-Layer Capacitors. Energy & fuels 2010, 24, 3334–3339. [Google Scholar] [CrossRef]
- Zubizarreta, L.; Arenillas, A.; Pirard, J.-P.; Pis, J.J.; Job, N. Tailoring the Textural Properties of Activated Carbon Xerogels by Chemical Activation with KOH. Microporous and mesoporous materials. 2008, 115, 480–490. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Arenillas, A.; Malaika, A.; Fernando, M.; Figueiredo, J.L. The Influence of the Surface Chemistry of Phosphorylated Carbon Xerogel Catalysts on the Production of HMF from Fructose in Water. Fuel 2023, 334, 126610. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Carbon Gels in Catalysis. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons: New York, 2008; pp. 373–400. [Google Scholar]
- Fairén-Jiménez, D.; Carrasco-Marín, F.; Moreno-Castilla, C. Adsorption of Benzene, Toluene, and Xylenes on Monolithic Carbon Aerogels from Dry Air Flows. 2007. 2007. [CrossRef]
- Carrales-Alvarado, D.H.; Leyva-Ramos, R.; Bailón-García, E.; Carrasco-Marín, F.; Villela-Martinez, D.E. Synthesis, Characterization, and Application of Pristine and Clay-Templated Carbon Xerogel Microspheres for Removing Diclofenac and Heavy Metals from Water Solution. Environmental Science and Pollution Research 2023, 30, 34684–34697. [Google Scholar] [CrossRef]
- Safri, A.; Fletcher, A.J.; Safri, A.; Fletcher, A.J. Concentration Dependence of TiO2 Nanoparticles in Carbon Xerogels on Adsorption–Photodegradation Applications. Gels 2023, Vol. 9, Page 468 2023, 9, 468. [Google Scholar] [CrossRef]
- Calvo, E.; Ferrera-Lorenzo, N.; Menéndez, J.; Arenillas, A. Microwave Synthesis of Micro-Mesoporous Activated Carbon Xerogels for High Performance Supercapacitors. Microporous and mesoporous materials. 2013, 168, 206–212. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and Properties of Resorcinol-Formaldehyde Organic and Carbon Gels. Advanced Materials 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.-P. Porous Carbon Xerogels with Texture Tailored by PH Control during Sol-Gel Process. Carbon N Y 2004, 42, 619–628. [Google Scholar] [CrossRef]
