1. Introduction
The multicomponent alloys contain several basic chemical elements. Each element plays an important role in achieving the declared properties of the material. In the classical soft magnetic nanocrystalline Fe
73.5Cu
1Nb
3Si
13.5B
9 alloy, boron and silicon contribute to the formation of an amorphous structure in a thin ribbon after rapidly quenching of the melt [
1]. At the initial stage of heat treatment, clustering of cooper atoms occurs. Copper clusters create concentration heterogeneity and contribute to the uniform distribution of nuclei of the α-Fe–Si crystalline phase. Niobium, which is insoluble in iron, inhibits crystal growth and promotes the formation of nanocrystalline grains. Some other elements can also be inhibitors, for example, Mo, W [
2]. After crystallization, a material is obtained with a crystal grain size of about 10 nm, a coercive force of less than 1 A/m and an initial permeability of about 100,000. Iron-soluble Ni and Co are introduced to increase the efficiency of heat treatment in a magnetic field, which ensures high linearity or rectangularity of the magnetic hysteresis loop [
3,
4].
Aluminum also has high solubility in iron, and the addition of aluminum leads to an increase in the initial permeability of the soft magnetic nanocrystalline material [
5]. On the other hand, aluminum has high chemical activity and, interacting with oxygen, forms aluminum oxide Al
2O
3. Aluminum also reacts with moisture to form aluminum oxide and hydrogen. A thin film of aluminum oxide protects against oxygen penetration deep into the metal. Without a protective film, aluminum turns into loose aluminum oxide powder. Aluminum may be present in the alloy as an undesirable impurity. The high affinity of aluminum for oxygen places high demands on the oxygen and moisture content at different stages of alloy production. Aluminum reacts with nitrogen at temperatures above 1100 K to form nitride AlN.
The production of a soft magnetic nanocrystalline alloy begins with smelting. At high temperatures in the liquid state, different atoms interact with each other, forming short-range order structures–clusters [
6]. The composition, structure and size of clusters, as well as their stability, depend on temperature, and their change is associated with the liquid–liquid structure transition (LLST) [
7,
8,
9,
10]. As a result of the LLST, the density, viscosity, electrical resistance and other macroscopic characteristics of the melt change [
9,
10,
11,
12]. After melting the alloy, rapidly quenching of the melt follows with the formation of a thin amorphous ribbon, which is a precursor for further heat treatment. The amorphous precursor inherits the short-range order structure of the metallic liquid. It is shown in [
13] that the structural heredity recorded in an amorphous ribbon Fe
72.5Cu
1Nb
2Mo
1.5Si
14B
9 after rapid quenching of the melt remains after several heating–cooling cycles of the melt.
Among all the macroscopic properties of liquid metals, viscosity is the most sensitive to structural transformations. In a liquid nanocrystalline iron-based alloy, the Arrhenius flow predominates. In this case, the Arrhenius plot, i.e., the dependence of the natural logarithm of the kinematic viscosity lnν on the reciprocal absolute temperature
T−1, is a linear function in a wide temperature range [
14]. Anomalies in fluid flow manifest themselves in changes in the activation energy of viscous flow during the transition from a low-temperature region to a high temperature and vice versa. Moreover, the activation energy increases with increasing size of clusters participating in the viscous flow of the melt [
15].
Arrhenius plots during heating and cooling can form a hysteresis loop [
10]. The nature of hysteresis is associated with a large number of metastable states. So the trajectory along which the melt structure will change can be very different. Also the activation energy of the viscous flow at the cooling stage and consequently the melt structure, depends on the overheating temperature of the melt [
16]. It follows from this that during solidification we can obtain a material with a different structure depending on the overheating temperature of the melt. Therefore, the main task of preparing the melt is to select the optimal temperature for overheating the melt before quenching.
To determine the viscosity of metallic liquids with a high melting point, an oscillating cup viscometer is most often used [
17,
18,
19]. In this viscometer, the sample is placed in a crucible suspended from a torsion wire that forms a pendulum. The pendulum is driven into torsional vibrations around a vertical axis. The movement of the pendulum gradually fades due to friction inside the viscous liquid. The viscosity of the liquid is calculated from the results of measuring the decrement and oscillation period. All studies using an oscillating cup viscometer refer to the temperature range in which the sample is in a liquid state. However, in multicomponent alloys there is a significant region of coexistence of liquid and solid phases. Therefore, the purpose of this article, along with studying the effect of small aluminum additions on the kinematic viscosity of multicomponent Fe-Cu-Nb-Mo-Si-B melts, is also to evaluate the applicability of the oscillating cup viscometer for studying the region of coexistence of liquid and solid phases during the melting and solidification.
