Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of the maximum of absorbance with the solvatochromic dyes in liquid samples
2.3. Determination of the maximum of absorbance with the solvatochromic dyes in solid thin film of polymer sample
3. Results
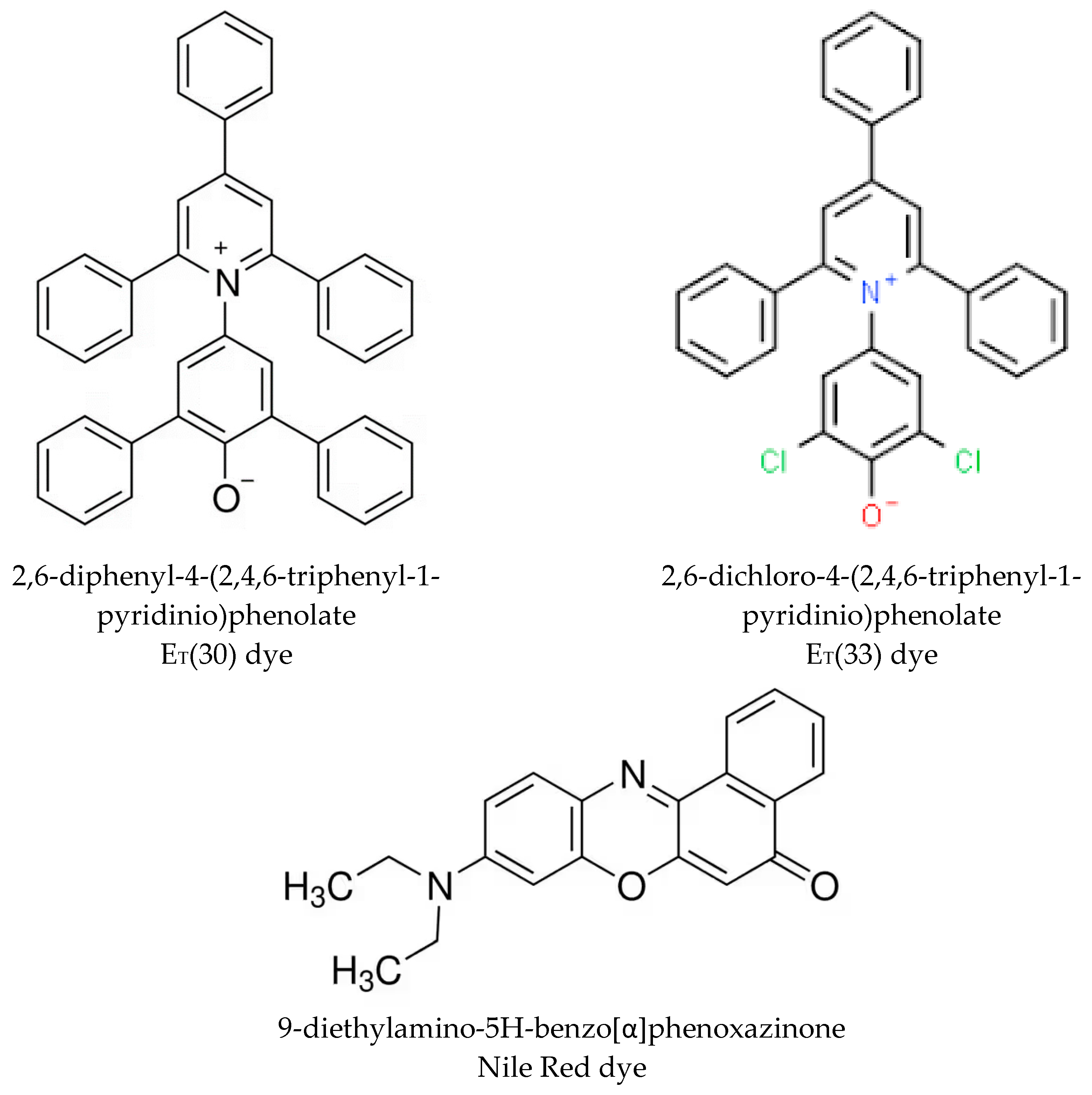
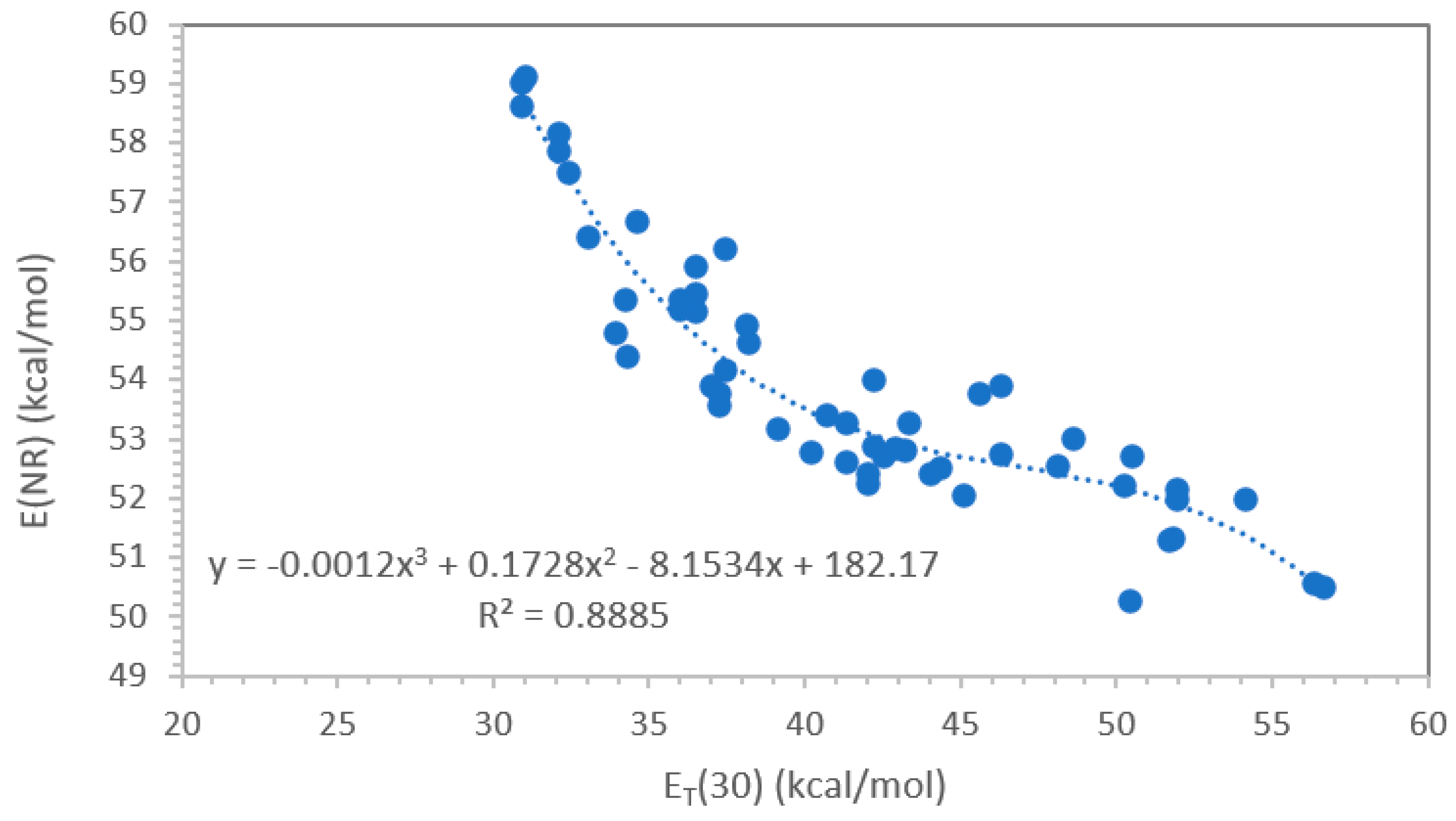
3.1. General aspects of Nile Red solvatochromic dye with respect to ET(30) dye.
3.2. Determination of rubber and polymer polarity with Nile Red solvatochromic dye
3.2. Determination of rubber plasticizers polarity with Nile Red solvatochromic dye
4. Discussion
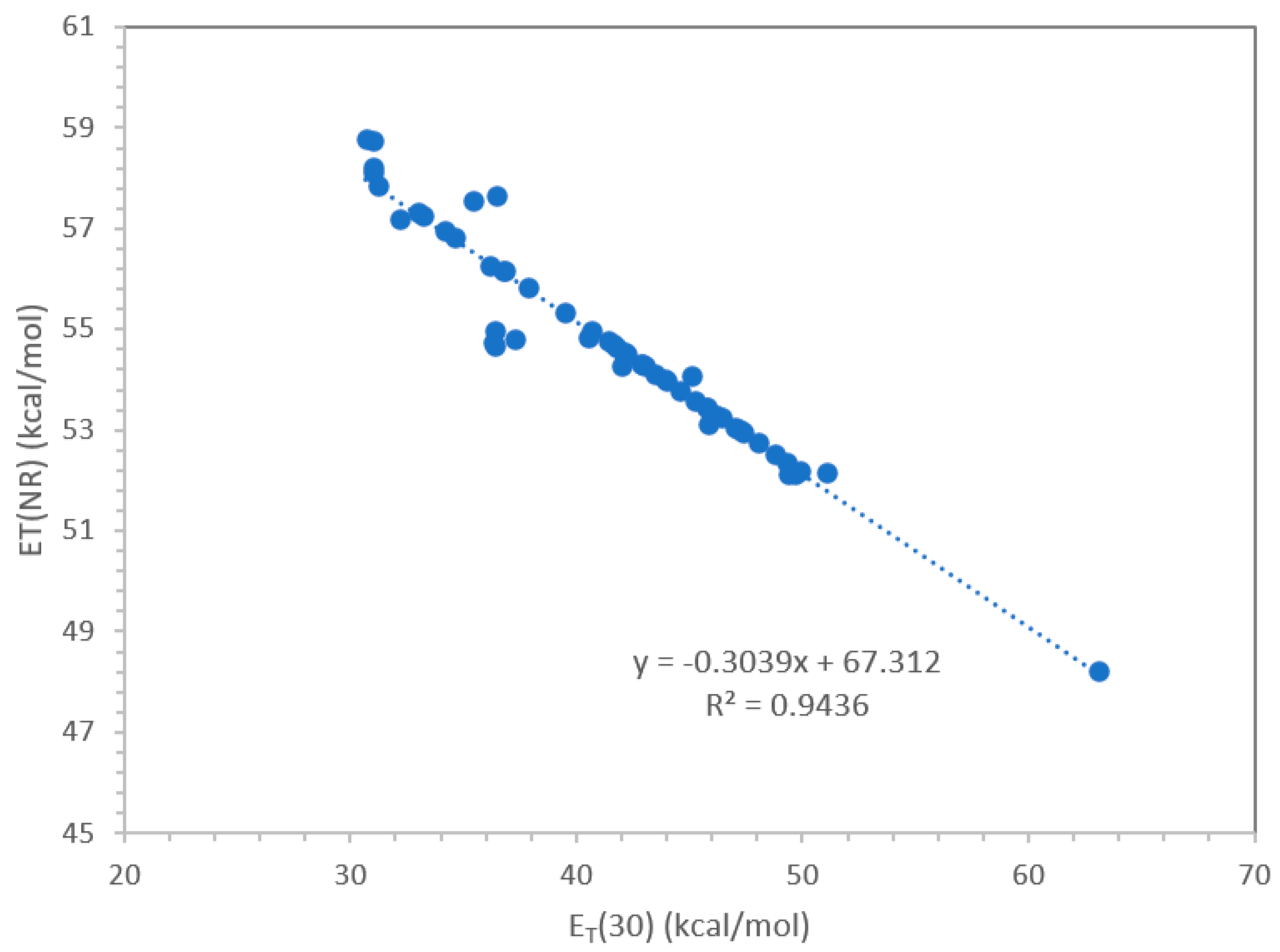
4.1. Correlation between the E(NR) scale and the ET(30) scale
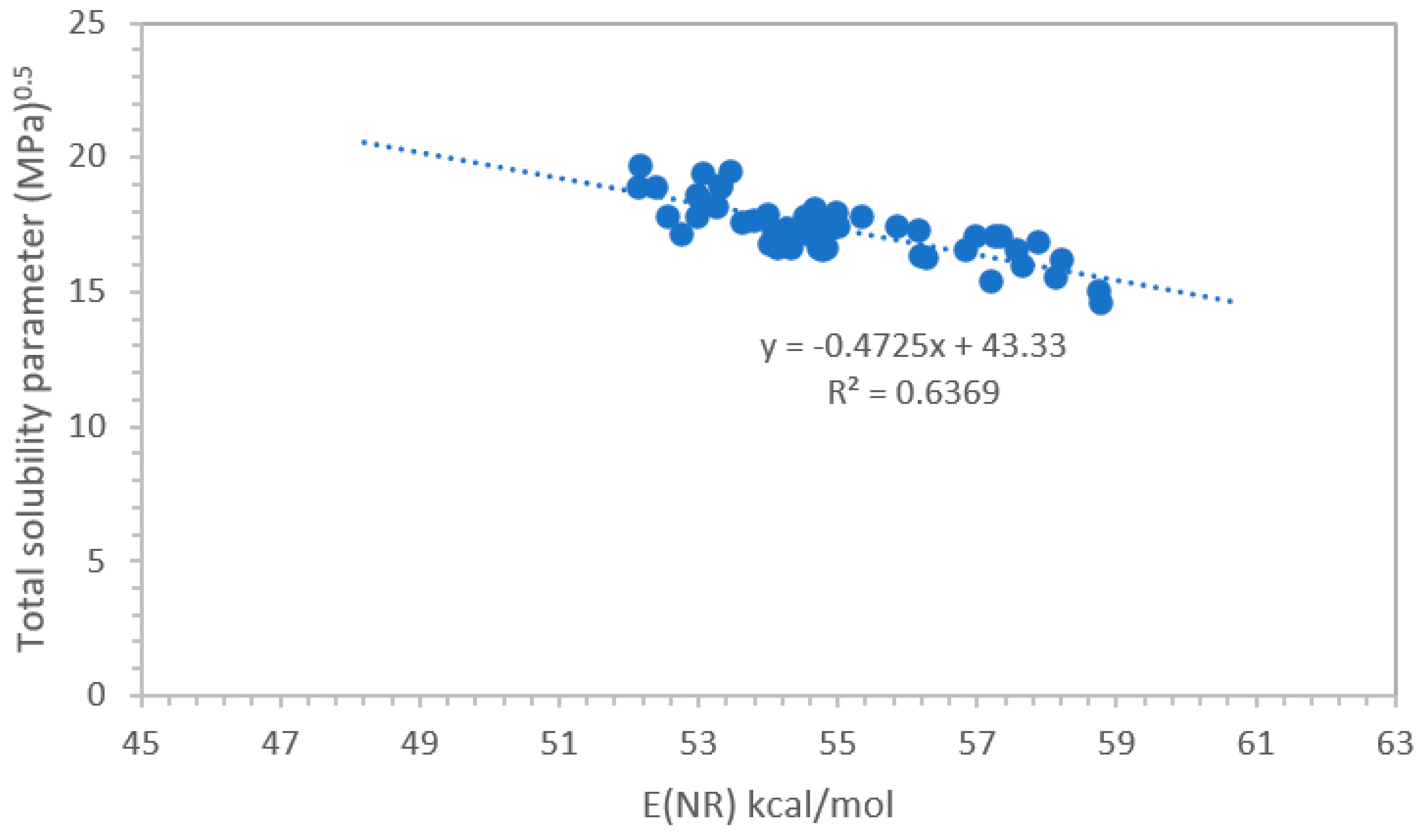
4.2. Correlation between the solubility parameter δt and the E(NR) scale
4.3. Some reflections on the diene rubber compatibility based on the E(NR) scale
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sears, J.K.; Darby, J.R. The Technology of Plasticizers; J. Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Stepek, J.; Daoust, H. Additives for Plastics; Springer Science: New York, NY, USA, 1983; pp. 7–33. [Google Scholar]
- Tager, A. The Physical Chemistry of Polymers; Mir Publishers: Moscow, USSR, 1978; pp. 547–567. [Google Scholar]
- Cadogan, D.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Elvers, B., Arpe, H.J. et al., et al., Eds.; VCH: Weinheim, Germany, 1992; Volume A20, pp. 439–458. [Google Scholar]
- Sears, J.F.; Touchette, N.W. Plasticizers. In Concise Encyclopedia of Polymer Science and Engineering; Kroschwitz, J., Ed.; John Wiley & Sons: New York, NY, USA, 1999; pp. 734–744. [Google Scholar]
