Submitted:
20 January 2024
Posted:
22 January 2024
Read the latest preprint version here
Abstract
Keywords:
1. INTRODUCTION
2. CURRENT PFAS DETECTION METHODS
2.1. PFAS Sample Preparation and Extraction
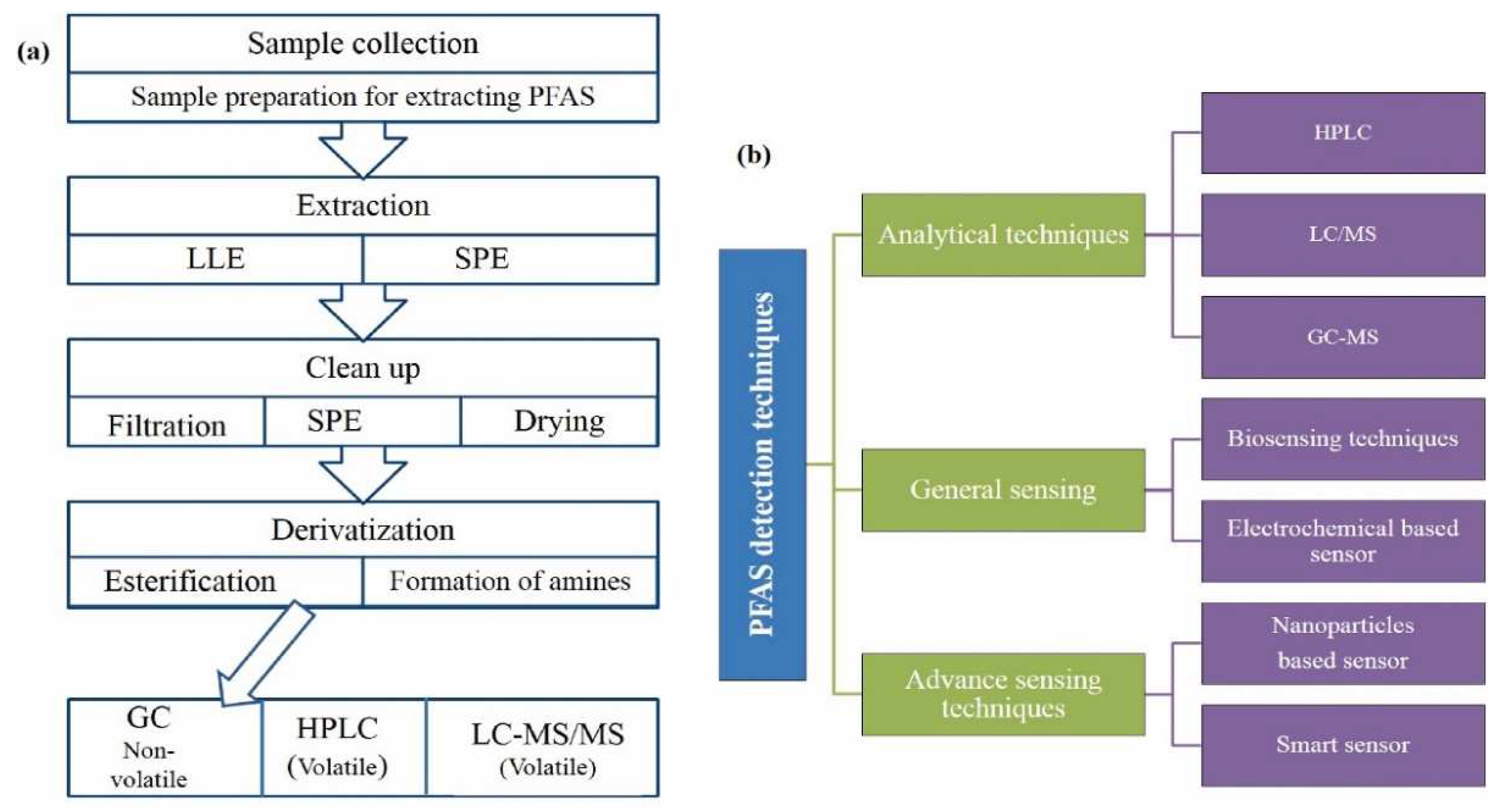
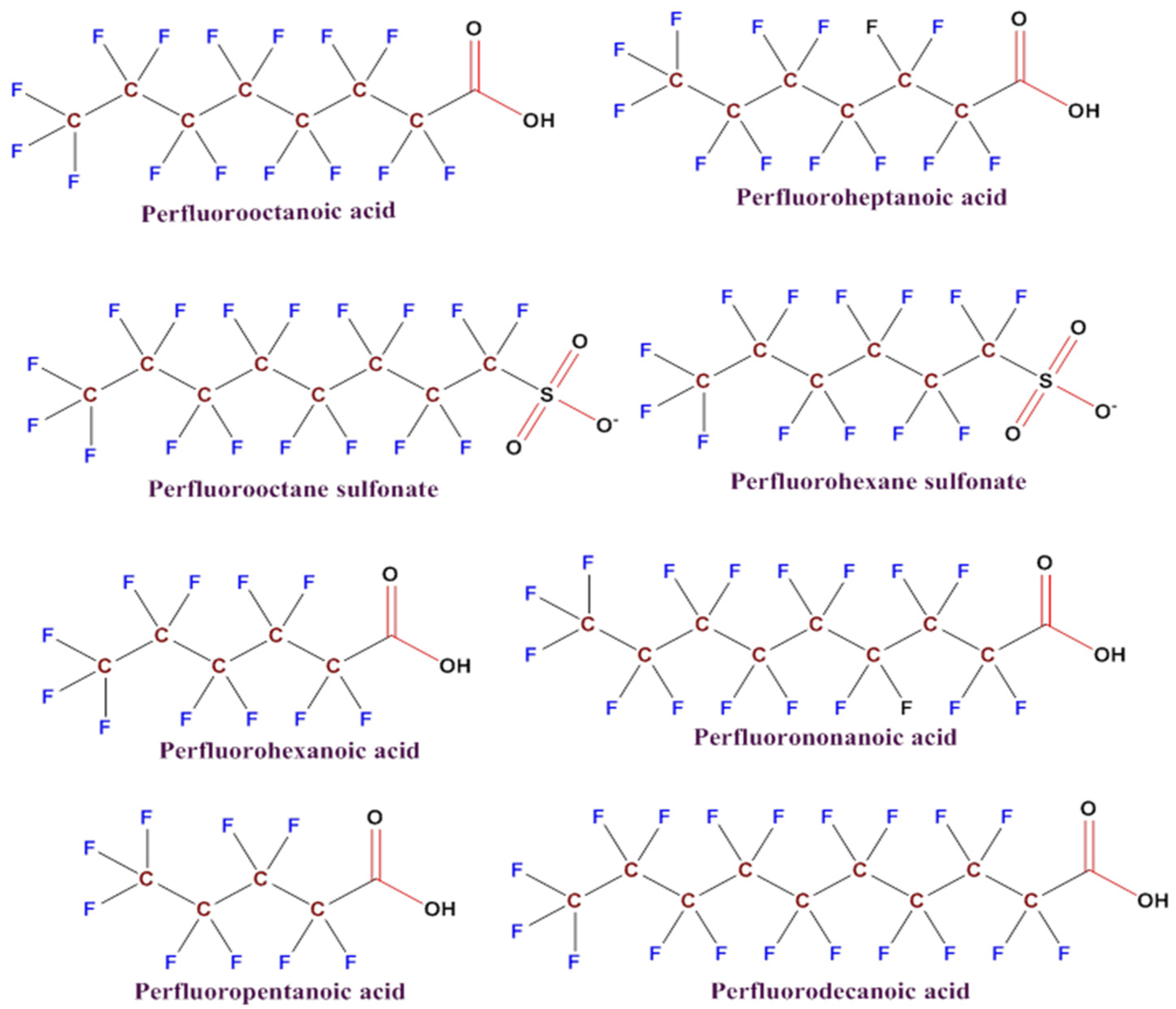
2.2. Analysis of PFAS via Chromatography
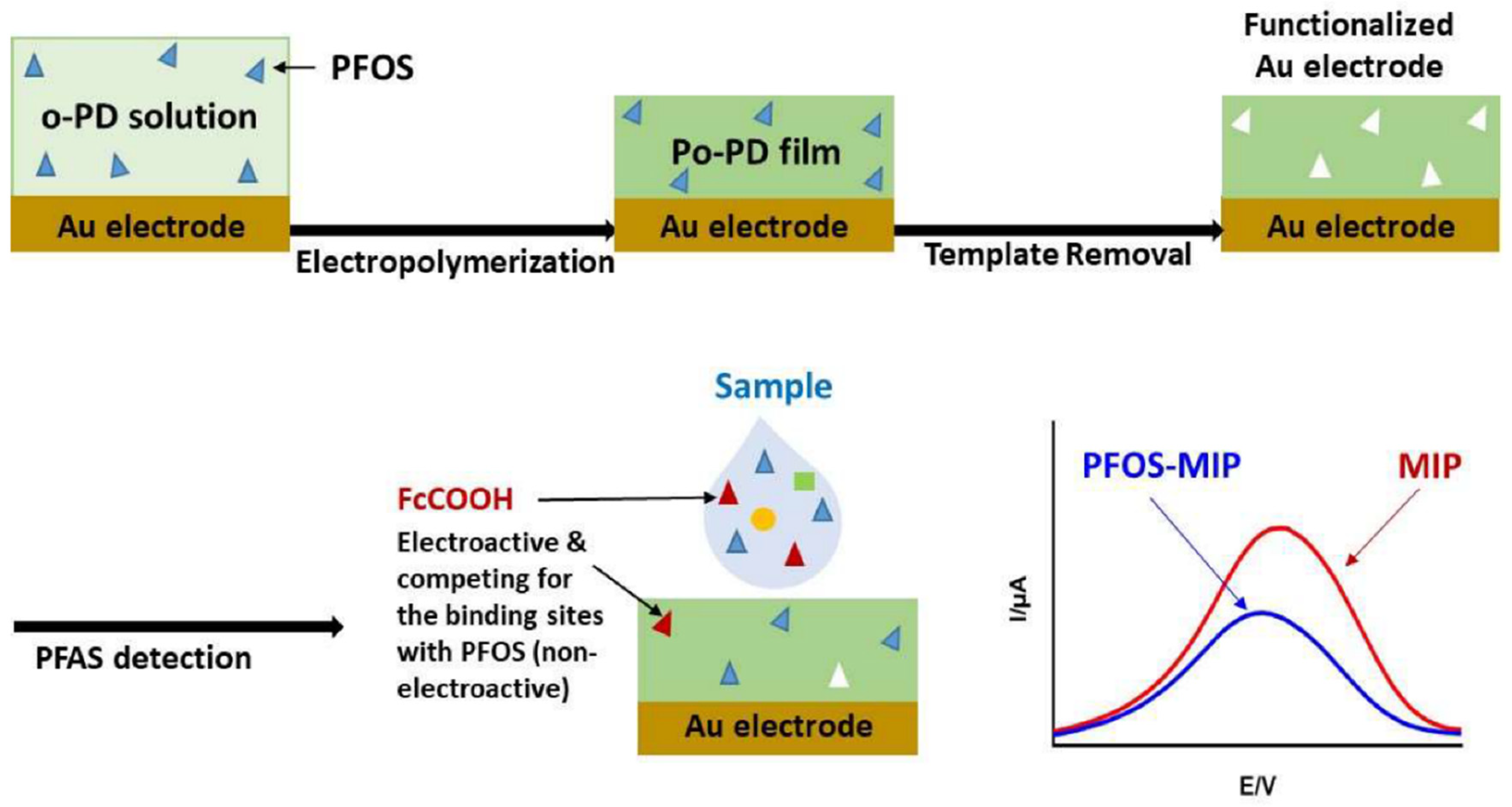
2.3. PFAS Detection by Sensors
2.4. Nanomaterial Based Sensor
2.5. PFAS Detection by Surface-Enhanced Raman Spectroscopy
3. CONCLUSIONS
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C.J.; Whittaker, A.K. Biological utility of fluorinated compounds: From materials design to molecular imaging, therapeutics and environmental remediation. Chem. Rev. 2021, 122, 167–208. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, F.; Shang, W.; Zhang, X.; Dong, W.; Liu, T.; Pang, W. Theoretical studies of perfluorochemicals (PFCs) adsorption mechanism on the carbonaceous surface. Chemosphere 2019, 235, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak-Kulikowska, J. Poly/Perfluorinated Alkyl Substances (PFASs)–Synthetic Methods, Properties and Applications. In Perfluoroalkyl Substances: Synthesis, Applications, Challenges and Regulations; Royal Society of Chemistry: London, UK, 2022. [Google Scholar]
- Dixit, F.; Munoz, G.; Mirzaei, M.; Barbeau, B.; Liu, J.; Duy, S.V.; Sauvé, S.; Kandasubramanian, B.; Mohseni, M. Removal of zwitterionic PFAS by MXenes: Comparisons with anionic, nonionic, and PFAS-specific resins. Environ. Sci. Technol. 2022, 56, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Militao, M.; Roddick, F.A.; Bergamasco, R.; Fan, L. Removing PFAS from aquatic systems using natural and renewable material-based adsorbents: A review. J. Environ. Chem. Eng. 2021, 9, 105271. [Google Scholar] [CrossRef]
- Mueller, R.; Yingling, V. History and use of per-and polyfluoroalkyl substances (PFAS); Interstate Technology & Regulatory Council: Washington, DC, USA, 2017. [Google Scholar]
- Bokkers, B.; Van de Ven, B.; Janssen, P.; Bil, W.; Van Broekhuizen, F.; Zeilmaker, M.; Oomen, A.G. Per-and polyfluoroalkyl substances (PFASs) in food contact materials. 2019. [CrossRef]