3. Results and Discussion
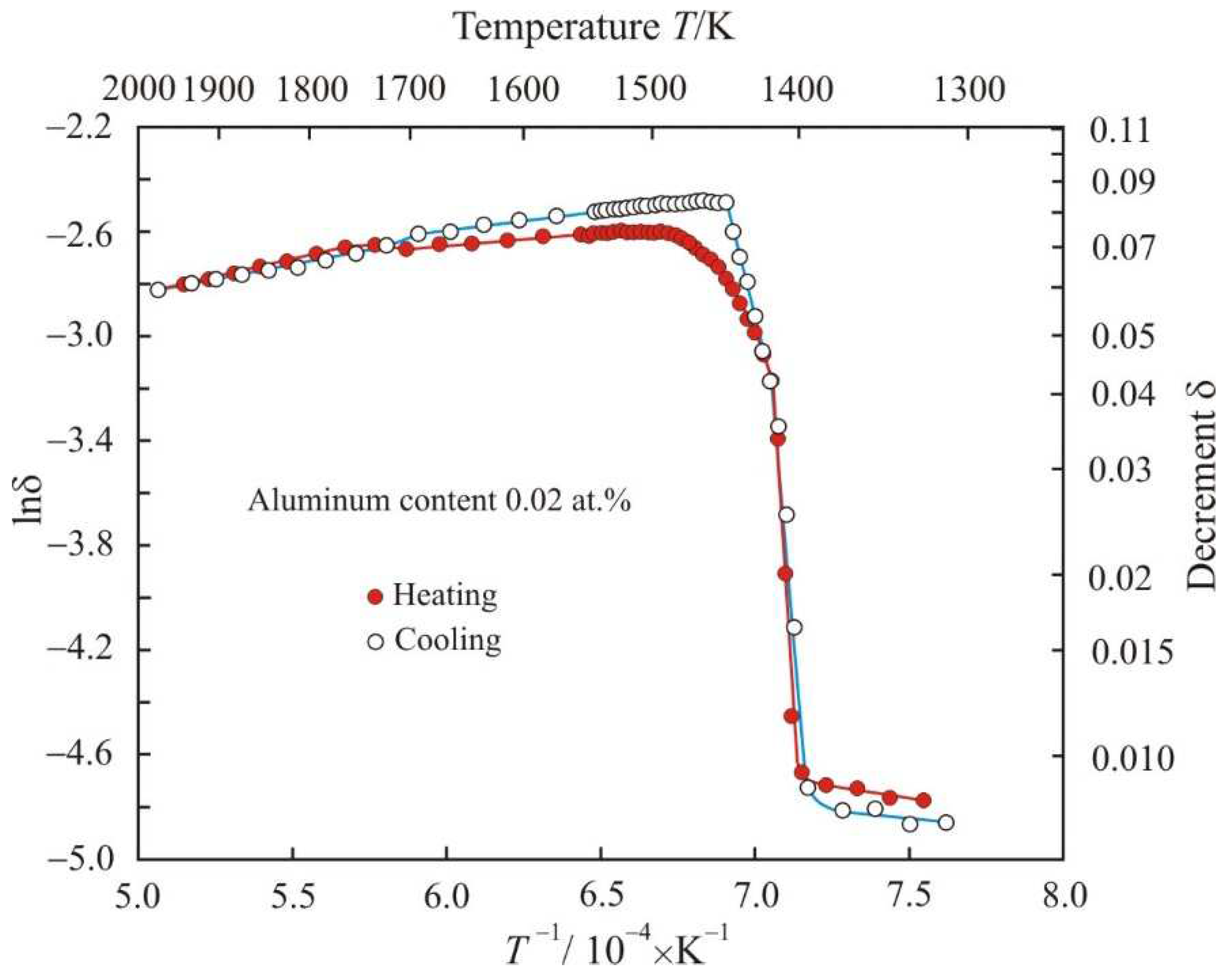
Figure 1 shows dependences of natural logarithm of decrement lnδ on the reciprocal temperature
T−1 when Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 alloy with aluminum content 0.02 at.% is heated to a temperature of 1975 K and cooled. In the low-temperature region, the decrement remains almost unchanged at the level of 0.008. When heated above 1400 K, the decrement sharply increases to δ = 0.04, and in this temperature range the relationship between lnδ and
T−1 is linear. Further, the decrement increases nonlinearly, reaching a maximum value at a temperature of about 1500 K. Starting from this temperature, the decrement, as well as the kinematic viscosity of the melt, decreases. A decrease in viscosity with temperature is a typical manifestation of the liquid state of a substance. Therefore, the temperature at which the decrement reaches its maximum value will be taken as the liquidus temperature of the multicomponent alloy
Tliq = 1523 K. This value is close to the liquidus temperature of 1520 K of the ternary Fe
80Si
10B
10 alloy [
22], in which the content of basic elements is comparable to our Fe
72.5Cu
1Nb
2Mo
1.5Si
14B
9 alloy. On the other hand, in the low-temperature region, the temperature at which the decrement almost does not change will be taken as the solidus temperature
Tsol = 1393 K. Then a sharp change in the decrement between these temperatures will correspond to the region of coexistence of the liquid and solid phases.
From
Figure 1 it follows that at the cooling stage, the liquidus temperature of the melt with aluminum content 0.02 at.% decreased to 1448 K, but the solidus temperature did not change. Noteworthy is the linear dependence of the logarithm of decrement lnδ on the reciprocal temperature
T−1 at the initial stage of solidification, in contrast to the exponential dependence at the final stage of melting. Both dependences during heating and cooling intersect at the point with coordinates lnδ = −3.2 (δ = 0.04) and
T = 1416 K. With a further decrease in temperature, the experimental points at the stages of heating and cooling lie on the same straight line:
with an adjusted coefficient of determination
R2adj = 0.991, and the decrement is related to temperature by an exponential function:
Figure 1.
Dependences of natural logarithm of decrement lnδ on the reciprocal temperature T−1 when Fe72.5−xAlxCu1Nb2Mo1.5Si14B9 alloy with aluminum content 0.02 at.% is heated to a temperature of 1975 K and cooled.
Figure 1.
Dependences of natural logarithm of decrement lnδ on the reciprocal temperature T−1 when Fe72.5−xAlxCu1Nb2Mo1.5Si14B9 alloy with aluminum content 0.02 at.% is heated to a temperature of 1975 K and cooled.
