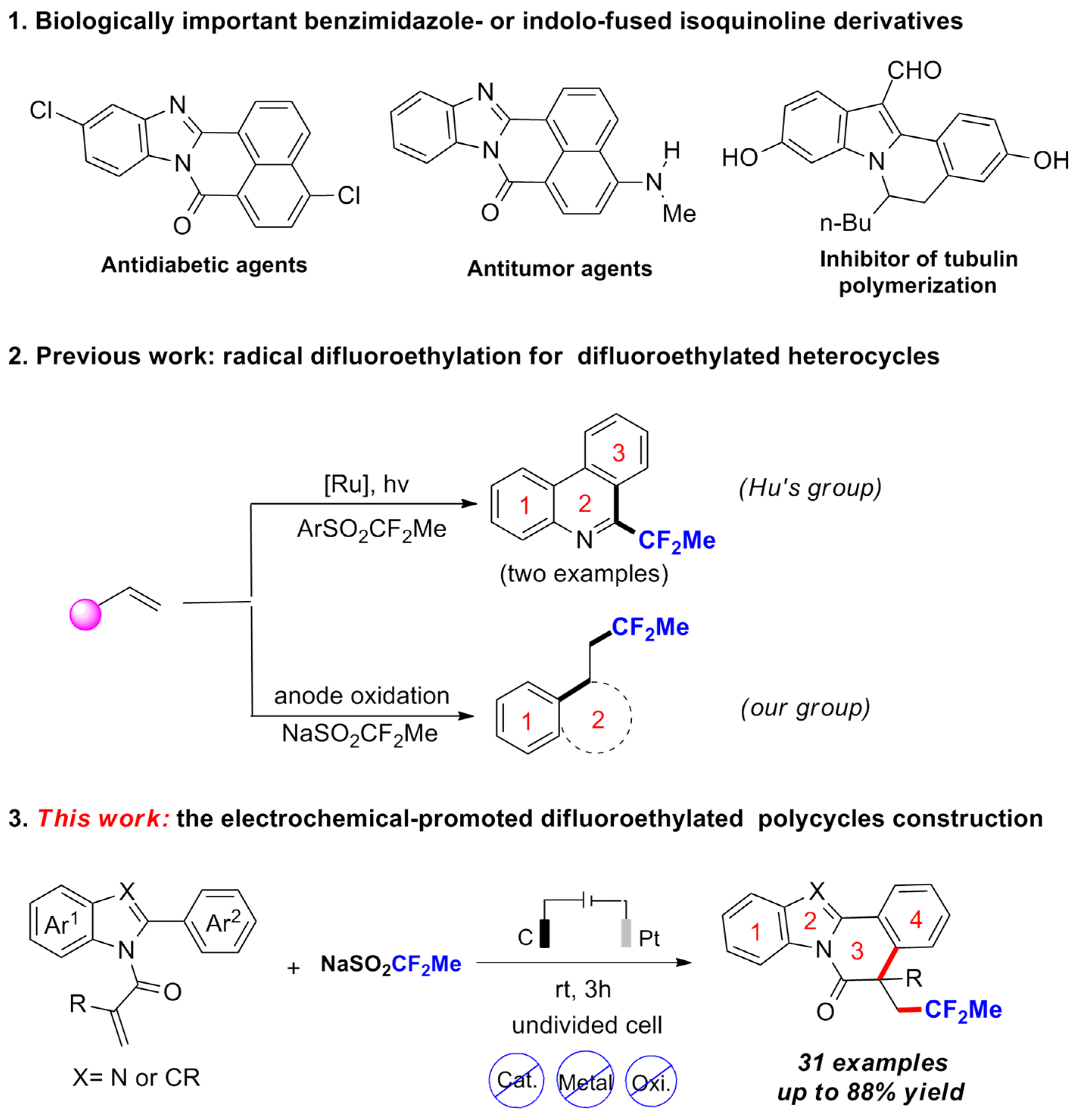
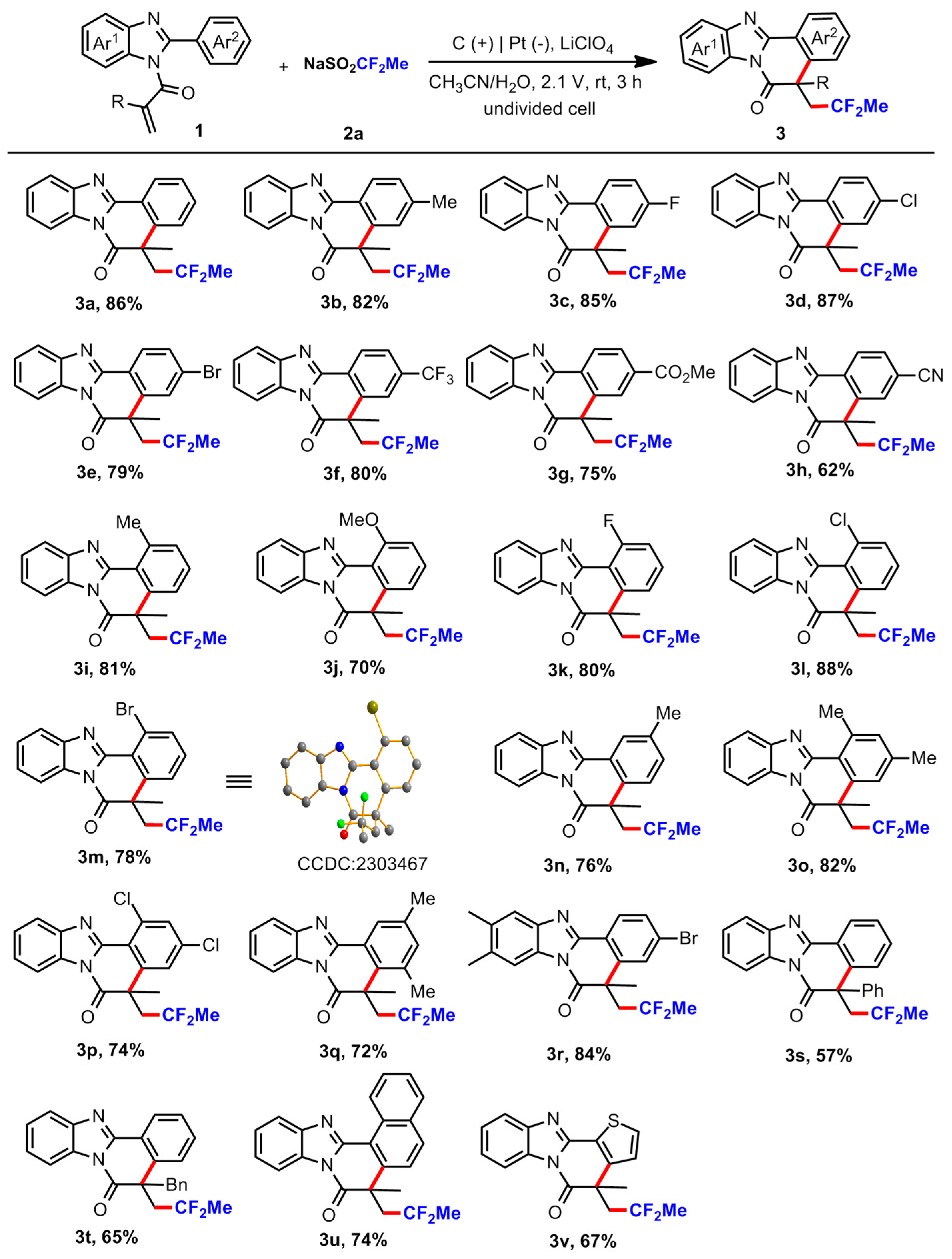
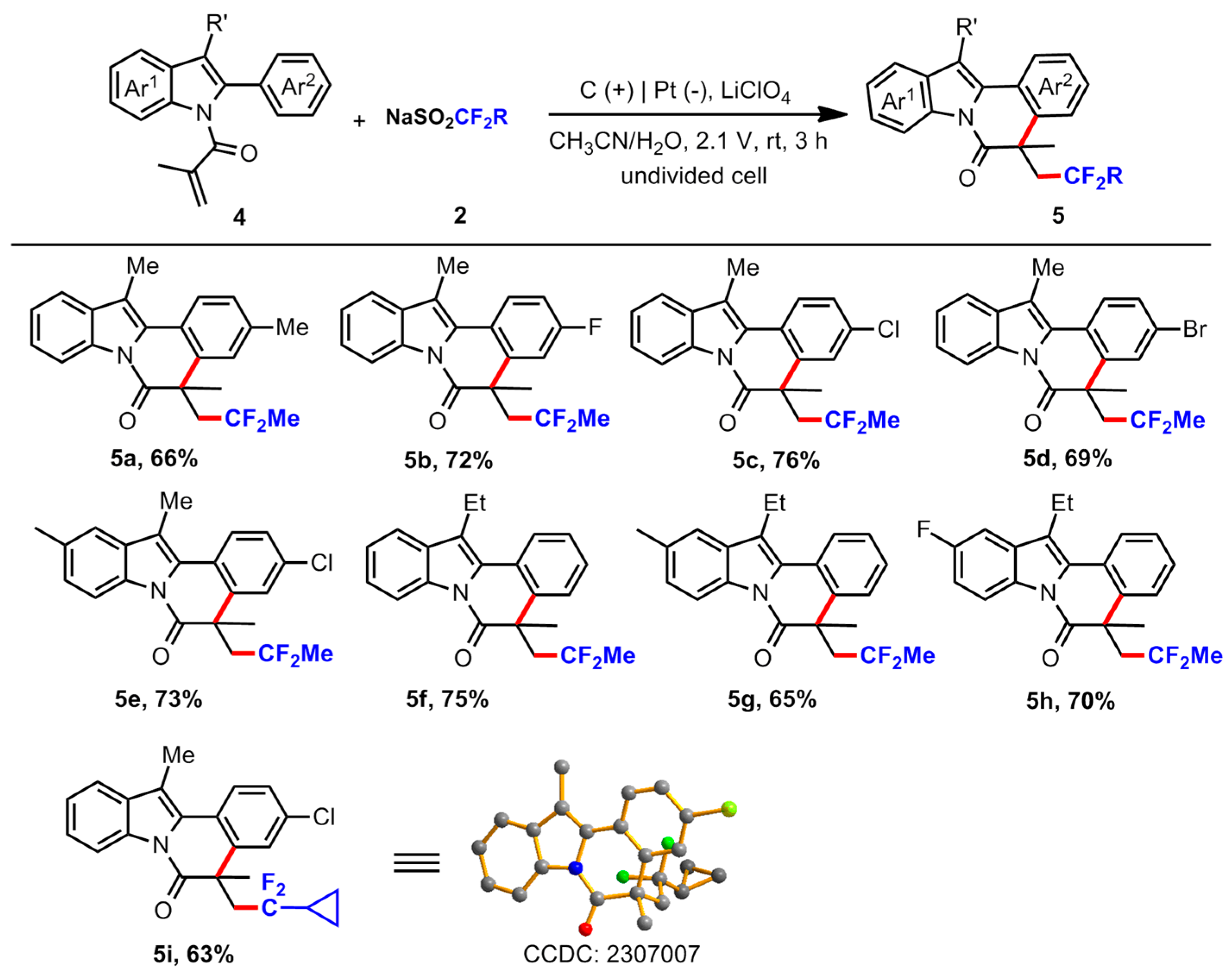
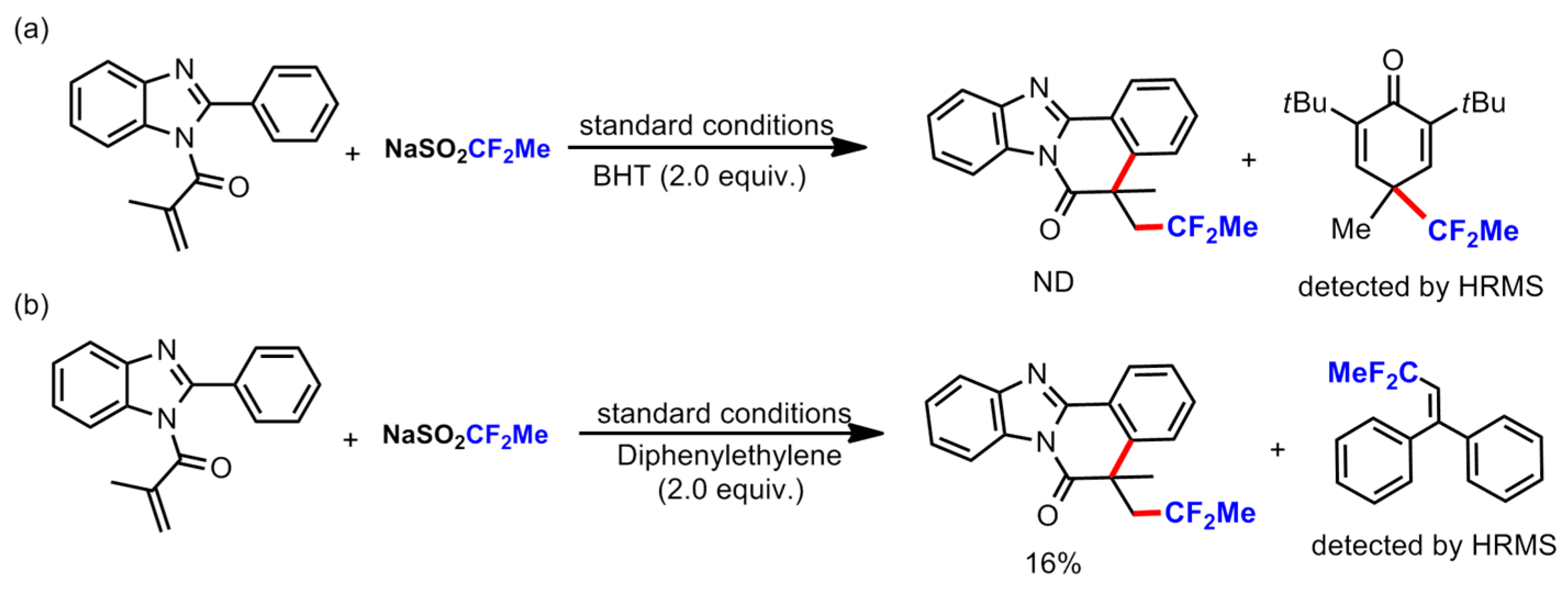
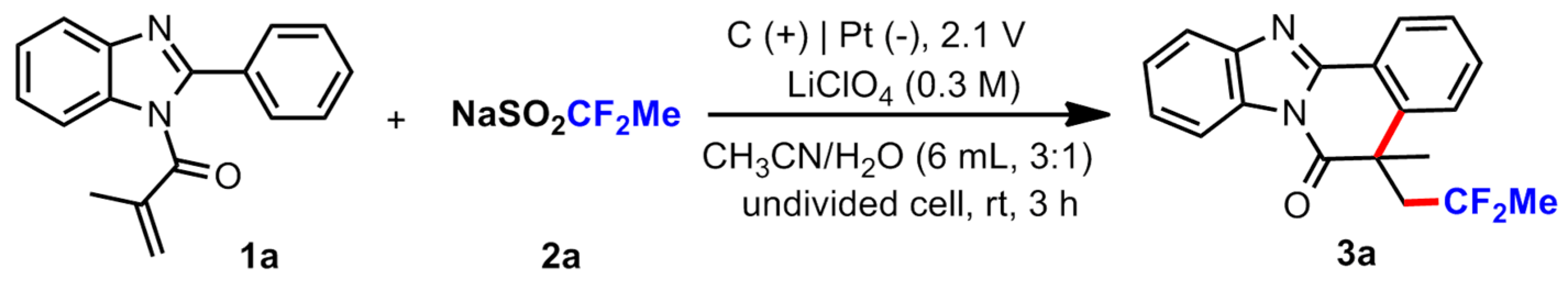
3.2. General Procedure for the Reaction
To a 20 mL test tube with a stir bar was charged with, 2-arylbenzimidazoles/2-arylindoles (1 equiv., 0.2 mmol), MeCF
2SO
2Na, or sodium cyclopropyldifluoromethylsulfinate (3 equiv., 0.6 mmol), LiClO
4 (0.3 M), MeCN (4.5 mL), H
2O (1.5 mL). The tube was equipped with a carbon plate (10 mm * 10 mm * 3 mm) as the anode and a platinum plate (10 mm × 10 mm × 0.2 mm) as the cathode. The reaction mixture was electrolyzed in an undivided cell at room temperature under a constant voltage of 2.1 V for 3 h. Upon completion, the mixture was extracted with EtOAc (10 mL × 3). The combined organic phases were dried over Na
2SO
4 and condensed under vacuum. The residue was purified by silica gel column chromatography to afford the final products. (
1H NMR,
19F NMR and
13C NMR of compounds (
3a–v and 5a-i ) are shown in
Supplementary Materials).