- Howick, C.J. Plasticizers. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Ley, C., Ed.; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Godwin, A.D. Plasticizers. In Applied Plastics and Engineering Handbook; Kutz, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 487–502. [Google Scholar]
- Marcilla, A.; Breltràn, A. Mechanism of plasticizers action. In Handbook of Plasticizers, 4th ed; Wypich, G., Ed.; ChemTec Publishing: Toronto, ON, Canada, 2023; pp. 139–158. [Google Scholar]
- Wypich, A. Compatibility of plasticizers. In Handbook of Plasticizers, 4th ed.; Wypich, G., Ed.; ChemTec Publishing: Toronto, ON, Canada, 2023; pp. 159–180. [Google Scholar]
- Morris, G. Plasticizers. In Developments in Rubber Technology – 1; Whelan, A., Lee, K.S., Eds.; Elsevier Applied Science: London, UK, 1979; pp. 207–225. [Google Scholar]
- Crouter, B.G. Processing aids and plasticizers. In Developments in Rubber Technology – 4; Whelan, A., Lee, K.S., Eds.; Elsevier Applied Science: London, UK, 1987; pp. 119–158. [Google Scholar]
- Brydson, J.A. Rubber Chemistry; Applied Science Publishers Ltd.: London, UK, 1978. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Van Krevelen, D.W. Properties of Polymers. Their correlation with chemical structure, their numerical estimation and prediction from additive group contributions, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Cataldo, F. On the solubility parameter of C60 and higher fullerenes. Fullerenes, Nanot Carbon Nanostruct 2009, 17, 79–84. [Google Scholar] [CrossRef]
- Cataldo, F. Solubility of fullerenes in fatty acids esters: a new way to deliver in vivo fullerenes. Theoretical calculations and experimental results”. In Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Cataldo, F., Da Ros, T., Eds.; Springer Science: Dordrecht, The Netherlands, 2008; Chapter 13. [Google Scholar]