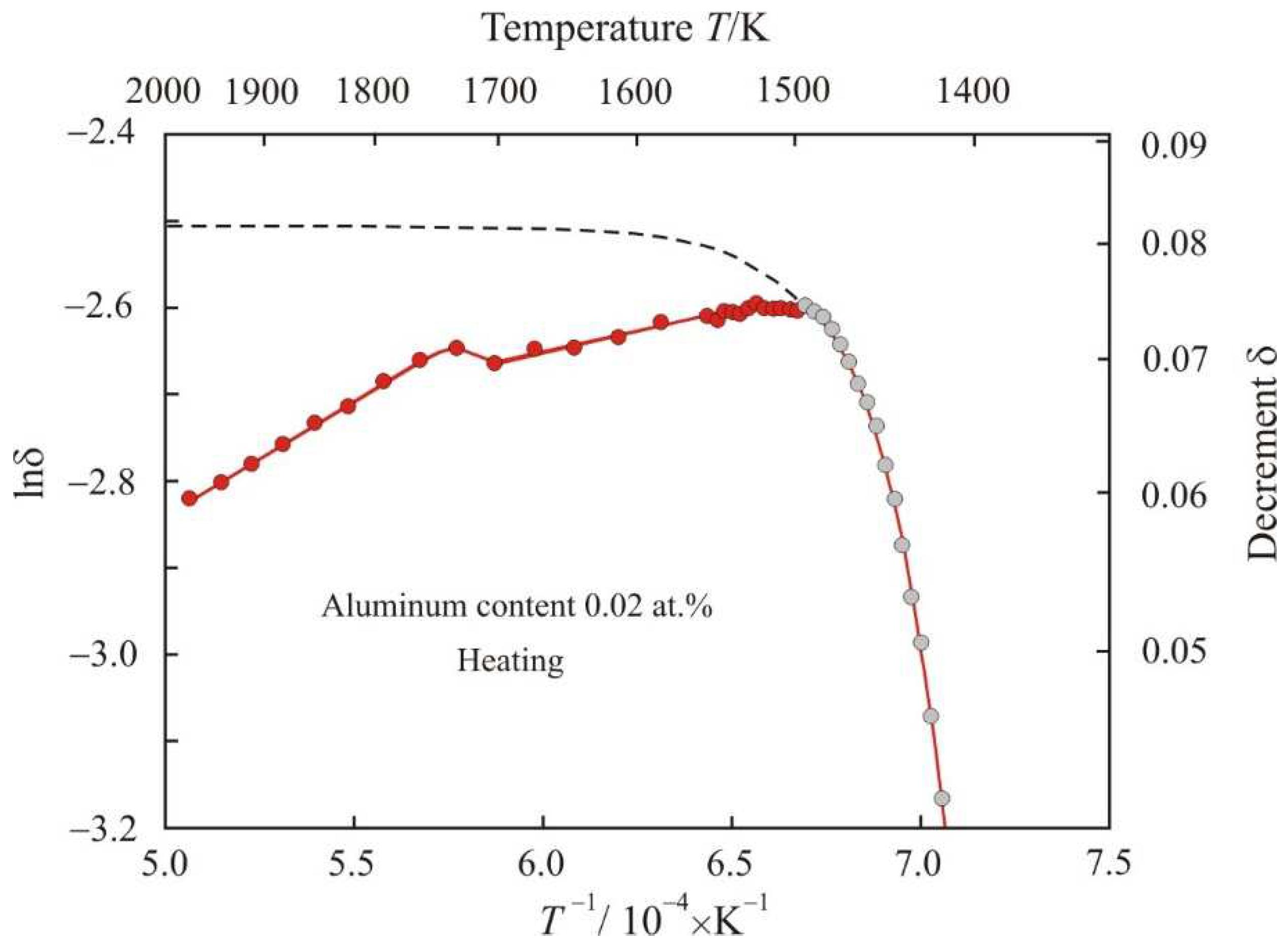
Figure 2 shows the dependence of natural logarithm of decrement lnδ on the reciprocal temperature
T−1 when Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 alloy with aluminum content 0.02 at.% is heated to a temperature of 1975 K.
Figure 2 is limited to the range of values lnδ = −3.2. Here at the initial stage of melting the fitting of the experimental gray points corresponds to the exponential function:
with an adjusted coefficient of determination
R2adj = 0.998. As temperature increases, the exponential Function (3) quickly approaches saturation lnδ = −2.51, this is shown by the dashed line in the
Figure 2.