5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3a). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 56.1 mg, 86 % yield. 1H NMR (400 MHz, CDCl3): δ 8.52 – 8.49 (m, 1H), 8.37 – 8.35 (m, 1H), 7.84 – 7.82 (m, 1H), 7.59 – 7.55 (m, 1H), 7.50 (t, J = 7.2 Hz, 2H), 7.47 – 7.40 (m, 2H), 3.30 – 3.18 (m, 1H), 2.79 – 2.67 (m, 1H), 1.72 (s, 3H), 1.34 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.2, 149.6, 144.0, 140.0, 131.5, 131.3, 127.9, 126.8, 126.0, 125.9, 125.6, 122.6 (t, J = 240.5 Hz), 122.3, 119.8, 115.7, 48.2 (t, J = 23.9 Hz), 45.5, 31.1, 24.7 (t, J = 27.3 Hz). 19F NMR (471 MHz, CDCl3): δ -86.07 – -86.30 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C19H16F2N2O (M+H)+ 327.1303; Found 327.1306.
5-(2,2-difluoropropyl)-3,5-dimethylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3b). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 55.7 mg, 82 % yield. 1H NMR (500 MHz, CDCl3): δ 8.37 (d, J = 8.0 Hz, 1H), 8.35 – 8.33 (m, 1H), 7.80 – 7.79 (m, 1H), 7.44 – 7.38 (m, 2H), 7.30 (d, J = 8.5 Hz, 1H), 7.26 (s, 1H), 3.21 (q, J = 15.0 Hz, 1H), 2.70 (q, J = 15.5 Hz, 1H), 2.45 (s, 3H), 1.70 (s, 3H), 1.32 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 172.3, 149.8, 144.1, 141.8, 140.1, 131.4, 129.0, 127.1, 126.0, 125.8, 125.3, 122.5 (t, J = 240.6 Hz), 119.7, 119.6, 115.6, 48.2 (t, J = 24.1 Hz), 45.5, 31.0, 24.7(t, J = 27.4 Hz), 21.8. 