- Barzen-Hanson, K.A. Per-and Polyfluoroalkyl Substances (PFASs) and Aqueous Film-Forming Foam Impacted Sites: New PFAS Discovery and Sorption of Anionic, Zwitterionic, and Cationic PFASs; Oregon State University: Corvallis, OR, USA, 2017. [Google Scholar]
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-chain per-and polyfluoroalkyl substances in aquatic systems: Occurrence, impacts and treatment. Chem. Eng. J. 2020, 380, 122506. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, X.; Dong, Z.; Wang, L.; Megharaj, M.; Naidu, R. Smartphone app-based/portable sensor for the detection of fluoro-surfactant PFOA. Chemosphere 2018, 191, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Radjenovic, J.; Duinslaeger, N.; Avval, S.S.; Chaplin, B.P. Facing the challenge of poly-and perfluoroalkyl substances in water: Is electrochemical oxidation the answer? Environ. Sci. Technol. 2020, 54, 14815–14829. [Google Scholar] [CrossRef]
- He, A.; Liang, Y.; Li, F.; Lu, Y.; Liu, C.; Li, J.; Zhou, Z.; Zhu, N.; Liao, C.; Wang, Y. Vital environmental sources for multitudinous fluorinated chemicals: New evidence from industrial byproducts in multienvironmental matrices in a fluorochemical manufactory. Environ. Sci. Technol. 2022, 56, 16789–16800. [Google Scholar] [CrossRef]
- Zhu, H.; Kannan, K. A pilot study of per-and polyfluoroalkyl substances in automotive lubricant oils from the United States. Environ. Technol. Innov. 2020, 19, 100943. [Google Scholar] [CrossRef]
- Vorst, K.L.; Saab, N.; Silva, P.; Curtzwiler, G.; Steketee, A. Risk assessment of per-and polyfluoroalkyl substances (PFAS) in food: Symposium proceedings. Trends Food Sci. Technol. 2021, 116, 1203–1211. [Google Scholar] [CrossRef]
- Zakaria, A.F.; Yahaya, N.; Raznisyafiq, M.; Loh, S.H.; Kamaruzaman, S. Recent advances in applications of hybrid natural polymers as adsorbent for perfluorinated compounds removal–review paper. J. Polym. Res. 2022, 29, 1–19. [Google Scholar] [CrossRef]
- Langberg, H.A.; Arp, H.P.H.; Breedveld, G.D.; Slinde, G.A.; Høiseter, Å.; Grønning, H.M.; Jartun, M.; Rundberget, T.; Jenssen, B.M.; Hale, S.E. Paper product production identified as the main source of per-and polyfluoroalkyl substances (PFAS) in a Norwegian lake: Source and historic emission tracking. Environ. Pollut. 2021, 273, 116259. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lai, W.W.