- Canal-Rodríguez, M.; Menéndez, J.A.; Arenillas, A. Carbon Xerogels: The Bespoke Nanoporous Carbons. In Porosity - Process, Technologies and Applications; Taher, G., Ed.; InTech: London, 2017. [Google Scholar]
- Zapata-Benabithe, Z.; Carrasco-Marín, F.; de Vicente, J.; Moreno-Castilla, C. Carbon Xerogel Microspheres and Monoliths from Resorcinol–Formaldehyde Mixtures with Varying Dilution Ratios: Preparation, Surface Characteristics, and Electrochemical Double-Layer Capacitances. LANGMUIR : ACS journal of surfaces and colloids 2013, 29, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.S.; Páez, C.A.; Zubizarreta, L.; Léonard, A.; Blacher, S.; Olivera-Fuentes, C.G.; Arenillas, A.; Pirard, J.-P.; Job, N. A Comparison of Physical Activation of Carbon Xerogels with Carbon Dioxide with Chemical Activation Using Hydroxides. Carbon N Y 2010, 48, 3157–3168. [Google Scholar] [CrossRef]
- Neme, I.; Gonfa, G.; Masi, C. Activated Carbon from Biomass Precursors Using Phosphoric Acid: A Review. Heliyon 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.G.; Hoon, G.G.; Sharain-Liew, Y.L.; Krishnaiah, D.; Massuanna, M. Preparation and Characterization of Activated Carbon Derived from Waste Rubber Tire via Chemical Activation with ZnCl2: Surface Area and Morphological Studies. Developments in Sustainable Chemical and Bioprocess Technology 2013, 371–380. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent Advances in Applications of Activated Carbon from Biowaste for Wastewater Treatment: A Short Review. J Clean Prod 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Durán-Valle, C.; Madrigal-Martínez, M.; Martínez-Gallego, M.; Fonseca, I.; Matos, I.; Botelho do Rego, A. Activated Carbon as a Catalyst for the Synthesis of N-Alkylimidazoles and Imidazolium Ionic Liquids. Catal Today 2012, 187, 108–114. [Google Scholar] [CrossRef]
- Perozo-Rondón, E.; Calvino-Casilda, V.; Martín-Aranda, R.M.; Casal, B.; Durán-Valle, C.J.; Rojas-Cervantes, M.L. Catalysis by Basic Carbons: Preparation of Dihydropyridines. Appl Surf Sci 2006, 252, 6080–6083. [Google Scholar] [CrossRef]