19F NMR (471 MHz, CDCl3): δ -86.01 – -86.20 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C20H18F2N2O (M+H)+ 341.1460; Found 341.1461.
5-(2,2-difluoropropyl)-3-fluoro-5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3c). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate =10/1), 58.5 mg, 85 % yield. 1H NMR (400 MHz, CDCl3): δ 8.50 (dd, J = 8.4, 6.0 Hz, 1H), 8.34 (d, J = 7.6 Hz, 1H), 7.80 (d, J = 7.6 Hz, 1H), 7.46 – 7.39 (m, 2H), 7.22 – 7.15 (m, 2H), 3.29 – 3.17 (m, 1H), 2.71 – 2.59 (m, 1H), 1.70 (s, 3H), 1.40 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.6, 164.6 (d, J = 252.5 Hz), 148.8, 144.0, 142.9 (d, J = 7.9 Hz), 131.4, 128.5 (d, J = 9.1 Hz), 125.7 (d, J = 37.5 Hz), 122.4 (t, J = 240.6 Hz), 119.7, 118.9, 115.9 (d, J = 22.4 Hz), 115.6, 113.7 (d, J = 23.2 Hz), 48.3 (t, J = 23.7 Hz), 45.7, 30.9, 24.7 (t, J = 27.3 Hz). 19F NMR (471 MHz, CDCl3): δ -85.51 – -86.22 (m, 1F), -87.32 – -88.02 (m, 1F), -106.94 – -106.99 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15F3N2O (M+H)+ 345.1209; Found 345.1211.