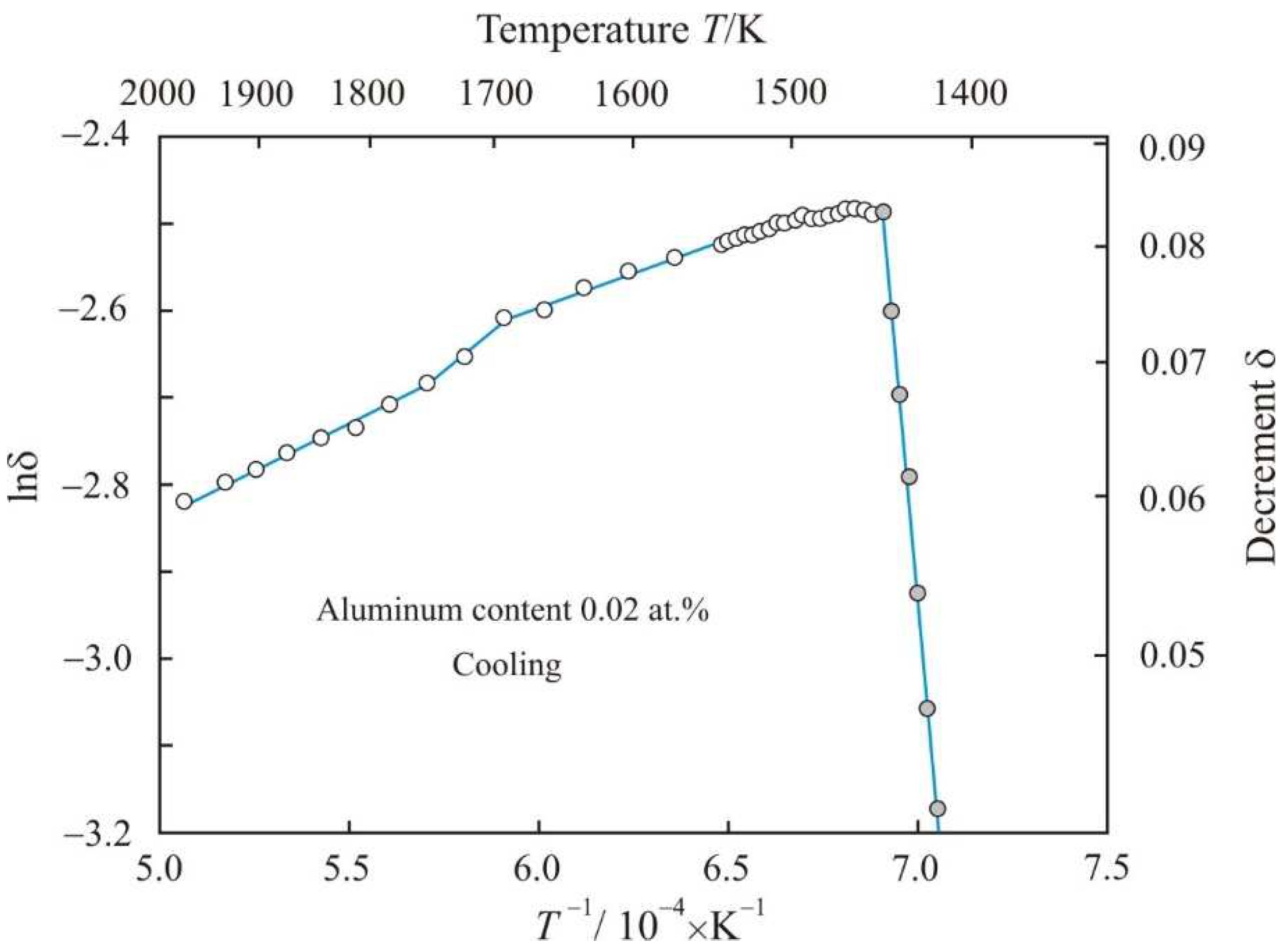
Figure 3 shows the dependence of natural logarithm of decrement lnδ on the reciprocal temperature
T−1 when Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 alloy with aluminum content 0.02 at.% is cooled from a temperature of 1975 K. During cooling, the liquidus temperature decreased by 55 K to 1448 K. At the initial stage of solidification, the fitting of the experimental gray points corresponds to the linear function:
with an adjusted coefficient of determination
R2adj = 0.997, so the decrement is related to the temperature exponential function:
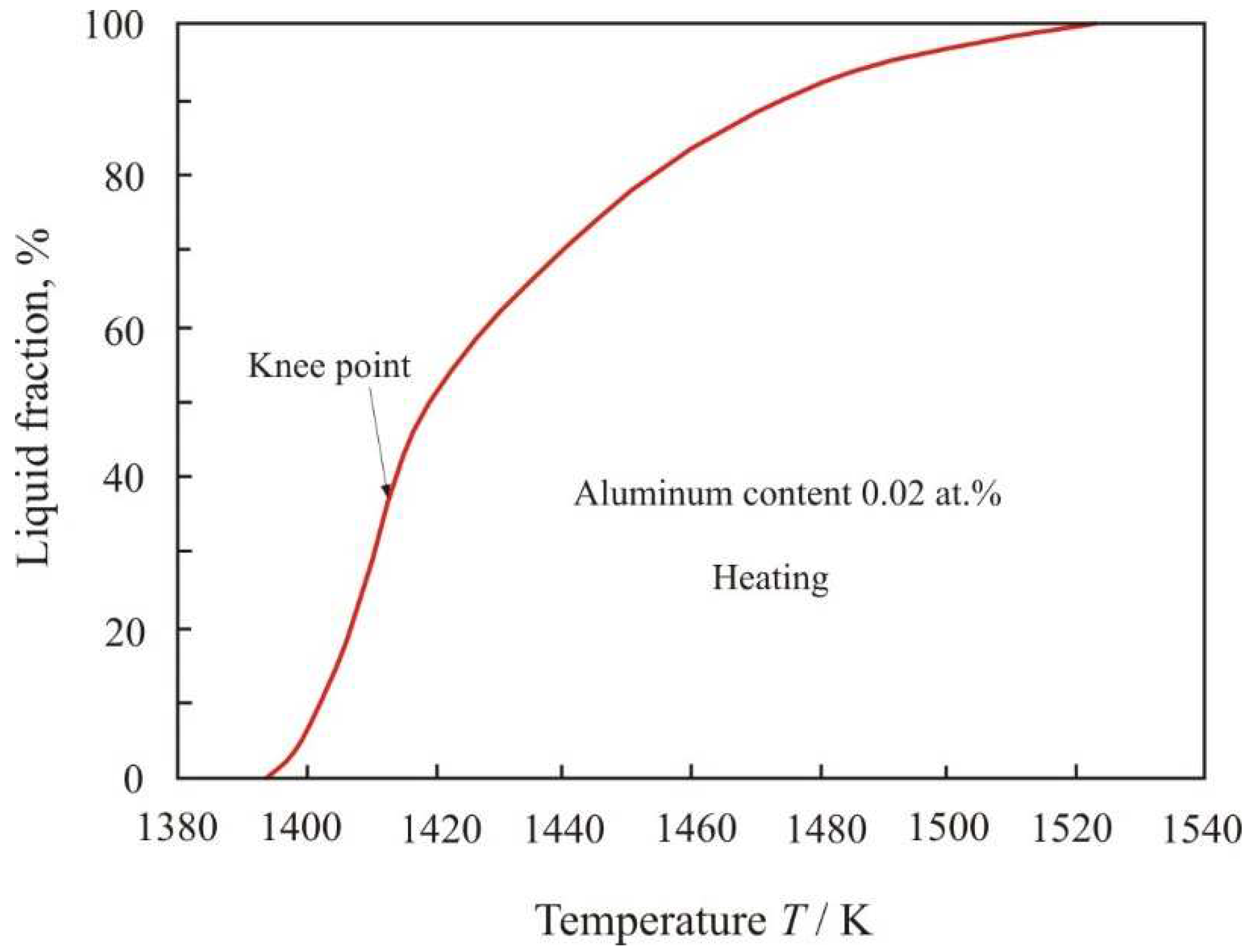
In a multicomponent alloy between the solidus and liquidus temperatures, there are solid and liquid phases. The decrement is proportional to the volume of the liquid phase, in which the movement of the pendulum is damped due to friction inside the viscous liquid. Using the temperature dependences of the decrement (1) and (3), it is possible to calculate the fraction of the liquid phase depending on the temperature.