- Ferrera-Escudero, S.; Perozo-Rondón, E.; Calvino-Casilda, V.; Casal, B.; Martín-Aranda, R.; López-Peinado, A.; Durán-Valle, C. The Effect of Ultrasound on the N-Alkylation of Imidazole over Alkaline Carbons: Kinetic Aspects. Appl Catal 2010, 378, 26–32. [Google Scholar] [CrossRef]
- Godino-Ojer, M.; Matos, I.; Bernardo, M.; Carvalho, R.; Olívia, O.S.; Durán-Valle, C.; Fonseca, I.M.; Mayoral, E.P. Acidic Porous Carbons Involved in the Green and Selective Synthesis of Benzodiazepines. Catal Today 2020, 357, 64–73. [Google Scholar] [CrossRef]
- Valente Nabais, J.M.; Carrott, P.J.M. Chemical Characterization of Activated Carbon Fibers and Activated Carbons. J Chem Educ 2006, 83, 436–438. [Google Scholar] [CrossRef]
- Lagergren, S.; Svenska, B.K. Zurtheorie Der Sogenannten Adsorption Geloesterstoffe. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochemistry 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solutions. Journal of the Sanitary Engineering Division, American Society of Civil Engineers 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J Am Chem Soc 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Zeitschrift für Physikalische Chemie 1906, 57, 471. [Google Scholar] [CrossRef]
- Lõpez-Sanz, J.; Pérez-Mayoral, E.; Soriano, E.; Omenat-Morán, D.; Durán, C.J.; Martín-Aranda, R.M.; Matos, I.; Fonseca, I. Acid-Activated Carbon Materials: Cheaper Alternative Catalysts for the Synthesis of Substituted Quinolines. ChemCatChem 2013, 5, 3736–3742. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; 2000; pp. 10815–10837. [Google Scholar]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds. Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Khaskheli, A.R.; Naz, S.; Ozul, F.; Aljabour, A.; Mahesar, S.A.; Patir, I.H.; Ersoz, M. Urchin-like Cobalt Nanostructures For Catalytic Degradation Of Nitro Anilines. Adv Mater Lett 2016, 7, 748–753. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Q.; Jiang, C.; Yang, D.; Liu, X.; Xu, S. One-Step Synthesis of Biocompatible Gold Nanoparticles Using Gallic Acid in the Presence of Poly-(N-Vinyl-2-Pyrrolidone). Colloids Surf A Physicochem Eng Asp 2007, 301, 73–79. [Google Scholar] [CrossRef]