3-chloro-5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3d). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 62.6 mg, 87 % yield. 1H NMR (400 MHz, CDCl3): δ 8.43 (d, J = 8.4 Hz, 1H), 8.34 (d, J = 6.8 Hz, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.47 – 7.42 (m, 4H), 3.28 – 3.16 (m, 1H), 2.73 – 2.61 (m, 1H), 1.71 (s, 3H), 1.40 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.5, 148.7, 143.9, 141.8, 137.5, 131.4, 128.5, 127.4, 127.0, 126.0, 125.8, 122.5 (t, J = 239.0 Hz), 120.9, 119.8, 115.6, 48.2, 45.5 (d, J = 3.3 Hz), 30.9, 24.8 (t, J = 27.2 Hz). 19F NMR (471 MHz, CDCl3): δ -85.56 – -86.27 (m, 1F), -87.33 – -88.03 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15ClF2N2O(M+H)+ 361.0914; Found 361.0915.
3-bromo-5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3e). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 64.0 mg, 79 % yield. 1H NMR (500 MHz, CDCl3): δ 8.37 – 8.33 (m, 2H), 7.82 – 7.80 (m, 1H), 7.63 – 7.61 (m, 2H), 7.46 – 7.41 (m, 2H), 3.27 – 3.17 (m, 1H), 2.72 – 2.63 (m, 1H), 1.71 (s, 3H), 1.41 (t, J = 18.5 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.4, 148.7, 143.9, 141.9, 131.4 (d, J = 7.8 Hz), 129.9, 127.5, 126.0, 125.8, 122.5 (t, J = 238.5 Hz), 121.4, 119.9, 115.6, 48.2 (t, J = 23.7 Hz), 45.5 (d, J = 3.3 Hz), 30.8, 24.8 (t, J = 27.2 Hz). 19F NMR (471 MHz, CDCl3): δ -85.57 – -86.28 (m, 1F), -87.26 – -87.96 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15BrF2N2O (M+H)+ 405.0408; Found 405.0409.
5-(2,2-difluoropropyl)-5-methyl-3-(trifluoromethyl)benzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3f). A light yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 63.0 mg, 80 % yield. 1H NMR (500 MHz, CDCl3): δ 8.63 (d, J = 8.0 Hz, 1H), 8.38 – 8.36 (m, 1H), 7.86 – 7.85 (m, 1H), 7.75 – 7.72 (m, 2H), 7.49 – 7.45 (m, 2H), 3.33 – 3.24 (m, 1H), 2.80 – 2.71 (m, 1H), 1.75 (s, 3H), 1.43 (t, J = 18.5 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.4, 148.1, 143.9, 140.7, 132.8 (q, J = 32.8 Hz), 131.5, 126.7, 126.24, 126.20, 125.6, 124.7 (q, J = 3.6 Hz), 124.0 – 123.9 (m), 122.5 (q, J = 271.1 Hz), 122.5 (t, J = 240.1 Hz), 120.2, 115.8, 48.1 (t, J = 23.5 Hz), 45.7 (d, J = 3.3 Hz), 30.8, 24.8 (t, J = 27.2 Hz). 19F NMR (471 MHz, CDCl3): δ -62.91 (s, 3F), -85.44 – -86.13 (m, 1F), -87.85 – -88.51 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C20H15F5N2O (M+H)+ 395.1177; Found 395.1179.
methyl 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carboxylate (3g). A light yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1), 57.6 mg, 75 % yield. 1H NMR (400 MHz, CDCl3): δ 8.57 (d, J = 8.0 Hz, 1H), 8.38 – 8.36 (m, 1H), 8.18 (s, 1H), 8.13 (dd, J = 8.4, 1.6 Hz, 1H), 7.86 – 7.84 (m, 1H), 7.49 – 7.44 (m, 2H), 3.98 (s, 3H), 3.31 – 3.19 (m, 1H), 2.87 – 2.75 (m, 1H), 1.76 (s, 3H), 1.40 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.7, 166.1, 148.6, 144.1, 140.3, 132.4, 131.5, 128.7, 128.3, 126.18, 126.16, 126.14, 126.12, 122.6 (t, J = 238.6 Hz), 120.1, 115.8, 52.5, 48.3 (t, J = 23.5 Hz), 45.7 (d, J = 3.4 Hz), 30.8, 24.8 (t, J = 27.2 Hz). 19F NMR (471 MHz, CDCl3): δ -85.83 – -86.54 (m, 1F), -87.26 – -87.96 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H18F2N2O3 (M+H)+ 385.1358; Found 385.1360.
5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile (3h). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 7/1), 43.5 mg, 62% yield. 1H NMR (500 MHz, CDCl3): δ 8.59 (d, J = 8.0 Hz, 1H), 8.36 – 8.34 (m, 1H), 7.86 – 7.84 (m, 1H), 7.78 (s, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.48 – 7.46 (m, 2H), 3.31 – 3.21 (m, 1H), 2.76 – 2.66 (m, 1H), 1.73 (s, 3H), 1.46 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 170.9, 147.6, 143.9, 141.0, 131.4, 131.0, 130.9, 126.7, 126.5, 126.3, 122.5 (t, J = 238.6 Hz), 120.3, 118.0, 115.8, 114.5, 48.1 (t, J = 23.3 Hz), 45.5 (d, J = 3.1 Hz), 30.6, 29.6, 24.8 (t, J = 27.1 Hz). 19F NMR (471 MHz, CDCl3): δ -85.22 – -85.93 (m, 1F), -88.52 – -89.22 (m, 1F) HRMS (ESI-TOF) m/z: Calcd for C20H15F2N3O (M+H)+ 352.1256; Found 352.1260.
5-(2,2-difluoropropyl)-1,5-dimethylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3i). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 55.1 mg, 81% yield. 1H NMR (400 MHz, CDCl3): δ 8.38 (d, J = 8.0 Hz, 1H), 8.36 – 7.34 (m, 1H), 7.81 – 7.79 (m, 1H), 7.45 – 7.38 (m, 2H), 7.31 (d, J = 8.0 Hz, 1H), 7.27 (s, 1H), 3.22 (q, J = 15.2 Hz, 1H), 2.72 (q, J = 15.2 Hz, 1H), 2.47 (s, 3H), 1.71 (s, 3H), 1.34 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.3, 149.8, 144.0, 141.8, 140.0, 131.4, 129.1, 127.2, 125.9, 125.8, 125.3, 122.6 (t, J = 240.6 Hz), 119.7, 119.6, 115.6, 48.2 (t, J = 23.9 Hz), 45.5, 31.1, 24.7 (t, J = 27.3 Hz), 21.9. 19F NMR (471 MHz, CDCl3): δ -85.99 – -86.18 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C20H18F2N2O (M+H)+ 341.1460; Found 341.1462.