-P.; Tung, H.; Lin, A.Y.-C. Occurrence of pharmaceuticals, hormones, and perfluorinated compounds in groundwater in Taiwan. Environ. Monit. Assess. 2015, 187, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.; Padilla-Sanchez, J.A.; Collins, C.D.; Cousins, I.T.; Covaci, A.; De Wit, C.A.; Leonards, P.E.; Voorspoels, S.; Thomsen, C.; Harrad, S. Sampling strategy for estimating human exposure pathways to consumer chemicals. Emerg. Contam. 2016, 2, 26–36. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Hossain, M.; Islam, M. Prediction of Solid Waste Generation Rate and Determination of Future Waste Characteristics at South-western Region of Bangladesh Using Artificial Neural Network; KUET: Khulna, Bangladesh, 2017. [Google Scholar]
- Stoiber, T.; Evans, S.; Naidenko, O.V. Disposal of products and materials containing per-and polyfluoroalkyl substances (PFAS): A cyclical problem. Chemosphere 2020, 260, 127659. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Chakrabarti, S.D. Scenario of existing solid waste management practices and integrated solid waste management model for developing country with reference to jhenaidah municipality, Bangladesh. In Proceedings of the 4th International Conference on Civil Engineering for Sustainable Development (ICCESD 2018), Khulna, Bangladesh, 9–11 February 2018. [Google Scholar]
- Garg, S.; Kumar, P.; Mishra, V.; Guijt, R.; Singh, P.; Dumée, L.F.; Sharma, R.S. A review on the sources, occurrence and health risks of per-/poly-fluoroalkyl substances (PFAS) arising from the manufacture and disposal of electric and electronic products. J. Water Process Eng. 2020, 38, 101683. [Google Scholar] [CrossRef]
- Page, D.; Vanderzalm, J.; Kumar, A.; Cheng, K.Y.; Kaksonen, A.H.; Simpson, S. Risks of perfluoroalkyl and polyfluoroalkyl substances (PFAS) for sustainable water recycling via aquifers. Water 2019, 11, 1737. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Moniruzzaman, S.M. A study on plastic waste recycling process in khulna city. In Proceedings of the 4th International Conference on Civil Engineering for Sustainable Development (ICCESD 2018), Khulna, Bangladesh, 9–11 February 2018. [Google Scholar]