- Hirun, N.; Dokmaisrijan, S.; Tantishaiyakul, V. Experimental FTIR and Theoretical Studies of Gallic Acid–Acetonitrile Clusters. Spectrochim Acta A Mol Biomol Spectrosc 2012, 86, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Calvino-Casilda, V.; López-Peinado, A.; Fierro, J.; Martίn-Aranda, R. Microwave Assisted N-Propargylation of Imidazole Using Alkaline Promoted Carbons. Appl Catal 2003, 240, 287–293. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, H.; Lee, S.; Cho, B.; Park, C. Effects of Sulfuric Acid Treatment on the Microstructure and Electrochemical Performance of a Polyacrylonitrile (PAN)-Based Carbon Anode. Carbon N Y 2005, 43, 163–169. [Google Scholar] [CrossRef]
- Oh, H.-J.; Lee, J.-H.; Ahn, H.-J.; Jeong, Y.; Kim, Y.-J.; Chi, C.-S. Nanoporous Activated Carbon Cloth for Capacitive Deionization of Aqueous Solution. Thin Solid Films 2006, 515, 220–225. [Google Scholar] [CrossRef]
- László, K.; Tombácz, E.; Josepovits, K. Effect of Activation on the Surface Chemistry of Carbons from Polymer Precursors. Carbon N Y 2001, 39, 1217–1228. [Google Scholar] [CrossRef]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Świątkowski, A. The Characterization of Activated Carbons with Oxygen and Nitrogen Surface Groups. Carbon N Y 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Terzyk, A.P. The Influence of Activated Carbon Surface Chemical Composition on the Adsorption of Acetaminophen (Paracetamol) in Vitro. Colloids and surfaces. 2001, 177, 23–45. [Google Scholar] [CrossRef]
- Gabriel, G.; Sauthier, G.; Fraxedas, J.; Moreno-Mañas, M.; Martínez, M.; Miravitlles, C.; Casabó, J. Preparation and Characterisation of Single-Walled Carbon Nanotubes Functionalised with Amines. Carbon N Y 2006, 44, 1891–1897. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Bulushev, D.A.; Kiwi-Minsker, L. Dynamic Behaviour of Activated Carbon Catalysts during Ozone Decomposition at Room Temperature. Appl Catal 2005, 61, 98–106. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van Der Waals Adsorption of Gases. J Am Chem Soc 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A. V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure and applied chemistry. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. 786. Studies in Adsorption. Part XI. A System of Classification of Solution Adsorption Isotherms, and Its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. Journal of the Chemical Society (Resumed) 1960, 3973–3993. [Google Scholar] [CrossRef]