- Cataldo, F.; Ursini, O.; Angelini, G. Biodiesel as a plasticizer of a SBR-based tire tread formulation. ISRN Polym Sci. 2013, 2013, 340426. [Google Scholar] [CrossRef]
- Cataldo, F. Application of Reichardt’s solvent polarity scale (ET(30)) in the selection of bonding agents for composite solid rocket propellants. Liquids 2022, 2, 289–302. [Google Scholar] [CrossRef]
- Sandstrom, P.H.; Rodewald, S.; Ramanathan, A. Rubber composition and tire with component comprised of polyisoprene rubber and soybean oil. US Patent N°20140135424A1, 2014. assigned to Goodyear Tyre & Rubber Co.. [Google Scholar]
- Brunelet, T.; Dinh, M.; Favrot, J.M.; Labrunie, P.; Lopitaux, G.; Royet, J.G. Plasticizing system for rubber composition. US Patent N°7834074B2, 2004. assigned to Michelin Recherche et Technique SA. [Google Scholar]
- Mohamed, N.R.; Othman, N.; Shuib, R.K. Synergistic effect of sunflower oil and soybean oil as alternative processing oil in the development of greener tyre tread compound. J. Rubber Res. 2022, 25, 239–249. [Google Scholar] [CrossRef]
- Reichardt, C. Pyridinium-N-phenolate betaine dyes as empirical indicators of solvent polarity: some new findings. Pure Appl. Chem. 2008, 80, 1415–1432. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Acree, W.E., Jr.; Lang, A.S. Reichardt’s Dye-Based Solvent Polarity and Abraham Solvent Parameters: Examining Correlations and Predictive Modeling. Liquids 2023, 3, 303–313. [Google Scholar] [CrossRef]
- Davis, M.M.; Helzer, H.B. Titrimetric and Equilibrium Studies Using Indicators Related to Nile Blue A. Anal. Chem. 1966, 38, 451–461. [Google Scholar] [CrossRef]