- Berg, C.; Crone, B.; Gullett, B.; Higuchi, M.; Krause, M.J.; Lemieux, P.M.; Martin, T.; Shields, E.P.; Struble, E.; Thoma, E.; Whitehill, A. Developing innovative treatment technologies for PFAS-containing wastes. J. Air Waste Manag. Assoc. 2022, 72, 540–555. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Roy, P.; Shah, M.H.; Argha, D.P.; Datta, D.; Ri, R.H. Recycling of cotton dust for organic farming is a pivotal replacement of chemical fertilizers by composting and its quality analysis. ERT 2021, 4, 2. [Google Scholar] [CrossRef]
- Roy, P.; Ahmed, M.A.; Shah, M.H. Biogas generation from kitchen and vegetable waste in replacement of traditional method and its future forecasting by using ARIMA model. Waste Dispos. Sustain. Energy 2021, 3, 165–175. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Zhu, Y.; Tan, X.; Gunjal, S.; Wang, Y.; Xin, R.; Fu, C.; Macintosh, K.; Sprague, L.; Leung, L. Turning Waste into Wealth: An Efficient Platform for Capturing, Recycling and Reusing Perfluorinated Compounds. 2023. [CrossRef]
- Ahmed, M.A.; Argha, D.B.P.; Rashid, M.R.; Riyad, R.H. Forms, Importance and Sources of Dissolved Organic Nitrogen (DON) in the Environment: A Review. SVU-Int. J. Eng. Sci. Appl. 2024, 5, 18–27. [Google Scholar] [CrossRef]
- Goukeh, M.N.; Alamdari, N. Removal of Contaminants in Stormwater via Subsurface-Flow Wetlands: A Review with Focus on Nutrients, Heavy Metals, and PFAS. J. Environ. Eng. 2024, 150, 03124001. [Google Scholar] [CrossRef]
- Sivagami, K.; Sharma, P.; Karim, A.V.; Mohanakrishna, G.; Karthika, S.; Divyapriya, G.; Saravanathamizhan, R.; Kumar, A.N. Electrochemical-based approaches for the treatment of forever chemicals: Removal of perfluoroalkyl and polyfluoroalkyl substances (PFAS) from wastewater. Sci. Total Environ. 2023, 861, 160440. [Google Scholar] [CrossRef]
- Kearns, J. The role of chemical exposures in reducing the effectiveness of water–sanitation–hygiene interventions in Bangladesh, Kenya, and Zimbabwe. Wiley Interdiscip. Rev. Water 2020, 7, e1478. [Google Scholar] [CrossRef]
- Roy, P.; Ahmed, M.A.; Kumer, A. An overview of hygiene practices and health risks related to street foods and drinking water from roadside restaurants of khulna city of Bangladesh. EJERE 2019, 3, 2. Available online: https://dergipark.org.tr/en/pub/ejere/issue/49620/590483 (accessed on 8 August 2023).