- Cagnon, B.; Chedeville, O.; Cherrier, J.F.; Caqueret, V.; Porte, C. Evolution of Adsorption Kinetics and Isotherms of Gallic Acid on an Activated Carbon Oxidized by Ozone: Comparison to the Raw Material. J Taiwan Inst Chem Eng 2011, 42, 996–1003. [Google Scholar] [CrossRef]
- Krasnova, T.A.; Gora, N. V.; Golubeva, N.S. Influence of Physical-Chemical Properties of Active Carbons on Gallic Acid Adsorption. Foods and Raw Materials 2015, 3, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Wang, S.G.; Fan, J.L.; Ma, G.H. Adsorption of Natural Organic Matter Surrogates from Aqueous Solution by Multiwalled Carbon Nanotubes. Journal of Physical Chemistry C 2012, 116, 25783–25789. [Google Scholar] [CrossRef]
- Han, F.; Xu, C.; Sun, W.Z.; Yu, S.T.; Xian, M. Effective Removal of Salicylic and Gallic Acids from Single Component and Impurity-Containing Systems Using an Isatin-Modified Adsorption Resin. RSC Adv 2017, 7, 23164–23175. [Google Scholar] [CrossRef]
- Ahmat, A.M.; Thiebault, T.; Guégan, R. Phenolic Acids Interactions with Clay Minerals: A Spotlight on the Adsorption Mechanisms of Gallic Acid onto Montmorillonite. Appl Clay Sci 2019, 180, 105188. [Google Scholar] [CrossRef]
- Levy, L.; Gurov, A.; Radian, A. The Effect of Gallic Acid Interactions with Iron-Coated Clay on Surface Redox Reactivity. Water Res 2020, 184, 116190. [Google Scholar] [CrossRef]
- Celestino, G.G.; Henriques, R.R.; Shiguihara, A.L.; Constantino, V.R.L.; De, R.; Melo, S.; Júnior, J.A. Adsorption of Gallic Acid on Nanoclay Modified with Poly(Diallyldimethylammonium Chloride). [CrossRef]
- Fan, S.; Huang, Z.; Zhang, Y.; Hu, H.; Liang, X.; Gong, S.; Zhou, J.; Tu, R. Magnetic Chitosan-Hydroxyapatite Composite Microspheres: Preparation, Characterization, and Application for the Adsorption of Phenolic Substances. Bioresour Technol 2019, 274, 48–55. [Google Scholar] [CrossRef]