- Green, F.J. The Sigma-Aldrich Handbook of Stains, Dyes and Indicators; Aldrich Chemical Company, Inc.: Milwaukee, WI, USA, 1990; pp. 519–520. [Google Scholar]
- Deye, J.F.; Berger, T.A.; Anderson, A.G. Nile Red as a solvatochromic dye for measuring solvent strength in normal liquids and mixtures of normal liquids with supercritical and near critical fluids. Anal. Chem. 1990, 62, 615–622. [Google Scholar] [CrossRef]
- Deye, J.F.; Berger, T.A.; Anderson, A.G. Errata Corrige. Anal. Chem. 1990, 62, 1552. [Google Scholar] [CrossRef]
- Ghoneim, N. Photophysics of Nile red in solution: steady state spectroscopy. Spectrochim Acta Part A Mol Biomol Spect 2000, 56, 1003–1010. [Google Scholar] [CrossRef]
- Dias Jr, L.C.; Custodio, R.; Pessine, F.B. Investigation of the Nile Red spectra by semi-empirical calculations and spectrophotometric measurements. Int. J. Quantum Chem. 2006, 106, 2624–2632. [Google Scholar] [CrossRef]
- Kawski, A.; Bojarski, P.; Kukliński, B. Estimation of ground-and excited-state dipole moments of Nile Red dye from solvatochromic effect on absorption and fluorescence spectra. Chem. Phys. Lett. 2008, 463, 410–412. [Google Scholar] [CrossRef]
- Guido, C.A.; Mennucci, B.; Jacquemin, D.; Adamo, C. Planar vs. twisted intramolecular charge transfer mechanism in Nile Red: new hints from theory. Phys. Chem. Chem. Phys. 2010, 12, 8016–8023. [Google Scholar] [CrossRef] [PubMed]
- Freidzon, A.Y.; Safonov, A.A.; Bagaturyants, A.A.; Alfimov, M.V. Solvatofluorochromism and twisted intramolecular charge-transfer state of the nile red dye. Int. J. Quantum Chem. 2012, 112, 3059–3067. [CrossRef]