5-(2,2-difluoropropyl)-1-methoxy-5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3j). A brown solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 49.8 mg, 70% yield. 1H NMR (500 MHz, CDCl3): δ 8.39 – 8.37 (m, 1H), 7.91 – 7.89 (m, 1H), 7.51 (t, J = 8.0 Hz, 1H), 7.43 – 7.39 (m, 2H), 7.12 (d, J = 8.0 Hz, 1H), 7.06 (d, J = 8.5 Hz, 1H), 4.14 (s, 3H), 3.23 (q, J = 15.0 Hz, 1H), 2.71 (q, J = 15.5 Hz, 1H), 1.72 (s, 3H), 1.32 (t, J = 18.5 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.1, 158.7, 147.7, 144.3, 142.6, 131.7, 130.3, 125.6, 124.8 (t, J = 463.0 Hz), 122.5, 120.5, 119.1, 115.5, 111.8, 110.4, 56.6, 48.6 (t, J = 23.9 Hz), 45.4, 31.5, 24.7 (t, J = 27.4 Hz). 19F NMR (471 MHz, CDCl3): δ -85.88 – -86.06 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C20H18F2N2O2 (M+H)+ 357.1409; Found 357.1410.
5-(2,2-difluoropropyl)-1-fluoro-5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3k). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 55.0 mg, 80% yield. 1H NMR (500 MHz, CDCl3): δ 8.38 – 8.37 (m, 1H), 7.94 – 7.92 (m, 1H), 7.51 (td, J = 8.0, 5.0 Hz, 1H), 7.47 – 7.43 (m, 2H), 7.31 (d, J = 8.0 Hz, 1H), 7.24 – 7.21 (m, 1H), 3.29 – 3.19 (m, 1H), 2.76 – 2.67 (m, 1H), 1.72 (s, 3H), 1.39 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.6, 160.4 (d, J = 262.1 Hz), 145.8 (d, J = 8.4 Hz), 144.2, 142.5, 131.8 (d, J = 9.6 Hz), 130.4, 126.0 (d, J = 14.0 Hz), 122.8, 122.5 (t, J = 240.6 Hz), 120.5, 115.8, 115.6, 115.5, 111.8 (d, J = 9.9 Hz), 48.5 (t, J = 23.7 Hz), 45.4, 31.3, 24.8 (t, J = 27.3 Hz). 19F NMR (471 MHz, CDCl3): δ -85.44 – -86.11 (m, 1F), -87.00 – -87.69 (m, 1F), -107.12 – -107.16 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15F3N2O (M+H)+ 345.1209; Found 345.1211.
1-chloro-5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3l). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 63.4 mg, 88% yield. 1H NMR (400 MHz, CDCl3): δ 8.39 (dd, J = 6.0, 3.2 Hz, 1H), 7.93 (dd, J = 6.0, 3.2 Hz, 1H), 7.58 – 7.56 (m, 1H), 7.47 – 7.42 (m, 4H), 3.30 – 3.19 (m, 1H), 2.77 – 2.65 (m, 1H), 1.72 (s, 3H), 1.38 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.4, 147.0, 143.9, 142.9, 133.5, 131.5, 130.6, 130.4, 126.3, 125.9, 125.7, 122.5 (t, J = 238.8 Hz), 120.7, 120.5, 115.7, 48.49 (t, J = 23.7 Hz), 45.76 (d, J = 3.5 Hz), 31.43 (s), 24.80 (t, J = 27.3 Hz). 19F NMR (471 MHz, CDCl3): δ -85.21 – -85.92 (m, 1F), -86.99 – -87.70 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15ClF2N2O (M+H)+ 361.0914; Found 361.0916.
1-bromo-5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3m). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 63.2 mg, 78% yield. 1H NMR (500 MHz, CDCl3): δ 8.38 (dd, J = 6.0, 3.0 Hz, 1H), 7.93 (dd, J = 6.0, 3.0 Hz, 1H), 7.82 (d, J = 8.0 Hz, 1H), 7.49 – 7.44 (m, 3H), 7.33 (t, J = 8.0 Hz, 1H), 3.29 – 3.20 (m, 1H), 2.76 – 2.67 (m, 1H), 1.72 (s, 3H), 1.38 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.3, 147.1, 143.5, 143.1, 135.3, 130.8, 130.6, 126.4, 126.3, 125.9, 122.5 (t, J = 240.7 Hz), 121.8, 121.3, 120.8, 115.7, 48.4 (t, J = 23.5 Hz), 45.9 (d, J = 3.2 Hz), 31.5, 24.8 (t, J = 27.3 Hz). 19F NMR (471 MHz, CDCl3): δ -85.15 – -85.86 (m, 1F), -86.99 – -87.69 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H15BrF2N2O (M+H)+ 405.0408; Found 405.0412.
5-(2,2-difluoropropyl)-2,5-dimethylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3n). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 51.7 mg, 76% yield. 1H NMR (500 MHz, CDCl3): δ 8.37 – 8.35 (m, 1H), 8.32 (s, 1H), 7.83 – 7.81 (m, 1H), 7.45 – 7.40 (m, 2H), 7.37 (s, 2H), 3.22 (q, J = 15.5 Hz, 1H), 2.70 (q, J = 15.5 Hz, 1H), 2.46 (s, 3H), 1.69 (s, 3H), 1.33 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.4, 149.8, 143.9, 137.9, 137.2, 132.4, 131.5, 126.7, 126.1, 125.8, 125.5, 122.6 (t, J = 240.5 Hz), 122.0, 119.7, 115.6, 48.2 (t, J = 24.0 Hz), 45.3 (d, J = 2.2 Hz), 31.1, 24.7 (t, J = 27.3 Hz), 20.9. 19F NMR (471 MHz, CDCl3): δ -86.02– -86.20 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C20H18F2N2O (M+H)+ 341.1460; Found 341.1462.
5-(2,2-difluoropropyl)-1,3,5-trimethylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3o). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 58.0 mg, 82% yield. 1H NMR (500 MHz, CDCl3): δ 8.39 – 8.38 (m, 1H), 7.83 – 7.81 (m, 1H), 7.44 – 7.39 (m, 2H), 7.15 (s, 2H), 3.27 – 3.18 (m, 1H), 3.02 (s, 3H), 2.76 – 2.67 (m, 1H), 2.42 (s, 3H), 1.71 (s, 3H), 1.32 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.5, 150.0, 144.2, 141.1, 140.2, 139.7, 132.3, 130.6, 125.5, 125.4, 125.3, 122.7 (t, J = 240.6 Hz), 119.9, 118.4, 115.6, 48.5 (t, J = 24.1 Hz), 45.4, 31.7, 24.7 (t, J = 27.3 Hz), 24.6, 21.6. 19F NMR (471 MHz, CDCl3): δ -85.66 – -85.96 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C21H20F2N2O (M+H)+ 355.1616; Found 355.1619.