- Song, X.; Chai, Z.; Zhu, Y.; Li, C.; Liang, X. Preparation and Characterization of Magnetic Chitosan-Modified Diatomite for the Removal of Gallic Acid and Caffeic Acid from Sugar Solution. Carbohydr Polym 2019, 219, 316–327. [Google Scholar] [CrossRef]
- Chai, Z.; Li, C.; Zhu, Y.; Song, X.; Chen, M.; Yang, Y.L.; Chen, D.; Liang, X.; Wu, J. Arginine-Modified Magnetic Chitosan: Preparation, Characterization and Adsorption of Gallic Acid in Sugar Solution. Int J Biol Macromol 2020, 165, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Kakkar, R. DFT Study of Structural and Electronic Properties of Gallic Acid and Its Anions in Gas Phase and in Aqueous Solution. [CrossRef]
- Radalla, A.M. Potentiometric Studies on Ternary Complexes Involving Some Divalent Transition Metal Ions, Gallic Acid and Biologically Abundant Aliphatic Dicarboxylic Acids in Aqueous Solutions. Beni Suef Univ J Basic Appl Sci 2015, 4, 174–182. [Google Scholar] [CrossRef]

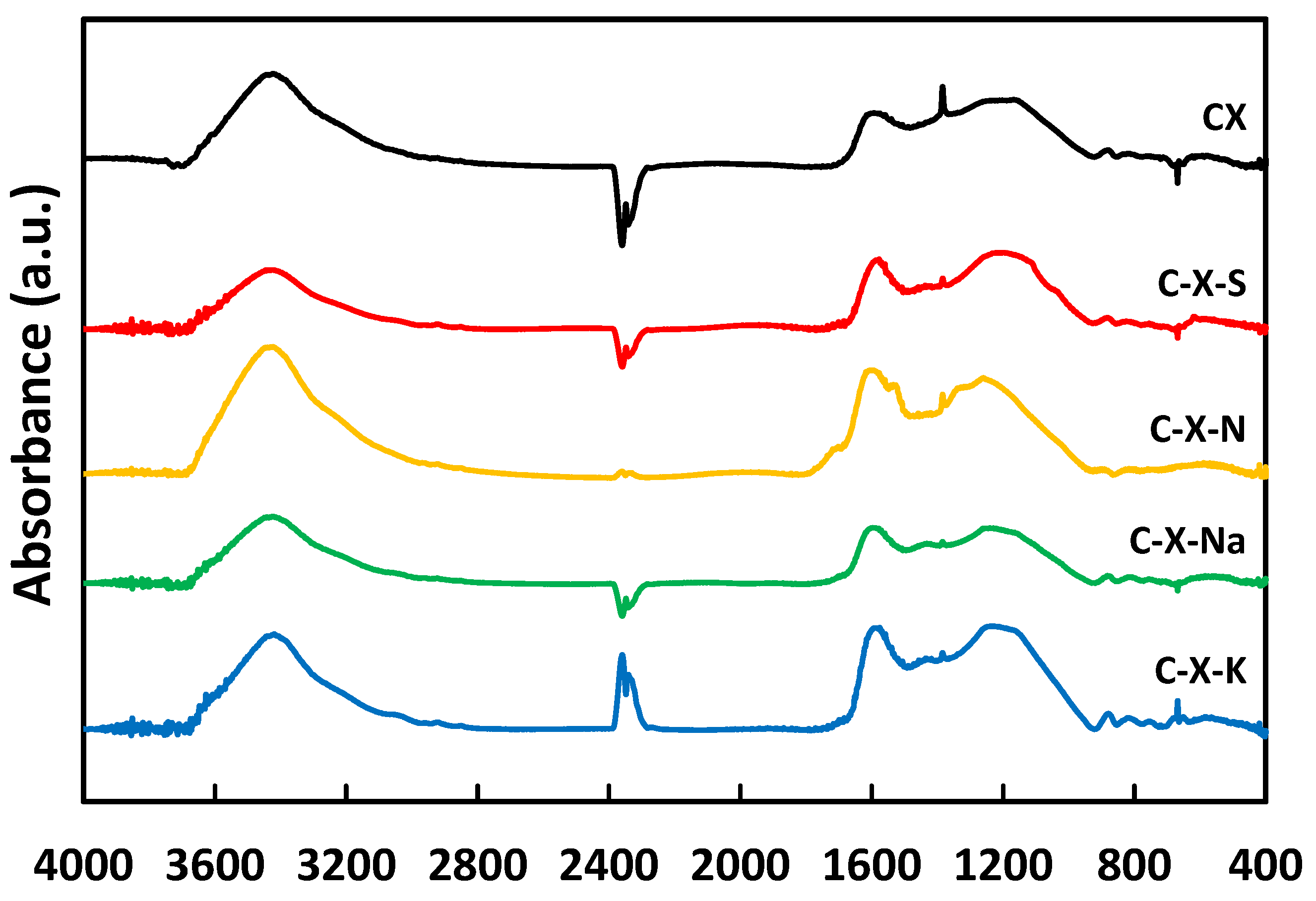
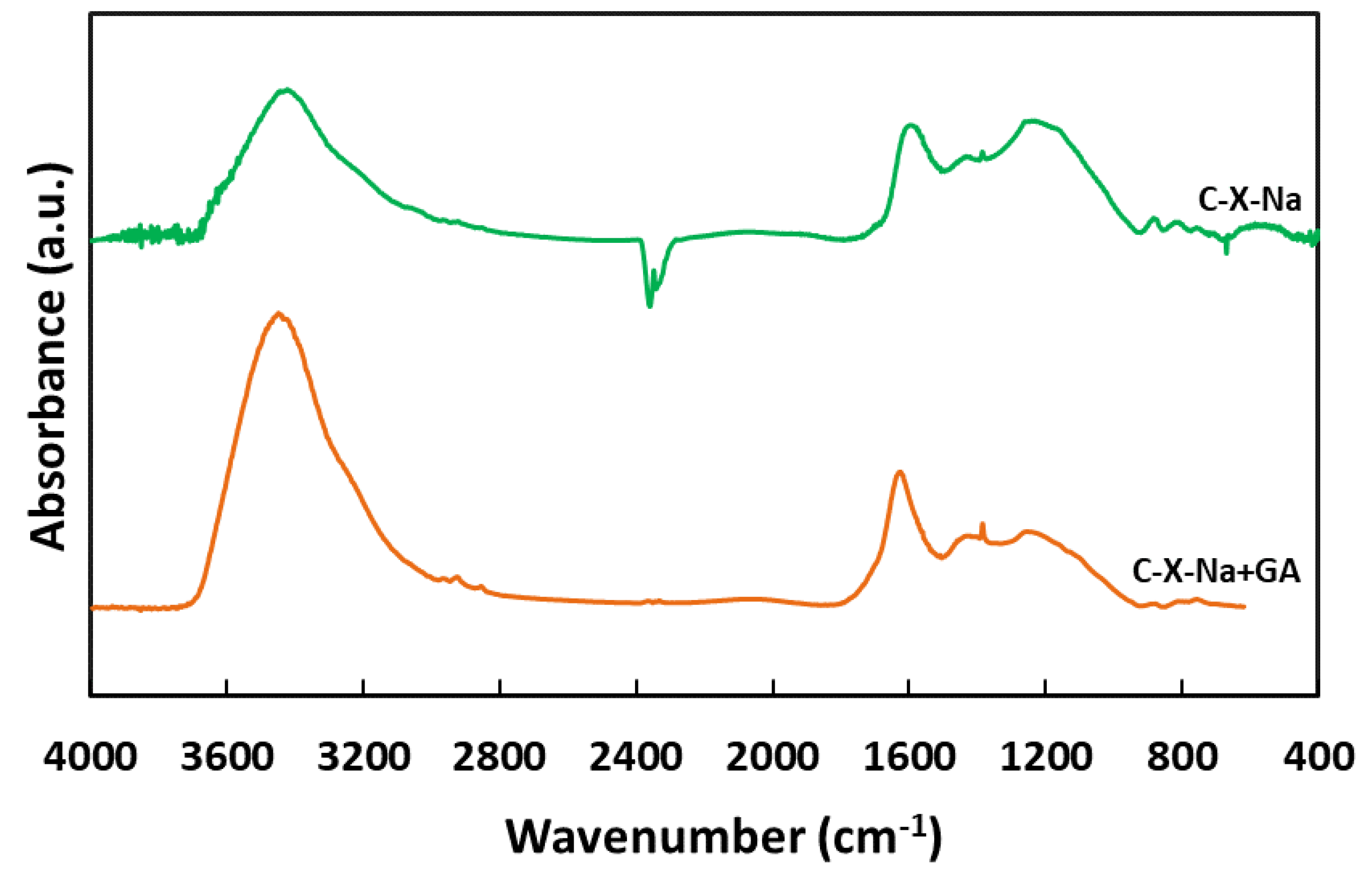
| Starting material | Reagent | Temperature, °C | Time, h | Code |
|---|---|---|---|---|
| CX-5 | - | - | - | C-X |
| 98% sulfuric acid | 25±2 | 1.5 | C-X-S | |
| 65% nitric acid | 25±2 | 1.5 | C-X-N | |
| 1M sodium chloride | 80±1 | 48 | C-X-Na | |
| 1M potassium chloride | 80±1 | 48 | C-X-K |
| Sample | %C | %H | %N | %S | %O* | %Na | %K | % others |
|---|---|---|---|---|---|---|---|---|
| C-X | 85.7 | 2.3 | 0.0 | 0.0 | 12.0 | N.M.** | N.M. | N.M. |
| C-X-S | 81.2 | 2.6 | 0.0 | 0.7 | 15.5 | N.M. | N.M. | N.M. |
| C-X-N | 74.7 | 2.1 | 1.4 | 0.0 | 21.8 | N.M. | N.M. | N.M. |
| C-X-Na | 85.7 | 2.6 | 0.0 | 0.0 | 10.9 | 0.5 | 0.0 | 0.8 (Cl, Cu) |
| C-X-K | 84.9 | 2.5 | 0.0 | 0.0 | 10.1 | 0.0 | 0.9 | 1.0 (Cl, Cu, Fe) |
| Sample | % C | % H | % N | % S |
|---|---|---|---|---|
| C-X | 92.8 | 7.2 | 0.0 | 0.0 |
| C-X-S | 90.6 | 8.5 | 0.0 | 0.9 |
| C-X-N | 86.1 | 12.2 | 1.7 | 0.0 |
| C-X-Na | 94.0 | 6.0 | 0.0 | 0.0 |
| C-X-K | 93.2 | 6.8 | 0.0 | 0.0 |
| Carbon | 284.8 eV | Approx. 286 eV | Approx. 289 eV |
|---|---|---|---|
| C-X | 53.8 | 29.5 | 16.7 |
| C-X-S | 57.1 | 22.7 | 20.2 |
| C-X-N | 53.0 | 23.0 | 24.0 |
| C-X-Na | 53.1 | 23.8 | 23.1 |