- Yablon, D.G.; Schilowitz, A.M. Solvatochromism of Nile Red in nonpolar solvents. Appl. Spectrosc. 2004, 58, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Zakerhamidi, M.S.; Sorkhabi, S.G. Solvent effects on the molecular resonance structures and photo-physical properties of a group of oxazine dyes. J. Lumin 2015, 157, 220–228. [Google Scholar] [CrossRef]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Gilani, A.G.; Moghadam, M.; Zakerhamidi, M.S. Solvatochromism of Nile red in anisotropic media. Dyes Pigments 2012, 92, 1052–1057. [Google Scholar] [CrossRef]
- Sarkar, N.; Das, K.; Nath, D.N.; Bhattacharyya, K. Twisted charge transfer processes of Nile red in homogeneous solutions and in faujasite zeolite. Langmuir, 1994, 10, 326–329. [Google Scholar] [CrossRef]
- Kreder, R.; Pyrshev, K.A.; Darwich, Z.; Kucherak, O.A.; Mély, Y.; Klymchenko, A.S. Solvatochromic Nile Red probes with FRET quencher reveal lipid order heterogeneity in living and apoptotic cells. ACS Chem. Biol. 2015, 10, 1435–1442. [Google Scholar] [CrossRef]
- Cser, A.; Nagy, K.; Biczók, L. Fluorescence lifetime of Nile Red as a probe for the hydrogen bonding strength with its microenvironment. Chem. Phys. Lett. 2002, 360, 473–478. [Google Scholar] [CrossRef]
- Dutta, A.K.; Kamada, K.; Ohta, K. Spectroscopic studies of nile red in organic solvents and polymers. J. Photochem. Photobiol. A Chem. 1996, 93, 57–64. [Google Scholar] [CrossRef]
- Cataldo, F. Aminoxyl (nitroxyl or nitroxide) radical formation by the action of ozone on squalene containing secondary aromatic amine antioxidants. J. Vinyl Addit. Technol. 2022, 28, 379–389. [Google Scholar] [CrossRef]
- Mariana, M.; Castelo-Branco, M.; Soares, A.M.; Cairrao, E. Phthalates’ exposure leads to an increasing concern on cardiovascular health. J. Hazard Mater. 2023, 457, 131680. [Google Scholar] [CrossRef]