- Dettori, M.; Arghittu, A.; Deiana, G.; Castiglia, P.; Azara, A. The revised European Directive 2020/2184 on the quality of water intended for human consumption. A step forward in risk assessment, consumer safety and informative communication. Environ. Res. 2022, 209, 112773. [Google Scholar] [CrossRef]
- del V, R.; Organization, W.H. Keeping our water clean: The case of water contamination in the Veneto Region, Italy. 2017.
- Modak, S.; Mokarizadeh, H.; Karbassiyazdi, E.; Hosseinzadeh, A.; Esfahani, M.R. The AI-assisted removal and sensor-based detection of contaminants in the aquatic environment. In Artificial Intelligence and Data Science in Environmental Sensing; Elsevier: Amsterdam, The Netherlands, 2022; pp. 211–244. [Google Scholar]
- Hosseinzadeh, A.; Altaee, A.; Li, X.; Zhou, J.L. Machine learning-based modeling and analysis of perfluoroalkyl and polyfluoroalkyl substances controlling systems in protecting water resources. Curr. Opin. Chem. Eng. 2023, 42, 100983. [Google Scholar] [CrossRef]
- Argha, D.B.P.; Ahmed, M.A. A Machine Learning Approach to Understand the Impact of Temperature and Rainfall Change on Concrete Pavement Performance Based on LTPP Data. SVU-Int. J. Eng. Sci. Appl. 2024, 5, 150–155. [Google Scholar] [CrossRef]
- Roostaei, J.; Colley, S.; Mulhern, R.; May, A.A.; Gibson, J.M. Predicting the risk of GenX contamination in private well water using a machine-learned Bayesian network model. J. Hazard. Mater. 2021, 411, 125075. [Google Scholar] [CrossRef]
- Lin, Z.; Chou, W.-C. Machine learning and artificial intelligence in toxicological sciences. Toxicol. Sci. 2022, 189, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Rainieri, S.; Conlledo, N.; Langerholc, T.; Madorran, E.; Sala, M.; Barranco, A. Toxic effects of perfluorinated compounds at human cellular level and on a model vertebrate. Food Chem. Toxicol. 2017, 104, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Longpré, D.; Lorusso, L.; Levicki, C.; Carrier, R.; Cureton, P. PFOS, PFOA, LC-PFCAS, and certain other PFAS: A focus on Canadian guidelines and guidance for contaminated sites management. Environ. Technol. Innov. 2020, 18, 100752. [Google Scholar] [CrossRef]
- US EPA. Drinking water health advisory for perfluorooctane sulfonate (PFOS); United States Environmental Protection Agency: Washington, DC, USA, 2016. [Google Scholar]
- Hagstrom, A.L.; Anastas, P.; Boissevain, A.; Borrel, A.; Deziel, N.C.; Fenton, S.E.; Fields, C.; Fortner, J.D.; Franceschi-Hofmann, N.; Frigon, R. Yale School of Public Health Symposium: An overview of the challenges and opportunities associated with per-and polyfluoroalkyl substances (PFAS). Sci. Total Environ. 2021, 778, 146192. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Quesada, I.; Tuduri, E.; Nogueiras, R.; Alonso-Magdalena, P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017, 13, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Koc, M. Recent developments in methods for analysis of perfluorinated persistent pollutants. Microchim. Acta 2013, 180, 957–971. [Google Scholar] [CrossRef]
- M.b., B.; Rhakho, N.; Jena, S.R.; Yadav, S.; Altaee, A.; Saxena, M.; Samal, A.K. Detection of PFAS via surface-enhanced Raman scattering: Challenges and future perspectives. Sustain. Chem. Environ. 2023, 3, 100031. [Google Scholar] [CrossRef]
- Miaz, L.T.; Plassmann, M.M.; Gyllenhammar, I.; Bignert, A.; Sandblom, O.; Lignell, S.; Glynn, A.; Benskin, J.P. Temporal trends of suspect-and target-per/polyfluoroalkyl substances (PFAS), extractable organic fluorine (EOF) and total fluorine (TF) in pooled serum from first-time mothers in Uppsala, Sweden, 1996–2017. Environ. Sci. Process. Impacts 2020, 22, 1071–1083. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Yang, L.; He, C.; Wu, W.; Sun, S. Liquid chromatography/mass spectrometry analysis of perfluoroalkyl carboxylic acids and perfluorooctanesulfonate in bivalve shells: Extraction method optimization. J. Chromatogr. A 2010, 1217, 436–442. [Google Scholar] [CrossRef]