| C-X-K | 69.0 | 17.2 | 13.8 |
| Carbon | Approx. 532.0 eV | Approx. 533.5 eV | Approx. 536.5 eV |
|---|---|---|---|
| C-X | 30.5 | 69.5 | - |
| C-X-S | 45.1 | 54.9 | - |
| C-X-N | 45.4 | 54.6 | - |
| C-X-Na | 12.2 | 73.8 | 14.0 |
| C-X-K | 53.9 | 35.7 | 10.4 |
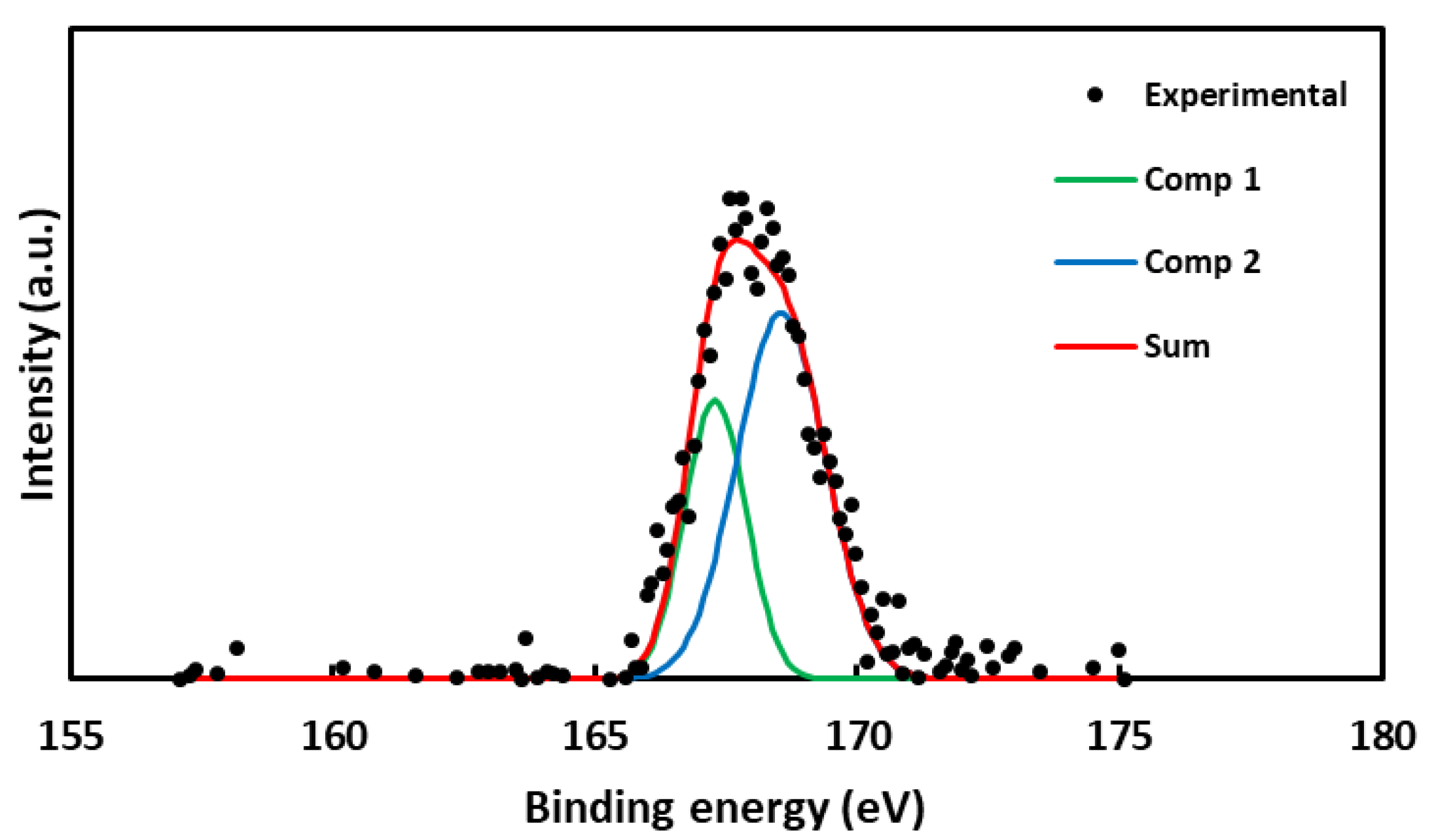
| S2p1/2 | S2p3/2 | |
|---|---|---|
| Carbon | 167.6 eV | 168.8 eV |
| C-X-S | 34.5 | 65.5 |
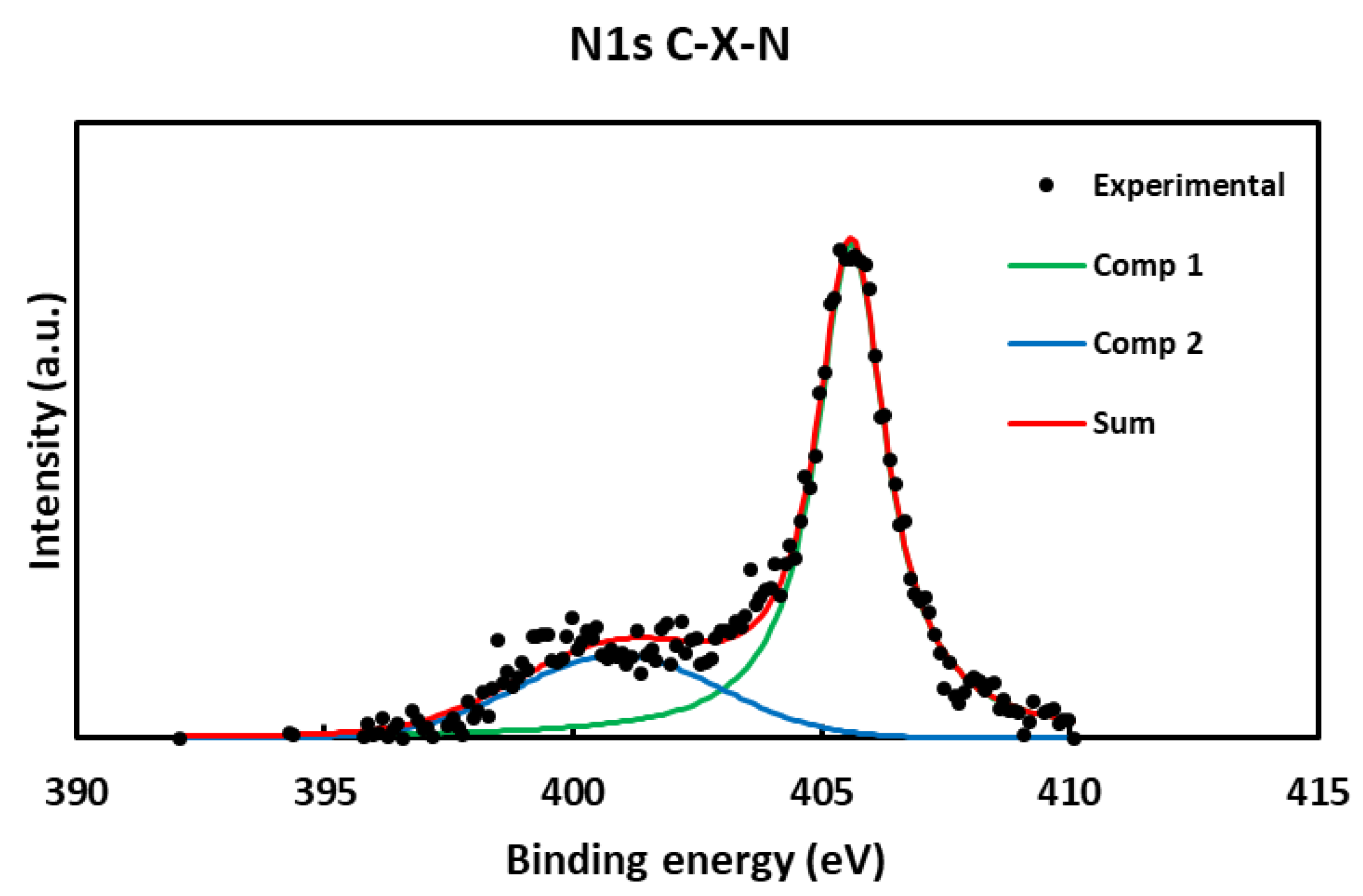
| Carbon | 401.2 eV | 405.9 eV |
| C-X-N | 24.2 | 75.8 |
| Sample | p.z.c. | Acidic groups meq g-1 | Basic groups meq g-1 |
|---|---|---|---|
| C-X | 7.20 | 0.20 | 0.85 |
| C-X-S | 2.49 | 0.58 | 0.80 |
| C-X-N | 3.42 | 0.85 | 0.81 |
| C-X-Na | 6.87 | 0.34 | 1.04 |
| C-X-K | 6.59 | 0.23 | 0.98 |
| Sample | SBET, m2 g-1 | Vmi DA, cm3 g-1 | Vmi DR, cm3 g-1 | Rporo DR, nm |
|---|---|---|---|---|
| C-X | 484 | 0.268 | 0.243 | 0.722 |
| C-X-S | 448 | 0.249 | 0.225 | 0.808 |
| C-X-N | 347 | 0.194 | 0.176 | 0.812 |
| C-X-Na | 508 | 0.284 | 0.257 | 0.725 |
| C-X-K | 563 | 0.291 | 0.267 | 0.528 |
| Sample | Vme-p, cm3 g-1 | Vma-p, cm3 g-1 |
|---|---|---|
| C-X | 0.79 | 0.70 |
| C-X-S | 0.75 | 0.39 |
| C-X-N | 0.74 | 0.36 |
| C-X-Na | 1.64 | 1.43 |
| C-X-K | 1.24 | 1.14 |
| Kinetic model | Parameter | C-X | C-X-S | C-X-N | C-X-Na | C-X-K |
|---|---|---|---|---|---|---|
| Pseudo-first order | Qe, mg g-1 | 31.29 | 19.83 | 21.61 | 26.22 | 29.74 |
| K1, h-1 | 0.329 | 0.253 | 0.176 | 0.220 | 0.267 | |
| R2 | 0.981 | 0.963 | 0.989 | 0.976 | 0.962 | |
| Pseudo-second order | Qe, mg g-1 | 48.54 | 47.62 | 40.32 | 47.62 | 47.85 |
| K2, h-1 | 0.021 | 0.035 | 0.020 | 0.019 | 0.018 | |
| R2 | 0.999 | 0.999 | 0.996 | 0.999 | 0.999 | |
| Weber and Morris | C, mg g-1 | 13.54 | 20.10 | 10.87 | 12.59 | 12.05 |
| Kid, mg min1/2 g-1 | 17.31 | 14.12 | 13.64 | 16.95 | 17.27 | |
| R2 | 0.771 | 0.647 | 0.838 | 0.797 | 0.804 |
| Isotherm model | Parameter | C-X | C-X-S | C-X-N | C-X-Na | C-X-K |
|---|---|---|---|---|---|---|
| Langmuir | Sm, mg g-1 | 62.89 | 83.33 | 68.49 | 72.46 | 71.94 |
| KL | 0.049 | 0.027 | 0.090 | 0.129 | 0.133 | |
| R2 | 0.954 | 0.889 | 0.973 | 0.839 | 0.969 | |
| Freundlich | n | 1.54 | 1.28 | 2.12 | 2.34 | 2.47 |