- Arrigo, F.; Impellitteri, F.; Piccione, G.; Faggio, C. Phthalates and their effects on human health: Focus on erythrocytes and the reproductive system. Compar. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 270, 109645. [Google Scholar] [CrossRef]
- Luís, C.; Algarra, M.; Câmara, J.S.; Perestrelo, R. Comprehensive insight from phthalates occurrence: From health outcomes to emerging analytical approaches. Toxics 2021, 9, 157. [Google Scholar] [CrossRef]
- Cataldo, F. unpublished results.
- Moreno, E.M.; Levy, D. Role of the comonomer GLYMO in ORMOSILs as reflected by nile red spectroscopy. Chem. Mater. 2000, 12, 2334–2340. [Google Scholar] [CrossRef]
| PLASTICIZER, SOLVENT OR POLYMER |
ET(30) Kcal/mol | E(NR) Kcal/mol | References or notes on the ET(30) values | References or notes on the E(NR) values | Solubility parameter δt in (MPa)0.5 calc. according ref. [14] |
|---|---|---|---|---|---|
| isooctane | 30.7 | 58.77 | ref. [23] | this work | 14.6 |
| n-hexane and cyclohexane | 31.0 | 58.75 | ref. [23] | this work | 15.1 |
| tetradecane | 31.0 | 58.21 | ref. [23] | this work | 16.2 |
| SQUALANE | 31.0 | 58.12 | estimated | this work | 15.6 |
| decalin | 31.2 | 57.87 | ref. [23] | this work | 16.9 |
| SQUALENE | 36.5 | 57.64 | this work fm ET(33) | this work | 16.0 |
| Liquid cis-POLYISOPRENE (Liq-IR) | 35.4 | 57.55 | this work fm ET(33) | this work | 16.6 |
| Liquid 1,2-POLYBUTADIENE | 33.0 | 57.32 | calculated (*) | this work | 17.1 |
| Liquid cis-POLYBUTADIENE (Liq-BR) | 33.2 | 57.25 | calculated (*) | this work | 17.1 |
| cyclohexene | 32.2 | 57.19 | ref. [23] | this work | 15.4 |