- Malinsky, M.D.; Jacoby, C.B.; Reagen, W.K. Determination of perfluorinated compounds in fish fillet homogenates: Method validation and application to fillet homogenates from the Mississippi River. Anal. Chim. Acta 2011, 683, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.; Nödler, K.; Brauch, H.-J.; Zwiener, C.; Lange, F.T. Robust trace analysis of polar (C2-C8) perfluorinated carboxylic acids by liquid chromatography-tandem mass spectrometry: Method development and application to surface water, groundwater and drinking water. Environ. Sci. Pollut. Res. 2019, 26, 7326–7336. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, S.; Cao, X.; Cao, Y.; Zhang, L.; Wang, H.; Liu, J. Perfluoroalkyl acids (PFAAs) in water and sediment from the coastal regions of Shandong peninsula, China. Environ. Monit. Assess. 2017, 189, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lee, C.; Davis, E.T.; Si, W.; Wang, F.; Trimpin, S.; Luo, L. 1000-fold preconcentration of per-and polyfluorinated alkyl substances within 10 minutes via electrochemical aerosol formation. Anal. Chem. 2019, 91, 14352–14358. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.X.L.; Lee, H.K. Automated bundled hollow fiber array-liquid-phase microextraction with liquid chromatography tandem mass spectrometric analysis of perfluorinated compounds in aqueous media. Anal. Chim. Acta 2018, 1019, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Moreta, C.; Tena, M.T. Determination of perfluorinated alkyl acids in corn, popcorn and popcorn bags before and after cooking by focused ultrasound solid–liquid extraction, liquid chromatography and quadrupole-time of flight mass spectrometry. J. Chromatogr. A 2014, 1355, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Curtzwiler, G.W.; Silva, P.; Hall, A.; Ivey, A.; Vorst, K. Significance of Perfluoroalkyl Substances (PFAS) in Food Packaging. Integr. Environ. Assess. Manag. 2021, 17, 7–12. [Google Scholar] [CrossRef]
- Moody, C.A.; Kwan, W.C.; Martin, J.W.; Muir, D.C.; Mabury, S.A. Determination of perfluorinated surfactants in surface water samples by two independent analytical techniques: Liquid chromatography/tandem mass spectrometry and 19F NMR. Anal. Chem. 2001, 73, 2200–2206. [Google Scholar] [CrossRef]
- Hebert, G.N.; Odom, M.A.; Bowman, S.C.; Strauss, S.H. Attenuated total reflectance FTIR detection and quantification of low concentrations of aqueous polyatomic anions. Anal. Chem. 2004, 76, 781–787. [Google Scholar] [CrossRef]
- Na, S.; Hai, R.; Wang, X.; Li, N.; Chen, D. Concentrations and seasonal variations of perfluorinated compounds in sludge from three wastewater treatment plants in China. Anal. Lett. 2020, 53, 2400–2412. [Google Scholar] [CrossRef]
- Wu, M.; Sun, R.; Wang, M.; Liang, H.; Ma, S.; Han, T.; Xia, X.; Ma, J.; Tang, L.; Sun, Y.; Xu, G. Analysis of perfluorinated compounds in human serum from the general population in Shanghai by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Chemosphere 2017, 168, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Junaid, M.; Wang, Z.; Sun, W.; Xu, N. Spatiotemporal distribution, sources and ecological risks of perfluorinated compounds (PFCs) in the Guanlan River from the rapidly urbanizing areas of Shenzhen, China. Chemosphere 2020, 245, 125637. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Cai, Y. A highly selective dispersive liquid–liquid microextraction approach based on the unique fluorous affinity for the extraction and detection of per-and polyfluoroalkyl substances coupled with high performance liquid chromatography tandem–mass spectrometry. J. Chromatogr. A 2018, 1544, 1–7. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, Y.; Luo, X.; Yang, J. Direct extraction of U(VI) from a simulated saline solution by alkali-activated collagen fiber. J. Radioanal. Nucl. Chem. 2018, 318, 1109–1118. [Google Scholar] [CrossRef]
- Park, N.; Kho, Y.; Kim, J. Levels of Perfluorinated Compounds in Liquid Milk Products in Korea. J. Food Hyg. Saf. 2021, 36, 310–315. [Google Scholar] [CrossRef]