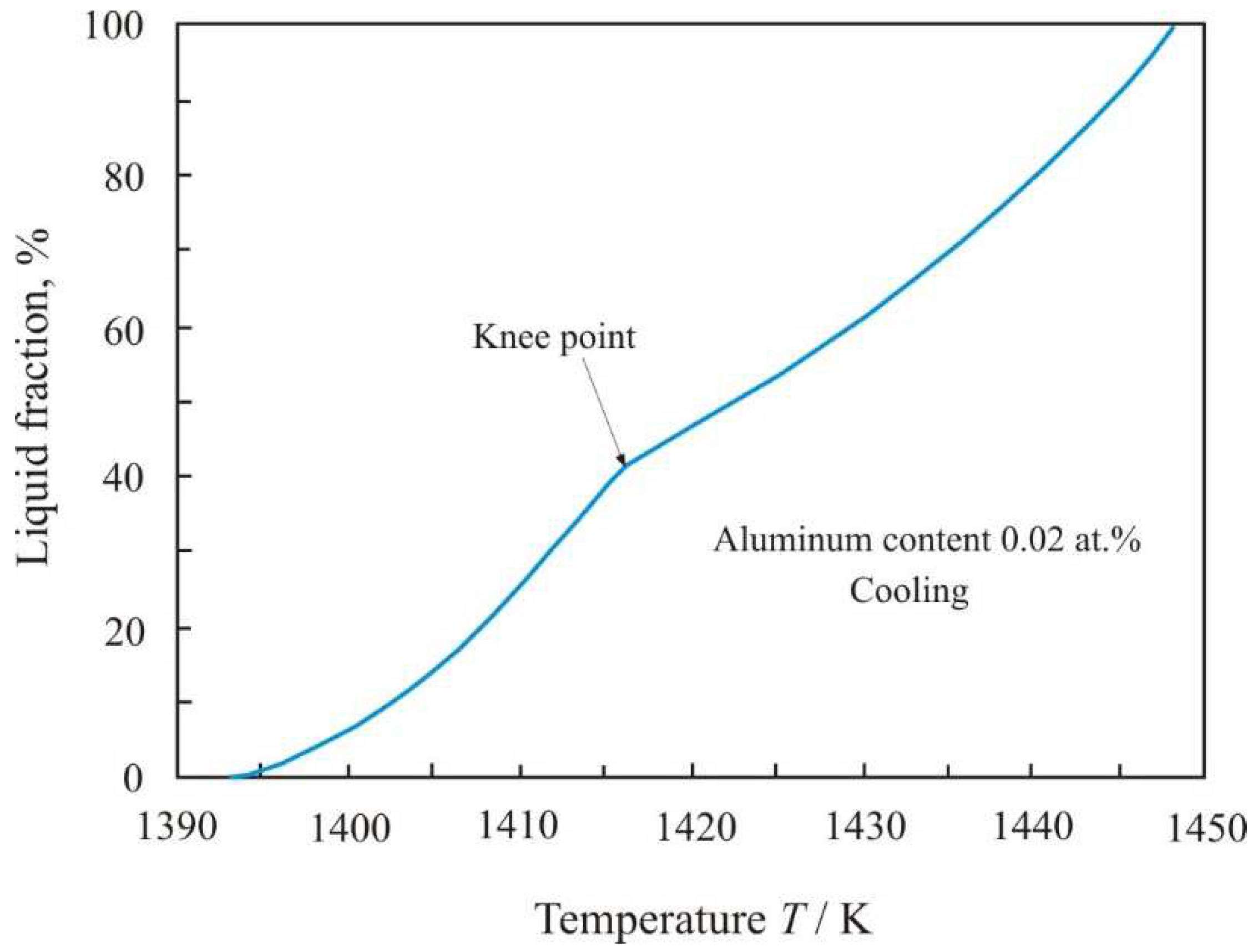
Figure 4 shows the dependence of the liquid fraction on temperature when Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 alloy with aluminum content 0.02 at.% heated within the temperature range from
Tsol = 1393 K to
Tliq = 1523 K. In knee point, which corresponds to a temperature of 1416 K, the functional dependence of the decrement changes during the heating process. Up to the knee point, the volume of the liquid phase grows rapidly, and then with increasing temperature the growth slows down.