| POLYBUTADIENE thin film (BR) | 34.2 | 56.96 | calculated (*) | this work | 17.1 |
| POLYISOPRENE thin film (IR) | 34.6 | 56.82 | calculated (*) | this work | 16.6 |
| Oleyl Oleate | 36.2 | 56.27 | this work fm ET(33) | this work | 16.3 |
| S-SBR with styrene 21% & vinyl 50% | 36.8 | 56.17 | calculated (*) | this work | 17.4 |
| T-DAE oil | 36.8 | 56.2 | this work fm ET(33) | calculated (**) | 17.3 |
| POLYSTYRENE thin film | 37.8 | 55.84 | calculated (*) | this work | 17.5 |
| di-n-butyl PHTHALATE | 39.5 | 55.3 | ref. [23] | calculated (**) | 17.8 |
| dimethyl PHTHALATE | 40.7 | 55.0 | ref. [23] | calculated (**) | 18.0 |
| Diisododecyl ADIPATE | 36.4 | 54.98 | this work fm ET(33) | this work | 17.5 |
| Ethyl OLEATE | 40.5 | 54.85 | this work fm ET(30) | this work | 17.3 |
| MES oil | 37.3 | 54.83 | this work fm ET(33) | this work | 16.7 |
| Biodiesel fm rapeseed oil | 41.4 | 54.77 | calculated (*) | this work | 16.6 |
| Diethylhexyl SEBACATE | 36.3 | 54.76 | this work fm ET(33) | this work | 17.0 |
| Cocconut methyl ester | 41.6 | 54.70 | calculated (*) | this work | 16.7 |
| dimethyl SEBACATE | 41.8 | 54.7 | this work fm ET(33) | calculated (**) | 18.1 |
| Diethylhexyl ADIPATE (DOA) | 36.4 | 54.67 | calculated (*) | this work | 17.8 |
| Dibutyl SEBACATE | 42.3 | 54.5 | this work fm ET(33) | calculated (**) | 17.8 |
| Soybean oil | 42.1 | 54.55 | calculated (*) | this work | 17.2 |
| Nytex BIO 6200 | 42.1 | 54.55 | calculated (*) | this work | 16.7 |
| Epoxidized Natural Rubber (ENR-25) | 42.9 | 54.32 | calculated (*) | this work | 17.2 |
| Dioctylterephthalate (DOTP) | 43.0 | 54.27 | calculated (*) | this work | 17.4 |
| Sunflower oil high oleic | 43.5 | 54.13 | calculated (*) | this work | 16.7 |