- Lockwood, T.E.; Talebi, M.; Minett, A.; Mills, S.; Doble, P.A.; Bishop, D.P. Micro solid-phase extraction for the analysis of per-and polyfluoroalkyl substances in environmental waters. J. Chromatogr. A 2019, 1604, 460495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, P.; Lu, Y.; Lu, X.; Zhang, A.; Liu, Z.; Zhang, Y.; Khan, K.; Sarvajayakesavalu, S. Bioaccumulation and human exposure of perfluoroalkyl acids (PFAAs) in vegetables from the largest vegetable production base of China. Environ. Int. 2020, 135, 105347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zheng, Y.; Liu, Y.; Long, S.; Du, L.; Liang, J.; Huang, C.; Swihart, M.T.; Tan, K. Core-shell quantum dots coated with molecularly imprinted polymer for selective photoluminescence sensing of perfluorooctanoic acid. Talanta 2019, 194, 1–6. [Google Scholar] [CrossRef]
- Xiang, L.; Chen, L.; Xiao, T.; Mo, C.-H.; Li, Y.-W.; Cai, Q.-Y.; Li, H.; Zhou, D.-M.; Wong, M.-H. Determination of trace perfluoroalkyl carboxylic acids in edible crop matrices: Matrix effect and method development. J. Agric. Food Chem. 2017, 65, 8763–8772. [Google Scholar] [CrossRef]
- Riviere, G.; Sirot, V.; Tard, A.; Jean, J.; Marchand, P.; Veyrand, B.; Le Bizec, B.; Leblanc, J. Food risk assessment for perfluoroalkyl acids and brominated flame retardants in the French population: Results from the second French total diet study. Sci. Total Environ. 2014, 491, 176–183. [Google Scholar] [CrossRef]
- Dalahmeh, S.; Tirgani, S.; Komakech, A.J.; Niwagaba, C.B.; Ahrens, L. Per-and polyfluoroalkyl substances (PFASs) in water, soil and plants in wetlands and agricultural areas in Kampala, Uganda. Sci. Total Environ. 2018, 631, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Zhang, C.; Sun, R.; Han, J.; Han, G.; Yang, W.; He, X. Occurrence and inputs of perfluoroalkyl substances (PFASs) from rivers and drain outlets to the Bohai Sea, China. Environ. Pollut. 2017, 221, 234–243. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Prasad, R.; Singh, J. Biological Biosensors for Monitoring and Diagnosis. In Microbial Biotechnology: Basic Research and Applications; Singh, J., Vyas, A., Wang, S., Prasad, R., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2020; pp. 317–335. ISBN 9789811528170. [Google Scholar]
- Herrera-Chacon, A.; Cetó, X.; Valle, M. del Molecularly imprinted polymers—Towards electrochemical sensors and electronic tongues. Anal. Bioanal. Chem. 2021, 413, 6117–6140. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Stortini, A.M.; Moretto, L.M.; Costantino, C.; Bogialli, S.; Ugo, P. Electrochemosensor for Trace Analysis of Perfluorooctanesulfonate in Water Based on a Molecularly Imprinted Poly(o-phenylenediamine) Polymer. ACS Sens. 2018, 3, 1291–1298. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, A.; Wang, N.; Zheng, G.; Zhu, L. Rapid fluorometric determination of perfluorooctanoic acid by its quenching effect on the fluorescence of quantum dots. J. Lumin. 2015, 161, 374–381. [Google Scholar] [CrossRef]
- Takayose, M.; Akamatsu, K.; Nawafune, H.; Murashima, T.; Matsui, J. Colorimetric Detection of Perfluorooctanoic Acid (PFOA) Utilizing Polystyrene-Modified Gold Nanoparticles. Anal. Lett. 2012, 45, 2856–2864. [Google Scholar] [CrossRef]
- Chen, S.; Li, A.; Zhang, L.; Gong, J. Molecularly imprinted ultrathin graphitic carbon nitride nanosheets–Based electrochemiluminescence sensing probe for sensitive detection of perfluorooctanoic acid. Anal. Chim. Acta 2015, 896, 68–77. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Porto, G.; Biasiolo, A.; Perri, C.; Arcadio, F.; Zeni, L. A Molecularly Imprinted Polymer on a Plasmonic Plastic Optical Fiber to Detect Perfluorinated Compounds in Water. Sensors 2018, 18, 1836. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, Y.; Li, Y.; Zhang, Q.; Xu, S.; Zhu, H.; Shu, B. A rapid and high-throughput quantum dots bioassay for monitoring of perfluorooctane sulfonate in environmental water samples. Environ. Pollut. 2011, 159, 1348–1353. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Barpaga, D.; Soltis, J.A.; Shutthanandan, V.; Kargupta, R.; Han, K.S.; McGrail, B.P.; Motkuri, R.K.; Basuray, S.; Chatterjee, S. Metal–Organic Framework-Based Microfluidic Impedance Sensor Platform for Ultrasensitive Detection of Perfluorooctanesulfonate. ACS Appl. Mater. Interfaces 2020, 12, 10503–10514. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Li, J.; Mo, L.; Liang, J.; Fan, H. A molecularly imprinted chitosan doped with carbon quantum dots for fluorometric determination of perfluorooctane sulfonate. Microchim. Acta 2018, 185, 473. [Google Scholar] [CrossRef] [PubMed]