The soft magnetic nanocrystalline Fe
73.5Cu
1Nb
3Si
13.5B
9 alloy has a chemical composition close to eutectic and consists of crystalline grains about 10 nm in size, which are separated by an amorphous matrix. The crystalline phase accounts for about 70 % of the volume [
1]. Nanocrystals have a composition close to Fe
80Si
20, and they contain elements soluble in iron. Insoluble elements such as boron and niobium remain at the periphery in the amorphous matrix. Melting begins along the grain boundaries [
23] with the formation of a liquid phase of eutectic composition. It can be assumed that above the knee point, stable phases melt, mainly Fe
2Si, Fe
2Si
0.4B
0.6 and Fe
2B [
22,
24].
Cooling of Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 melt with aluminum content 0.02 at.% begins at a high temperature of 1975 K. At this temperature, the atoms are weakly bonded to each other. The melt is characterized by a large number of metastable states. Therefore, melt solidification pathways with temperature can vary greatly. When cooling from a high temperature, the metastable phases Fe
3B and Fe
23B
6 [
25] have an advantage, and they effectively suppress the formation of stable phases and lead to undercooling of the melt.
Figure 5 shows the dependence of liquid fraction on the temperature
T when Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 alloy with aluminum content 0.02 at.% is cooled in the range from
Tliq = 1448 K to
Tsol = 1393 K. The form of the obtained dependences is typical for alloys with a wide region of coexistence of liquid and solid phases [
26], and knee points on these curves coincide with the crystallization of stable phases. From
Figure 5 it follows that upon cooling, the volume of the liquid phase decreases more significantly at the final stage of solidification.
Table 1 shows melting and solidification parameters in the range from liquidus temperature to knee point 1416 K for Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 alloys with aluminum content 0.02; 0.22 and 0.42 at.%. Coefficients
A0, and
B correspond to the approximation of experimental values in the form of an exponential function at the final stage of melting, starting from the knee point 1416 K:
and coefficient
b corresponds to the approximation of experimental values in the form of an linear function at the initial stage of solidification up to knee point 1416 K:
The
Table 1 shows that with increasing aluminum content, the liquidus and solidus temperatures, as well as the coefficients
B and
b, increase. An increase in the coefficients indicates that the temperature dependences are becoming steeper. Consequently, small additions of aluminum accelerate the melting and solidification processes.
The temperature dependence of kinematic viscosity can be represented as an Arrhenius-type equation:
where ν is the kinematic viscosity (m
2·s
−1), ν
0 is a pre-exponential factor with the dimension of the kinematic viscosity,
Ea is the activation energy of the viscous flow (J·mol
−1),
R is the gas constant (J·K
−1·mol
−1),
T is the absolute temperature (K). Taking the logarithm of Equation (1) we obtain Arrhenius plot:
where
Ea·(
RT)
−1 is the reduced activation energy, which compares the activation energy
Ea with thermal energy
RT. At constant of
Ea and ν
0, the logarithm of the kinematic viscosity is a linear function of the inverse absolute temperature.
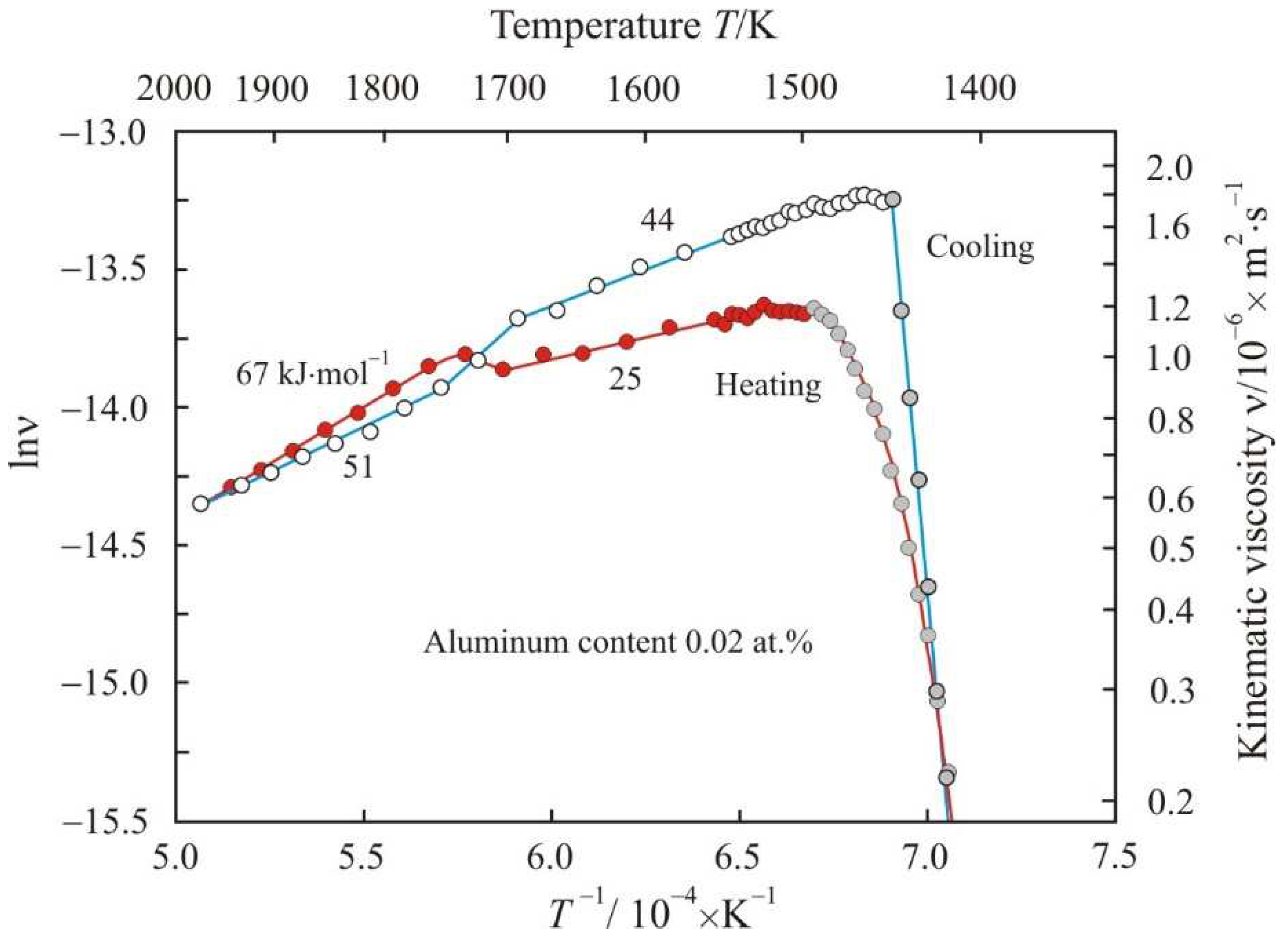
Figure 6 shows Arrhenius plots as the dependences of the natural logarithm of the kinematic viscosity lnν on the reciprocal temperature
T−1 when Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 alloy with aluminum content 0.02 at.% is heated to a temperature of 1975 K and cooled. The numbers next to the curves show the activation energy of the viscous flow
Ea in the corresponding linear sections of the Arrhenius plots. It follows from the
Figure 6 that when heated in a low-temperature region, the activation energy has a low value of 25 kJ·mol