1,3-dichloro-5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3p). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 58.5 mg, 74% yield. 1H NMR (500 MHz, CDCl3): δ 8.38 – 8.36 (m, 1H), 7.92 – 7.91 (m, 1H), 7.58 (d, J = 2.0 Hz, 1H), 7.48 – 7.44 (m, 2H), 7.40 (d, J = 1.5 Hz, 1H), 3.29 – 3.20 (m, 1H), 2.72 – 2.63 (m, 1H), 1.73 (s, 3H), 1.45 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 170.7, 146.3, 144.1, 143.8, 136.2, 134.4, 131.3, 130.5, 126.5, 126.1, 122.4 (t, J = 240.8 Hz), 120.8, 119.2, 115.6, 48.4 (t, J = 23.4 Hz), 45.8 (d, J = 3.0 Hz), 31.3, 24.9 (t, J = 27.1 Hz). 19F NMR (471 MHz, CDCl3): δ -85.15 – -85.86 (m, 1F), -87.94 – -88.64 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C19H14Cl2F2N2O (M+H)+ 395.0524; Found 395.0527.
5-(2,2-difluoropropyl)-2,4,5-trimethylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3q). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 51.0 mg, 72% yield. 1H NMR (400 MHz, CDCl3): δ 8.35 – 8.33 (m, 2H), 7.81 (dd, J = 7.0, 1.6 Hz, 1H), 7.42 (pd, J = 7.2, 1.6 Hz, 2H), 7.18 (s, 1H), 3.34 – 3.11 (m, 2H), 2.62 (s, 3H), 2.41 (s, 3H), 1.80 (s, 3H), 1.38 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 173.5, 150.4, 144.1, 137.7, 137.5, 136.4, 134.6, 131.5, 125.9, 125.2, 122.9 (t, J = 240.3 Hz), 122.9, 119.6, 115.7, 46.6, 44.9 (t, J = 23.5 Hz), 27.2, 24.5 (t, J = 27.5 Hz), 22.8, 20.6. 19F NMR (471 MHz, CDCl3): δ -88.43 – -88.61 (m, 1F), -88.66 – -88.85 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H20F2N2O (M+H)+ 355.1616; Found 355.1619.
3-bromo-5-(2,2-difluoropropyl)-5,9,10-trimethylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3r). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 72.7 mg, 84% yield. 1H NMR (500 MHz, CDCl3): δ 8.31 (d, J = 9.0 Hz, 1H), 8.12 (s, 1H), 7.60 – 7.58 (m, 2H), 7.56 (s, 1H), 3.26 – 3.16 (m, 1H), 2.70 – 2.61 (m, 1H), 2.41 (s, 3H), 2.39 (s, 3H), 1.70 (s, 3H), 1.39 (t, J = 18.5 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.3, 148.0, 142.4, 141.7, 135.2, 135.0, 131.2, 129.9, 129.7, 127.2, 125.3, 122.4 (t, J = 240.7 Hz), 121.7, 120.0, 115.9, 48.2 (t, J = 23.8 Hz), 45.4 (d, J = 3.3 Hz), 30.8, 24.7 (t, J = 27.2 Hz), 20.5, 20.4. 19F NMR (471 MHz, CDCl3): δ -85.57 – -86.28 (m, 1F), -87.17 – -87.87 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H19BrF2N2O (M+H)+ 433.0721; Found 433.0723.
5-(2,2-difluoropropyl)-5-phenylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3s). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 44.2 mg, 57% yield. 1H NMR (400 MHz, CDCl3): δ 8.58 (dd, J = 8.0, 1.2 Hz, 1H), 8.26 (d, J = 7.6 Hz, 1H), 7.84 (d, J = 7.6 Hz, 1H), 7.56 – 7.37 (m, 4H), 7.32 – 7.24 (m, 4H), 7.22 – 7.17 (m, 3H), 3.98 – 3.07 (m, 1H), 3.18 – 3.07 (m, 1H), 1.47 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 170.3, 149.7, 144.1, 142.3, 139.8, 131.6, 131.2, 129.3, 129.1, 128.2, 128.1, 126.8, 125.93, 125.87, 125.7, 123.6, 122.8 (t, J = 239.8 Hz), 119.9, 115.7, 53.4, 46.0 (t, J = 23.5 Hz), 25.3 (t, J = 27.5 Hz). 19F NMR (471 MHz, CDCl3): δ -84.84 – -85.02 (m, 1F), -85.04 – -85.22 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C24H18F2N2O (M+H)+ 389.1460; Found 389.1463.
5-benzyl-5-(2,2-difluoropropyl)benzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (3t). A white solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 52.3 mg, 65% yield. 1H NMR (500 MHz, CDCl3): δ 8.36 – 8.34 (m, 1H), 8.29 (d, J = 8.0 Hz, 1H), 7.68 – 7.66 (m, 1H), 7.63 – 7.62 (m, 2H), 7.49 – 7.46 (m, 1H), 7.41 – 7.36 (m, 2H), 6.87 (t, J = 7.5 Hz, 1H), 6.77 (t, J = 7.5 Hz, 2H), 6.49 (d, J = 7.5 Hz, 2H), 3.53 (d, J = 12.5 Hz, 1H), 3.49 – 3.41 (m, 1H), 3.17 (d, J = 12.5 Hz, 1H), 2.99 – 2.90 (m, 1H), 1.43 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.2, 149.2, 143.6, 137.4, 133.2, 130.9, 130.8, 129.1, 128.0, 127.8, 127.3, 125.72, 125.69, 125.4, 124.1, 122.6 (t, J = 240.8 Hz), 119.6, 115.4, 51.9, 50.7, 46.5 (t, J = 23.8 Hz), 25.1 (t, J = 27.3 Hz). 19F NMR (471 MHz, CDCl3): δ -83.32 – -84.00 (m, 1F), -84.83 – -85.51 (m, 1F). HRMS (ESI-TOF) m/z: C25H20F2N2O (M+H)+ 403.1616; Found 403.1617.
7-(2,2-difluoropropyl)-7-methylbenzo[h]benzo[4,5]imidazo[2,1-a]isoquinolin-8(7H)-one (3u). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 55.6 mg, 74% yield. 1H NMR (500 MHz, CDCl3): δ 10.56 (d, J = 8.5 Hz, 1H), 8.47 – 8.45 (m, 1H), 8.01 (d, J = 8.5 Hz, 1H), 7.96 – 7.94 (m, 1H), 7.91 (d, J = 8.0 Hz, 1H), 7.84– 7.81 (m, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.56 (d, J = 8.5 Hz, 1H), 7.50 – 7.46 (m, 2H), 3.37 – 3.27 (m, 1H), 2.90 – 2.81 (m, 1H), 1.78 (s, 3H), 1.33 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.3, 149.7, 144.0, 140.6, 132.7, 132.0, 130.4, 130.3, 128.7, 128.4, 128.2, 126.9, 125.9, 125.8, 122.5 (d, J = 240.5 Hz), 123.7, 120.1, 117.6, 115.7, 47.9 (t, J = 24.1 Hz), 45.9, 31.0, 24.6 (t, J = 27.3 Hz). 19F NMR (471 MHz, CDCl3): δ -86.07 – -86.30 (m, 1F), -86.32 – -86.46 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C23H18F2N2O (M+H)+ 377.1460; Found 377.1462.