- Suarasan, S.; Focsan, M.; Potara, M.; Soritau, O.; Florea, A.; Maniu, D.; Astilean, S. Doxorubicin-Incorporated Nanotherapeutic Delivery System Based on Gelatin-Coated Gold Nanoparticles: Formulation, Drug Release, and Multimodal Imaging of Cellular Internalization. ACS Appl. Mater. Interfaces 2016, 8, 22900–22913. [Google Scholar] [CrossRef] [PubMed]
- Pistocchi, A.; Loos, R. A map of European emissions and concentrations of PFOS and PFOA. Environ. Sci. Technol. 2009, 43, 9237–9244. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Yin, M.; Zhang, Z.; Chen, Y.; Deng, Q.; Wang, S. Surfactant-Sensitized Covalent Organic Frameworks-Functionalized Lanthanide-Doped Nanocrystals: An Ultrasensitive Sensing Platform for Perfluorooctane Sulfonate. ACS Omega 2019, 4, 15947–15955. [Google Scholar] [CrossRef] [PubMed]
- Tran, T, T.; Li, J.; Feng, H.; Cai, J.; Yuan, L.; Wang, N.; Cai, Q. Molecularly imprinted polymer modified TiO2 nanotube arrays for photoelectrochemical determination of perfluorooctane sulfonate (PFOS). Sens. Actuators B Chem. 2014, 190, 745–751. [Google Scholar] [CrossRef]
- Ganesan, S.; Chawengkijwanich, C.; Gopalakrishnan, M.; Janjaroen, D. Detection methods for sub-nanogram level of emerging pollutants—Per and polyfluoroalkyl substances. Food Chem. Toxicol. 2022, 168, 113377. [Google Scholar] [CrossRef]
- Huang, T.; McClelland, A.; Zeng, T.H. Trace PFAS Detection in Water Sources Using Silver Nanoparticles for Surface-Enhanced Raman Spectroscopy (SERS). In Proceedings of the 2022 IEEE 22nd International Conference on Nanotechnology (NANO); 2022; pp. 342–345. [Google Scholar]
- Keskin, B.; Üzer, A.; Apak, R. Colorimetric sensing of ammonium perchlorate using methylene Blue−Modified gold nanoparticles. Talanta 2020, 206, 120240. [Google Scholar] [CrossRef]
| Techniques | Samples Type | Limit of Detection (LOD) | Extraction Methods | Detected PFASs | References |
| LC-MS/MS | Water | 0.6 – 8.7 ng/L | Liquid-liquid extraction | PFOA, PFOS, PFHxA, PFODA, PFHpS, PFDS | [36] |
| HPLC-MS/MS | Water | 0.01-1.15 ng/L | Solid phase extraction | PFOS, PFOA, PFNA, PFHpA, PFDA, PFHxS, | [37] |
| LC-MS/MS | Milk | 0.057 ng/L (PFOA), 0.021 ng/L (PFOS) | Liquid-liquid extraction | PFNA, PFDA, PFOA, PFHpA, PFBS, PFHxS, PFOS, PFUnA | [38] |
| LC-MS/MS | Liquid Sample | 0.29 - 6.6 ng/L | Solid phase extraction | PFOS, PFOA, PFNA, PFDA, PFBA, PFUnA, | [39] |
| LC-MS/MS | Fruits and Vegetables | 0.07 ng/g (PFOS) | Solid phase extraction | PFOS, PFOA | [40] |
| HPLC-MS/MS | Sediment | 1.5 – 10.9 ng/L | Liquid-liquid extraction | PFOS, PFDA, PFOA, PFDoA, PFHxS, PFNA | [41] |
| HPLC-MS/MS | Cabbage, lettuce, mustard leaf, | 0.017-0.180 ng/g | Ultrasonic extraction | PFOS, PFHxS | [42] |
| LC-MS/MS | Vegetables | 0.002 – 3.73 ng/g | Liquid-liquid extraction | PFOS, PFOA, PFDA, PFBA, PFBS | [43] |
| HPLC-MS/MS | Surface water | 50 – 1790 ng/L | Solid phase extraction | FOSA, MeFOSA, EtFOSA, MeFOSE | [44] |
| HPLC-MS/MS | Different Water Samples | 0.05–0.22 ng/L | Solid phase extraction | PFOS and PFOA | [45] |
| Detection System | Matrix | Limit of Detection (ng/L) | Detection Range (ng/L) | References |
| Spectrofluorometer | MPA-CdS QDs | 124200 | 207000–16563000 | [50] |
| Colorimetric detection | Gold nanoparticles | - | - | [51] |
| Electro chemiluminescence | Ultrathin nanosheets of carbon nitride | 10 | 20–4000 | [52] |
| Optical | Novel SPR | 210 | 0 - 200000 | [53] |
| Optical density | Bio-Gold Nanoparticles | 2.5 | 2.5–75 | [54] |
| Smartphone camera | Smart sensor | 0.5 | 10000–1000000 | [6] |
| Impedimetric | PFOS | 0.0005 | 0.00005–50000 | [55] |
| Note: MPA = 3-mercaptopropionic acid, CdS = Cadmium sulfide, QDs = Quantum Dots | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