−1. At a temperature of about 1700 K, the Arrhenius plot switches to a new trajectory with a high activation energy of 67 kJ·mol
−1. The cooling trajectory of the melt from a temperature of 1975 K does not coincide with the heating trajectory. During cooling, the transition to a new trajectory also occurs at a temperature of about 1700 K, and the activation energy in the low-temperature region is equal 44 kJ·mol
−1. Thus, in the low-temperature region, the activation energy at the cooling stage is higher than during heating.
Previously [
10,
16] it was shown that at a constant temperature the activation energy is higher when larger clusters participate in the viscous flow of a liquid. An increase in activation energy with increasing cluster size is typical not only for viscous fluid flow, but for other transport phenomena [
28,
29,
30]. Thus, when heated in a low-temperature region with the lowest activation energy, small clusters contribute to the viscous flow of the melt. For example, in a Co-B melt, an activation energy of 25 kJ·mol
−1 corresponds to clusters with a size of about 0.15 nm, i.e., on the order of atomic size [
10].
Figure 6 shows that immediately after melting at a temperature of about 1500 K, the slope of the Arrhenius plot corresponds to an activation energy of even less than 25 kJ·mol
−1, and it is comparable to the thermal energy
RT = 12.5 kJ·mol
−1 at this temperature. High thermal energy increases the mobility of atoms, which freely overcome the potential barrier
Ea. As temperature increases, the number of free atoms increases. Free atoms can move over significant distances and, interacting with each other, form new clusters.
Electronegativity on the Luo–Benson scale for Fe and B are 1.77 and 3.66, respectively [
31]. Boron atoms are most strongly associated with iron atoms since this pair of elements has the distinct electronegativity.
Table 2 shows design parameters of the compounds Fe-B. Stable Fe
2B boride with high formation energy predominates in the structure of the Fe
72.5Cu
1Nb
2Mo
1.5Si
14B
9 alloy at the initial stage of melting. Therefore, when heated in a low-temperature region, the melt contains Fe
2B-based clusters [
22,
24] along with free atoms of other elements. In the Fe
72.5Cu
1Nb
2Mo
1.5Si
14B
9 alloy, the ratio of iron atoms to boron atoms is 89:11. This ratio is much greater than that for the Fe
2B compound in which iron and boron atoms are bonded. As temperature increases, the mobility of atoms increases and the bonds between atoms weaken. But at the same time, the probability of free iron atoms approaching boron increases. In a cluster based on Fe
3B, the ratio of bound iron atoms to boron atoms is 3:1. In a cluster based on Fe
23B
6, the ratio is 79:21, and it’s much closer to Fe
72.5Cu
1Nb
2Mo
1.5Si
14B
9. Thus, in the cluster structure based on Fe
23B
6, boron atoms bind almost all iron atoms in Fe
72.5Cu
1Nb
2Mo
1.5Si
14B
9 melt, thereby ensuring a more uniform distribution of atoms in the melt at high temperature.
The direction of clustering from clusters based on Fe
2B to large clusters based on Fe
23B
6 is a consequence of the tendency of a multicomponent melt to a homogeneous structure at high temperature. This LLST occurs at a temperature of about 1700 K, and is manifested in
Figure 6 as an increase in the activation energy of viscous flow. Note that in [
35], using differential scanning calorimetry, an endothermic peak was discovered at 1687 K in liquid Fe
78Si
9B
13, which confirms the structural transition in the melt at this temperature.
At the cooling stage at a temperature of about 1700 K, a reverse structural transition occurs with a decrease in the activation energy to 44 kJ·mol
−1, see
Figure 6. A slight decrease in the activation energy indicates that in this temperature region clusters based on Fe
23B
6 are retained in the melt. The preservation of such clusters confirms the discovery of the metastable Fe
23B
6 phase in undercooled eutectic Fe
83B
17 alloy in the solid state [
25].