4-(2,2-difluoropropyl)-4-methylbenzo[4,5]imidazo[1,2-a]thieno[2,3-c]pyridin-5(4H)-one (3v). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 44.5 mg, 67% yield. 1H NMR (400 MHz, CDCl3): δ 8.33 – 8.30 (m, 1H), 7.78 – 7.75 (m, 1H), 7.59 (d, J = 4.8 Hz, 1H), 7.44 – 7.38 (m, 2H), 7.11 (d, J = 5.2 Hz, 1H), 3.24 – 3.12 (m, 1H), 2.66 – 2.54 (m, 1H), 1.67 (s, 3H), 1.38 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 172.6, 146.4, 145.9, 143.9, 130.9, 130.4, 125.9, 125.8, 125.5, 123.6, 122.4 (t, J = 240.5 Hz), 119.7, 115.2, 48.2 (t, J = 24.3 Hz), 45.6 – 45.5 (m), 29.9, 24.5 (t, J = 27.3 Hz). 19F NMR (471 MHz, CDCl3): δ -86.86 – -87.18 (m, 2F). HRMS (ESI-TOF) m/z: Calcd for C17H14F2N2OS (M+H)+333.0868; Found 333.0870.
5-(2,2-difluoropropyl)-3,5,12-trimethylindolo[2,1-a]isoquinolin-6(5H)-one (5a). A white gummy after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 46.6 mg, 66% yield. 1H NMR (400 MHz, CDCl3): δ 8.62 (d, J = 7.2 Hz, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.60 – 7.58 (m, 1H), 7.41 – 7.34 (m, 3H), 7.23 (d, J = 8.4 Hz, 1H), 3.28 – 3.16 (m, 1H), 2.71 – 2.60 (m, 1H), 2.65 (s, 3H), 2.44 (s, 3H), 1.69 (s, 3H), 1.31 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 172.0, 137.3, 137.0, 134.0 (d, J = 30.9 Hz), 132.6, 129.7, 129.4, 128.3, 127.4, 125.2 (d, J = 48.0 Hz), 124.2, 123.2, 122.9 (t, J = 240.2 Hz), 118.2, 116.7, 113.6, 48.1 (t, J = 24.2 Hz), 45.0 – 44.8 (m), 31.4, 24.5 (t, J = 27.4 Hz), 21.5, 11.5. 19F NMR (471 MHz, CDCl3): δ -84.11 – -84.82 (m, 1F), -85.19 – -85.89(m, 1F). HRMS (ESI-TOF) m/z: Calcd for C22H21F2NO (M+H)+ 354.1664; Found 354.1666.
5-(2,2-difluoropropyl)-3-fluoro-5,12-dimethylindolo[2,1-a]isoquinolin-6(5H)-one (5b). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 51.4 mg, 72 % yield. 1H NMR (500 MHz, CDCl3): δ 8.61 (d, J = 8.0 Hz, 1H), 8.03 (dd, J = 8.8, 6.0 Hz, 1H), 7.59 (d, J = 7.0 Hz, 1H), 7.42 – 7.35 (m, 2H), 7.17 – 7.11 (m, 2H), 3.28 – 3.18 (m, 1H), 2.63 (s, 3H), 2.62 – 2.55 (m, 1H), 1.69 (s, 3H), 1.38 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.2, 161.8 (d, J = 248.4 Hz), 139.8 (d, J = 6.9 Hz), 134.2, 132.4, 128.9, 126.9 (d, J = 8.3 Hz), 125.7, 124.3, 122.7 (t, J = 240.6 Hz), 122.4 (d, J = 2.5 Hz), 118.3, 116.7, 114.9 (d, J = 21.8 Hz), 114.1, 113.9, 48.1 (t, J = 24.1 Hz), 45.1, 31.2, 24.6 (t, J = 27.4 Hz), 11.4. 19F NMR (471 MHz, CDCl3): δ -84.17 – -84.88 (m, 1F), -86.70 – -87.41 (m, 1F), -112.80 – -112.85 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H18F3NO (M+H)+ 358.1413; Found 358.1415.
3-chloro-5-(2,2-difluoropropyl)-5,10,12-trimethylindolo[2,1-a]isoquinolin-6(5H)-one (5c). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 56.8 mg, 76 % yield. 1H NMR (400 MHz, CDCl3): δ 8.62– 8.59 (m, 1H), 7.97 (d, J = 8.4 Hz, 1H), 7.61 – 7.58 (m, 1H), 7.43 (d, J = 1.6 Hz, 1H), 7.41 – 7.35 (m, 3H), 3.28 – 3.16 (m, 1H), 2.63 (s, 3H), 2.67 – 2.55 (m, 1H), 1.69 (s, 3H), 1.38 (t, J = 18.8 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 171.2, 138.9, 134.2, 133.1, 132.3, 128.6, 127.6, 127.4, 126.2, 125.9, 124.5, 124.4, 122.8 (t, J = 240.4 Hz), 118.4, 116.7, 114.9, 48.0 (t, J = 23.9 Hz), 44.9, 31.2, 24.7 (t, J = 27.3 Hz), 11.5. 19F NMR (471 MHz, CDCl3): δ -84.35 – -85.06 (m, 1F), -86.79 – -87.49 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H18ClF2NO (M+H)+ 374.1118; Found 374.1120.
3-bromo-5-(2,2-difluoropropyl)-5,12-dimethylindolo[2,1-a]isoquinolin-6(5H)-one (5d). A yellowish liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 57.7 mg, 69% yield. 1H NMR (500 MHz, CDCl3): δ 8.60 (d, J = 7.5 Hz, 1H), 7.90 (d, J = 8.5 Hz, 1H), 7.60 – 7.58 (m, 2H), 7.52 (dd, J = 8.5, 2.0 Hz, 1H), 7.43 – 7.35 (m, 2H), 3.27 – 3.17 (m, 1H), 2.63 (s, 3H), 2.65 – 2.56 (m, 1H), 1.68 (s, 3H), 1.38 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.1, 139.2, 134.3, 132.3, 130.5, 130.3, 128.7, 126.4, 126.0, 124.9, 124.4, 122.7 (t, J = 240.5 Hz), 121.2, 118.5, 116.8, 115.1, 48.1 (t, J = 23.9 Hz), 44.9, 31.2, 24.7 (t, J = 27.3 Hz), 11.5. 19F NMR (471 MHz, CDCl3): δ -84.40 – -85.07 (m, 1F), -86.79 – -87.47 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C21H18BrF2NO (M+H)+ 420.0594; Found 420.0598.