| KF | 4.254 | 2.709 | 9.998 | 13.932 | 14.273 | |
| R2 | 0.941 | 0.975 | 0.993 | 0.857 | 0.978 |
| Type of adsorbent | pH | T /ºC | Q0 /mg g-1 | Reference |
|---|---|---|---|---|
| Commercial activated carbon (Picachem 150) | - | 5-50 | 239.9 -318.2 | [58] |
| Activated carbons | - | 25 | 20.4 - 27.2 | [59] |
| Activated carbon from date stones | 4.5 | 25 | 156.1 | [1] |
| Powdered AC | - | 25 | 57.5 | [60] |
| Multiwalled C-nanotubes | - | 25 | 17.6 - 23.5 | |
| TiO2-coated AC | 3 | 25 | 4.9 | [5] |
| Olive pomace AC in alginate beads: granular-powdered | 4.4 | 25 | 62.5 - 208.3 | [6] |
| Polymeric adsorbent WJN-09 | - | 30 | 97 | [2] |
| HF-02 (resin) | - | 15-45 | 141.6 - 171.3 | [61] |
| Montmorillonite | 5 | 25 | 20.2 | [62] |
| Iron-oxide coated montmorillonite clay | 4.2 | - | 88.5 | [63] |
| Nanoclay modified with poly (diallyldimethylammonium) | 4 | 15-45 | 39.15 - 80.58 | [64] |
| Chitosan-HAP composite | 6 | 30 | 23.3 | [65] |
| Chitosan-diatomite | 6 | 30 | 22.9 | [66] |
| Magnetic chitosan | - | 30-50 | 27.2 - 48.4 | [67] |
| Carbon xerogels | ≈4 | 30 | 62.89 - 83.33 | This study |
| Sample | ∆S, KJ K-1 mol-1 | ∆H, KJ mol-1 | ∆G298K, KJ mol-1 |
|---|---|---|---|
| C-X | 0.063 | 16.10 | -2.57 |
| C-X-S | 0.051 | 13.97 | -1.09 |
| C-X-N | 0.070 | 21.21 | 0.28 |
| C-X-Na | 0.088 | 22.34 | -3.80 |
| C-X-K | 0.084 | 21.46 | -3.55 |
| Adsorption | Desorption | ||||||
|---|---|---|---|---|---|---|---|
| [GA]0, ppm |
[GA]F, ppm | GA ads., % |
qe, mg g-1 |
[GA]ext, ppm | GA ext., % |
qext, mg g-1 |
|
| Cycle 1 | 105.84 | 27.94 | 73.60 | 77.9 | 25.10 | 32.22 | 25.1 |
| Cycle 2 | 105.84 | 49.56 | 53.17 | 56.3 | 29.56 | 52.52 | 29.6 |
| Cycle 3 | 107.64 | 44.48 | 58.68 | 63.2 | 30.44 | 48.20 | 30.4 |
| Adsorption | Desorption | ||||||
|---|---|---|---|---|---|---|---|
| [GA]0, ppm |
[GA]F, ppm | GA ads., % |
qe, mg g-1 |
[GA]ext, ppm | GA ext., % |
qext, mg g-1 |
|
| Cycle 1 | 105.84 | 27.94 | 73.60 | 77.9 | 7.34 | 9.42 | 7.3 |
| Cycle 2 | 105.84 | 45.14 | 57.35 | 60.7 | 16.16 | 26.62 | 16.2 |
| Cycle 3 | 107.64 | 50.24 | 53.33 | 57.4 | 18.28 | 31.85 | 18.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