| Ethyl PALMITATE | 43.9 | 54.0 | this work fm ET(30) | calculated (**) | 16.8 |
| Diethyl AZELATE | 45.1 | 54.08 | this work fm ET(33) | this work | 17.3 |
| methyl undecenoate | 44.0 | 53.99 | calculated (*) | this work | 17.9 |
| PEG dioleate | 44.6 | 53.79 | calculated (*) | this work | 17.7 |
| PEG monooleate | 45.2 | 53.60 | calculated (*) | this work | 17.6 |
| Polymethylmethacrylate film (PMMA) | 45.8 | 53.44 | calculated (*) | ref. [41] | |
| Poly(Lactic acid) film (PLLA) | 45.8 | 53.44 | calculated (*) | this work | 19.5 |
| Bis(THFA) ADIPATE | 46.2 | 53.30 | this work fm ET(33) | calculated (**) | 19.0 |
| Ethyl levulinate | 46.2 | 53.30 | calculated (*) | this work | 18.9 |
| Dioctylphthalate (ethylhexyl) (DOP) | 46.4 | 53.24 | calculated (*) | this work | 18.2 |
| Nitrile Rubber with 33% ACN film | 47.0 | 53.07 | calculated (*) | this work | 19.4 |
| Bis(THFA) SEBACATE | 47.1 | 53.0 | this work fm ET(33) | calculated (**) | 18.4 |
| Bis(THFA) AZELATE | 47.3 | 53.0 | this work fm ET(33) | calculated (**) | 18.6 |
| THFA OLEATE | 47.4 | 53.0 | this work fm ET(33) | calculated (**) | 17.8 |
| THFA LAURATE (30°C) | 48.0 | 52.8 | this work fm ET(33) | calculated (**) | 17.2 |
| THFA PELARGONATE (30°C) | 49.3 | 52.4 | this work fm ET(33) | calculated (**) | 18.9 |
| Tetrahydrofurfuryl alcohol | 49.9 | 52.2 | this work fm ET(30) | calculated (**) | |
| L-(-) ethyl lactate | 51.1 | 52.16 | ref. [23] | this work | |
| Polyethylene glycol (PEG-400) | 49.7 | 52.15 | this work fm ET(30) | this work | 19.7 |
| Polytetrahydrofuran (PolyTHF)low Mw | 49.3 | 52.12 | this work fm ET(30) | this work | 18.9 |
| Water | 63.1 | 48.21 | ref. [23] | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