3-chloro-5-(2,2-difluoropropyl)-5,10,12-trimethylindolo[2,1-a]isoquinolin-6(5H)-one (5e). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 56.6 mg, 73% yield. 1H NMR (500 MHz, CDCl3): δ 8.34 (d, J = 8.5 Hz, 1H), 7.84 (d, J = 8.5 Hz, 1H), 7.30 (s, 1H), 7.25 (d, J = 9.0 Hz, 1H), 7.15 (s, 1H), 7.11 (d, J = 8.0 Hz, 1H), 3.14 – 3.04 (m, 1H), 2.50 (s, 3H), 2.52 – 2.43 (m, 1H), 2.38 (s, 3H), 1.56 (s, 3H), 1.25 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 170.9, 139.0, 134.1, 133.0, 132.5, 132.5, 128.8, 127.6, 127.4, 127.2, 126.1, 124.6, 122.7 (t, J = 240.4 Hz), 118.5, 116.4, 114.7, 48.1 (t, J = 24.0 Hz), 44.9, 31.2, 24.6 (t, J = 27.4 Hz), 21.6, 11.5. 19F NMR (471 MHz, CDCl3): δ -84.19 – -84.90 (m, 1F), -86.72 – -87.42 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C22H20ClF2NO (M+H)+ 388.1274; Found 388.1275.
5-(2,2-difluoropropyl)-12-ethyl-5-methylindolo[2,1-a]isoquinolin-6(5H)-one (5f). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 52.9 mg, 75% yield. 1H NMR (500 MHz, CDCl3): δ 8.64 (d, J = 7.5 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.61 (d, J = 7.0 Hz, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.44 – 7.35 (m, 4H), 3.26 – 3.13 (m, 3H), 2.70 – 2.61 (m, 1H), 1.70 (s, 3H), 1.42 (t, J = 7.5 Hz, 3H), 1.31 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 172.0, 137.1, 134.5, 131.8, 128.8, 127.5, 127.4, 127.3, 125.7, 124.7, 124.3, 122.9 (t, J = 240.2 Hz), 121.1, 118.2, 116.9, 48.1 (t, J = 24.3 Hz), 44.9, 31.3, 24.5 (t, J = 27.4 Hz), 18.6, 13.3. 19F NMR (471 MHz, CDCl3): δ -84.21 – -84.92 (m, 1F), -85.41 – -86.12 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C22H21F2NO (M+H)+ 354.1664; Found 354.1667.
5-(2,2-difluoropropyl)-12-ethyl-5,10-dimethylindolo[2,1-a]isoquinolin-6(5H)-one (5g). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 47.7 mg, 65% yield. 1H NMR (500 MHz, CDCl3): δ 8.50 (d, J = 8.5 Hz, 1H), 7.98 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 7.5 Hz, 1H), 7.45 – 7.35 (m, 3H), 7.22 (d, J = 8.0 Hz, 1H), 3.25 – 3.16 (m, 1H), 3.13 (q, J =7.5 Hz, 2H), 2.69 – 2.60 (m, 1H), 2.51 (s, 3H), 1.69 (s, 3H), 1.41 (t, J = 7.5 Hz, 3H), 1.30 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.7, 137.0, 133.9, 132.6, 131.9, 128.9, 127.4, 127.3, 127.0, 125.8, 124.6, 122.9 (t, J = 240.2 Hz), 121.0, 118.2, 116.6, 48.1 (t, J = 24.4 Hz), 44.8, 31.2, 24.4 (t, J = 27.4 Hz), 21.6, 18.6, 13.3. 19F NMR (471 MHz, CDCl3): δ -84.10 – -84.77 (m, 1F), -85.39 – -86.06 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C23H23F2NO (M+H)+ 368.1820; Found 368.1824.
5-(2,2-difluoropropyl)-12-ethyl-10-fluoro-5-methylindolo[2,1-a]isoquinolin-6(5H)-one (5h). A yellow liquid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 51.9 mg, 70% yield. 1H NMR (500 MHz, CDCl3): δ 8.57 (dd, J = 9.0, 5.0 Hz, 1H), 7.98 (d, J = 7.5 Hz, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.44 – 7.37 (m, 2H), 7.23 (dd, J = 9.0, 2.5 Hz, 1H), 7.09 (td, J = 9.0, 2.5 Hz, 1H), 3.24 – 3.15 (m, 1H), 3.12 – 3.07 (m, 2H), 2.69 – 2.60 (m, 1H), 1.69 (s, 3H), 1.40 (t, J = 7.5 Hz, 3H), 1.31 (t, J = 19.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.8, 160.4 (d, J = 241.1 Hz), 137.3, 133.2 (d, J = 9.4 Hz), 130.7, 130.4, 127.65 (d, J = 41.6 Hz), 127.3, 125.4, 124.8, 122.8 (d, J = 240.4 Hz), 120.6 (d, J = 4.1 Hz), 118.0 (d, J = 9.0 Hz), 113.1 (d, J = 24.6 Hz), 104.0 (d, J = 24.0 Hz), 48.3 (t, J = 24.2 Hz), 44.8, 31.2, 24.6 (t, J = 27.4 Hz), 18.7, 13.2. 19F NMR (471 MHz, CDCl3): δ -84.78 – -85.42 (m, 1F), -85.86 – -86.52 (m, 1F), -118.11 – -118.16 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C22H20F3NO (M+H)+ 372.1570; Found 372.1575.
3-chloro-5-(2-cyclopropyl-2,2-difluoroethyl)-5,12-dimethylindolo[2,1-a]isoquinolin-6(5H)-one (5i). A yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 20/1), 50.4 mg, 63% yield. 1H NMR (500 MHz, CDCl3): δ 8.61 (d, J = 7.5 Hz, 1H), 7.97 (d, J = 8.5 Hz, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.44 (s, 1H), 7.42 – 7.36 (m, 3H), 3.40 – 3.30 (m, 1H), 2.76 – 2.67 (m, 1H), 2.63 (s, 3H), 1.69 (s, 3H), 1.02 – 0.95 (m, 1H), 0.44 – 0.23 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.2, 139.2, 134.3, 133.1, 132.3, 128.8, 127.5, 126.1, 125.9, 124.4, 124.3, 122.4 (t, J = 242.3 Hz), 118.4, 116.8, 114.7, 47.7 (t, J = 25.9 Hz), 45.0, 31.7, 16.6 (t, J = 28.9 Hz), 11.5, 1.25 (dt, J = 6.3, 3.1 Hz). 19F NMR (471 MHz, CDCl3): δ -94.49 – -95.10 (m, 1F), -99.95 – -100.56 (m, 1F). HRMS (ESI-TOF) m/z: Calcd for C23H20ClF2NO (M+H)+ 400.1274; Found 400.1279.