In the eutectic melt Co
81.4B
18.6, LLST was also recorded with a transition to a short-range order of Co
23B
6 with a high activation energy of about 70 kJ·mol
−1 [
10]. A decrease in melt density was also detected in this region. This confirms the presence of clusters based on Co
23B
6, which have the largest molar volume. However, in the high-temperature region, the activation energy in the Co
81.4B
18.6 melt again drops to 19 kJ·mol
−1, and this drop indicates the predominance of atomic-sized clusters in the melt. In Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 alloy with aluminum content 0.02 at.%, the temperature range with high activation energy is maintained up to a maximum temperature of 1975 K. This can be explained by the greater stability of iron borides compared to cobalt borides. This follows from a comparison of the formation energy of the compounds Co
23B
6, Co
3B, Co
2B, which is −0.089; −0.133; −0.175 eV·atom
−1 [
10], with data for iron borides in
Table 2.
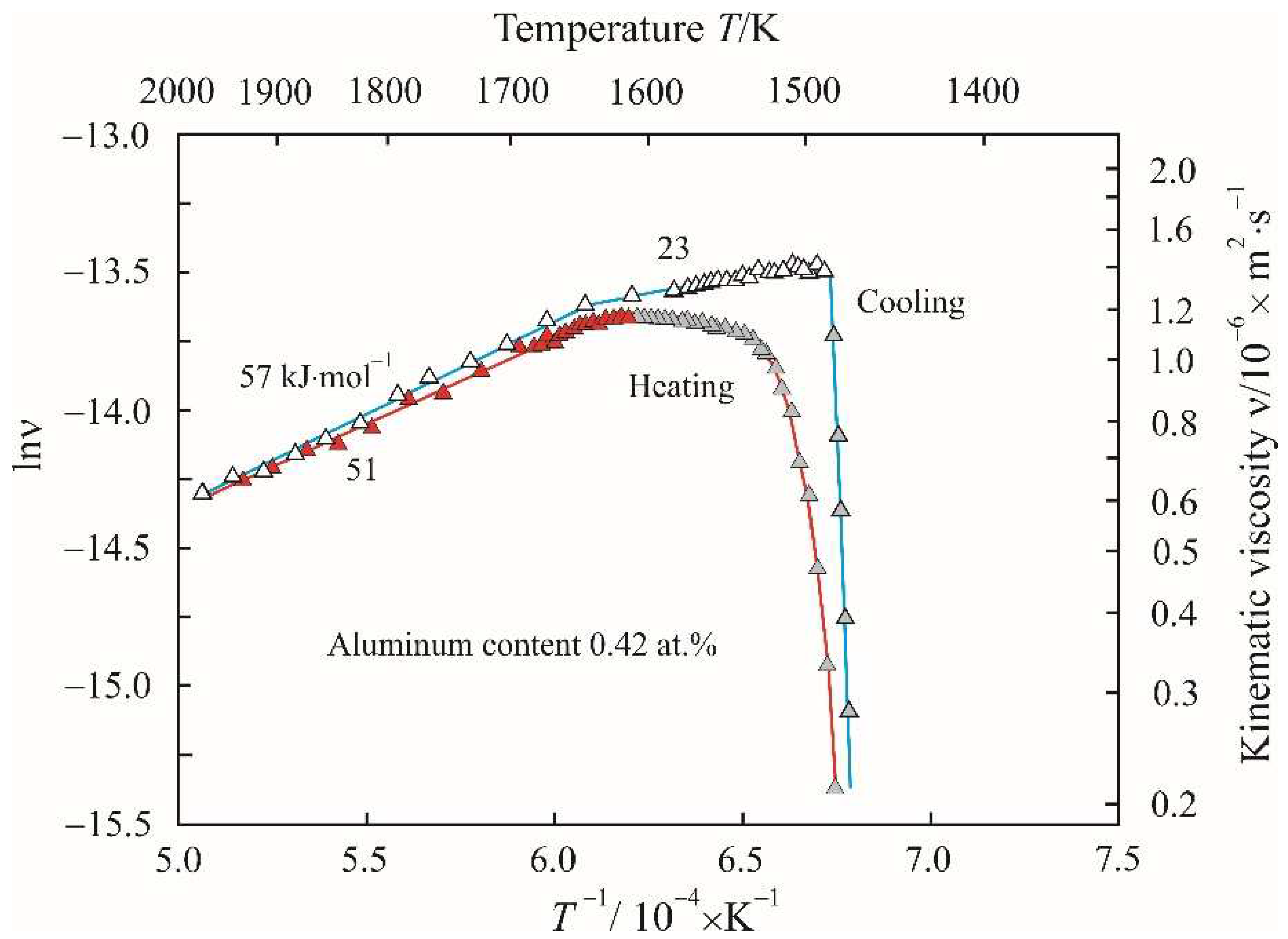
Figure 7 shows Arrhenius plots as the dependences of the natural logarithm of the kinematic viscosity lnν on the reciprocal temperature
T−1 when Fe
72.5−xAl
xCu
1Nb
2Mo
1.5Si
14B
9 alloy with aluminum content 0.42 at.% is heated to a temperature of 1975 K and cooled. The numbers next to the curves show the activation energy of the viscous flow
Ea in the corresponding linear sections of the Arrhenius plots. From a comparison of
Figure 6 and
Figure 7 it follows that the addition of aluminum almost did not change the viscosity of the melt at a maximum temperature of 1975 K, but the trajectory of the Arrhenius plots noticeably changed.
Figure 7 shows that a transition to high activation energy is observed above 1650 K during heating and cooling. The constancy of the activation energy indicates an insignificant change in the structure of the melt over a wide temperature range. The electronegativity of aluminum on the Luo–Benson scale is 2.40 and is midway between iron and boron. Therefore, aluminum atoms attract both iron atoms and boron atoms with the formation of clusters based on the stable ternary phase Fe
2AlB
2 [
33,
34] with high formation energy, see
Table 2, or higher-order metastable phases Fe
3AlB
4 and Fe
4AlB
6 [
36].